Abstract
The female hormonal profile is of utmost importance for the assessment of the endocrinological functional status and the diagnosis of diseases. The analysis must delimit their normality intervals based on the manufacturer's cut-off points. Due to not all intervals can be evaluated before use, it is imperative to verify the reference intervals to achieve uniformity in the interpretation of results in the female population. We determine the reference intervals of five female sex hormones [Follicle Stimulating hormone (FSH), Estradiol, Luteinizing Hormone (LH), Prolactin, and progesterone] using electrochemiluminescence in the Cobas e411 (Roche). We included female patients >18 years old, between the 3rd and 15th day of the menstrual cycle (follicular phase) and had no previous medical history or recent medication. For reference intervals analysis, we followed the recommendations of the CLSI C28-A3 guideline. The average concentration for FSH, progesterone, LH, prolactin and estradiol were 11.48 ± 21.10 mIU/ml, 8.19 ± 11.90 ng/ml, 10.98 ± 11.55 ng/ml, 25.05 ± 32.74 ng/mL, and 147.08 ± 473.8 pmol/mL, respectively. Eighty per cent of parameters showed a satisfactory transfer for the manufacturer's reference intervals, except for estradiol, which had 85.5% of transferred values. Our results suggest that 4/5 sex hormones were found within the manufacturer's reference intervals and can be quantified in Peruvian women, ensuring the quality of their results. However, it is necessary to determine the estradiol with other reagents and assays since we show errors in the transfer of intervals.
Keywords: Reference intervals, Sex hormones, Lutein hormone, Progesterone, Estrogen, Folic stimulant hormone, Prolactin, Chemiluminescence, Peru
Reference intervals; Sex hormones; Lutein hormone; Progesterone; Estrogen; Folic stimulant hormone; Prolactin; Chemiluminescence; Peru.
1. Introduction
The clinical laboratory is a crucial complement to medical decision-making in clinical practice, so serum markers permit the establishment of a sequence of pathophysiological phenomena that ultimately affect the population. Clinical biochemistry is a paramount technique for determining whether circulating female hormones are normal or abnormal in the female population. These assessments allow the interpretations of pathologic events in the endocrine disorders and illnesses, such as diabetes, sex hormone changes, thyroid disorders, among others [1, 2].
One of the most frequently changes in the adult population is the imbalances of sexual hormones that lead to the loss of libido, behavioural changes, infertility, and other endocrine syndromes such as hypothyroidism [3]. An accurate understanding of these alterations, treating and controlling possible significant imbalances in the feminine population, is vital in obtaining confidence results and very close to reality. It can undoubtedly be achieved by ensuring the quality of the results, as an error in quality management can lead to a misinterpretation of results triggering medically relevant affectations in the treatment and health of women [4, 5].
To provide high-quality results, clinical laboratories must establish a quality management process to ensure reliable results [6]. One of the principal quality assurance processes is the timely evaluation of reference intervals that commercial houses attribute to the reagents to interpret the estimated values in the user population and appreciate their normality [7, 8].
As per ISO 15189:2012, the Clinical and Laboratory Standard Institute (CLSI), and the Clinical Chemistry International Federation (IFCC), these intervals must be verifiable periodically and demandingly when new analysis tests are incorporated into a population that has not gotten previous quality evaluations. Therefore, clinical decision values have not been correctly positioned [9, 10]. Since the 1960s, verification processes and transfer of trade reference ranges for several markers in clinical chemistry, immunology, and haematology have been used with greater eagerness and uniformity [7, 8, 11, 12, 13, 14]. Nevertheless, many countries have not yet regulated the reference intervals analysis for all the markers; for example, in Peru, the thyroid or feminine hormone profiles have not yet been verified despite their constant demand.
The knowledge of commercial reference intervals could be transferable or not to the Peruvian population. It will provide consistency in the clinical interpretation of hormonal analysis results, ensuring the quality of medical attention to patients who present changes in their hormonal levels, especially in this context of the government's promotion of quality and the decadence of clinical analysis offered by Peruvian laboratories [15].
