Abstract
Purpose:
In RE-MIND2 (NCT04697160), patient-level outcomes from the L-MIND study (NCT02399085) of tafasitamab plus lenalidomide were retrospectively compared with patient-level matched observational cohorts treated with National Cancer Care Network (NCCN)/European Society for Medical Oncology (ESMO)-listed systemic therapies for relapsed/refractory diffuse large B-cell lymphoma (DLBCL).
Patients and Methods:
Data were collected from health records of eligible patients aged ≥18 years with histologically confirmed DLBCL who had received ≥2 systemic therapies for DLBCL (including ≥1 anti-CD20 therapy). Patients from L-MIND were matched with patients from the RE-MIND2 observational cohort using estimated propensity score-based 1:1 nearest-neighbor matching, balanced for nine covariates. The primary analysis compared tafasitamab plus lenalidomide with patients who received any systemic therapy for R/R DLBCL (pooled in one cohort) or bendamustine plus rituximab (BR) or rituximab plus gemcitabine and oxaliplatin (R-GemOx; as two distinct cohorts). The primary endpoint was overall survival (OS). Secondary endpoints included treatment response and time-to-event outcomes.
Results:
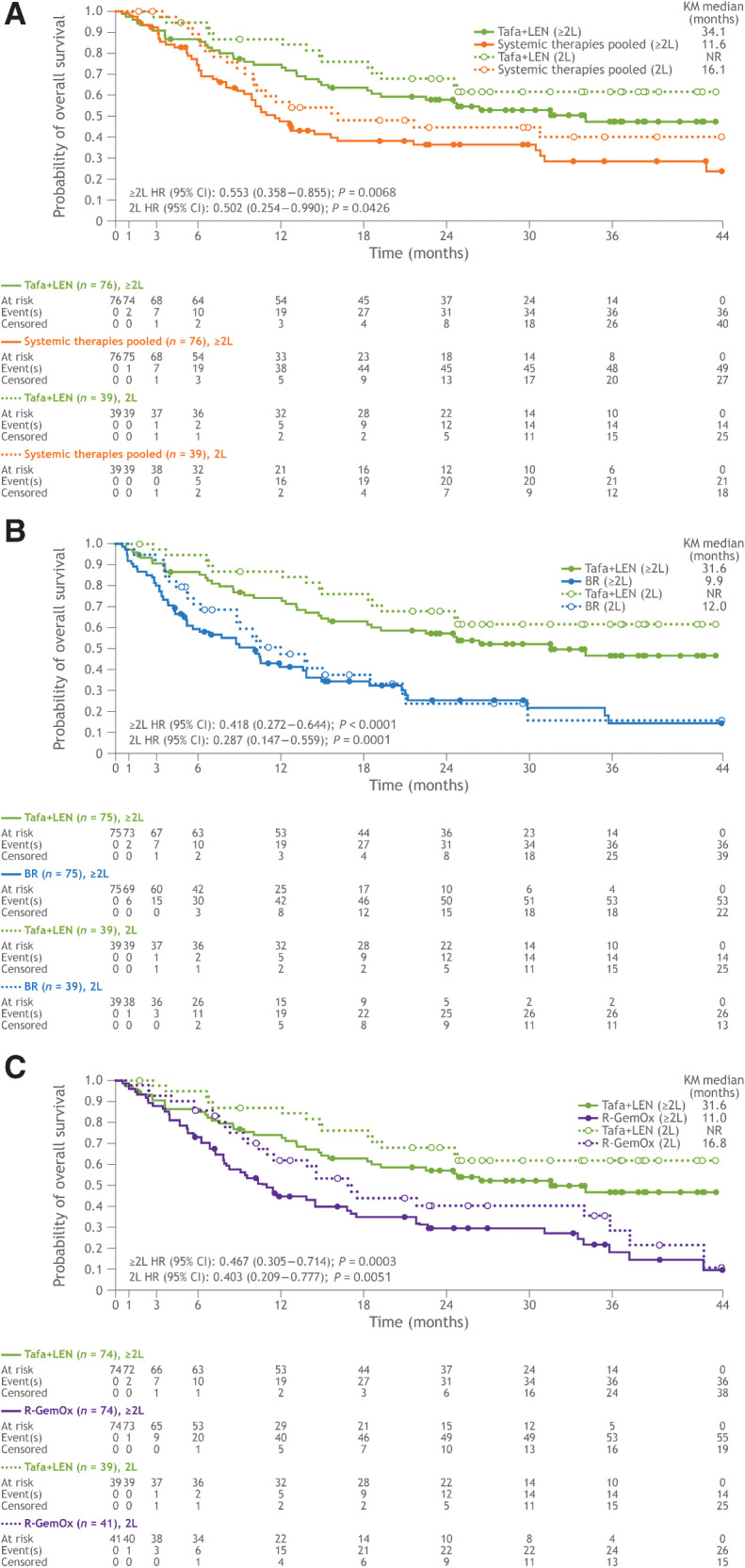
In RE-MIND2, 3,454 patients were enrolled from 200 sites in North America, Europe, and Asia-Pacific. Strictly matched pairs of patients consisted of tafasitamab plus lenalidomide versus systemic therapies pooled (n = 76 pairs), versus BR (n = 75 pairs), and versus RGemOx (n = 74 pairs). Significantly prolonged OS was reported with tafasitamab plus lenalidomide versus systemic pooled therapies [hazard ratios (HR): 0.55; P = 0.0068], BR (HR: 0.42; P < 0.0001), and R-GemOx (HR: 0.47; P = 0.0003).
Conclusions:
RE-MIND2, a retrospective observational study, met its primary endpoint, demonstrating prolonged OS with tafasitamab plus lenalidomide versus BR and R-GemOx.
Translational Relevance.
Tafasitamab plus lenalidomide was recently granted accelerated approval in the United States and conditional marketing authorization in the EU as second-line therapy for autologous stem-cell transplant (ASCT)-ineligible adult patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL). The single-arm L-MIND study (NCT02399085) established efficacy and safety; a retrospective lenalidomide monotherapy control arm (NCT04150328) determined tafasitamab's contribution. Comparisons with other NCCN/ESMO-listed therapies for R/R DLBCL will provide physicians insight that may support treatment decisions. RE-MIND2 established matched cohorts of ASCT-ineligible patients receiving any systemic therapy for R/R DLBCL (pooled in one cohort) or bendamustine plus rituximab or rituximab plus gemcitabine and oxaliplatin (as distinct cohorts) in a real-world setting, with patients receiving tafasitamab and lenalidomide in the L-MIND trial. Overall survival, plus other time-to-event outcomes, and treatment response improved with tafasitamab plus lenalidomide versus comparators. The retrospective, observational, matched cohort approach adopted in RE-MIND2 demonstrates the value of real-world data to contextualize patient outcomes for a nonrandomized study.
Introduction
As the most common non-Hodgkin lymphoma subtype, diffuse large B-cell lymphoma (DLBCL) represents 25% to 45% of new lymphoma cases (1). For over 20 years, first-line treatment comprised six cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy. Although curative in 60% to 70% of patients (2, 3), 30% to 40% experience a relapsed/refractory (R/R) disease course (4, 5).
One of the preferred treatment options for R/R DLBCL is high-dose chemotherapy and autologous stem-cell transplant (ASCT; ref. 2). Although ASCT can provide a sustained remission, 40% to 65% of patients relapse, depending on initial risks (6–8), and prolonged survival is achieved by only 20% to 30% of patients (4). Moreover, patients are often ineligible for intensive treatment due to advanced age or comorbidities (5, 9).
Treatment options for ASCT-ineligible patients include chemoimmunotherapy regimens, such as bendamustine and rituximab (BR) and rituximab plus gemcitabine and oxaliplatin (R-GemOx; ref. 2). However, the treatment landscape is expanding, with recent therapies such as polatuzumab vedotin plus BR (pola-BR) listed in National Cancer Care Network (NCCN) guidelines as second- and subsequent-line therapy for nontransplant eligible patients (2, 10). The antibody–drug conjugate loncastuximab tesirine, the single-agent drug selinexor, and chimeric antigen receptor T-cell (CAR-T) therapy are recent third- and subsequent-line options (2, 11–15).
Tafasitamab, an Fc-modified humanized anti-CD19 monoclonal antibody, combined with the immunomodulatory drug lenalidomide, is the only approved second-line therapy for adult patients who progress during or after first-line treatment for DLBCL. The FDA granted accelerated approval in July 2020 for patients with R/R DLBCL not eligible for ASCT (16), and the European Medicines Agency and Health Canada granted conditional approval in August 2021 (17, 18). The NCCN treatment guidelines also recommend this regimen as second-line therapy for patients who are not candidates for ASCT (2).
Tafasitamab plus lenalidomide is effective and well tolerated in the R/R DLBCL patient population, as demonstrated in the single-arm, phase II L-MIND study (NCT02399085; ref. 19). The objective response rate (ORR) was 58%, with a complete response (CR) in 40% of patients. Responses were durable, with a 44-month median duration of response (DoR). At a median follow-up of 43 months, the median overall survival (OS) was 34 months, indicating long-lasting benefit (20). The most frequent treatment-emergent adverse events (TEAE) were neutropenia (51%) and anemia (37%; ref. 20). After 12 cycles of combination therapy, the incidence of all AEs declined in the tafasitamab monotherapy phase, following lenalidomide cessation, during which no patients discontinued treatment due to TEAEs (20).
Combining real-world data (RWD) with clinical trial data helps researchers assess the comparative effectiveness of different therapies without conducting time-consuming and expensive head-to-head trials (21–23). The wider availability of electronic health records for data collection, combined with statistical methods that facilitate strict patient-level matching according to predefined baseline covariates, allows outcomes to be compared between real-world and clinical trial patient populations (21, 24–26).
