Abstract
Early life adversity (ELA) is a major risk factor for the development of pathology, including anxiety disorders. Neurodevelopmental and behavioral outcomes following ELA are multifaceted and are influenced heavily by the type of adversity experienced and sex of the individual experiencing ELA. It remains unclear what properties of ELA portend differential neurobiological risk and the basis of sex-differences for negative outcomes. Predictability of the postnatal environment has emerged as being a core feature supporting development, with the most salient signals deriving from parental care. Predictability of parental care may be a distinguishing feature of different forms of ELA, and the degree of predictability afforded by these manipulations may contribute to the diversity of outcomes observed across models. Further, questions remain as to whether differing levels of predictability may contribute to differential effects on neurodevelopment and expression of genes associated with risk for pathology. Here, we tested the hypothesis that changes in maternal behavior in mice would be contingent on the type of ELA experienced, directly comparing predictability of care in the limited bedding and nesting (LBN) and maternal separation (MS) paradigms. We then tested whether the predictability of the ELA environment altered the expression of corticotropin-releasing hormone (Crh), a sexually-dimorphic neuropeptide that regulates threat-related learning, in the amygdala of male and female mice. The LBN manipulation reliably increased the entropy of maternal care, a measure that indicates lower predictability between sequences of dam behavior. LBN and MS rearing similarly increased the frequency of nest sorties and licking of pups but had mixed effects on other aspects of dam-, pup-, and nest-related behaviors. Increased expression of Crh-related genes was observed in pups that experienced ELA, with gene expression measures showing a significant interaction with sex and type of ELA manipulation. Specifically, MS was associated with increased expression of Crh-related genes in males, but not females, and LBN primarily increased expression of these genes in females, but not males. The present study provides evidence for predictability as a distinguishing feature of models of ELA and demonstrates robust consequences of these differing experience on sex-differences in gene expression critically associated with stress responding and sex differences in risk for pathology.
Keywords: Early life adversity, Entropy, Maternal behavior, Amygdala, Sex-differences, Corticotropin-releasing hormone
Highlights
-
•
Type of early life adversity differentially altered quantity of maternal behavior.
-
•
Limited bedding and nesting increased unpredictable dam behavior.
-
•
Amygdalar Crh expression in male and female pups were dependent on the type rearing.
1. Introduction
Early postnatal life represents a sensitive period during which brain maturation is highly influenced by sensory signals from the environment (Khazipov et al., 2004; Hensch 2005; Barkat et al. 2011; Espinosa and Stryker 2012; Short and Baram 2019). A great deal is known about the impact of the early environment on sensory and motor development. However, despite compelling evidence linking early life adversity (ELA) with long-term disruption in the activity and structure of stress-circuits in both humans (Tottenham et al., 2010; Dylan G. Gee et al., 2014; Godoy et al., 2018) and rodents (Bolton et al., 2018; Manzano Nieves et al., 2020; Goodwill et al., 2018; Demaestri et al., 2020; Bath et al. 2016; Kangas et al., 2022; Levis et al., 2021; Honeycutt et al., 2020), much less information is available with regard to environmental effects on the development of neuronal populations and circuits that process signals related to stress (Birnie and Baram 2022). Improving our understanding of the nature, type, and timing of the signals that influence neurodevelopmental trajectories of these circuits will inform the development of individualized treatments and interventions.
In humans (Nelson et al., 2007), primates (Pryce et al., 2005; Sánchez et al., 1998), and rodent models (Levine 1957; Demaestri et al., 2020; Chen and Baram 2016), exposure to physical and psychosocial stress early in life has a profound impact on neural and behavioral development. In prior work, ELA has been shown to increase the risk for adverse outcomes and these effects appear to derive more from the ‘psychosocial’ rather than physical aspects of the adversity (Bolton et al., 2019; Herzog and Schmahl 2018). The primary caregiver during early postnatal development is often the most potent source of social and physiological signals in the early, proximate environment. Thus, an extensive amount of work has focused on the elements of care that might constitute ELA. Indeed, the quantity and quality of postnatal care influences neurodevelopmental outcomes, especially with regard to the programing of stress circuits (Norman et al., 2012; Glod and Teicher 1996; Herzog and Schmahl 2018; Nelson et al., 2020; Short et al., 2021).
To date, the majority of work has centered on the valence of the care signals from the caregiver to human infant or to the rodent pup such as the quality and quantity of behaviors. In recent years, the focus has begun to shift to the patterns of caregiver behaviors, with findings from recent reports identifying a strong impact of alterations in the pattern of care on structural and functional brain circuit development across species (Davis et al., 2017, 2019; Molet et al., 2016a, Molet et al., 2016b; Manzano Nieves et al., 2020; Gallo et al., 2019; Bath et al. 2016; Demaestri et al., 2020; Granger et al., 2021; Glynn and Baram 2019; Birnie and Baram 2022). Unpredictable sequences of caregiving behaviors, regardless of the quality or quantity of those behaviors, were predictive of significant shifts in the timing of cognitive and emotional development, as well as with enduring consequences on behavioral outcomes. Specifically, models that induce fragmented patterns of care have been causally linked with effects on brain maturation (Ivy et al., 2008; Molet et al., 2016a; Molet et al., 2016b; Walker et al., 2017; Gallo et al., 2019; Bath et al. 2016).
The type of adversity experienced, as well as the sex of the individual experiencing the adversity, can also significantly influence neurodevelopmental outcomes (Demaestri et al., 2020; Norman et al., 2012; Herzog and Schmahl 2018; Nelson et al., 2020; Baram et al., 2012; Luby et al., 2020). In humans, the effects of the type, timing and duration of adversities experienced have been difficult to isolate and directly test due to significant heterogeneity and overlap in these variables (Luby et al., 2020; Malter Cohen et al., 2013; Tottenham and Sheridan 2010; VanTieghem and Tottenham, 2018) and sex differences in the patterns of adversity experienced (Haahr-Pedersen et al., 2020). Experimental models have allowed for the direct manipulation of the type of adversity, as well as tight control over its timing, severity and duration, and measurements of specific outcomes on a uniform genetic background in males and females. Two widely used models of ELA in rodents are the limited bedding and nesting (LBN) and the maternal separation (MS) paradigms which were designed to simulate adversity associated with loss of resources and absence of parental care, respectively. The type of adversity experienced leads to unique consequences on developmental processes and outcomes (Demaestri et al., 2020; Peña et al. 2019; Granata et al., 2021). However, questions remain regarding the impact of each on maternal care and whether these changes are associated with differential outcomes in males and females.
The mechanisms supporting enduring consequences of a transiently experienced form of ELA are not fully understood. One identified candidate mechanism involves long-lasting molecular changes, which alter the level and timing of expression of crucial genes supporting neurodevelopment (Fiori and Turecki, 2016; Nestler 2012; Vaiserman and Koliada 2017; Peña et al. 2019; Bolton et al., 2020). Indeed, significant effects of ELA on the transcriptome have been established (Short et al., 2021; Bolton et al., 2020). One key molecular target that has been identified to be impacted by maternal signals is corticotropin releasing hormone (Crh). The expression of the Crh family of genes and receptors in the neonate amygdala reflects the initiation of threat learning (Moriceau et al., 2006, Moriceau et al., 2009) and are particularly sensitive to changes in the early environment (Bolton et al., 2018, 2019; Dubé et al., 2015). Crh release in the amygdala acts through its two receptors, Crh receptor 1 (CrhR1) and CrhR2, and is thought to be regulated by Crh binding protein (CrhBP) (Kalin 2018). While the unique developmental roles of CrhR1 and CrhR2 are still being established, evidence supports an involvement of both receptors in the development of stress-related emotional dysfunction (Reul and Holsboer 2002; Eghbal-Ahmadi et al., 1997).
As maternal-derived sensory signals to the pups may drive enduring changes in gene expression and brain circuit development, the current study sought to address two related questions: First, how do two widely used models of ELA, limited bedding and nesting (LBN) and maternal separation (MS), influence patterns of maternal care. Second, what is the influence of such changes on gene expression in a key brain region supporting future responses to stress, the amygdala, considering the important role of sex in modulating the impact of ELA. We tested the hypothesis that different forms of ELA distinctly alter the predictability of maternal care and promote elevated expression of Crh-related genes in the amygdala, modulated by the type of ELA and sex.
2. Methods
2.1. Mice and housing
C57BL/6N mice were bred in house and maintained on a 12 h:12 h light cycle (lights on at 7AM) with ad libitum access to food and water. Mice were housed in 31 × 12 × 14 cm cages with cob bedding and 4 × 4 cm cotton nestlet. Litters were composed of both male and female pups and ranged from 4 to 8 pups per litter. A total of 16 litters were used for the maternal behavior recordings (control:6, LBN:5, MS:5) and a total of 23 separate litters were used for brain extraction at either PD8, PD16 or PD21 (control:8, LBN: 7, MS, 8). Virgin dams were not used for the current studies. Pups were weaned and sex segregated at postnatal day (PD) 21. All animal procedures were approved by the Brown University Institutional Animal Care and Use Committee and consistent with the guide for the care and use of animals in research.
2.2. Maternal separation paradigm
A breeder female and male were housed in a standard home cage with cob bedding and a 4 × 4 cm cotton nestlet. All mice were bred in house and checked daily for the birth of pups (PD0). On PD4, prior to the start of MS, the male was permanently separated from dam and pups. From PD4 to PD11, the pups were removed daily from their home cage and dam for 3-h per day between the hours of ∼9 a.m. and ∼12 p.m. (Fig. 1A). During this separation, the pups were each individually placed in separate cups that contained soiled home cage bedding and that were secured in cup holders in a circulating water bath maintained at 35–37 °C to support pup thermoregulation. On PD11, the pups were returned to their standard housing conditions after the 3-h separation where they remained with the dam until weaning at PD21.
