This randomized clinical trial compares cure rates, treatment durations, safety profiles, and relapse rates of itraconazole, 100, 200, and 400 mg/d, for the treatment of tinea corporis/cruris in adult patients.
Key Points
Question
What are the efficacy, treatment durations, safety, and relapse rates of 100, 200, and 400 mg/d of itraconazole in treatment of tinea corporis/cruris?
Findings
In this randomized clinical trial of 149 adult patients, there was high overall drug efficacy but need for prolonged treatment. There was no statistically significant difference observed in efficacy or treatment durations between the 100- and 200-mg groups, while treatment with the 400-mg dose led to significantly higher cure rates and shorter treatment durations, though relapse rates were similar across groups.
Meaning
Itraconazole is highly efficacious at all 3 doses but requires prolonged treatment, and a substantial number of patients relapsed after successful treatment.
Abstract
Importance
With worldwide emergence of recalcitrant and resistant dermatophytosis, itraconazole is increasingly being used as the first-line drug for treatment of tinea corporis/cruris (TCC). Apparent inadequacy with low doses has led to empirical use of higher doses and antifungal combinations.
Objective
To compare cure rates, treatment durations, safety profiles, and relapse rates of itraconazole 100, 200, and 400 mg/d for the treatment of TCC.
Design, Setting, and Participants
This double-blind randomized clinical trial included adult patients with treatment-naive TCC involving at least 5% body surface area. Patients were recruited from the dermatology outpatient department of a tertiary care hospital in New Delhi, India between March 1, 2020, and August 31, 2021.
Interventions
Patients were randomized to 1 of the 3 treatment groups. Biweekly blinded assessments were performed until cure or treatment failure. Posttreatment follow-up of at least 8 weeks was conducted to detect relapses.
Main Outcome and Measures
Cure rates, treatment durations, safety profiles, and relapse rates were assessed. Secondary outcomes included comparison of rapidity of clinical response and cost-effectiveness between groups.
Results
Of the 149 patients assessed, the mean (SD) age was 34.3 (12.2) years, 69 patients (46.4%) were women, and 80 patients (53.6%) were men. The difference in cure rate between the 100- and 200-mg groups was statistically nonsignificant (hazard ratio [HR], 1.44; 95% CI, 0.91-2.30; P = .12), while the difference between the 100- and 400-mg groups (HR, 2.87; 95% CI, 1.78-4.62; P < .001) and between the 200- and 400-mg groups (HR, 1.99; 95% CI, 1.28-3.09; P = .002) was statistically significant. Mean (SD) treatment durations were statistically significantly different between the 100- and 400-mg groups (7.7 [4.7] weeks vs 5.2 [2.6] weeks; P = .03) and between the 200- and 400-mg groups (7.2 [3.8] weeks vs 5.2 [2.6] weeks; P = .004), but the difference between the 100- and 200-mg groups was not statistically significant. A total of 55 patients (47.4%) relapsed after treatment. Relapse rates were comparable across groups. No patient discontinued treatment due to adverse effects. Treatment with the 200-mg dose incurred a 63% higher cost and 400 mg a 120% higher cost over 100 mg in achieving cure.
Conclusions and Relevance
In this randomized clinical trial, high overall efficacy was observed among the 3 itraconazole doses for treatment of TCC, but with prolonged treatment durations and considerable relapse rates. Treatment with the 200- and 100-mg doses did not differ significantly in efficacy or treatment durations, while 400 mg scored over the other 2 on these outcomes. Considerable additional cost is incurred in achieving cure with the 200- and 400-mg doses.
Trial Registration
Clinical Trials Registry of India Identifier: CTRI/2020/03/024326
Introduction
Dermatophytosis is the most common fungal infection affecting humans and a considerable health care concern. Inadequate clinical response to fluconazole and griseofulvin has long been reported,1 and more recently, increasing in vitro resistance to terbinafine has been reported from various countries. Reports of a novel, highly terbinafine-resistant species belonging to the Trichophyton mentagrophytes–Trichophyton interdigitale complex started appearing from India in late 2017,2,3,4,5 followed by numerous similar reports from Asia (Japan,6,7 Iran,8,9 and Vietnam10), Europe (Germany,11 Denmark,12 Belgium,13 France,14,15 Finland,16 Greece,17 Switzerland,18 and Poland19), and recently from North America (Canada20). To avoid confusion in the taxonomy, Kano et al have suggested a new name—Trichophyton indotineae sp. nov— for this species.6 A multination study involving 135 isolates from India, China, Australia, Germany, and the Netherlands reported a predominance of T indotineae (64 isolates), with 53% of these isolates showing high terbinafine minimum inhibitory concentrations (MICs).21 The origin of this species is postulated to be zoonotic, and its emergence is likely related to widespread misuse of antifungals.22 Thus, with rising terbinafine resistance, itraconazole is being increasingly used as the first-line systemic antifungal drug for tinea corporis/cruris (TCC).1 Previous literature on itraconazole in the treatment of TCC demonstrates efficacy of a 100-mg/d dosage over 50 mg/d and documents lack of added efficacy with itraconazole 200mg/d, except for a faster clearance.23,24 Itraconazole 400 mg/d has been extensively used for tinea pedis/manuum25and onychomycosis26 but not for TCC. Due to the sustained skin levels beyond treatment cessation, short fixed-duration regimens of itraconazole 100 to 200 mg/d for 1 to 2 weeks were shown to have high efficacy (≥80%) and thus became the standard recommended treatment for TCC.27,28 However, in the current scenario, these regimens seem to have become obsolete, and prolonged treatment durations and higher doses are empirically prescribed by clinicians.29 There are also unfounded claims of lack of clinical response to itraconazole and an unfortunate trend toward using systemic antifungal combinations and third-generation azoles for TCC.30,31,32,33,34,35 This not only entails considerable additional health care costs, but is also a risk to antifungal stewardship programs. The situation calls for a reappraisal of itraconazole in management of TCC and a reassessment of its efficacy and the optimum dose and duration of treatment.
This randomized clinical trial addressed the primary objective of comparing cure rates, treatment durations, safety profiles, and relapse with 3 dosages of itraconazole (100, 200, and 400 mg/d). The secondary objectives were to compare rapidity of clinical clearance, perform a cost-effectiveness analysis among the groups, and to document “persistent sites” of infection, necessitating prolongation of treatment.
