Abstract
In Enterococcus gallinarum SC1, a low-level vancomycin-resistant strain, only monomeric muropentapeptides with a C-terminal d-alanine were detected after growth without vancomycin. In contrast, in SC1 induced by vancomycin, as well as in AIB39, a constitutive vancomycin-resistant strain, monomeric and dimeric muropentapeptides with a C-terminal d-serine were detected.
VanC-type Enterococcus gallinarum is intrinsically resistant to low levels of vancomycin but not to teicoplanin (9). The basis of this resistance, which is inducible by vancomycin or constitutive (3, 9, 13, 14), is the replacement of d-Ala by d-Ser at the C-terminal position of the peptidoglycan precursor, which results in its lower affinity for vancomycin (3). E. gallinarum produces two ligases, Ddl and VanC1, which synthesize the dipeptides d-Ala–d-Ala and d-Ala–d-Ser, respectively (8, 13). Two additional enzymes are necessary for the expression of the resistance and the production of the precursor pentapeptide with a C-terminal d-Ser (1, 12, 13): VanT, a membrane-bound serine racemase which converts l-Ser to d-Ser (1), and VanXYc, a dd-dipeptidase which preferentially hydrolyzes the dipeptide d-Ala–d-Ala and the C terminus of the pentapeptide peptidoglycan precursor ending in d-Ala (12).
In this study we have analyzed the peptidoglycan structures of two nonisogenic E. gallinarum clinical isolates, one of which expresses the vancomycin resistance inducibly while the other expresses it constitutively.
Strains, susceptibility testing, and growth conditions.
Two clinical isolates of E. gallinarum were used in this study: SC1, an inducible vancomycin-resistant strain (16), and AIB39, a constitutively vancomycin-resistant strain (14). Cultures were grown at 37°C without shaking in brain heart infusion broth (5). The MICs of vancomycin on brain heart infusion agar for SC1 and AIB39 were 8 and 16 μg/ml, respectively.
Muropeptide composition of SC1 not induced by vancomycin.
Muropeptides were prepared from cell walls as described previously (4, 5) except that hydrofluoric acid was used during the peptidoglycan purification (6) and cellosyl (a generous gift from Hoechst) at 250 μg/ml was added to mutanolysin (Sigma, Saint Quentin Fallavier, France) at 250 μg/ml in phosphate buffer (25 mM, pH 6.5) containing MgCl2 (10 mM) during the hydrolysis step. The structures of the muropeptides were determined by mass spectrometry (MS) analysis (4, 10, 11). For some muropeptides the structures, deduced by MS, were confirmed by fragmentation using an MS-MS system performed on singly and doubly charged protonated molecules using argon as a collision gas (4, 11).
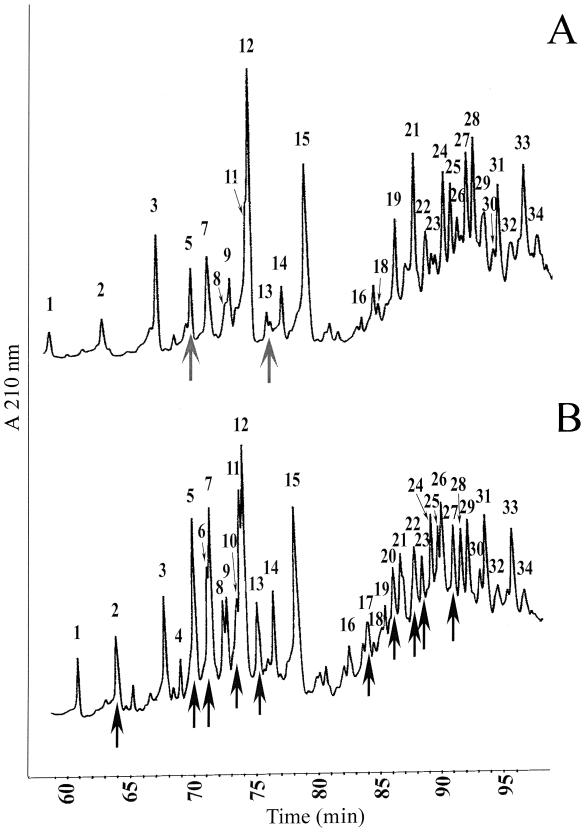