This approach was used in RE-MIND (NCT04150328) to determine tafasitamab's contribution when added to lenalidomide. The retrospective, observational study generated a patient-level matched control cohort of patients treated with lenalidomide monotherapy (27). From 76 matched patient pairs, significantly improved outcomes [ORR, CR, DoR, progression-free survival (PFS) and OS] were observed for the L-MIND combination cohort versus the observational lenalidomide monotherapy cohort (28). Although the efficacy of tafasitamab plus lenalidomide versus lenalidomide monotherapy was demonstrated by RE-MIND, assessing the combination's efficacy in the context of other NCCN and/or European Society for Medical Oncology (ESMO)-listed routine therapies for R/R DLBCL will provide physicians further insight that may support treatment decisions (2, 29). Here we report the primary analysis of RE-MIND2 (NCT04697160), a retrospective, observational cohort study designed to generate a historical control of routinely administered therapies to compare with the tafasitamab plus lenalidomide combination from the L-MIND trial.
Patients and Methods
RE-MIND2 was conducted according to the International Conference on Harmonization Good Pharmacoepidemiology Practice guidelines (30) and the Declaration of Helsinki. Where applicable, written informed consent [approved by an independent ethics committee (IEC)/institutional review board (IRB)] was obtained from patients prior to data collection. A waiver of consent was obtained from the responsible IEC/IRB in accordance with local laws or regulations for the following countries: Australia, Austria, Canada, Denmark, Republic of Korea, Taiwan, and the United States. In accordance with the French declaration of conformity MR-004 for research that reuses data, a Patient Information Sheet was used to inform patients.
Data collection and patients
Data were collected for patients diagnosed with DLBCL between 2010 and 2020 from study sites (academic hospitals, public hospitals, and private practices) across Europe, North America, and the Asia-Pacific region. Sites were selected based on completeness of data and number of patients with R/R DLBCL in their health records. Data were collected using electronic data capture (Medidata RAVE electronic case report form, Cardinal Health electronic survey tool). To identify potential duplicate patient health records, a deduplication algorithm was applied to deidentify (coded) patient data, prior to statistical analysis.
Eligibility criteria were based on the patient population enrolled in the L-MIND study (19). Therefore, for the observational cohort, data were collected for patients with R/R DLBCL ages ≥18 years at the initial DLBCL diagnosis, with histologically confirmed diagnosis of DLBCL and who had received at least two systemic anti-DLBCL regimens (including at least one anti-CD20 containing therapy). Noneligibility criteria for enrolment in the observational cohort were central nervous system (CNS) involvement at initial DLBCL diagnosis, prior allogenic transplant, prior treatment with CD19-targeted therapy or immunomodulatory drugs (e.g., thalidomide, lenalidomide) as a first-line DLBCL therapy, a history of malignancies other than DLBCL (unless disease free ≥5 years prior to inclusion) patients who previously received tafasitamab as any line of therapy, and human immunodeficiency virus-positive status (sites in Taiwan only).
Study design
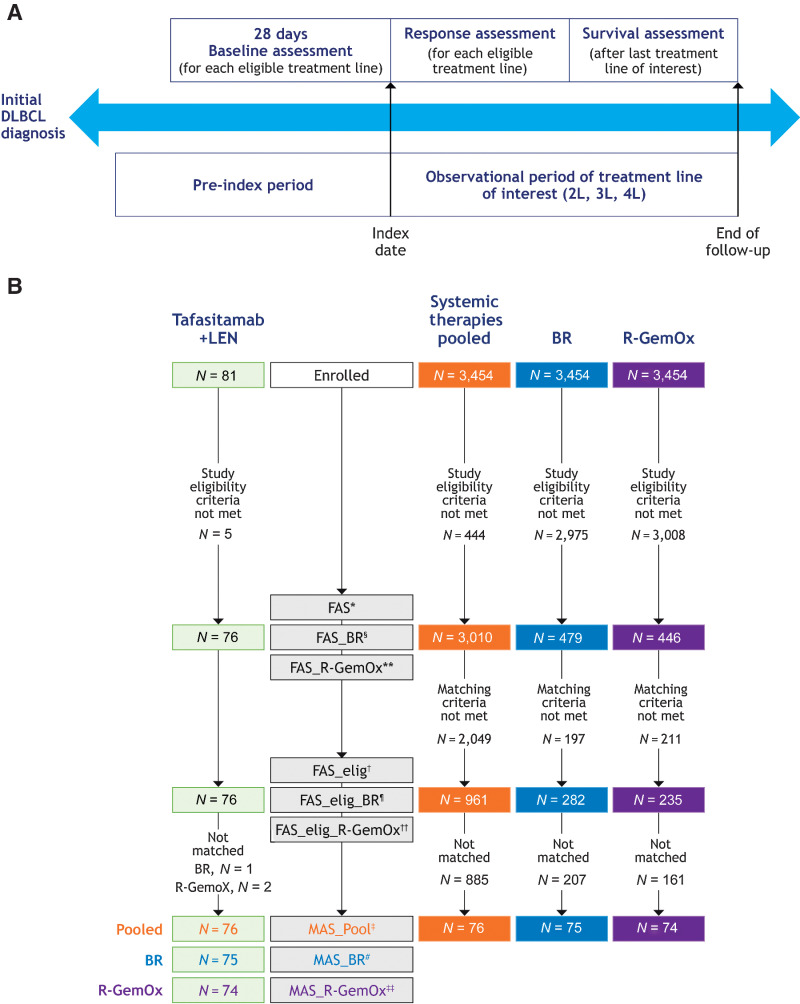
Following the assessment of eligibility criteria, the data collected comprised date and histologic subtype of initial DLBCL diagnosis, demographics, information for baseline covariates (see following), history of cancers other than DLBCL, DLBCL therapies administered and their efficacy outcomes, treatment details (i.e., start date, stop date or discontinuation and reason; e.g., AE), reasons for ASCT ineligibility, response assessment criteria used [e.g., Cheson 1999 (31), 2007 (32), 2014 (33)], Eastern Cooperative Oncology Group (ECOG) performance status (when available), patient survival information, bone marrow involvement, and tumor biopsy information. Following data collection, efficacy outcomes with tafasitamab plus lenalidomide from L-MIND were compared with outcomes from an observational cohort, comprising matched patient populations who received treatment for R/R DLBCL in a real-world setting (29, 34). In the observational cohort, an index date was assigned for each patient per therapy line, based on the first record of a systemically administered therapy for R/R DLBCL (start of second-, third-, or fourth-line treatment). For patients in L-MIND, the index date was the date of the first dose of either tafasitamab or lenalidomide. The duration of survival follow-up was defined as the interval between the index date and data cutoff date (or equivalent duration for the observational cohort; Fig. 1A). Tafasitamab plus lenalidomide administration was followed by tafasitamab monotherapy until disease progression in L-MIND (19), whereas comparator therapies in the observational cohort were administered as per their prescribing information. Patient data from L-MIND were included with a cutoff of 2 years after the last patient enrolled in the trial (November 2019; Fig. 1A).
Figure 1.
RE-MIND2 study design. A, Assessment periods. Patients who received at least two therapy lines for DLBCL were assigned an index date (index date 2L, 3L, or 4L) for each eligible therapy line. Pre-index period, time between initial DLBCL diagnosis and index date of treatments (2L, 3L, or 4L). Index date, start of R/R DLBCL treatment (2L, 3L, or 4L). Observational period, time between index date and end of follow-up including survival assessment. Baseline, 28 days of baseline assessment prior to index date. B, Patient flow and disposition of enrolled patients into the MAS for tafasitamab plus lenalidomide versus systemic therapies pooled, BR, and R-GemOx. *, FAS included patients who met the eligibility/noneligibility criteria of RE-MIND2 and patients from the L-MIND study who received at least one dose of tafasitamab and one dose of LEN; all patients had a minimum of 6 months' follow-up. †, FAS_elig included a subset of patients from FAS who were eligible for matching. ‡, MAS_Pool included 1:1 matched patients from the L-MIND study and the observational cohort using baseline covariates. §, FAS_BR included patients who met the eligibility/noneligibility criteria of RE-MIND2 and received BR, and patients from the L-MIND study who received at least one dose of tafasitamab and one dose of LEN; all patients had a minimum of 6 months' follow-up. ¶, FAS_elig_BR included a subset of patients from FAS_BR who were eligible for matching. #, MAS_BR included 1:1 matched patients from the L-MIND study and those who received BR. **, FAS_R-GemOx included patients who met the eligibility/noneligibility criteria of RE-MIND2 and received R-GemOx, and patients from the L-MIND study who received at least one dose of tafasitamab and one dose of LEN; all patients had a minimum of 6 months' follow-up. ††, FAS_elig_R-GemOx included a subset of patients from FAS_R-GemOx who were eligible for matching. ‡‡, MAS_R-GemOx included 1:1 matched patients from the L-MIND study and those who received R-GemOx. BR, bendamustine + rituximab; DLBCL, diffuse large B-cell lymphoma; elig, eligible; ePS, estimated propensity score; FAS, full analysis set; L, therapy line; LEN, lenalidomide; MAS, matched analysis set; R-GemOx, rituximab + gemcitabine + oxaliplatin; R/R, relapsed/refractory.
Cohort balancing
Cohorts were balanced by using nine clinically relevant prognostic outcome and laboratory parameters as baseline characteristics (covariates): age (<70 vs. ≥70; refs. 5, 10, 11), Ann Arbor stage (I/II versus III/IV; refs. 5, 10, 11), refractory to last therapy line (Yes vs. No; ref. 5, 11; see Supplementary Methods for definition), number of prior lines of therapy (1 vs. 2/3; ref. 10), history of primary refractoriness (Yes vs. No; 5, 11; see Supplementary Methods for definition), prior ASCT (Yes vs. No; ref. 10), elevated lactate dehydrogenase [LDH; LDH >upper limit of normal (ULN) versus LDH ≤ ULN; ref. 35], neutropenia (absolute neutrophil count <1.5×109/L versus ≥1.5×109/L; ref. 36), and anemia (hemoglobin <10 g/dL versus ≥10 g/dL; ref. 36).
