Abstract
Increasing effort has been devoted to understanding the neural mechanisms underlying decision making during risk, yet little is known about the effect of voluntary choice on risk taking. The Balloon Analog Risk Task (BART), in which subjects inflate a virtual balloon that can either grow larger or explode (Lejuez et al., J. Exp. Psychol. Appl., 2002, 8, 75–84), provides an ecologically valid model to assess human risk taking propensity and behaviour. In the present study, we modified this task for use during functional magnetic resonance imaging (fMRI) and administered it in both an active choice mode and a passive no-choice mode in order to examine the neural correlates of voluntary and involuntary risk taking in the human brain. Voluntary risk in the active choice task is associated with robust activation in mesolimbic-frontal regions, including the midbrain, ventral and dorsal striatum, anterior insula, dorsal lateral prefrontal cortex (DLPFC), and anterior cingulate/medial frontal cortex (ACC/MFC), in addition to activation in visual pathway regions. However, these mesolimbic-frontal activation patterns were not observed for involuntary risk in the passive no-choice task. Decision making was associated with neural activity in the right DLPFC. These findings demonstrate the utility of the modified BART paradigms for using during fMRI to assess risk taking in the human brain, and suggest that recruitment of the brain mesolimbic-frontal pathway during risk-taking is contingent upon the agency of the risk taker. The present paradigm may be extended to pathological populations to determine the specific neural components of their impaired risk behavior.
Keywords: Risk, Choice, Striatum, Frontal, fMRI
Introduction
Risk is ubiquitous in the natural world and human life. Although some amount of risk-taking behaviour is desirable and essential for human survival and advancement, excessive risk-taking may underlie pathological conditions such as drug-abuse and compulsive gambling (Bechara & Damasio, 2002; Weintraub & Potenza, 2006). Over the past century, multiple theories and models have been developed to understand how people assess risk and make decisions. For example, standard expected utility (EU) theory assumes people are perfect rational machines for utility maximization and largely ignores the influence of emotion in decision-making. However, human decisions are not always rational, and converging empirical evidence has challenged the rational assumption model (e.g., Allais & Hagan, 1979). Prospect theory (Kahneman & Tversky, 1979; Tversky & Kahneman, 1992) provides an alternative psychologically realistic model. This theory includes additional psychological factors, such as status quo, loss aversion, framing effects, and editing effects, and neatly describes attitudes toward risk in ecologically valid settings. More recently, the somatic marker hypothesis (Damasio, 1996; Bechara & Damasio, 2005) posits that every stimulus is associated with an affective weight, and emotion-based somatic signals arising from changes in the autonomic nervous system are integrated in higher brain areas to regulate risky decision-making. Furthermore, some investigators have argued that decision-making is dominated by affective heuristics (Finucane et al., 2000; Peters & Slovic, 2000) and may be conceptualized as a general feeling state (Loewenstein et al., 2001).
Converging evidence from neuroimaging and brain lesion studies supports the critical role of emotion processing in decision-making (for a review, see Bechara, 2004; Ernst and Paulus, 2005; Trepel et al., 2005). It is now established that decision-making under risk or uncertainty involves a distributed subcortical-cortical network including multiple prefrontal, parietal, limbic, and subcortical regions (Ernst and Paulus, 2005; Trepel et al., 2005; Krain et al., 2006). Within this network, dopamine rich mesolimbic regions including the midbrain, striatum, and their reciprocally connected frontal cortex, have been suggested to play a particular role in processing reward or motivational stimulus salience during decision-making (Bozarth, 1991; Schultz et al., 1997; Delgado, 2007).
However, not all risks are predicated on decisions people make. Some risks, such as those of accidental injury or illness, are inherent in human life and may occur regardless of people’s choices. In many instances, people have no option to choose between situations, and may subsequently be forced to accept the consequences and outcomes of passive risk conditions. Conversely, many decisions people make on a daily basis have no implication of risk or reward. These empirical observations suggest that risk and decision-making may be dissociable, and raise the following questions: Which brain regions mediate risk regardless of the involvement of decision making? Which brain regions mediate decision-making regardless of the involvement of risk? Specifically, is the human dopamine system activated by risk or decision-making alone, or must risk and decision-making be combined in order to activate the dopamine system in the human brain? Although numerous previous studies have consistently demonstrated the involvement of mesolimbic-frontal regions in decision-making (Matthews et al., 2004; Hsu et al., 2005; Kuhnen and Knutson 2005; Huettel et al., 2006; Preuschoff et al., 2006), few if any studies have dissociated the neural substrates of risk from decision-making.
To address these questions, we modified the Balloon Analog Risk Task (BART) for use during functional magnetic resonance imaging (fMRI). The BART is a laboratory-based behavioural measure of risk taking behaviour developed by Lejeuz and colleagues (Lejeuz, et al., 2002). In this task, participants are required to sequentially inflate a balloon that could either grow larger or explode. A larger balloon is naturally associated with an increased probability of explosion. Unlike other risk tasks, such as the IOWA gambling task (Bechara, et al., 1994), in which risk was defined and manipulated by arbitrary connections from stimuli to outcomes, risk in the BART was more directly and ecologically defined as the probability of explosion for each balloon. During the task, participants are repeatedly given the option to continue or discontinue inflating a virtual balloon. This provides participants the voluntary choice to determine the risk level for each balloon. The larger the balloon participants inflated, the greater risk level participants were willing to take. Therefore the average number of inflations participants made for the balloons provides an objective assessment of risk preference. Behavioural studies (Lejeuz, et al., 2002; 2003a; 2003b; 2007; Hunt et al., 2005) have consistently shown that participants’ risk preferences measured by the BART correlate with scores on risk-related constructs (e.g., sensation seeking and impulsivity) as well as the self-reported occurrence of risk behaviours (e.g., drug addiction, smoking and delinquency). Although the BART provides an ecologically valid model for the assessment of risk taking propensity and behaviour, no fMRI study has been published using this task to examine the neural bases underlying risk taking in the human brain to date. Another appealing feature of the BART as an fMRI task is the parametric relationship between risk level and balloon size for successive inflations. This feature allows risk in this task to be manipulated and modeled as a covariate that is independent of other task components. In the original BART task, the reward increase for successive inflations was constant. However, in order to encourage inflation over a greater range of balloon sizes for improved parametric modeling of risk, we increased the virtual monetary reward for continued inflation commensurate with the increased probability of explosion in this task for our fMRI application.
