Abstract
Objective
Multiple long-term conditions (MLTCs) are prevalent in rheumatoid arthritis (RA) and associated with worse outcomes and greater economic burden. However, little is known about the impact of MLTCs on the cost-of-illness (COI) in early RA, including direct and indirect costs. The objective of this study was to quantify this impact on COI.
Methods
The Scottish Early Rheumatoid Arthritis study is a national cohort of adults with new-onset RA. Direct costs were estimated applying relevant unit costs to health resource utilisation; indirect costs were measured by productivity loss due to health conditions. Two-part models were used, adjusting for age, gender, baseline functional disability and health-related quality of life. The Charlson Comorbidity Index score was calculated using ICD-10 diagnoses. Individuals were defined as ‘RA alone’, ‘RA plus LTC’ and ‘RA plus MLTCs’ according to the number of coexisting LTCs.
Results
Data were available for 818 participants. Average annualised direct costs incurred by people with early RA plus MLTCs (£4444; 95% CI £3100 to £6371) were twice as, and almost five times higher than, those with a single LTC (£2184; 95% CI £1596 to £2997) and those without LTC (£919; 95% CI £694 to £1218), respectively. Indirect costs incurred by RA plus MLTCs (£842; 95% CI £377to £1521) were 3.1 times higher than RA alone (£530; 95% CI £273to £854). The relative proportion of direct costs increased with LTC category, ranging from 77.2% to 84.1%. In addition to increased costs with LTCs, costs also increased with age and were higher for men regardless of LTC category.
Conclusions
MLTCs impact on COI early in the course of RA. The presence of LTCs is associated with significant increases in both direct and indirect costs among people with early RA.
Keywords: Arthritis, Rheumatoid; Economics; Epidemiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
While specific long-term conditions (LTCs) in established rheumatoid arthritis (RA) are known to incur additional healthcare costs, little is known about the impact of multiple LTCs (MLTCs) on the cost-of-illness in early RA, particularly for indirect costs.
WHAT THIS STUDY ADDS
Among people with early RA, people with MLTCs incurred direct costs almost five times higher and indirect costs three times higher than those with RA alone.
The six comorbidities highlighted in the European Alliance of Associations for Rheumatology RA recommendations are useful in characterising distinct MLTC burden and can serve as a potential framework when multiple data sources are available.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Future research is needed to develop validated methods to assess MLTCs and further understand the economic impact beyond direct medical costs and which clusters of LTCs contribute most to costs, and the impact of strategies to prevent or minimise MLTCs in RA.
Introduction
Traditionally, coexisting long-term conditions (LTCs) or comorbidity has been defined as the ‘existence or occurrence of any additional entity during the clinical course of a patient who has the index disease under study’.1 In contrast, multiple LTCs (MLTCs) have been defined as the coexistence of two or more LTCs in the same individual.2 The accumulation of LTCs within an individual is associated with worse outcomes.3 MLTCs are now an established priority for both research and clinical practice4 5 owing to the high prevalence of coexisting diseases among patients, particularly with ageing populations.
In the management of rheumatoid arthritis (RA), LTCs such as cardiovascular diseases, infections, gastrointestinal diseases, malignancies, osteoporosis and depression are prevalent and remain an important issue to consider.6 Having coexisting conditions in RA is associated with worse health and quality-of-life outcomes for patients.7–9 It has a significant negative impact on functional ability and all-cause mortality, independent of disease activity.10–13 MLTCs, therefore, need to be considered in strategies to optimise outcomes and minimise adverse outcomes and costs in RA.14 15
Existing studies have focused on the added economic burden associated with specific LTCs16 17 or selected LTCs in people with established RA.18–20 However, very little is known about the impact of MLTCs on costs in early RA. This study aims to describe and quantify the impact of MLTCs on the cost-of-illness (COI), including direct and indirect costs, for people with early RA. In addition, it evaluates variations in costs by age and gender using two validated comorbidity indices.
Methods
Data
The Scottish Early Rheumatoid Arthritis (SERA) study is a national multicentre, prospective inception cohort of people with newly diagnosed RA or undifferentiated arthritis. Participants were recruited from rheumatology departments in 20 hospitals across Scotland between September 2011 and April 2015.21 RA was clinically diagnosed by a rheumatologist and the participants selected for this COI study additionally met the 2010 American College of Rheumatology (ACR)/European Alliance of Associations for Rheumatology (EULAR) RA classification criteria22 at their baseline visit. Data were collected at baseline, 6 monthly intervals until year 2 and annually thereafter until year 5. Information on demographic characteristics, employment status, imaging and laboratory examinations was obtained during face-to-face study visits. Functional disability and health-related quality-of-life (HRQoL) were assessed by the Health Assessment Questionnaire Disability Index (HAQ-DI) and EuroQol-5 Dimensions-3 Level (EQ5D), respectively. SERA participants were asked to consent to linkage with their National Health Service (NHS) records for research purposes by the electronic Data Research and Innovation Service team (part of Public Health Scotland (PHS)). Socioeconomic status, measured by the Scottish Index of Multiple Deprivation (SIMD),23 was linked to participants’ records. SIMD quintiles reflect multiple deprivation ranked from the most (quintile 1) to the least deprived (quintile 5) areas in Scotland. Linked records were available from the start of recruitment (September 2011) up to November 2019.
LTCs were identified from hospital admission records through data linkage. The Charlson Comorbidity Index (CCI) is one of the most widely used comorbidity indices.24 The CCI score was calculated using the R-package comorbidity25 to identify relevant the International Classification of Diseases, Tenth Revision (ICD-10) codes in all hospital records throughout the follow-up period. Once a condition occurred, it was considered to be prevalent throughout the remainder of the follow-up. The number of LTCs was categorised into the following three distinct groups: ‘RA alone’, ‘RA plus single LTC’ and ‘RA plus MLTCs (>1 LTCs)’.