The resulting primary analysis sets included patients who met the eligibility criteria, were ASCT ineligible for the given therapy line, were not double-hit/triple-hit patients, had no CNS involvement in the prior therapy line, had complete data on all nine covariates, and had ≥ 6 months’ follow-up, and baseline tumor assessment. A minimum follow-up of 6 months for the comparative analysis was applied to prevent overestimating the rate of nonresponders in the observational cohort; this avoided bias in favor of the L-MIND (tafasitamab plus lenalidomide) cohort, in which responses to treatment may have been more adequately captured in a clinical trial setting. Propensity scores were estimated for patients with complete information on all baseline covariates, using a logistic regression model (26). The resulting estimated propensity score (ePS) indicated the probability of a patient being assigned to the tafasitamab plus lenalidomide cohort.
Using nearest neighbor (NN) 1:1 matching, each L-MIND patient was randomly selected for matching with one patient from the observational cohort according to ePS. To assess the balance achieved between the L-MIND and observational cohorts, the absolute standardized difference was used to estimate the difference in covariate proportions, using units of the pooled standard deviation (26). To achieve a high quality of covariate balance, a matched analysis set was attained when an absolute standardized difference of <0.2 was achieved for each covariate.
Comparator therapies
A cohort was created from pooled patients who received any systemic therapy for R/R DLBCL (the systemic therapies pooled cohort; Supplementary Table S1). Additionally, cohorts were generated for prespecified NCCN/ESMO-listed treatments for R/R DLBCL, contingent on data availability for the nine baseline covariates. These prespecified therapies of interest were BR (listed in NCCN guidelines only), R-GemOx, rituximab plus lenalidomide (R2), pola-BR, and CAR-T therapies.
Sample size
No minimum sample size was set prior to data collection. However, in general, including approximately 2,800 patients who received any systemic therapy for R/R DLBCL was expected to be necessary. This would sufficiently balance treatment arms for each covariate to enable a valid comparison of efficacy endpoints.
Endpoints
The primary endpoint was OS, defined as time (in months) from the index date (start of a given therapy) until death due to any cause. Secondary endpoints were ORR, CR rate, DoR, event-free survival (EFS), PFS, time to next treatment (TTNT), and treatment discontinuation due to AEs (in the tafasitamab plus lenalidomide cohort, this was discontinuation of combination therapy). See Supplementary Methods for definitions of time-to-event secondary endpoints. Response assessments in the observational cohort followed the 1999, 2007, and 2014 International Working Group (IWG) response criteria (31–33), and in the tafasitamab plus lenalidomide cohort, the 2007 IWG response criteria were applied (32).
Statistical analyses
The time-to-event endpoints of OS (primary endpoint), PFS, EFS, DoR, and TTNT were analyzed using the standard Kaplan–Meier methodology. Hazard ratios (HR) with 95% confidence intervals (CI) were estimated using a Cox proportional hazard model; P values were reported using the log-rank test. ORR and CR rate were compared between matched cohorts using Fisher exact tests with P values reported. Exploratory subgroup analyses were performed for OS and PFS by age (<70 vs. ≥70 years), primary refractoriness (Yes vs. No), number of prior therapy lines (1 vs. ≥ 2), prior ASCT (Yes vs. No), and refractoriness to last therapy line (Yes vs. No). See Supplementary Methods for a description of the analysis window.
Sensitivity analysis
A sensitivity analysis was conducted to demonstrate the robustness of the comparisons, using 11 baseline covariates to balance comparator cohorts. The covariate “history of primary refractoriness,” which used a balancing covariate in the main analysis, was omitted and the following three covariates were included: ECOG performance status (0 to 1 vs. ≥ 2; ref. 37), history of primary progressive disease (Yes vs. No), and history of early relapse (Yes vs. No; ref. 35). The history of primary refractoriness covariate was replaced to facilitate a stricter definition (vs. the L-MIND definition) of R/R disease history. Primary progressive disease was defined as patients having no complete or partial tumor response. Early relapse was defined as relapse within 6 months of completing first-line therapy. A sensitivity analysis of patients with a minimum of 18 months survival follow-up was performed to address the potential imbalance in the follow-up period for OS analysis. An additional sensitivity analysis of OS by applying multiple imputation was conducted to alleviate the potential problems of bias from systematically missing data.
Data availability
Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors upon reasonable request.
Results
Patient disposition
Data from 200 individual sites were collected. Data from 2,688 patients across 158 sites (23 in North America, 118 in Europe, and 17 in Asia-Pacific) were captured using Medidata RAVE electronic case reports. Data from 766 patients from an additional 36 US sites were collected by the healthcare company Cardinal Health using an electronic survey tool. Supplementary Table S2 presents descriptive statistics of patient volume from study sites. In total, 3,454 patients who met the eligibility criteria and had a valid index date in the analysis window were enrolled in the observational cohort.
In addition to the cohort of systemic therapies pooled, two comparator cohorts were created for the prespecified treatments BR and R-GemOx based on data availability for the nine baseline covariates. Comparisons with other prespecified treatments (pola-BR, R2, and CAR-T therapies) were not performed as a part of the primary analysis, as the number of patients eligible for matching was too low, as per the study criteria. However, these comparisons will be presented in a subsequent paper utilizing an expanded methodology.
When applying the eligibility and matching criteria (as described in the “Cohort balancing” section), 961, 282, and 235 patients treated with systemic therapies pooled, BR, and R-GemOx, respectively, were eligible for matching (Fig. 1B). From L-MIND, 76 of 81 patients were eligible for matching. From these populations, matching was performed between the L-MIND (tafasitamab plus lenalidomide) and the above-specified comparator cohorts, resulting in 76, 75, and 74 matched pairs in the systemic therapies pooled, BR, and R-GemOx cohorts, respectively (Fig. 1B). A high degree of covariate balance was achieved for the matched analysis set for systemic therapies pooled, with an absolute standardized difference between 0 and 0.08 and between 0 and 0.19 for the matched analysis set for BR and R-GemOx (Table 1). The index date timeframe for the tafasitamab plus lenalidomide cohort was from March 2016 to November 2017, from April 2010 to November 2020 for the systemic therapies pooled cohort, from May 2010 to September 2020 for the BR cohort, and from October 2010 to September 2020 for R-GemOx cohort. Demographic and baseline characteristics for the matched analysis sets are presented in Table 1.
Table 1.
Demographics and baseline characteristics for the matched analysis sets for systemic therapies pooled, BR, and R-GemOx with the absolute standardized difference for covariates used for 1:1 NN matching.