The objective of this study was to determine the reference values of female hormonal profiles in healthy adults in Peru.
2. Methods
2.1. Study design and location
A retrospective single-centre study was conducted in 2019. Patient selection and hormone profile analysis were performed in the Suiza Lab in Miraflores, Lima (Peru). This laboratory carries out clinical analysis of hospitals, clinics, and research centres nationwide. It also has a comprehensive quality system supervised every semester by the American College of Pathologists.
2.2. Samples, volunteers and inclusion criteria
All patients enrolled voluntarily in this study, with previous approval and after filling up the informed consent form. We included female patients >18 years of age, between the 3rd and 15th day of the menstrual cycle (follicular phase) and had no previous medical history or recent medication. We excluded pregnant women, patients with neoplasms, cardiovascular and autoimmune diseases, chromosomopathies, anabolic consumption, hormonal therapy for infertility, and those with a personal and family history of the endocrinological disease.
2.3. Hormonal assessment
The collection of samples has been performed by venipuncture with the BD Vacutainer system (Leax Point, France) according to the specific date for the sampling following the Standard Operational Process (SOP) of the laboratory. Samples were processed with the electrochemiluminescence method in the automated system of the fourth generation of Cobas e411 (Roche Diagnostics, Chicago, USA). The female hormonal profile included the simultaneous determination of Stimulating Follicle (FSH), Luteinizing Hormone (LH), Estradiol (E2), progesterone, and prolactin.
Three levels of quality control materials provided by the manufacturer (PreciControl ISD from Roche Diagnostics) were used to check the precision of the study. The results of these controls were measured double daily, within the established threshold (10%) and monthly reported to the CAP external quality assessment (EQA) program. Within this programme, the analytical sensitivity of each marker and performance measures (i.e., the limit of quantification) were also verified using CAP EQA materials and acceptance criteria that laboratory met monthly. This hormonal evaluation system participates in Roche’s external quality control program, which ensures the traceability of the results, as well, as internal control.
2.4. Reference intervals and data analysis
The analysis results were codified directly into the data matrix in IBM SPSS v24.0 (Armonk, US) for macOS. After patients were enrolled in the study, their clinical criteria were reviewed in their medical record to consider them as part of the sample. All those who did not meet the requirements were discarded. After this, the normality of the results with the Kolgomorov-Smirnov test was evaluated.
Then, following the recommendations of the CLSI C28-A3 guideline [16], a descriptive analysis and a reference interval analysis were developed. This analysis included the detection of marginal values with the Tukey y Dixon- Reed method [17, 18] considering a significance threshold of 5% and excluding outliers that were outside three standard deviations of the mean or had extreme values at or outside the normal limit. Estimation of the lower reference limit (2,5% percentiles) and the upper reference limit (97,5% percentiles), the comparison of obtained intervals and estimation of transfer of values in front of commercial intervals for each test of the female hormonal profile, considering a cut-off of 95% for successful transfer. For all analyses, the p-value < 0.05 and a 95% confidence interval (CI) were considered significant. Finally, we used BoxPlotR (Tyers and Rappsilber labs, Berlin) for figure designs.
2.5. Ethical aspects
This study had the approval of the Laboratory Management (Nº SL-O1-046-2019) and for the Etics Research Committee of the Universidad Norbert Wiener (UNW-VRI-Nº 054-2019, Date: Jan 15th, 2019).