Estimation of COI
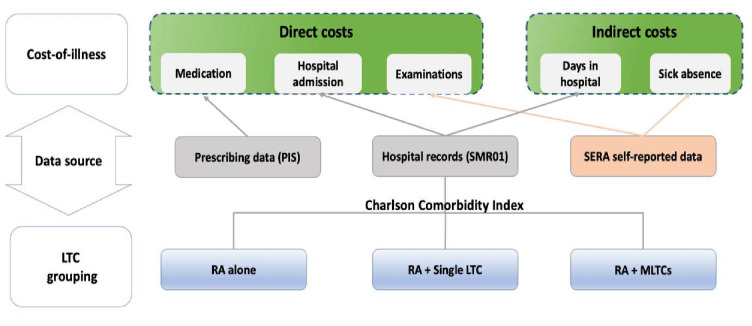
Average annualised direct and indirect costs per person were calculated, stratified by LTC group to describe the impact on COI. Data sources for estimating the COI and those used to define LTC groups are presented in figure 1. In this study, we intended to show the relative difference in magnitude of COI across LTC groups. Therefore, unit costs were derived from the most recent base year for prices of 2019–2020 for this COI.
Figure 1.
Data source for estimating cost-of-illness and MLTCs grouping. LTCs, long-term conditions; MLTCs, multiple LTCs, PIS, Prescription Information Service; RA, rheumatoid arthritis; SERA, Scottish Early Rheumatoid Arthritis; SMR01, Scottish Morbidity Records-general/acute inpatient daycase; ICD-10: International Classification of Diseases, Tenth Revision.
Direct costs
Direct costs were defined as health resource utilisation from the perspective of NHS Scotland, using a bottom-up microcosting approach. As the NHS in Scotland provides universal coverage, the linkage allows for the creation of a comprehensive data source relating to hospital admissions, community prescription encashment, cancer registry. Prescribing data were derived from the Prescription Information Service (PIS) database. The PIS database covers all NHS prescriptions prescribed, dispensed and reimbursed within the community setting. The quality of PIS data is guaranteed by an electronic system, which eliminates errors related to manual data entry processes.26 Costs for prescriptions in primary care, hospitalisations, imaging and laboratory examinations were included. Unit costs were obtained from the Scottish Drug Tariff published by PHS.27 Speciality-specific unit costs for hospitalisations (per bed day and day case) were obtained from PHS for the financial year 2019–2020.28 Mean unit costs for X-rays and blood tests were obtained from the same source (online supplemental table S1, unit costs).
rmdopen-2022-002454supp001.pdf (300.7KB, pdf)
Indirect costs
The human capital approach was applied, reflecting lost productive potential.29–31 Indirect costs were estimated from self-reported sickness absence of participants aged under 65 years. In addition, participants who were hospitalised were assumed to be absent from work for the duration of their hospital stay. Length of stay in hospital was added to self-reported sickness absence and multiplied by age and gender-specific average weekly wages (Office for National Statistics [ONS], table 6.1a, weekly pay rate, gross (£) for all employee jobs in the UK at 2020 prices) to generate indirect costs.32
Statistical analyses
Direct costs were estimated using a generalised linear model (GLM). Due to the skewness of cost data, a log-link function with a gamma distribution was chosen, rendering the data symmetric to evaluate effects on COI associated with RA. A two-part model (probit followed by GLM) was employed to estimate indirect costs accounting for zero values. The first part models the probability of incurring indirect costs different from zero, while the second part determines the level of indirect costs conditional on individuals incurring costs. Annualised total costs were calculated by combining direct and indirect costs and stratifying by LTC group. For all econometric models, ‘LTC group’ was the independent variable, with ‘RA alone’ used as the reference category. Participants’ age (updated each year of follow-up) and gender were adjusted for, with the youngest age group and male sex serving as the reference category. EQ5D responses were converted into utility values with UK tariffs using the R-package EQ5D.33 HAQ-DI and EQ5D scores at baseline were used as continuous measures to adjust for functional disability and HRQoL. Socioeconomic status was controlled for using SIMD quintiles, where the most deprived category was used as the reference category. Lastly, because treatment-related costs may differ over the RA disease course, follow-up period was included in the estimation of direct costs, with the first year for each participant as the reference category.
Sensitivity analyses
Indirect costs
The first sensitivity analysis was conducted due to the shortcoming of data availability of indirect costs. In SERA, primary data on sick leave were collected during nurse visits and only provided a snapshot of sickness absence in the week preceding the nurse visit. Therefore, we intended to validate our findings by using external data collected by a specifically designed health economics questionnaire (TIRA2).34 TIRA2 comprised 463 early RA patients recruited between 2006 and 2009 in Sweden, with comparable demographics to RA participants in SERA (TIRA2 vs SERA: 67% vs 65% of female; mean age: 58±14 vs 59±14 years). The number of days of sick leave was reported during all outpatient visits and hospital admissions. The same age-specific and gender-specific average weekly wages (ONS) were employed to generate indirect costs.
EULAR list of comorbidities
As the CCI is a generic measure, it may not capture all LTCs frequently found in people with RA, and only focuses on hospitalised morbidities. Therefore, sensitivity analysis was performed using an alternative LTC grouping algorithm, which consists of the six comorbidities highlighted in the EULAR recommendations for screening and managing chronic inflammatory rheumatic diseases.6 Comorbidities were selected based on frequency and severity (impact on mortality and disease outcome).