Methods
Inclusion and Exclusion Criteria
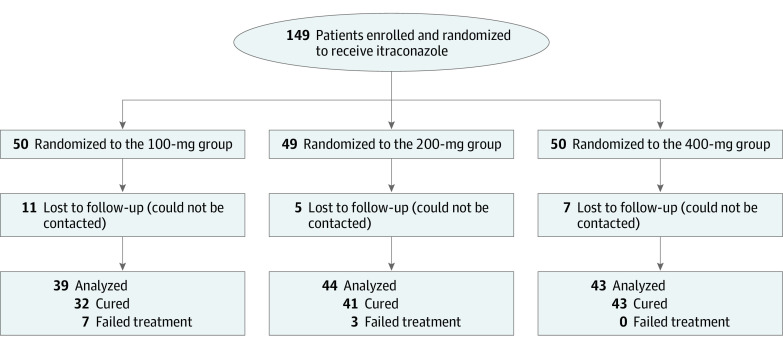
Patients with TCC who presented to the dermatology outpatient department of Dr Ram Manohar Lohia Hospital, a tertiary care center in New Delhi, India, were eligible for trial inclusion. Consecutive adult patients (>18 years old) with TCC involving at least 5% body surface area (BSA) with no nail/scalp/palm/sole involvement and no history of intake of any oral antifungal in the preceding 4 weeks or of a topical antifungal/steroid in the preceding 2 weeks were recruited. Pregnant and lactating patients, those receiving systemic drugs with major pharmacokinetic interactions with itraconazole, and those with liver enzymes elevation of more than 2 times the upper limit of normal at baseline were excluded. Patient randomization and group allocation is presented in Figure 1 and the eMethods in Supplement 1.
Figure 1. CONSORT Diagram.
This trial was carried out with prior protocol approval from the institutional ethics committee of the Atal Bihari Vajpayee Institute of Medical Sciences and the Dr Ram Manohar Lohia Hospital (Supplement 2). This trial also followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Baseline Assessment
Written informed consent was obtained in Hindi or English, per the patients’ choice. Diagnosis was made clinically and confirmed by direct microscopic examination using 10% potassium hydroxide (KOH) mount performed in clinic. Skin scrapings were sent to the microbiology department for further processing. Recording of lesions and BSA estimation was completed,36 and clinical photographs were taken. Patients were asked to score their pruritus on a visual analog scale (VAS) from 0 (minimum) to 10 (maximum). A detailed history of disease onset, duration, course, family history, and previous treatments was also recorded.
Drug Dispensing, Follow-ups, and Outcomes Assessed
The chosen brand performed comparably with the innovator brand in terms of serum levels achieved with the 3 assessed doses, as per a serum level analysis previously performed,37 and in terms morphometric assessment of pellets (a visual indicator of the quality of manufacturing process, as previously noted38). No topical drugs were allowed. For more details on drug dispensing, see the eMethods in Supplement 1. Follow-up assessments were performed every 2 weeks until the primary end points were achieved and 2 weeks thereafter to detect relapses (for cured patients). Clinical assessment at baseline and follow-ups were performed in a blinded manner (A.K., A.A., D.A., and Khushboo Sethia). This included recording clinical clearance (as percentage improvement), VAS score for pruritus and any adverse effects, and a KOH examination from remaining lesions. Persistent sites, defined as site(s) in which clinical/mycological signs of infection persisted for more than 4 weeks after clinical/mycological clearance at all other sites, leading consequently to prolongation of treatment, were also recorded. Liver function tests were performed at baseline and every 4 weeks thereafter.
Primary and Secondary End Points
The treatment was stopped when either of the following end points were reached:
1. Complete cure, defined as clinical cure with negative KOH smears. Clinical cure was defined as complete clinical clearance, and mycological cure as negative KOH mount from multiple sites. Hereafter, cure refers to complete cure unless otherwise specified.
2. Treatment failure, defined as an absence of significant (>50%) clinical improvement by 8 weeks or appearance of new lesions/extension of original lesions anytime during treatment.
Patients were followed up for a minimum of 8 weeks beyond cure to detect relapses (recorded as present/absent). Relapse was termed minor if it involved less than 25% of the initially involved BSA and major if involving more than 25% of the initial BSA. Secondary outcomes included comparison of rapidity of clinical response, cost-effectiveness among groups, and persistent sites of infection.
Mycological Investigations
The skin scales were used to culture the organism per standard protocols (eMethods in Supplement 1). Antifungal susceptibility testing was performed using the Clinical and Laboratory Standards Institute broth-microdilution method.39 Internal transcribed spacer (ITS) gene sequencing for further identification was performed on a few culture isolates.
Statistical Analysis
Statistical analysis was performed using Excel (Microsoft) and SPSS, version 16.0 (IBM).40,41 The qualitative variables were taken as percent or proportions and the quantitative variables as mean and standard deviation. Cure rates for each group were estimated as the percentage of patients who attained cure from among the total patients who completed treatment within that group during the study period. A 2-sided P < .05 was considered statistically significant. See the eMethods in Supplement 1 for additional details on sample size estimation and statistical analysis.
Results
Patients
A total of 149 patients were recruited between March 1, 2020, and August 31, 2021. Demographic and baseline clinical features among groups were comparable and are summarized in Table 1. Mycological and antifungal susceptibility testing results are summarized in Table 2.
Table 1. Demographic and Baseline Clinical Characteristics of the 3 Treatment Groups.
| Parameter | No. (%) | ||
|---|---|---|---|
| 100 mg (n = 50) | 200 mg (n = 49) | 400 mg (n = 50) | |
| Age, mean (SD), y | 34.14 (11.69) | 33.88 (12.72) | 34.8 (12.36) |
| Gender | |||
| Female | 22 (44.0) | 25(51.0) | 22 (44.0) |
| Male | 28 (56.0) | 24 (48.9) | 28 (56.0) |
| Education status | |||
