Tissue-specific chromatin accessibility identifies active genomic regions, integrates genetic information with exposure history, provides unique signatures that remain stable over longer timescales than gene transcription, and requires limited input material1. Indeed, chromatin accessibility profiles have enabled precise molecular subtyping of a wide variety of tumors2. For cardiomyopathies, however, tissue biopsy is not routinely performed, and etiology is most often determined by clinical information alone. Nevertheless, cardiologists are commonly faced with diagnostic dilemmas, such as a cardiomyopathy patient with obstructive coronary artery disease (CAD) involving less than three vessels. Here, knowledge of cardiomyopathy etiology would likely impact prognosis3 and guide management4. A more precise molecular diagnosis could also help clinicians avoid incorrect diagnoses based on clinical criteria alone, as recently described for hypertrophic cardiomyopathy (HCM)5. Here we propose to leverage the rich information content of chromatin accessibility to improve the accuracy of cardiomyopathy classification.
Human heart specimens were obtained from the Southwest Transplant Alliance, the UTSW Cardiac Tissue Biobank (STU 092010–193), and the HCM patient registry (STU 082017–072) following approval by the local Institutional Review Board (IRB) and proper informed consent. Healthy specimens were obtained from donor hearts rejected for cardiac transplantation, and neither the underlying diagnosis nor genetic etiology was established in NICM patients. Disease specimens were matched with healthy controls, although one unmatched healthy specimen was retained in our final analysis due to quality control (QC) failure of individual cardiomyopathy samples. The assay for transposase-accessible chromatin with sequencing (ATAC-seq) was performed on cardiomyocyte nuclei2. Sequencing reads were processed and mapped to the reference GRCh38 human genome. A Random Forest Classifier was built using uniquely accessible regions with the number of trees set to 1000. Leave-one-out cross-validation (LOOCV) was performed with 100 iterations of leaving out one replicate pair for testing and using the remaining samples to build the classifier. Anonymized data have been made publicly available at dbGaP (https://www.ncbi.nlm.nih.gov/gap/).
We profiled chromatin accessibility (Figure [A–B]) in 21 individuals (6 healthy; 5 ischemic cardiomyopathy [ICM]; 5 non-ischemic cardiomyopathy [NICM]; 5 HCM). Uniform Manifold Approximation and Projection (UMAP) analysis of chromatin accessibility resolved healthy and cardiomyopathy samples by etiology (Figure [C]). In a two-way comparison of healthy versus cardiomyopathy samples, 1066 regions were differentially accessible (Figure [D]). Focusing on uniquely accessible genomic regions, we generated a heatmap to highlight differential accessibility between healthy tissue and individual cardiomyopathy subtypes (Figure [E]). Analysis of co-accessible loci from each group yielded transcription factor (TF) motifs and potential downstream target genes (Figure [F]). Based on this analysis, we speculate that MEF2 transcription factors activate contractile gene expression in ICM, nuclear hormone receptors influence cellular adhesion programs in NICM, and ETS TFs activate aberrant developmental processes in HCM.