| MAS for systemic therapies pooled | MAS for BR | MAS for R-GemOx | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tafasitamab + lenalidomide | Systemic therapies pooled | Absolute standardized difference | Tafasitamab + lenalidomide | BR | Absolute standardized difference | Tafasitamab + lenalidomide | R-GemOx | Absolute standardized difference | ||
| (n = 76) | (n = 76) | (n = 75) | (n = 75) | (n = 74) | (n = 74) | |||||
| Cohort balancing characteristics | ||||||||||
| Age, n (%) | Age < 70 years | 33 (43.4) | 31 (40.8) | 0.05 | 33 (44.0) | 33 (44.0) | 0.00 | 31 (41.9) | 26 (35.1) | 0.14 |
| Age ≥ 70 years | 43 (56.6) | 45 (59.2) | 42 (56.0) | 42 (56.0) | 43 (58.1) | 48 (64.9) | ||||
| Ann Arbor stage, n (%) | I+II | 19 (25.0) | 19 (25.0) | 0.00 | 18 (24.0) | 19 (25.3) | 0.03 | 18 (24.3) | 15 (20.3) | 0.10 |
| III+IV | 57 (75.0) | 57 (75.0) | 57 (76.0) | 56 (74.7) | 56 (75.7) | 59 (79.7) | ||||
| Refractoriness to last prior therapy, n (%) | Yes | 34 (44.7) | 35 (46.1) | 0.03 | 33 (44.0) | 32 (42.7) | 0.03 | 33 (44.6) | 29 (39.2) | 0.11 |
| No | 42 (55.3) | 41 (53.9) | 42 (56.0) | 43 (57.3) | 41 (55.4) | 45 (60.8) | ||||
| Number of prior systemic treatment lines, n (%) | 1 | 39 (51.3) | 39 (51.3) | 0.00 | 39 (52.0) | 39 (52.0) | 0.00 | 39 (52.7) | 41 (55.4) | 0.05 |
| 2/3 | 37 (48.7) | 37 (48.7) | 36 (48.0) | 36 (48.0) | 35 (47.3) | 33 (44.6) | ||||
| History of primary refractoriness, n (%) | Yes | 14 (18.4) | 12 (15.8) | 0.07 | 14 (18.7) | 19 (25.3) | 0.16 | 14 (18.9) | 14 (18.9) | 0.00 |
| No | 62 (81.6) | 64 (84.2) | 61 (81.3) | 56 (74.7) | 60 (81.1) | 60 (81.1) | ||||
| Prior ASCT, n (%) | Yes | 9 (11.8) | 10 (13.2) | 0.04 | 9 (12.0) | 14 (18.7) | 0.19 | 8 (10.8) | 8 (10.8) | 0.00 |
| No | 67 (88.2) | 66 (86.8) | 66 (88.0) | 61 (81.3) | 66 (89.2) | 66 (89.2) | ||||
| Elevated LDH (> ULN), n (%) | LDH > ULN | 41 (53.9) | 44 (57.9) | 0.08 | 41 (54.7) | 37 (49.3) | 0.11 | 41 (55.4) | 48 (64.9) | 0.19 |
| LDH < ULN | 35 (46.1) | 32 (42.1) | 34 (45.3) | 38 (50.7) | 33 (44.6) | 26 (35.1) | ||||
| Neutropenia (cutoff < 1.5×109/L), n (%) | ANC < 1.5×109/L | 2 (2.6) | 2 (2.6) | 0.00 | 2 (2.7) | 4 (5.3) | 0.14 | 2 (2.7) | 5 (6.8) | 0.19 |
| ANC ≥ 1.5×109/L | 74 (97.4) | 74 (97.4) | 73 (97.3) | 71 (94.7) | 72 (97.3) | 69 (93.2) | ||||
| Anemia (cutoff hemoglobin < 10 g/dL), n (%) | Hb < 10 g/dL | 6 (7.9) | 5 (6.6) | 0.05 | 6 (8.0) | 5 (6.7) | 0.05 | 6 (8.1) | 5 (6.8) | 0.05 |
| Hb > 10 g/dL | 70 (92.1) | 71 (93.4) | 69 (92.0) | 70 (93.3) | 68 (91.9) | 69 (93.2) | ||||
| Other characteristics | ||||||||||
| Sex, n (%) | Female | 36 (47.4) | 32 (42.1) | 36 (48.0) | 30 (40.0) | 35 (47.3) | 38 (51.4) | |||
| Male | 40 (52.6) | 44 (57.9) | 39 (52.0) | 45 (60.0) | 39 (52.7) | 36 (48.6) | ||||
| Age at index date, years | Mean (SD) | 69.1 (9.71) | 68.7 (11.88) | 69.0 (9.75) | 69.3 (9.23) | 69.5 (9.59) | 71.0 (9.64) | |||
| Median (Q1–Q3) | 71.5 (62.0–76.0) | 72.0 (60.0–77.0) | 71.0 (62.0–76.0) | 71.0 (62.0–76.0) | 72.0 (62.0–76.0) | 73.5 (66.0–77.0) | ||||
| Range (min–max) | 41–86 | 37–87 | 41–86 | 48–86 | 41–86 | 40–87 | ||||
| ECOG PS, n (%) | 0 | 29 (38.2) | 17 (22.4) | 29 (38.7) | 11 (14.7) | 28 (37.8) | 15 (20.3) | |||
| 1 | 41 (53.9) | 27 (35.5) | 40 (53.3) | 29 (38.7) | 40 (54.1) | 22 (29.7) | ||||
| 2 | 6 (7.9) | 18 (23.7) | 6 (8.0) | 20 (26.7) | 6 (8.1) | 22 (29.7) | ||||
| 3 | 0 | 3 (3.9) | 0 | 3 (4.0) | 0 | 5 (6.8) | ||||
| 4 | 0 | 0 | 0 | 0.00 | 0 | 1 (1.4) | ||||
| Race, n (%) | Black or African American | 0 | 6 (7.9) | 0 | 12 (16.0) | 0 | 10 (13.5) | |||
| American Indian | 0 | 1 (1.3) | 0 | 0.00 | 0 | 1 (1.4) | ||||
| Asian | 0 | 9 (11.8) | 0 | 13 (17.3) | 0 | 2 (2.7) | ||||
| Native Hawaiian or other Pacific Islander | 0 | 0 | 0 | 0 | 0 | 1 (1.4) | ||||
| White | 70 (92.1) | 49 (64.5) | 69 (92.0) | 36 (48.0) | 68 (91.9) | 49 (66.2) | ||||
| Unknown | 0.00 | 4 (5.3) | 0.00 | 11 (14.7) | 0.00 | 3 (4.1) | ||||
| Other | 1 (1.3) | 7 (9.2) | 1 (1.3) | 3 (4.0) | 1 (1.4) | 8 (10.8) | ||||
| Missing | 5 (6.6) | 0 | 5 (6.7) | 0 | 5 (6.8) | 0.00 | ||||
| Primary progressive disease, n (%) | Yes | 2 (2.6) | 5 (6.6) | 2 (2.7) | 8 (10.7) | 2 (2.7) | 6 (8.1) | |||
| No | 74 (97.4) | 71 (93.4) | 73 (97.3) | 67 (89.3) | 72 (97.3) | 68 (91.9) | ||||
| Early relapse, n (%) | Yes | 12 (15.8) | 7 (9.2) | 12 (16.0) | 11 (14.7) | 12 (16.2) | 8 (10.8) | |||
| No | 64 (84.2) | 69 (90.8) | 63 (84.0) | 64 (85.3) | 62 (83.8) | 66 (89.2) | ||||
| Imaging modalities used for best tumor response assessment | Patients with best tumor response assessment performeda | 70 (92.1) | 71 (93.4) | 69 (92.0) | 68 (90.7) | 68 (91.9) | 72 (97.3) | |||
| No radiologic assessment doneb | 0 | 1 (1.4) | 0 | 0 | 0 | 0 | ||||
| PET/CT b | 14 (20.0) | 36 (50.7) | 14 (20.3) | 45 (66.2) | 14 (20.6) | 39 (54.2) | ||||
| PET/MRIb | 0 | 1 (1.4) | 0 | 0 | 0 | 0 | ||||
| PET onlyb | 0 | 1 (1.4) | 0 | 0 | 0 | 1 (1.4) | ||||
| MRIb | 2 (2.9) | 2 (2.8) | 2 (2.9) | 0 | 2 (2.9) | 1 (1.4) | ||||
| CTb | 54 (77.1) | 24 (33.8) | 53 (76.8) | 15 (22.1) | 52 (76.5) | 28 (38.9) | ||||
| Unknownb | 0 | 1 (1.4) | 0 | 1 (1.5) | 0 | 0 | ||||
| Otherb | 0 | 5 (7.0) | 0 | 7 (10.3) | 0 | 3 (4.2) | ||||
Abbreviations: ANC, absolute neutrophil count; ASCT, autologous stem-cell transplant; BR, bendamustine + rituximab; CT, computed tomography; ECOG PS, Eastern Cooperative Oncology Group performance status; Hb, hemoglobin; LDH, lactate dehydrogenase; MAS, matched analysis set; N, total number of patients in that cohort; MRI, magnetic resonance imaging; n, number of patients with nonmissing values for that variable; PET, positron emission tomography; Q1, lower quartile; Q3, upper quartile; R-GemOx, rituximab + gemcitabine + oxaliplatin; SD, standard deviation; ULN, upper limit of normal.
aPercentages were calculated based on the number of patients in each cohort with best response.
bPercentages were calculated based on the number of patients with assessments in each cohort with best response.
Outcomes: Primary endpoint
The median duration of follow-up for OS in the systemic therapies pooled, BR, and R-GemOx cohorts was 33.3, 25.0, and 33.2 months, respectively. In the matched analysis sets for systemic therapies pooled, BR, and R-GemOx, median duration of follow-up for OS in the tafasitamab plus lenalidomide cohort was 31.8, 32.9, and 32.9 months, respectively. The study met its primary endpoint. A significant difference in OS was observed with the tafasitamab plus lenalidomide combination versus systemic therapies pooled (HR: 0.553; 95% CI, 0.358–0.855; log-rank test, P = 0.0068), BR (HR: 0.418; 95% CI, 0.272–0.644; log-rank test, P < 0.0001), and R-GemOx (HR: 0.467; 95% CI, 0.305–0.714; log-rank test, P = 0.0003). Median OS in the tafasitamab plus lenalidomide cohorts was 34.1, 31.6, and 31.6 months, compared with 11.6, 9.9, and 11.0 months in the systemic therapies pooled, BR, and R-GemOx cohorts, respectively (Table 2; Fig. 2). In the comparative observational cohort, median OS for patients receiving second-line therapy with systemic therapies pooled, BR, and R-GemOx was 16.1, 12.0, and 16.8 months, respectively. Whereas in the matched tafasitamab plus lenalidomide cohorts, median OS for second-line patients was not reached, signifying an >50% OS rate by the end of the follow-up period (Kaplan–Meier method estimate). Consequently, a significant improvement in OS was observed with tafasitamab plus lenalidomide as second-line therapy compared with systemic therapies pooled, BR, and R-GemOx (Table 2; Fig. 2).
Table 2.
Comparative analysis results for primary and secondary endpoints for tafasitamab plus lenalidomide compared with systemic therapies pooled, BR, and R-GemOx.