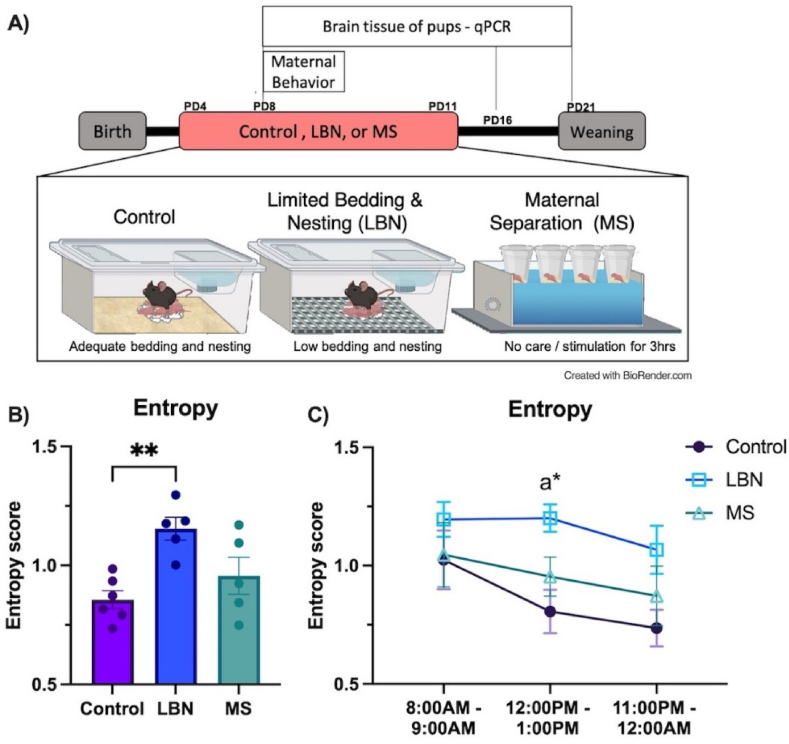
Fig. 1.
Experimental Timeline and Entropy Score. A) From postnatal day (PD)4-PD11, pups and dams were exposed to either control rearing, limited bedding and nesting (LBN) rearing or 3hr/day maternal separation (MS). Maternal behavior was analyzed from three, 1 h-long sessions starting at 8AM, 12PM, and 11PM on PD8 and brain tissue was collected at PD8, PD16 and PD21. B) Entropy score averaged across three timepoints. LBN rearing increased dam entropy score compared to control. C) Entropy score at 8AM, 12PM, and 11PM on PD8. LBN increased entropy score relative to controls at 12PM. Pup reunion to MS dams at 12PM did not change entropy score. n/group = 5–6. Plots depict mean ± SEM; circles represent individual dams; “a” represents LBN different to control at that time point. (p* <0.05, **<0.01).
2.3. Limited bedding and nesting paradigm
A breeder female and male were pair-housed in a standard home cage with bedding and a 4 × 4 cm cotton nestlet for nest building. All mice were bred in house and checked daily for the birth of pups. At PD4, the dam and pups were transferred from their standard home cage, to a cage with a wire mesh floor, no bedding, and approximately 3 × 4 cm cotton nestlet (Fig. 1A). Mice remained in the LBN housing conditions for seven days. On PD11, the dam and pups were returned to their standard housing conditions with nesting and bedding material where they remained until weaning at PD21.
2.4. Home cage video monitoring
Mice in LBN, MS or control conditions were maintained in a specialized housing room in the vivarium, housed in 31 x 31 × 14 cm cages with a clear plexiglass cage topper with ventilation holes. Mice were transferred to the recording room in the vivarium when pups were PD6, allowing two days for habituation to the recording room prior to PD8 recording. Mice remained in this room for 4 consecutive days (PD6–PD10). Throughout the home-cage video monitoring, LBN and control cages were unhandled and continuously recorded with the aid of an overhead camera. MS dams were handled for daily pup separations at ∼9:00AM and return at ∼12:00PM. Groups were completed in cohorts of 2–3 dams per recording and MS recording did not overlap with LBN or control recordings. Using Ethovision, maternal behaviors were manually scored on PD8 from the overhead video. To sample maternal behaviors throughout PD8, three 1 h-long epochs were analyzed: 8:00 a.m.-9:00 a.m. (prior to MS separation), 12:00 p.m.-1:00 p.m. (immediately after MS pup return), 11:00 p.m. - 12:00 a.m. (dark-phase). The 11:00PM timepoint was chosen so as to assess maternal behavior during the dark cycle that had stabilized post lights-off. The remainder of videos were cataloged and can be made available upon request for further evaluation. On PD10, cages were returned to the vivarium, where they continued either one additional day of ELA rearing or control conditions.
2.5. Maternal behavior
On PD8, home-cage recordings were analyzed during three 1-h time points starting at 8AM, 12PM, and 11PM. The following behaviors were manually scored based on overhead recordings: frequency of nest sorties, on-nest duration, nest fluffing, licking and grooming of pups, nursing of pups, self-grooming (dam), and eating and drinking. The frequency of nest sorties was calculated by quantifying the frequency of dam entries and then exits from the nest. Nest entries were defined by the dam having at least two paws on nest and physically touching at least one pup. The overall duration that the dam spent on the nest was measured as well as the length of the nest sortie bouts (duration that animal remained on the nest following an entry). The average nest sortie duration was calculated by dividing the total time spent on the nest by the frequency of nest sorties. Nest fluffing was defined as the dam building or moving portions of the nest, which can (but do not always) co-occur with the time spent on-nest. Nursing of pups was defined by the overall position of the dam, inclusive of both arched-back and passive nursing, and at least one pup in clear proximity and position to be latched onto dam. Thus, the time spent nursing always co-occurred with time on-nest. Licking and grooming of pups was determined based on the head movement and paw position of the dam, in which contact between dam nose and paws (to orient pup) were made with pup body. The time spent licking and grooming typically coincided with time on nest, with the few exceptions of dam licking and grooming a pup that had been moved off the nest, and sometimes coincided with time spent nursing. The time spent self-grooming was defined by dams in upright position exhibiting stroke-like arm motions towards the face and body. The time spent eating or drinking was defined as active licking of the water spout or active ingesting of food at food hopper and elsewhere.
2.6. Entropy score
The entropy score is a summary measure of randomness or unpredictability of a probability distribution. The entropy rate can be used to measure the unpredictability of a sequence of observed behaviors, in this case the 7 maternal behaviors described above. The dam's sequence of behaviors was characterized using the empirical transition matrix <pij> i,j = 1 … 7 of conditional probabilities of moving from one behavior (behavior i) to another (behavior j). The entropy rate of each dam was calculated from the transition matrix as previously described (Molet et al., 2016a, Molet et al., 2016b; Vegetabile et al., 2019) and as follows:
where pij is the conditional probability that maternal behavior j is observed next after a mother is observed performing behavior i, πi is the frequency with which behavior i is observed, and M (=7) is the number of different behaviors. Entropy was calculated using R (version 4.0.3; code available at https://github.com/bvegetabile/entropyrate).
2.7. Brain tissue extraction, RNA purification, and cDNA synthesis
In separate cohorts of mice that did not undergo maternal behavior recordings, brain punches were collected from the amygdala in order to measure relative abundance of gene transcripts that could serve as markers of the levels of Crh-related gene expression in response to ELA. At predefined timepoints (PD8, PD16, PD21), mice were euthanized between 8:30AM and 9:30AM by rapid decapitation and the full brain was immediately extracted and hand-dissected using Allen Brain Atlas as a guide (Franklin and Paxinos, 2019). To eliminate potential effects due to a reduction in litter size, litters were maintained at ∼70% of the initial size such that either a maximum of two pups were taken in litters that would continue to develop to later time points or whereby all pups were euthanized at that timepoint, with two pups per sex (4 total) being included in these analyses, to eliminate potential cohort effects. This led to a total of 23 litters (control:8, LBN: 7, MS, 8) for collection of 112 pups for an n = 6–7 per group. Amygdala punches were collected and homogenized in RNAzol (Molecular Research Center, Cincinnati, OH) and stored at −20 °C until processing. Total RNA was isolated using the manufacturer's protocol. After precipitation, the RNA pellet was washed 3x in 75% ethanol, solubilized in 30 μl of RNAsecure resuspension solution (Thermo Fisher Scientific, CAT: AM7010) and stored in −20 °C until further processing. Single stranded cDNA was synthesized using random priming (N7 primers) with the aid of New England Biolabs MmULV protocols (NEB, Ipswitch, MA). cDNA was solubilized in 25 μl of 10 mM Tris-HCl, pH 7.5. 1 mM EDTA. Due to inadequate RNA or cDNA extractions, a total of 16 samples were excluded from the analysis, leading to a total of 96 pups composed of n = 5–7 per group, with the exception of LBN male PD8 and LBN male PD21 groups which included n = 4.
2.8. Realtime qPCR
Predesigned and pre-validated Taqman assays from Applied Biosystems (Life Technologies, Norwalk, CT) were run in triplicates with the housekeeping gene 18s rRNA (Thermo Fisher Scientific, CAT: 4310893E). Relative gene expression was calculated for Crh (Mm01293920_S1), CrhBP (Mm01283832_m1), CrhR1 (Mm00432670_m1), and CrhR2 (Mm00438308_m1) at PD8, PD16 and PD21 using the fold change method (2−ΔΔCt). The triplicate cycle threshold (Ct) values were averaged for the gene of interest and 18s for each sample to calculate the CTΔ between the gene of interest and 18s and then Δ from the control group. Female controls at each time point were used as the control group, to test for effects of rearing and rearing x sex interactions. All gene expression profiling was completed using CFX384 RT-qPCR system (Biorad, Hercules, CA).
