Purpose of review
Antibody-mediated rejection (AMR) has emerged as the leading cause of late graft loss in kidney transplant recipients. Donor-specific antibodies are an independent risk factor for AMR and graft loss. However, not all donor-specific antibodies are pathogenic. AMR treatment is heterogeneous due to the lack of robust trials to support clinical decisions. This review provides an overview and comments on practical but relevant dilemmas physicians experience in managing kidney transplant recipients with AMR.
Recent findings
Active AMR with donor-specific antibodies may be treated with plasmapheresis, intravenous immunoglobulin and corticosteroids with additional therapies considered on a case-by-case basis. On the contrary, no treatment has been shown to be effective against chronic active AMR. Various biomarkers and prediction models to assess the individual risk of graft failure and response to rejection treatment show promise.
Summary
The ability to personalize management for a given kidney transplant recipient and identify treatments that will improve their long-term outcome remains a critical unmet need. Earlier identification of AMR with noninvasive biomarkers and prediction models to assess the individual risk of graft failure should be considered. Enrolling patients with AMR in clinical trials to assess novel therapeutic agents is highly encouraged.
Keywords: antibody-mediated rejection, dilemma, donor-specific antibodies, kidney transplantation, treatment
INTRODUCTION
Antibody-mediated rejection (AMR) is the most common cause of late allograft loss after kidney transplantation [1–3]. Banff 2019 classification recognizes three diagnostic AMR categories: active AMR, chronic active AMR and chronic (inactive) AMR (Table 1) [4]. Active AMR requires three diagnostic criteria: histologic evidence of microvascular inflammation (MVI) (e.g. glomerulitis and peritubular capillaritis), evidence of current or recent antibody interaction with the endothelium (usually C4d-positive staining) and serologic evidence of donor-specific antibody (DSA), although C4d staining or validated endothelium transcripts may substitute for DSA [5]. Chronic active AMR has similar criteria but with histologic evidence of chronic MVI, such as transplant glomerulopathy. Chronic (inactive) AMR shows histologic evidence of chronic tissue injury, including basement membrane duplication, without MVI and without C4d deposition in peritubular capillaries [4]. Patients with active AMR are at an increased risk for subsequent rejection, chronic AMR and graft loss [2,5–7]. Similarly, those with chronic AMR have a higher risk for graft loss and patient death [1–3]. There are no FDA-approved treatments for AMR [8], resulting in significant heterogeneity in AMR treatment across the transplant centres. In this review, we focus on specific clinical dilemmas encountered by physicians in preventing, monitoring and managing kidney transplantation recipients with AMR, including highlights about novel potential treatments in the pipeline.
Table 1.
Antibody-mediated rejection spectrum according to Banff 2019 Classification Report
| Active AMR | Chronic active AMR | Chronic (inactive) AMR | |
| Histopathologya | Acute tissue injury, including one or more of the following: Microvascular inflammation (glomerulitis and/or peritubular capillaritis) in the absence of recurrent or de-novo glomerulonephritis Intimal or transmural arteritis Acute TMA, in the absence of any other apparent cause Acute tubular injury, in the absence of any other apparent cause | Chronic tissue injury, including one or more of the following: Transplant glomerulopathy (glomerular basement membrane duplication in the absence of subendothelial immune complex deposits) if no evidence of chronic TMA in the absence of recurrent or de-novo glomerulonephritis Severe peritubular capillary basement membrane multilayering (requires EM) Transplant arteriopathy (arterial intimal fibrosis of new onset) AND Mild to moderate acute tissue injury (microvascular inflammation) | Chronic tissue injury: Transplant glomerulopathy, and/or Severe peritubular capillary basement membrane multilayering (requires EM) Significant loss of peritubular capillaries (capillaries simply no longer exist to show capillaritis) |
| Evidence of antibody interaction with the endotheliumb | C4d deposition in peritubular capillaries, OR At least moderate microvascular inflammation, OR Molecular markers of endothelial activation | C4d deposition in peritubular capillaries, OR At least moderate microvascular inflammation, OR Molecular markers of endothelial activation | C4d negative There may be prior evidence of antibody interaction with the endothelium |
| DSAc,d | Detectable serum anti-HLA DSA If anti-HLA DSA is undetectable, non-HLA antibody testing should be considered | Detectable serum anti-HLA DSA If anti-HLA DSA is undetectable, non-HLA antibody testing should be considered | Anti-HLA DSA may be undetectable However, there should be prior evidence of anti-HLA or non-HLA DSA |
| Clinical presentation | Acute kidney injury, hypertension ± proteinuria | Subacute. Commonly observed on for-cause biopsies in patients with deteriorating renal function and proteinuria, or on protocol biopsies from patients with normal graft function, with or without proteinuria, ranging from 3 months to 5 years posttransplant | Progressive kidney allograft dysfunction, progressive proteinuria, hypertension |
| Prognosis | It may respond to prompt therapy | Typically, more guarded prognosis | Poor prognosis with almost universal graft lost |
| Treatment | Under investigation in clinical trials Plasma exchange, intravenous immunoglobulin, and corticosteroids | Under investigation in clinical trials It is unclear how patients with microvascular inflammation, with or without early transplant glomerulopathy, should be treated | Optimization of baseline immunosuppression |
At one end of the spectrum, active AMR is characterized by microvascular inflammation, endothelial injury and serological evidence of DSA, and may respond to current therapeutic strategies. At the other end of the spectrum, chronic AMR is characterized by transplant glomerulopathy, a form of advanced glomerular injury and remodelling, and is unlikely to be ameliorated by current therapies.
AMR, antibody-mediated rejection; DSA, donor-specific antibodies; EM, electron microscopy; TMA, thrombotic microangiopathy.
a,b,cAll three criteria should be met for diagnosis of AMR.
dC4d staining and validated molecular assays could serve as potential alternatives to DSAs in the diagnosis of AMR.
Box 1.
no caption available
WHAT IS THE BEST STRATEGY TO PREVENT THE DEVELOPMENT OF DE-NOVO DONOR-SPECIFIC ANTIBODIES?
Donor-specific antibody is an independent risk factor for active AMR and graft loss [9]. Given that most centres avoid preformed DSA by listing unacceptable donor human leukocyte antigen (HLA), the most prevalent form of AMR encountered in clinical practice today is associated with de-novo DSA (dnDSA) [2]. DnDSA develops in 15–25% of kidney transplantation recipients within the first 5 years posttransplant [10], with an incidence of 2% per year in adherent patients [11]. Risk factors for dnDSA formation are nonadherence or reduced immunosuppression, higher eplet mismatch, younger age and preceding T-cell mediated rejection (TCMR) [12–15].
Optimizing maintenance immunosuppression
Immunosuppressive medication nonadherence has emerged as the primary cause of dnDSA formation [11]. Maintaining adequate baseline immunosuppression, particularly a calcineurin inhibitor (CNI), is a key to preventing dnDSA formation [13,16,17]. When comparing CNIs, recipients treated with cyclosporin-based therapy have a 2.7-fold higher incidence of dnDSA development compared with tacrolimus-based therapy [13]. DnDSA may be prevented by maintaining tacrolimus trough levels (Tac C0) at least 7 ng/ml in the first year posttransplant [13] and at least 5 ng/ml beyond the first year posttransplant [13,18].