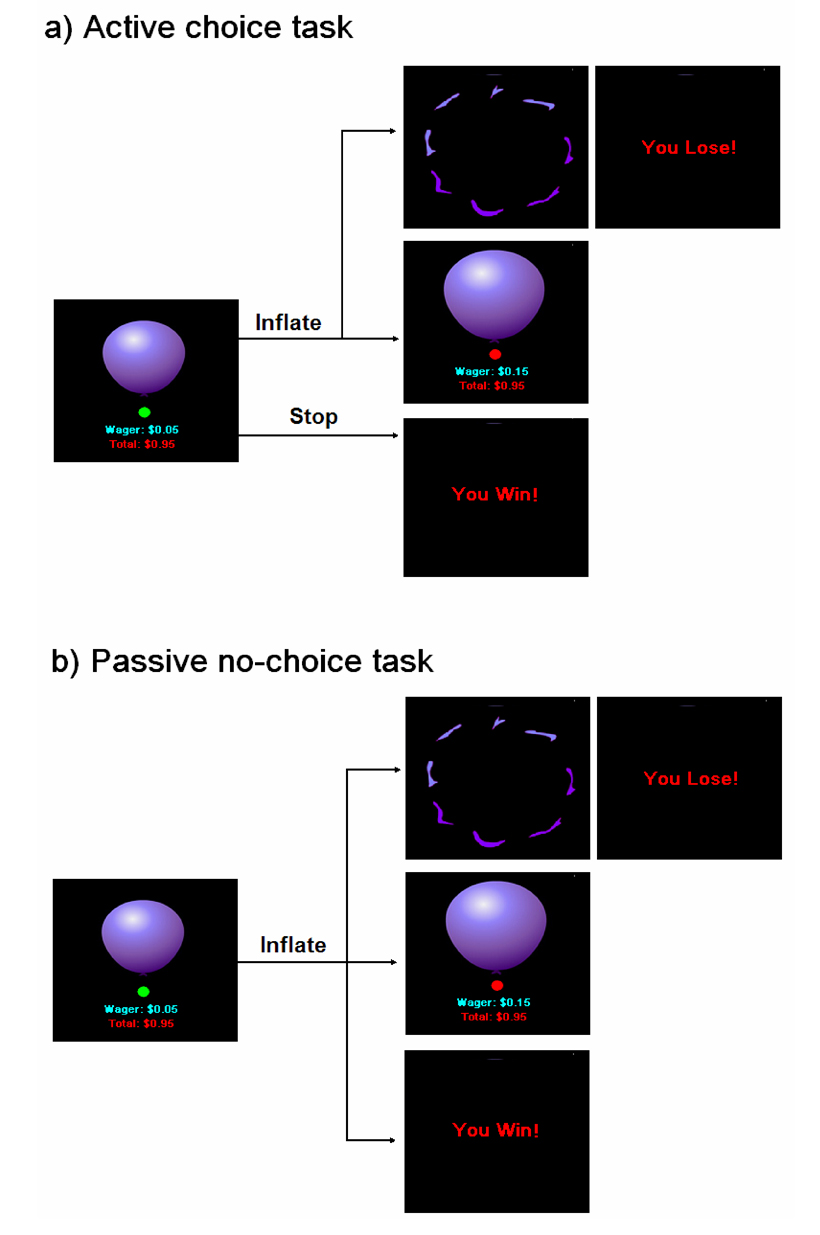
For this study, we also administered the BART both in an active mode where participants had the choice to discontinue inflating the balloon at any time and in a passive “Russian Roulette” mode where participants had no choice and were forced to continue inflating at each turn while the computer randomly determined the end size and corresponding monetary reward outcome for each balloon (Fig. 1). Except for the choice to discontinue inflation, the two tasks were identical in all other experimental parameters. In both active and passive tasks, increasing the probability of explosion with balloon size produces a parametric variation in the level of risk with successive inflations as the trial proceeds. Thus, the neural substrates underlying both voluntary and involuntary risk could be identified by directly correlating the explosion probability of each balloon (i.e., risk level) with regional brain activity. Moreover, the neural substrates underlying decision making could be isolated by comparing the actively chosen inflations in one task to the forced no-choice inflations in the other task.
Figure 1.
The balloon inflation risk tasks in the active choice mode (a) and the passive no-choice mode (b). In the active choice task, participants could choose to continue or discontinue inflating the balloon at each turn. In the passive no-choice task, participants were required to continue inflating the balloon at each turn and the computer program determined the win or loss outcomes for each balloon.
The primary goal of this study was to image and dissociate the neural correlates of voluntary and involuntary risk taking in the human brain. Using active and passive BART conditions with a parametric fMRI design, the present study was designed to dissociate the neural substrates meditating risk taking with and without choice, and examine how voluntary choice, i.e., the agency of risk taker, influences risk processing in the brain. Based on the critical role of dopamine rich mesolimbic structures in both reward and emotion processing (Bozarth, 1991; Delgado, 2007; Jentsch et al., 2007; Schultz 2007) as well as the consistent activation in these regions associated with risk and reward (McClure et al., 2004; Hsu et al., 2005; Montague et al., 2006; Preuschoff et al., 2006), we predicted that risk in the active choice task would correlate with neural activity in the mesolimbic pathway, specifically the midbrain, striatum, and their reciprocally interconnected frontal areas. Moreover, previous studies have shown that voluntary choice or agency modulated striatum activity associated with win and reward outcomes (Tricomi et al., 2004; Zink et al., 2004; Coricelli et al., 2005). By using both voluntary and involuntary BART paradigms, the present study provides an opportunity to examine the effect of agency on risk in addition to outcomes. We predicted that agency would highlight striatal activity for both risk and outcomes.
The second goal of this study was to dissociate the neural substrates mediating the component of active choice or voluntary decision making in the human brain. Although numerous decision-making imaging studies have been conducted, few if any studies have isolated and directly visualized the area underlying decision making in the human brain. By comparing the two tasks which were identical in stimuli, risk, and reward parameters with the sole exception of the voluntary choice component, the current design minimized experimental confounds and localized the brain area mediating voluntary as opposed to involuntary decision making. Based on the critical role of the dorsal lateral prefrontal cortex (DLPFC) in executive control, goal maintenance and impulsivity inhibition (Miller and Cohen et al., 2001) and the causal relationship between disrupted DLPFC activity and risky decision making (Knoch et al., 2006a, 2006b), we predicted that activation in the DLPFC would mediate voluntary decision making during risk.