2.9. Statistical analysis
We first screened for outliers, removing values that were more than 3 standard deviations away from the mean. We then performed one-way analysis of variance (ANOVAs) to test for effects of rearing group on maternal behaviors. Maternal behaviors were assessed as a cumulative score on PD8 where three 1-h blocks of data were sampled across the 24hr period (8:00AM, 12:00PM, 11:00PM). For this cumulative data point, entropy, nest bout length, duration spent on nest, fluffing, nursing, self-grooming, and eating and drinking were averaged across the three timepoints and frequency of nest sorties and pup licking were summed across three time points. To test for changes specific to the time of day, a repeated measures ANOVA was used to test for rearing x time interactions for each behavior. Significant main effects (alpha = 0.05) were followed up with post-hoc tests using Tukey's correction for multiple comparisons. In addition, due to a-priori hypotheses regarding changes associated with pup reunion in MS groups and prior results from our lab indicating circadian effects of LBN on maternal behavior (Gallo et al., 2019), post-hoc tests included comparisons at each of the 3 h-long timepoints. Two-way ANOVAs were used to test for changes as a result of rearing, sex and a sex x rearing interaction on the expression of Crh, CrhBP, CrhR1, and CrhR2 at PD8, PD16 and PD21. Effect sizes were estimated by calculating the value of Partial η2 (SSeffect + SSerror/SSeffect) and power analysis were calculated using G*Power 3.1 tool.
3. Results
3.1. LBN rearing increased dam entropy score, indexing unpredictable sequences of dam behavior
The type of rearing experienced during early life altered the predictability of maternal care (F2,13 = 7.581, p = 0.006, η2partial = 0.538, 1-β = 0.938) on PD8, as indexed by an elevation in the entropy score in the LBN manipulation (Fig. 1B). The average entropy score at PD8 of dams in LBN cages was higher compared to controls (p = 0.005). The entropy score in LBN dams was also higher than MS, but this difference did not reach statistical significance (p = 0.070). To determine if either the positive effect of treatment (or lack of effect) on entropy score were driven by a particular time point, a rearing x time interaction was tested for, using entropy scores calculated at 8:00AM, 12:00PM, and 11:00PM. The effect of treatment on entropy did not interact with the time of day (rearing*time: F4,26 = 0.355, p = 0.838, η2partial = 0.051, 1-β = 0.082; Fig. 1C), suggesting that the overall effect of rearing was not dependent on a particular timepoint. Due to the return of MS pups at 12:00PM, additional post-hoc comparisons were conducted. MS did not change entropy score compared to controls when pups were returned at 12:00PM (p = 0.656). LBN dams had a higher entropy score than controls dams at the 12:00PM timepoint (p = 0.022).
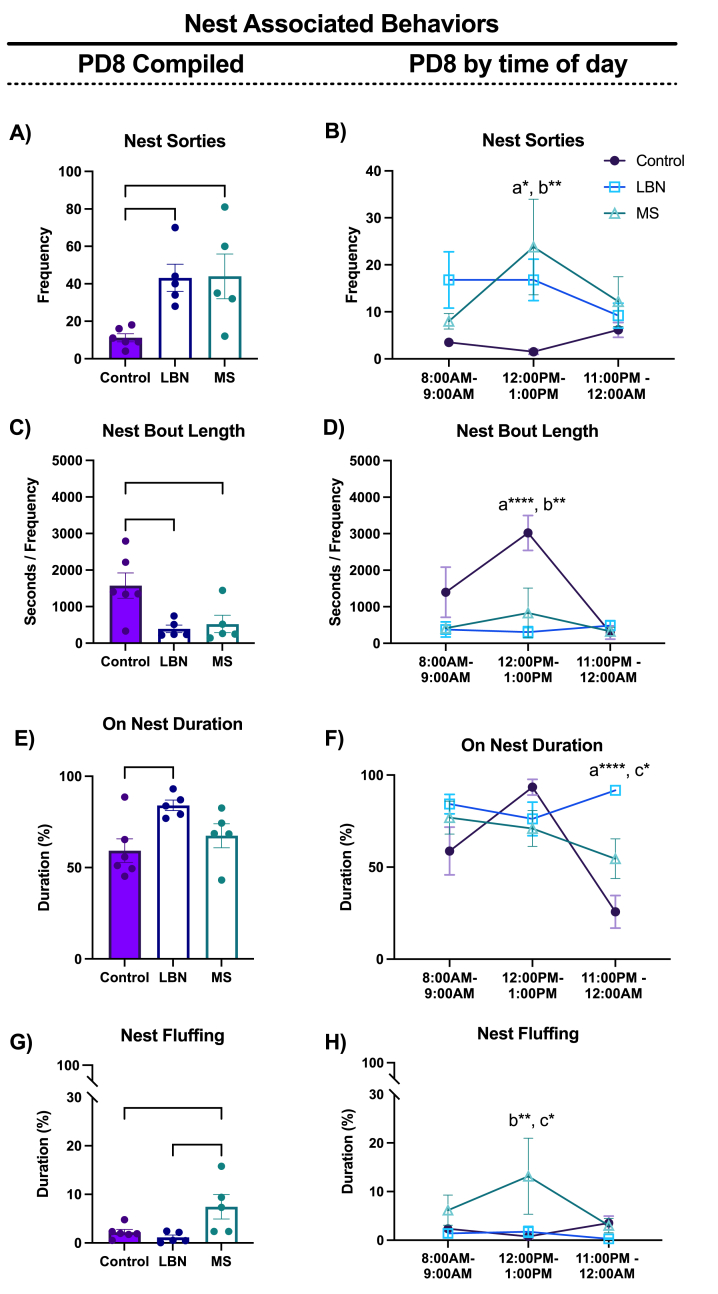
3.2. LBN and MS increased the frequency of dam sorties from the nest and decreased the length of time on nest
The type of rearing condition significantly influenced all nest-directed behaviors measured at PD8: frequency of nest sorties (F2,13 = 6.162, p = 0.013, η2partial = 0.486, 1-β = 0.880; Fig. 2A), the length of on-nest bouts (F2,13 = 6.199, p = 0.012, η2partial = 0.488, 1-β = 0.882; Fig. 2C), the time that the dam spent on nest (F2,13 = 4.811, p = 0.027, η2partial = 0.425, 1-β = 0.785; Fig. 2E), and nest fluffing (F2,13 = 5.394, p = 0.019, η2partial = 0.453, 1-β = 0.832; Fig. 2G). LBN dam nest sortie frequency was increased (p = 0.029) and resulted in shorter length of bouts on the nest (p = 0.018), a well-documented consequence of LBN (Rice et al., 2008; Gallo et al., 2019; Heun-Johnson and Levitt 2016). LBN dams also spent more time on the nest compared to controls (p = 0.026). MS also led to an increase frequency of nest sorties (p = 0.029) and decreased on-nest bout length (p = 0.036) but did not alter the time spent on nest compared to control dams (p = 0.580). Nest fluffing was the only nest-directed behavior that was altered by MS, but not by LBN, with MS dams spending greater time fluffing the nest compared to both control (p = 0.049) and LBN (p = 0.023) dams.
Fig. 2.
Nest Associated Behaviors: Nest associated behaviors were sampled across three 1-h timepoints on PD8. Behaviors were analyzed cumulatively and also at each timepoint to assess for changes associated with time of day and MS pup reunion. A) LBN and MS increased the frequency of nest sorties. B) The observed increase in nest sorties in LBN and MS dams occurred at 12PM. C) LBN and MS dams spent shorter periods of time on nest relative to controls. D) The nest bout lengths in LBN and MS dams were shorter relative to controls at 12PM. E) LBN dams spent more time on the nest compared to controls. F) The observed LBN increase in on-nest duration occurred at 11PM and was greater compared to controls and MS. G) MS dams spent more time fluffing their nest. H) MS dams increased their nest fluffing compared to controls and LBN at 12PM, when pups are returned from 3hr separation. n/group = 5–6. Plots depict mean ± SEM; circles represent individual dams; “a” represents LBN different to control at that time point. “b” represents MS different to control at that time point. “c” represents LBN and MS different to eachother at that timepoint. (p* <0.05, **<0.01, ****<0.0001).
Due to diurnal fluctuations of behavior of mice, the levels of maternal behavior have the potential to vary across the time points measured (Butler-Struben et al. 2022; Gallo et al., 2019). Further, MS pups were returned to the dam just prior to the 12:00PM time point, which could influence behaviors measured at this time point. To test for effects of rearing condition on maternal behaviors across time, we tested for interaction between rearing condition x time of day. The effect of rearing on nest sorties were not dependent on time of day (rearing x time: F4,26 = 1.879, p = 0.144, η2partial = 0.224, 1-β = 0.265). Follow-up post-hoc analysis at 12:00PM was conducted to directly assess the contribution of pup return to MS dams on nest sorties which revealed that MS dams did show increased nest sorties compared to controls dams immediately following being reunited with pups (p = 0.002; Fig. 2B). At this same time point we also observed higher levels of nest sorties in LBN dams (p = 0.050; Fig. 2B). The effect of rearing on nest bout length was dependent on the time of day the measure was collected (rearing x time: F4,26 = 4.634, p = 0.005, η2partial = 0.416, 1-β = 0.605). Both LBN and MS dams exhibited a shorter length of nest bouts compared to controls (p < 0.0001 and p = 0.001, respectively) at 12:00PM timepoint, but not at either 8:00AM or 11:00PM timepoints (p > 0.05; Fig. 2D). A rearing x time interaction (F4,26 = 6.729, p < 0.001, η2partial = 0.508, 1-β = 0.785) was also observed for the duration of time spent on nest, with LBN dams spending significantly more time on the nest compared to both control (p < 0.0001) and MS (p = 0.018) at 11:00PM timepoint (Fig. 2F). Further, while a rearing x time interaction was not observed for the duration of nest fluffing (F4,26 = 1.444, p < 0.247, η2partial = 0.181, 1-β = 0.208), post-hoc analysis at 12:00PM was conducted to directly assess the contribution of pup return to MS dams on nest sorties. Nest fluffing was higher in MS compared to controls following pup reunion when MS dams spent longer fluffing their nest than controls (p = 0.007) and LBN (p = 0.021) dams (Fig. 2H).