CNI withdrawal has been attempted in low-immunological risk patients who received T-cell depletion induction therapy. However, the rate of dnDSA was more than 40% within first-year posttransplant, leading to the early termination of the study [19]. Although T-cell depletion has a potent effect in lowering acute TCMR rates in high immunological risk patients, it can favour preferential expansion of T follicular helper (Tfh) cells and be associated with a higher risk of DSA generation [20▪]. Therefore, delaying initiation, dose minimization or withdrawal of CNI may favour the development of an antibody-mediated alloimmune response [21,22].
Alternatives to CNI-based therapy are being sought to eliminate its side effects for kidney transplantation recipients (e.g. nephrotoxicity and neurotoxicity). The alloimmune response requires signalling through the costimulatory pathway for optimal T cell activation and proliferation. Belatacept, a selective T cell costimulation blocker [23], has been FDA-approved based on noninferiority for biopsy-proven acute rejection relative to cyclosporine. Data from BENEFIT and BENEFIT-EXT trials showed significantly lower cumulative event rates for dnDSA development and better kidney function with belatacept-based vs. cyclosporine-based immunosuppression, at 7 years posttransplant [24,25]. A recent randomized control trial comparing belatacept with tacrolimus did not observe any difference in dnDSA formation or AMR rates at 1 year [26]. The estimated glomerular filtration rate (eGFR) was significantly higher with belatacept compared with tacrolimus, but so was the incidence of biopsy-proven TCMR [27▪]. However, the short-term follow-up may limit any conclusive interpretation with respect to dnDSA development or long-term outcomes [26,28–30] and follow-up of at least 5 years may be needed.
Why is the rate of dnDSA generation with belatacept lower compared with tacrolimus despite higher rates of TCMR? Tfh cells are a subset of specialized CD4+ T cells that provide critical help to B cells through the costimulation pathway B7/CD28, enabling B cell activation and differentiation into memory B cells and plasma cells that secrete high-affinity antibodies [31]. Therefore, targeting Tfh–B cells costimulation signal by belatacept may prevent dnDSA and AMR [32]. Although Tfh cells are primarily located in secondary lymphoid organs, circulating Tfh cells may represent a biomarker of humoral alloreactivity [33]. In a retrospective study in kidney transplantation recipients, the expansion of the circulating Tfh cells was predictive of IgG3 DSA generation, more severe allograft injury and a higher rate of allograft loss [34]. Further studies are needed to investigate if monitoring Tfh cells may have a role in the early detection of AMR.
Greater human leukocyte antigen class II matching
Antibodies against HLA class II donor antigens are predominant in the posttransplant period [2]. Class II antibodies are more likely to persist compared with class I (HLA-DRβ3/4/5, HLA-DQα1β1 or HLA-DRβ1>Class I), which may explain the poor outcome observed in patients with class II antibodies [35]. Donor-recipient HLA-DQ and HLA-DR matching can potentially minimize the risk of dnDSA posttransplant [36–38]. HLA class II matching can also decrease the risk for TCMR, AMR, transplant glomerulopathy and graft loss [10,12,13]. In retransplantation, class II repeat mismatches seem to increase the risk of graft loss [39]. Nonetheless, HLA class II matching on a large scale is currently challenging to implement in organ allocation algorithms due to the organ shortage and significant polymorphism of HLA class II antigens.
Decreasing human leukocyte antigen eplet mismatches
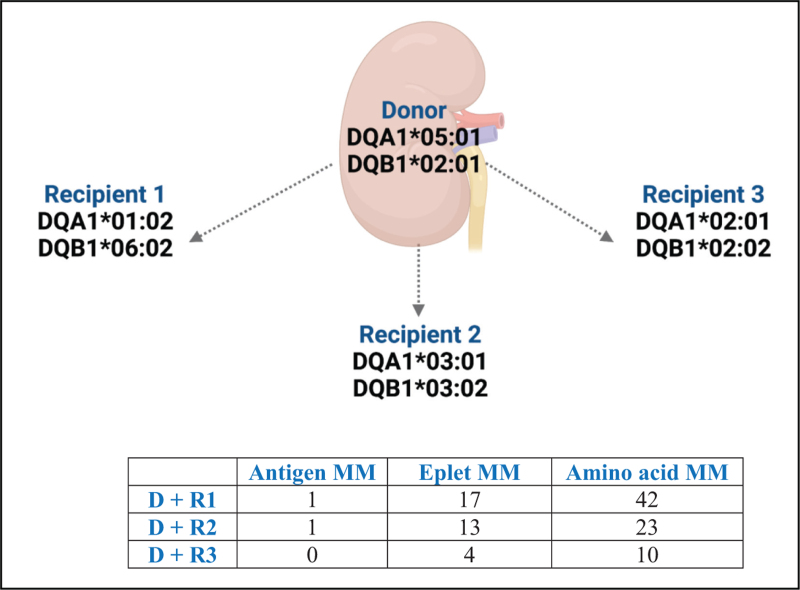
HLA antibodies recognize polymorphic, three-dimensional structures of the HLA antigen, called epitopes, rather than the complete antigen [40]. Each HLA antigen contains a unique set of epitopes (private epitopes) as well as epitopes that are present in other HLA antigens (shared/public epitopes). An eplet, or functional epitope, is a patch of amino acids within a 3 Å radius of polymorphic residues located on the surface of HLA molecules within the larger 15–22 amino acids of an HLA epitope that are recognized by anti-HLA antibodies. Scientific advances permit assessment of donor-recipient HLA mismatch at the eplet level [13,41,42]. The eplet-mismatch load is determined by counting the number of eplets that are mismatched between a recipient and the potential donor. Several observational studies have shown that a higher number of mismatched eplets is associated with a higher risk of developing DSA posttransplantation [10,43,44], transplant glomerulopathy [45] and graft loss [44]. Recipients of a low-risk HLA-DR/DQ molecular mismatch appear to tolerate lower CNI trough levels while maintaining minimal acute rejection rates and lower rates of dnDSA [19,46]. Nevertheless, universal CNI minimization based on low-risk eplet mismatch cannot be currently endorsed on a large scale in the absence of clinical trials supporting this strategy. Moreover, not all eplet mismatches result in the development of alloantibody responses. The immunogenicity of a donor HLA eplet is a consequence of several physicochemical factors (e.g. polarity, size, solubility, amino acid sequence). Thus, two donor-recipient combinations that have an identical eplet mismatch load may exhibit different immunogenicity (Fig. 1) [47]. HLA eplet mismatch calculation may represent a valuable tool for risk stratification at the population level, although prospective validation is needed [48].
FIGURE 1.