To construct the EULAR score, additional data sources were employed beyond hospital records, including prescribing and cancer registry data. For prescribing data, medications were assigned by therapeutic uses for the six comorbidities within relevant British National Formulary chapters (online supplemental table S2). Subsequently, the Proportion of Days Covered (PDC) approach was adopted to identify medications with a PDC over 0.8 in the last year of follow-up (1 December 2018–30 November 2019). For hospital records, relevant ICD-10 codes were assigned to related LTCs from all primary and secondary diagnostic codes throughout the follow-up period. In addition, the cancer registry35 was used to identify cancer patients. Finally, the prescribing, hospital and cancer records were combined to count the number of LTCs (online supplemental figure S1).
Results
The majority (68.8%) of the 818 participants with RA in SERA had no LTC, while 18% and 13.2% had a single LTC and MLTCs, respectively. Females accounted for 69.9% among the RA alone group; however, the proportion of females decreased with LTC category. More than a half of people with MLTCs lived in the most deprived areas (quintiles 1 and 2). Baseline functional ability and HRQoL at time of RA onset were already decreased with increasing LTC category (table 1). At baseline, individuals with RA plus MLTCs were older and less likely to report either paid or unpaid work than those with RA alone or with a single LTC.
Table 1.
Baseline demographics and clinical outcomes by LTC group at study recruitment
| RA alone | RA+single LTC | RA+MLTCs | |
| Participants | 563 | 147 | 108 |
| Age (years) | 56.4±13.4 | 63.3±12.6 | 67.6±9.9 |
| Age groups (n) | |||
| 45 and younger | 18.7% (120) | 6.1% (9) | 0% (0) |
| 45–54 | 25.3% (149) | 19.7% (29) | 15.7% (17) |
| 55–64 | 28.3% (153) | 25.2% (37) | 19.4% (21) |
| 65–74 | 19.9% (107) | 29.3% (43) | 43.5% (47) |
| Over 75 | 7.8% (34) | 19.7% (29) | 21.3% (23) |
| Gender (n) | |||
| Male | 30.1% (172) | 36.1% (53) | 47.2% (51) |
| Female | 69.9% (391) | 63.9% (94) | 52.8% (57) |
| SIMD (n) | |||
| 1 (most deprived) | 19.2% (107) | 18.4% (27) | 26.9% (29) |
| 2 | 21.3% (120) | 26.5% (39) | 25.9% (28) |
| 3 | 20.1% (113) | 17.0% (25) | 13.0% (14) |
| 4 | 22.7% (128) | 19.0% (28) | 24.1% (26) |
| 5 (least deprived) | 16.7% (94) | 17.7% (26) | 10.2% (11) |
| Missing | 0.2% (1) | 1.4% (2) | 0.0% (0) |
| HAQ-DI score | 1.21±0.78 | 1.31±0.79 | 1.41±0.84 |
| EQ5D score | 0.53±0.21 | 0.53±0.23 | 0.49±0.24 |
| Employment status* (n) | |||
| Paid work | 59.3% (334) | 39.5% (58) | 26.9% (29) |
| Unpaid work | 4.6% (26) | 3.4% (5) | 1.9% (2) |
| Retired | 29.1% (164) | 49.0% (72) | 66.7% (72) |
| Unemployment | 5.9% (33) | 8.2% (12) | 3.7% (4) |
| Student | 1.1% (6) | 0% (0) | 0.9% (1) |
Data are presented as mean±SD or % (n).
*Paid work includes full-time and part-time employment and self-employed; unpaid work refers to those answered ‘homemaker’.
EQ5D, EuroQol-5 Dimensions; HAQ-DI, Health Assessment Questionnaire Disability Index; LTC, long-term condition; MLTCs, multiple LTCs; RA, rheumatoid arthritis; SIMD, Scottish Index of Multiple Deprivation.
Average annualised cost per person, by LTC group
Regression results for direct and indirect costs are presented in online supplemental table S3 and S4, respectively. For direct costs, the coefficients indicate a gradual increase with advancing age. Participants living in the most deprived areas incurred the highest direct costs, followed by a gradual decrease with decreasing socioeconomic status. Direct costs in the subsequent years were higher compared with the index year. Overall, individuals having single and multiple LTCs were strongly associated with higher direct and indirect costs than RA alone.
Average annualised direct costs for all RA participants were estimated to be (£1636; 95% CI £1262 to £2121) (table 2). Among people with MLTCs, average annualised direct costs (£4444; 95% CI £3100 to £6367) were twice as high as in participants with a single LTC (£2184; 95% CI £1596 to £2997), and 4.8 times higher than in people with RA alone (£919; 95% CI £694 to £1218). Similarly, average annualised indirect costs increased with LTC category. People with RA plus MLTCs incurred average annualised indirect costs 3.1 times higher (£842; 95% CI £377 to £1521) than people with RA alone (£271; 95% CI £98 to £517) and 1.6 times the cost incurred by people with RA plus a single LTC.
Table 2.
Average annualised costs per person, by LTC group
| All | LTC group £ (95% CI) | |||||||
| Cost components | %* | RA alone | % | RA + single LTC |
% | RA + MLTCs |
% | |
| Direct costs | 1636 (1262 to 2121) |
81.9 | 919 (694 to 1218) |
77.2 | 2184 (1596 to 2997) |
80.5 | 4444 (3100 to 6371) |
84.1 |
| Indirect costs | 362 (123 to 728) |
18.1 | 271 (98 to 517) |
23.8 | 530 (273 to 854) |
19.5 | 842 (377 to 1,521) |
15.9 |
| Total costs† | 1998 | 100 | 1190 | 100 | 2714 | 100 | 5286 | 100 |
*% of the costs in that LTC category.
†Total costs were calculated by combining direct and indirect costs and stratifying by LTC group.