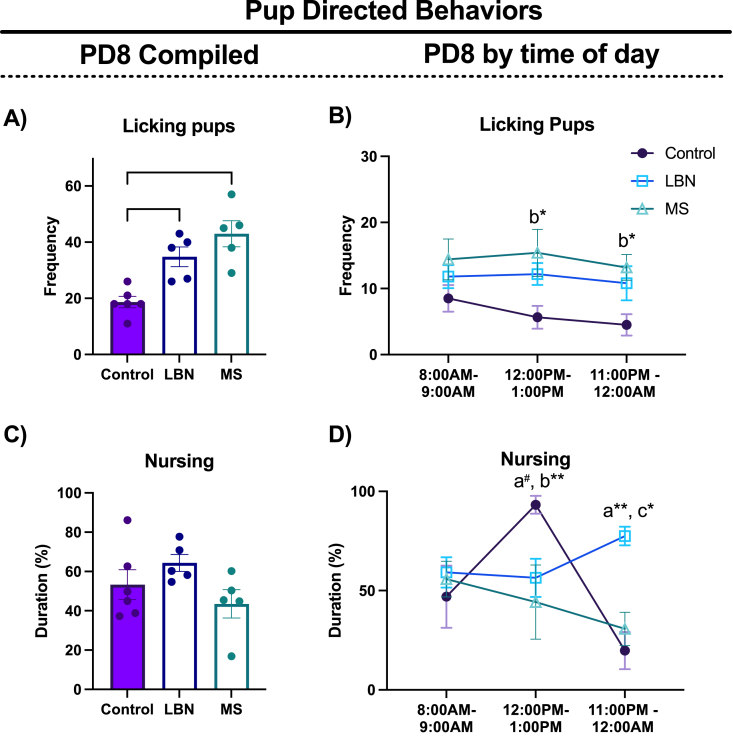
3.3. LBN and MS increased the frequency of licking pups and differentially changed diurnal pattern of nursing behavior
Rearing condition differentially altered pup-directed behaviors of the dam. On PD8, the frequency of licking pups (F2,13 = 13.890, p = 0.0006, η2partial = 0.682, 1-β = 0.997; Fig. 3A), but not duration of nursing (F2,13 = 2.254, p = 0.144, η2partial = 0.257, 1-β = 0.451; Fig. 3C) differed across conditions. Both MS (p = 0.008) and LBN (p = 0.012) dams spent more time licking pups compared to controls. To determine if the effects were driven by a particular timepoint, interaction between rearing condition and time on pup directed behaviors were tested for. For frequency of licking pups, the interaction between rearing condition and time was not significant (rearing x time: F4,26 = 2.95, p = 0.878, η2partial = 0.043, 1-β = 0.077; Fig. 3B). Due to the manipulation returning pups to the dam in the MS condition at 12PM, planned post-hoc tests were carried out for this time point and revealed that MS dams showed increased frequency of licking pups compared to controls at 12:00PM (p = 0.011), and that this effect was also present at the 11:00PM timepoint (p = 0.026; Fig. 3B). A significant interaction between rearing and time of day was found for time spent nursing (rearing x time: F4,26 = 6.583, p = 0.0008, η2partial = 0.502, 1-β = 0.773; Fig. 3D). MS dams nursed less compared to controls at the 12:00PM timepoint (p = 0.006), and a similar trend was observed for the LBN dams (p = 0.051; Fig. 3D). At 11:00PM, no differences between MS dams and controls were found for duration of nursing (p = 0.825); in contrast, LBN dams spent more time nursing compared to controls (p = 0.001) suggesting that, although the time spent nursing may have occurred at different times of day, their overall nursing time over the day was comparable to that of controls (Molet et al., 2016a, Molet et al., 2016b).
Fig. 3.
Pup Directed Behaviors: Pup directed behaviors were analyzed cumulatively across three 1-h long sessions and analyzed at each time point. A) LBN and MS increased the frequency of dams licking their pups. B) When analyzed across time of day, MS dams increased their frequency compared to controls at 12PM, when pups were reunited, and at 11PM. C) Type of rearing did not alter cumulative duration nursing on PD8. D) LBN and MS dams spent less time nursing relative to controls at 12PM. At 11PM, LBN dams increased their duration nursing compared to controls and MS dams. n/group = 5–6. Plots depict mean ± SEM; circles represent individual dams; “a” represents LBN different to control at that time point. “b” represents MS different to control at that time point. “c” represents LBN and MS different to each other at that timepoint. (p# = 0.051, * <0.05, **<0.01).
3.4. Rearing condition differentially changed quantity of dam self-directed behaviors
Dam self-directed behaviors were also altered as a result of rearing condition. There was a significant main effect of rearing on overall time spent self-grooming (F2,13 = 3.968, p = 0.045, η2partial = 0.379, 1-β = 0.701; Fig. 4A) and eating and drinking (F2,13 = 4.484, p = 0.030, η2partial = 0.408, 1-β = 0.755; Fig. 4C) on PD8. LBN dams trended towards spending more time self-grooming than MS dams (p = 0.055) and decreased time eating and drinking compared to controls dams (p = 0.033). To test if these changes were driven by a particular time of day, we tested for interactions between time of day and the measures of self-grooming, eating, and drinking. We found that the effects of rearing on self-grooming were not dependent on time of day (rearing x time: F4,26 = 2.207, p = 0.096, η2partial = 0.253, 1-β = 0.308; Fig. 4B). A significant interaction was observed between time of day and time spent eating and drinking (rearing x time: F4,26 = 7.610, p = 0.0003, η2partial = 0.539, 1-β = 0.837; Fig. 3D). Post-hoc tests were conducted for both self-grooming and eating and drinking to directly test for changes associated with pup reunion to MS dams and circadian cycle, which we have observed previously (Gallo et al., 2019). MS rearing did not change the duration of self-directed behaviors upon pup reunion (p > 0.05). LBN dams spent more time self-grooming compared to controls at 11:00PM, during the dark cycle (p = 0.009), at which point both LBN and MS dams spent less time eating and drinking compared to controls (p < 0.0001 and p = 0.014, respectively).
Fig. 4.
Self Directed Behaviors: The duration spent self-grooming and eating and drinking was analyzed cumulatively on PD8 and across the sampled 1-h long time points. A) LBN dams trended towards increased time spent self-grooming compared to MS dams. B) At 11PM, LBN dams spent longer time self-grooming compared to control dams. C) LBN dams spent less time eating and drinking relative to control dams. D) Both LBN and MS decreased the amount of time eating and drinking relative to controls at 11PM and LBN dams spent less time eating and drinking compared to MS dams. Plots depict mean ± SEM; circles represent individual dams; “a” represents LBN different to control at that time point. “b” represents MS different to control at that time point. “c” represents LBN and MS different to each other at that timepoint. (p* <0.05, **<0.01, ****<0.0001).
3.5. Expression of Crh-related genes in the amygdala in male and female mice are differentially influenced by LBN and MS rearing
The expression, and potentially function, of Crh and its receptors are influenced by early life experiences (Eghbal-Ahmadi et al., 1997; Bolton et al., 2020; Short et al., 2021). Therefore, we tested for effects of rearing condition on the expression of Crh-related genes in the amygdala. Specifically, the expression of Crh, CrhBP, CrhR1 and CrhR2 mRNA was quantified at three developmental time points. This included PD8, four days following the start of differential rearing, PD16, five days following the end of adversity rearing, and PD21, prior to weaning. Sex was considered as an important parameter that could moderate the effects of ELA on developmental gene expression. Thus, we tested for main effects of rearing condition in addition to main effects of sex and sex x rearing interactions.
No significant main effect of rearing was found for the expression of Crh at PD8, PD16 or PD21 (Fig. .5A–C). However, a significant sex x rearing interaction was found for the levels of Crh at PD16 (F2,27 = 6.138, p = 0.006, η2partial = 0.312, 1-β = 0.915; Fig. 5B). Higher expression of Crh was found in males who experienced MS compared to both control (p = 0.002) and LBN males (p = 0.008). The expression of CrhBP was also found to be altered at PD16, leading to both a main effect of rearing (F2,30 = 4.375, p = 0.021, η2partial = 0.225, 1-β = 0.791) and a sex x rearing interaction (F2,30 = 5.313, p = 0.011, η2partial = 0.261, 1-β = 0.868; Fig. 5E). The type of ELA experienced increased CrhBP expression levels, with effects being sex-dependent. LBN led to higher levels of CrhBP in females (p = 0.028), but not males (p = 0.991) and MS increased CrhBP levels in males (p = 0.011), but not females (p = 0.261). By PD21, CrhBP expression levels were equivalent across all rearing groups in females. However, for males, levels of CrhBP in MS reared mice remained elevated compared to both control males (p = 0.007) and LBN males (p = 0.006), evidenced by a sexrearing interaction (F2,28 = 6.194, p = 0.005, η2partial = 0.306, 1-β = 0.933, Fig. 5F). Rearing condition also led increased CrhR1 expression, with effects emerging as early as PD8 (F2,28 = 5.449, p = 0.009, η2partial = 0.254, 1-β = 0.876; Fig. 5G), persisting through PD16 (F2,28 = 6.021, p = 0.006, η2partial = 0.301, 1-β = 0.909; Fig. 5H) and lasting into PD21 (F2,27 = 3.708, p = 0.037, η2partial = 0.215, 1-β = 0.721; Fig. 5I). At PD8, MS reared mice had elevated CrhR1 expression levels compared to controls (p = 0.008, Fig. 5G). At PD16 a sex x rearing interaction (F2,28 = 3.77, p = 0.035, η2partial = 0.212, 1-β = 0.713; Fig. 5H) revealed increased CrhR1 expression in LBN females compared to controls females (p = 0.028) and higher CrhR1 levels in MS males compared to both control (p = 0.010) and LBN males (p = 0.026, Fig. 5H). The expression of CrhR2 was also altered by rearing. At PD8, for CrhR2 we observed both a main effect of rearing condition (F2,31 = 3.435, p = 0.044, η2partial = 0.182, 1-β = 0.685) and sex x rearing interaction (F2,31 = 4.125, p = 0.025, η2partial = 0.201, 1-β = 0.741), with MS males having elevated CrhR2 expression compared to control males (p = 0.007, Fig. 5J). The effect of rearing on CrhR2 expression at PD16 and PD21 appeared altered but did not reach statistical significance (PD16: F2,27 = 3.256, p = 0.054, η2partial = 0.194, 1-β = 0.682; PD21: F2,26 = 3.019, p = 0.055, η2partial = 0.199, 1-β = 0.676, Fig. 5K-L). In summary, the type of adversity experienced led to changes in the expression of Crh-related genes that were often dependent on the sex of the pup.