Antigen, eplet and amino acid mismatch loads for DQA1∗ DQB1∗ between a kidney donor and three potential recipients. Despite the kidney donor and potential recipients 1 and 2 exhibiting the same antigen mismatch load for DBA1-DQB1, they have different eplet mismatch loads (17 and 13, respectively). Furthermore, although the kidney donor and potential recipient 3 have no antigen mismatches, they exhibit 4 eplet mismatches and 10 amino acid mismatches. For this example, the donor and potential recipients are assumed to share one haplotype. www.epitopes.net and www.histocheck.org were used to estimate eplet and amino acid mismatches, respectively. D, donor; MM, mismatch; R, recipient.
Preventing T-cell mediated rejection
TCMR, dnDSA and AMR are on the continuum of the alloimmune response. TCMR frequently precedes the development of DSA [11]. Furthermore, reports have documented that dnDSA-associated AMR occurs later, has a higher rate of graft loss, and is frequently manifested as a mixed TCMR/AMR rejection, as compared to memory-associated AMR, which typically displays a pure AMR phenotype, occurs early posttransplant and is more responsive to therapy [17,49]. Thus, the alloimmune response cannot be separated into cellular or antibody-mediated, but should be considered a continuous process with the dominance of different components at various time-points posttransplant.
SHOULD ANTI-HUMAN LEUKOCYTE ANTIGEN ANTIBODIES BE MONITORED POST-TRANSPLANTATION?
There is controversy regarding the clinical utility of routine posttransplant DSA monitoring and management of patients with dnDSA, especially in the absence of allograft dysfunction. Monitoring for dnDSA is primarily recommended when immunosuppression reduction is advised by the physician (e.g. infection, cancer), known or suspected medication nonadherence, or at the time of a rejection episode [50]. Some transplant centres also perform annual anti-HLA testing as a monitoring strategy in stable patients. Patients with a pretransplant DSA undergo protocol kidney biopsies at months 3 and 12 posttransplant at some transplant centers or are followed by frequent DSA monitoring posttransplant (e.g. at 1 week, 2 weeks, 1, 3 and 6 months). If there is no concomitant rejection on biopsy at the onset of dnDSA, it appears that most centres would only optimize the maintenance immunosuppression [8]. A relevant question that remains unanswered is whether screening for dnDSA followed by an intervention (i.e. a kidney biopsy and treatment if subclinical rejection is identified) could minimize the subsequent development of chronic AMR and transplant glomerulopathy.
HOW SHOULD DE-NOVO DONOR-SPECIFIC ANTIBODIES BE MANAGED IN KIDNEY TRANSPLANT RECIPIENTS WITH STABLE GRAFT FUNCTION? IS DE-NOVO DONOR-SPECIFIC ANTIBODIES PATHOGENIC OR AN INNOCENT BYSTANDER?
Not all DSAs are pathogenic or associated with AMR [51,52]. Interestingly, 24–75% of kidney transplant recipients with dnDSA exhibit no evidence of clinical or subclinical rejection on biopsy [11,53,54]. This, together with the lack of demonstrated effect of antibody depletion on allograft rejection in preclinical models [55], brings into question DSA pathogenicity in at least a subset of cases with AMR. Several characteristics of DSA are associated with worse outcomes, such as certain IgG subclasses, higher titers and complement-binding ability [56,57]. In addition, antibody characteristics that are not routinely assessed could affect its pathogenicity, such as the antibody glycosylation pattern, its antigen affinity and the HLA antigen target expression on the donor kidney [58,59]. AMR in kidney transplant recipients with dnDSA is associated with higher levels of proteinuria, more transplant glomerulopathy lesions and worse eight-year allograft survival compared with kidney transplant recipients with AMR and pretransplant DSA [49]. Higher DSA mean fluorescent intensity (MFI) measured by single-antigen bead assay, IgG3 subclass of immunodominant DSA and C1q-binding ability of DSA have been associated with a higher risk of AMR and allograft loss [56]. DSA Fc glycosylation may modulate antibody pathogenicity and AMR risk through differential activation of Fc receptors on natural killer (NK) and myeloid cells [60], though further studies are needed to validate these findings. Despite these characteristics, there are no methods to reliably predict who will develop AMR in kidney transplant recipients with dnDSA and stable kidney function. As about half of the kidney transplant recipients with dnDSA and stable allograft function have subclinical AMR on allograft biopsy [54], we recommend performing a kidney allograft biopsy in patients with a significant rise in DSA or who develop a dnDSA to evaluate whether AMR is present.
WHAT IS THE BEST TREATMENT STRATEGY FOR PATIENTS WITH DONOR-SPECIFIC ANTIBODY POSITIVE ANTIBODY-MEDIATED REJECTION AND HOW SHOULD TREATMENT RESPONSE BE MEASURED?
There are several published randomized clinical trials evaluating treatment regimens in AMR in kidney transplant recipients [61–68]. However, most had small sample sizes and were underpowered to find differences between treatment regimens. As a result, there are no FDA-approved treatments for AMR in kidney transplant recipients. Current treatment strategies based on the understanding of AMR pathophysiology include a combination of antibody removal [61–65], glucocorticoids [61,64], intravenous immunoglobulins (IVIg) [69], anti-CD20 antibodies [66], proteasome inhibitors [67] and/or complement blockade [68]. Two clinical trials showed that adding antibody removal to AMR treatment was associated with an improvement in allograft survival [61,62], but other trials of antibody removal did not show a significant benefit [63,64]. Evidence for the combination of IVIg and plasmapheresis to improve allograft outcomes in AMR only comes from observational studies [69]. Regarding anti-CD20 therapy, the largest randomized clinical trial showed that addition of a single dose of rituximab (375 mg/m2) to glucocorticoids, plasma exchange and IVIg was not associated with improvement in allograft function or survival [66]. Remarkably, the coadministration of IVIg with rituximab can shorten the half-life of anti-CD20 mAb and lead to quicker recovery of B cells, potentially affecting anti-CD20 efficacy [70]. In terms of proteasome inhibitors, the largest randomized clinical trial of bortezomib in late AMR showed no improvement in allograft function [67]. Larger studies that are powered to detect differences between treatment groups and test new therapeutics for AMR in kidney transplant recipients are urgently needed. Given the lack of high-quality data, most recommendations are based on low-quality evidence and expert opinion [50].
We agree with the recommendations from the Transplantation Society that active AMR in the setting of pretransplant DSA should be treated with plasmapheresis, IVIg and corticosteroids with additional therapies considered on a case-by-case basis [50]. We are also in agreement that chronic active AMR in the setting of detectable DSA should be treated with optimization of maintenance immunosuppression [50]. Enrolling these patients in mechanistic studies to identify new therapeutic targets and in clinical trials evaluating novel treatments for chronic active AMR is highly encouraged.