LTC, long-term condition; MLTCs, multiple LTCs; RA, rheumatoid arthritis.
Average total costs were highest for those with MLTCs, with direct costs accounting for 84.1%. For people with a single LTC and RA alone, 80.5% and 77.2% of total costs were attributable to direct costs, respectively.
Average annualised cost per person, by age group
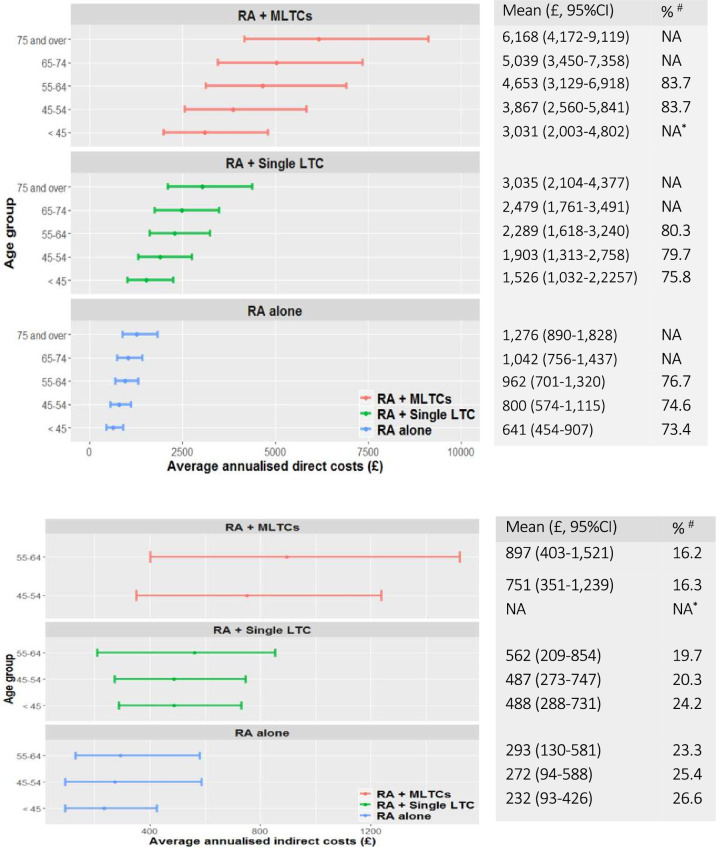
Average annualised direct and indirect costs by age group and LTC category are shown in figure 2A, B. Increasing LTC category was associated with increased average direct costs in age groups. Moreover, the proportion of direct costs gradually increased with age within each LTC category. Indirect costs were only captured for those under 65 years (below retirement age). There was no clear association between indirect costs and age in any LTC category (figure 2B).
Figure 2.
(A) Average annualised direct costs by age and LTC category. #The proportion of direct costs in total (direct and indirect) costs for those aged under 65 years within each age group; *No observation under 45 years was found within RA+MLTCs. (B) Average annualised indirect costs by age and LTC category. #The proportion of indirect costs in total (direct and indirect) costs for those aged under 65 years within each age group due to work issue; *No observation under 45 years was found within RA+MLTCs. LTC, long-term condition; MLTCs, multiple LTC; RA, rheumatoid arthritis.
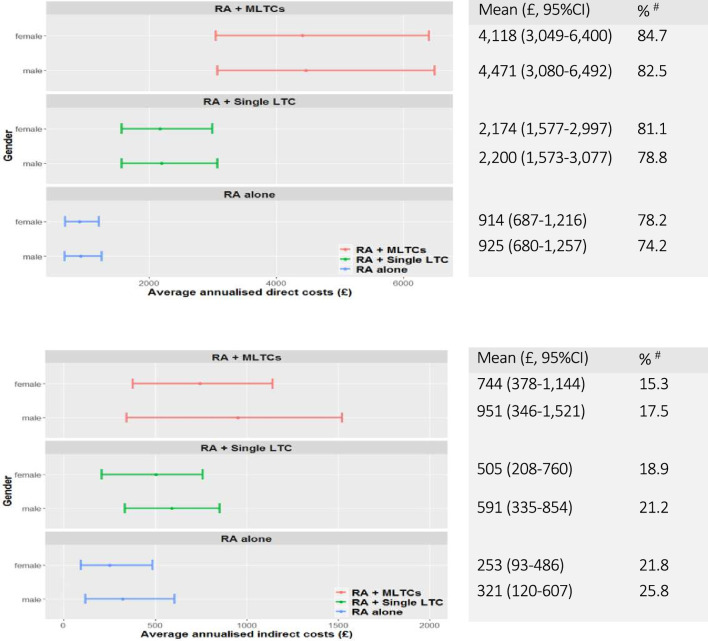
Average annualised cost per person, by gender
Figure 3A, B shows average annualised direct and indirect costs by gender. Direct costs were comparable between men and women, while indirect costs incurred by men were slightly higher than women regardless of LTC category.
Figure 3.
(A) Average annualised direct costs per person by gender and LTC category. #The proportion of direct costs in total (direct and indirect) costs within each gender group. (B) Average annualised indirect costs per person by gender and LTC category. #The proportion of indirect costs in total (direct and indirect) costs within each gender group. LTC, long-term condition; MLTCs, multiple LTC; RA, rheumatoid arthritis.
Sensitivity analyses
On average, 55.1 days for men and 34.5 days for women of sick leave were reported in TIRA2. Estimated average annualised total costs were £6206 for all participants when combining direct and indirect costs, of which 73.6% were attributable to indirect costs.