Materials and Methods
Participants
Fourteen healthy individuals (8 male, 6 female, mean age 25.1 yrs, range 21–35 yrs) participated in this study. All participants had normal or corrected-to-normal vision, were right-handed and had no history of neurological or psychiatric disorders. Written consent was obtained from all participants according to the University of Pennsylvania Institutional Review Board. Each participant was paid $35 compensation for participating in the study.
Tasks and Paradigms
The tasks used in this study included two versions of balloon inflation paradigms (see Figure 1) modified from the original BART (Lejeuz et al., 2002). In both tasks, participants were presented with a realistic image of a balloon in the center of the screen and were required to press a button to sequentially inflate the balloon. Participants were instructed that the balloon could either grow larger or explode, and their goal in the study was maximize the virtual monetary reward during both tasks. However, participants were instructed that the monetary amount was a “virtual reward” which did not consist of actual money. Participants were also instructed that the balloon could explode at any size, and larger balloons were associated with greater risk of explosion as well as higher virtual monetary reward. The maximum number of inflations participants could make for each balloon was 12. In order to encourage participants to make multiple inflation attempts for one balloon, the wager size and probability of explosion both monotonically increased with the number of inflations. From the smallest balloon to the largest balloon, the probability of explosion was set to monotonically increase from 0 to 89.6%, and the wager increased from 0 to 5.15 dollars (Table 1). The actual percentage of explosion for a given inflation level occurring in the task, calculated by dividing the sum of the number of explosions by the sum of the number of inflations across all subjects and over both task conditions, reasonably approximated the pre-determined probabilities of explosion (Table 1). The value of wagers in corresponding to the various balloon sizes and the cumulative earnings for the tasks were explicitly displayed underneath the balloon stimuli, whereas the maximum number of inflations and the exact probability of explosion associated with a given inflation were unknown to participants.
Table 1.
The risk of explosion (the probability set by the program), the actual probability of explosion, the value of wager, and the reward variance associated with each balloon inflation in the tasks.
| Number of Inflation | Risk of explosion (%) | Actual probability of explosion (%) | Wager ($) | Reward Variance ($) |
|---|---|---|---|---|
| 1 | 0.0 | 0 | 0.00 | 0.00 |
| 2 | 2.1 | 0 | 0.05 | 0.0001 |
| 3 | 4.2 | 5.1 | 0.15 | 0.0009 |
| 4 | 6.3 | 8.0 | 0.25 | 0.0037 |
| 5 | 14.6 | 19.4 | 0.55 | 0.0377 |
| 6 | 23.9 | 25.8 | 0.95 | 0.1641 |
| 7 | 31.3 | 43.8 | 1.45 | 0.4521 |
| 8 | 43.8 | 58.7 | 2.05 | 1.0345 |
| 9 | 56.3 | 66.7 | 2.75 | 1.8606 |
| 10 | 68.8 | 69.3 | 3.45 | 2.5549 |
| 11 | 79.2 | 76.0 | 4.25 | 2.9755 |
| 12 | 89.6 | 83.6 | 5.15 | 2.4715 |
The balloon inflation tasks included an active choice mode and a passive no-choice mode. In the active choice mode, participants were repeatedly given two options: to press the right button to continue inflating the balloon or to press the left button to discontinue inflation. The participants’ choice to inflate the balloon led to two possible outcomes that were immediately fed back to them: a no-explosion balloon inflation event in which a larger balloon with an increased wager were displayed, or a loss event in which a picture of a balloon explosion and the text “You Lose!” were displayed. If the balloon exploded, participants lost the wager and the lost virtual monetary amount was subtracted from the cumulative earnings as the penalty. If the participants’ choice to discontinue inflation led to an immediate win event in which the text “You Win!” was displayed, participants won the wager and the amount of reward was added to the cumulative earnings. During the active choice condition, participants could determine the level of risk and the amount of virtual monetary reward for each balloon. However, in the passive no-choice mode, participants merely pressed the right button to continue inflating the balloon at each turn while the computer determined the end point as well as the win or loss outcomes for each balloon. In this condition, participants were forced to accept the risk level and corresponding amount of virtual monetary reward the computer determined for each balloon. With the exception of the voluntary choice to discontinue inflation and collect the virtual monetary reward by pressing the left button in the active condition, the two versions of the task were identical in all other respects.
The timing of inflation during both tasks was controlled by a cue, which consisted of a small circle that changed color from red to green with a jittered time interval. Participants could press a button to continue or discontinue inflation only when the color of the cue was green. After participants successfully pressed a button and inflated the balloon, the cue immediately turned red for a random interval between 1.5–2.5 seconds, and then the cue turned green again to indicate the next inflation. After the end of previous balloon, there was also a jittered 2–4 second interval prior to the beginning of next balloon. The number of balloons participants completed during the scan was not pre-determined in either active or passive tasks. Instead, the number depended on the response speed, which varied between subjects.
Data Acquisition
Task stimuli were projected onto a display screen at the back of the magnet’s bore and participants viewed the stimuli through a mirror. Button-press responses on an fMRI-compatible response box were made using the left and right thumbs. Prior to the scan, participants received instructions for the active and passive task conditions and practiced several balloons for each task. They experienced both loss and win events during practice and were instructed to maximize the virtual reward during performing the same tasks in the scanner.
Functional imaging was conducted on a Siemens 3.0T Trio whole-body scanner (Siemens AG, Erlangen, Germany), using a product 8-channel array coil. Functional images, which measured the blood oxygenation level dependent (BOLD) signal, were acquired using a standard echo-planar imaging sequence (TR = 1500 ms, TE = 30 ms, flip angle = 90°, 25 interleaved axial slices with 5 mm thickness, in-plane resolution = 3.44 mm × 3.44 mm). Each participant completed two 8 minute functional runs, one for each task. The scanning order of the two tasks was counterbalanced across subjects. After the functional scans, high-resolution T1-weighted anatomic images were obtained using 3D MPRAGE (TR = 1620 ms, TI = 950 ms, TE = 3 ms, flip angle = 15°, 160 contiguous slices of 1.0 mm thickness, in-plane resolution = 1 mm × 1 mm).