AMR treatment responses can be monitored by evaluating changes in serum creatinine, proteinuria, DSA levels, histopathologic findings and other new potential approaches. Studies have shown that an improvement in serum creatinine to less than 1.5 mg/dl after treatment in kidney transplant recipients with rejection and allograft dysfunction is associated with better allograft survival. However, this should not be surprising as any improvement in function will portend better allograft survival regardless of the underlying cause of injury [71]. A decrease in the DSA MFI values after treatment, as measured by the semi-quantitative assay of Luminex, is associated with lower odds of allograft loss [17]. Nonetheless, experts caution against mistakenly concluding that the antibody removal treatment has been ineffective based on serial MFI alone [8], as anti-HLA antibodies are rarely eradicated [72], even in patients with clinical improvement [73]. Treating with incremental regimens for the goal of DSA elimination implies a significant risk of overimmunosuppression and toxicity. None of the noninvasive clinical assays are sensitive or specific enough to detect the resolution of injury in the allograft. A repeat kidney biopsy can be considered [74]. Potential novel approaches to assess response to AMR treatment include incorporating donor-derived cell-free DNA levels [75]. Deciding which patients to treat more aggressively could also be based on the use of novel allograft-failure prediction models, such as the iBox score [76] and the dynamic integrative system for predicting outcome (DISPO) [77▪]. DISPO is a risk prediction tool for death-censored allograft failure that includes eGFR, proteinuria, recipient's immunological profile and allograft biopsy findings. Longitudinal changes in DISPO scores were associated with risk of kidney allograft failure [77▪], making it an attractive tool to assess response to AMR treatment in kidney transplant recipients.
HOW SHOULD WE MANAGE PATIENTS WITH DONOR-SPECIFIC ANTIBODY NEGATIVE ANTIBODY-MEDIATED REJECTION?
The correlation between DSA and chronic AMR is poor, as chronic AMR frequently develops in individuals with no detectable DSA [58]. MVI in the kidney biopsy, suggestive of AMR but without DSA in serum, represents a challenge for diagnosis and patient management. This is a condition in which molecular diagnostics could be helpful. Treatment of such lesions could be recommended if the molecular scores were higher than a prespecified cut-off value [78]. An alternative explanation for such cases is the possible occurrence of non-HLA antibodies such as antiangiotensin II type 1 (AT1) receptor antibodies [79], antiendothelial antibodies [80], perlecan fragment LG3 [81], anti-Ro/SS-A, anti-CENP-B [82▪] and several others. The prevalence of pretransplantation non-HLA antibodies is unknown, due to the use of distinct methods for non-HLA antibody detection [83]. In the absence of universally established and validated clinical assays to detect these antibodies, their overall prevalence and importance are difficult to study systematically. The immunosuppressive treatment of AMR in such patients is generally similar to that of patients with AMR and an anti-HLA DSA. Anti-AT1 receptor antibodies should be suspected in patients with severe hypertension and notable vascular lesions (e.g. endarteritis), with or without anti-HLA DSA [79]. Patients who are found to have an anti-AT1 receptor antibody should receive, in addition to immunosuppressive therapy, an angiotensin II receptor blocker, which inhibits AT1-receptor antibody-mediated effects [79,84].
Groups have reported that in the absence of preformed DSA, highly sensitized individuals have graft survival equivalent to unsensitized recipients [85,86]. DSA-negative histologic AMR had an outcome equivalent to unsensitized patients without AMR as compared to DSA-positive AMR [87]. However, in a series of 180 kidney transplant recipients with moderate MVI with or without DSA, it was found that MVI, even in the absence of DSA, was associated with poor patient and graft survival compared to those with DSA-positive AMR [88]. Moreover, a recent study demonstrated that transcriptional changes in kidney allograft with histology of AMR showed overexpression of transcripts mostly related to IFNɣ-induced pathways and activation of NK cells and endothelial cells irrespective of DSA status [89]. NK cells have been identified to play a central role in the pathophysiology and graft failure in AMR [90]. In sum, even in the absence of DSA, MVI should likely be treated as AMR to prevent chronic injury and graft function deterioration.
WHAT ARE THE NOVEL TREATMENTS FOR PATIENTS WITH REFRACTORY CHRONIC ANTIBODY-MEDIATED REJECTION?
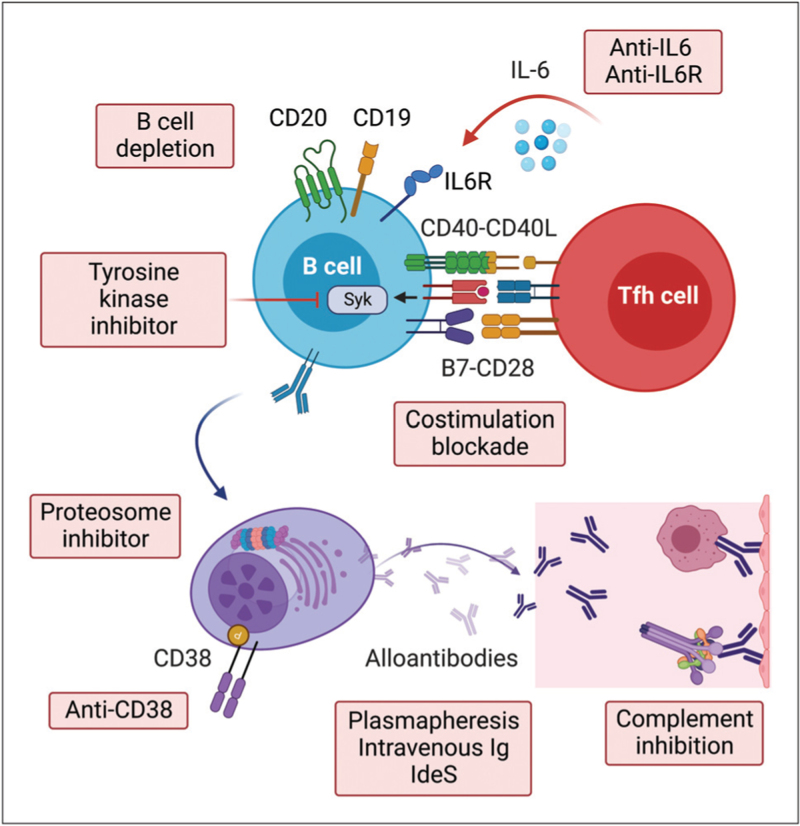
Novel treatments for AMR include IgG endopeptidases (i.e. imilfidase) [91], newer generation anti-CD20 antibodies (e.g. obinutuzumab) [92], anti-CD38 antibodies [93], proteasome inhibitors [94], complement inhibitors [95], anti-IL-6/IL-6-receptor antibodies [96,97▪▪] and tyrosine kinase inhibitors [98] (Fig. 2). Many of these agents are being actively evaluated in ongoing clinical trials (Table 2). Given the complex pathophysiology of AMR that involves alloantibodies [99], the complement system [100] and multiple immune cell types [100–103], it is likely that a multitargeted treatment approach aimed at reducing antibody production by B and plasma cells, enhancing antibody removal and inhibiting the pathways underlying antibody-mediated allograft injury (e.g. complement-mediated injury) is needed to effectively treat AMR. A multitargeted approach has the potential to treat multiple components of the pathophysiology of the disease with additive and/or synergistic therapeutic actions, likely superior to a single-target approach [104].
FIGURE 2.