Figure. Identification of cardiomyopathy etiology based on unique chromatin accessibility signatures.
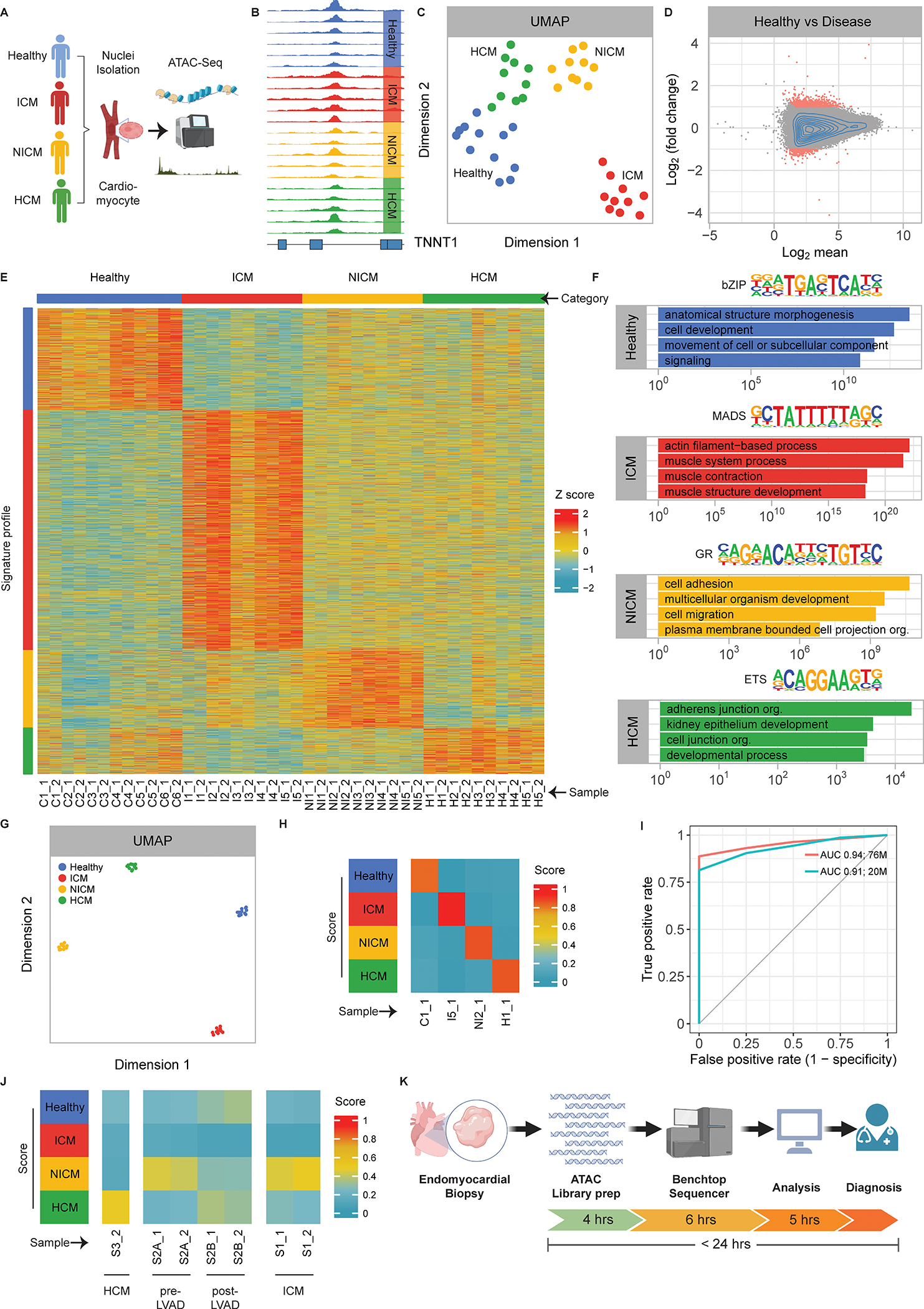
A. Steps involved in processing human heart specimens for ATAC-seq.
B. Genome browser tracks across samples for the heart-enriched TNNT11 gene.
C. Uniform Manifold Approximation and Projection (UMAP) for all ATAC-seq samples using the entire genome.
D. MA-plot demonstrating differentially accessible peaks in healthy versus cardiomyopathy samples. X-axis: Log2 mean; Y-axis: Log2 (fold change).
E. Heatmap of genomic peaks uniquely accessible in each patient category. Columns represent individual samples, and rows show genomic loci. Etiology groupings are indicated at the top and individual patients at the bottom. Color scale indicates z-scores for peak accessibility as shown.
F. Transcription factor sequence logos with nearest match are shown for each group at the top. The top four gene ontology (GO) classifications using genomic regions enrichment of annotations tool (GREAT) analysis of unique chromatin accessibility peaks to highlight patterns of putatively regulated genes are shown at the bottom. Y-axis: -log(p-value). bZIP: basic leucine zipper domain; MADS: MCM1/AG/DEFA/SRF domain; GR: glucocorticoid receptor; ETS: E twenty-six domain.
G. UMAP for all ATAC-seq samples using the refined set of peaks from (E).
H. Diagnostic heatmap showing representative examples of applying Random Forest Classifier to each patient group using maximum available sequencing depth. Color scale indicates the percentage of trees in favor of each diagnosis.
I. Area under the curve (AUC) for LOOCV performed on all samples in the original patient cohort. Red line: AUC using original sequencing depth; Blue line: AUC using randomly downsampled sequencing depth of 20 million reads. X-axis: False positive rate (1-specificity); Y-axis: True positive rate.
J. Diagnostic heatmap using downsampled sequencing depth (20 million reads) for a singleton external HCM patient sample (left), a NICM patient (pre- and post-LVAD) (middle), and a clinically diagnosed ICM patient (right). Color scale indicates the percentage of trees in favor of each diagnosis.
K. Proposed rapid diagnostic strategy for cardiomyopathy etiology.
Next, we sought to improve cardiomyopathy discrimination by using only the focused set of chromatin accessibility peaks from Figure [E]. UMAP analysis showed that the refined subset of peaks improved cardiomyopathy classification (Figure [G]; compare with Figure [C]). Consequently, we trained a Random Forest Classifier to nominate a cardiomyopathy diagnosis based on the informative subset of chromatin accessibility peaks (Figure [H]). LOOCV was performed across all samples, and the area under the receiver-operator curve (AUC) demonstrated excellent algorithm performance (Figure [I]; red line). Given that clinical benchtop sequencers can generate 20 million reads within hours, we repeated the LOOCV analysis after random selection of 20 million reads from each sample (i.e. downsampling) and observed little drop-off in algorithm performance (Figure [I]; blue line).