Fig. 5.
Gene expression of Crh-related genes in the amygdala. Separate cohorts of male and female mouse pups who experienced either control, LBN, or MS rearing were sacrificed at postnatal day (PD)8, PD16, and PD21 for RTqPCR. Relative gene expression was quantified for corticotropin releasing hormone (Crh), Crh binding protein (CrhBP), Crh receptor 1 (CrhR1), and CrhR2 using the ΔΔCt method. Ct values for each sample were used to calculate CTΔ from the control gene (18s) and Δ from the control group (female controls). A-B) MS in males increased Crh relative to control and LBN males at PD16. D-F) At PD16, LBN in females increase CrhBP compared to control females and LBN males. MS in males increased CrhBP at PD16 compared to LBN males and at PD21 relative to control and LBN males. G-I) MS in males increased CrhR1 at PD8 relative to control males and at PD16 relative to control and LBN males. LBN in females increased CrhR1 relative to control females at PD16. J-L) MS in males increased CrhR2 at PD8 relative to control males and MS females. Plots depict mean ± SEM; circles represent individual pups. (p* <0.05, **p < 0.01).
We also tested for a main effect of sex on gene expression, as these differences could provide insight into differential responses to adversity in males and females. For females, Crh gene expression was elevated relative to males at PD8 (F1,26 = 3.871, p = 0.059, η2partial = 0.129, 1-β = 0.788, Fig. 5A) and levels in males were significantly higher than females by PD21 (F1,26 = 9.417, p = 0.005, η2partial = 0.265, 1-β = 0.904, Fig. 5C). CrhBP expression also differed based on the sex of the pup. At PD16 we observed a main effect of sex (F1,30 = 6.522 p = 0.005, η2partial = 0.227, 1-β = 0.882) with LBN females having higher expression of CrhBP compared to LBN males (p = 0.004, Fig. 5E). CrhR1 was not altered by sex at any developmental timepoint (Fig. .5G–I). CrhR2 expression was higher in males at PD8 compared to females (F1,31 = 12.41, p = 0.017, η2partial = 0.168, 1-β = 0.754; Fig. 5J), but not other time points measured. That effect at PD8 appeared to be driven primarily by male MS mice having higher expression than female MS mice (p = 0.002).
4. Discussion
In the current report, the effects of the LBN and MS models of ELA on maternal care behaviors were compared, with a particular focus on entropy score as a quantitative measure of the predictability of dam behavior. Both models of ELA altered maternal behavior compared to control dams. A key distinguishing feature of the two paradigms was the predictability of dam behavior, with LBN dams engaging in more unpredictable sequences of behavior compared to controls. Testing for changes in gene expression in the amygdala, a region particularly vulnerable to postnatal adversity (VanTieghem and Tottenham, 2018; Donnici et al., 2021; D. G. Gee et al., 2013), the type of ELA experienced led to altered expression patterns of Crh-related genes. LBN and MS led to distinct effects on expression profiles of Crh-related genes that emerged in a sex-dependent manner, an indication that alterations in maternal signals resulting from the two different forms of ELA can have disparate effects on male and female brain development.
4.1. LBN provokes unpredictable dam behavior
Higher entropy scores (unpredictable maternal care) were apparent in the LBN but not MS model of ELA. Consistent with prior findings in rats (Molet et al., 2016a, Molet et al., 2016b), higher entropy scores were observed in LBN dams compared to control reared dams, with more robust effects observed during the light phase. The current findings add to the existing literature identifying predictability of care, in addition to fragmentation of care, as a consistent feature of LBN rearing (Gallo et al., 2019; Rice et al., 2008; Baram et al., 2012; Molet et al., 2016a, Molet et al., 2016b). A conflicting report failed to find a similar heightened entropy score using the LBN model in rats (Granata et al., 2021). The single time point, and shorter bout of observation used in that report may not have provided sufficient sampling to observe alterations in entropy. In humans, unpredictable maternal care is associated with poor regulation of the stress response in infants (Noroña-Zhou et al., 2020), with cognitive dysfunction later in life (Davis et al., 2017, 2019; Luby et al., 2020) and with poorer effortful control, a key predictor of emotional health (Davis et al., 2019). Given the cross-species influence of unpredictable maternal care on emotional dysfunction, the LBN model may provide a useful translational model to understand the mechanistic effects of early life unpredictability on human pathology.
4.2. LBN and MS lead to fragmented dam behavior
Both LBN and MS rearing led to increased nest sorties and a decrease in the duration of on-nest bouts. It has been well established that LBN leads to fragmented maternal care as indexed by increased nest sorties and decreased nest bout length (Gallo et al., 2019; Rice et al., 2008; Baram et al., 2012; Orso et al., 2019; Heun-Johnson and Levitt 2016). Our results confirm this feature of the LBN manipulation and add to the literature identifying a fragmentation in maternal care provided during the MS ELA manipulations in mice. The fragmentation in care resulting from MS was primarily observed immediately following the return of pups to the dam, with behavior largely normalized to the levels observed in controls across the other timepoints. Thus, while both LBN and MS do disrupt continuity of care, these effects are more persistent throughout the manipulation in the LBN paradigm and are more restricted and time sensitive (and possibly predictable) in the MS manipulation. We also observed that LBN led to increased quantity of care but these patterns of care were more erratic (shorter bouts and higher entropy score).
To determine if LBN and MS changed pup-directed and self-directed care and if these differences were dependent on time of day or MS pup reunion, several behaviors were quantified across three, 1 h-long timepoints. Consistent with prior work in our lab, LBN rearing influenced normative circadian patterns of dam behavior (Gallo et al., 2019; Ivy et al., 2008). Notably, while the overall duration that the LBN dams spent nursing pups was not different from control reared dams, the pattern of nursing exhibited during the 12PM and 11PM time points were shifted. Developing offspring rely on maternal signals for circadian entrainment (Agorastos et al., 2019; Thomas et al., 2014). A disrupted circadian pattern of care during a sensitive developmental window can lead to lasting consequences such as disrupted circadian patterns as adults that are indicative of depressive-like pathology and avoidance behaviors indicative of anxiety-like pathology (Smarr et al., 2017; Goodwill et al., 2018). Changes observed in MS dams were primarily pup and nest directed at 12PM when pups were reunited with the dam, with the exception of licking and grooming which lasted into the 11PM timepoint. The current observation of increased pup-directed behaviors following 3hrs of maternal separation are consistent with others who have reported increased maternal care on reunion (Own and Patel 2013; Singh-Taylor et al., 2018; Fenoglio et al., 2005). While evidence has shown that licking and grooming is a critical programmer of neurodevelopment and can be protective against later stressors (Caldji et al., 1998; Liu et al., 1997; D. L. Champagne et al., 2008; Meaney and Szyf, 2005; de Medeiros et al., 2009; F. Champagne et al., 2001), it is still unclear if ELA-associated increases in licking and grooming portends similar effects (Berman et al. 2014). Conflicting reports as to the resiliency of MS-induced increased care upon reunion may be due to individual differences in care received by pups. For example, dam licking post-MS has been shown to be biased towards male pups and the types of alarm calls produced by pups are both sex and experience-dependent (Granata et al., 2021). Individual differences in maternal care, such as licking and grooming and nursing, contribute to alterations in the expression of Crh-associated genes in the amygdala (Caldji et al., 1998; Francis et al., 1999a; Francis et al., 1999b; Dubé et al., 2015). Continued work addressing factors such as sex, and environment, and analysis of maternal care on individual pups will be critical in understanding how these individual differences confer differential long-term consequences.
4.3. ELA interacts with sex and rearing type to drive differential effects on Crh-related gene expression
Crh-expressing neurons in the amygdala play a critical role in integrating sensory signals that provide stress-related information and for processing the predictability of external threats. Crh expression is first observed in the neonate amygdala during the first postnatal week and peaks in the second postnatal week, making its developmental trajectory sensitive to maternal signals and changes in the environment. Here, changes in Crh-related gene expression in the amygdala were found following ELA, but differed based on the type of ELA manipulation, sex, or developmental timepoint measured. For example, LBN in females, but not males, increased the expression of CrhBP and CrhR1 at PD16, four days following the end of the LBN manipulation. While the unique developmental roles of the receptors and CrhBP are still being established, evidence supports that heightened expression in the neonate amygdala is indicative of a transition from a hypo-functional to a hyper-functional amygdala and supports accelerated aversive learning (Moriceau and Sullivan 2004, 2006; Moriceau et al., 2004, Moriceau et al., 2006; Vazquez et al., 2006; Korosi and Baram 2008). Further, it has been postulated that CrhR1 and CrhR2 play opposing roles whereby the former is anxiogenic and the latter is anxiolytic, mediating stress recovery (Reul and Holsboer 2002), and both receptors are altered by a variety of early-life experiences (Vazquez et al., 2006; Wang et al., 2011; Eghbal-Ahmadi et al., 1997). The observed changes in CrhR1 but not in CrhR2 as a result of LBN in females may reflect their opposing roles. While the current experimental design did not allow us to assess the direct relationship between gene expression and entropy score, we can speculate that these maternal signals were critical in influencing offspring gene expression profiles, indicating that female LBN mice may be more responsive to early life unpredictability than male LBN mice. In fact, the current results fit well with prior work showing that unpredictable foot shock early in life leads to female-specific changes in the expression Crh-related genes in the adult amygdala (Prusator and Greenwood-Van Meerveld, 2017). A possibility is that unpredictable sensory-input, both maternally-derived and/or environmentally-derived (i.e. unpredictable foot shock), influences the maturation of systems supporting the processing and regulation of emotions. Continued work pursuing (un)predictability as a potential mediator of normative Crh-related expression in both sexes will be a strong target for interventions that are specific to the sex of the individual.
