Abstract
In this study we investigated the relationship between the MATα locus of Cryptococcus neoformans and several MATα-specific mitogen-activated protein (MAP) kinase signal transduction cascade genes, including STE12α, STE11α, and STE20α. To resolve the location of the genes, we screened a cosmid library of the MATα strain B-4500 (JEC21), which was chosen for the C. neoformans genome project. We isolated several overlapping cosmids spanning a region of about 71 kb covering the entire MATα locus. It was found that STE12α, STE11α, and STE20α are imbedded within the locus rather than closely linked to the locus. Furthermore, three copies of MFα, the mating type α-pheromone gene, a MATα-specific myosin gene, and a pheromone receptor (CPRα) were identified within the locus. We created a physical map, based on the restriction enzyme BamHI, and identified both borders of the MATα locus. The MATα locus of C. neoformans is approximately 50 kb in size and is one of the largest mating type loci reported among fungi with a one-locus, two-allele mating system.
Cryptococcus neoformans is the etiologic agent of cryptococcosis, which is one of the most serious fungal diseases encountered worldwide. Although C. neoformans primarily affects patients with impaired immune systems, people with no known underlying immunodeficiencies are also affected (9).
C. neoformans is a bipolar heterothallic fungus in which completion of the meiotic cycle is dependent upon interaction between cells of MATα and MATa types. Although segregation patterns of the meiotic products yield MATα and MATa progeny in the expected 1:1 ratio for a bipolar heterothallic fungus (12), MATα strains are found far more frequently than MATa strains in clinical as well as environmental isolates among serotype D strains (10). Among serotype A strains, MATα has thus far been the only mating type to be found regardless of isolate source (13). To investigate the relationship between mating type and virulence, Kwon-Chung et al. (11) constructed a pair of congenic MATα (B-4500) and MATa (B-4476) strains of serotype D for genetic analysis. In a mouse systemic infection model, both the parental strain and F1 progeny of MATα type were found to be significantly more virulent than the MATa parental strain and its F1 progeny (11). Therefore, molecular and pathobiological studies of C. neoformans serotype D isolates have since been carried out mostly with MATα strains (3, 20).
In 1993, Moore and Edman (16) identified the MATα locus by employing a difference cloning method using the congenic strains B-4500 (JEC21, MATα) and B-4476 (JEC20, MATa). The MATα locus was marked by the presence of the MFα gene, which encodes a pheromone precursor. Analysis of MATα-specific phage and cosmid clones led them to conclude that the MATα locus was 35 to 45 kb in size. Subsequently, Wickes et al. discovered haploid fruiting to be a MATα-specific phenomenon in C. neoformans (26). Molecular analysis of haploid fruiting in MATα strains resulted in the cloning of the STE12α gene, a homolog of the Saccharomyces cerevisiae transcriptional activator STE12, and the STE11α gene (26), a homolog of the S. cerevisiae STE11 (MEKK) gene (19, 22). Recently, Wang and Heitman isolated STE20α (accession no. AF162330), a homolog of S. cerevisiae STE20, from H99, a serotype A MATα strain of C. neoformans. Although these genes were reported to be specific for MATα strains, their genomic locations have not been clearly determined. Additionally, STE12α was not found in the MATα-specific region (26) previously reported by Moore and Edman (16), nor was a MATα-specific receptor of the MATa pheromone found, a gene which should be mating type specific.
The STE12α gene recently has been deleted from both serotype D and serotype A strains, and the gene was found to be essential for haploid fruiting but not for mating (4, 28). Furthermore, STE12α was reported to regulate several virulence associated genes in serotype D C. neoformans (4). Mating type-specific mitogen-activated protein (MAP) kinase genes of the signal transduction cascade have not been reported in any other fungi. Since the importance of these genes in the pathobiology of C. neoformans is becoming increasingly evident (4; D. L. Clarke, U. Edman, G. L. Woodlee, C. M. McClelland, T. S. Seymour, J. C. Edman, and B. L. Wickes, unpublished data), we have attempted to determine their genomic locations.
In this paper, we present a physical map of the B-4500 MATα locus which was obtained by analysis of overlapping subclones isolated from a cosmid library of B-4500. The MATα locus, which spans 50 kb, contained several mating type α-specific homologs of the pheromone response MAP kinase signal transduction cascade genes, multiple copies of MFα, and one copy of the pheromone receptor gene, CPRα. Unexpectedly, a myosin gene and a homolog of S. cerevisiae translation initiation factor, PRT1, specific to the MATα strain, were also discovered within the locus.