Data Analysis
Functional data processing and analyses were conducted using Statistical Parametric Mapping software (SPM2, Wellcome Department of Cognitive Neurology, UK, implemented in Matlab 6.5, Math Works, Natick, MA). For each subject, functional images were realigned to correct head motion, corrected for slice acquisition time differences, coregistered with the anatomical image, smoothed in space with a three-dimensional, 10 mm FWHM (Full Width at Half Maximum) Gaussian kernel, and entered into a voxel-wise analysis using the general linear model (GLM). A high-pass filter with a cut-off at 128s was used to remove low frequency fluctuations. An event-related design was used and the BOLD time series data were modelled using a standard hemodynamic response function (HRF) with time derivative.
During the tasks, three types of events resulted from a button press: an inflation of balloon (i.e., a larger balloon), a win outcome, or a loss outcome. Thus, the GLM for each task included three regressors representing these types of events, respectively. The risk level associated with each inflation (i.e., the probability of explosion, orthogonalized by mean central correction) was also entered into the model as a linear parametric modulation of the balloon inflation regressor. For each subject, a contrast of risk for each task was defined in order to examine the brain activations that covaried with the parametric level of risk (i.e. active risk, and passive risk). Four between-task contrasts were also defined to compare the activation differences associated with each regressor in the active and passive tasks (i.e., active inflation vs. passive inflation; active risk vs. passive risk, active win vs. passive win, and active loss vs. passive loss). We also defined two contrasts to compare loss and win outcomes within each task (i.e., active loss vs. active win, and passive loss vs. passive win). All contrast images (beta maps) were calculated from individual-level GLM analysis, normalized to the standard Montreal Neurological Institute brain template with a 2 × 2 × 2 mm3 voxel size, and then entered into one-sample t-tests for the group-level random-effect analyses. A threshold of whole brain false discovery rate (FDR) corrected p < 0.05 and cluster size larger than 50 voxels was used to identify activation areas associated with the contrasts of risk. No activation areas survived the whole brain corrected threshold for the other contrasts, which may be due to the small number of loss and win events in both tasks. Thus, a more liberal threshold of uncorrected p < 0.005 and cluster size larger than 50 voxels was applied.
To identify the neural substrates associated with active choice selection, we compared the inflation events with choice in the active task to the same events without choice in the passive task. In both tasks, participants pressed the right button which resulted in either an inflated balloon or a loss outcome. Choice selection was the common component of the contrasts of active inflation vs. passive inflation and active loss vs. passive loss. Therefore, a conjunction analysis using the SPM minimum T-statistic (Friston et al., 2005) was conducted on these two contrasts. At the group level, an uncorrected threshold of p < 0.005 was used to form a mask from one comparison (e.g., active inflation vs. passive inflation) and applied to the other comparison (e.g., active loss vs. passive loss). This mask procedure was repeated with swapped order of the two contrasts. Voxels that exceeded the small volume corrected threshold of p < 0.05 in both procedures were considered significant for conjunction analysis.
Results
Behavioral Data
The behavioral results on average were similar in the two tasks. Participants completed 20.1 (SD = 4.5) balloons in the active task and 22.1 (SD = 2.1) balloons in the passive task. For each balloon, participants inflated 7.6 (SD = 0.9) times in the active task and 7.5 (SD = 0.7) times in the passive task. The number of balloons and the number of inflations did not differ between tasks (both p > 0.1). In both tasks, the number of trials participants won was greater than the number of trials participants lost. During the active task, participants won 12.8 ± 4.2 balloons and lost 7.8 ± 2.5 balloons (p = 0.002). Similarly, during the passive task, participants won 12.7 ± 2.4 balloons and lost 9.4 ± 2.1 balloons (p = 0.01). There was no significant interaction (p > 0.2) between task condition (i.e., active choice or passive no-choice) and outcome valence (i.e., loss or win). No significant difference was found for mean reaction time between the two tasks (389 ± 146 ms in the active task vs. 353 ± 90 ms in the passive task, p > 0.2). In addition, reaction times for each balloon did not change during inflation in both tasks (p > 0.2).
Imaging Results
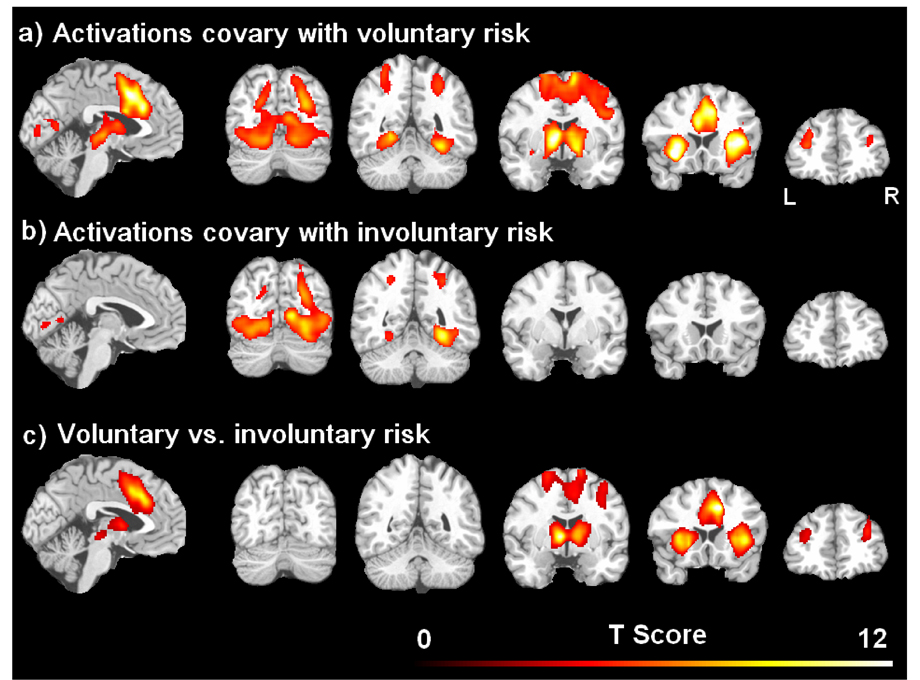
The brain activations that co-varied with the parametric risk levels in the active choice and passive no-choice tasks are illustrated in Figure 2 and listed in Table 2. Consistent with our hypotheses, risk in the active choice mode showed robust activation in a network consisting of dopamine rich mesolimbic structures and frontal target regions (Figure 2a). The activated regions include the midbrain (ventral tegmental area, VTA), striatum (nucleus accumbens, globus pallidus nucleus caudate, and putamen), anterior insula, dorsal lateral prefrontal cortex (DLPFC) and anterior cingulate cortex/medial frontal cortex (ACC/MFC). Risk in the active choice condition also activated a posterior brain network consisting of bilateral visual pathway areas, including occipital, fusiform, and parietal cortices. In contrast, no risk-related activation was observed in the midbrain, striatum, ACC/MFC, or DLPFC in the passive no-choice task. Notably, risk in the no-choice condition only activated visual pathway areas (Figure 2b). Direct comparisons between the two tasks confirmed that activation in the midbrain, striatum, anterior insula, DLPFC, and ACC/MPFC co-varied with the voluntary risk but not the involuntary risk (Figure 2c and Table 2). There was no activation that covaried with the involuntary risk but not the voluntary risk.