In contrast, MS led to male-specific increased expression of Crh, CrhBP, and CrhR1 at PD16, four days following the termination of ELA exposure, with the effects on CrhR1 emerging as early as four days after the onset of the MS treatment. These results are consistent with some prior reports showing an enhancement of Crh expression with daily 15 min maternal separation in the male amygdala (Fenoglio et al., 2004) that is proposed to primarily occur due to augmented maternal sensory input, such as licking and grooming (Sánchez et al., 2001). Further, some reports show male-specific consequences on cognitive functioning and anxiety-like behaviors, as a result of this form of ELA (Xu et al., 2018; Demaestri et al., 2020), which may result from Crh-related changes in males associated with MS rearing. One may also speculate that ELA interacts with sex bidirectionally via a change in the way that the dam interacts with pups and in the way that pups interact with the dam. Specifically, pup vocalizations play an important role in facilitating dam maternal care (Muller et al. 2010). A recent study revealed sex dependent effects of type of ELA on the developmental trajectory of pup vocalization, where altered patterns of vocalizations were observed in MS males, but not females, and in LBN females, but not males (Granata et al., 2021).
The observed sex x rearing interaction in gene expression of Crh-related genes in the neonate amygdala provides evidence for sexually dimorphic responsivity to different early life conditions and highlights the complex nature of the effects of adversity in driving outcomes. Additional work exploring the functional role of these genes in mediating early brain maturation will inform how each may be differentially regulated by different types of experiences and will be critical to understanding sex-specific vulnerability to pathology.
4.4. Limitations
The current results should be interpreted in the context of some limitations. (1) In order to limit cannibalism associated with cage disturbance of newly born pups, the litter size and sex distribution of the litters were not directly manipulated. However, rearing groups were assigned randomly and gestational housing conditions were maintained constant across groups, such that factors that could influence litter size, such as the size and age of the dam and nutrition (R. J. Berry 1970; C.A. Finn 1963; Holt et al. 2004), would be due to chance. Furthermore, while some evidence indicates that dams rearing all male litters show increased licking and grooming compared to all female litters (Alleva et al. 1989), others fail to observe maternal behavior changes associated with litter sex composition (Namikas and Wehmer 1978; Musi et al. 1993). These mixed reports may partially result from experimental manipulation of culling litters, cross-fostering, and comparing litters composed fully of one sex to the other. Therefore, additional work carefully evaluating maternal care directed to either males or females within mixed litters is needed to assess its impact on maternal behavior. (2) The maternal behavior and gene expression studies were not completed in the same groups of mice and, thus, we were unable to directly test for a relationship between maternal behaviors and the expression of Crh-related genes in pups. Future studies assessing the relationship between maternal behavior received by individual pups and gene expression changes will uncover whether individual differences in gene expression profiles are influenced by specific patterns of maternal care.
4.5. Conclusion
The current work details the impact of two different forms of ELA (MS and LBN) on maternal behaviors predictability and quantity and tested the impact of these forms of ELA on Crh-gene expression in the amygdala. LBN rearing led to unpredictable and fragmented care that altered the Crh expression profiles in the amygdala in female pups. MS rearing led to fragmented maternal care, altered pup-directed care driven primarily when pups and dam were reunited and led to male-specific changes of Crh-related genes in the amygdala. Continued work investigating the interactions between early experience and sex as a result of environmental manipulations will be crucial to understand individual differences in risk for negative mental health outcomes and informing ways to improve the timing and type of treatments and interventions.
CRediT authorship contribution statement
Camila Demaestri: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization, Project administration. Meghan Gallo: Conceptualization, Methodology, Validation, Investigation, Resources, Writing – review & editing. Elisa Mazenod: Validation, Investigation, Data curation. Alexander T. Hong: Investigation, Data curation. Hina Arora: Formal analysis, Methodology, Data curation. Annabel K. Short: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Hal Stern: Conceptualization, Methodology, Supervision. Tallie Z. Baram: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Supervision. Kevin G. Bath: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
No competing financial interests or any other conflicts of interest exist.
Acknowledgements
The work was supported by the National Institutes of Health RO1-MH115914 (KGB), RO1-MH115049 (KGB) and F31-MH127888 (CD). We would also like to acknowledge and thank Dayshalis Ofray, Madalyn Critz, and Tracy Pan for their support in various technical aspects of the project and Dr. Jocelyn Breton for thoughtful discussions.
Data availability
Data will be made available on request.
References
- Agorastos Agorastos, Pervanidou Panagiota, Chrousos George P., Baker Dewleen G. Developmental trajectories of early life stress and trauma: a narrative review on neurobiological aspects beyond stress system dysregulation. Front. Psychiatr. 2019;10(March):118. doi: 10.3389/fpsyt.2019.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleva E., Caprioli A., Laviola G. Litter gender composition affects maternal behavior of the primiparous mouse dam (Mus Musculus) J. Compar. Psychol. 1989;103(1):83–87. doi: 10.1037/0735-7036.103.1.83. 1983. [DOI] [PubMed] [Google Scholar]
- Baram Tallie Z., Davis Elysia P., Andre Obenaus. Sandman Curt A., Small Steven L., Ana Solodkin, Stern Hal. Fragmentation and unpredictability of early-life experience in mental disorders. Am. J. Psychiatr. 2012;169(9):907–915. doi: 10.1176/appi.ajp.2012.11091347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkat Tania Rinaldi, Polley Daniel B., Hensch Takao K. A critical period for auditory thalamocortical connectivity. Nat. Neurosci. 2011;14(9):1189–1194. doi: 10.1038/nn.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath K.G., Manzano-Nieves G., Goodwill H. Early life stress accelerates behavioral and neural maturation of the Hippocampus in male mice. Horm. Behav. 2016;82(June):64–71. doi: 10.1016/j.yhbeh.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman Ariel Kupfer, Lott Rhonda B., Tiffany Donaldson S. Periodic maternal deprivation may modulate offspring anxiety-like behavior through mechanisms involving neuroplasticity in the amygdala. Brain Res. Bull. 2014;101(February):7–11. doi: 10.1016/j.brainresbull.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry R.J. The natural history of the house mouse. Fld. Stud. 1970;3:219–262. [Google Scholar]
- Birnie Matthew T., Baram Tallie Z. Principles of emotional brain circuit maturation. Science. 2022;376(6597):1055–1056. doi: 10.1126/science.abn4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton Jessica L., Jenny Molet, Regev Limor, Chen Yuncai, Rismanchi Neggy, Haddad Elizabeth, Yang Derek Z., Obenaus Andre, Tallie Z., Baram Anhedonia following early-life adversity involves aberrant interaction of reward and anxiety circuits and is reversed by partial silencing of amygdala corticotropin-releasing hormone gene. Biol. Psychiatr. 2018;83(2):137–147. doi: 10.1016/j.biopsych.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton Jessica L., Katherine Short Annabel, Simeone Kristina A., Daglian Jennifer, Baram Tallie Z. Programming of stress-sensitive neurons and circuits by early-life experiences. Front. Behav. Neurosci. 2019;13:30. doi: 10.3389/fnbeh.2019.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton Jessica L., Anton Schulmann, Garcia-Curran Megan M., Regev Limor, Chen Yuncai, Kamei Noriko, Shao Manlin, et al. Unexpected transcriptional programs contribute to hippocampal memory deficits and neuronal stunting after early-life adversity. Cell Rep. 2020;33(11) doi: 10.1016/j.celrep.2020.108511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler-Struben, Hanna M., Kentner Amanda C., Trainor Brian C. What's wrong with my experiment?: the impact of hidden variables on neuropsychopharmacology research. Neuropsychopharmacology. 2022;47(7):1285–1291. doi: 10.1038/s41386-022-01309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C., Tannenbaum B., Sharma S., Francis D., Plotsky P.M., Meaney M.J. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc. Natl. Acad. Sci. USA. 1998;95(9):5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne Frances, Diorio Josie, Sharma Shakti, Michael J., Meaney Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc. Natl. Acad. Sci. USA. 2001;98(22):12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne D.L., Bagot R.C., van Hasselt F., Ramakers G., Meaney M.J., de Kloet E.R., Joels M., Krugers H. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J. Neurosci. 2008;28(23):6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Yuncai, Baram Tallie Z. Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology. 2016;41(1):197–206. doi: 10.1038/npp.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis Elysia Poggi, Stout Stephanie A., Jenny Molet, Vegetabile Brian, Glynn Laura M., Sandman Curt A., Heins Kevin, Stern Hal, Tallie Z., Baram Exposure to unpredictable maternal sensory signals influences cognitive development across species. Proc. Natl. Acad. Sci. USA. 2017;114(39):10390–10395. doi: 10.1073/pnas.1703444114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis Elysia Poggi, Korja Riikka, Karlsson Linnea, Glynn Laura M., Sandman Curt A., Vegetabile Brian, Kataja Eeva-Leena, et al. Across continents and demographics, unpredictable maternal signals are associated with children's cognitive function. EBioMedicine. 2019;46(August):256–263. doi: 10.1016/j.ebiom.2019.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaestri Camila, Pan Tracy, Critz Madalyn, Ofray Dayshalis, Gallo Meghan, Kevin G., Bath Type of early life adversity confers differential, sex-dependent effects on early maturational milestones in mice. Horm. Behav. 