The peptidoglycan structure of the inducible vancomycin-resistant strain SC1 was first analyzed after growth in the absence of vancomycin. Under such conditions, this strain was previously shown to exclusively produce the cytoplasmic peptidoglycan precursor ending in d-Ala (3, 14). The high-performance liquid chromatography (HPLC) muropeptide profile is presented in Fig. 1A. The structures of muropeptides from 34 peaks (Table 1) were deduced from the molecular masses, the retention times, and coelution with other structures previously identified in Enterococcus faecium and Lactobacillus casei (4, 5, 7). The relative amounts of the muropeptides are expressed as percentages of all identified muropeptides (Table 1). Monomers (peaks 1 to 15) accounted for about 54.3% of all muropeptides, versus 45.7% for the dimers (peaks 16 to 34). The structures present in peaks 5 and 12 differed from those in peaks 1 and 3, respectively, by a molecular mass of +114 units, which indicates, as previously found for E. faecium and L. casei, the presence of one asparagine residue branched to the ɛ-amino group of the third lysine (4, 5, 11). The presence of a branched aspartate residue in peaks 4 and 9, instead of an asparagine residue, was deduced, since they eluted before peaks 5 and 12, respectively, and each differed by a molecular mass of +1 unit (5, 11). Peaks 2, 7, 8, 11, 14, and 15 eluted after peaks 1, 3, 4, 5, 9, and 12, respectively, and differed by an apparent molecular mass of −42 units, corresponding to the loss of the N-acetyl residue from the N-acetylglucosaminyl moiety of the disaccharide. Loss of this residue, which is thought to play a role in autolysis control (2), occurred in 64.9% of all muropeptides identified in SC1.
FIG. 1.
Separation of E. gallinarum cell wall muropeptides by reverse-phase HPLC. (A) Inducible strain SC1 not induced by vancomycin; (B) inducible strain SC1 induced by vancomycin. The arrows in panel A indicate the presence of pentapeptide/Ala; the arrows in panel B indicate the presence of pentapeptide/Ser.
TABLE 1.
Molecular masses and compositions of muropeptides from E. gallinarum
| Peak | Structurec | m/z (observed)a | % of all peaksb
|
|
|---|---|---|---|---|
| SC1 NI | SC1 I | |||
| Monomers | 54.3 | 59.4 | ||
| 1 | DS-tri | 826.1 | 1.4 | 2.1 |
| 2 | DS-tri (−42) | 784.1 | 2.2 | 2.7 |
| DS-penta/Ser | 984.5 | 0 | 0.5 | |
| 3 | DS-tetra | 897.5 | 5.9 | 4.1 |
| 4 | DS-D-tri | 941.5 | 0 | 0.9 |
| 5 | DS-N-tri | 940.4 | 2 | 5.4 |
| DS-penta/Ala | 968.4 | 0.4 | 0 | |
| DS-D-penta/Ser | 1,099.5 | 0 | 1 | |
| 6 | DS-N-penta/Ser | 1,098.6 | 0 | 3.3 |
| 7 | DS-tetra (−42) | 855.3 | 4.8 | 4.8 |
| 8 | DS-D-tri (−42) | 899.3 | 1.4 | 2.2 |
| 9 | DS-D-tetra | 1,012.5 | 2.8 | 2.2 |
| 10 | DS-D-penta/Ser (−42) | 1,057.6 | 0 | 3.3 |
| 11 | DS-N-tri (−42) | 898.4 | 5.8 | 5.7 |
| 12 | DS-N-tetra | 1,011.6 | 11.6 | 6.8 |
| 13 | DS-N-penta/Ala | 1,082.6 | 1 | 0 |
| DS-N-penta/Ser (−42) | 1,056.7 | 0 | 2.5 | |
| 14 | DS-D-tetra (−42) | 970.6 | 2.8 | 2.4 |
| 15 | DS-N-tetra (−42) | 969.0 | 12.2 | 9.5 |
| Dimers | 45.7 | 40.6 | ||
| 16 | Bis-DS-N-tetra-D-trid | 1,934.2 | 0.3 | 1.2 |