Figure 2.
Brain activations covaried with the parametric level of voluntary risk in the active choice task (a) and the parametric level of involuntary risk in the passive no-choice task (b), respectively. The brain activation difference between voluntary and involuntary risk were shown in (c). The threshold was set as the whole brain corrected p < 0.05.
Table 2.
Brain activations covaried with the parametric level of voluntary risk, involuntary risk, and differed between voluntary and involuntary risk. The threshold was set as the whole brain corrected p < 0.05 and cluster size larger than 50 voxels.
| Brain Regions | Peak MNI Coordinates | Z Scores | P Values (corrected) | Cluster size | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Activations covaried with voluntary risk | ||||||
| R. Insula | 38 | 10 | −2 | 6.28 | <0.001 | 16859 |
| L. Insula | −34 | 18 | −6 | 6.23 | <0.001 | |
| B. ACC/MFC | 0 | 12 | 42 | 5.83 | <0.001 | |
| L. Striatum | −10 | 2 | 4 | 5.76 | <0.001 | |
| R. Striatum | 14 | 2 | −2 | 5.18 | <0.001 | |
| R. Midbrain/Thalamus | 6 | −16 | −2 | 5.00 | <0.001 | |
| L. Midbrain/Thalamus | −6 | −12 | −4 | 4.29 | 0.007 | |
| L. Fusiform | −22 | −54 | −4 | 5.88 | <0.001 | 13888 |
| R. Occipital | 30 | −76 | 24 | 5.28 | <0.001 | |
| R. Fusiform | 30 | −52 | −16 | 5.09 | <0.001 | |
| L. Occipital | −28 | −80 | 20 | 4.53 | <0.001 | |
| R. Parietal | 30 | −46 | 46 | 4.22 | <0.001 | |
| L. DLPFC | −34 | 46 | 16 | 4.21 | <0.001 | 375 |
| L. Parietal | −52 | −36 | 38 | 4.00 | 0.001 | 282 |
| R. DLPFC | 30 | 36 | 20 | 3.74 | 0.001 | 185 |
| Activations covaried with involuntary risk | ||||||
| L. Occipital | −42 | −80 | 4 | 5.29 | <0.001 | 4430 |
| R. Fusiform | 28 | −50 | −10 | 5.27 | <0.001 | 5692 |
| R. Occipital | 10 | −68 | 6 | 5.26 | <0.001 | |
| R. Parietal | 30 | −50 | 48 | 3.92 | 0.002 | 150 |
| L. Parietal | −28 | −48 | 50 | 3.89 | 0.002 | 50 |
| Activations differed between voluntary and involuntary risk | ||||||
| R. ACC/MFC | 12 | 18 | 38 | 6.09 | <0.001 | 1316 |
| L. Striatum | −8 | 0 | 4 | 5.64 | <0.001 | 1379 |
| R. Insula | 36 | 16 | 0 | 5.40 | <0.001 | |
| L. Insula | −28 | 18 | 6 | 5.16 | <0.001 | 1577 |
| R. Striatum | 12 | 4 | 4 | 5.12 | <0.001 | |
| R. DLPFC | 32 | 46 | 26 | 3.79 | 0.005 | 207 |
| L. DLPFC | −32 | 46 | 22 | 3.31 | 0.02 | 119 |
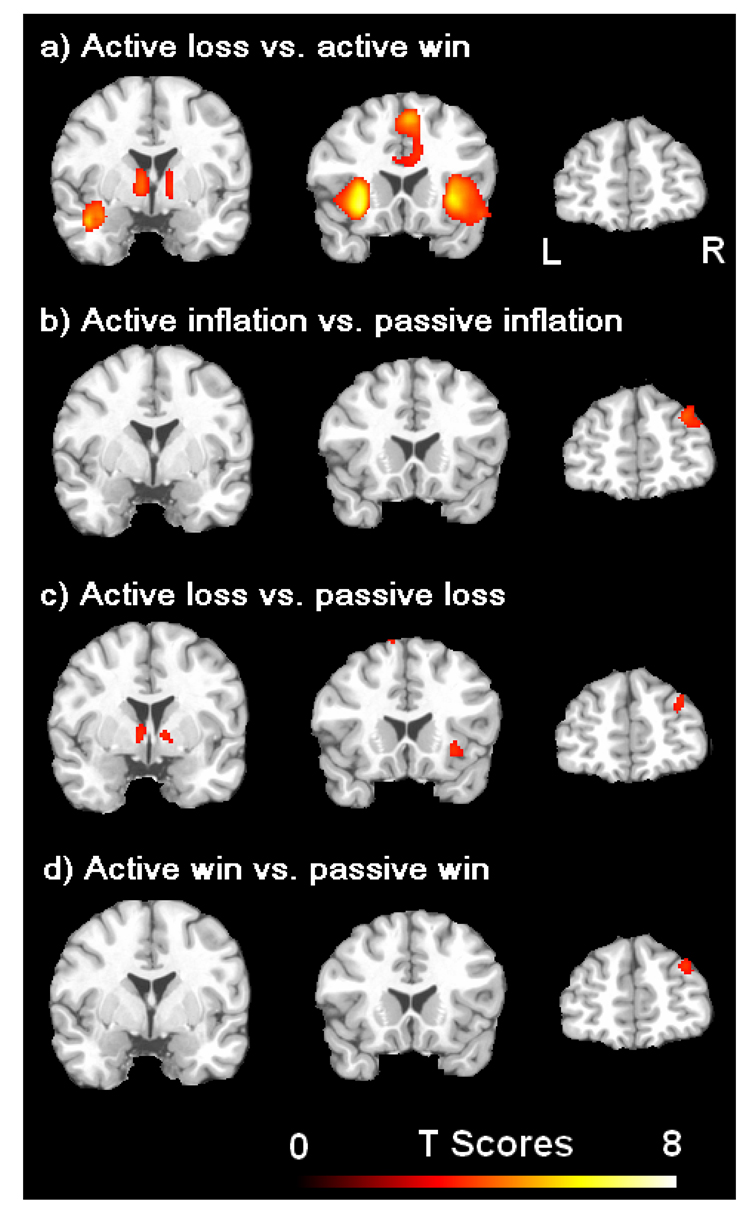
Using a more liberal threshold of uncorrected p < 0.005, the comparisons between the active and passive tasks for the three regressors of balloon inflation, loss outcome, and win outcome consistently showed a greater activation in right DLPFC for the active task compared to the passive task (Figure 3, Table 3). This right DLPFC activation survived the conjunction analysis and was identified as the neural substrate associated with voluntary decision making. Except the right DLPFC, greater activations in bilateral striatum and right insula were found for the contrast of active loss vs. passive loss, whereas no activation difference was found in these mesolimbic regions for the contrast of active win vs. passive win (Figure 3c, Table 3). Greater activations in bilateral striatum and insula were also found for the contrast of loss vs. win in the active task (Table 3), whereas no activation difference was found in these regions for the contrast of loss vs. win in the passive task.