| MAS for systemic therapies pooled | MAS for BR | MAS for R-GemOx | ||||
|---|---|---|---|---|---|---|
| Tafasitamab + lenalidomide | Systemic therapies pooled | Tafasitamab + lenalidomide | BR | Tafasitamab + lenalidomide | R-GemOx | |
| (n = 76) | (n = 76) | (n = 75) | (n = 75) | (n = 74) | (n = 74) | |
| OS, median (mo) (95% CI) | 34.1 (18.3–NR) | 11.6 (8.8–16.1) | 31.6 (18.3–NR) | 9.9 (5.3–13.7) | 31.6 (18.3–NR) | 11.0 (7.9–16.8) |
| HR for OS (95% CI) | 0.553 (0.358–0.855) | 0.418 (0.272–0.644) | 0.467 (0.305–0.714) | |||
| P a | 0.0068 | <0.0001 | 0.0003 | |||
| 2L OS, median (mo) (95% CI) | NR (24.6–NR) | 16.1 (10.0–NR) | NR (24.6–NR) | 12.0 (8.8–18.5) | NR (24.6–NR) | 16.8 (11.0–35.8) |
| HR for 2L OS (95% CI) | 0.502 (0.254–0.990) | 0.287 (0.147–0.559) | 0.403 (0.209–0.777) | |||
| P a | 0.0426 | 0.0001 | 0.0051 | |||
| TTNT, median (mo) (95% CI) | 12.5 (7.6–24.7) | 6.3 (3.3–8.3) | 12.1 (7.3–24.7) | 6.9 (4.2–10.6) | 12.5 (7.6–28.0) | 5.7 (4.0–7.2) |
| HR for TTNT (95% CI) | 0.461 (0.314–0.676) | 0.527 (0.357–0.780) | 0.423 (0.289–0.619) | |||
| P a | <0.0001 | 0.0011 | <0.0001 | |||
| ORR, n (%) (95% CI) | 51 (67.1) | 37 (48.7) | 50 (66.7) | 41 (54.7) | 51 (68.9) | 34 (45.9) |
| (55.4–77.5) | (37.0–60.4) | (54.8–77.1) | (42.7–66.2) | (57.1–79.2) | (34.3–57.9) | |
| P b | 0.032 | 0.181 | 0.008 | |||
| Difference of ORR (%) (95% CI) | 18.42 (1.905–34.204) | 12.00 (−4.657 to 28.173) | 22.91 (6.285–38.722) | |||
| P c | 0.0323 | 0.1810 | 0.0076 | |||
| CR rate d , n (%) (95% CI) | 29 (38.2) | 16 (21.1) | 29 (38.7) | 21 (28.0) | 29 (39.2) | 17 (23.0) |
| (27.2–50.0) | (12.5–31.9) | (27.6–50.6) | (18.2–39.6) | (28.0–51.2) | (14.0–34.2) | |
| P b | 0.032 | 0.225 | 0.050 | |||
| Difference of CR rated (%) (95% CI) | 17.11 (0.579–32.952) | 10.67 (−5.987 to 26.891) | 16.22 (−0.548 to 32.318) | |||
| P c | 0.0324 | 0.2252 | 0.050 | |||
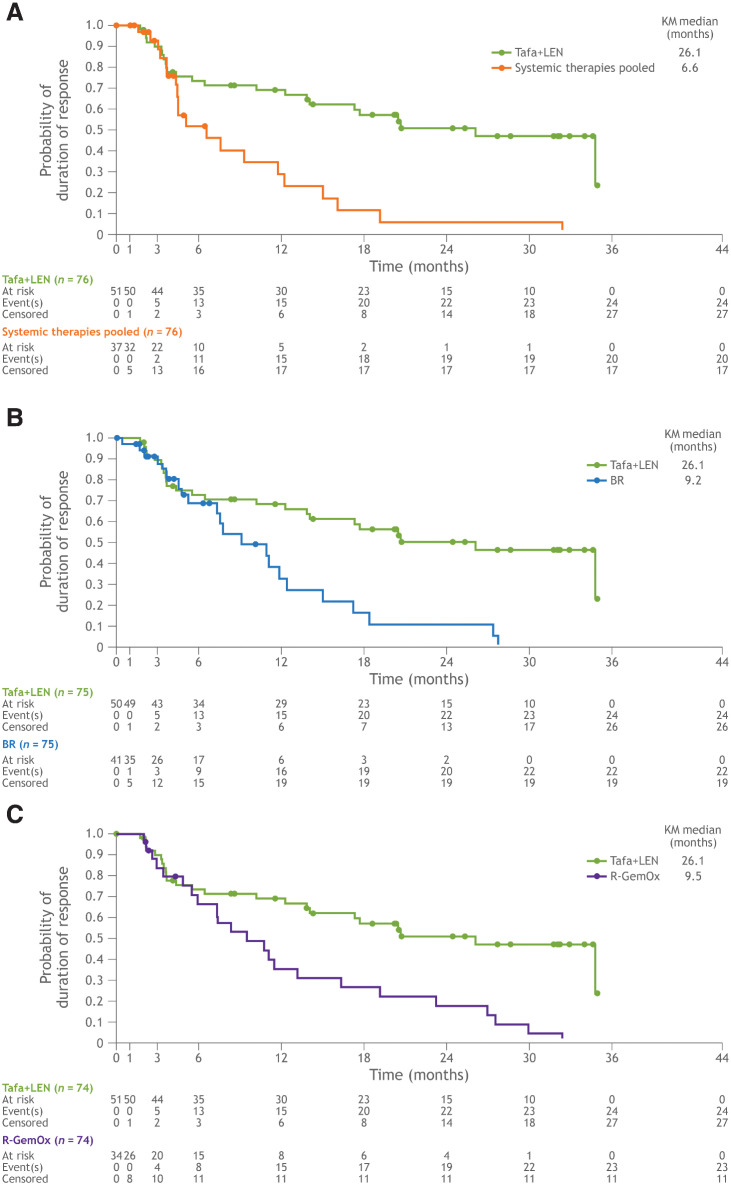
| DoR, median (mo) (95% CI)) | 26.1 (13.9–NR) | 6.6 (4.4–11.8) | 26.1 (13.9–NR) | 9.2 (5.3–12.5) | 26.1 (13.9–NR) | 9.5 (5.5–13.2) |
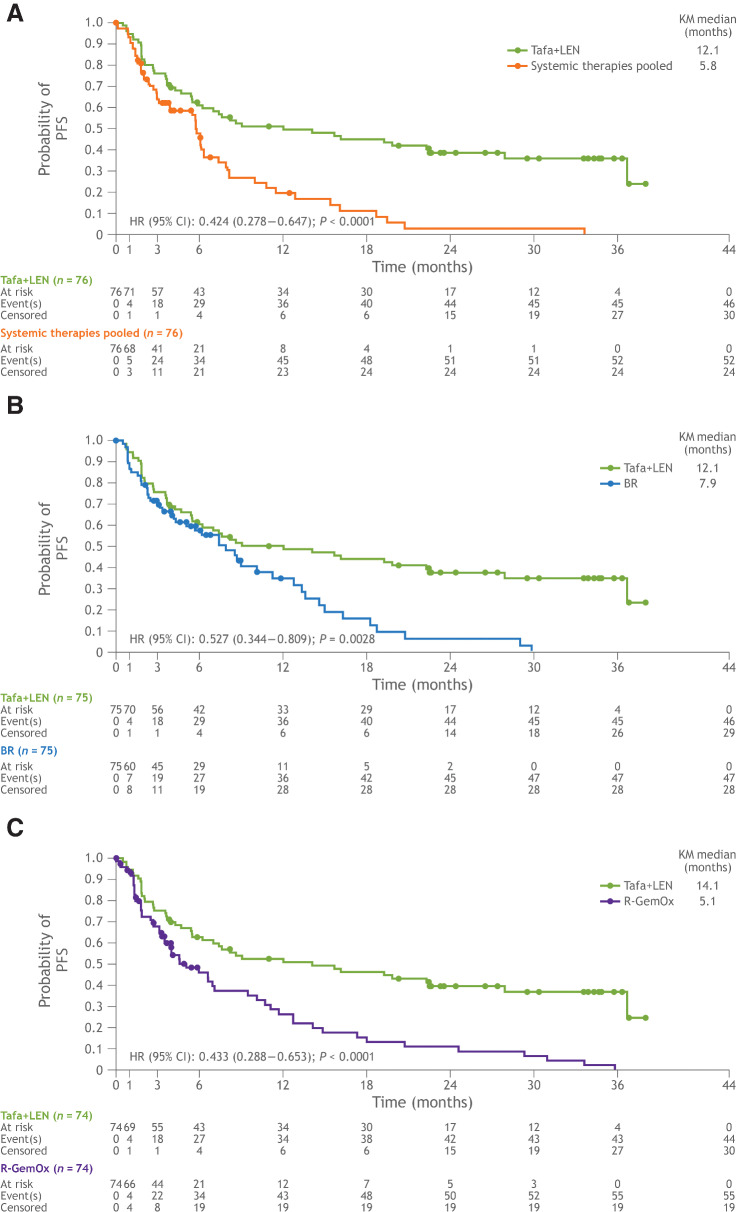
| PFS, median (mo) (95% CI) | 12.1 (5.9–22.5) | 5.8 (3.1–6.4) | 12.1 (5.5–22.5) | 7.9 (4.3–11.3) | 14.1 (6.3–28.0) | 5.1 (3.5–9.5) |
| HR for PFS (95% CI) | 0.424 (0.278–0.647) | 0.527 (0.344–0.809) | 0.433 (0.288–0.653) | |||
| P a | < 0.0001 | 0.0028 | < 0.0001 | |||
| 2L PFS, median (mo) (95% CI) | 16.2 (7.0–NR) | 8.0 (5.8–11.5) | 16.2 (7.0–NR) | 8.8 (5.8–12.8) | 16.2 (7.0–NR) | 7.1 (6.0–12.8) |
| HR for 2L PFS (95% CI) | 0.452 (0.251–0.814) | 0.475 (0.260–0.868) | 0.466 (0.262–0.831) | |||
| P a | 0.0068 | 0.0134 | 0.0081 | |||
Abbreviations: 2L, second line; BR, bendamustine + rituximab; CI, confidence interval; CR, complete response; DoR, duration of response; HR, hazard ratio; MAS, matched analysis set; mo, months; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; R-GemOx, rituximab + gemcitabine + oxaliplatin; TTNT, time to next treatment.
aCalculated using the log-rank test.
bCalculated using the Fisher exact test.
cCalculated using the Chang-Zang method.
dAs best response.
Figure 2.
Kaplan–Meier plot of overall survival in patients who received one (dotted lines) and one or more (solid lines) prior lines of therapy. A, Tafasitamab plus lenalidomide versus systemic therapies pooled. B, Tafasitamab plus lenalidomide versus BR. C, Tafasitamab plus lenalidomide versus R-GemOx. BR, bendamustine + rituximab; CI, confidence interval; HR, hazard ratio; KM; Kaplan–Meier; L, line; LEN; lenalidomide; NR, not reached; R-GemOx, rituximab + gemcitabine + oxaliplatin; Tafa, tafasitamab.
Outcomes: Secondary endpoints
ORR and CR rate were significantly higher for tafasitamab plus lenalidomide compared with systemic therapies pooled and R-GemOx (Table 2). For the comparison with BR, ORR and CR rate for tafasitamab plus lenalidomide were more than 10% higher but did not reach statistical significance with the given sample size of 75 patient pairs (Table 2). A meaningful improvement in DoR was observed with tafasitamab plus lenalidomide versus the comparator therapies (Table 2; Fig 3). In all three matched comparisons, median DoR in the tafasitamab plus lenalidomide cohorts was 26.1 months; the median DoR observed in the systemic therapies pooled, BR and R-GemOx cohorts was 6.6, 9.2, and 9.5 months, respectively (Table 2; Fig 3).
Figure 3.
Kaplan–Meier plot of duration of response. A, Tafasitamab plus lenalidomide versus systemic therapies pooled. B, Tafasitamab plus lenalidomide versus BR. C, Tafasitamab plus lenalidomide versus R-GemOx. BR, bendamustine + rituximab; KM; Kaplan–Meier; LEN; lenalidomide; R-GemOx, rituximab + gemcitabine + oxaliplatin; Tafa, tafasitamab.
