ABSTRACT
Objectives:
With a relatively high percentage of type I fibers in the vastus medialis (VM), its fatigability may be more sensitive to the effects of muscle activity in the quadriceps. However, sex-related differences in the muscle fatigability of the VM remain unknown. The purpose of the present study was to assess the differences in fatigability of the VM between healthy adult men and women.
Methods:
Surface electromyographic (EMG) activities of VM oblique (VMO) and VM long (VML) were recorded during sustained isometric contraction on a leg press machine. The results of EMG power spectral analysis were compared between healthy adult men and women. The decline in the median frequency (MF), defined as MF slope, was calculated using spectrum analysis after fast Fourier transform of the raw EMG signals of VMO and VML.
Results:
The endurance time and the MF slopes of the VMO and VML were significantly longer and lower, respectively, in women than in men. The present results demonstrated that both VMO and VML are more fatigue-resistant in women than in men.
Conclusions:
Understanding the sex differences in fatigability could help to design more effective exercise regimens for VMO and VML in healthy individuals. A similar approach should be considered when prescribing practical exercise regimens for patients with muscle atrophy.
Keywords: electromyography, muscle fatigue, sex differences, vastus medialis long, vastus medialis oblique
INTRODUCTION
Differences in the fatigability of skeletal muscles between sexes have been reported previously.1,2,3,4,5,6) In general, these studies suggested that the fatigability of muscles is less in women than in men. Furthermore, histological studies reported that type I muscle fibers were more predominant in women than in men.7,8,9,10,11,12,13)
In humans, the quadriceps femoris muscle plays an important role in the activities of daily life and in sports. The quadriceps femoris muscle generates knee-extensor torque and maintains knee stability during extension. Among the quadriceps femoris muscles, the vastus medialis (VM) muscle is the major contributor to knee joint stabilization during extension, thus reducing the risk of knee injuries during physical activity.14) The VM has significant involvement in maintain knee stability, and the importance of the VM oblique (VMO) has recently been recognized.14) The VM consists of the following two components: the VM long and the VM oblique.15) Differences in the morphological and anatomical profiles of the muscle fibers of the VMO and VML, such as musculoaponeurotic geometry, attachment sites, and structural parameters, were revealed previously; the fiber bundles were shorter, proximal and distal pennation angles were greater, and the physiologic cross-sectional area was smaller in the VMO than those in the VML.16) In addition, the VML was found to be connected to the fascia lata via thin fascial bands, but that kind of structure was not observed in the VMO. The posteromedially oriented line of action of the VMO and its connection to the medial aspect of the patella via the medial patellar retinaculum indicate that the VMO contributes to patellar stabilization from the medial aspect. Conversely, given that the VML originates from the linea aspera of the femur, faces a more vertical direction than the VMO, and attaches to the patellar base via the quadriceps tendon, it contributes to force generation for knee extension. Moreover, the percentage of type 1 muscle fibers in the VML was higher than in the VMO.17) Therefore, the fatigabilities of the VMO and the VML may be different. In previous studies, it was reported that the percentage of type I muscle fibers was decreased by a reduction in muscle activity.18) Therefore, the VM that has a relatively high percentage of type I fibers among the quadriceps may be more sensitive to the effect of reduced muscle activity. In fact, among the entire quadriceps, the VML is likely to atrophy during recovery after reconstruction of the anterior cruciate ligament,19) while VMO atrophy is observed considerably more in patients with the patellofemoral pain syndrome.20) Therefore, the differences in the properties of the VMO and VML between healthy adult women and men need to be determined to understand the knee-related symptoms and to design separate training programs for men and women. However, no study has focused on the differences in the fatigability of the VMO and VML between healthy adult women and men.
According to previous studies using electromyographic (EMG) power spectral analysis, the fatigability of skeletal muscle is associated with a shift in the median frequency (MF) toward a lower-frequency side.21,22,23,24,25) A change in MF is also correlated with the composition of type I and II muscle fibers; thus, MF has been used as a marker of local muscle fatigability.26) Previous studies reported that dynamometric values could be used to assess quadriceps fatigue and suggested that the intraclass correlation coefficient (ICC) of the MF slope calculated from the surface EMG signal was low (ICC=0.04–0.41)27,28,29,30) or moderate (ICC=0.50–0.68).31,32) In contrast, Minoshima et al.33) reported the reproducibility of the MF slope for the VMO and VML during moderate isometric contraction with 90° flexion of the hip and knee to be high (ICC=0.70–0.86).