Figure 3.
Brain activations associated with the comparison of loss versus win in the active choice task (a), and the comparisons of balloon inflation (b), loss (c), and win (d) in the active choice task versus those in the passive no choice task, respectively. The threshold was set as uncorrected p < 0.005. Note the right dorsal lateral prefrontal cortex (DLPFC) activation was consistently observed in all comparisons between the two tasks (b–d) but not in the comparison within the active choice task (a).
Table 3.
Brain activations associated with the contrasts of active inflation vs. passive inflation, active loss vs. passive loss, active win vs. passive win, and active loss vs. passive win, respectively. The threshold was set as uncorrected p < 0.005 and cluster size larger than 50 voxels.
| Brain Regions | Peak MNI Coordinates | Z Scores | P Values (uncorrected) | Cluster size | ||
|---|---|---|---|---|---|---|
| X | Y | Y | ||||
| Active inflation vs. passive inflation | ||||||
| R. DLPFC | 30 | 50 | 32 | 3.20 | 0.001 | 146 |
| Active loss vs. passive loss | ||||||
| L. MFC | −14 | 10 | 68 | 3.29 | <0.001 | 79 |
| R. DLPFC | 30 | 54 | 26 | 3.03 | 0.001 | 120 |
| R. insula | 30 | 22 | −6 | 2.96 | 0.002 | 177 |
| L. striatum | −8 | 2 | 0 | 2.89 | 0.002 | 121 |
| R. striatum | 8 | 4 | 2 | 2.88 | 0.002 | 71 |
| Active win vs. passive win | ||||||
| R. DLPFC | 32 | 52 | 32 | 2.96 | 0.002 | 95 |
| R. MFC | 10 | 12 | 70 | 2.80 | 0.003 | 93 |
| Active loss vs. active win | ||||||
| L. insula/superior temporal | −32 | 18 | −6 | 4.45 | 54 | 1735 |
| R. insula/superior temporal | 32 | 20 | −2 | 4.08 | 54 | 1383 |
| B. ACC/MFC | 4 | 22 | 52 | 3.79 | 894 | 2149 |
| L. striatum | −8 | 2 | 2 | 3.35 | 894 | 477 |
| R. striatum | 10 | 4 | 0 | 3.31 | 140 | 203 |
Discussion
In the present study, we modified the BART for use during fMRI as a paradigm for examining risk induced activation patterns in the decision network, particularly dopamine rich mesolimbic and frontal regions. We manipulated not only the level of risk but also the agency of the risk taker in BART in order to measure and compare neural activation patterns associated with both voluntary and involuntary risk taking. In both conditions, increasing risk correlated with neural activity in bilateral visual pathway areas, which may reflect increased attention to or processing of visual information as the size of the balloon increases. Moreover, in the active choice condition, increasing risk also correlated with robust neural activity in the mesolimbic-frontal pathway, including the VTA, striatum, insula, ACC and DLPFC. These results provide direct evidence supporting the specific role of dopamine rich mesolimbic and frontal regions mediating risk during active or voluntary decision-making. This relationship was not observed for involuntary risk during the passive no-choice condition, suggesting that risk alone cannot engage the mesolimbic-frontal pathway. These results support the critical role of voluntary choice in risk processing in the human brain, and suggest that recruitment of mesolimbic-frontal regions is contingent upon the active volitional control or agency of the risk taker.
The effect of agency on striatal activation associated with reward outcomes has been consistently reported. For example, Zink et al. (2004) showed greater striatal activations for performance dependent rewards than performance independent rewards. Similarly, Tricomi et al. (2004) observed robust caudate activation only for outcomes that were contingent upon participants’ button press responses, and Coricelli et al (2005) found significant striatal activation for win outcomes only in choice conditions but not in no-choice conditions. The present finding that robust activation in the midbrain and striatum was only observed for risk in the active choice mode but not the passive no-choice task is consistent with these reports and further extends the influence of agency on outcomes to risk.
However, in the present study, neither the active nor passive tasks showed any striatal activation for win than loss outcomes, a pattern that has been consistently reported in literature (e.g., Coricelli et al., 2005; Montague et al., 2006; Tom et al., 2007). Instead, greater striatal and midbrain activations were observed for loss than win outcomes in the active choice task but not in the passive no-choice task. A possible explanation for this inconsistency may be the differences in tasks and paradigms used in the present and previous studies. In previous studies, subjects were required to choose a single action which would produce a loss or win outcome directly following their action. There were prediction errors for both loss and win outcomes. However, in the present study, subjects were required to sequentially inflate the balloon, but inflations would not produce a loss or win outcome until the end of the balloon trial. When subjects decided to continue inflating the balloon in the active task, they expected the balloon to grow larger. An unexpected balloon explosion, that is, a loss event following this action might produce a strong prediction error, which has been associated with significant striatal activation (Schultz et al., 1997; Pagnoni et al., 2002; Jensen et al., 2007; Schultz, 2007). In contrast, when subjects chose to discontinue inflation, the expectation of a win outcome following their action would occur without any prediction error, hence the lack of observed striatal activation for win outcomes in the present study. Similarly, during the passive no-choice task, the outcomes were not determined by subjects thus there was no need for prediction and hence no striatal activation for either loss or win outcomes. These findings support the view that human striatal activity is associated with processing stimulus salience rather than value or hedonic feelings of reward (Zink et al., 2004, 2006; Jensen et al., 2007).