MATERIALS AND METHODS
C. neoformans strains, Escherichia coli plasmids and cosmids, and growth conditions.
The C. neoformans strains B-4500 (JEC21, MATα) and B-4476 (JEC20, MATa) are isogenic strains (11). The strains were maintained on YEPD agar medium (1% yeast extract, 2% peptone, 2% dextrose, 1.5% agar) and grown on MIN medium (0.67% yeast nitrogen base without amino acids, 2% glucose) before DNA was extracted. E. coli plasmids and cosmids used in this study are listed in Table 1.
TABLE 1.
E. coli plasmids and cosmids used in this study
| Plasmid or cosmid | Construction | Source or reference |
|---|---|---|
| pNH7 | Rescued plasmid with 8.5-kb insert, containing STE12α | 26 |
| pC12-1.3 | 1.3-kb BamHI fragment in pBluescript KS(−) | This work |
| pC12-6.5 | 6.5-kb BamHI fragment in pBluescript KS(−) | This work |
| pC12-17 | 12-kb BamHI fragment in pBC KS− | This work |
| pC12-4.5 | 4.5-kb BamHI fragment in pBC KS− | This work |
| pSCC93-3.8 | 3.8-kb BamHI fragment in pBluescript KS(−) | This work |
| pC12-13 | 13-kb BamHI fragment in pBC KS− | This work |
| pSCC93-20 | 21-kb BamHI fragment in pBC KS− | This work |
Molecular techniques.
Standard methods described by Sambrook et al. (21) were used for transformation of E. coli and DNA analysis. Genomic DNA was extracted from C. neoformans essentially as described by Pitkin et al. (18). All Southern blots (except the myosin blot) were hybridized and washed under stringent conditions at 65°C. The myosin blot was hybridized under less stringent conditions at 45°C. For subcloning of DNA fragments >10 kb from the isolated cosmids (containing an ampicillin resistance cassette), 5 μg of cosmid DNA was digested to completion, ethanol precipitated, washed, and resuspended in 20 μl of H2O. The DNA fragments were cloned into a linearized and dephosphorylated plasmid (pBC KS−) containing a cassette for chloramphenicol resistance (Stratagene, La Jolla, Calif.). This cloning strategy eliminated clones derived from religation of the cosmid backbone, since the cosmid selectable marker is ampicillin and recircularized vectors will not grow on chloramphenicol. Positive clones were identified by using colony hybridization and an appropriate DNA sequence as a probe. For sequencing, 0.5 to 1.0 μg of DNA was used with the DNA Sequencing Kit ABI PRISM and an ABI377 DNA sequencer from Perkin-Elmer (PE Biosystems, Warrington, England).
CHEF analysis.
Contour-clamped homogeneous electric field (CHEF) blots were prepared as previously described (27) and analyzed by Southern hybridization using probes generated from various regions of the MAT locus. The probes of MATα and MATa have previously been described (5). The probes FUR and MK were generated by PCR, using primers derived from partial sequence analysis of regions III and VI, respectively (see Fig. 4). The oligonucleotide pairs 5′-AATGGGGGAAAACGCACGAG-3′ with 5′-CTTCTAAGGACTTGCGGTTCTCAACTC-3′ and 5′-CCTGCACGGAAAATATCCAC-3′ with 5′-GCAAGATATATGGACCCCTG-3′ were used to isolate FUR and MK, respectively.
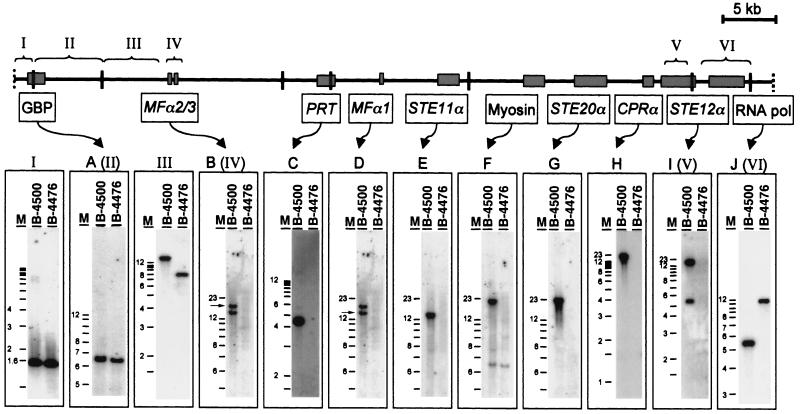
FIG. 4.