Currently used and investigational drugs for kidney transplant recipients with antibody-mediated rejection.
Table 2.
Ongoing clinical trials for the treatment of antibody-mediated rejection
| Trial ID numbera | Intervention arm | Target/Mechanism | Control arm | AMR type | Phase |
| NCT03380377 | Clazakizumab (anti-IL-6 antibody) | Plasma cells | N/A (single-arm study) | Chronic Active AMR | 1/2 |
| NCT03744910 | Clazakizumab (anti-IL-6 antibody) | Plasma cells | Placebo | Chronic Active AMR | 3 |
| NCT04561986 | Tocilizumab (anti-IL-6 receptor-α antibody) | Plasma cells | Placebo | Chronic Active AMR | 3 |
| NCT03737136 | Bortezomib (proteasome inhibitor) in addition to PLEX, IVIg and rituximab | Plasma cells, antibody removal, immunomodulation, and B cells | PLEX, IVIg and rituximab | Chronic Active AMR | N/A |
| NCT05021484 | Felzartamab (anti-CD38 antibody) | B cells and plasma cells | Placebo | Late AMR | 2 |
| NCT03994783 | Rituximab (anti-CD20 antibody) in addition to PLEX, IVIg and corticosteroids | B cells, antibody removal, and immunomodulation | PLEX, IVIg and corticosteroids | Active AMR | 3 |
| NCT03991780 | Fostamatinib (spleen tyrosine kinase inhibitor) | T and B cells | N/A (single-arm study) | Chronic Active AMR | 1/2 |
| NCT05156710 | BIVV020 (anti-C1s antibody) in addition to PLEX, IVIg and corticosteroids | Proximal complement inhibition, antibody removal, immunomodulation | PLEX, IVIg and corticosteroids | Active AMR | 2 |
| NCT03897205 | Imilfidase (IgG endopeptidase) | Antibody cleavage | PLEX or immunoadsorption | Active AMR | 2 |
AMR, antibody-mediated rejection; IL, interleukin; IVIg, intravenous immunoglobulins; N/A, not available; PLEX, plasma exchange.
Trials were identified from searching ‘antibody-mediated rejection’ in www.clinicaltrials.gov.
CONCLUSION
AMR is the leading cause of kidney allograft failure. Despite a relatively large number of observational studies, it is unclear which combination therapy is the safest and most effective. In the context of a heterogeneous kidney transplant population, the challenge is to administer the right treatment to the right patient and personalize the degree of immunosuppression in proportion to the patient's alloimmune risk to minimize drug toxicity while maintaining therapeutic efficacy. In addition, to permit individualized treatment and immune monitoring strategies, an essential requirement is the availability of reliable prognostic or predictive biomarkers. Several potential therapeutic agents for AMR are currently being investigated in clinical trials. Similarly, diverse biomarkers and prediction models to assess the risk of individual graft failure and response to AMR treatment have shown promising findings. Efforts to phenotype kidney transplant patients better, identify new disease mechanisms and therapeutic targets, and evaluate them in clinical trials should lead to more successful prevention, monitoring and management of kidney transplant recipients with AMR.
Acknowledgements
The authors thank Dr Kathryn Tinckam (Medical Director of the HLA Laboratory and transplant nephrologist at University Health Network, Toronto, Ontario, Canada) for her assistance in the design of Fig. 1 . Cartoons were created with BioRender.com.
Financial support and sponsorship
This work was supported by the following by the National Institute of Health (NIH) grant R01 AI143887 (to L.V.R.). This work was supported in part by the Assistant Secretary of Defense and Health Affairs, through the Reconstructive Transplant Research, under award W81XWH2010758 and W81XWH2110904 (to L.V.R.). Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. This work was also supported by funding from the Canadian Institutes for Health Research (CIHR) Project grant 469957 to A.K., Canadian Donation and Transplantation Research Program Innovation Grant 2021, and the Ajmera Transplant Centre/Toronto General and Western Hospital Foundation to A.K.
Conflicts of interest
L.V.R. had received research grant support from Bristoll-Meiers-Squibb, Caredx and Natera. For the remaining authors, there are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Sellarés J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 2012; 12:388–399. [DOI] [PubMed] [Google Scholar]
- 2.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant 2012; 12:1157–1167. [DOI] [PubMed] [Google Scholar]
- 3.El-Zoghby ZM, Stegall MD, Lager DJ, et al. Identifying specific causes of kidney allograft loss. Am J Transplant 2009; 9:527–535. [DOI] [PubMed] [Google Scholar]
- 4.Loupy A, Haas M, Roufosse C, et al. The Banff 2019 Kidney Meeting Report (I): updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant 2020; 20:2318–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant 2018; 18:293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan SC, Pescovitz MD. Presensitization: the problem and its management. Clin J Am Soc Nephrol 2006; 1:421–432. [DOI] [PubMed] [Google Scholar]
- 7.Amico P, Hönger G, Mayr M, et al. Clinical relevance of pretransplant donor-specific HLA antibodies detected by single-antigen flow-beads. Transplantation 2009; 87:1681–1688. [DOI] [PubMed] [Google Scholar]
- 8.Velidedeoglu E, Cavaillé-Coll MW, Bala S, et al. Summary of 2017 FDA Public Workshop: antibody-mediated rejection in kidney transplantation. Transplantation 2018; 102:257–264. [DOI] [PubMed] [Google Scholar]
- 9.Willicombe M, Brookes P, Sergeant R, et al. De novo DQ donor-specific antibodies are associated with a significant risk of antibody-mediated rejection and transplant glomerulopathy. Transplantation 2012; 94:172–177. [DOI] [PubMed] [Google Scholar]
- 10.Wiebe C, Pochinco D, Blydt-Hansen TD, et al. Class II HLA epitope matching: a strategy to minimize de novo donor-specific antibody development and improve outcomes. Am J Transplant 2013; 13:3114–3122. [DOI] [PubMed] [Google Scholar]
- 11.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Rates and determinants of progression to graft failure in kidney allograft recipients with de novo donor-specific antibody. Am J Transplant 2015; 15:2921–2930. [DOI] [PubMed] [Google Scholar]
- 12.Wiebe C, Kosmoliaptsis V, Pochinco D, et al. HLA-DR/DQ molecular mismatch: a prognostic biomarker for primary alloimmunity. Am J Transplant 2019; 19:1708–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiebe C, Rush DN, Nevins TE, et al. Class II eplet mismatch modulates tacrolimus trough levels required to prevent donor-specific antibody development. J Am Soc Nephrol 2017; 28:3353–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girerd S, Schikowski J, Girerd N, et al. Impact of reduced exposure to calcineurin inhibitors on the development of de novo DSA: a cohort of nonimmunized first kidney graft recipients between 2007 and 2014. BMC Nephrol 2018; 19:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigo E, Segundo DS, Fernández-Fresnedo G, et al. Within-patient variability in tacrolimus blood levels predicts kidney graft loss and donor-specific antibody development. Transplantation 2016; 100:2479–2485. [DOI] [PubMed] [Google Scholar]
- 16.Liefeldt L, Brakemeier S, Glander P, et al. Donor-specific HLA antibodies in a cohort comparing everolimus with cyclosporine after kidney transplantation. Am J Transplant 2012; 12:1192–1198. [DOI] [PubMed] [Google Scholar]
- 17.Haas M, Mirocha J, Reinsmoen NL, et al. Differences in pathologic features and graft outcomes in antibody-mediated rejection of renal allografts due to persistent/recurrent versus de novo donor-specific antibodies. Kidney Int 2017; 91:729–737. [DOI] [PubMed] [Google Scholar]
- 18.Davis S, Gralla J, Klem P, et al. Lower tacrolimus exposure and time in therapeutic range increase the risk of de novo donor-specific antibodies in the first year of kidney transplantation. Am J Transplant 2018; 18:907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hricik DE, Formica RN, Nickerson P, et al. Adverse outcomes of tacrolimus withdrawal in immune-quiescent kidney transplant recipients. J Am Soc Nephrol 2015; 26:3114–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪.Benedetti-Gassen R, Borges TJ, Pérez-Sáez MJ, et al. T cell depletion increases humoral response by favoring T follicular helper cells expansion. Am J Transplant 2022; 22:1766–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that antithymyocyte globulin depletion in the absence of maintenance immunosuppression with tacrolimus or rapamycin is associated with an increase in the percentage of circulating T follicular helper cells following T cell depletion and greater alloantibody generation in a mouse model of kidney transplantation.