3. Results
3.1. Participants
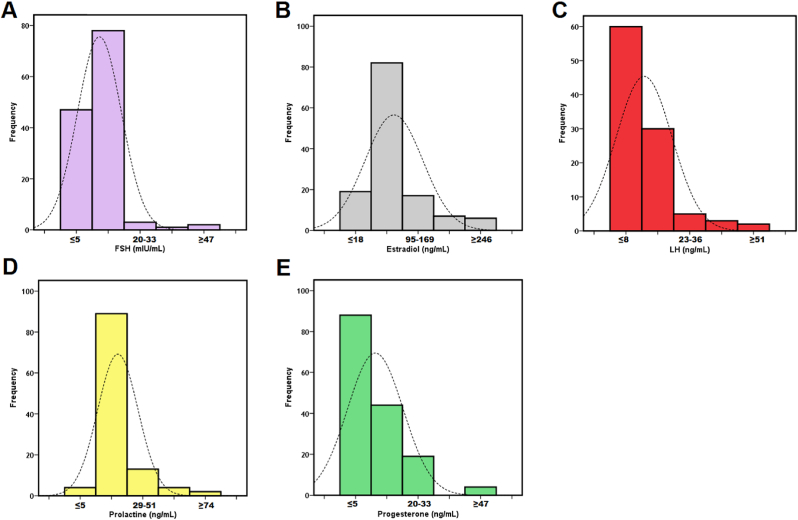
We included a total of 707 patients who met the inclusion criteria, but 48 patients were excluded due to extreme values. In the analysis of 659 samples included in the study, the normal distribution of all-female markers was determined (all p > 0.05). The average age of the participants was 29.9 ± 6.1 years (range 18–40 years) and all the women were from Lima, Peru. Figure 1A–E shows the distribution of each hormonal marker.
Figure 1.
Histograms and distribution of female sex hormones. Stimulating Follicle Stimulating hormone (A), Estradiol (B), Luteinizing Hormone (C), Prolactin (D) and progesterone (E).
3.2. Main results
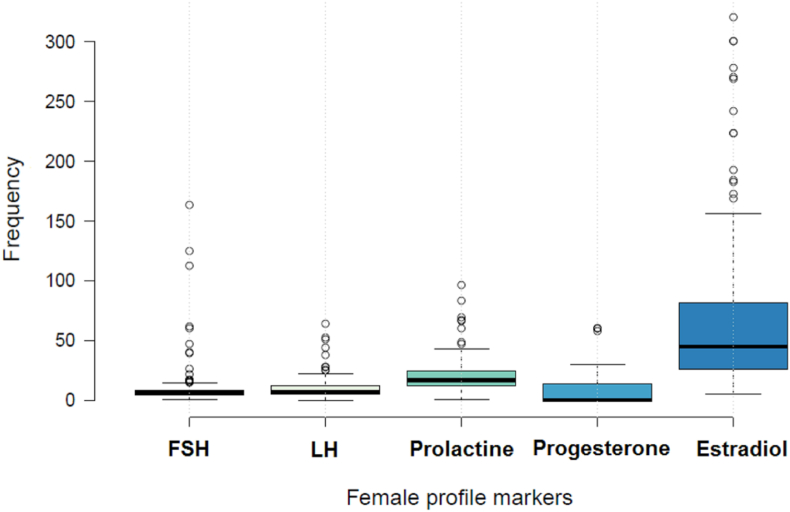
Amongst the hormonal markers, progesterone was the one with the highest number of analyses (n = 156). The average concentration of FSH was 11.48 ± 21.1 mlU/mL (95% CI, 7.9 to 15.1) and LH was 10.58 ± 11.6 ng/mL (95% CI, 9.1 to 12.9) (Figure 2). While the average serum concentration of progesterone, prolactin and estradiol was 8.19 ± 11.9 ng/mL (95% CI, 6.3 to 10.1), 24.29 ± 32.7 ng/mL (95% CI, 19.5 to 30.6), and 147.1 ± 473.8 ng/mL (95% CI 66.3 to 227.9), respectively (Table 1).
Figure 2.
Distribution of the analysis of sex hormones in Peruvian women.
Table 1.