Overview of the MATα locus as well as identification and localization of the genes within the locus. Overlapping cosmids covering the whole mating type locus were isolated and analyzed. Using Southern blot techniques and several probes, the mating type-specific (between IV and V) and shared (I to III, VI) sequences were identified. The brackets mark the regions of the probes, indicated by roman letters. Specific probes were used to hybridize with BamHI-digested genomic DNA of B-4500 (MATα) and B-4476 (MATa) to confirm their mating type specificity. Probes A (GTP binding protein) and J (RNA polymerase) are located outside the mating type locus which hybridized with the DNA of both mating types. (B) MFα2/3 are located on the 17-kb BamHI fragment, followed by a mating type-specific translation initiation factor, a homolog of the S. cerevisiae PRT1 (C). Previously described MFα1 (D) is located on the same 13-kb BamHI fragment as STE11α (E). The largest BamHI fragment is ∼21 kb in size and contains a myosin gene (F), STE20α (G), the pheromone receptor CPRα (H), and STE12α (I).
RESULTS
Creation of a physical map and localization of STE12α within the MATα locus.
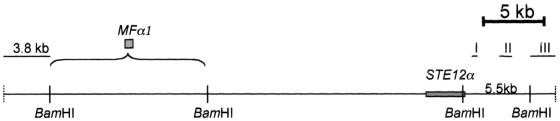
Previous studies suggested that the location of STE12α and STE11α was closely linked to the MATα locus (26). Subsequently, STE20α, an α-specific homolog of S. cerevisiae STE20, was cloned by Wang and Heitman (accession no. AF162330) although its location in relation to the MATα locus has not been reported. To establish the physical relationship between these MAP kinase signal transduction cascade genes and the MATα locus in C. neoformans, the sequence downstream of STE12α was first used as a probe (Fig. 1, probe I) to screen a cosmid library of B-4500. Several overlapping cosmids were isolated. A physical map of these cosmids was created by using the restriction enzyme BamHI (Fig. 1). Southern blot analysis using a PCR-derived probe of MFα1, the original marker for the MATα locus described by Moore and Edman (16), detected a 13-kb BamHI band in several of our cosmids (data not shown). These data indicated that STE12α is physically linked to the MFα1 gene, although the exact position of MFα1 could not be determined at this stage. To investigate the neighboring sequence of STE12α, we subcloned and sequenced the 5.5-kb BamHI fragment (Fig. 1) which contained the downstream sequence of the STE12α gene. We identified a 3.4-kb sequence about 1 kb downstream of STE12α with high similarity to the gene encoding a mitochondrial RNA polymerase of S. cerevisiae (RPO41, accession no. M17539) (7). To determine whether this sequence was shared in both mating types, BamHI-digested genomic DNAs from B-4500 and B-4476 were each hybridized with a probe from this gene (Fig. 1, probe II). The hybridization pattern revealed a single band with different sizes in each mating type (see Fig. 4J). Another probe derived from a 3-kb BamHI fragment, which was located next to the 5.5-kb fragment outside the conserved RPO41 gene (Fig. 1, probe III), also showed a signal in both mating types (data not shown). When several different regions upstream of STE12α (left side of STE12α in Fig. 1) were used as probes, they all showed an α-specific pattern (see below). It was therefore clear that not only was STE12α physically associated with the MFα1 gene but it was actually located at one end of the MATα locus, close to the boundary.
FIG. 1.
Physical map of the overlapping cosmids digested by BamHI. Cosmids were isolated by using probe I to screen a cosmid library of B-4500 (JEC21). STE12α is located on the right side of the restriction map, and MFα1 is localized on the 13-kb BamHI fragment at the left side. The exact position of MFα1 could not be determined at this stage. Shaded bars are locations of genes. I, II, and III represent the regions used as probes, hybridizing to genomic DNA of both mating types.