| Illiterate | 8 (16.0) | 9 (18.4) | 10 (20.0) |
| Primary school | 14 (28.0) | 19 (38.7) | 15 (30.0) |
| Middle school | 12 (24.0) | 11 (22.4) | 14 (28.0) |
| Higher secondary school | 6 (12.0) | 3 (6.1) | 4 (8.0) |
| Graduate school | 9 (18.0) | 6 (12.2) | 6 (12.0) |
| Postgraduate school | 1 (2.0) | 1 (2.0) | 0 |
| Duration of illness, mean (SD), mo | 15.5 (17.98) | 12.64 (13.24) | 14.4 (14.65) |
| Family history of tinea corporis/cruris | |||
| Yes | 26 (52.0) | 22 (44.9) | 25 (50.0) |
| No | 24 (48.0) | 27 (55.5) | 25 (50.0) |
| Sharing of towels/clothes | |||
| Yes | 19 (38.0) | 19 (38.7) | 18 (36.0) |
| No | 31 (62.0) | 30 (61.2) | 32 (64.0) |
| Pets in house | |||
| Yes | 6 (1.2) | 4 (8.2) | 7 (14.0) |
| No | 44 (88.0) | 45 (91.8) | 43 (86.0) |
| Bathing frequency | |||
| Twice a day | 9 (18.0) | 7 (14.2) | 7 (14.0) |
| Once a day | 38 (76.0) | 39 (79.6) | 33 (66.0) |
| Alternate day | 3 (6.0) | 3 (6.1) | 8 (16.0) |
| Less than alternating days | 0 | 0 | 2 (4.0) |
| Outdoor sports activity | |||
| Yes | 9 (18.0) | 4 (8.0) | 7 (14.0) |
| No | 41 (82.0) | 45 (91.8) | 43 (86.0) |
| Working in hot, humid environment | |||
| Yes | 35 (70.0) | 28 (57.1) | 31 (62.0) |
| No | 15 (10.0) | 21 (42.8) | 19 (38.0) |
| Prior antifungal usea | |||
| Yes | 18 (36.0) | 15 (30.6) | 16 (32,0) |
| No | 15 (30.0) | 18 (36.7) | 19 (38.0) |
| Yes, but nature not known | 17 (34.0) | 16 (32.6) | 15 (30.0) |
| Prior topical steroid useb | |||
| Yes | 22 (44.0) | 24 (48.9) | 24 (48.0) |
| No | 28 (56.0) | 25 (51.0) | 26 (52.0) |
| History of tinea corporis/cruris | |||
| Yes | 8 (16.0) | 4 (8.2) | 9 (18.0) |
| No | 42 (84.0) | 45 (91.8) | 41 (82.0) |
| Presence of other skin conditionsc | |||
| Yes | 7 (14.0) | 6 (12.2) | 6 (12.0) |
| No | 43 (86.0) | 43 (87.7) | 44 (8.8) |
| Presence of any systemic diseased | |||
| Yes | 7 (14.0) | 6 (12.2) | 6 (12.0) |
| No | 43 (86.0) | 43 (87.7) | 44 (88.0) |
| Body surface area involved, mean (SD), % | 8.3 (4.3) | 8.5 (3.7) | 9.4 (5.1) |
| No. of lesions | |||
| 1-5 | 21 (42.0) | 15 (30.6) | 17 (34.0) |
| 6-10 | 23 (46.0) | 22 (44.9) | 18 (36.0) |
| 11-20 | 4 (8.0) | 8 (16.3) | 11(22.0) |
| >20 | 2 (4.0) | 4 (8.2) | 4 (8.0) |
| Baseline VAS score for pruritus, mean (SD) [range, 0-10] | 7.86 (2.30) | 8.33 (2.28) | 7.32 (2.28) |
| Cutaneous signs of topical steroid abuse | |||
| Yes | 6 (12.0) | 11 (22.4) | 3 (6.0) |
| No | 44 (88.0) | 38 (77.5) | 47 (94.0) |
Abbreviation: VAS, visual analog scale.
Prior oral antifungals used include itraconazole (n = 26), terbinafine (n = 13), fluconazole (n = 12), griseofulvin (n = 4), and ketoconazole (n = 2). Topical formulations used include miconazole (n = 33), clotrimazole (n = 17), luliconazole (n = 16), terbinafine (n = 11), ketoconazole (n = 10), sertaconazole (n = 5), tolnaftate (n = 2), oxiconazole (n = 1), ciclopirox (n = 1) and itraconazole (n = 1).
Topical steroids used include clobetasol, 0.05% (n = 43); betamethasone valerate, 0.1% (n = 28); beclomethasone (n = 7); flucinolone (n = 3); mometasone (n = 2); and triamcinolone acetonide (n = 1). Medications were prescribed by chemists without prescription (n = 42), general practitioners (n = 14), dermatologists (n = 20), and alternate medical practitioners (n = 2).
Other skin conditions include acne (n = 3), seborrheic dermatitis (n = 3), eczema (n = 3), scabies (n = 2), chronic spontaneous urticaria (n = 2), vitiligo (n = 1), polymorphous light eruption (n = 1), lentigines (n = 1), erythema ab igne (n = 1), acanthosis nigricans (n = 1), and pityriasis rosea (n = 1).
Systemic diseases include type 2 diabetes (n = 10), hypertension (n = 5), chronic suppurative otitis media (n = 2), hypothyroidism (n = 3), asthma (n = 3), migraine (n = 1), epilepsy (n = 1), kidney stones (n = 1), multivitamin use after bariatric surgery (n = 1), and knee pain (n = 1).
Table 2. Minimum Inhibitory Concentrations (MICs) of 62 Isolates to the 6 Antifungals Tested.
| Species | MIC, μg/mL | |||||
|---|---|---|---|---|---|---|
| Fluconazole | Ketoconazole | Voriconazole | Terbinafine | Luliconazole | Itraconazole | |
| Trichophyton mentagrophytes (n = 34)a | ||||||
| Range | 0.125-32 | 0.25-4 | 0.0625-0.25 | 0.25-8 | 0.0625 | 0.125-0.5 |
| GM MIC | 1.37 | 1.2 | 0.073 | 1.303 | 0.0625 | 0.235 |
| MIC 50 | 4 | 1 | 0.0625 | 1 | 0.0625 | 0.125 |
| MIC 90 | 8 | 2 | 0.125 | 8 | 0.0625 | 0.5 |
| Trichophyton interdigitale (n = 13)b | ||||||
| Range | 1-8 | 0.5-4 | 0.0625-1 | 0.25-8.25 | 0.0625-0.5 | 0.125-1 |
| GM | 3.792 | 1.798 | 0.125 | 1.057 | 0.073 | 0.278 |
| MIC 50 | 4 | 2 | 0.125 | 1 | 0.0625 | 0.5 |
| MIC 90 | 8 | 4 | 0.25 | 8 | 0.0625 | 0.5 |
| Nannizzia gypsea (n = 6) a , c | ||||||
| Range | 0.5-4 | 0.0625-0.125 | 0.0625-0.125 | 0.0625-0.25 | 0.0625 | 0.125 |
| GM | 1.122 | 0.088 | 0.0702 | 0.079 | 0.0625 | 0.125 |
| MIC 50 | 0.5 | 0.0625 | 0.0625 | 0.0625 | 0.0625 | 0.125 |
| MIC 90 | 4 | 0.125 | 0.125 | 0.25 | 0.0625 | 0.125 |
| Trichophyton rubrum (n = 5)a | ||||||
| Range | 0.5-2 | 2-4 | 0.5 | 0.25-4 | 0.0625 | 0.125-1 |
| GM | 1.741 | 2.639 | 0.5 | 1 | 0.0625 | 0.435 |
| MIC 50 | 2 | 2 | 0.5 | 0.5 | 0.0625 | 0.5 |
| MIC 90 | 4 | 4 | 0.5 | 4 | 0.0625 | 1 |
| Trichophyton tonsurans (n = 2)a | ||||||
| Range | 1 | 4-8 | 0.0625-0.25 | 0.25-1 | 0.0625 | 0.125 |
| GM | 1 | 5.657 | 0.125 | 0.5 | 0.0625 | 0.125 |
| MIC 50 | 1 | 4 | 0.0625 | 0.25 | 0.0625 | 0.125 |
| MIC 90 | 1 | 8 | 0.25 | 1 | 0.0625 | 0.125 |
| Epidermophyton (n = 2)a | ||||||
| Range | 4 | 0.0625-0.125 | 0.0625-0.125 | 0.0625 | 0.0625 | 0.125 |
| GM | 4 | 0.088 | 0.088 | 0.0625 | 0.0625 | 0.125 |
| MIC 50 | 4 | 0.0625 | 0.0625 | 0.0625 | 0.0625 | 0.125 |
| MIC 90 | 4 | 0.125 | 0.125 | 0.0625 | 0.0625 | 0.125 |
Abbreviation: GM, geometric mean.