Based on the above background, we hypothesized that the VMO and VML in women would have less fatigability than that in men. To test our hypothesis, we assessed the declines in the MF slope of the VMO and VML during sustained isometric contraction of the quadriceps in women and men using EMG power spectral analysis.
MATERIALS AND METHODS
Subjects
Sixteen healthy women [age, 22.8 ± 1.5 years (mean ± standard deviation); range, 21–26 years] and 16 men (age 25.4 ± 2.6 years; range, 22–30 years) participated in this cross-sectional study. The participants had no history of knee symptoms. Participants who habitually performed regular exercises were excluded because the results of the study could have varied considerably depending on their sports history. We determined the sample size as 26 (13 women and 13 men), using Gpower 3.1.9, based on a study performed by Tsuboi et al.24) The effective size was 1.36 while assuming a type 1 error rate of 5% and a type 2 error rate of 10% (90% power). Taking into account the possible missing data and participant drop-out, we included 16 healthy women and 16 men. Table 1 shows the age, height, body weight, and body mass index of each group. The participants were instructed to refrain from strenuous exercise on the day before and on the day of the experiment to avoid any effects of accumulative muscle fatigue. The procedures of this study conformed to the guidelines in the Declaration of Helsinki and were approved by the Human Ethics Committee of Wakayama Medical University (approval number: 1616). Before participating in the study, each participant provided written informed consent after receiving a thorough explanation of the study protocol. This study followed a test protocol as previously described by the authors.33)
Table 1. Anthropometric characteristics of the participants.
| Men (n=16) | Women (n=16) | |
| Age (years) | 25.4 ± 2.6 | 22.8 ± 1.5 |
| Height (cm) | 173.8 ± 5.8 | 161.0 ± 4.6 |
| Body weight (kg) | 67.6 ± 7.0 | 53.5 ± 5.4 |
| Body mass index (kg/m2) | 22.4 ± 2.2 | 20.6 ± 1.6 |
Data given as mean ± standard deviation
Position Task
The strength of the knee extensor muscle was measured using a leg press machine (SYGNUM80, Gym80, Gelsenkirchen, Germany). The participants rested for 15 min while seated in a chair before taking a position on the leg press machine with their right hip and knee flexed at 90° (Fig. 1). Maximal voluntary contraction (MVC) was defined as the maximal load that the participant could withstand with the knee at 90° flexion; the process of weight loading was performed in increments of 2.5 kg. The test was terminated when the subject could no longer maintain the knee at 90° flexion (defined as >90° flexion for 5 s) despite strong verbal encouragement. Knee flexion angle was measured using a goniometer (Todai style goniometer with an overall length of 60 cm; TTM-KO, Sakai Medical, Tokyo, Japan) and the angle of the knee joint was manually evaluated. The machine was calibrated to apply 60% of MVC, with the right hip and knee at 90° flexion (position task). After assuming a sitting position for 15 min, the participants were instructed to press and sustain the same posture as that maintained during the measurement of MVC for as long as possible (position task with knee at 90° flexion; Fig. 1). The test was completed when the participants were no longer able to maintain knee flexion of over 90° for 5 s, even with strong verbal cheering. Thereafter, the endurance time was noted as one of the markers of knee extensor isometric endurance. The MVC was obtained by dividing by body weight to compensate for differences in body size between men and women.
Fig. 1.
Body position during the static knee extensor strength and position task.
Recording and Analyzing Electromyographic Signal
The EMG signal was monitored during the position task for the knee at 90° flexion. Before placing the electrodes, the area selected for electrode placement was prepared by shaving (when required) and wiping with an alcohol swab. Two 10-mm Ag-AgCl surface electrodes were placed 2 cm apart on the VMO and VML by a single examiner. The active electrodes were adhered to the VML according to the guidelines provided by Surface ElectroMyoGraphy for the Non-Invasive Assessment of Muscles.34) The electrode on the VMO was attached at a distance of approximately 50 mm from the superior-medial aspect of the patella, along a line inclined 50° with respect to the anterior superior iliac spine.35) The inter-electrode axis of the electrodes was aligned with the assumed direction of the muscle fibers. Based on the Standards for Reporting EMG Data, endorsed by the International Society of Electrophysiology and Kinesiology,36) a signal in the range of 8–500 Hz was transmitted through a bandpass filter, amplified using an MQ16 transmitter (Marq-Medical, Farum, Denmark), digitized by an A/D converter (Vital Recorder2, Kissei Comtec, Matsumoto, Japan), and then stored on a computer at a sampling rate of 2000 Hz. The MF indices were obtained after Fourier transform for every 1-s window of the raw EMG signal and were plotted against the time [s] during the test using a spectrum analysis program (BIMUTASRII-A, Kissei Comtec). Linear regression analysis was performed to calculate the declining slope of MF at a given time [s], defined as the MF slope. To allow comparison between participants, values during each test were expressed as a percentage of baseline.