| 17 | Bis-DS-N-tetra-D-penta/Serd | 2,092.0 | 0 | 0.8 |
| 18 | Bis-DS-N-tetra-N-tri | 1,933.2 | 1.3 | 1 |
| 19 | Bis-DS-N-tetra-D-tetrad | 2,005.2 | 3.8 | 1.2 |
| 20 | Bis-DS-D-tetra-N-tri (−42)d | 1,892.2 | 0 | 2.8 |
| Bis-DS-D-tetra-N-penta/Ser (−42)d | 2,050.2 | 0 | 0.6 | |
| 21 | Bis-DS-N-tetra-N-tetra | 2,004.2 | 4.6 | 0.9 |
| Bis-DS-N-tetra-D-tri (−42)d | 1,892.0 | 1.6 | 3 | |
| 22 | Bis-DS-N-tetra-N-tri (−42) | 1,891.0 | 2.3 | 2.1 |
| Bis-DS-N-tetra-D-penta/Ser (−42)d | 2,049.8* | 0 | 1.3 | |
| 23 | Bis-DS-tetra-D-tetra (−42) | 1,849.2 | 0.5 | 0.8 |
| Bis-DS-N-tetra-N-penta/Ser (−42) | 2,049.2 | 0 | 1.0 | |
| 24 | Bis-DS-D-tetra-N-tetra (−42)d | 1,963.2 | 2.8 | 3.4 |
| 25 | Bis-DS-N-tetra-D-tetra (−42)d | 1,963.0 | 1.9 | 2.6 |
| 26 | Bis-DS-D-tetra-N-tri (−42)×2d | 1,850.2 | 0.8 | 3 |
| 27 | Bis-DS-N-tetra-N-tetra (−42) | 1,962.2 | 2.9 | 1.7 |
| Bis-DS-D-tetra-D-penta/Ser (−42)×2 | 2,009.8* | 0 | 0.3 | |
| 28 | Bis-DS-N-tetra-N-tetra (−42) | 1,962.3 | 3.3 | 1.8 |
| 29 | Bis-DS-D-tetra-N-tri (−42)×2d | 1,850.1 | 2.1 | 2.3 |
| 30 | Bis-DS-N-tetra-N-tri (−42)×2 | 1,849.2 | 0.7 | 1 |
| 31 | Bis-DS-D-tetra-D-tetra (−42)×2 | 1,922.2 | 3.4 | 2.8 |
| 32 | Bis-DS-D-tetra-N-tetra (−42)×2d | 1,921.4 | 2.2 | 0.9 |
| 33 | Bis-DS-N-tetra-D-tetra (−42)×2d | 1,921.2 | 8.3 | 3 |
| 34 | Bis-DS-N-tetra-N-tetra (−42)×2 | 1,920.6 | 2.9 | 1.1 |
[M+H]+ ion of the reduced muropeptide. The observed values were different from the calculated values by 0.3 or less, with only two exceptions marked by asterisks.
The values shown are percentages of the sums of the integrated areas of all peaks present in the table. SC1 NI, inducible strain not induced by vancomycin; SC1 I, inducible strain SC1 induced by vancomycin.
DS, disaccharide (GlcNAc-MurNAc); Bis-DS, dimeric form; di, dipeptide (l-Ala–d-iGln); tri, tripeptide (l-Ala–d-iGln–l-Lys); tetra, tetrapeptide (l-Ala–d-iGln–l-Lys–d-Ala); penta/Ala, pentapeptide (l-Ala–d-iGln–l-Lys–d-Ala–d-Ala); penta/Ser, pentapeptide (l-Ala–d-iGln–l-Lys–d-Ala–d-Ser); (−42), monomer without acetyl group on GlcNAc; (−42)×2, dimer without acetyl group on both GlcNAc of the dimer; D, aspartic acid; N, asparagine. DS- or bis-DS-containing pentapeptides with a C-terminal d-alanine or d-serine are shown in boldface.
Assignment of the D and N residues to either stem peptide is arbitrary.
Two monomeric disaccharide-pentapeptides with molecular masses compatible with a C-terminal d-alanine were identified (Table 1). One of the compounds present in peak 5, with an [M + H]+ of 968.4 (DS-penta/Ala), coeluted with an asparagine branched tripeptide (DS-N-tri). The other monomeric pentapeptide present in peak 13 had an [M + H]+ of 1,082.6, which differs by 114 units from that of the DS-penta/Ala, indicating the presence of an additional asparagine residue branched to the ɛ-amino group of the lysine. No molecular mass compatible with a disaccharide-pentapeptide ending in d-Ser (DS-penta/Ser) was detected.