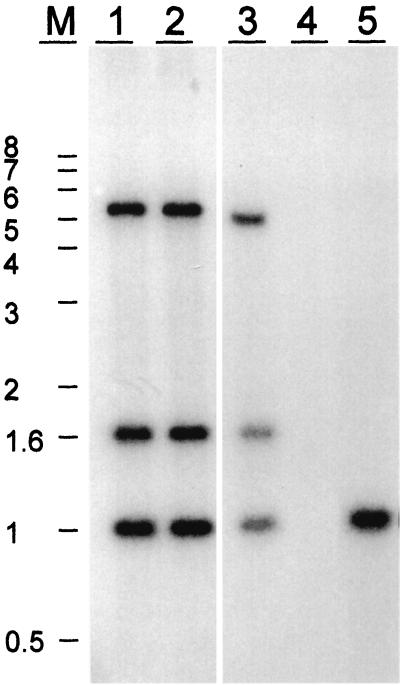
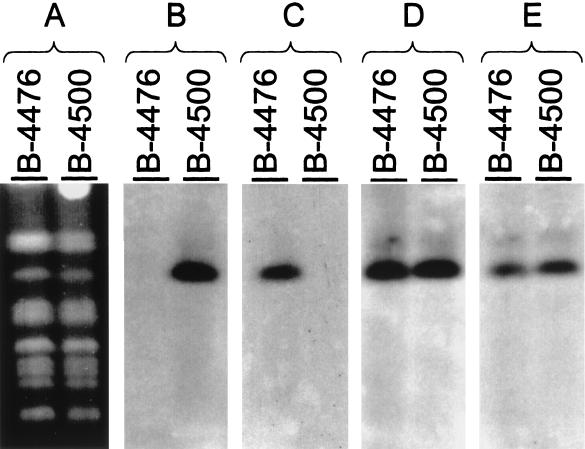
After the identification of one end of the MATα locus, a physical map of the entire region was created in order to determine the location of other α-specific genes within the locus. Using the 3.8-kb BamHI fragment located at the left side of the map (Fig. 1) as a hybridization probe to genomic B-4500 and B-4476 DNA, we realized that this probe hybridized to a 4.5-kb fragment present only in the α-mating type (data not shown). These data suggested that our initial cosmids did not contain the sequence present in the other end of the MATα locus. Another indication for the lack of the entire MATα locus in our first cosmids was that the banding patterns of HaeII-digested cosmid DNA and B-4500 genomic DNA hybridized with MFα1 were not identical. While three distinct bands of 1, 1.6, and 5 kb were present in the genomic DNA of B-4500, only a single band of 1 kb was detected in the cosmid DNA (Fig. 2). To isolate overlapping cosmids which covered the entire MATα locus, we performed a second screening of the cosmid library by using the 3.8-kb BamHI probe. Several cosmids extending to the left side of our previous map were identified. Southern blot analysis identified two new cosmids having the same hybridization pattern as B-4500 genomic DNA when the MFα1 coding region was used as a probe (Fig. 2, lanes 1 and 2). A new map containing all the overlapping cosmids is depicted in Fig. 4. With the information on one border of the MATα locus in hand, an attempt was made to identify the opposite border. We generated several probes using DNA fragments starting from the left side of the new map and hybridized them against genomic DNA of B-4500 and B-4476 digested with BamHI (see Fig. 4). Fragments I, II, and III hybridized to both mating types, whereas fragment IV hybridized only to the DNA of the α-mating type. When several other different regions from the new extended map (right side from fragment IV) were used as probes, they all showed a MATα-specific pattern (see below). These data suggested that we identified the other boundary of the MATα locus. Confirmation of the mating type junction location was obtained by hybridization to CHEF blots of C. neoformans chromosomal DNA isolated from the two strains B-4476 and B-4500. Each pheromone probe, MFα1 and MFa (5), hybridized exclusively to its corresponding 2.5-Mb MAT chromosome (Fig. 3). Probes adjacent to the locus junction hybridized to the MAT chromosomes from both mating types. These data, in conjunction with the cosmid hybridizations, demonstrate that the α-specific probes as well as the common a/α junction probes reside on the MAT locus-containing chromosome in both mating types.
FIG. 2.
Southern blot analysis of MFα genes. Cosmid DNA and genomic DNA of B-4500 and B-4476 digested with HaeII were hybridized with the MFα1 probe. Lanes 1 and 2 show three MFα bands detected in the DNA of the cosmids C12 and C18. The same banding pattern was observed with genomic DNA of B-4500 (lane 3), whereas no signal was obtained with the genomic DNA of B-4476 (lane 4). A single band of 1 kb was detected with DNA of cosmid C5-3 (lane 5).
FIG. 3.
Southern hybridization with various probes to a CHEF panel of strains B-4476 and B-4500. Shown are the karyotype (A), hybridizations with the MATα probe (B) and the MATa probe (C) recently described by Chaturvedi et al. (5), and hybridizations with FUR (D) and MK (E) (see Materials and Methods).