Statistical Analysis
Data were shown as mean ± standard deviation. Differences in the endurance time and the MF slope between women and men were assessed by the Student’s t-test. The null hypothesis was rejected at a P value <0.05. Statistical analysis was performed using SPSS for Windows, version 23.0 (IBM SPSS, Armonk, NY, USA).
RESULTS
Maximal Voluntary Contraction and Endurance Time
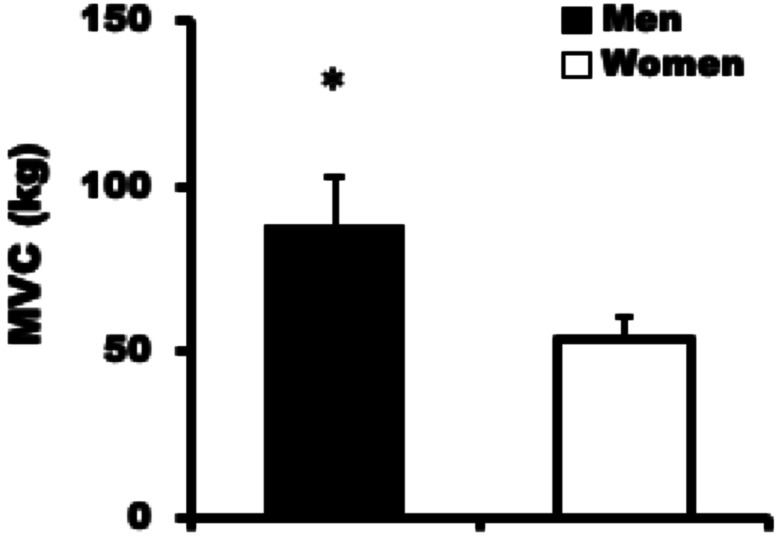
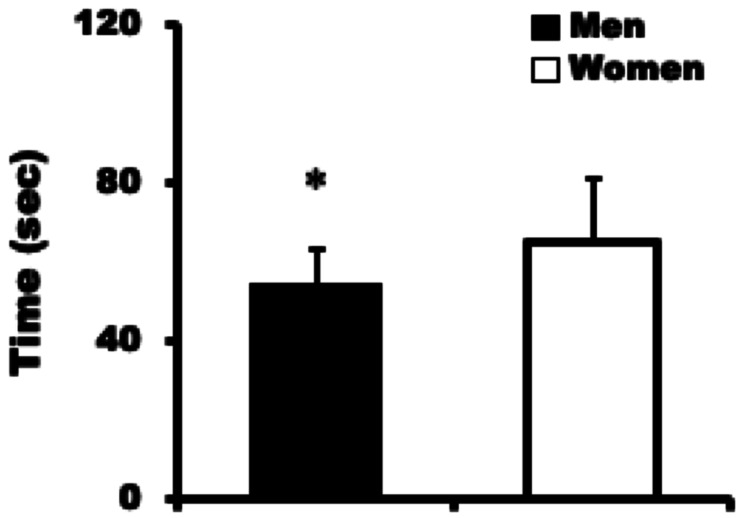
The MVC of women (54.2 ± 6.3 kg; range, 41.0–65.0 kg) was significantly lower than that of men (88.5 ± 14.7 kg; range, 65.0–117.0 kg; P <0.01) (Fig. 2). Moreover, the normalized MVC by body weight for women (1.02 ± 0.14 kg/kg; range, 0.78–1.32) was significantly lower than that of men (1.32 ± 0.26 kg/kg; range, 0.95–2.04; P <0.01). The endurance time of women (64.8 ± 16.1 s; range, 43.0–102.0 s) was significantly longer than that of men (54.3 ± 9.2 s; range, 42.0–74.0 s; P <0.05) (Fig. 3).
Fig. 2.
Maximal voluntary contraction (MVC) in men and women for the knee extensor test. Values are mean ± standard deviation. Asterisk indicates significant difference vs. women (P<0.01).
Fig. 3.
Endurance time during the static leg press test in men and women. Values are mean ± standard deviation. Asterisk indicates significant difference vs. women (P<0.05).