All of the deduced dimeric structures showed an interpeptide bridge with an asparagine or an aspartate residue branched to the ɛ-amino group of the l-Lys3 of the acceptor. Muropeptides present in peaks 21 to 34 showed molecular mass differences of −42 or −84 units, corresponding to the mono- or bidesacetylated form of the muropeptides present in peaks 16 to 21. Overall, the peptidoglycan structure of E. gallinarum resembles that of E. faecium, with an A4α-type cross bridge (15).
Muropeptide compositions of SC1 induced by vancomycin and of the constitutively vancomycin-resistant strain AIB39.
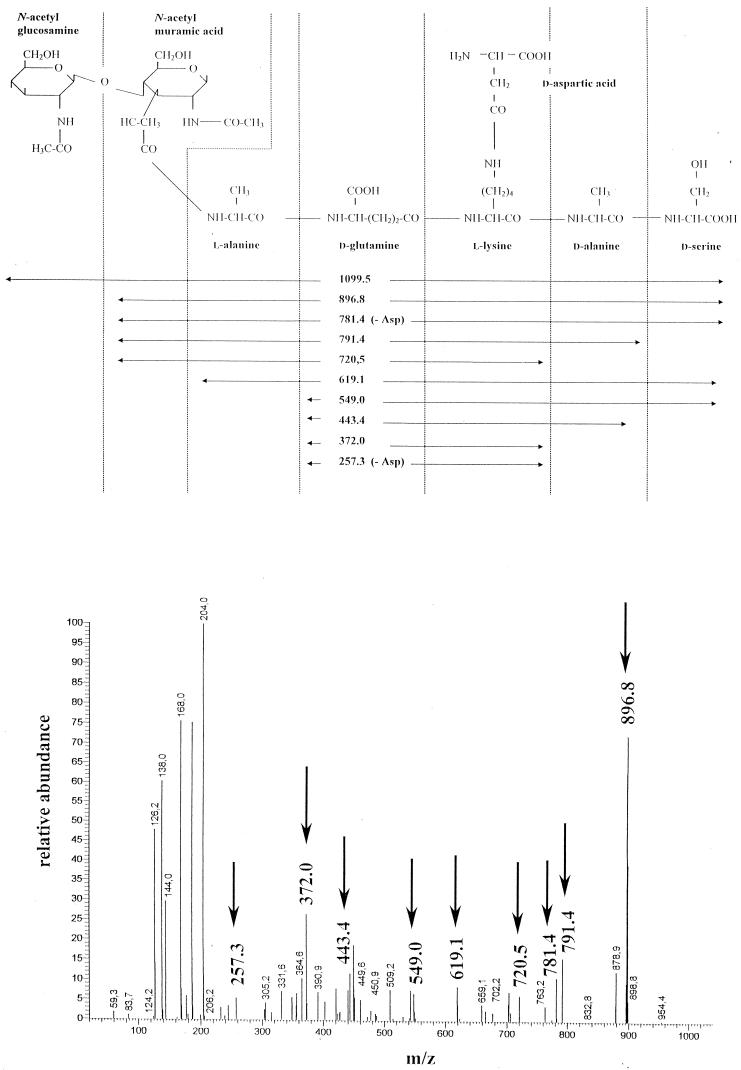
To determine the consequences of the expression of the metabolic pathway leading to vancomycin resistance on the peptidoglycan structure, and in particular the possible presence of muropeptides with a penta/Ser stem peptide, two sets of experiments were designed. First, the inducible SC1 strain was grown in the presence of 2 μg of vancomycin per ml, a concentration which allows the synthesis of the penta/Ser precursor (3). Second, to inhibit the penicillin-binding proteins (PBPs) with a dd-carboxypeptidase activity (4) which would convert muropeptide penta/Ser to murotetrapeptide, strain AIB39, which constitutively produces the penta/Ser precursor (14), was grown in the presence of penicillin (at one-fourth of the MIC). The profile and the relative amounts (percentages) of the muropeptides of SC1 induced by vancomycin are presented in Fig. 1B and Table 1. The profiles of the muropeptides of SC1 induced by vancomycin and AIB39 grown in the presence of penicillin (data not shown) were similar. In comparison with SC1 grown in the absence of vancomycin, a moderate increase in the amounts of monomers, from 54 to 59%, was observed for the two strains. The striking difference was the absence of penta/Ala monomers and the identification of new structures in peaks 2, 5, 6, 10, and 13 (Table 1), which corresponded to monomer penta/Ser with or without asparagine or aspartate branched on the stem peptide. MS-MS performed on all of these peaks confirmed the presence of a C-terminal serine. As an example, the MS-MS done on the structure with an [M + H]+ of 1,099.5 present in peak 5 (Fig. 2) showed that this muropeptide was a penta/Ser monomer with a d-aspartate branched on the ɛ-amino group of lysine (DS-d-penta/Ser).