Culture-based identification.
Internal transcribed spacer sequencing–based identification.
Formerly known as Microsporum gypseum.
Efficacy
Of 149 patients enrolled initially, 126 patients completed the study protocol, while 23 were lost to follow-up, mostly during the national lockdown for the COVID-19 pandemic (Figure 1). There was no statistically significant difference in those lost to follow-up among the 100-, 200-, and 400-mg groups (11 of 50 patients, 5 of 49 patients, and 7 of 50 patients, respectively; P = .24). All following analyses are on patients who completed the protocol.
Of the 126 patients, 116 were cured while 10 failed treatment (7 in the 100-mg group and 3 in the 200-mg group), giving an overall cure rate of 92.1%. The cure rates in the 100-, 200-, and 400-mg groups thus were 82.0% (32 of 39 patients), 93.2% (41 of 44 patients), and 100% (43 of 43 patients), respectively. Thirty-seven patients were recorded to have persistent sites, including buttocks (n = 22), groin (n = 8), axillae (n = 4), waistline (n = 4), abdomen (n = 4), ear (n = 3), arms (n = 2), hand (n = 2), thighs (n = 2), chest (n = 1), inframammary region (n = 1), and face (n = 1).
For comparing the cure rates among the 3 groups, a Cox proportional hazard model was used, and hazard ratios (HRs) and 95% CIs were calculated. The overall model was statistically significant. Considering the 100-mg group as reference, a statistically nonsignificant HR of 1.44 (95% CI, 0.91-2.30; P = .12) was observed when compared with the 200-mg dose group. When comparing the 100-mg group with the 400-mg group, a statistically significant HR of 2.87 (95% CI, 1.78-4.62; P < .001) was observed, suggesting that the chances of cure are 2.87 times higher with a dose of 400 mg compared with a dose of 100 mg. Similarly, when comparing the 200-mg and 400-mg groups, a statistically significant HR of 1.99 (95% CI, 1.28-3.09; P = .002) was observed, suggesting that the chances of cure with a dose of 400 mg are 1.98 times compared with a dose of 200 mg (eTable 1 in Supplement 1). Patients who were cured had a 93.4% clinical clearance by 8 weeks, while those who failed treatment had a 75.0% clinical clearance by this time point (P = .001; eFigure in Supplement 1). For differential cutoff clinical clearance at other points of time during treatment, see the eFigure in Supplement 1.
Statistically significant risk of treatment failure was found among patients engaged in outdoor sports and in those with any persistent site recorded. The odds of treatment failure in such settings were 4.86 (95% CI, 1.22-19.36) and 7.33 (95% CI, 1.77-30.27), respectively. Higher risk of treatment failure was observed in those with buttocks as the persistent site (odds ratio, 5.82; 95% CI, 1.52-22.29; P = .01). The cure rate, however, was not statistically significantly related to age, gender, disease duration, family history of tinea, sharing of towels, having pets, education status, bathing frequency, previous treatment taken, previous steroid or antifungal use, history of tinea, or presence of other systemic diseases. It was also not statistically significantly associated with number of lesions, presence of cutaneous signs of topical steroid use, or BSA involved (Figure 2 and eTable 2 in Supplement 1).
Figure 2. Survival Graphs Depicting Proportion of Patients Achieving Cure Over Time With Respect to Various Clinical and Demographic Factors.
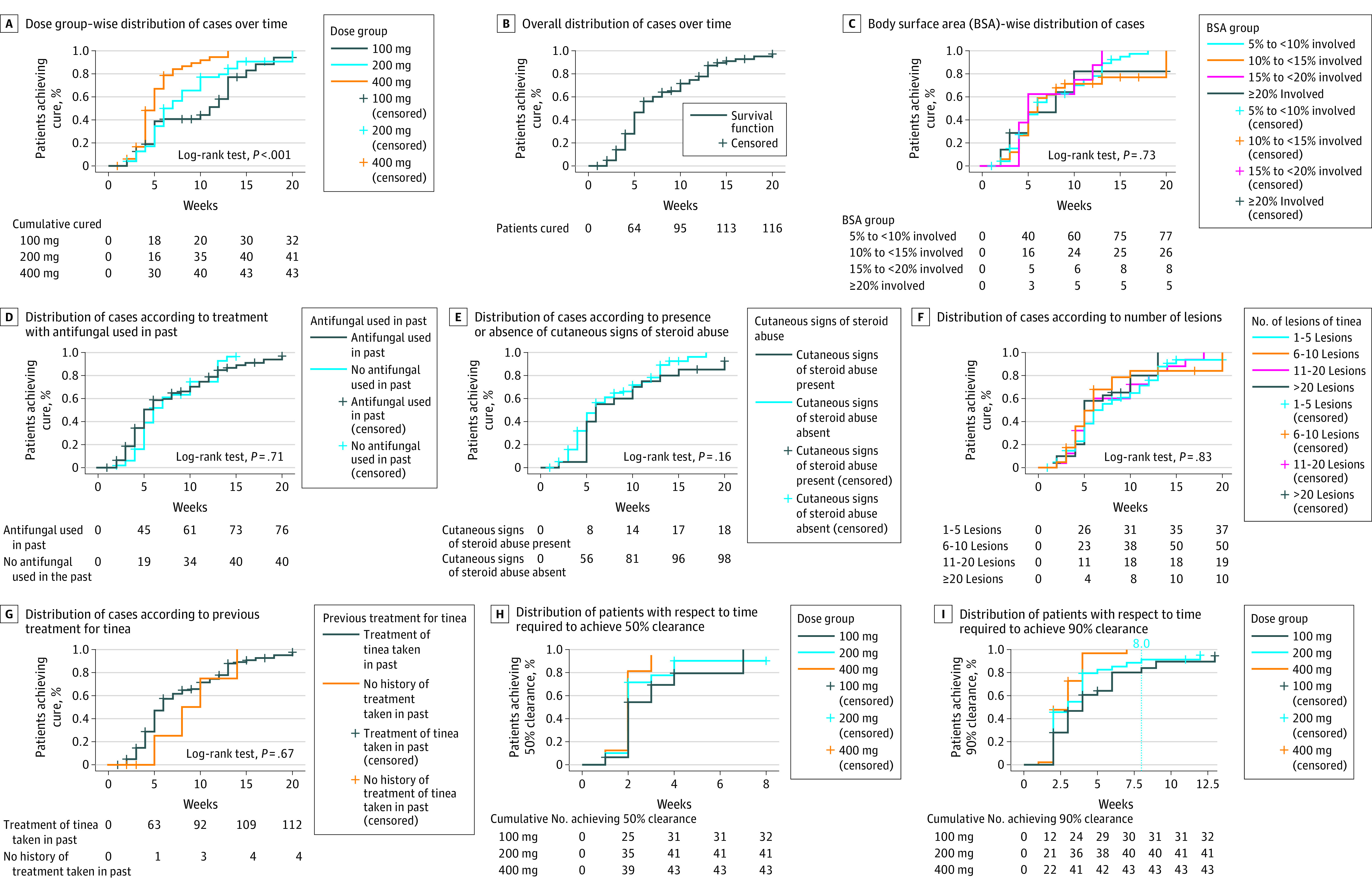
A total of 13 patients (40.6%) in the 100-mg group, 27 (65.8%) in the 200-mg group, and 27 (64.3%) in the 400-mg group achieved a VAS score of zero during the treatment. This difference among groups was not statistically significant.