Baseline characteristics of the five female sexual markers of Peruvian patients.
| Sex hormones | N | X ± DS | Range | 95% CI | p-value∗ |
|---|---|---|---|---|---|
| FSH (mUI/ml) | 131 | 11.48 ± 21.10 | 0.33–162.8 | 7.89–15.08 | 0.072 |
| Progesterone (ng/mL) | 155 | 8.19 ± 11.90 | 0.05–60 | 6.31–10.07 | 0.095 |
| LH (ng/mL) | 121 | 10.58 ± 11.55 | 0.1–63.85 | 9.01–12.95 | 0.857 |
| Prolactine (ng/mL) | 120 | 24.29 ± 32.74 | 0.26–339.8 | 19.46–30.63 | 0.094 |
| Estradiol (ng/mL) | 131 | 147.08 ± 473.8 | 5–500 | 66.25–227.92 | 0.077 |
Results of Kolgomorov-Smirnov test. Abbreviation: FSH: Stimulating Follicle Hormone, LH: Luteinizing Hormone.
Four of the five markers of hormonal function showed 100% of their values in the manufacturer’s reference intervals. Only estradiol did not have a satisfactory verification or transfer of normal values in the Peruvian population, with an average of 85,5% with the electrochemiluminescence method. In Table 2, we show the validation and transfer of the estimated reference interval in the study population.
Table 2.
Comparison between study range and manufacturer's intervals for five female sex markers.
| Sex hormones | N | Study intervals (2.5–97.5%) | MRI | Transfer (%) | Verification |
|---|---|---|---|---|---|
| FSH (mUI/ml) | 131 | 1.30–96.70 | 0.100–200 | 100 | Yes |
| Progesterone (ng/mL) | 156 | 0.01–58.20 | 0.05–60 | 100 | Yes |
| LH (ng/mL) | 121 | 0.30–52.0 | 0.100–200 | 100 | Yes |
| Prolactine (ng/mL) | 120 | 3.30–92.10 | 0.0470–470 | 100 | Yes |
| Estradiol (pmol/mL) | 131 | 5.00–2603.4 | 18.4–11010 | 85.5 | No |
Abbreviation: FSH: Stimulating Follicle Hormone, MRI: Manufacturer reference intervals, LH: Luteinizing Hormone.
4. Discussion
This is the first study in Peru to assess the status of female hormones and found that 100% of the data on the four sex hormones (FSH, LH, progesterone, and prolactin) of women of childbearing age have been transferred. Estradiol did not have a successful transfer when analyzed in Cobas e411 with the electrochemiluminescence method.
Even if the hormonal parameter depends on a large number of factors that include the technique and the reagents used for its determination, the preanalytic phase of the study, as well as the adequacy of the sample, lack of evaluation, unrecognized and little investment in quality assurance are critical factors [19, 20]. A recent study [21] determined that the specific reference range for estrogen (E1) and estradiol (E2) in women of childbearing age is 3–12 pmol. A recent study [21] determined that the specific reference range for estrogen (E1) and estradiol (E2) in women of childbearing age is 3–12 pmol. Our findings are inconsistent with this study because our interval is much longer (5.0–2603.4 pmol/L). These marked differences could be attributed to changes in the analysis methods because this study was related to liquid chromatography-mass spectrometry (LC-MS/MS), which is more sensitive than electrochemiluminescence essays [22]. Likewise, the results could vary due to the inclusion criteria of the sample. Moreover, these values can increase with age, puberty stage. and change during the menstrual cycle, as in mini puberty (<100 pmol/L) and childhood (≤20 pmol/L) have been recently proven [21].
Other results with LC-MS/MS in the Dutch population established that the estradiol reference ranges during the early follicular phase and late were 31–771 pmol/L and 104–1742 pmol/L, respectively; while during the LH peak, the estradiol levels increased to 275–2864 pmol/L [22]. Our results revealed a significant difference from the follicular phase; however, they are slightly close to the upper estradiol limit (2603.4 vs 275–2864 pmol/L) during the LH peak period. Although in this study the estradiol levels have not been estimated according to the menstrual cycle (follicular phase and early and tardy luteal), the intervals found have not achieved a satisfactory transfer that prevents the application of this interval as in the female population of childbearing age analyzed in Lima, Peru.