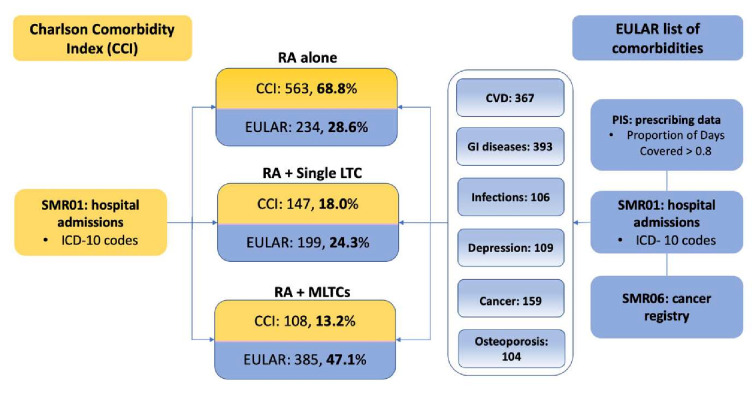
Using the EULAR list of LTCs, 28.6% of participants were recorded with RA alone, 24.3% had a single LTC, and 47.1% were categorised as RA plus MLTCs (figure 4). Average annualised direct and indirect costs increased with LTC category using the EULAR list of comorbidities (table 3). Although monetary values were substantially lower across all categories compared with the CCI grouping, average annualised direct costs incurred by people with MLTCs were 2.4 and 4.6 times higher than RA plus a single LTC and RA alone, respectively. In contrast, indirect costs incurred by those with RA plus MLTCs were 3.1 times higher than in those with RA alone.
Figure 4.
LTC groups adopting the CCI and EULAR list of comorbidities. CVD, cardiovascular diseases; EULAR, European Alliance of Associations for Rheumatology; GI, gastrointestinal; LTC, long-term conditions; MLTCs, multiple LTCs; PIS, Prescription Information Service; RA, rheumatoid arthritis; SMR01, SMR01, Scottish Morbidity Records.
Table 3.
Average annualised costs per person, by LTC group (EULAR)
| LTC group £ (95% CI) | ||||||
| Cost components | RA alone | %† | RA + Single LTC |
%† | RA + MLTCs |
%† |
| Direct costs | 539 (396 to 735) |
75.6 | 1027 (749 to 1,410) |
75.6 | 2491 (1,871 to 3,316) |
82.3 |
| Indirect costs | 174 (68 to 378) |
24.4 | 332 (150 to 639) |
24.4 | 537 (266 to 946) |
17.7 |
| Total costs* | 713 | 100 | 1359 | 100 | 3028 | 100 |
*Total costs were calculated by combining direct and indirect costs and stratifying by LTC group
†% of the costs in that LTC category.
EULAR, European Alliance of Associations for Rheumatology; LTC, long-term condition; MLTCs, multiple LTC; RA, rheumatoid arthritis.
Annualised direct and indirect costs across age groups and gender using the alternative EULAR grouping approach are presented in online supplemental figures S2 and S3. For direct costs, increasing LTC category was associated with increased average direct costs, in particular for those aged over 75 years. Narrower 95% CIs were found across age groups and LTC categories compared with the CCI grouping. Similarly, men incurred higher indirect costs than women, regardless of LTC category.
Discussion
In this study, we quantified the economic impact of a single LTC and MLTCs on the COI in people with newly diagnosed RA. Our findings show that total annualised direct and indirect costs increased with increasing LTC category. Average annualised direct costs incurred by people with early RA plus MLTCs were twice and 4.8 times higher than in people with RA plus a single LTC and RA alone, respectively. Indirect costs incurred by people with RA plus MLTCs were 3.1 times higher than in people with RA alone. In addition to increased costs with LTC, costs also generally increased with age and were higher for men, regardless LTC group.
Average annualised total costs in this study (direct costs: £1636; 95% CI £1262 to £2121; indirect costs: £362; 95% CI £123 to £728) were lower than those reported in other recent studies, ranging from £2987 to £3742 for people with established RA.36–38 In addition to differences in countries and health systems, the discrepancy may be due to data availability for cost items. Moreover, our study focused on early RA, when costs may be different to established disease. Direct costs for outpatient visits could not be estimated as outpatient records were unavailable. Second, prescribing costs were only available for primary care, which will include most prescriptions, including conventional synthetic disease-modifying antirheumatic drugs (DMARDs), but not biological therapies as information on medication prescribed in the hospital setting was unavailable. In line with national guidelines and standard clinical practice, patients with RA have to fail at least two conventional synthetic DMARDs before starting a biological DMARD. As SERA is an inception cohort, all participants started on conventional DMARDs21 prescribed in primary care, with only 8% of participants receiving biologics after a mean follow-up period of 18 months.39 This could lead to an underestimation of absolute costs across LTC groups since biologics are much more expensive than conventional DMARDs.
Published studies investigating the added economic burden of selected LTCs in RA used varying approaches for categorising LTC group. For example, one study assessed the difference in direct costs between the presence and absence of LTCs,20 while other studies have been more granular in their categories, for example, 1–25 LTCs; groups of 0/1–2/3–4/≥5 LTCs; groups of 0/1/2/3/≥4 LTCs.18 19 40 However, except for one study investigating the impact of MLTCs on absenteeism, presenteeism and employment status,40 the existing evidence is limited to direct medical costs in established RA. Our study evaluated and quantified the impact of MLTCs on both direct and indirect costs in people with early RA. Compared with indirect costs, direct costs increased more substantially with LTC group. In addition to increased costs with LTC, there appeared to be more effect of increasing age associated with the category of LTC in direct costs, while there was no clear association with age for indirect costs. As a result, the proportion of direct costs increased with age within each LTC group. Nevertheless, wide confidence intervals were observed in particular for those with MLTCs, suggesting that the level of MLTCs (eg, severity and number of LTCs) was very heterogeneous within this group.