FIG. 2.
Schematic representation of the MS-MS daughter spectrum of DS-D-penta/serine with an [M+H]+ ion at m/z 1,099.5.
Proportions of the different monomers and dimers present in the different strains.
For SC1 not induced by vancomycin, the monomer pentapeptides represented only 2.6% of the total monomers, while for SC1 induced by vancomycin and the constitutive strain AIB39, the monomer penta/Ser represented 17.8 and 30.9% of the total monomers, respectively. The increased proportion of muropeptide penta/Ser in AIB39 exposed to penicillin would relate to the inhibition of the penicillin-susceptible dd-carboxypeptidases, since examination of the HPLC profile of AIB39 peptidoglycan grown in the absence of penicillin showed that the proportion of monomer penta/Ser was comparable to that found in SC1 grown in the presence of vancomycin (data not shown). The absence of detectable monomer penta/Ala in SC1 induced by vancomycin and in AIB39 was explained by the elimination of the cytoplasmic precursors penta/Ala (references 3 and 13 and data not shown) due to the production of the VanXYc dipeptidase (12).
The presence of dimers, with penta/Ser stem peptides, detected in peaks 17, 20, 22, and 27, which account for 9.9 and 11.5% of the total dimers present in SC1 induced and AIB39, respectively, indicate that the dd-transpeptidases can use these penta/Ser pentapeptide monomers not only as donors but also as acceptors.
The relative proportion of monomer penta/Ala and tetrapeptides (2.6 and 73.8%, respectively) present in SC1 in the absence of induction compared to the proportion of monomer penta/Ser and tetrapeptides (17.8 and 50.2%, respectively) present after induction suggests that the PBPs with a dd-carboxypeptidase activity may not hydrolyze the monomer penta/Ser as well as the monomer penta/Ala.
Different proportions of monomeric and dimeric muropeptides containing tri- and tetrapeptides were observed in SC1 not induced or induced. In SC1 induced by vancomycin, the proportion of monomer tetrapeptides was decreased by 31%, while that of the monomer tripeptides was increased by 35%. This was associated with a 37.9% decreased proportion of tetrapeptide-tetrapeptide dimers and a very significant increased proportion of the tetrapeptide-tripeptide dimers (103%). This suggests that in these strains expressing the vancomycin resistance, monomeric tripeptides could substitute for monomeric tetrapeptides as acceptors by the dd-transpeptidases. However, a question as to the origin of the increased proportion of monomeric tripeptide remains. Finally, the specificity of transpeptidation could differ between the noninduced and induced strain SC1. In the absence of induction and in the presence of penta/Ala as a donor, the transpeptidases may well prefer a tetrastem peptide as an acceptor, while after induction, in the presence of the pentapeptide/Ser as a donor, they may well prefer a tristem peptide as an acceptor. If this is indeed the case, one could hypothesize that in the induced strain, some PBPs would preferentially be involved in the transpeptidation of the monomer penta/Ser donor, as suggested previously by the synergistic effect observed between vancomycin and some β-lactam antibiotics on strain SC1 (16).
Acknowledgments
We thank M. Arthur for critically reading the manuscript.
This work was supported by grants CRI 95060 and EMI 0004.