- 21.Knechtle SJ, Pirsch JD, Fechner JH, et al. Campath-1H induction plus rapamycin monotherapy for renal transplantation: results of a pilot study. Am J Transplant 2003; 3:722–730. [DOI] [PubMed] [Google Scholar]
- 22.Flechner SM, Friend PJ, Brockmann J, et al. Alemtuzumab induction and sirolimus plus mycophenolate mofetil maintenance for CNI and steroid-free kidney transplant immunosuppression. Am J Transplant 2005; 5:3009–3014. [DOI] [PubMed] [Google Scholar]
- 23.Kim EJ, Kwun J, Gibby AC, et al. Costimulation blockade alters germinal center responses and prevents antibody-mediated rejection. Am J Transplant 2014; 14:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med 2016; 374:333–343. [DOI] [PubMed] [Google Scholar]
- 25.Durrbach A, Pestana JM, Florman S, et al. Long-term outcomes in belatacept- versus cyclosporine-treated recipients of extended criteria donor kidneys: final results from BENEFIT-EXT, a phase III randomized study. Am J Transplant 2016; 16:3192–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodle ES, Kaufman DB, Shields AR, et al. Belatacept-based immunosuppression with simultaneous calcineurin inhibitor avoidance and early corticosteroid withdrawal: a prospective, randomized multicenter trial. Am J Transplant 2020; 20:1039–1055. [DOI] [PubMed] [Google Scholar]
- 27▪.Kaufman DB, Woodle ES, Shields AR, et al. Belatacept for simultaneous calcineurin inhibitor and chronic corticosteroid immunosuppression avoidance: two-year results of a prospective, randomized multicenter trial. Clin J Am Soc Nephrol 2021; 16:1387–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]; This randomized clinical trial showed that compared with tacrolimus-based immunosuppression, belatacept-based immunosuppression in kidney transplant recipients is associated with a higher proportion of patients having an eGFR more than 45 ml/min/1.73 m2, a higher incidence of acute cellular rejection and similar rates of antibody-mediated rejection at 2 years.
- 28.Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant 2010; 10:535–546. [DOI] [PubMed] [Google Scholar]
- 29.Durrbach A, Pestana JM, Pearson T, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study). Am J Transplant 2010; 10:547–557. [DOI] [PubMed] [Google Scholar]
- 30.Bray RA, Gebel HM, Townsend R, et al. De novo donor-specific antibodies in belatacept-treated vs cyclosporine-treated kidney-transplant recipients: post hoc analyses of the randomized phase III BENEFIT and BENEFIT-EXT studies. Am J Transplant 2018; 18:1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity 2014; 41:529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leibler C, Matignon M, Moktefi A, et al. Belatacept in renal transplant recipient with mild immunologic risk factor: a pilot prospective study (BELACOR). Am J Transplant 2019; 19:894–906. [DOI] [PubMed] [Google Scholar]
- 33.La Muraglia GM, Wagener ME, Ford ML, et al. Circulating T follicular helper cells are a biomarker of humoral alloreactivity and predict donor-specific antibody formation after transplantation. Am J Transplant 2020; 20:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Louis K, Macedo C, Bailly E, et al. Coordinated circulating T follicular helper and activated B cell responses underlie the onset of antibody-mediated rejection in kidney transplantation. J Am Soc Nephrol 2020; 31:2457–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Senev A, Lerut E, Van Sandt V, et al. Specificity, strength, and evolution of pretransplant donor-specific HLA antibodies determine outcome after kidney transplantation. Am J Transplant 2019; 19:3100–3113. [DOI] [PubMed] [Google Scholar]
- 36.KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009; 9: (Suppl 3): S1–S155. [DOI] [PubMed] [Google Scholar]
- 37.Neuberger JM, Bechstein WO, Kuypers DRJ, et al. Practical recommendations for long-term management of modifiable risks in kidney and liver transplant recipients: a guidance report and clinical checklist by the Consensus on Managing Modifiable Risk in Transplantation (COMMIT) Group. Transplantation 2017; 101: (4S Suppl 2): S1–56. [DOI] [PubMed] [Google Scholar]
- 38.Tambur AR. HLA-DQ antibodies: are they real? Are they relevant? Why so many? Curr Opin Organ Transplant 2016; 21:441–446. [DOI] [PubMed] [Google Scholar]
- 39.Tinckam KJ, Rose C, Hariharan S, et al. Re-examining risk of repeated HLA mismatch in kidney transplantation. J Am Soc of Nephrol 2016; 27:2833–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tambur AR, Rosati J, Roitberg S, et al. Epitope analysis of HLA-DQ antigens: what does the antibody see? Transplantation 2014; 98:157–166. [DOI] [PubMed] [Google Scholar]
- 41.Wiebe C, Nickerson P. Acceptable mismatching at the class II epitope level: the Canadian experience. Curr Opin Organ Transplant 2014; 19:442–446. [DOI] [PubMed] [Google Scholar]
- 42.Sypek MP, Hughes P, Kausman JY. HLA epitope matching in pediatric renal transplantation. Pediatr Nephrol 2017; 32:1861–1869. [DOI] [PubMed] [Google Scholar]
- 43.Wiebe C, Nickerson P. Strategic use of epitope matching to improve outcomes. Transplantation 2016; 100:2048–2052. [DOI] [PubMed] [Google Scholar]
- 44.Senev A, Coemans M, Lerut E, et al. Eplet mismatch load and de novo occurrence of donor-specific anti-HLA antibodies, rejection, and graft failure after kidney transplantation: an observational cohort study. J Am Soc Nephrol 2020; 31:2193–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sapir-Pichhadze R, Tinckam K, Quach K, et al. HLA-DR and -DQ eplet mismatches and transplant glomerulopathy: a nested case-control study. Am J Transplant 2015; 15:137–148. [DOI] [PubMed] [Google Scholar]
- 46.Wiebe C, Nevins TE, Robiner WN, et al. The synergistic effect of class II HLA epitope-mismatch and nonadherence on acute rejection and graft survival. Am J Transplant 2015; 15:2197–2202. [DOI] [PubMed] [Google Scholar]
- 47.Tambur AR. HLA-epitope matching or eplet risk stratification: the devil is in the details. Front Immunol 2018; 9:2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tambur AR, Campbell P, Claas FH, et al. Sensitization in transplantation: assessment of risk (STAR) 2017 Working Group Meeting Report. Am J Transplant 2018; 18:1604–1614. [DOI] [PubMed] [Google Scholar]