Median Frequency Slopes During the Test
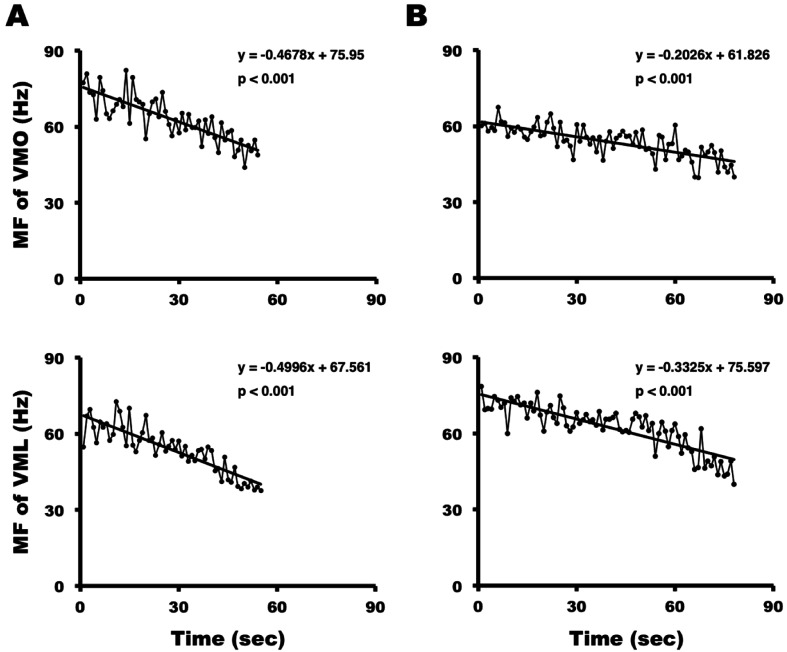
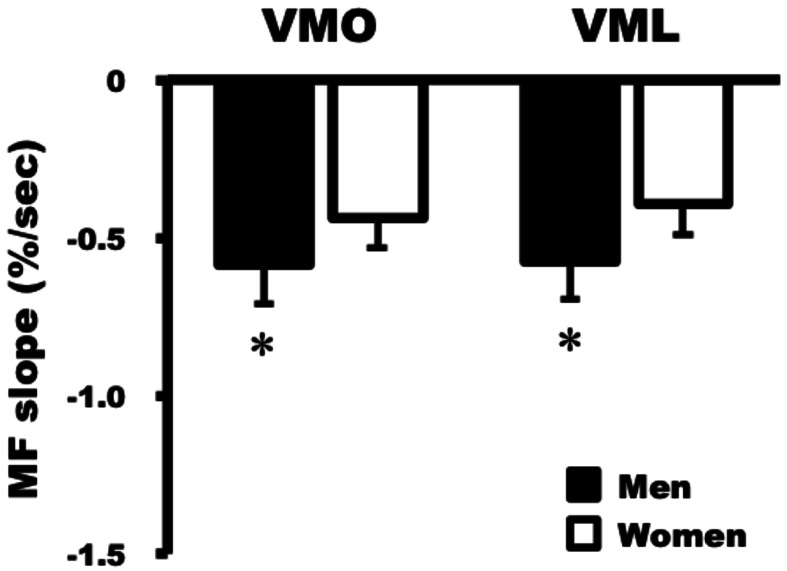
Figure 4 shows representative changes in the MF slope of VMO and VML recorded for a 30-year-old man and a 23-year-old woman. Analysis of data of the entire group showed a linear decrease in MF during the test time in all participants (P <0.001). The magnitudes of the MF slopes for the VMO and VML were significantly lower in women than in men (VMO, −0.59 ± 0.15 vs. −0.44 ± 0.12%/s, P <0.01; VML, −0.57 ± 0.22 vs. −0.39 ± 0.12%/s, P <0.01) (Fig. 5).
Fig. 4.
Changes in median frequency (MF) of VMO (upper) and VML (lower) in (A) a representative 30-year-old man and (B) a 23-year-old woman. MF decreased linearly with test time in both subjects.
Fig. 5.
Comparison of MF slopes for VMO and VML in men and women. VMO, −0.59 ± 0.15 (men) vs. −0.44 ± 0.12%/s (women), P <0.01; VML, −0.57 ± 0.22 (men) vs. −0.39 ± 0.12%/s (women), P <0.01). Asterisk indicates significant difference vs. women (P<0.01).