Robust activations were also observed in the ACC and insula both for risk and for loss versus win outcomes in the active choice task. The ACC has been linked to monitoring performance conflict and error as well as relating action to consequences (Botvinick et al., 1999; 2001; Rushworth et al., 2004). Enhanced ACC activity during the loss trials supports the role of ACC in error monitoring during decision-making. With higher risk levels, greater ACC activity was found in the active task but not in the passive task, suggesting that ACC activity is also contingent upon the combination of risk and active choice selection.
The insula has been associated with multimodal sensory integration (Calvert, 2001; Craig, 2003) and aversive emotion processing such as pain and disgust (Price, 2000; Wicker et al., 2003). Previous studies have consistently reported insula activation during decision-making and suggested that the insula may be the critical brain region to instantiate anticipatory aversive somatic markers that regulate risky decision-making (Paulus et al., 2003; Bechara & Damasio, 2005; Kuhnen and Knutson, 2005; Preuschoff et al., 2006). Enhanced insula activity associated with loss versus win outcomes in the active task supports the role of the insula in aversive somatic regulation. Risk-induced activity in the insula may reflect enhanced processing of aversive emotion associated with increased risk when the balloon is inflated over time. The absence of observed insula activation in the passive task suggests that risk alone, in the absence of voluntary choice, is not sufficiently capable of inducing somatic markers in the human brain.
Greater activity was consistently observed in the right DLPFC when comparing the inflation events in the active choice task with those in the passive no-choice task. However, no DLPFC activity was observed when comparing different outcomes in the same task. These findings support our hypothesis that DLPFC activity is specifically associated with the difference between the two tasks and independent of outcome valence. The DLPFC plays an important role in the maintenance and manipulation of cognitive representations and planning of future actions (Miller and Cohen et al., 2001; Trepel et al., 2005). Patients with lesions in the DLPFC have demonstrated impairments in making optimal choices during risky situations (Manes et al., 2002; Fellows and Farah, 2003). During decision-making, DLPFC activity may mediate representations of prospects, balance possible outcomes and calculate subsequent utilities (Trepel et al., 2005). The DLPFC activity observed in the present study supports this view. Furthermore, the observed laterality of right as opposed to left DLPFC activity mediating risky decision-making is consistent with findings from recent transcranial magnetic stimulation (TMS) studies (Knoch et al., 2006a, 2006b) demonstrating that disruptions in the right DLFPC but not the left DLFPC impairs decision-making.
This study was not intended to explicitly examine any decision theory, and the conceptual and mathematical definitions of risk used in the present study are slightly different from those used by decision theorists. In decision theory, risk may be defined as the increasing variance in the probability distribution of possible outcomes (Trepel et al., 2005), whereas in the present study, risk was defined as the increasing probability of potential loss (i.e. explosion) for a balloon during inflation. Calculating reward variances for each balloon size, however, showed that reward variances indeed increased monotonically with balloon size with the exception of the final inflation (see Table 1). The correlation between the probabilities of explosion and reward variances is almost ideal (R = 0.96, P < 10−6), which means that in practice these definitions of risk are interchangeable, at least for the specific task paradigm used.
There are several limitations to the present study. First, agency might alter the physiological engagement of participants (Coricelli et al. 2005) and affect attention and arousal levels. Because we did not record subjects’ physiological responses, we could not exclude the possible effect of physiological responses on observed brain activation patterns. However, it is unlikely that the observed activation differences between the two tasks are simply due to variations in attention or arousal levels between the tasks, since behavioural reaction time data showed no difference and suggested similar attentional states across the active and passive conditions. Direct physiological measures such as heart rate and galvanic skin response need to be included in future studies to examine the dynamic changes of both physiological and cerebral activity during risk taking.
Second, people naturally choose larger risk in a manner that is commensurate with higher reward. In the original BART, the number of balloon inflations ranges from 1–128, and the risk of probability nonlinearly increases from 0.8% (1/128) to 100%, while the monetary reward linearly increases a fixed small value (e.g., 5 cents) for each inflation. Due to the time limitation inherent in the fMRI experimental design, the number of possible balloon inflations in our modified BART tasks is necessarily reduced and ranges from 1–12. To encourage subjects to make multiple inflation attempts for each balloon so that a greater range of risk level can be obtained during an 8-minute scanning for each task, the monetary reward values associated with each balloon were set to monotonically increase with balloon inflations. Thus, the effects of risk and reward are confounded in this study. Future studies using a paradigm in which risk and reward change independently are needed to further dissociate the processing of risk and reward in the human brain.
Third, although all participants were explicitly instructed that there was no choice to discontinue balloon inflation in the passive task, we could not exclude the possibility that participants might have made a covert decision in their mind. In this case, there might be an alternative explanation that the observed right DLPFC activation may reflect its role in the control over the choice rather than the choice selection per se. Future studies including structural interviews or questionnaires to assess the extent to which participants made a decision in their mind in the passive condition are needed to overcome this limitation.
Finally, the present experimental paradigm used a brief ISI between 2–4 seconds and required continuous balloon inflations throughout the task. Thus it is difficult to temporally dissociate different states of decision-making, such as the assessment of preferences, the selection and execution of an action, and the experience of an outcome (Ernst and Paulus, 2005). A paradigm using a longer ISI and delayed feedback may be implemented in future studies to replicate and further demonstrate the temporal characteristics of neural activity observed in the present study.
In summary, the present study modified the BART in both active and passive modes for use during fMRI and the findings provide direct visualization of voluntary and involuntary risk processing in the human brain. Regardless of the involvement of voluntary decision making, risk in this task is processed in visual pathway regions in the occipital and parietal lobes. However, during active decision-making, risk is associated with additional robust activation in dopamine rich mesolimbic (VTA-striatum) and frontal regions (insula, ACC/MFC, and DLPFC). Voluntary decision making per se, is associated with activation in the right DLPFC, which is absent in the involuntary no-choice condition. These results contribute to understanding the neural basis of normal and high risk behavior. Extending this paradigm to pathological populations characterized by impaired decision-making, such as patients with drug addition and compulsive gambling, may allow the specific neural components of impaired risk behavior to be distinguished, and may ultimately inform more effective clinical treatment interventions.