Indirect costs in the main analysis accounted for 18.1% of total costs for all participants under 65 years. While different sick leave regulations in Sweden may impact on the results, 73.6% of total costs were attributable to indirect costs when adopting external data on sickness absence from the TIRA2 study. In this study, indirect costs were unlikely to be truly representative as we were using routine data with no detailed health economic questionnaire, our findings give an indication of the relative impact of LTC and MLTCs in early RA, using two measures of LTCs. Both the CCI and EULAR grouping showed that indirect costs incurred by patients with MTLCs were approximately two to three times higher than in those with a single LTC and RA alone.
Using the CCI, 68.8% of study participants were categorised as RA alone, while 18% had a single LTC and 13.2% had MLTCs. Comorbidity rates reported in the literature are between 60% and 75%,41–43 and on average people with RA have 1.8 to 2.3 additional conditions, although these were in people with established RA.10 12 Generic comorbidity measures such as CCI are easy to use and compare across disease areas, but some diseases might be underrepresented due to the focus on hospitalisations. In contrast, 47.1% of RA participants in SERA had MLTCs using the targeted six item EULAR list, with 24.3% having a single LTC and 28.6% RA alone, which are more comparable with existing literature. The excess costs across LTC categories and higher proportion of direct costs when using the CCI may indicate that participants were more ill, as the CCI is driven by hospitalisation data. However, a similarly increasing direct and indirect costs trend with LTC group was consistent when using either CCI or the EULAR grouping. The six EULAR comorbidities can easily be used to give an estimate of the MLTC burden, and therefore the impact on costs and outcomes, and can also serve as a potential framework to consider for future studies in RA when detailed data on all LTCs is not available.
A major strength of our study is the linkage between routinely collected healthcare records to a representative RA inception patient registry in Scotland to conduct an analysis that neither data source alone could accommodate. More importantly, our findings demonstrate that the impact of LTCs on the COI occurs early in the disease course, when there may still be opportunities to intervene. A limitation of our study lies in the potential underestimation of the COI due to data availability for outpatient attendance and hospital prescribing. In line with national treatment guidelines, patients with a new diagnosis of RA are treated with conventional DMARDs which are captured by standard prescribing datasets, so the latter is more relevant in later disease when biological DMARDs are more likely to be prescribed by the hospital; future linkage to hospital and homecare prescribing datasets would capture this. Furthermore, only absenteeism was included in indirect costs; other societal costs such as presenteeism, informal care, impact on unpaid work or caregiver were not included as these require a dedicated health economic questionnaire to collect. Although the evolution of routinely collected electronic data within care services provides new opportunities for collecting data without burdening patients or caregivers, self-reported methods will still be required when a societal perspective is required for the intended analysis.44 Moreover, there is still a need to improve the methods for collecting, measuring and valuing indirect costs. Lastly, we do not have the data on RA phenotype/severity so we cannot say whether the extra costs of illness found in the groups with one or more comorbidities are due to a more severe RA phenotype. Other limitations are primarily inherent to the nature of administrative data, such as missing records or incomplete data.
Conclusions
In people with early RA, those with MLTCs incurred higher direct and indirect costs than those with RA alone. The findings provide additional support for the importance of early and active screening and early intervention to mitigate or prevent the progression of MLTCs in people with RA. Future research is needed for developing validated methods to assess MLTCs and further understand the economic impact beyond direct medical costs and which clusters of LTCs contribute most to costs, and the impact of strategies to prevent or minimise MLTCs in early RA.
Acknowledgments
This study is based on a PhD thesis (https://theses.gla.ac.uk/82985/1/2022HsiehPhD.pdf). The authors acknowledge the invaluable contribution of all participants, clinical and nursing colleagues who have contributed their time and support to the SERA study. In particular, the authors would like to thank Prof Duncan Porter, Caron Patterson, Fraser Morton and Allen Tervit (RCB) for their advice and technical support to access the data. "SERA Investigators: Cosimo de Bari, University of Aberdeen; Margaret Duncan, Ayr Hospital; Carl Goodyear, University of Glasgow; Lisa Hutton, Inverclyde Royal Hospital; John Harvie, Forth Valley; Vinod Kumar, Ninewells Hospital, Dundee; Iain McInnes*, University of Glasgow; Mike McMahon, Dumfries & Galloway Royal Infirmary; Robin Munro, Wishaw General Hospital; John Larkin, Victoria Infirmary Glasgow; Neil McKay, Western General Hospital, Edinburgh; John McLaren, Whyteman's Brae Hospital, Fife; David M Reid, Aberdeen Royal Infirmary; Duncan Porter*, Gartnavel General Hospital, Glasgow; Stuart Ralston, University of Edinburgh; Ruth Richmond, Borders General Hospital, Melrose; Gillian Roberts, Vale of Leven Hospital; Sarah Saunders, Glasgow Royal Infirmary; Anne McEntegart, Stobhill Hospital, Glasgow. Study management team: Caron Paterson, Jane Hair, Sharon Kean ".
Footnotes
Contributors: Conception and design: PH-H, OW, CG and EM. Analysis and interpretation of data: PH-H, OW, CG, EM and SS. Acquisition of data: PH-H and CG. First draft of the manuscript: PH-H. Critical revisions of the manuscript for important intellectual content: CG, OW, EM and SS. PH-H is responsible for the overall content as guarantor. The guarantor accepts full responsibility for the finished work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: ICMJE disclosure forms uploaded during the submission process.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. The SERA outcome data are stored and managed by the University of Glasgow and are available for analysis by bona fides external researchers. Applications to access the resources are reviewed by the SERA Access Committee and application details are available on the SCAR website (www.scarnetwork.org).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Scottish Early Rheumatoid Arthritis (SERA) study was approved by the West of Scotland Research Ethics Committee 4 (reference 10/S0704/20). This study was approved by the SERA Steering Committee (applicant number: 2018082901). Participants gave informed consent to participate in the study before taking part.