DISCUSSION
The main findings of the position task were that longer endurance time and lower MF slopes of the VMO and VML were noted in women than in men, suggesting that the VMO and VML in women have less fatigability than in men. A previous study reported that muscle strength correlates with muscle cross-sectional area, and the cross-sectional area of quadriceps in women is lower than that in men.37) In the present study, the muscle force-generating capacity was lower in women than in men, which is consistent with the previous study. To examine motor unit recruitment during isometric lower-limb contraction using power spectrum analysis, previous studies have recommended that the load of contraction be more than 40% of MVC.38) However, other studies have used a load set below 40% of MVC because of the difficulty in applying higher load.27,28,29,30) Recently, we reported the successful application of identical VMO and VML isometric muscle contraction at 60% of MVC.33) The study revealed high within-day and between-day reproducibility in the MF slopes of the VMO and VML. Therefore, the present study was designed to quantify the contraction level relative to 60% of MVC. The present assessment of differences in the fatigabilities of the VMO and VML between sexes was conducted properly and indicated that the VMO and VML are more fatigue-resistant in women than in men.
The results of the present study are consistent with the results of previous studies that evaluated the sex difference in fatigabilities of the erector spinae muscles,24,25) which showed longer endurance time (duration of unsupported trunk holding test) and lower MF slopes in young adult women than those in age-matched men. Muscle fibers are categorized into type I and type II fibers. Generally, aerobic capacity is associated with the relative volume of type I muscle fibers,39) while maximal isometric strength is dependent on the superiority of type II muscle fibers.40) As previously described, EMG power spectral analysis was combined with histochemical analysis to determine the types of muscle fibers26,41,42); muscles with a greater ratio of type II fibers exhibited a greater decline in MF slope during sustained contraction.26) The relative area of type I fibers in the erector spinae is larger and correlates with lower MF slopes as per the trunk holding test.41) Given that both the VM and erector spinae contain relatively higher proportions of type I fibers, they are beneficial for use as antigravity muscles.17,43) As previously suggested, the ratios of the type I fibers in the erector spinae and VM would be lower in healthy adult men than those in women.11,17,43) The results of previous studies are consistent with the MF slopes observed in the present study, and the proportions of type I fibers in the VMO and VML are relatively larger in women than in men.
In our previous studies that evaluated erector spinae muscle fatigabilities using surface EMG, the erector spinae muscles were more fatigue-resistant in prepubertal boys and girls than in young adults, and there were sex differences in muscle fatigability in prepubertal children, similar to healthy adults.44) However, the age-related change in fatigability occurred in only men, and there were no sex differences in muscle fatigability in healthy older people.24) Moreover, the specific differences found in athletes with lumbar spondylolysis25) and patients with Parkinson’s disease45) compared with age, sex, and anthropometric characteristics matched healthy individuals; MF slope was higher in patients with Parkinson’s disease than healthy individuals25), however, MF slope in athletes with terminal stage lumbar spondylolysis was lower than athletes without lumbar spondylolysis45), Sato et al.46) revealed that the fatigabilities of the VMO and VML on the involved side differed in muscle strength recovery in adult men who had undergone anterior cruciate ligament reconstruction. Based on that knowledge, we believe that physical therapists need to understand the differences in muscle according to age, sex, and disease when conducting effective strength training for patients; however, it has been unclear whether all muscles are affected.
The sex-related differences in the fatigability found in the present study may serve as supportive information to design effective strategies when performing strength training of the VMO and VML for women and men. Moreover, specific changes in the VMO, VML, or both may occur in various knee disorders. For example, selective atrophy of type II muscle fibers occurs in the VM in cases of knee osteoarthritis.47) Therefore, further studies are needed to determine the properties of the VMO and VML, to collect information about knee symptoms, and to help design training programs based on these sex differences.
CONCLUSION
This is the first study to evaluate differences in the fatigabilities of the VMO and VML between different sexes using EMG power spectral analysis. The results suggest that the VMO and VML are less fatigable in women than in men.
ACKNOWLEDGMENTS
We thank Hideaki Tanina and Ryo Koyanagi for their excellent technical assistance. We also thank Dr. Faiq G. Issa (www.word-medex.com.au) for careful reading and editing of the manuscript.
Footnotes
CONFLICTS OF INTEREST: The authors report no conflicts of interest.