Treatment Duration
Treatment duration to achieve cure ranged from 2 to 20 weeks, with a mean (SD) of 6.63 (3.85) weeks. The mean (SD) duration of treatment for clinical cure was 6.30 (3.69) weeks, while it was 5.62 (4.07) weeks for mycological cure. The mean (SD) duration of treatment required to achieve cure was lower in the 400-mg group (5.20 [2.60] weeks) compared with both the 100-mg (7.70 [4.73] weeks) and 200-mg (7.20 [3.81] weeks) groups, whereas there was no statistically significant difference among the 100-mg and 200-mg groups (eTable 3 in Supplement 1). Twelve patients (37.5%) in the 100-mg group, 16 (39.0%) in the 200-mg group, and 20 (46.5%) in the 400-mg group achieved cure within 8 weeks of treatment, while 20 (62.5%), 25 (61.0%), and 23 (53.5%) patients, respectively, required treatment beyond 8 weeks (Figure 2); however, the difference among groups was not statistically significant (P = .68). Fifty-four patients achieved mycological cure before clinical cure and required treatment for a mean (SD) of 2.04 (1.44) weeks (range, 1-9 weeks) beyond mycological cure to achieve complete cure, while 17 patients achieved clinical cure before mycological cure and required an additional 1.82 (0.81) weeks of treatment (range, 1-4 weeks) to achieve complete cure. However, the difference among groups, on these parameters, was not significant.
Mean treatment duration was not statistically significantly associated with age; gender; disease duration; family history of tinea; education status; sharing of towels; working in hot, humid environments; history of outdoor sports activities; bathing frequency; previous treatments taken; previous antifungal or steroid use; history of tinea; or presence of other skin or systemic diseases. It was also not statistically significantly correlated with BSA, number of lesions, or signs of topical steroid–induced skin damage; however, it was statistically significantly longer in those for whom any persistent site was noted (eTable 8 in Supplement 1).
Rapidity of Clinical Response
No statistically significant difference was observed in time to achieve 50% clinical clearance among the 3 groups (eTable 4 in Supplement 1). The duration required to achieve 90% clinical clearance was, however, statistically significantly different among groups (eTable 4 in Supplement 1), with duration required in the 400-mg group being significantly shorter compared with the 100-mg group, whereas there was no statistically significant difference with the 200-mg group or between the 100- and 200-mg groups.
Safety
The number of patients reporting an adverse event was statistically significantly different among groups; however, all observed adverse events were mild (eTable 5 in Supplement 1) and none warranted treatment discontinuation. No patient developed more than 2 times elevation in liver enzymes. The most common adverse events in the 100-, 200-, and 400-mg groups were acidity (8.0%, 10.2%, and 14.0%, respectively), abdominal discomfort/pain (2.0%, 8.2%, and 10.0%, respectively), constipation (4.0%, 4.1%, and 10.0%, respectively), and loose stools (4.0%, 8.2%, and 2.0%, respectively) (eTable 5 in Supplement 1).
Relapse Rates
A total of 55 patients (47.4%) relapsed following successful treatment completion. The group-wise values were 19 relapses in the 100-mg group (59.4% of total cured), 17 in the 200-mg group (41.5% of total cured), and 19 in the 400-mg group (44.2% of the cured); the difference in relapse rates among the groups was not statistically significant (P = .33). The time in weeks to relapse was statistically significantly longer in the 400-mg group (eTable 6 in Supplement 1). Furthermore, on comparison of relapses occurring within 4 weeks (termed as early relapses for the analysis) and beyond 4 weeks (late relapses), a statistically significant difference was observed among groups (eTable 9 in Supplement 1). This distribution between groups was 13 early and 6 late relapses in the 100-mg group, 10 early and 7 late relapses in the 200-mg group, and 3 early and 29 late relapses in the 400-mg group. The incidence of relapses was statistically significantly higher in female patients, with odds of relapse being 3 times more than male patients (P = .006). There was also a statistically significant difference in occurrence of relapse with age (those 21-30 and 31-40 years of age were affected more than other age groups) and education status (more in illiterate, primary school–educated, and middle school–educated groups). The occurrence of relapse, however, had no statistically significant association with family history nor history of tinea, presence of other skin or systemic diseases, duration of illness, previous topical steroid use, presence of any persistent site during treatment, number of lesions, or cutaneous signs of topical steroid–induced damage. Furthermore, treatment duration or initial BSA involved also had no statistically significant correlation with occurrence of relapse (eTable 9 in Supplement 1). Fourteen of the relapses were classified as minor, 17 as major, and 24 as minor, though later progressing to major.
Cost Analysis
To achieve cure, the cost of treatment increased by 63% in the 200-mg group and by 120% in the 400-mg group, over and above the cost to cure with 100 mg (eTable 7 in Supplement 1). The additional cost incurred for treatment of relapsed infections could not be considered for this analysis because complete follow-up records were not available for all relapsed patients.
Discussion
We observed a high overall cure rate with itraconazole treatment, comparable with historical literature on the drug, but with much longer treatment required for achieving the same.42,43 There was no statistically significant difference in cure rates between 100- and 200-mg doses of itraconazole, which is also as previously reported, albeit with much shorter treatment durations.24,28,43 Notably, itraconazole, 200 mg, is most often used by clinicians empirically.44 The MICs for 3 treatment failures could be obtained and were all less than 0.5 μg/mL, the recently proposed upper limit of wild-type MIC of the prevalent Indian strain.45 The reason for these failures may thus be interindividual variations in itraconazole serum levels achieved, translating to low drug concentrations at the site of action. The same has been highlighted in a recent report.37
Although the cure rates were considerably higher, and treatment duration considerably shorter, in the 400-mg group compared with both lower-dose groups, increasing the dose of itraconazole from 100 to 400 mg incurs 1.2 times increase in cost of treatment. While we did not find a statistically significant difference between the 100- and 200-mg doses on parameters of efficacy and treatment duration, the dose of 200 mg incurred an additional cost of 63% over the 100-mg dose in achieving cure. It is worthwhile to mention that serum levels achieved with 100 and 200 mg of itraconazole are also not statistically significantly different, which lends support to the findings of this study.37
Of patients in the 100-, 200-, and 400-mg groups, 62.5%, 61.0%, and 53.5% of patients, respectively, required treatment beyond 8 weeks. This brings forth the importance of patient counseling and compliance for a successful treatment outcome. All groups performed comparably in term of achieving a nil VAS score for pruritus. Notably, 42.3% of patients did not achieve a nil VAS score by the time of complete cure. An alteration in barrier function by dermatophytes has been proposed and may play a role here.46
It is alarming that with complete elimination of compliance factor, too, we observed a 47.4% relapse rate, with no statistically significant difference between groups. We believe this may be related to an inherent fungal virulence factor, because high relapses have been reported with other drugs as well. 47 However, we could not find any literature detailing virulence factors of this novel species and hope that this is taken up by future studies. Greater relapses in those with lower education status may be related to compliance with hygiene practices. We are, however, unable to explain the higher relapses seen in women. A longer time to relapse in the 400-mg group is unlikely to be related to persistent skin levels of drug, as itraconazole is not detectable in the stratum corneum after 2 weeks of treatment discontinuation, except in the highly seborrheic areas.48
In 37 patients, infection persisted at some sites beyond clearance at all others, leading to a meaningful prolongation of treatment duration. In most of these patients (n = 22), buttocks was the persistent site. This brings forth the importance of examining all sites, and especially the buttocks (if involved), for clearance before treatment discontinuation because such sites may be the source for autoinoculation and recurrences. The reasoning for persistence at this site specifically may be the occlusion effect promoting fungal growth, or possibly lower itraconazole skin levels at these sites, but the same would need to be verified in future studies.