References
- 1.Feinstein AR. The pre-therapeutic classification of co-morbidity in chronic disease. J Chronic Dis 1970;23:455–68. 10.1016/0021-9681(70)90054-8 [DOI] [PubMed] [Google Scholar]
- 2.van den Akker M, Buntinx F, Knottnerus JA. Comorbidity or multimorbidity: what’s in a name? A review of literature. Eur J Gen Pract 1996;2:65–70. [Google Scholar]
- 3.MacMahon S, et al. Multimorbidity: a priority for global health research. 127. London, UK: The Academy of Medical Sciences, 2018. [Google Scholar]
- 4.Whitty CJM, MacEwen C, Goddard A, et al. Rising to the challenge of multimorbidity. BMJ 2020:l6964. 10.1136/bmj.l6964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NICE . Multimorbidity: clinical assessment and management, 2016. Nice guideline. Available: https://www.nice.org.uk/guidance/ng56/resources/multimorbidity-clinical-assessment-and-management-1837516654789
- 6.Baillet A, Gossec L, Carmona L, et al. Points to consider for reporting, screening for and preventing selected comorbidities in chronic inflammatory rheumatic diseases in daily practice: a EULAR initiative. Ann Rheum Dis 2016;75:965–73. 10.1136/annrheumdis-2016-209233 [DOI] [PubMed] [Google Scholar]
- 7.Nurmohamed MT, Heslinga M, Kitas GD. Cardiovascular comorbidity in rheumatic diseases. Nat Rev Rheumatol 2015;11:693–704. 10.1038/nrrheum.2015.112 [DOI] [PubMed] [Google Scholar]
- 8.Peters MJL, Symmons DPM, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. 69, 2010: 325–31. 10.1136/ard.2009.113696 [DOI] [PubMed] [Google Scholar]
- 9.Kanis JA, Burlet N, Cooper C, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporosis International 2008;19:1103–4. 10.1007/s00198-008-0599-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norton S, Koduri G, Nikiphorou E, et al. A study of baseline prevalence and cumulative incidence of comorbidity and extra-articular manifestations in RA and their impact on outcome. Rheumatology 2013;52:99–110. 10.1093/rheumatology/kes262 [DOI] [PubMed] [Google Scholar]
- 11.Radner H, Smolen JS, Aletaha D. Impact of comorbidity on physical function in patients with rheumatoid arthritis. Ann Rheum Dis 2010;69:536–41. 10.1136/ard.2009.118430 [DOI] [PubMed] [Google Scholar]
- 12.Michaud K, Wolfe F. Comorbidities in rheumatoid arthritis. Best Pract Res Clin Rheumatol 2007;21:885–906. 10.1016/j.berh.2007.06.002 [DOI] [PubMed] [Google Scholar]
- 13.McQueenie R, Nicholl BI, Jani BD, et al. Patterns of multimorbidity and their effects on adverse outcomes in rheumatoid arthritis: a study of 5658 UK Biobank participants. BMJ Open 2020;10:e038829. 10.1136/bmjopen-2020-038829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radner H, Yoshida K, Smolen JS, et al. Multimorbidity and rheumatic conditions-enhancing the concept of comorbidity. Nat Rev Rheumatol 2014;10:252–6. 10.1038/nrrheum.2013.212 [DOI] [PubMed] [Google Scholar]
- 15.Dey M, Busby A, Elwell H, et al. The use and context of the term 'multimorbidity' in rheumatoid arthritis: a systematic literature review. Rheumatology 2021;60:3058–71. 10.1093/rheumatology/keab214 [DOI] [PubMed] [Google Scholar]
- 16.Joyce AT, Smith P, Khandker R, et al. Hidden cost of rheumatoid arthritis (rA): estimating cost of comorbid cardiovascular disease and depression among patients with RA. J Rheumatol 2009;36:743–52. 10.3899/jrheum.080670 [DOI] [PubMed] [Google Scholar]
- 17.Hitchon CA, Walld R, Peschken CA, et al. Impact of psychiatric comorbidity on health care use in rheumatoid arthritis: a population-based study. Arthritis Care Res 2021;73:90–9. 10.1002/acr.24386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han G-M, Han X-F. Comorbid conditions are associated with healthcare utilization, medical charges and mortality of patients with rheumatoid arthritis. Clin Rheumatol 2016;35:1483–92. 10.1007/s10067-016-3277-y [DOI] [PubMed] [Google Scholar]
- 19.An J, Nyarko E, Hamad MA. Prevalence of comorbidities and their associations with health-related quality of life and healthcare expenditures in patients with rheumatoid arthritis. Clin Rheumatol 2019;38:2717–26. 10.1007/s10067-019-04613-2 [DOI] [PubMed] [Google Scholar]
- 20.Osiri M, Sattayasomboon Y. Prevalence and out-patient medical costs of comorbid conditions in patients with rheumatoid arthritis. Joint Bone Spine 2013;80:608–12. 10.1016/j.jbspin.2013.01.013 [DOI] [PubMed] [Google Scholar]
- 21.Dale J, Paterson C, Tierney A, et al. The Scottish early rheumatoid arthritis (sera) study: an inception cohort and Biobank. BMC Musculoskelet Disord 2016;17:461. 10.1186/s12891-016-1318-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funovits J, Aletaha D, Bykerk V, et al. The 2010 American College of Rheumatology/European League against rheumatism classification criteria for rheumatoid arthritis: methodological report phase I. Ann Rheum Dis 2010;69:1589–95. 10.1136/ard.2010.130310 [DOI] [PubMed] [Google Scholar]
- 23.Scottish index of multiple deprivation, 2020. Available: https://www.gov.scot/collections/scottish-index-of-multiple-deprivation-2020/ [Accessed cited 2020 January].