REFERENCES
- 1.Clark BC,Manini TM,Thé DJ,Doldo NA,Ploutz-Snyder LL: Gender differences in skeletal muscle fatigability are related to contraction type and EMG spectral compression. J Appl Physiol 2003;94:2263–2272. 10.1152/japplphysiol.00926.2002 [DOI] [PubMed] [Google Scholar]
- 2.Fulco CS,Rock PB,Muza SR,Lammi E,Cymerman A,Butterfield G,Moore LG,Braun B,Lewis SF: Slower fatigue and faster recovery of the adductor pollicis muscle in women matched for strength with men. Acta Physiol Scand 1999;167:233–239. 10.1046/j.1365-201x.1999.00613.x [DOI] [PubMed] [Google Scholar]
- 3.Hunter SK,Enoka RM: Sex differences in the fatigability of arm muscles depends on absolute force during isometric contractions. J Appl Physiol 2001;91:2686–2694. 10.1152/jappl.2001.91.6.2686 [DOI] [PubMed] [Google Scholar]
- 4.Russ DW,Kent-Braun JA: Sex differences in human skeletal muscle fatigue are eliminated under ischemic conditions. J Appl Physiol 2003;94:2414–2422. 10.1152/japplphysiol.01145.2002 [DOI] [PubMed] [Google Scholar]
- 5.Semmler JG,Kutzscher DV,Enoka RM: Gender differences in the fatigability of human skeletal muscle. J Neurophysiol 1999;82:3590–3593. 10.1152/jn.1999.82.6.3590 [DOI] [PubMed] [Google Scholar]
- 6.West W,Hicks A,Clements L,Dowling J: The relationship between voluntary electromyogram, endurance time and intensity of effort in isometric handgrip exercise. Eur J Appl Physiol Occup Physiol 1995;71:301–305. 10.1007/BF00240408 [DOI] [PubMed] [Google Scholar]
- 7.Gerdle B,Wretling ML,Henriksson-Larsén K: Do the fibre-type proportion and the angular velocity influence the mean power frequency of the electromyogram? Acta Physiol Scand 1988;134:341–346. 10.1111/j.1748-1716.1988.tb08501.x [DOI] [PubMed] [Google Scholar]
- 8.Gerdle B,Karlsson S,Crenshaw AG,Fridén J: The relationships between EMG and muscle morphology throughout sustained static knee extension at two submaximal force levels. Acta Physiol Scand 1997;160:341–351. 10.1046/j.1365-201X.1997.00167.x [DOI] [PubMed] [Google Scholar]
- 9.Gerdle B,Karlsson S,Crenshaw AG,Elert J,Fridén J: The influences of muscle fibre proportions and areas upon EMG during maximal dynamic knee extensions. Eur J Appl Physiol Occup Physiol 2000;81:2–10. 10.1007/PL00013792 [DOI] [PubMed] [Google Scholar]
- 10.Lexell J,Taylor CC: Variability in muscle fibre areas in whole human quadriceps muscle. How much and why? Acta Physiol Scand 1989;136:561–568. 10.1111/j.1748-1716.1989.tb08702.x [DOI] [PubMed] [Google Scholar]
- 11.Miller AE,MacDougall JD,Tarnopolsky MA,Sale DG: Gender differences in strength and muscle fiber characteristics. Eur J Appl Physiol Occup Physiol 1993;66:254–262. 10.1007/BF00235103 [DOI] [PubMed] [Google Scholar]
- 12.Simoneau JA,Lortie G,Boulay MR,Thibault MC,Thériault G,Bouchard C: Skeletal muscle histochemical and biochemical characteristics in sedentary male and female subjects. Can J Physiol Pharmacol 1985;63:30–35. 10.1139/y85-005 [DOI] [PubMed] [Google Scholar]
- 13.Simoneau JA,Bouchard C: Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am J Physiol Endocrinol Metab 1989;257:E567–E572. 10.1152/ajpendo.1989.257.4.E567 [DOI] [PubMed] [Google Scholar]
- 14.Smillie IS: The quadriceps in relation to recovery from injuries of the knee-joint. Physiotherapy 1949;35:53–57. [PubMed] [Google Scholar]
- 15.Lieb FJ,Perry J: Quadriceps function. An anatomical and mechanical study using amputated limbs. J Bone Joint Surg Am 1968;50:1535–1548. 10.2106/00004623-196850080-00003 [DOI] [PubMed] [Google Scholar]
- 16.Castanov V,Hassan SA,Shakeri S,Vienneau M,Zabjek K,Richardson D,McKee NH,Agur AM: Muscle architecture of vastus medialis obliquus and longus and its functional implications: a three‐dimensional investigation. Clin Anat 2019;32:515–523. 10.1002/ca.23344 [DOI] [PubMed] [Google Scholar]