REFERENCES
- 1.Arias C A, Martin-Martinez M, Blundell T L, Arthur M, Courvalin P, Reynolds P E. Characterization and modelling of VanT: a novel, membrane-bound, serine racemase from vancomycin-resistant Enterococcus gallinarum BM4174. Mol Microbiol. 1999;31:1653–1664. doi: 10.1046/j.1365-2958.1999.01294.x. [DOI] [PubMed] [Google Scholar]
- 2.Atrih A, Bacher G, Allmaier G, Williamson M P, Foster S J. Analysis of peptidoglycan structure from vegetative cells of Bacillus subtilis 168 and role of PBP 5 in peptidoglycan maturation. J Bacteriol. 1999;181:3956–3966. doi: 10.1128/jb.181.13.3956-3966.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billot-Klein D, Gutmann L, Sable S, Guittet E, van Heijenoort J. Modification of peptidoglycan precursors is a common feature of the low-level vancomycin-resistant VANB-type Enterococcus strain D366 and of the naturally glycopeptide-resistant species Lactobacillus casei, Pediococcus pentosaceus, Leuconostoc mesenteroides, and Enterococcus gallinarum. J Bacteriol. 1994;176:2398–2405. doi: 10.1128/jb.176.8.2398-2405.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billot-Klein D, Legrand R, Schoot B, van Heijenoort J, Gutmann L. Peptidoglycan structure of Lactobacillus casei, a species highly resistant to glycopeptide antibiotics. J Bacteriol. 1997;179:6208–6212. doi: 10.1128/jb.179.19.6208-6212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billot-Klein D, Shlaes D, Bryant D, Bell D, van Heijenoort J, Gutmann L. Peptidoglycan structure of Enterococcus faecium expressing vancomycin resistance of the VanB type. Biochem J. 1996;313:711–715. doi: 10.1042/bj3130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jonge B L, Chang Y S, Gage D, Tomasz A. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain. The role of penicillin binding protein 2A. J Biol Chem. 1992;267:11248–11254. [PubMed] [Google Scholar]
- 7.de Jonge B L, Gage D, Handwerger S. Peptidoglycan composition of vancomycin-resistant Enterococcus faecium. Microb Drug Resist. 1996;2:225–229. doi: 10.1089/mdr.1996.2.225. [DOI] [PubMed] [Google Scholar]
- 8.Dutka-Malen S, Molinas C, Arthur M, Courvalin P. Sequence of the vanC gene of Enterococcus gallinarum BM4174 encoding a d-alanine:d-alanine ligase-related protein necessary for vancomycin resistance. Gene. 1992;112:53–58. doi: 10.1016/0378-1119(92)90302-6. [DOI] [PubMed] [Google Scholar]
- 9.Leclercq R, Dutka-Malen S, Duval J, Courvalin P. Vancomycin resistance gene vanC is specific to Enterococcus gallinarum. Antimicrob Agents Chemother. 1992;36:2005–2008. doi: 10.1128/aac.36.9.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mainardi J L, Billot-Klein D, Coutrot A, Legrand R, Schoot B, Gutmann L. Resistance to cefotaxime and peptidoglycan composition in Enterococcus faecalis are influenced by exogenous sodium chloride. Microbiology. 1998;144:2679–2685. doi: 10.1099/00221287-144-10-2679. [DOI] [PubMed] [Google Scholar]
- 11.Mainardi J L, Legrand R, Arthur M, Schoot B, van Heijenoort J, Gutmann L. Novel mechanism of beta-lactam resistance due to by-pass of dd-transpeptidation in Enterococcus faecium. J Biol Chem. 2000;275:16490–16496. doi: 10.1074/jbc.M909877199. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds P E, Arias C A, Courvalin P. Gene vanXYc encodes d,d-dipeptidase (VanX) and d,d-carboxypeptidase (VanY) activities in vancomycin-resistant Enterococcus gallinarum BM4174. Mol Microbiol. 1999;34:341–349. doi: 10.1046/j.1365-2958.1999.01604.x. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds P E, Snaith H A, Maguire A J, Dutka-Malen S, Courvalin P. Analysis of peptidoglycan precursors in vancomycin-resistant Enterococcus gallinarum BM4174. Biochem J. 1994;301:5–8. doi: 10.1042/bj3010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahm D F, Free L, Handwerger S. Inducible and constitutive expression of vanC-1-encoded resistance to vancomycin in Enterococcus gallinarum. Antimicrob Agents Chemother. 1995;39:1480–1484. doi: 10.1128/aac.39.7.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schleifer K H, Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972;36:407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincent S, Minkler P, Bincziewski B, Etter L, Shlaes D M. Vancomycin resistance in Enterococcus gallinarum. Antimicrob Agents Chemother. 1992;36:1392–1399. doi: 10.1128/aac.36.7.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]