The physical maps of all isolated cosmids covered about 71 kb of the B-4500 genome. To rule out the presence of artifacts which could have been generated during the cloning process, Southern blot analysis containing all the BamHI-digested overlapping cosmids and B-4500 genomic DNA was carried out. DNA fragments representing each BamHI fragment of the cosmid map were used as probes in these analyses. In each case, excluding the 1.3-kb fragment on the left side, we obtained a matching signal with the predicted size in both genomic and cosmid DNA (data not shown). Using the 1.3-kb fragment from the left border (Fig. 4, probe I), we detected a 1.6-kb band in the genomic DNA. These data indicated that the genomic sequence was truncated around the 1.3-kb region in the cosmid clone. Each BamHI fragment of the overlapping cosmids was subcloned, and both ends were sequenced. PCR primers designed adjacent to each BamHI site were used to amplify DNA across each BamHI site using cosmid and genomic DNA as template. Matching PCR fragments were obtained in all cases (data not shown). The arrangement of the BamHI fragments in the cosmids, therefore, is free from any artifact and reflects the actual position in the genome.
Localization of other mating type α strain-specific genes in the MATα locus.
After isolation of overlapping cosmids spanning the entire MATα locus, further analysis of the locus was made in relation to other α-specific genes. We characterized regions near all the BamHI sites in the map. Southern blottings were used to demonstrate the mating type specificity of each characterized region (Fig. 4). The positions of already known mating type-specific genes inside the locus were determined, and new genes were discovered. Sequence data suggested the existence of a sequence encoding a putative GTP-binding protein (accession no. AC019018) between the 1.3- and 6.5-kb BamHI fragments (Fig. 4A) located in the genomes of B-4500 and B-4476. From the HaeII-digested genomic DNA of B-4500 (Fig. 2), we predicted that there were three copies of the MFα gene in the MATα locus. By analyzing our cosmid clones, we located all three copies of the MFα genes. The previously reported MFα gene (16) was located on the 13-kb BamHI fragment (Fig. 4D) and was renamed MFα1 (accession no. S56460). The other two MFα genes were adjacent to each other and were located in the 17-kb BamHI fragment near the left boundary of the MATα locus (Fig. 4B). We designated these two genes MFα2/3. A putative eukaryotic translation initiation factor (accession no. J02674), a homolog of S. cerevisiae PRT1, was located between the 4.5- and 13-kb fragments (Fig. 4C). The STE11α gene (accession no. AF294841) was found (Fig. 4E) in the 13-kb fragment where MFα1 is located (Fig. 4D). The genes on the 21-kb BamHI fragment that have been thus far identified include a MATα-specific myosin (accession no. AF267642) gene (Fig. 4F) and STE20α (accession no. AF162330) (Fig. 4G). In the case of myosin, an additional band was detected in both MATα and MATa strains, under low stringency and washing conditions (Fig. 4F). We also identified the CPRα gene (accession no. AF259519), a homolog of the Coprinus cinereus pheromone receptor (Fig. 4H) located about 1 kb upstream of STE12α (accession no. AF012924) (Fig. 4I). The 5.5-kb fragment beyond the right border of the MATα locus contained an RNA polymerase gene (accession no. AF295125) (Fig. 4J). Therefore, the entire MATα locus of C. neoformans var. neoformans appears to span about 50 kb on chromosome 3 (27).
DISCUSSION
It is known for S. cerevisiae that STE20, STE11, and STE12, components of the MAP kinase pathway that signal the mating pheromone response, are also involved in filamentous morphogenesis in diploid as well as haploid cells (reviewed in reference 15). These genes are required for mating since mutations in these genes cause sterility. Unlike those in C. neoformans, however, the components of the S. cerevisiae MAP kinase pathway which respond to pheromone have no association with mating type. The link between mating type, virulence, and haploid (monokaryotic) fruiting in C. neoformans is unique to this species. It is even more unusual to find that some genes of the MAP kinase pathway are mating type specific. Recent studies have shown that STE12α (4) and STE11α (D. L. Clarke, U. Edman, G. L. Woodlee, C. M. McClelland, T. S. Seymour, J. C. Edman, and B. L. Wickes, unpublished data) play important roles in virulence in the mouse model. In order to clarify the physical relationship between the α-specific genes and the MATα locus, we cloned the entire MATα locus and constructed its physical map.