Acknowledgements
We thank Dr. M.A. Fernandez-Seara for her help in data acquisition, Drs M.J. Farah, J. Wang, D. Weintraub, D. Kimberg, and M.M. Botvinick for their helpful comments on the earlier version of this manuscript. This research was supported by NSF Grant BCS-0224007, NIH Grants P30 NS045839, R01 DA015149, and Chinese NSF Grant 30470571.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allais M, Hagan O. The so-called Allais Paradox and rational decisions under uncertainty. In: Allais OHM, editor. Expected Utility Hypothesis and the Allais Paradox. Dordrecht, The Netherlands: Reidel Publishing Company; 1979. pp. 434–698. [Google Scholar]
- Bechara A. The role of emotion in decision-making: evidence from neurological patients with orbitofrontal damage. Brain Cogn. 2004;55:30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR. The somatic marker hypothesis: a neural theory of economic decision. Games Econ. Behav. 2005;52:336–372. [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Bozarth MA. The mesolimbic dopamine system as a model brain reward system. In: Willner P, Scheel-Krüger J, editors. The mesolimbic dopamine system: From motivation to action. London: John Wiley & Sons; 1991. pp. 301–330. [Google Scholar]
- Calvert GA. Crossmodal processing in the human brain: insights from functional neuroimaging studies. Cereb. Cortex. 2001;11:1110–1123. doi: 10.1093/cercor/11.12.1110. [DOI] [PubMed] [Google Scholar]
- Coricelli G, Critchley HD, Joffily M, O'Doherty JP, Sirigu A, Dolan RJ. Regret and its avoidance: a neuroimaging study of choice behavior. Nat. Neurosci. 2005;8:1255–1262. doi: 10.1038/nn1514. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Ann. N. Y. Acad. Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision-making: a selective review from a neurocognitive and clinical perspective. Biol. Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Finucane ML, Alhakami A, Slovic P, Johnson SM. The affect heuristic in judgments of risks and benefits. J. Behav. Decis. Mak. 2000;13:1–17. [Google Scholar]
- Friston KJ, Penny WD, Glaser DE. Conjunction revisited. NeuroImage. 2005;25:661–667. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310:1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Stowe CJ, Gordon EM, Warner BT, Platt ML. Neural signatures of economic preferences for risk and ambiguity. Neuron. 2006;49:765–775. doi: 10.1016/j.neuron.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Hunt MK, Hopko DR, Bare R, Lejuez CW, Robinson EV. Construct validity of the Balloon Analog Risk Task BART.: associations with psychopathy and impulsivity. Assessment. 2005;12:416–428. doi: 10.1177/1073191105278740. [DOI] [PubMed] [Google Scholar]
- Jensen J, Smith AJ, Willeit M, Crawley AP, Mikulis DJ, Vitcu I, Kapur S. Separate brain regions code for salience vs. valence during reward prediction in humans. Hum. Brain Mapp. 2007;28:294–302. doi: 10.1002/hbm.20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;4:263–291. [Google Scholar]
- Knoch D, Gianotti LR, Pascual-Leone A, Treyer V, Regard M, Hohmann M, Brugger P. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. J. Neurosci. 2006a;26:6469–6472. doi: 10.1523/JNEUROSCI.0804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science. 2006b;314:829–832. doi: 10.1126/science.1129156. [DOI] [PubMed] [Google Scholar]
- Krain AL, Wilson AM, Arbuckle R, Castellanos FX, Milham MP. Distinct neural mechanisms of risk and ambiguity: A meta-analysis of decision-making. NeuroImage. 2006;32:477–484. doi: 10.1016/j.neuroimage.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task BART. J. Exp. Psychol. Appl. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Jones HA, Richards JB, Strong DR, Kahler CW, Read JP. The Balloon Analogue Risk Task BART. differentiates smokers and nonsmokers. Exp. Clin. Psychopharmacol. 2003a;11:26–33. doi: 10.1037//1064-1297.11.1.26. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Zvolensky MJ, Pedulla CM. Evaluation of the Balloon Analogue Risk Task BART. as a predictor of adolescent real-world risk-taking behaviours. J. Adolesc. 2003b;26:475–479. doi: 10.1016/s0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Daughters S, Zvolensky M, Kahler C, Gwadz M. Reliability and validity of the youth version of the Balloon Analogue Risk Task BART-Y. in the assessment of risk-taking behavior among inner-city adolescents. J. Clin. Child. Adolesc. Psychol. 2007;36:106–111. doi: 10.1080/15374410709336573. [DOI] [PubMed] [Google Scholar]
- Loewenstein GF, Weber EU, Hsee CK, Welch N. Risk as feelings. Psychol. Bull. 2001;127:267–286. doi: 10.1037/0033-2909.127.2.267. [DOI] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, Robbins T. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Matthews SC, Simmons AN, Lane SD, Paulus MP. Selective activation of the nucleus accumbens during risk-taking decision-making. NeuroReport. 2004;15:2123–2127. doi: 10.1097/00001756-200409150-00025. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Montague PR, King-Casas B, Cohen JD. Imaging Valuation Models in Human Choice. Annu. Rev. Neurosci. 2006;29:417–448. doi: 10.1146/annurev.neuro.29.051605.112903. [DOI] [PubMed] [Google Scholar]
- Pagnoni G, Zink CF, Montague PR, Berns GS. Activity in human ventral striatum locked to errors of reward prediction. Nat. Neurosci. 2002;5:97–98. doi: 10.1038/nn802. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. NeuroImage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Peters E, Slovic P. The springs of action: affective and analytical information processing in choice. Pers. Soc. Psychol. Bull. 2000;26:1465–1475. [Google Scholar]
- Preuschoff K, Bossaerts P, Quartz SR. Neural differentiation of expected reward and risk in human subcortical structures. Neuron. 2006;51:381–390. doi: 10.1016/j.neuron.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn. Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- Trepel C, Fox CR, Poldrack RA. Prospect theory on the brain? Toward a cognitive neuroscience of decision under risk. Cogn. Brain Res. 2005;23:34–50. doi: 10.1016/j.cogbrainres.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41:281–292. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- Tversky A, Kahneman D. Advances in prospect theory: Cumulative representation of uncertainty. J. Risk Uncertain. 1992;5:297–323. [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42:509–517. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Chappelow J, Martin-Skurski M, Berns GS. Human striatal activation reflects degree of stimulus saliency. NeuroImage. 2006;29:977–983. doi: 10.1016/j.neuroimage.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]