On the other hand, the transfer of reference intervals for other markers of the female profile, such as FSH and LH, has been successfully determined. This transference achievement is consistent with previous studies of the Italian population [23], Australian [24], and Australian [25] where, although congruent intervals have been estimated, a national-wide study is necessarily required to propose a consensus according to the user population, the method employed, and the reagents used.
Prolactin is a pleiotropic globular protein with a hormonal function of 199 amino acids secreted by the adenohypophysis in response to a necessary stimulus for milk secretion in the breasts. Its role in some physiological processes is critical, and its male and female quantifications are linked to diseases [26]. Given its clinical significance, we establish a reference interval of 25.5 ± 32.7 ng/mL in our findings, which differs from the range estimated in other people, such as healthy females in India (11.6 ± 2.8 ng/m) [27, 28]. These differences highlight the importance of establishing reference intervals specific to each population since manufacturers provide general ones with a wide range based on their commercial validation study. Nevertheless, a comparison of different brands has proved that four of the six ranges of total prolactin have values outside the 2.5th lower percentile [29].
A multicenter study of 1,043 Chinese men showed that hormone levels increased significantly with age while prolactin decreased [30]. For their part, Schüring et al. have demonstrated that the reference interval of prolactin is affected by hormonal status in 139 reproductive women and 103 postmenopausal women [12]. Both studies allow us to understand the importance of the choice of the study population and the monitoring of patients. Although in this study, the monitoring of the volunteers was not according to the menstrual cycle. Our results have demonstrated the verification and transparency of reference intervals of the five feminine hormones in Peru. These results cannot generalized due to possible fluctuations in the values during the menstrual cycle. We prove that the intervals for FSH, LH, progesterone, and prolactin were satisfactorily transferred and can be applied as general ranges in the Peruvian population in the absence of other reports.
The limitations of the study were 1) the study developed an evaluation in a single-centre, being necessary to carry out multicenter studies considering multiculturalism and Peruvian biological variability; 2) we verify the reference intervals on the Cobas e411 electrochemiluminescence analyzer. However, it is necessary to evaluate the transfer of intervals in other platforms widely used in Peru such as Enzyme-Linked ImmunoSorbent Assay, chemiluminescence, and LC-MS/MS, finally 3) the intervals of reference were verified for fertile women population, further studies are necessary to evaluate the analysis of women of menopausal, pre-menopausal age, pre-pubertal stage, in replacement therapy, transgender community, men and child population.
In conclusion, this study shows the verification of 4/5 parameters of the female hormonal profile evaluated in the Peruvian population of fertile age in the follicular phase. Estradiol transfer to the manufacturer’s reference intervals defined in the study using the electrochemiluminescence method was unsuccessful.
It is the first verification study of the reference intervals of sexual hormones in Peruvian women. In further studies, we suggest that it is necessary to continue qualitative research to understand changes in hormonal intervals and design a consensus report on clinical use.
Declarations
Author contribution statement
Jeel Moya-Salazar, Sandra P. Cerda: Conceived and designed the experiments, Performed the experiments, Analyzed and interpreted the data, Contributed reagents, materials, analysis tools or data, Wrote the paper.
Marcia M. Moya-Salazar: Conceived and designed the experiments, Performed the experiments, Contributed reagents, materials, analysis tools or data, Wrote the paper.
Hans Contreras-Pulache: Conceived and designed the experiments, Analyzed and interpreted the data, Wrote the paper.