We also noted that clinical and mycological cure may not occur concurrently and, thus, it is important to evaluate for both before discontinuing treatment. There is a renewed interest in using KOH smear examination for treatment monitoring, and its low cost and quick turnaround time makes it feasible for use as a routine clinic procedure.49
Notably, neither the cure rates nor the treatment durations were appreciably affected by the BSA involved, number of lesions, previous topical steroid (and its cutaneous sequelae), antifungal use, or history of recurring tinea infections. These have often been discussed as possible reasons for treatment failure or prolongation,50 but it seems that itraconazole’s high in vitro susceptibility scores over these factors. However, the washout period of 2 and 4 weeks for topical and systemic drugs, respectively, could also have contributed to lack of association with prior steroid/antifungal use. We found low MICs to itraconazole, voriconazole, and luliconazole, and high MICs to terbinafine, fluconazole, and ketoconazole in the isolates (Table 2), as per the recently described upper limit of wild-type MICs for the Indian strain.45
Limitations
We could perform ITS sequencing only on 13 isolates, owing to logistic constraints, and all of these were identified as T interdigitale. The correct identification of the highly virulent and multidrug-resistant species T indotineae has, however, been a much debated issue,51,52 and distinguishing it from T mentagrophyes s str and T interdigitale s str requires a multilocus approach combining ITS and Tef1-α genes with mating type genes HMG and α box,22 thus unfortunately making its precise identification out of purview of most laboratories. However, a near complete dominance of T indotineae has been reported in isolates from our center and from other centers in the country previously.2,3,4,5 In addition, the reported results are likely to be specific for the Indian strain and may not be generalizable to Trichophyton rubrum spp. and T mentagrophytes spp., the predominant species in most parts of the world as yet. However, the rapid spread of T indotineae with global travel and migration, as discussed before, warrants an awareness among clinicians worldwide of the nuances in management of TCC caused by this species.
Conclusions
In this randomized clinical trial, we report high efficacy and safety of itraconazole at all studied doses and note that prolonged treatment durations are needed for achieving cure. High relapse rates noted across all groups are disconcerting and call for more research focused on organism virulence factors. There was no statistically significant difference observed in cure rate or treatment duration among the 100- and 200-mg groups, although 63% of incremental cost is incurred in obtaining cure with the 200-mg dose. The itraconazole 400-mg dose achieves a higher cure rate than the 100- and 200-mg doses, but an almost 120% incremental cost in achieving cure with this dose may not justify its routine use.
eMethods
eTable 1. Cox proportional hazard model using drug dosage
eTable 2. Cure rate comparison between different Body Surface Area (BSA) segregated groups: overall analysis and subgroup analysis
eTable 3. Comparison of duration of treatment among cured patients in the 3 groups
eTable 4. Rapidity of response: time to achieve 50% and 90% clinical clearance
eTable 5. Adverse events stratified by dose groups
eTable 6. Comparison of weeks to relapse between dose groups
eTable 7. Incremental cost of cure per unit effectiveness (C/E value)
eTable 8. Comparison of duration of treatment with baseline demographic and clinical parameters and presence of “persistent sites” noted during treatment
eTable 9. Occurrence of relapse as compared with treatment duration, body surface area involved, and dose groups, and comparison of early (<4 weeks) and late (>4 weeks) relapses among groups
eFigure. ROC curves depicting the different areas of clearance at different time intervals among the cases who ultimately got cured or failed treatment
Trial Protocol
Data Sharing Statement
References
- 1.Khurana A, Sardana K, Chowdhary A. Antifungal resistance in dermatophytes: recent trends and therapeutic implications. Fungal Genet Biol. 2019;132:103255. doi: 10.1016/j.fgb.2019.103255 [DOI] [PubMed] [Google Scholar]
- 2.Rudramurthy SM, Shankarnarayan SA, Dogra S, et al. Mutation in the squalene epoxidase gene of Trichophyton interdigitale and Trichophyton rubrum associated with allylamine resistance. Antimicrob Agents Chemother. 2018;62(5):e02522-e17. doi: 10.1128/AAC.02522-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh A, Masih A, Khurana A, et al. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses. 2018;61(7):477-484. doi: 10.1111/myc.12772 [DOI] [PubMed] [Google Scholar]
- 4.Khurana A, Masih A, Chowdhary A, et al. Correlation of in vitro susceptibility based on MICs and squalene epoxidase mutations with clinical response to terbinafine in patients with tinea corporis/cruris. Antimicrob Agents Chemother. 2018;62(12):e01038-e18. doi: 10.1128/AAC.01038-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh A, Masih A, Monroy-Nieto J, et al. A unique multidrug-resistant clonal Trichophyton population distinct from Trichophyton mentagrophytes/Trichophyton interdigitale complex causing an ongoing alarming dermatophytosis outbreak in India: genomic insights and resistance profile. Fungal Genet Biol. 2019;133:103266. doi: 10.1016/j.fgb.2019.103266 [DOI] [PubMed] [Google Scholar]
- 6.Kano R, Kimura U, Kakurai M, et al. Trichophyton indotineae sp. nov.: a new highly terbinafine-resistant anthropophilic dermatophyte species. Mycopathologia. 2020;185(6):947-958. doi: 10.1007/s11046-020-00455-8 [DOI] [PubMed] [Google Scholar]
- 7.Kimura U, Hiruma M, Kano R, et al. Caution and warning: arrival of terbinafine-resistant Trichophyton interdigitale of the Indian genotype, isolated from extensive dermatophytosis, in Japan. J Dermatol. 2020;47(5):e192-e193. doi: 10.1111/1346-8138.15300 [DOI] [PubMed] [Google Scholar]
- 8.Fattahi A, Shirvani F, Ayatollahi A, et al. Multidrug-resistant Trichophyton mentagrophytes genotype VIII in an Iranian family with generalized dermatophytosis: report of four cases and review of literature. Int J Dermatol. 2021;60(6):686-692. doi: 10.1111/ijd.15226 [DOI] [PubMed] [Google Scholar]