- 49.Aubert O, Loupy A, Hidalgo L, et al. Antibody-mediated rejection due to preexisting versus de novo donor-specific antibodies in kidney allograft recipients. J Am Soc Nephrol 2017; 28:1912–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schinstock CA, Mannon RB, Budde K, et al. Recommended treatment for antibody-mediated rejection after kidney transplantation: the 2019 Expert Consensus from the Transplantion Society Working Group. Transplantation 2020; 104:911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parajuli S, Joachim E, Alagusundaramoorthy S, et al. Donor-specific antibodies in the absence of rejection are not a risk factor for allograft failure. Kidney Int Rep 2019; 4:1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lefaucheur C, Viglietti D, Bentlejewski C, et al. IgG donor-specific anti-human HLA antibody subclasses and kidney allograft antibody-mediated injury. J Am Soc Nephrol 2016; 27:293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schinstock CA, Cosio F, Cheungpasitporn W, et al. The value of protocol biopsies to identify patients with de novo donor-specific antibody at high risk for allograft loss. Am J Transplant 2017; 17:1574–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamamoto T, Watarai Y, Takeda A, et al. De novo anti-HLA DSA characteristics and subclinical antibody-mediated kidney allograft injury. Transplantation 2016; 100:2194–2202. [DOI] [PubMed] [Google Scholar]
- 55.Zeng Q, Ng YH, Singh T, et al. B cells mediate chronic allograft rejection independently of antibody production. J Clin Invest 2014; 124:1052–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lefaucheur C, Loupy A, Hill GS, et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol 2010; 21:1398–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loupy A, Lefaucheur C, Vernerey D, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 2013; 369:1215–1226. [DOI] [PubMed] [Google Scholar]
- 58.Chong AS, Rothstein DM, Safa K, et al. Outstanding questions in transplantation: B cells, alloantibodies, and humoral rejection. Am J Transplant 2019; 19:2155–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clotet-Freixas S, McEvoy CM, Batruch I, et al. Extracellular matrix injury of kidney allografts in antibody-mediated rejection: a proteomics study. J Am Soc Nephrol 2020; 31:2704–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bharadwaj P, Shrestha S, Pongracz T, et al. Afucosylation of HLA-specific IgG1 as a potential predictor of antibody pathogenicity in kidney transplantation. medRxiv 2022; 1:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonomini V, Vangelista A, Frascà GM, et al. Effects of plasmapheresis in renal transplant rejection. A controlled study. Trans Am Soc Artif Intern Organs 1985; 31:698–703. [PubMed] [Google Scholar]
- 62.Böhmig GA, Wahrmann M, Regele H, et al. Immunoadsorption in severe C4d-positive acute kidney allograft rejection: a randomized controlled trial. Am J Transplant 2007; 7:117–121. [DOI] [PubMed] [Google Scholar]
- 63.Allen NH, Dyer P, Geoghegan T. Plasma exchange in acute renal allograft rejection. A controlled trial. Transplantation 1983; 35:425–428. [DOI] [PubMed] [Google Scholar]
- 64.Kirubakaran MG, Disney APS, Norman J, et al. A controlled trial of plasmapheresis in the treatment of renal allograft rejection. Transplantation 1981; 32:164–165. [DOI] [PubMed] [Google Scholar]
- 65.Vangelista A, Frascà GM, Nanni Costa A, et al. Value of plasma exchange in renal transplant rejection induced by specific anti-HLA antibodies. Trans Am Soc Artif Intern Organs 1982; 28:599–603. [PubMed] [Google Scholar]
- 66.Sautenet B, Blancho G, Büchler M, et al. One-year results of the effects of rituximab on acute antibody-mediated rejection in renal transplantation: RITUX ERAH, a multicenter double-blind randomized placebo-controlled trial. Transplantation 2016; 100:391–399. [DOI] [PubMed] [Google Scholar]
- 67.Eskandary F, Regele H, Baumann L, et al. A randomized trial of bortezomib in late antibody-mediated kidney transplant rejection. J Am Soc Nephrol 2018; 29:591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kulkarni S, Kirkiles-Smith NC, Deng YH, et al. Eculizumab therapy for chronic antibody-mediated injury in kidney transplant recipients: a pilot randomized controlled trial. Am J Transplant 2017; 17:682–691. [DOI] [PubMed] [Google Scholar]
- 69.Lee CY, Lin WC, Wu MS, et al. Repeated cycles of high-dose intravenous immunoglobulin and plasmapheresis for treatment of late antibody-mediated rejection of renal transplants. J Formos Med Assoc 2016; 115:845–852. [DOI] [PubMed] [Google Scholar]
- 70.Laws LH, Parker CE, Cherala G, et al. Inflammation causes resistance to anti-CD20-mediated B cell depletion. Am J Transplant 2016; 16:3139–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vereerstraeten P, Abramowicz D, de Pauw L, et al. Absence of deleterious effect on long-term kidney graft survival of rejection episodes with complete functional recovery. Transplantation 1997; 63:1739–1743. [DOI] [PubMed] [Google Scholar]
- 72.Everly MJ, Everly JJ, Arend LJ, et al. Reducing de novo donor-specific antibody levels during acute rejection diminishes renal allograft loss. Am J Transplant 2009; 9:1063–1071. [DOI] [PubMed] [Google Scholar]
- 73.Konvalinka A, Tinckam K. Utility of HLA antibody testing in kidney transplantation. J Am Soc Nephrol 2015; 26:1489–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parajuli S, Mandelbrot DA, Muth B, et al. Rituximab and monitoring strategies for late antibody-mediated rejection after kidney transplantation. Transplant Direct 2017; 3:e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bloom RD, Bromberg JS, Poggio ED, et al. Cell-free DNA and active rejection in kidney allografts. J Am Soc Nephrol 2017; 28:2221–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loupy A, Aubert O, Orandi BJ, et al. Prediction system for risk of allograft loss in patients receiving kidney transplants: international derivation and validation study. BMJ 2019; 366:l4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77▪.Raynaud M, Aubert O, Divard G, et al. Dynamic prediction of renal survival among deeply phenotyped kidney transplant recipients using artificial intelligence: an observational, international, multicohort study. Lancet Digit Health 2021; 3:e795–805. [DOI] [PubMed] [Google Scholar]; This study examined a large number of kidney transplant recipients and developed a dynamic artificial intelligence tool to predict risk of death-censored allograft failure using clinical, immunological and histological characteristics.