Betsy Cañari: Performed the experiments, Analyzed and interpreted the data, Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Austin R.C., Bussey T.J., Cortes K.L. American Chemical Society Press; Washington D.C.: 2019. Biochemistry education: from theory to practice. (ACS Symposium Series 1337). [Google Scholar]
- 2.Berg J. The approach to pathology harmony in the UK. Ann. Clin. Biochem. 2012;33(3):89–93. [PMC free article] [PubMed] [Google Scholar]
- 3.Hausmann M. Why sex hormones matter for neuroscience: a very short review on sex, sex hormones, and functional brain asymmetries. J. Neurosci. Res. 2017;95(1-2):40–49. doi: 10.1002/jnr.23857. [DOI] [PubMed] [Google Scholar]
- 4.Aakre K.M., Langlois M.R., Watine J., Barth J.H., Baum H., Collinson P., et al. Critical review of laboratory investigations in clinical practice guidelines: proposals for the description of investigation. Clin. Chem. Lab. Med. 2013;51(6):1217–1226. doi: 10.1515/cclm-2012-0574. [DOI] [PubMed] [Google Scholar]
- 5.Schork N.J., Weder A.B., Schork M.A. On the asymmetry of biological frequency distributions. Genet. Epidemiol. 1990;7:427–446. doi: 10.1002/gepi.1370070605. [DOI] [PubMed] [Google Scholar]
- 6.Westgard J.O. Design of internal quality control for reference value studies. Clin. Chem. Lab. Med. 2004;42(7):863–867. doi: 10.1515/CCLM.2004.141. [DOI] [PubMed] [Google Scholar]
- 7.Lazo C.Y., López P.A. Facultad de Medicina, Universidad Peruana Cayetano Heredia; 2018. Verificación de intervalos de referencia de analitos más frecuentes en el área de Química Clínica en el laboratorio del Centro Médico Naval. [Thesis] Lima. [Google Scholar]
- 8.Marrero S.J., Lárez C.R., Avilés Y.M., Segovia J.A., Chirinos A.Y., Romero M.A., et al. Verificación y transferencia de intervalos de referencia del perfil tiroideo y PSA total en individuos masculinos de la ciudad de Valencia, Venezuela. Rev. Latinoam. Patol. Clin. Med. Lab. 2017;64(2):94–99. [Google Scholar]
- 9.Schneider F., Maurer C., Friedberg R.C. International organization for standardization (ISO) 15189. Ann. Lab. Med. 2017;37(5):365–370. doi: 10.3343/alm.2017.37.5.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Federation of Clinical Chemistry . third ed. C28-A3. Clinical and Laboratory Standards Institute; Wayne, PA: 2008. Defining, establishing, and verifying reference intervals in the clinical laboratory. (Approved Guideline). [Google Scholar]
- 11.Ayetekin M., Emerk K. Accurate reference intervals are required for accurate diagnosis and monitoring of patients. eJIFCC. 2008;19(2):1–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Dighea A.S., Moya J.H., Hayes F.J., Sluss P.M. High-resolution reference ranges for estradiol, luteinizing hormone, and follicle-stimulating hormone in men and women using the AxSYM assay system. Clin. Biochem. 2005;38:175–179. doi: 10.1016/j.clinbiochem.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Greene D.N., Schmidt R.L., McPherson G.W., Rongitsch J., Imborek K.L., Dickerson J.A., et al. Reproductive endocrinology reference intervals for transgender women on stable hormone therapy. J. Appl. Lab. Med. 2021;6(1):15–26. doi: 10.1093/jalm/jfaa028. [DOI] [PubMed] [Google Scholar]
- 14.Schüring A.N., Kelsch R., Pierściński G., Nofer J.R. Establishing reference intervals for sex hormones on the analytical platforms advia centaur and immulite 2000XP. Ann. Lab. Med. 2016;36(1):55–59. doi: 10.3343/alm.2016.36.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moya-Salazar J., Pio-Davila L. Evaluation of inter-batch variability in establishing and quality control of glucose. Med. Univ. 2016;18(71):85–90. [Google Scholar]
- 16.Clinical and Laboratory Standards Institute . third ed. CLSI document C28-A3; Wayne, PA.: 2008. Defining Establishing and Verifying Reference Intervals in Clinical Laboratory; Approved Guidelines. [Google Scholar]