- 9.Taghipour S, Shamsizadeh F, Pchelin IM, et al. Emergence of terbinafine resistant Trichophyton mentagrophytes in Iran, harboring mutations in the squalene epoxidase (Sqle) gene. Infect Drug Resist. 2020;13:845-850. doi: 10.2147/IDR.S246025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngo TMC, Ton Nu PA, Le CC, Ha TNT, Do TBT, Tran Thi G. First detection of Trichophyton indotineae causing tinea corporis in central Vietnam. Med Mycol Case Rep. 2022;36:37-41. doi: 10.1016/j.mmcr.2022.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nenoff P, Verma SB, Ebert A, et al. Spread of terbinafine-resistant Trichophyton mentagrophytes type VIII (India) in Germany—“the tip of the iceberg?” J Fungi (Basel). 2020;6(4):207. doi: 10.3390/jof6040207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Astvad KMT, Hare RK, Jørgensen KM, Saunte DML, Thomsen PK, Arendrup MC. Increasing terbinafine resistance in Danish Trichophyton isolates 2019-2020. J Fungi (Basel). 2022;8(2):150. doi: 10.3390/jof8020150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sacheli R, Harag S, Dehavay F, et al. Belgian national survey on tinea capitis: epidemiological considerations and highlight of terbinafine-resistant T. mentagrophytes with a mutation on SQLE gene. J Fungi (Basel). 2020;6(4):195. doi: 10.3390/jof6040195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jabet A, Brun S, Normand AC, et al. Extensive dermatophytosis caused by terbinafine-resistant Trichophyton indotineae, France. Emerg Infect Dis. 2022;28(1):229-233. doi: 10.3201/eid2801.210883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dellière S, Joannard B, Benderdouche M, et al. Emergence of difficult-to-treat tinea corporis caused by Trichophyton mentagrophytes complex isolates, Paris, France. Emerg Infect Dis. 2022;28(1):224-228. doi: 10.3201/eid2801.210810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Järv H, Uhrlaß S, Simkin T, et al. Terbinafine resistant Trichophyton mentagrophytes genotype VIII, Indian type, isolated in Finland. J Fungi (Basel). 2019;5:39. [Google Scholar]
- 17.Siopi M, Efstathiou I, Theodoropoulos K, Pournaras S, Meletiadis J. Molecular epidemiology and antifungal susceptibility of Trichophyton isolates in Greece: emergence of terbinafine-resistant Trichophyton mentagrophytes type VIII locally and globally. J Fungi (Basel). 2021;7(6):419. doi: 10.3390/jof7060419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh A, Quenan S, Riat A, Toutous-Trellu L, Fontao L. A new mutation in the SQLE gene of Trichophyton mentagrophytes associated to terbinafine resistance in a couple with disseminated tinea corporis. J Mycol Med. 2019;29(4):352-355. doi: 10.1016/j.mycmed.2019.100903 [DOI] [PubMed] [Google Scholar]
- 19.Łagowski D, Gnat S, Nowakiewicz A, Osińska M, Dyląg M. Intrinsic resistance to terbinafine among human and animal isolates of Trichophyton mentagrophytes related to amino acid substitution in the squalene epoxidase. Infection. 2020;48(6):889-897. doi: 10.1007/s15010-020-01498-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Posso-De Los Rios CJ, Tadros E, Summerbell RC, Scott JA. Terbinafine resistant Trichophyton indotineae isolated in patients with superficial dermatophyte infection in Canadian patients. J Cutan Med Surg. 2022;26(4):371-376. doi: 10.1177/12034754221077891 [DOI] [PubMed] [Google Scholar]
- 21.Kong X, Tang C, Singh A, et al. Antifungal susceptibility and mutations in the squalene epoxidase gene in dermatophytes of the Trichophyton mentagrophytes species complex. Antimicrob Agents Chemother. 2021;65(8):e0005621. doi: 10.1128/AAC.00056-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang C, Kong X, Ahmed SA, et al. Taxonomy of the Trichophyton mentagrophytes/T. interdigitale species complex harboring the highly virulent, multiresistant genotype T. indotineae. Mycopathologia. 2021;186(3):315-326. doi: 10.1007/s11046-021-00544-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cauwenbergh G, De Doncker P. Itraconazole (R 51 211): a clinical review of its antimycotic activity in dermatology, gynecology, and internal medicine. Drug Dev Res. 1986;8(1-4):317-323. doi: 10.1002/ddr.430080136 [DOI] [Google Scholar]
- 24.Boonk W, de Geer D, de Kreek E, Remme J, van Huystee B. Itraconazole in the treatment of tinea corporis and tinea cruris: comparison of two treatment schedules. Mycoses. 1998;41(11-12):509-514. doi: 10.1111/j.1439-0507.1998.tb00714.x [DOI] [PubMed] [Google Scholar]
- 25.Schuller J, Remme JJ, Rampen FH, Van Neer FC. Itraconazole in the treatment of tinea pedis and tinea manuum: comparison of two treatment schedules. Mycoses. 1998;41(11-12):515-520. doi: 10.1111/j.1439-0507.1998.tb00715.x [DOI] [PubMed] [Google Scholar]
- 26.Doncker PD, Gupta AK, Marynissen G, Stoffels P, Heremans A. Itraconazole pulse therapy for onychomycosis and dermatomycoses: an overview. J Am Acad Dermatol. 1997;37(6):969-974. doi: 10.1016/S0190-9622(97)70074-4 [DOI] [PubMed] [Google Scholar]
- 27.Katsambas A, Antoniou C, Frangouli E, et al. Itraconazole in the treatment of tinea corporis and tinea cruris. Clin Exp Dermatol. 1993;18(4):322-325. doi: 10.1111/j.1365-2230.1993.tb02207.x [DOI] [PubMed] [Google Scholar]
- 28.Parent D, Decroix J, Heenen M. Clinical experience with short schedules of itraconazole in the treatment of tinea corporis and/or tinea cruris. Dermatology. 1994;189(4):378-381. doi: 10.1159/000246883 [DOI] [PubMed] [Google Scholar]
- 29.Rajagopalan M, Inamadar A, Mittal A, et al. Expert Consensus on The Management of Dermatophytosis in India (ECTODERM India). BMC Dermatol. 2018;18(1):6. doi: 10.1186/s12895-018-0073-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh S, Chandra U, Anchan VN, Verma P, Tilak R. Limited effectiveness of four oral antifungal drugs (fluconazole, griseofulvin, itraconazole and terbinafine) in the current epidemic of altered dermatophytosis in India: results of a randomized pragmatic trial. Br J Dermatol. 2020;183(5):840-846. doi: 10.1111/bjd.19146 [DOI] [PubMed] [Google Scholar]
- 31.Chen E, Ghannoum M, Elewski BE. Treatment-resistant tinea corporis, a potential public health issue. Br J Dermatol. 2021;184(1):164-165. doi: 10.1111/bjd.19420 [DOI] [PubMed] [Google Scholar]
- 32.Sardana K, Mathachan SR. The science and rationale of arriving at the correct drug and dosimetry of griseofulvin, fluconazole, terbinafine and itraconazole in superficial dermatophyte infections: an important step before a pragmatic trial. Br J Dermatol. 2021;184(2):376-377. doi: 10.1111/bjd.19562 [DOI] [PubMed] [Google Scholar]