2020;124 doi: 10.1016/j.yhbeh.2020.104763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnici Claire, Long Xiangyu, Dewey Deborah, Letourneau Nicole, Bennett Landman, Huo Yuankai, Lebel Catherine. Prenatal and postnatal maternal anxiety and amygdala structure and function in young children. Sci. Rep. 2021;11(1):4019. doi: 10.1038/s41598-021-83249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubé Céline M., Jenny Molet, Singh-Taylor Akanksha, Ivy Autumn, Maras Pamela M., Baram Tallie Z. Hyper-excitability and epilepsy generated by chronic early-life stress. Neurobiol. Stress. 2015;2:10–19. doi: 10.1016/j.ynstr.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghbal-Ahmadi M., Hatalski C.G., Avishai-Eliner S., Baram T.Z. Corticotropin releasing factor receptor type II (CRF2) messenger ribonucleic acid levels in the hypothalamic ventromedial nucleus of the infant rat are reduced by maternal deprivation. Endocrinology. 1997;138(11):5048–5051. doi: 10.1210/endo.138.11.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa J. Sebastian, Stryker Michael P. Development and plasticity of the primary visual cortex. Neuron. 2012;75(2):230–249. doi: 10.1016/j.neuron.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio Kristina A., Brunson Kristen L., Avishai-Eliner Sarit, Chen Yuncai, Baram Tallie Z. Region-specific onset of handling-induced changes in corticotropin-releasing factor and glucocorticoid receptor expression. Endocrinology. 2004;145(6):2702–2706. doi: 10.1210/en.2004-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio Kristina A., Brunson Kristen L., Avishai-Eliner Sarit, Stone Blake A., Kapadia Bhumika J., Baram Tallie Z. Enduring, handling-evoked enhancement of hippocampal memory function and glucocorticoid receptor expression involves activation of the corticotropin-releasing factor type 1 receptor. Endocrinology. 2005;146(9):4090–4096. doi: 10.1210/en.2004-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn C.A. Reproductive capacity and litter size in mice: effect of age and environment. J. Reproduct. Fertility. 1963;6:205–214. doi: 10.1530/jrf.0.0060205. [DOI] [PubMed] [Google Scholar]
- Fiori Laura M., Turecki Gustavo. Investigating epigenetic consequences of early-life adversity: some methodological considerations. Eur. J. Psychotraumatol. 2016;7(November) doi: 10.3402/ejpt.v7.31593. 10.3402/ejpt.v7.31593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis Darlene D., Caldji Christian, Champagne Frances, Plotsky Paul M., Meaney Michael J. The role of corticotropin-releasing factor–norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biol. Psychiatr. 1999;46(9):1153–1166. doi: 10.1016/S0006-3223(99)00237-1. [DOI] [PubMed] [Google Scholar]
- Francis Darlene D., Champagne Frances A., Dong Liu, Meaney Michael J. Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann. N. Y. Acad. Sci. 1999;896(1):66–84. doi: 10.1111/j.1749-6632.1999.tb08106.x. [DOI] [PubMed] [Google Scholar]
- Franklin Keith, Paxinos George. 5th Edition. Academic Press; 2019. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates.https://www.elsevier.com/books/paxinos-and-franklins-the-mouse-brain-in-stereotaxic-coordinates-compact/franklin/978-0-12-816159-3 [Google Scholar]
- Gallo Meghan, Shleifer Daniel G., Godoy Livea D., Ofray Dayshalis, Olaniyan Aliyah, Campbell Talia, Kevin G., Bath Limited bedding and nesting induces maternal behavior resembling both hypervigilance and abuse. Front. Behav. Neurosci. 2019;13(July) doi: 10.3389/fnbeh.2019.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H., Hare T.A., Bookheimer S.Y., Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. USA. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee Dylan G., Gabard-Durnam Laurel, Telzer Eva H., Humphreys Kathryn L., Goff Bonnie, Shapiro Mor, Flannery Jessica, et al. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol. Sci. 2014;25(11):2067–2078. doi: 10.1177/0956797614550878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glod Carol A., Teicher Martin H. Relationship between early abuse, posttraumatic stress disorder, and activity levels in prepubertal children. J. Am. Acad. Child Adolesc. Psychiatry. 1996;35(10):1384–1393. doi: 10.1097/00004583-199610000-00026. [DOI] [PubMed] [Google Scholar]
- Glynn Laura M., Baram Tallie Z. The influence of unpredictable, fragmented parental signals on the developing brain. Front. Neuroendocrinol. 2019;53(April) doi: 10.1016/j.yfrne.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy Lívea Dornela, Rossignoli Matheus Teixeira, Delfino-Pereira Polianna, Garcia-Cairasco Norberto, Eduardo Henrique de Lima Umeoka A comprehensive overview on stress neurobiology: basic concepts and clinical implications. Front. Behav. Neurosci. 2018;12 doi: 10.3389/fnbeh.2018.00127. https://www.frontiersin.org/article/10.3389/fnbeh.2018.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwill Haley L., Manzano-Nieves Gabriela, Gallo Meghan, Hye-In Lee, Oyerinde Esther, Serre Thomas, Kevin G., Bath Early life stress leads to sex differences in development of depressive-like outcomes in a mouse model. Neuropsychopharmacology. 2018 doi: 10.1038/s41386-018-0195-5. September. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granata Lauren, Valentine Alissa, Hirsch Jason L., Honeycutt Jennifer, Brenhouse Heather. Trajectories of mother-infant communication: an experiential measure of the impacts of early life adversity. Front. Hum. Neurosci. 2021;15:72. doi: 10.3389/fnhum.2021.632702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger Steven J., Glynn Laura M., Sandman Curt A., Small Steven L., Andre Obenaus. Keator David B., Baram Tallie Z., Stern Hal, Yassa Michael A., Davis Elysia Poggi. Aberrant maturation of the uncinate fasciculus follows exposure to unpredictable patterns of maternal signals. J. Neurosci. 2021;41(6):1242–1250. doi: 10.1523/JNEUROSCI.0374-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haahr-Pedersen Ida, Perera Camila, Hyland Philip, Vallières Frédérique, Murphy David, Hansen Maj, Spitz Pernille, Hansen Pernille, Cloitre Marylène. Females have more complex patterns of childhood adversity: implications for mental, social, and emotional outcomes in adulthood. Eur. J. Psychotraumatol. 2020;11(1) doi: 10.1080/20008198.2019.1708618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch Takao K. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 2005;6(11):877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Herzog Julia I., Schmahl Christian. Adverse childhood experiences and the consequences on neurobiological, psychosocial, and somatic conditions across the lifespan. Front. Psychiatr. 2018;9(September):420. doi: 10.3389/fpsyt.2018.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heun-Johnson Hanke, Levitt Pat. Early-life stress paradigm transiently alters maternal behavior, dam-pup interactions, and offspring vocalizations in mice. Front. Behav. Neurosci. 2016;10(July):142. doi: 10.3389/fnbeh.2016.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt M., Vangen O., Farstad W. Components of litter size in mice after 110 generations of selection. Reproduction. 2004;127(5):587–592. doi: 10.1530/rep.1.00118. [DOI] [PubMed] [Google Scholar]
- Honeycutt Jennifer A., Demaestri Camila, Peterzell Shayna, Silveri Marisa M., Cai Xuezhu, Kulkarni Praveen, Cunningham Miles G., Ferris Craig F., Brenhouse Heather C. In: ELife. Shackman Alexander., editor. vol. 9. 2020. Altered corticolimbic connectivity reveals sex-specific adolescent outcomes in a rat model of early life adversity. January. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy Autumn S., Brunson Kristen L., Sandman Curt, Baram Tallie Z. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154(3):1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin Ned H. Corticotropin-releasing hormone binding protein (CRHBP): stress, psychopathology, and antidepressant treatment response. Am. J. Psychiatr. 2018;175(3):204–206. doi: 10.1176/appi.ajp.2018.18010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas Brian D., Short Annabel K., Oanh T Luc, Stern Hal S., Baram Tallie Z., Pizzagalli Diego A. A cross-species assay demonstrates that reward responsiveness is enduringly impacted by adverse, unpredictable early-life experiences. Neuropsychopharmacology. 2022;47(3):767–775. doi: 10.1038/s41386-021-01250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazipov Rustem, Sirota Anton, Leinekugel Xavier, Holmes Gregory L., Ben-Ari Yehezkel, Buzsáki György. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432(7018):758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- Korosi Aniko, Baram Tallie Z. The central corticotropin releasing factor system during development and adulthood. Eur. J. Pharmacol. 2008;583(2–3):204–214. doi: 10.1016/j.ejphar.2007.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine Seymour. Infantile experience and resistance to physiological stress. Science. 1957;126(3270) doi: 10.1126/science.126.3270.405. 405–405. [DOI] [PubMed] [Google Scholar]
- Levis Sophia C., Bentzley Brandon S., Jenny Molet, Bolton Jessica L., Perrone Christina R., Baram Tallie Z., Mahler Stephen V. On the early life origins of vulnerability to opioid addiction. Mol. Psychiatr. 2021;26(8):4409–4416. doi: 10.1038/s41380-019-0628-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Dong, Diorio Josie, Tannenbaum Beth, Caldji Christian, Francis Darlene, Freedman Alison, Sharma Shakti, Pearson Deborah, Plotsky Paul M., Meaney Michael J. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Luby Joan L., Baram Tallie Z., Rogers Cynthia E., Barch Deanna M. Neurodevelopmental optimization after early-life adversity: cross-species studies to elucidate sensitive periods and brain mechanisms to inform early intervention. Trends Neurosci. 2020;43(10):744–751. doi: 10.1016/j.tins.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malter Cohen M., Jing D., Yang R.R., Tottenham N., Lee F.S., Casey B.J. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc. Natl. Acad. Sci. USA. 2013;110(45):18274–18278. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano Nieves Gabriela, Bravo Marilyn, Baskoylu Saba, Bath Kevin G. In: Hill Matthew N., Kate M Wassum, Walker Claire-Dominique., editors. vol. 9. ELife; 2020. (Early Life Adversity Decreases Pre-adolescent Fear Expression by Accelerating Amygdala PV Cell Development). July. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney Michael J., Szyf Moshe. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin. Neurosci. 2005;7(2):103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros Cynthia B. de, Fleming Alison S., Johnston Celeste C., Walker Claire-Dominique. Artificial rearing of rat pups reveals the beneficial effects of mother care on neonatal inflammation and adult sensitivity to pain. Pediatr. Res. 2009;66(3):272–277. doi: 10.1203/PDR.0b013e3181b1be06. [DOI] [PubMed] [Google Scholar]