- 17.Reed A.H., Henry R.J., Mason W.B. Influence of statistical method used on the resulting estimate of normal range. Clin. Chem. 1971;7(4):275–284. [PubMed] [Google Scholar]
- 18.Tukey J.W. Addison-Wesley; Reading MA: 1997. Exploratory Data Analysis. [Google Scholar]
- 19.Haddad A.R., Giacherio D., Barkan A.L. Interpretation of common endocrine laboratory tests: technical pitfalls, their mechanisms and practical considerations. Clin. Diabetes Endocrinol. 2019;5:12. doi: 10.1186/s40842-019-0086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vesper H.W., Botelho J.C., Wang Y. Challenges and improvements in testosterone and estradiol testing. Asian J. Androl. 2014;16(2):178–184. doi: 10.4103/1008-682X.122338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frederiksen H., Johannsen T.H., Andersen S.E., Albrethsen J., Landersoe S.K., Petersen J.H., et al. Sex-specific estrogen levels and reference intervals from infancy to late adulthood determined by LC-MS/MS. J. Clin. Endocrinol. Metab. 2020;105(3):dgz196. doi: 10.1210/clinem/dgz196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verdonk S.J., Vesper H.W., Martens F.D., Sluss P.M., Hillebrand J.J., Heijboer A.C. Estradiol reference intervals in women during the menstrual cycle, postmenopausal women and men using an LC-MS/MS method. Clin. Chim. Acta. 2019;495:198–204. doi: 10.1016/j.cca.2019.04.062. [DOI] [PubMed] [Google Scholar]
- 23.Radicioni A., Lenzi A., Spaziani M., Anzuini A., Ruga G., Papi G., et al. A multicenter evaluation of immunoassays for follicle-stimulating hormone, luteinizing hormone and testosterone: concordance, imprecision and reference values. J. Endocrinol. Invest. 2013;36:739–744. doi: 10.1007/BF03347112. [DOI] [PubMed] [Google Scholar]
- 24.Bogner B., Schwenoha K., Vogl M., Weghuber D., Roth C., Kipman U., et al. Evaluation of reference intervals of haematological and biochemical markers in an Austrian adolescent study cohort. Clin. Chem. Lab. Med. 2019;57(6):891–900. doi: 10.1515/cclm-2018-0715. [DOI] [PubMed] [Google Scholar]
- 25.Mewton L., Champion K., Kay-Lambkin F., Sunderland M., Thornton L., Teesson M. Lifestyle risk indices in adolescence and their relationships to adolescent disease burden: findings from an Australian national survey. BMC Publ. Health. 2019;19:60. doi: 10.1186/s12889-019-6396-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Chalabi M., Bass A.N., Alsalman I. StatPearls Treasure Island. StatPearls Publishing; 2021. Physiology, prolactin. [Google Scholar]
- 27.Lahiri K.D., Baruah M., Ghosh J., Sengupta S. Establishment of reference interval of serum prolactin in an Indian population. J. Clin. Diagn. Res. 2014;8(7):CC08–CC10. doi: 10.7860/JCDR/2014/8400.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitehead S.J., Cornes M.P., Ford C., Gama R. Reference ranges for serum total and monomeric prolactin for the current generation Abbott Architect assay. Ann. Clin. Biochem. 2015;52(Pt 1):61–66. doi: 10.1177/0004563214547779. [DOI] [PubMed] [Google Scholar]
- 29.Overgaard M., Pedersen S.M. Serum prolactin revisited: parametric reference intervals and crossplatform evaluation of polyethylene glycol precipitation-based methods for discrimination between hyperprolactinemia and macroprolactinemia. Clin. Chem. Lab. Med. 2017;55(11):1744–1753. doi: 10.1515/cclm-2016-0902. [DOI] [PubMed] [Google Scholar]
- 30.Yu S., Qiu L., Liu M., Li S., Tao Z., Zhang Q., et al. Establishing reference intervals for sex hormones and SHBG in apparently healthy Chinese adult men based on a multicenter study. Clin. Chem. Lab. Med. 2018;56(7):1152–1160. doi: 10.1515/cclm-2017-0749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.