A previous report by Moore and Edman (16) indicated that the entire MATα locus may span between 35 and 70 kb and that one MFα gene (MFα1) was located within the locus. The present study revealed that the locus is approximately 50 kb in size. We detected 5 to 7 kb of flanking sequence at both ends of the locus which is shared between strains of MATα and MATa, reflecting the boundary of the MAT locus. The MATα locus harbored two additional MFα genes, MFα2 and MFα3, and a putative α-pheromone receptor, CPRα. The presence of the pheromone genes and pheromone receptor(s) in the mating type locus has been frequently recognized in several heterothallic fungi, such as C. cinereus, Schizophyllum commune, and Ustilago maydis (2, 17, 25).
The three homologs of the S. cerevisiae pheromone response MAP kinase signal transduction cascade genes, STE11α, STE12α, and STE20α, were found to be within a 24-kb region near MFα1. Between STE20α and STE11α, a MATα-specific sequence homologous to myosin was identified. The existence of MAP kinase signal transduction cascade genes, a mating type-specific gene of a molecular motor, and a translation initiation factor in a MAT locus have never been reported for any other fungi. These MAP kinase signal transduction cascade genes may play important roles in pathogenicity in addition to the mating of the fungus. In fact, a recent study of the role of STE12α suggested that this gene regulates virulence-associated genes, such as the CAP genes and CNLAC1 gene in serotype D strains of C. neoformans (4). What sets STE12α apart from STE12 of S. cerevisiae is that STE12α is not essential for mating but essential for haploid fruiting (4). Such a phenomenon was not expected since STE12α is part of the MATα locus. While STE12α is not essential for mating, ste12α mutants are reduced in mating efficacy by approximately 100-fold (4).
Characterization of the remaining MAP kinase signal transduction genes within the MATα locus should clarify whether STE12α functions in the same pathway as the other two kinases. Sequence analysis of the pheromone receptor which was found adjacent to STE12α showed a high degree of homology to seven previously characterized transmembrane pheromone receptors from other fungi (e.g., C. cinereus, U. maydis, S. commune) (2, 17, 25). The role of CPRα in mating as well as in cryptococcal pathogenicity is presently being characterized in our laboratory.
Discovery of MATα-specific myosin in the MAT locus is unprecedented and unexpected. Since myosin is an important protein for maintenance of morphological structure, this gene may be responsible for the ability to form hyphae during mating or during haploid fruiting, which is specific to mating type α strains. An additional smaller band was detected in both mating types with the myosin probe, perhaps due to cross-hybridization, since low-stringency conditions were used. These bands may suggest the presence of an additional non-mating type-specific myosin gene. Functional analysis of the mating type-specific myosin gene should reveal its role in morphogenesis.
The cloning of the complete MATα locus not only will allow us to analyze the function of remaining genes located within the locus, such as the translation initiation factor, but also will allow us to identify their counterparts in the MATa locus. A comparison of the genomic arrangement between the two loci may lead to a further understanding of the mating system and its role in pathobiology of C. neoformans. Our recent characterization of the a mating type-specific pheromone receptor gene, CPRa (M. Karos, Y. C. Chang, and K. J. Kwon-Chung, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. F76, 2000), and STE12a (Y. C. Chang and K. J. Kwon-Chung, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. F63, 1999) revealed that the relative arrangement of these two genes in the MATa locus is different from that of the MATα homologs (data not shown). It is known from the genomic arrangement of mating loci in other organisms that rearrangement of homologous genes in opposite mating types serves as a preventive measure for recombination or gene conversion, thus protecting the genetic organization of the locus (6). Another important feature of mating type loci is to make sure that the locus is inherited as a single intact unit in order to maintain heterothallism. In fact, conservation of mating type genes within and between species has been well documented for other heterothallic fungi, such as Pyrenopeziza brassicae and Tapesia yallundae, which are plant pathogenic discomycetes (23).
It is possible that an essential gene within the locus may ensure the stable inheritance of the locus. The PRT1 homolog, present in the C. neoformans MATα locus, may serve this function since this gene is essential in S. cerevisiae (8). If this assumption is correct, there must be a gene in the MATa locus which has the same function as the PRT1 gene. Analysis of the MATa locus will clarify this question.