- 17.Travnik L,Pernus F,Erzen I: Histochemical and morphometric characteristics of the normal human vastus medialis longus and vastus medialis obliquus muscles. J Anat 1995;187:403–411. [PMC free article] [PubMed] [Google Scholar]
- 18.Vikne H,Strøm V,Pripp AH,Gjøvaag T: Human skeletal muscle fiber type percentage and area after reduced muscle use: a systematic review and meta‐analysis. Scand J Med Sci Sports 2020;30:1298–1317. 10.1111/sms.13675 [DOI] [PubMed] [Google Scholar]
- 19.Gerber C,Hoppeler H,Claassen H,Robotti G,Zehnder R,Jakob RP: The lower-extremity musculature in chronic symptomatic instability of the anterior cruciate ligament. J Bone Joint Surg Am 1985;67:1034–1043. 10.2106/00004623-198567070-00007 [DOI] [PubMed] [Google Scholar]
- 20.Pattyn E,Verdonk P,Steyaert A,Vanden Bossche L,Van den Broecke W,Thijs Y,Witvrouw E: Vastus medialis obliquus atrophy: does it exist in patellofemoral pain syndrome? Am J Sports Med 2011;39:1450–1455. 10.1177/0363546511401183 [DOI] [PubMed] [Google Scholar]
- 21.De Luca CJ: Myoelectrical manifestations of localized muscular fatigue in humans. Crit Rev Biomed Eng 1984;11:251–279. [PubMed] [Google Scholar]
- 22.Merletti R,Knaflitz M,De Luca CJ: Myoelectric manifestations of fatigue in voluntary and electrically elicited contractions. J Appl Physiol 1990;69:1810–1820. 10.1152/jappl.1990.69.5.1810 [DOI] [PubMed] [Google Scholar]
- 23.Umezu Y,Shiba N,Tajima F,Mizushima T,Okawa H,Ogata H,Nagata K,Basford JR: Muscle endurance and power spectrum of the triceps brachii in wheelchair marathon racers with paraplegia. Spinal Cord 2003;41:511–515. 10.1038/sj.sc.3101495 [DOI] [PubMed] [Google Scholar]
- 24.Tsuboi H,Nishimura Y,Sakata T,Ohko H,Tanina H,Kouda K,Nakamura T,Umezu Y,Tajima F: Age-related sex differences in erector spinae muscle endurance using surface electromyographic power spectral analysis in healthy humans. Spine J 2013;13:1928–1933. 10.1016/j.spinee.2013.06.060 [DOI] [PubMed] [Google Scholar]
- 25.Tsuboi H,Nishimura Y,Sakata T,Tanina H,Arakawa H,Nakamura T,Umezu Y,Tajima F: Properties of paraspinal muscles in Japanese high school baseball players with terminal-stage lumbar spondylolysis. PM R 2018;10:175–182. 10.1016/j.pmrj.2017.06.018 [DOI] [PubMed] [Google Scholar]
- 26.Kupa EJ,Roy SH,Kandarian SC,De Luca CJ: Effects of muscle fiber type and size on EMG median frequency and conduction velocity. J Appl Physiol 1995;79:23–32. 10.1152/jappl.1995.79.1.23 [DOI] [PubMed] [Google Scholar]
- 27.Kollmitzer J,Ebenbichler GR,Kopf A: Reliability of surface electromyographic measurements. Clin Neurophysiol 1999;110:725–734. 10.1016/S1388-2457(98)00050-9 [DOI] [PubMed] [Google Scholar]
- 28.McCarthy CJ,Oldham JA: The reliability, validity and responsiveness of an aggregated locomotor function (ALF) score in patients with osteoarthritis of the knee. Br J Rheumatol 2004;43:514–517. 10.1093/rheumatology/keh081 [DOI] [PubMed] [Google Scholar]
- 29.McCarthy CJ,Callaghan MJ,Oldham JA: The reliability of isometric strength and fatigue measures in patients with knee osteoarthritis. Man Ther 2008;13:159–164. 10.1016/j.math.2006.12.003 [DOI] [PubMed] [Google Scholar]
- 30.Zech A,Witte K,Pfeifer K: Reliability and performance-dependent variations of muscle function variables during isometric knee extension. J Electromyogr Kinesiol 2008;18:262–269. 10.1016/j.jelekin.2006.08.013 [DOI] [PubMed] [Google Scholar]
- 31.Mathur S,Eng JJ,MacIntyre DL: Reliability of surface EMG during sustained contractions of the quadriceps. J Electromyogr Kinesiol 2005;15:102–110. 10.1016/j.jelekin.2004.06.003 [DOI] [PubMed] [Google Scholar]
- 32.Callaghan MJ,McCarthy CJ,Oldham JA: The reliability of surface electromyography to assess quadriceps fatigue during multi joint tasks in healthy and painful knees. J Electromyogr Kinesiol 2009;19:172–180. 10.1016/j.jelekin.2007.05.004 [DOI] [PubMed] [Google Scholar]