A significant improvement in PFS was observed with the tafasitamab plus lenalidomide combination in all three matched comparisons (Table 2; Fig 4). Median PFS was 12.1 months in both the tafasitamab plus lenalidomide cohorts matched to the systemic therapies pooled and BR cohorts and was 14.1 months in the R-GemOx matched cohort. Median PFS in the systemic therapies pooled, BR, and R-GemOx cohorts was 5.8, 7.9, and 5.1 months, respectively (Table 2; Fig 4). For patients receiving tafasitamab plus lenalidomide as second-line treatment, median PFS was 16.2 months in each matched cohort. Median PFS for patients receiving second-line treatment in the systemic therapies pooled, BR, and R-GemOx cohorts was 8.0, 8.8, and 7.1 months, respectively (Table 2; Fig 4). Accordingly, a significant improvement in PFS was observed with tafasitamab plus lenalidomide compared with systemic therapies pooled, BR, and R-GemOx (Table 2; Fig 4).
Figure 4.
Kaplan–Meier plot of progression-free survival. A, Tafasitamab plus lenalidomide versus systemic therapies pooled. B, Tafasitamab plus lenalidomide versus BR. C, Tafasitamab plus lenalidomide versus R-GemOx. BR, bendamustine + rituximab; CI, confidence interval; HR, hazard ratio; KM; Kaplan–Meier; LEN; lenalidomide; R-GemOx, rituximab + gemcitabine + oxaliplatin; Tafa, tafasitamab.
A significant improvement in TTNT was observed with the tafasitamab plus lenalidomide combination in the three matched comparisons. Median TTNT in the three matched tafasitamab plus lenalidomide cohorts was 12.5, 12.1, and 12.5 months, versus 6.3, 6.9, and 5.7 months in the cohorts of systemic therapies pooled, BR, and R-GemOx, respectively (Table 2). EFS was significantly higher in the tafasitamab plus lenalidomide cohort versus the cohorts of systemic therapies pooled, BR, and R-GemOx (Supplementary Table S3).
Eight patients discontinued combination therapy due to AEs in the tafasitamab plus lenalidomide cohort. On account of different denominators in the matched analysis sets, this represented 14.5%, 14.5%, and 15.1% of patients in the cohorts matched with systemic therapies pooled, BR, and R-GemOx, respectively. Five patients (6.8%) in the systemic therapies pooled cohort, two (2.8%) in BR, and four (5.4%) in the R-GemOx cohort had AEs leading to treatment discontinuation. Thirty-six patients (48%) died in the tafasitamab plus lenalidomide cohort; for systemic therapies pooled, BR, and R-GemOx, 49 (64.5%), 53 (70.7%), and 55 (74.3%) died, respectively. The most common reason for death across all cohorts was disease progression.
Sensitivity analyses
Results from the sensitivity analysis, balanced for 11 baseline covariates, supported the primary analysis of OS in comparisons of tafasitamab plus lenalidomide with systemic therapies pooled and R-GemOx. An improvement in OS with tafasitamab plus lenalidomide compared with BR was observed, although it was less pronounced than in the primary analysis. Overall, the sensitivity analyses of the secondary endpoints using the 11 covariates favored tafasitamab plus lenalidomide versus the systemic therapies pooled, BR, and R-GemOx cohorts (Supplementary Table S4). The sensitivity analysis of OS in patients with a minimum of 18 months’ follow-up favored tafasitamab plus lenalidomide compared with systemic therapies pooled, BR, and R-GemOx (P < 0.0005 for each comparison; Supplementary Table S5). Results from the sensitivity analysis of OS by applying multiple imputation technique (data not shown) further significantly confirmed the conclusion from the primary analysis.
Subgroup analyses
Overall, a comparative analysis of OS by the subgroups age (<70 vs. ≥70), primary refractory patients (Yes vs. No), number of prior therapies (1 vs. ≥2), prior ASCT (Yes vs. No), and refractoriness to last therapy line (Yes vs. No) significantly supported the primary analysis favoring tafasitamab plus lenalidomide in matched comparisons against systemic therapies pooled, BR, and R-GemOx for each subgroup (Supplementary Fig. S1; Supplementary Table S6A). Similarly, a comparative analysis of PFS in the same subgroups significantly favored tafasitamab plus lenalidomide in matched comparisons against the systemic therapies pooled, BR, and R-GemOx cohorts within each subgroup (Supplementary Fig. S2; Supplementary Table S6B).
Discussion
RE-MIND2 generated a real-world, synthetic control for the L-MIND phase II trial to compare the effectiveness of tafasitamab plus lenalidomide with other systemic therapies listed in NCCN/ESMO guidelines for the treatment of patients with R/R DLBCL. The primary endpoint of the analysis was met, with a significant and clinically meaningful difference in OS observed with tafasitamab plus lenalidomide treatment in a clinical trial, compared with matched observational cohorts of systemic therapies pooled, BR, and R-GemOx. Consistent and significantly improved outcomes with tafasitamab plus lenalidomide, relative to the comparator cohorts, were also reported for the secondary endpoints.
The results from our analysis are notable in the context of outcomes associated with routine therapies administered for patients with R/R DLBCL. Median OS was 10.4 months in a subgroup of 23 ASCT-ineligible patients with R/R DLBCL from a large observational study (1,039 enrolled patients) by Farooq and colleagues (38). These patients relapsed after each of their two prior lines of therapy (including BR, R-GemOx, and single-agent rituximab), and less than 20% proceeded to ASCT; their median age was 72 years (38). The OS reported in this subgroup is in line with the median OS (11.6 months) observed in the cohort of systemic therapies pooled in RE-MIND2. By comparison, a median OS of 34.1 months was observed in the tafasitamab plus lenalidomide cohort in the matched analysis set for systemic therapies pooled, highlighting the treatment's benefit on survival relative to other systemic therapies for R/R DLBCL (2, 29). Additionally, in the matched analysis sets for BR and R-GemOx, OS with tafasitamab plus lenalidomide was significantly longer (31.6 vs. 9.9 and 11.0 months, respectively).
The sensitivity analysis for the primary endpoint of OS, balancing for 11 matched covariates, supported the main analysis, suggesting a benefit of tafasitamab plus lenalidomide compared with the systemic therapies pooled and R-GemOx cohorts. The lack of a significant difference between the matched tafasitamab plus lenalidomide and BR cohorts in the sensitivity analysis might reflect the lower number of patients in this analysis (65 vs. 75 in the primary analysis). In the systemic therapies pooled and R-GemOx cohorts, patients had a similar median duration of follow-up for OS (33.3 vs. 33.2 months, respectively) relative to the tafasitamab plus lenalidomide cohort (32.9 months). By comparison, patients in the BR cohort had a median follow-up duration of 25 months. However, the median follow-up duration for OS between the tafasitamab plus lenalidomide and BR cohorts was comparable in the additional sensitivity analysis of OS in patients with a minimum of 18 months' follow-up. This analysis supported the primary analysis favoring tafasitamab plus lenalidomide versus systemic therapies pooled, BR, and R-GemOx (Supplementary Table S5).
The results for the endpoints ORR, CR rate, DoR, and PFS should be interpreted with caution and are exploratory in nature due to a variation in the criteria applied to determine treatment response and progression in routine clinical care, compared with the more uniform criteria applied in a clinical trial. Apart from these limitations, our analyses indicated notable differences in treatment response rates with the tafasitamab plus lenalidomide combination relative to systemic therapies pooled and R-GemOx (P < 0.05). The ORR (45.9%) and CR rate (23%) for R-GemOx in RE-MIND2 were similar to that reported in the literature (ORR, 38%–78%; CR rate, 33%–50%; refs. 39–41). OS and PFS with R-GemOx observed in RE-MIND2 (11.0 and 5.1 months, respectively) were also comparable with prior studies (OS, 10–11 months; PFS, 5 months; refs. 39, 41), suggesting that responses to treatment with R-GemOx were short lived as suggested by the observed DoR. We observed ORR and CR rate as both being about 10% higher with tafasitamab plus lenalidomide versus the BR cohort, but this difference did not reach statistical significance with the given sample size of 75 patient pairs. A broad range of outcomes with BR have been reported for ORR (17.5%–62.7%), CR rate (15.3%–37.3%), and PFS (3.7–6.7 months; refs. 10, 42–44), which may be attributed to a variation in patients’ baseline characteristics and the study conduct. The outcomes with BR in the RE-MIND2 primary analysis (ORR, 54.7%; CR rate, 28.0%; median PFS, 7.9 months) were within the range of previously reported outcomes (10, 42–44); however, results from the sensitivity analyses with 11 matched covariates suggested more favorable outcomes with BR for ORR (67.7%), CR rate (46.2%), and PFS (median, 11.5 months), possibly due to a higher proportion of patients (69.2%) in the sensitivity analysis who received BR as a second-line therapy than in the primary analysis (52.0%). The additional time-to-event endpoints assessed, DoR, PFS, EFS, and TTNT, supported the primary analysis of OS.
Safety in RE-MIND2 was assessed by the rate of treatment discontinuation due to AEs. In the matched tafasitamab plus lenalidomide cohorts, ∼15% of patients discontinued treatment with the combination due to AEs versus systemic therapies pooled (6.8%), BR (2.8%), and R-GemOx cohorts (5.4%); however, higher rates of treatment discontinuation due to toxicities with BR (10.3%) and R-GemOx (8%) have been reported (10, 39). We note the higher treatment discontinuation rate due to AEs in the tafasitamab plus lenalidomide cohort, which is likely attributed to the recording of safety data under the stringent conditions of a clinical trial, relative to the collection of safety information in clinical practice. Additionally, patients in the tafasitamab plus lenalidomide cohort had a longer treatment exposure (median: ∼10 months) relative to the shorter durations of exposure to systemic therapies pooled (median: 2.4 months), BR (3.2 months), and R-GemOx (median: 2.9 months), which allowed more safety data to be collected. It is also important to consider that most patients in the RE-MIND2 observational cohort who discontinued treatment did so due to disease progression/death (1,495/3,454; 44.7%) rather than due to an AE (156/3,454; 4.7%).