The MATα locus in C. neoformans is one of the largest MAT loci among fungi with a one-locus, two-allele heterothallism. It is known that the MAT locus of Ustilago hordei, a bipolar fungus, is even larger, spanning a region of about 500 kb (14). Though bipolar in phenotype, the MAT locus of U. hordei, however, is composed of two distinct but closely linked loci, a and b. The a locus encodes mating-type specific pheromones (Uhmfa) as well as the pheromone receptors (Uhpra). The b locus is multiallelic and contains two divergently transcribed genes, bE (bEast) and bW (bWest). Furthermore, the b locus governs pathogenicity and completion of the life cycle. The a and b loci are separated by a spacer region in which recombination is probably suppressed, and thus, the bipolarity of the species is maintained (14). The MAT loci in U. maydis or S. commune are much more complex. U. maydis has, in contrast, a tetrapolar mating system because the a and b loci are on separate chromosomes and therefore segregate independently during meiosis (1), whereas S. commune has, in addition to its tetrapolar mating system, a multiallelic Bα locus (24). Therefore, the MATα locus in C. neoformans is not only different from MAT loci in these fungi in its organization but also is one of the largest known mating type loci. The presence of numerous mating type-specific genes in the MAT locus distinguishes C. neoformans from all other heterothallic fungi thus far investigated.
ACKNOWLEDGMENTS
We thank L. Penoyer for technical assistance.
B.L.W. is a Burroughs-Wellcome New Investigator in Molecular Pathogenic Mycology and is supported by U.S. Public Health Service Grant R29AI43522 from the National Institutes of Health.
REFERENCES
- 1.Bakkeren G, Kronstad J W. Conservation of the b mating-type gene complex among bipolar and tetrapolar smut fungi. Plant Cell. 1993;5:123–136. doi: 10.1105/tpc.5.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bölker M, Urban M, Kahmann R. The a mating type locus of U. maydis specifies cell signaling components. Cell. 1992;68:441–450. doi: 10.1016/0092-8674(92)90182-c. [DOI] [PubMed] [Google Scholar]
- 3.Chang Y C, Kwon-Chung K J. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol Cell Biol. 1994;14:4912–4919. doi: 10.1128/mcb.14.7.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang Y C, Wickes B L, Miller G F, Penoyer L A, Kwon-Chung K J. Cryptococcus neoformans STE12α regulates virulence but is not essential for mating. J Exp Med. 2000;191:871–882. doi: 10.1084/jem.191.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaturvedi S, Rodeghier B, Fan J, McClelland C M, Wickes B L, Chaturvedi V. Direct PCR of Cryptococcus neoformans MATα and MATa pheromones to determine mating type, ploidy, and variety: a tool for epidemiological and molecular pathogenesis studies. J Clin Microbiol. 2000;38:2007–2009. doi: 10.1128/jcm.38.5.2007-2009.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferris P J, Goodenough U W. The mating-type locus of Chlamydomonas reinhardtii contains highly rearranged DNA sequences. Cell. 1994;76:1135–1145. doi: 10.1016/0092-8674(94)90389-1. [DOI] [PubMed] [Google Scholar]
- 7.Greenleaf A L, Kelly J L, Lehman I R. Yeast RPO41 gene product is required for transcription and maintenance of the mitochondrial genome. Proc Natl Acad Sci USA. 1986;83:3391–3394. doi: 10.1073/pnas.83.10.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanic-Joyce P J, Singer R A, Johnston G C. Molecular characterization of the yeast PRT1 gene in which mutations affect translation initiation and regulation of cell proliferation. J Biol Chem. 1987;262:2845–2851. [PubMed] [Google Scholar]
- 9.Kwon-Chung K J, Bennet J E. Medical mycology. Philadelphia, Pa: Lea & Febiger; 1992. pp. 397–446. [Google Scholar]
- 10.Kwon-Chung K J, Bennett J E. Distribution of α and a mating types of Cryptococcus neoformans among natural and clinical isolates. Am J Epidemiol. 1978;108:337–340. doi: 10.1093/oxfordjournals.aje.a112628. [DOI] [PubMed] [Google Scholar]