- 24.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 25.Gasparini A. Comorbidity: an R package for computing comorbidity scores. J Open Source Softw 2018;3:648. 10.21105/joss.00648 [DOI] [Google Scholar]
- 26.Alvarez-Madrazo S, McTaggart S, Nangle C, et al. Data resource profile: the Scottish national prescribing information system (PIs). Int J Epidemiol 2016;45:714–5. 10.1093/ije/dyw060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scottish drug tariff, 2020. Available: https://www.isdscotland.org/health-topics/prescribing-and-medicines/scottish-drug-tariff/ [Accessed cited 2020 October].
- 28.Reports for financial year 2019 to 2020, 2020. Available: https://beta.isdscotland.org/topics/finance/file-listings-fy-2019-to-2020/
- 29.Rice DP. Estimating the cost of illness. Am J Public Health Nations Health 1967;57:424–40. 10.2105/AJPH.57.3.424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodgson TA, Meiners MR. Cost-of-Illness Methodology: A Guide to Current Practices and Procedures. Milbank Memorial Fund quarterly. Health and society 1982;60:429–62. [PubMed] [Google Scholar]
- 31.Koopmanschap MA, Rutten FF, van Ineveld BM, et al. The friction cost method for measuring indirect costs of disease. J Health Econ 1995;14:171–89. 10.1016/0167-6296(94)00044-5 [DOI] [PubMed] [Google Scholar]
- 32.Employee earnings in the UK: 2020, 2020. Available: https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/bulletins/annualsurveyofhoursandearnings/2020 [Accessed cited 2020 November].
- 33.Fraser, M. and S.N. Jagtar . Calculating EQ-5D indices and summarising profiles with eq5d, 2021. Available: https://cran.r-project.org/web/packages/eq5d/vignettes/eq5d.html [Accessed cited 2022 February].
- 34.Hallert E, Husberg M, Kalkan A, et al. Rheumatoid arthritis is still expensive in the new decade: a comparison between two early RA cohorts, diagnosed 1996-98 and 2006-09. Scand J Rheumatol 2016;45:371–8. 10.3109/03009742.2015.1126344 [DOI] [PubMed] [Google Scholar]
- 35.Information Services Division, N.S.S. Scottish Cancer Registry cited 2021 February]; Available from:. Available: https://www.isdscotland.org/Health-Topics/Cancer/Scottish-Cancer-Registry/
- 36.Leon L, et al. Direct medical costs and its predictors in EMAR-II cohort. 29. Reumatologia Clinica., 2016. [DOI] [PubMed] [Google Scholar]
- 37.Barnabe C, Thanh NX, Ohinmaa A, et al. Healthcare service utilisation costs are reduced when rheumatoid arthritis patients achieve sustained remission. Ann Rheum Dis 2013;72:1664–8. 10.1136/annrheumdis-2012-201918 [DOI] [PubMed] [Google Scholar]
- 38.Ohinmaa AE, Thanh NX, Barnabe C, et al. Canadian estimates of health care utilization costs for rheumatoid arthritis patients with and without therapy with biologic agents. Arthritis Care Res 2014;66): :1319–27. 10.1002/acr.22293 [DOI] [PubMed] [Google Scholar]
- 39.Fragoulis GE, Paterson C, Gilmour A, et al. Neutropaenia in early rheumatoid arthritis: frequency, predicting factors, natural history and outcome. RMD Open 2018;4:e000739. 10.1136/rmdopen-2018-000739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bradshaw A, Bosworth A, Walker-Bone K, et al. SAT0072 the impact of comorbidities on absenteeism, presenteeism and employment status in people living with rheumatoid arthritis. Ann Rheum Dis 2020;79:970.2–1. 10.1136/annrheumdis-2020-eular.5467 [DOI] [Google Scholar]
- 41.Grøn KL, Ornbjerg LM, Hetland ML. The association of fatigue, comorbidity burden, disease activity, disability and gross domestic product in patients with rheumatoid arthritis. results from 34 countries participating in the Quest-RA program. Clin Exp Rheumatol 2014;32:869. [PubMed] [Google Scholar]
- 42.Luque Ramos A, et al. , Comorbidities in Patients with Rheumatoid Arthritis and Their Association with Patient-reported Outcomes . Results of claims data linked to questionnaire survey. The Journal of Rheumatology 2019;46:564. [DOI] [PubMed] [Google Scholar]
- 43.Espiño-Lorenzo P, Manrique-Arija S, Ureña I, et al. Baseline comorbidities in patients with rheumatoid arthritis who have been prescribed biological therapy: a case control study. Reumatol Clin 2013;9:18–23. 10.1016/j.reuma.2012.05.012 [DOI] [PubMed] [Google Scholar]
- 44.Franklin M, Thorn J. Self-Reported and routinely collected electronic healthcare resource-use data for trial-based economic evaluations: the current state of play in England and considerations for the future. BMC Med Res Methodol 2019;19:8. 10.1186/s12874-018-0649-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsieh PH. Improving the estimation of cost-of-illness in rheumatoid arthritis. University of Glasgow, 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002454supp001.pdf (300.7KB, pdf)
Data Availability Statement
Data are available on reasonable request. The SERA outcome data are stored and managed by the University of Glasgow and are available for analysis by bona fides external researchers. Applications to access the resources are reviewed by the SERA Access Committee and application details are available on the SCAR website (www.scarnetwork.org).