To ensure robust and accurate comparisons, RE-MIND2 included several measures to reduce bias and ensure that the identified observational cohort provided an authentic comparator for the tafasitamab plus lenalidomide cohort. As the patient groups from the L-MIND and real-world observational study did not arise from a single, randomized clinical trial, there was a potential for treatment-selection bias. To address this, eligibility criteria for the observational cohort were aligned with those from the L-MIND study. Patient-level data collection, to balance the tafasitamab plus lenalidomide cohort with the treatment regimens in the observational cohorts, was performed using 1:1 NN matching according to nine baseline covariates. The absolute standardized difference was predefined to be <0.2 to ensure a high level of balance in each baseline covariate between the tafasitamab plus lenalidomide and comparator cohorts. The potential presence of bias, arising from excluding patients with missing data, was assessed by sensitivity analyses.
Randomized controlled trials (RCT) are generally considered by regulatory authorities to be the gold standard for establishing the causal relationship between treatments and patient outcomes. However, the high costs, long duration, and limited generalizability of RCTs have prompted a need for alternative approaches to inform regulatory decision-making (45). RE-MIND2 demonstrates the use of RWD to compare the efficacy outcomes of therapies used in routine clinical care for patients with R/R DLBCL with those from a clinical trial population. As there are multiple therapeutic approaches in the R/R DLBCL landscape, performing a range of head-to-head phase III trials comparing novel therapies to standard-of-care is not practical. In support of this scenario, the FDA has released guidance on utilizing RWD to supplement efficacy results from clinical trials (46). Consequently, the benefits of comparing the efficacy of different treatment regimens through the use of secondary databases and matched patient populations are evident. Large patient cohorts can be followed over long time periods and, compared with clinical trial populations, research questions can be answered in a relatively short time frame (the fastest studies may take a year, depending on access to health records and data collected; ref. 47). Propensity scores are frequently used to compare real-world and clinical trial data. For example, as in RE-MIND2, ePS matching has been used to compare outcomes for R/R DLBCL with the CAR T-cell therapy axicabtagene ciloleucel between the ZUMA-1 and SCHOLAR-1 studies (two characteristics were used to match populations between studies; ref. 22). Furthermore, patient-level matching using ePS has been used to assess adjuvant treatment effects in stage II colon cancer (21). The method has also been used for comparing outcomes from a clinical trial of blinatumomab, for R/R Philadelphia chromosome–positive B-precursor acute lymphoblastic leukemia, with an external cohort receiving standard of care (48). A more widespread adoption of ePS-based matching approaches for comparative effectiveness studies remains to be seen. However, as demonstrated in RE-MIND2, there is clear potential for this approach to facilitate comparisons between novel treatments assessed in clinical trial populations and real-world observational cohorts who received standard-of-care regimens.
The authors duly acknowledge the limitations associated with observational, retrospective analyses such as the present RE-MIND2 study. However, measures that were taken to minimize bias and the effects of potential confounding factors, as well as the consistent outcomes reported via various sensitivity analyses, have enabled a robust comparison of important patient outcomes in this therapeutic setting. These analyses help contextualize the outcomes from the novel tafasitamab plus lenalidomide immunotherapy combination with those from routinely administered, mostly chemoimmunotherapies in this patient population.
In conclusion, a significant clinical advantage in OS was observed in the cohort treated with tafasitamab plus lenalidomide in a clinical trial versus matched observational cohorts treated with pooled systemic therapies, BR, and R-GemOx. In the context of current treatments, these data further highlight the clinical value of the tafasitamab plus lenalidomide combination in patients with R/R DLBCL.
Supplementary Material
Acknowledgments
The authors would like to thank the patients and their families, clinical researchers, and their teams and hospitals that have participated in this study. This study was sponsored by MorphoSys AG. Medical writing assistance was provided by Eoin Duffy, PhD, of Syneos Health and funded by MorphoSys AG.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
G.S. Nowakowski reports grants and nonfinancial support from MorphoSys AG during the conduct of the study as well as grants and other support from BMS/Celgene and Roche and other support from Fate Therapeutics, Incyte, Kyte, AbbVie, Genmab, TG Therapeutics, Blueprint Medicines, Karyopharm, Ryvu, Kymera, Zai, and Daiichi outside the submitted work. D.H. Yoon reports grants and personal fees from Celltrion, Janssen, Boryung, Samyang, and Kirin Pharma and personal fees from Amgen, Roche, Celgene, and Takeda outside the submitted work. A. Peters reports advisory board payment from Incyte. E. Joffe reports personal fees from Epizyme and AstraZeneca outside the submitted work. I. Fleury reports personal fees from Incyte during the conduct of the study as well as personal fees from Novartis, Gilead, BMS, AstraZeneca, Janssen, Roche, Merck, and Seattle Genetics outside the submitted work. R. Greil reports personal fees from Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, BMS, MSD, Sandoz, AbbVie, Gilead, Daiichi Sankyo, and Sanofi during the conduct of the study as well as personal fees from Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, BMS, MSD, Sandoz, AbbVie, Gilead, Daiichi Sankyo, and Sanofi outside the submitted work. M. Ku reports other support from Roche and Antengene outside the submitted work. R. Marks reports personal fees from Incyte, Kite/Gilead, and Novartis outside the submitted work. K. Kim reports grants from MorphoSys AG during the conduct of the study as well as grants from AstraZeneca and Grail outside the submitted work. J. Trotman reports other support from MorphoSys AG during the conduct of the study as well as other support from Roche, Celgene/BMS, PCYC, Janssen, Takeda, and BeiGene outside the submitted work. M. Winderlich reports other support from Syneos Health during the conduct of the study as well as other support from MorphoSys AG outside the submitted work; in addition, M. Winderlich has a patent for WO2017207574A1 issued to MorphoSys AG. N.C. Kurukulasuriya reports personal fees from MorphoSys AG during the conduct of the study as well as personal fees from MorphoSys AG outside the submitted work. S. Ambarkhane reports other support from MorphoSys AG outside the submitted work. G. Hess reports grants and personal fees from AbbVie, BMS-Celgene, Incyte, Janssen, Kite/Gilead, MorphoSys AG, and Roche; personal fees from ADC-Therapeutics, AstraZeneca, Genmab, Takeda, and Novartis; and grants from Pfizer during the conduct of the study. G. Salles reports other support from MorphoSys AG during the conduct of the study as well as personal fees from AbbVie, Bayer, BeiGene, BMS/Celgene, Epizyme, Genentech/Roche, Genmab, Incyte, Janssen, Kite/Gilead, Loxo, Milteniy, Molecular Partners, MorphoSys AG, Nordic Nanovector, Novartis, Rapt, Regeneron, and Takeda outside the submitted work; in addition, G. Salles is coauthor of a patent issued (WO2012010561A1: characterization of another anti-CD19 monoclonal antibody with antibody-dependent cell-mediated cytotoxicity, developed in collaboration with IDD-biotech); this antibody and the company have no relationship with the anti-CD19 antibody described in the current paper (tafasitamab), and the antibody has not been licensed to any third party. No disclosures were reported by the other authors.
Authors' Contributions
G.S. Nowakowski: Conceptualization, resources, data curation, supervision, validation, investigation, writing–original draft, writing–review and editing. D.H. Yoon: Resources, data curation, investigation, writing–original draft, writing–review and editing. A. Peters: Resources, data curation, investigation, writing–original draft, writing–review and editing. P. Mondello: Resources, data curation, investigation, writing–original draft, writing–review and editing. E. Joffe: Resources, data curation, investigation, writing–original draft, writing–review and editing. I. Fleury: Resources, data curation, investigation, writing–original draft, writing–review and editing. R. Greil: Resources, data curation, investigation, writing–original draft, writing–review and editing. M. Ku: Resources, data curation, investigation, writing–original draft, writing–review and editing. R. Marks: Resources, data curation, investigation, writing–original draft, writing–review and editing. K. Kim: Resources, data curation, investigation, writing–original draft, writing–review and editing. P.L. Zinzani: Resources, data curation, investigation, writing–original draft, writing–review and editing. J. Trotman: Resources, data curation, investigation, writing–original draft, writing–review and editing. D. Huang: Conceptualization, resources, data curation, software, formal analysis, supervision, validation, investigation, visualization, methodology, writing–original draft, project administration, writing–review and editing. E.E. Waltl: Conceptualization, resources, data curation, supervision, validation, investigation, visualization, methodology, writing–original draft, project administration, writing–review and editing. M. Winderlich: Conceptualization, resources, data curation, formal analysis, supervision, validation, investigation, visualization, methodology, writing–original draft, project administration, writing–review and editing. N.C. Kurukulasuriya: Conceptualization, resources, data curation, supervision, validation, investigation, methodology, writing–original draft, project administration, writing–review and editing. S. Ambarkhane: Conceptualization, resources, data curation, supervision, validation, investigation, visualization, methodology, writing–original draft, project administration, writing–review and editing. G. Hess: Resources, data curation, investigation, writing–original draft, writing–review and editing. G. Salles: Conceptualization, resources, data curation, supervision, validation, investigation, writing–original draft, writing–review and editing.
About Tafasitamab
Tafasitamab is a humanized Fc-modified cytolytic CD19 targeting monoclonal antibody.
In 2010, MorphoSys licensed exclusive worldwide rights to develop and commercialize tafasitamab from Xencor, Inc.
Tafasitamab incorporates an XmAb® engineered Fc domain, which mediates B-cell lysis through apoptosis and immune effector mechanism including antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP).
In January 2020, MorphoSys and Incyte entered into a collaboration and licensing agreement to further develop and commercialize tafasitamab globally. Following accelerated approval by the U.S. Food and Drug Administration in July 2020, tafasitamab is being co-commercialized by MorphoSys and Incyte in the United States. Incyte has exclusive commercialization rights outside the United States.
XmAb® is a registered trademark of Xencor Inc.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. World Health Organization. Cancer research for cancer prevention. Lyon, France: World Cancer Rep. IARC Press; 2020. [Google Scholar]
- 2. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: B-cell lymphomas v4.2021.