- 33.Sharma P, Bhalla M, Thami GP, Chander J. Evaluation of efficacy and safety of oral terbinafine and itraconazole combination therapy in the management of dermatophytosis. J Dermatolog Treat. 2020;31(7):749-753. doi: 10.1080/09546634.2019.1612835 [DOI] [PubMed] [Google Scholar]
- 34.Chandrashekar BS, Poojitha DS. Evaluation of efficacy and safety of oral voriconazole in the management of recalcitrant and recurrent dermatophytosis. Clin Exp Dermatol. 2022;47(1):30-36. doi: 10.1111/ced.14799 [DOI] [PubMed] [Google Scholar]
- 35.Sardana K, Mathachan SR, Sachdeva S, Khurana A. Is there a rationale for the use of voriconazole in dermatophytosis in the absence of mycological and mutational data? an urgent need for antifungal stewardship. Clin Exp Dermatol. 2021;46(8):1621-1623. doi: 10.1111/ced.14824 [DOI] [PubMed] [Google Scholar]
- 36.Murari A, Singh KN. Lund and Browder chart-modified versus original: a comparative study. Acute Crit Care. 2019;34(4):276-281. doi: 10.4266/acc.2019.00647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khurana A, Agarwal A, Singh A, et al. Predicting a therapeutic cut-off serum level of itraconazole in recalcitrant tinea corporis and cruris-A prospective trial. Mycoses. 2021;64(12):1480-1488. doi: 10.1111/myc.13367 [DOI] [PubMed] [Google Scholar]
- 38.Sardana K, Khurana A, Singh A, Gautam RK. A pilot analysis of morphometric assessment of itraconazole brands using dermoscopy and its relevance in the current scenario. Indian Dermatol Online J. 2018;9(6):426-431. doi: 10.4103/idoj.IDOJ_339_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clinical and Laboratory Standards Institute . Reference Method for Broth Dilution Antifungal Susceptibility Testing Of Filamentous Fungi: Approved Standard M38-A2. 2nd ed. Clinical and Laboratory Standards Institute; 2010. [Google Scholar]
- 40.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175-191. doi: 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 41.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149-1160. doi: 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- 42.Nuijten ST, Schuller JL. Itraconazole in the treatment of tinea corporis: a pilot study. Rev Infect Dis. 1987;9(suppl 1):S119-S120. doi: 10.1093/clinids/9.Supplement_1.S119 [DOI] [PubMed] [Google Scholar]
- 43.Degreef H, Mariën K, De Veylder H, Duprez K, Borghys A, Verhoeve L. Itraconazole in the treatment of dermatophytoses: a comparison of two daily dosages. Rev Infect Dis. 1987;9(suppl 1):S104-S108. doi: 10.1093/clinids/9.Supplement_1.S104 [DOI] [PubMed] [Google Scholar]
- 44.Rengasamy M, Shenoy MM, Dogra S, et al. Indian Association of Dermatologists, Venereologists and Leprologists (IADVL) Task Force against Recalcitrant Tinea (ITART) Consensus on the Management of Glabrous Tinea (INTACT). Indian Dermatol Online J. 2020;11(4):502-519. doi: 10.4103/idoj.IDOJ_233_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaw D, Singh S, Dogra S, et al. MIC and upper limit of wild-type distribution for 13 antifungal agents against a Trichophyton mentagrophytes-Trichophyton interdigitale complex of Indian origin. Antimicrob Agents Chemother. 2020;64(4):e01964-e19. doi: 10.1128/AAC.01964-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sardana K, Gupta A, Mathachan SR. Immunopathogenesis of dermatophytoses and factors leading to recalcitrant infections. Indian Dermatol Online J. 2021;12(3):389-399. doi: 10.4103/idoj.IDOJ_503_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Majid I, Sheikh G, Kanth F, Hakak R. Relapse after oral terbinafine therapy in dermatophytosis: a clinical and mycological study. Indian J Dermatol. 2016;61(5):529-533. doi: 10.4103/0019-5154.190120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cauwenbergh G, Degreef H, Heykants J, Woestenborghs R, Van Rooy P, Haeverans K. Pharmacokinetic profile of orally administered itraconazole in human skin. J Am Acad Dermatol. 1988;18(2 pt 1):263-268. doi: 10.1016/S0190-9622(88)70037-7 [DOI] [PubMed] [Google Scholar]
- 49.Khurana A, Agarwal A, Agrawal D, Sethia K. Re-emerging role of KOH smear examination in the era of recalcirant dermatophytoses. Dermatol Ther. 2021;34(2):e14891. doi: 10.1111/dth.14891 [DOI] [PubMed] [Google Scholar]
- 50.Khurana A, Gupta A, Sardana K, et al. A prospective study on patterns of topical steroids self-use in dermatophytoses and determinants predictive of cutaneous side effects. Dermatol Ther. 2020;33(4):e13633. doi: 10.1111/dth.13633 [DOI] [PubMed] [Google Scholar]
- 51.Nenoff P, Verma SB, Uhrlaß S, Burmester A, Gräser Y. A clarion call for preventing taxonomical errors of dermatophytes using the example of the novel Trichophyton mentagrophytes genotype VIII uniformly isolated in the Indian epidemic of superficial dermatophytosis. Mycoses. 2019;62(1):6-10. doi: 10.1111/myc.12848 [DOI] [PubMed] [Google Scholar]
- 52.Chowdhary A, Singh A, Singh PK, Khurana A, Meis JF. Perspectives on misidentification of Trichophyton interdigitale/Trichophyton mentagrophytes using internal transcribed spacer region sequencing: urgent need to update the sequence database. Mycoses. 2019;62(1):11-15. doi: 10.1111/myc.12865 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTable 1. Cox proportional hazard model using drug dosage
eTable 2. Cure rate comparison between different Body Surface Area (BSA) segregated groups: overall analysis and subgroup analysis
eTable 3. Comparison of duration of treatment among cured patients in the 3 groups
eTable 4. Rapidity of response: time to achieve 50% and 90% clinical clearance
eTable 5. Adverse events stratified by dose groups
eTable 6. Comparison of weeks to relapse between dose groups
eTable 7. Incremental cost of cure per unit effectiveness (C/E value)
eTable 8. Comparison of duration of treatment with baseline demographic and clinical parameters and presence of “persistent sites” noted during treatment
eTable 9. Occurrence of relapse as compared with treatment duration, body surface area involved, and dose groups, and comparison of early (<4 weeks) and late (>4 weeks) relapses among groups
eFigure. ROC curves depicting the different areas of clearance at different time intervals among the cases who ultimately got cured or failed treatment
Trial Protocol
Data Sharing Statement