Finally, we present three simulated applications using the diagnostic algorithm with 20 million randomly selected reads per sample. First, chromatin accessibility was profiled in a single replicate for a HCM patient with limited tissue availability (Figure [J]), which was consistent with internal validations (Figure [H]). Second, we analyzed specimens from a NICM patient before and after left ventricular assist device (LVAD) implantation. Interestingly, this example confirmed the etiology in the pre-LVAD sample and suggested that LVAD treatment alters chromatin accessibility (Figure [J]). Third, we highlight a case in which ICM was diagnosed by coronary angiography prior to bypass surgery, yet chromatin accessibility at transplant favored a NICM classification (Figure [J]). Consistent with this observation, the patient developed four chamber enlargement without regional wall motion abnormalities between bypass surgery and heart transplant, suggesting a superimposed NICM, although definitive clinical evidence is lacking.
In conclusion, our study establishes proof-of-concept for a cardiomyopathy diagnostic algorithm using chromatin accessibility signatures at a sequencing depth achievable by benchtop instruments. Nonetheless, two important limitations deserve mention. First, all specimens were taken from the left ventricle to ensure matched anatomical locations, rather than the conventional right ventricular biopsy site. Second, a small number of samples were included in the study, so future external validation is necessary to confirm these findings. If replicated, however, the proposed strategy could enable rapid diagnosis (Figure [K]) and facilitate precision medicine approaches for cardiomyopathy patients.
Sources of Funding
This work was supported by the AHA (17PRE33670730), NIH (HL136604, HL133642, and HL135217 to N.V.M; DP2GM128203 to G.C.H.; UM1HG011996 to N.V.M. and G.C.H.; R01HL102478 and P50HD087351 to P.P.A.M.), the Burroughs Wellcome Fund (1009838 to N.V.M.; 1019804 to G.C.H.), the March of Dimes Foundation (#5-FY13–203 to N.V.M.), the Department of Defense (PR172060 to N.V.M. and G.C.H.), CPRIT (G.C.H.), and the Green Center for Reproductive Biology (G.C.H.).
Footnotes
Disclosures
None.
ARTICLE INFORMATION
Research materials, experimental procedures, and protocols are available from the corresponding authors upon reasonable request.
REFERENCES
- 1.Klemm SL, Shipony Z and Greenleaf WJ. Chromatin accessibility and the regulatory epigenome. Nat Rev Genet. 2019;20:207–220. [DOI] [PubMed] [Google Scholar]
- 2.Corces MR, Granja JM, Shams S, Louie BH, Seoane JA, Zhou W, Silva TC, Groeneveld C, Wong CK, Cho SW, Satpathy AT, Mumbach MR, Hoadley KA, Robertson AG, Sheffield NC, Felau I, Castro MAA, Berman BP, Staudt LM, Zenklusen JC, Laird PW, Curtis C, Cancer Genome Atlas Analysis Network, Greenleaf WJ and Chang HY. The chromatin accessibility landscape of primary human cancers. Science. 2018;362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pecini R, Moller DV, Torp-Pedersen C, Hassager C and Kober L. Heart failure etiology impacts survival of patients with heart failure. Int J Cardiol. 2011;149:211–215. [DOI] [PubMed] [Google Scholar]
- 4.Bozkurt B, Colvin M, Cook J, Cooper LT, Deswal A, Fonarow GC, Francis GS, Lenihan D, Lewis EF, McNamara DM, Pahl E, Vasan RS, Ramasubbu K, Rasmusson K, Towbin JA, Yancy C, American Heart Association Committee on Heart Failure and Transplantation of the Council on Clinical Cardiology, Council on Cardiovascular Disease in the Young, Council on Cardiovascular and Stroke Nursing, Council on Epidemiology and Prevention, and Council on Quality of Care and Outcomes Research. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement From the American Heart Association. Circulation. 2016;134:e579–e646. [DOI] [PubMed] [Google Scholar]
- 5.Alashi A, Desai RM, Khullar T, Hodges K, Rodriguez ER, Tan C, Popovic ZB, Thamilarasan M, Wierup P, Lever HM, Smedira NG and Desai MY. Different Histopathologic Diagnoses in Patients With Clinically Diagnosed Hypertrophic Cardiomyopathy After Surgical Myectomy. Circulation. 2019;140:344–346. [DOI] [PubMed] [Google Scholar]