- 3. Coiffier B, Thieblemont C, Van E, Neste D, Lepeu GR, Plantier I, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood 2010;116:2040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sarkozy C, Sehn LH. New drugs for the management of relapsed or refractory diffuse large B-cell lymphoma. Ann Lymphoma 2019;3:10. [Google Scholar]
- 5. Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 2017;130:1800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. González-Barca E, Boumendil A, Blaise D, Trněný M, Masszi T, Finel H, et al. Outcome in patients with diffuse large B-cell lymphoma who relapse after autologous stem cell transplantation and receive active therapy. A retrospective analysis of the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant 2020;55:393–9. [DOI] [PubMed] [Google Scholar]
- 7. Chihara D, Izutsu K, Kondo E, Sakai R, Mizuta S, Yokoyama K, et al. High-dose chemotherapy with autologous stem cell transplantation for elderly patients with relapsed/refractory diffuse large B cell lymphoma: a nationwide retrospective study. Biol Blood Marrow Transplant 2014;20:684–9. [DOI] [PubMed] [Google Scholar]
- 8. Gisselbrecht C, Glass B, Mounier N, Gill DS, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 2010;28:4184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sarkozy C, Coiffier B. Diffuse large B-cell lymphoma in the elderly: a review of potential difficulties. Clin Cancer Res 2013;19:1660–9. [DOI] [PubMed] [Google Scholar]
- 10. Sehn LH, Herrera AF, Matasar MJ, Kamdar M, Assouline S, Hertzberg M, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 2019;38:155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 2020;396:839–52. [DOI] [PubMed] [Google Scholar]
- 13. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019;380:45–56. [DOI] [PubMed] [Google Scholar]
- 14. Kalakonda N, Maerevoet M, Cavallo F, Follows G, Goy A, Vermaat JSP, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol 2020;7:e511–22. [DOI] [PubMed] [Google Scholar]
- 15. Hamadani M, Radford J, Carlo-Stella C, Caimi PF, Reid E, O'Connor OA, et al. Final results of a phase 1 study of loncastuximab tesirine in relapsed/refractory B-cell non-Hodgkin lymphoma. Blood 2021;137:2634–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. US Food & Drug Administration. FDA grants accelerated approval to tafasitamab-cxix for diffuse large B-cell lymphoma. 2020. [cited 2021 Nov 23]:1–2. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-tafasitamab-cxix-diffuse-large-b-cell-lymphoma.
- 17. European Medicines Agency. Committee for Orphan Medicinal Products: Orphan Maintenance Assessment Report for Minjuvi. 2021. [cited 2021 Sep 11]. Available from: https://www.ema.europa.eu/en/documents/orphan-maintenance-report/minjuvi-orphan-maintenance-assessment-report_en.pdf.
- 18. Government of Canada, Health Canada, Public Affairs C and RB. Drug Product Database: Minjuivi. 2021. [cited 2021 Sep 21]. Available from: https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=100793.
- 19. Salles G, Duell J, Barca EG, Tournilhac O, Jurczak W, Liberati AM, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol 2020;21:978–88. [DOI] [PubMed] [Google Scholar]
- 20. Duell J, Maddocks KJ, González-Barca E, Jurczak W, Liberati AM, De Vos S, et al. Long-term outcomes from the phase II L-MIND study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. Haematologica 2021;106:2417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jongeneel G, Klausch T, van Erning FN, Vink GR, Koopman M, Punt CJA, et al. Estimating adjuvant treatment effects in stage II colon cancer: comparing the synthesis of randomized clinical trial data to real-world data. Int J Cancer 2020;146:2968–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Neelapu SV, Locke FL, Bbartlett NL, Lekakis LJ, Reagon PM. A comparison of one year outcomes in ZUMA-1 (axicabtagene ciloleucel) and SCHOLAR-1 in patients with refractory, aggressive non-Hodgkin lymphoma (NHL). Blood 2017;130:579. [Google Scholar]
- 23. Mullard A. How much do phase III trials cost? Nat Rev Drug Discov 2018;17:777. [DOI] [PubMed] [Google Scholar]
- 24. Ramsey SD, Adamson BJ, Wang X, Bargo D, Baxi SS, Ghosh S, et al. Using electronic health record data to identify comparator populations for comparative effectiveness research. J Med Econ 2020;23:1618–22. [DOI] [PubMed] [Google Scholar]
- 25. Miriovsky BJ, Shulman LN, Abernethy AP. Importance of health information technology, electronic health records, and continuously aggregating data to comparative effectiveness research and learning health care. J Clin Oncol 2012;30:4243–8. [DOI] [PubMed] [Google Scholar]
- 26. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nowakowski G, Rodgers T, Marino D, Frezzato M, Barbui AM, Castellino C, et al. RE-MIND study: a propensity score-based 1:1-matched comparison of tafasitamab + lenalidomide (L-MIND) versus lenalidomide monotherapy (real-world data) in transplant-ineligible patients with relapsed/refractory diffuse large B-cell lymphoma. J Clin Oncol 2020;38:8020. [Google Scholar]
- 28. Zinzani PL, Rodgers T, Marino D, Frezzato M, Barbui AM, Castellino C, et al. RE-MIND study: comparison of tafasitamab + lenalidomide (L-MIND) vs lenalidomide monotherapy (real-world data) in transplant-ineligible patients with relapsed/refractory diffuse large B-cell lymphoma. Presented at European Hematology Association EHA25 Virtual Meeting; 2020 Jun 11-21. Abstract S238. [Google Scholar]
- 29. Tilly H, Gomes da Silva M, Vitolo U, Jack A, Meignan M, Lopez-Guillermo A, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v116–25. [DOI] [PubMed] [Google Scholar]
- 30. International Society of Pharmacoepidemiology. Guidelines for good pharmacoepidemiology practice (GPP). Pharmacoepidemiol Drug Saf 2016;25:2–10. [DOI] [PubMed] [Google Scholar]
- 31. Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. J Clin Oncol 1999;17:1244–53. [DOI] [PubMed] [Google Scholar]
- 32. Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579–86. [DOI] [PubMed] [Google Scholar]
- 33. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: B-Cell Lymphomas v5.2019.
- 35. Hamlin PA, Zelenetz AD, Kewalramani T, Qin J, Satagopan JM, Verbel D, et al. Age-adjusted international prognostic index predicts autologous stem cell transplantation outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. Blood 2003;102:1989–96. [DOI] [PubMed] [Google Scholar]
- 36. Jurczak W, Zinzani PL, Gaidano G, Goy A, Provencio M, Nagy Z, et al. Phase IIa study of the CD19 antibody MOR208 in patients with relapsed or refractory B-cell non-Hodgkin's lymphoma. Ann Oncol 2018;29:1266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peng GL, Yao W-K, Zheng Y, Liu Y, Duan X-M, et al. Identification of prognostic factors in patients with diffuse large B-cell lymphoma. Indian J Pathol Microbiol 2017;60:87. [DOI] [PubMed] [Google Scholar]
- 38. Farooq U, Maurer MJ, Thompson CA, Thanarajasingam G, Inwards DJ, Micallef I, et al. Clinical heterogeneity of diffuse large B cell lymphoma following failure of front-line immunochemotherapy. Br J Haematol 2017;179:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cazelles C, Belhadj K, Vellemans H, Camus V, Poullot E, Gaulard P, et al. Rituximab plus gemcitabine and oxaliplatin (R-GemOx) in refractory/relapsed diffuse large B-cell lymphoma: a real-life study in patients ineligible for autologous stem-cell transplantation rituximab plus gemcitabine and oxaliplatin (R-GemOx) in refractory. Leuk Lymphoma 2021;62:2161–8. [DOI] [PubMed] [Google Scholar]
- 40. Corazzelli G, Capobianco G, Arcamone M, Ballerini PF, Iannitto E, Russo F, et al. Long-term results of gemcitabine plus oxaliplatin with and without rituximab as salvage treatment for transplant-ineligible patients with refractory/relapsing B-cell lymphoma. Cancer Chemother Pharmacol 2009;64:907–16. [DOI] [PubMed] [Google Scholar]
- 41. Mounier N, El Gnaoui T, Tilly H, Canioni D, Sebban C, Casasnovas R-O, et al. Rituximab plus gemcitabine and oxaliplatin in patients with refractory/relapsed diffuse large B-cell lymphoma who are not candidates for high-dose therapy: a phase II lymphoma study association trial. Haematologica 2013;98:1726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sehn LH, Kamdar M, Herrera AF, McMillan A, Flowers C, Kim WS, et al. Randomized phase 2 trial of polatuzumab vedotin (pola) with bendamustine and rituximab (BR) in relapsed/refractory (r/r) FL and DLBCL. J Clin Oncol 2018;36:7507. [Google Scholar]
- 43. Ohmachi K, Niitsu N, Uchida T, Kim SJ, Ando K, Takahashi N, et al. Multicenter phase II study of bendamustine plus rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 2013;31:2103–9. [DOI] [PubMed] [Google Scholar]
- 44. Vacirca JL, Acs PI, Tabbara IA, Rosen PJ, Lee P, Lynam E. Bendamustine combined with rituximab for patients with relapsed or refractory diffuse large B cell lymphoma. Ann Hematol 2014;93:403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Use of real-world evidence to support regulatory decision-making for medical devices | FDA. [cited 2021 Jul 1]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-real-world-evidence-support-regulatory-decision-making-medical-devices.
- 46. FDA. Submitting documents using real-world data and real-world evidence to FDA for drugs and biologics guidance for industry. Draft guidance. 2019.
- 47. Schneeweiss S. Developments in post-marketing comparative effectiveness research. Clin Pharmacol Ther 2007;82:143–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rambaldi A, Ribera JM, Kantarjian HM, Dombret H, Ottmann OG, Stein AS, et al. Blinatumomab compared with standard of care for the treatment of adult patients with relapsed/refractory Philadelphia chromosome–positive B-precursor acute lymphoblastic leukemia. Cancer 2020;126:304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors upon reasonable request.