- Molet Jenny, Heins K., Zhuo X., Mei Y.T., Regev L., Baram Tallie Z., Stern Hal S. Fragmentation and high entropy of neonatal experience predict adolescent emotional outcome. Transl. Psychiatry. 2016;6(1) doi: 10.1038/tp.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet Jenny, Maras Pamela M., Kinney-Lang Eli, Harris Neil G., Rashid Faisal, Ivy Autumn S., Ana Solodkin, Obenaus Andre, Tallie Z., Baram MRI uncovers disrupted hippocampal microstructure that underlies memory impairments after early-life adversity. Hippocampus. 2016;26(12):1618–1632. doi: 10.1002/hipo.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau Stephanie, Sullivan Regina M. Corticosterone influences on mammalian neonatal sensitive-period learning. Behav. Neurosci. 2004;118(2):274–281. doi: 10.1037/0735-7044.118.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau Stephanie, Sullivan Regina M. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat. Neurosci. 2006;9(8):1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau Stephanie, Roth Tania L., Okotoghaide Terri, Sullivan Regina M. Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. Int. J. Dev. Neurosci. : Off. J. Int. Soc. Develop. Neurosci. 2004;22(5–6):415–422. doi: 10.1016/j.ijdevneu.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau Stephanie, Wilson Donald A., Levine Seymour, Sullivan Regina M. Dual circuitry for odor–shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. J. Neurosci. 2006;26(25):6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau Stephanie, Shionoya Kiseko, Jakubs Katherine, Sullivan Regina M. Early-life stress disrupts attachment learning: the role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. J. Neurosci. 2009;29(50):15745–15755. doi: 10.1523/JNEUROSCI.4106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller Jeff, Brunelli Susan, Shair Harry. In: Brudzynski Stefan M., editor. vol. 19. Elsevier; 2010. Chapter 6.4 - rat infant isolation vocalizations and their modulation by social cues as a model of expression of infantile emotionality. (Handbook of Behavioral Neuroscience). 227–40. Handbook of Mammalian Vocalization. [DOI] [Google Scholar]
- Musi Barbara, de Acetis Luigi, Alleva Enrico. Influence of litter gender composition on subsequent maternal behaviour and maternal aggression in female house mice. Ethology. 1993;95(1):43–53. doi: 10.1111/j.1439-0310.1993.tb00455.x. [DOI] [Google Scholar]
- Namikas Jessie, Wehmer Francine. Gender composition of the litter affects behavior of male mice. Behav. Biol. 1978;23(2):219–224. doi: 10.1016/S0091-6773(78)91830-8. [DOI] [PubMed] [Google Scholar]
- Nelson Charles A., Zeanah Charles H., Fox Nathan A., Marshall Peter J., Smyke Anna T., Guthrie Donald. Cognitive recovery in socially deprived young children: the bucharest early intervention project. Science (New York, N.Y.) 2007;318(5858) doi: 10.1126/science.1143921. 1937–40. [DOI] [PubMed] [Google Scholar]
- Nelson Charles A., Bhutta Zulfiqar A., Harris Nadine Burke, Andrea Danese, Samara Muthanna. Adversity in childhood is linked to mental and physical health throughout life. BMJ. 2020;October doi: 10.1136/bmj.m3048. m3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler Eric J. Stress makes its molecular mark. Nature. 2012;490(7419):171–172. doi: 10.1038/490171a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman Rosana E., Byambaa Munkhtsetseg, De Rumna, Alexander Butchart, Scott James, Vos Theo. The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Med. 2012;9(11) doi: 10.1371/journal.pmed.1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noroña-Zhou Amanda N., Alyssa Morgan, Glynn Laura M., Sandman Curt A., Baram Tallie Z., Stern Hal S., Davis Elysia Poggi. Unpredictable maternal behavior is associated with a blunted infant cortisol response. Dev. Psychobiol. 2020;62(6):882–888. doi: 10.1002/dev.21964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orso Rodrigo, Kerstin Camile Creutzberg. Eduardo Wearick-Silva Luis, Wendt Viola Thiago, Gantes Tractenberg Saulo, Benetti Fernando, Grassi-Oliveira Rodrigo. How early life stress impact maternal care: a systematic review of rodent studies. Front. Behav. Neurosci. 2019;13 doi: 10.3389/fnbeh.2019.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Own Lawrence S., Patel Paresh D. Maternal behavior and offspring resiliency to maternal separation in C57bl/6 mice. Horm. Behav. 2013;63(3):411–417. doi: 10.1016/j.yhbeh.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Peña Catherine Jensen, Nestler Eric J., Bagot Rosemary C. Environmental programming of susceptibility and resilience to stress in adulthood in male mice. Front. Behav. Neurosci. 2019;13 doi: 10.3389/fnbeh.2019.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusator Dawn K., Greenwood-Van Meerveld Beverley. Amygdala-mediated mechanisms regulate visceral hypersensitivity in adult females following early life stress: importance of the glucocorticoid receptor and corticotropin-releasing factor. Pain. 2017;158(2):296–305. doi: 10.1097/j.pain.0000000000000759. [DOI] [PubMed] [Google Scholar]
- Pryce Christopher, Dettling Andrea, Spengler Marianne, Spaete Corinne, Feldon Joram. Annals of the New York Academy of Sciences; 2005. Evidence for Altered Monoamine Activity and Emotional and Cognitive Disturbance in Marmoset Monkeys Exposed to Early Life Stress; pp. 245–249. 1032 (January) [DOI] [PubMed] [Google Scholar]
- Reul Johannes M.H. M., Holsboer Florian. On the role of corticotropin-releasing hormone receptors in anxiety and depression. Dialogues Clin. Neurosci. 2002;4(1):31–46. doi: 10.31887/DCNS.2002.4.1/jreul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice Courtney J., Sandman Curt A., Lenjavi Mohammed R., Baram Tallie Z. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149(10):4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez M.M., Hearn E.F., Do D., Rilling J.K., Herndon J.G. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 1998;812(1–2):38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Sánchez M.Mar, Ladd Charlotte O., Plotsky Paul M. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev. Psychopathol. 2001;13(3):419–449. doi: 10.1017/S0954579401003029. [DOI] [PubMed] [Google Scholar]
- Short Annabel K., Baram Tallie Z. Early-life adversity and neurological disease: age-old questions and novel answers. Nat. Rev. Neurol. 2019;15(11):657–669. doi: 10.1038/s41582-019-0246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short Annabel K., Thai Christina W., Chen Yuncai, Kamei Noriko, Pham Aidan L., Birnie Matthew T., Bolton Jessica L., Ali Mortazavi, Baram Tallie Z. Single-cell transcriptional changes in hypothalamic corticotropin-releasing factor–expressing neurons after early-life adversity inform enduring alterations in vulnerabilities to stress. Biol. Psychiatr. Global Open Sci. 2021 doi: 10.1016/j.bpsgos.2021.12.006. December. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Taylor A., Molet J., Jiang S., Korosi A., Bolton J.L., Noam Y., Simeone K., et al. NRSF-dependent epigenetic mechanisms contribute to programming of stress-sensitive neurons by neonatal experience, promoting resilience. Mol. Psychiatr. 2018;23(3):648–657. doi: 10.1038/mp.2016.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smarr Benjamin L., Grant Azure D., Perez Luz, Zucker Irving, Kriegsfeld Lance J. Maternal and early-life circadian disruption have long-lasting negative consequences on offspring development and adult behavior in mice. Sci. Rep. 2017;7(1):3326. doi: 10.1038/s41598-017-03406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas Karen A., Burr Robert L., Susan Spieker. Lee Jungeun, Chen Jessica. Mother-infant circadian rhythm: development of individual patterns and dyadic synchrony. Early Hum. Dev. 2014;90(12):885–890. doi: 10.1016/j.earlhumdev.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham Nim, Sheridan Margaret. A review of adversity, the amygdala and the Hippocampus: a consideration of developmental timing. Front. Hum. Neurosci. 2010;3 doi: 10.3389/neuro.09.068.2009. https://www.frontiersin.org/article/10.3389/neuro.09.068.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham Nim, Hare Todd A., Quinn Brian T., McCarry Thomas W., Nurse Marcella, Gilhooly Tara, Millner Alexander, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev. Sci. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaiserman Alexander M., Koliada Alexander K. Early-life adversity and long-term neurobehavioral outcomes: epigenome as a bridge? Hum. Genom. 2017;11(1):34. doi: 10.1186/s40246-017-0129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTieghem Michelle R., Tottenham Nim. Neurobiological programming of early life stress: functional development of amygdala-prefrontal circuitry and vulnerability for stress-related psychopathology. Curr. Topics Behav. Neurosci. 2018;38:117–136. doi: 10.1007/7854_2016_42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez Delia M., Bailey Charles, Dent Gersham W., Okimoto Darren K., Amy Steffek, López Juan F., Seymour Levine. Brain corticotropin-releasing hormone (CRH) circuits in the developing rat: effect of maternal deprivation. Brain Res. 2006;1121(1):83–94. doi: 10.1016/j.brainres.2006.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegetabile Brian G., Stout-Oswald Stephanie A., Davis Elysia Poggi, Baram Tallie Z., Stern Hal S. Estimating the entropy rate of finite Markov chains with application to behavior studies. J. Educ. Behav. Stat. 2019;44(3):282–308. doi: 10.3102/1076998618822540. [DOI] [Google Scholar]
- Walker Claire-Dominique, Kevin G., Bath, Marian Joels, Korosi Aniko, Larauche Muriel, Lucassen Paul J., Morris Margaret J., et al. Chronic early life stress induced by limited bedding and nesting (LBN) material in rodents: critical considerations of methodology, outcomes and translational potential. Stress. 2017;20(5):421–448. doi: 10.1080/10253890.2017.1343296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Xiao-Dong, Rammes Gerhard, Kraev Igor, Wolf Miriam, Liebl Claudia, Scharf Sebastian H., Rice Courtney J., et al. Forebrain CRF1 modulates early-life stress-programmed cognitive deficits. J. Neurosci. 2011;31(38):13625–13634. doi: 10.1523/JNEUROSCI.2259-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Hongyu, Ye Yuqin, Yelu Hao, Shi Fei, Yan Zhiqiang, Yuan Guohao, Yang Yuefan, Zhou Fei, He Xiaosheng. Sex differences in associations between maternal deprivation and alterations in hippocampal calcium-binding proteins and cognitive functions in rats. Behav. Brain Funct. : BBF. 2018;14(May):10. doi: 10.1186/s12993-018-0142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.