- 33.Minoshima Y,Nishimura Y,Tsuboi H,Satou H,Kamijo Y,Arakawa H,Umezu Y,Tajima F: Reliability of power spectral analysis of surface electromyogram recorded during sustained vastus medialis isometric contraction in assessment of muscle fatigability. Open J Ther Rehabil 2017;5:43–52. 10.4236/ojtr.2017.52005 [DOI] [Google Scholar]
- 34.Hermens HJ,Freriks B,Disselhorst-Klug C,Rau G: Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 2000;10:361–374. 10.1016/S1050-6411(00)00027-4 [DOI] [PubMed] [Google Scholar]
- 35.Rainoldi A,Bullock-Saxton JE,Cavarretta F,Hogan N: Repeatability of maximal voluntary force and of surface EMG variables during voluntary isometric contraction of quadriceps muscles in healthy subjects. J Electromyogr Kinesiol 2001;11:425–438. 10.1016/S1050-6411(01)00022-0 [DOI] [PubMed] [Google Scholar]
- 36.Merletti R.International Society of Electrophysiology and Kineiology. Standards for reporting EMG data. . Accessed 9 September 2022. [Google Scholar]
- 37.Kanehisa H,Ikegawa S,Fukunaga T: Comparison of muscle cross-sectional area and strength between untrained women and men. Eur J Appl Physiol Occup Physiol 1994;68:148–154. 10.1007/BF00244028 [DOI] [PubMed] [Google Scholar]
- 38.Bernardi M,Solomonow M,Baratta RV: Motor unit recruitment strategy of antagonist muscle pair during linearly increasing contraction. Electromyogr Clin Neurophysiol 1997;37:3–12. [PubMed] [Google Scholar]
- 39.Costill DL,Fink WJ,Pollock ML: Muscle fiber composition and enzyme activities of elite distance runners. Med Sci Sports Exerc 1976;8:96–100. 10.1249/00005768-197600820-00015 [DOI] [PubMed] [Google Scholar]
- 40.Tesch P,Karlsson J: Isometric strength performance and muscle fibre type distribution in man. Acta Physiol Scand 1978;103:47–51. 10.1111/j.1748-1716.1978.tb06189.x [DOI] [PubMed] [Google Scholar]
- 41.Mannion AF,Dumas GA,Stevenson JM,Cooper RG: The influence of muscle fiber size and type distribution on electromyographic measures of back muscle fatigability. Spine 1998;23:576–584. 10.1097/00007632-199803010-00010 [DOI] [PubMed] [Google Scholar]
- 42.Crossman K,Mahon M,Watson PJ,Oldham JA,Cooper RG: Chronic low back pain-associated paraspinal muscle dysfunction is not the result of a constitutionally determined “adverse” fiber-type composition. Spine 2004;29:628–634. 10.1097/01.BRS.0000115133.97216.EC [DOI] [PubMed] [Google Scholar]
- 43.Mannion AF,Dumas GA,Cooper RG,Espinosa FJ,Faris MW,Stevenson JM: Muscle fibre size and type distribution in thoracic and lumbar regions of erector spinae in healthy subjects without low back pain: normal values and sex differences. J Anat 1997;190:505–513. 10.1046/j.1469-7580.1997.19040505.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanina H,Nishimura Y,Tsuboi H,Sakata T,Nakamura T,Murata K,Arakawa H,Umezu Y,Tajima F: Fatigue-related differences in erector spinae between prepubertal children and young adults using surface electromyographic power spectral analysis. J Back Musculoskeletal Rehabil 2016;30:1–9. 10.3233/BMR-160705 [DOI] [PubMed] [Google Scholar]
- 45.Nishimura Y,Tsuboi H,Murata KY,Minoshima Y,Sato H,Umezu Y,Tajima F: Comparison of erector spinae fatigability between female patients with Parkinson’s disease and healthy individuals: a cross sectional pilot study. BMC Neurol 2022;22:189. 10.1186/s12883-022-02719-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sato H,Nishimura Y,Tsuboi H,Minoshima Y,Sakata T,Umezu Y,Tajima F: Differences in fatigability of vastus medialis muscle between patients with limb symmetry index of <90% and ≥90% after chronic anterior cruciate ligament reconstruction. Knee 2021;31:39–45. 10.1016/j.knee.2021.05.005 [DOI] [PubMed] [Google Scholar]
- 47.Fink B,Egl M,Singer J,Fuerst M,Bubenheim M,Neuen-Jacob E: Morphologic changes in the vastus medialis muscle in patients with osteoarthritis of the knee. Arthritis Rheum 2007;56:3626–3633. 10.1002/art.22960 [DOI] [PubMed] [Google Scholar]