- 78.Sellarés J, Reeve J, Loupy A, et al. Molecular diagnosis of antibody-mediated rejection in human kidney transplants. Am J Transplant 2013; 13:971–983. [DOI] [PubMed] [Google Scholar]
- 79.Hilbrands L, Hoitsma A, Wetzels J, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med 2005; 352:2027–2028. [DOI] [PubMed] [Google Scholar]
- 80.Sun Q, Liu Z, Yin G, et al. Detectable circulating antiendothelial cell antibodies in renal allograft recipients with C4d-positive acute rejection: a report of three cases. Transplantation 2005; 79:1759–1762. [DOI] [PubMed] [Google Scholar]
- 81.Cardinal H, Dieudé M, Hébert MJ. The emerging importance of non-HLA autoantibodies in kidney transplant complications. J Am Soc of Nephrol 2017; 28:400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82▪.Clotet-Freixas S, Kotlyar M, McEvoy CM, et al. Increased autoantibodies against Ro/SS-A, CENP-B, and La/SS-B in patients with kidney allograft antibody-mediated rejection. Transplant Direct 2021; 7: [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed increased pretransplant levels of anti-Ro and anti-CENP antibody levels in kidney transplant recipients with antibody-mediated rejection and mixed rejection compared with those with acute cellular rejection.
- 83.Zhang Q, Reed EF. The importance of non-HLA antibodies in transplantation. Nat Rev Nephrol 2016; 12:484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fuss A, Hope CM, Deayton S, et al. C4d-negative antibody-mediated rejection with high antiangiotensin II type I receptor antibodies in absence of donor-specific antibodies. Nephrology 2015; 20:467–473. [DOI] [PubMed] [Google Scholar]
- 85.Bray RA, Nolen JDL, Larsen C, et al. Transplanting the highly sensitized patient: the emory algorithm. Am J Transplant 2006; 6:2307–2315. [DOI] [PubMed] [Google Scholar]
- 86.Heidt S, Haasnoot GW, Witvliet MD, et al. Allocation to highly sensitized patients based on acceptable mismatches results in low rejection rates comparable to nonsensitized patients. Am J Transplant 2019; 19:2926–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Senev A, Coemans M, Lerut E, et al. Histological picture of antibody-mediated rejection without donor-specific anti-HLA antibodies: clinical presentation and implications for outcome. Am J Transplant 2019; 19:763–780. [DOI] [PubMed] [Google Scholar]
- 88.Parajuli S, Redfield RR, Garg N, et al. Clinical significance of microvascular inflammation in the absence of anti-HLA DSA in kidney transplantation. Transplantation 2019; 103:1468–1476. [DOI] [PubMed] [Google Scholar]
- 89.Callemeyn J, Lerut E, de Loor H, et al. Transcriptional changes in kidney allografts with histology of antibody-mediated rejection without anti-HLA donor-specific antibodies. J Am Soc of Nephrol 2020; 31:2168–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yazdani S, Callemeyn J, Gazut S, et al. Natural killer cell infiltration is discriminative for antibody-mediated rejection and predicts outcome after kidney transplantation. Kidney Int 2019; 95:188–198. [DOI] [PubMed] [Google Scholar]
- 91.Jordan SC, Lorant T, Choi J, et al. IgG endopeptidase in highly sensitized patients undergoing transplantation. N Engl J Med 2017; 377:442–453. [DOI] [PubMed] [Google Scholar]
- 92.Redfield RR, Jordan SC, Busque S, et al. Safety, pharmacokinetics, and pharmacodynamic activity of obinutuzumab, a type 2 anti-CD20 monoclonal antibody for the desensitization of candidates for renal transplant. Am J Transplant 2019; 19:3035–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spica D, Junker T, Dickenmann M, et al. Daratumumab for treatment of antibody-mediated rejection after ABO-incompatible kidney transplantation. Case Rep Nephrol Dial 2019; 9:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jain D, Rajab A, Young JS, et al. Reversing donor-specific antibody responses and antibody-mediated rejection with bortezomib and belatacept in mice and kidney transplant recipients. Am J Transplant 2020; 20:2675–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tan EK, Bentall A, Dean PG, et al. Use of eculizumab for active antibody-mediated rejection that occurs early postkidney transplantation: a consecutive series of 15 cases. Transplantation 2019; 103:2397–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Choi J, Aubert O, Vo A, et al. Assessment of tocilizumab (Anti-Interleukin-6 Receptor Monoclonal) as a potential treatment for chronic antibody-mediated rejection and transplant glomerulopathy in HLA-sensitized renal allograft recipients. Am J Transplant 2017; 17:2381–2389. [DOI] [PubMed] [Google Scholar]
- 97▪▪.Doberer K, Duerr M, Halloran PF, et al. A randomized clinical trial of anti-IL-6 antibody clazakizumab in late antibody-mediated kidney transplant rejection. J Am Soc Nephrol 2021; 32:708–722. [DOI] [PMC free article] [PubMed] [Google Scholar]; This phase II randomized clinical trial showed that clazakizumab use in kidney transplant recipients with late antibody-mediated rejection was associated with a significant reduction in donor-specific antibody levels and less decline in estimated GFR.
- 98.Tempest-Roe S, Prendecki M, McAdoo SP, et al. Inhibition of spleen tyrosine kinase decreases donor specific antibody levels in a rat model of sensitization. Sci Rep 2022; 12:3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lúcia M, Luque S, Crespo E, et al. Preformed circulating HLA-specific memory B cells predict high risk of humoral rejection in kidney transplantation. Kidney Int 2015; 88:874–887. [DOI] [PubMed] [Google Scholar]
- 100.Lefaucheur C, Viglietti D, Hidalgo LG, et al. Complement-activating anti-HLA antibodies in kidney transplantation: allograft gene expression profiling and response to treatment. J Am Soc Nephrol 2018; 29:620–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sablik KA, Jordanova ES, Pocorni N, et al. Immune cell infiltrate in chronic-active antibody-mediated rejection. Front Immunol 2020; 10:3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miyairi S, Baldwin WM, Valujskikh A, et al. Natural killer cells: critical effectors during antibody-mediated rejection of solid organ allografts. Transplantation 2021; 105:284–290. [DOI] [PubMed] [Google Scholar]
- 103.Venner JM, Hidalgo LG, Famulski KS, et al. The molecular landscape of antibody-mediated kidney transplant rejection: evidence for NK involvement through CD16a Fc receptors. Am J Transplant 2015; 15:1336–1348. [DOI] [PubMed] [Google Scholar]
- 104.Proschak E, Stark H, Merk D. Polypharmacology by design: a medicinal chemist's perspective on multitargeting compounds. J Med Chem 2019; 62:420–444. [DOI] [PubMed] [Google Scholar]