- 11.Kwon-Chung K J, Edman J C, Wickes B L. Genetic association of mating types and virulence in Cryptococcus neoformans. Infect Immun. 1992;60:602–605. doi: 10.1128/iai.60.2.602-605.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon-Chung K J, Hill W B. Sexuality and pathogenicity of Filobasidiella neoformans (Cryptococcus neoformans) In: Vanbreuseghem R, De Vroey C, editors. Sexuality and pathogenicity of fungi. New York, N.Y: Masson; 1981. pp. 243–250. [Google Scholar]
- 13.Kwon-Chung K J, Lazera M, Chang Y, Kang B S. Is Cryptococcus neoformans evolving into an asexual organism? 1999. p. 93. , abstr. I-37. In Proceedings of the 4th International Conference on Cryptococcus and Cryptococcosis, London, United Kingdom. [Google Scholar]
- 14.Lee N, Bakkeren G, Wong K, Sherwood J E, Kronstad J W. The mating-type and pathogenicity locus of the fungus Ustilago hordei spans a 500-kb region. Proc Natl Acad Sci USA. 1999;96:15026–15031. doi: 10.1073/pnas.96.26.15026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madhani H D, Fink G R. The control of filamentous differentiation and virulence in fungi. Trends Cell Biol. 1998;8:348–353. doi: 10.1016/s0962-8924(98)01298-7. [DOI] [PubMed] [Google Scholar]
- 16.Moore T D, Edman J C. The α-mating type locus of Cryptococcus neoformans contains a peptide pheromone gene. Mol Cell Biol. 1993;13:1962–1970. doi: 10.1128/mcb.13.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Shea S F, Chaure P T, Halsall J R, Olesnicky N S, Leibbrandt A, Connerton I F, Casselton L A. A large pheromone and receptor gene complex determines multiple B mating type specificities in Coprinus cinereus. Genetics. 1998;148:1081–1090. doi: 10.1093/genetics/148.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pitkin J W, Panaccione D G, Walton J D. A putative cyclic peptide efflux pump encoded by the TOXA gene of the plant-pathogenic fungus Cochliobolus carbonum. Microbiology. 1996;142(Pt. 6):1557–1565. doi: 10.1099/13500872-142-6-1557. [DOI] [PubMed] [Google Scholar]
- 19.Rhodes N, Connell L, Errede B. STE11 is a protein kinase required for cell-type-specific transcription and signal transduction in yeast. Genes Dev. 1990;4:1862–1874. doi: 10.1101/gad.4.11.1862. [DOI] [PubMed] [Google Scholar]
- 20.Salas S D, Bennett J E, Kwon-Chung K J, Perfect J R, Williamson P R. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J Exp Med. 1996;184:377–386. doi: 10.1084/jem.184.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 22.Shuster J R. Mating-defective ste mutations are suppressed by cell division cycle start mutations in Saccharomyces cerevisiae. Mol Cell Biol. 1982;2:1052–1063. doi: 10.1128/mcb.2.9.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh G, Dyer P S, Ashby A M. Intra-specific and inter-specific conservation of mating-type genes from the discomycete plant-pathogenic fungi Pyrenopeziza brassicae and Tapesia yallundae. Curr Genet. 1999;36:290–300. doi: 10.1007/s002940050503. [DOI] [PubMed] [Google Scholar]
- 24.Wendland J, Kothe E. Allelic divergence at Bα1 pheromone receptor genes of Schizophyllum commune. FEMS Microbiol Lett. 1996;145:451–455. doi: 10.1111/j.1574-6968.1996.tb08615.x. [DOI] [PubMed] [Google Scholar]
- 25.Wendland J, Vaillancourt L J, Hegner J, Lengeler K B, Laddison K J, Specht C A, Raper C A, Kothe E. The mating-type locus B alpha 1 of Schizophyllum commune contains a pheromone receptor gene and putative pheromone genes. EMBO J. 1995;14:5271–5278. doi: 10.1002/j.1460-2075.1995.tb00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wickes B L, Edman U, Edman J C. The Cryptococcus neoformans STE12α gene: a putative Saccharomyces cerevisiae STE12 homologue that is mating type specific. Mol Microbiol. 1997;26:951–960. doi: 10.1046/j.1365-2958.1997.6322001.x. [DOI] [PubMed] [Google Scholar]
- 27.Wickes B L, Moore T D, Kwon-Chung K J. Comparison of the electrophoretic karyotypes and chromosomal location of ten genes in the two varieties of Cryptococcus neoformans. Microbiology. 1994;140(Pt. 3):543–550. doi: 10.1099/00221287-140-3-543. [DOI] [PubMed] [Google Scholar]
- 28.Yue C, Cavallo L M, Alspaugh J A, Wang P, Cox G M, Perfect J R, Heitman J. The STE12α homolog is required for haploid filamentation but largely dispensable for mating and virulence in Cryptococcus neoformans. Genetics. 1999;153:1601–1615. doi: 10.1093/genetics/153.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]