Abstract
Objective
One of the most important risk factors for developing a glaucomatous optic neuropathy is elevated intraocular pressure. Moreover, mechanisms such as altered perfusion have been postulated to injure the optical path. In a mouse model, we compare first negative effects of cerebral perfusion/reperfusion on the optic nerve structure versus alterations by elevated intraocular pressure. Second, we compare the alterations by isolated hypoperfusion-reperfusion and isolated intraocular pressure to the combination of both.
Methods and analysis
Mice were divided in four groups: (1) controls; (2) perfusion altered mice that underwent transient bi-common carotid artery occlusion (BCCAO) for 40 min; (3) glaucoma group (DBA/2J mice); (4) combined glaucoma and altered perfusion (DBA/2J mice with transient BCCAO). Optic nerve sections were stereologically examined 10–12 weeks after intervention.
Results
All experimental groups showed a decreased total axon number per optic nerve compared with controls. In DBA/2J and combined DBA/2J & BCCAO mice the significant decrease was roughly 50%, while BCCAO leaded to a 23% reduction of axon number, however reaching significance only in the direct t-test. The difference in axon number between BCCAO and both DBA/2J mice was almost 30%, lacking statistical significance due to a remarkably high variation in both DBA/2J groups.
Conclusion
Elevated intraocular pressure in the DBA/2J mouse model of glaucoma leads to a much more pronounced optic nerve atrophy compared with transient forebrain hypoperfusion and reperfusion by BCCAO. A supposed worsening effect of an altered perfusion added to the pressure-related damage could not be detected.
Keywords: Glaucoma, Anatomy, Experimental & animal models, Optic Nerve
WHAT IS ALREADY KNOWN ON THIS TOPIC
Beside intraocular pressure (IOP), an altered perfusion has been supposed to be a risk factor for glaucoma progression in patients. The DBA/2J mouse is a well-described model for IOP induced optic neuropathy.
WHAT THIS STUDY ADDS
This study shows that DBA/2J leads to more pronounced optic neuropathy than a hypoperfusion, reperfusion damage by transient bi-common carotid artery occlusion (BCCAO) alone. There is no worsening effect of BCCAO to high IOP in DBA/2J mice.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The results of this study support the view that different mechanisms might play a role in disease progression in patients with high IOP versus patients with normal-tension glaucoma.
Introduction
Glaucoma is a major cause of blindness worldwide and defined as a progressive loss of retinal ganglion cells (RGCs) with typical changes of the optic nerve (ON) head.1 2 The progressive disease is characterised by visual field changes. Several risk factors for the aetiology and the progression of glaucoma have been described in literature. One of the most important risk factors is an elevated intraocular pressure (IOP)3 and most current therapies aim to lower and maintain a target IOP. Lowering IOP may also help to prevent vision loss in 30% of glaucoma patients with low or normal IOP.4 Nevertheless, lowering of the IOP is not always sufficient to prevent progressive optic disc damage or visual field loss.5 This suggests that other risk factors such as age, race, genetics, decreased corneal thickness, increased cup-to-disc ratio and cardiovascular disorders may contribute to the development of glaucoma.6
Recent research shows increasing evidence for generalised vascular dysfunction in glaucoma patients with decreased local and cerebral blood flow. Thus, a deficient blood supply of the choroid, the retina and the ON head has been shown,7 as well as a relationship between the mean flow in the middle cerebral artery and the central visual function, in open-angle glaucoma patients.8
Taking these findings into account, the observation of MRI changes in the brain in glaucoma patients such as small cerebral infarcts, corpus callosum atrophy9 and lateral geniculate nucleus (LGN) atrophy10 could be caused by an elevated IOP or by other risk factors, such as altered blood flow.11
In experimental models of glaucoma with elevated IOP, RGC loss with visible alteration of their axons leads to anterograde degeneration of neurons in the LGN.12–14 Memantine, an N-methyl-D-aspartate (NMDA) glutamate channel blocker used in neurodegenerative diseases like Alzheimer’s disease, can prevent this transsynaptic degeneration.15 However, transsynaptical retrograde degeneration of the ON and RGCs occurs following injury of brain structures such as the striate cortex.16 Therefore, both local alterations in the eye and ON as well as changes in the brain might be responsible for a further progression in glaucoma once the IOP is lowered sufficiently.
The purpose of this study was to investigate by means of stereological assessment of axonal damage in murine ON of (1) incomplete global cerebral perfusion, reperfusion damage to the ON, of (2) elevated IOP, and the combination effect of hypoperfusion, reperfusion damage and IOP, versus controls and with each other.
Methods
Animals and animal background
The project and the procedures involved were accepted by the animal ethics committee of the Canton Bern approval number: BE87/13. The animals used for this study were female SV-129 mice (controls) and female DBA/2J mice. Both mice strains were chosen because of patent posterior communicating arteries in the circle of Willis.17 The presence of the posterior communicating artery in the mice used in this particular study was confirmed by injecting dye into the aorta after sacrifice and anatomical preparation of the circle of Willis (figure 1A).
Figure 1.
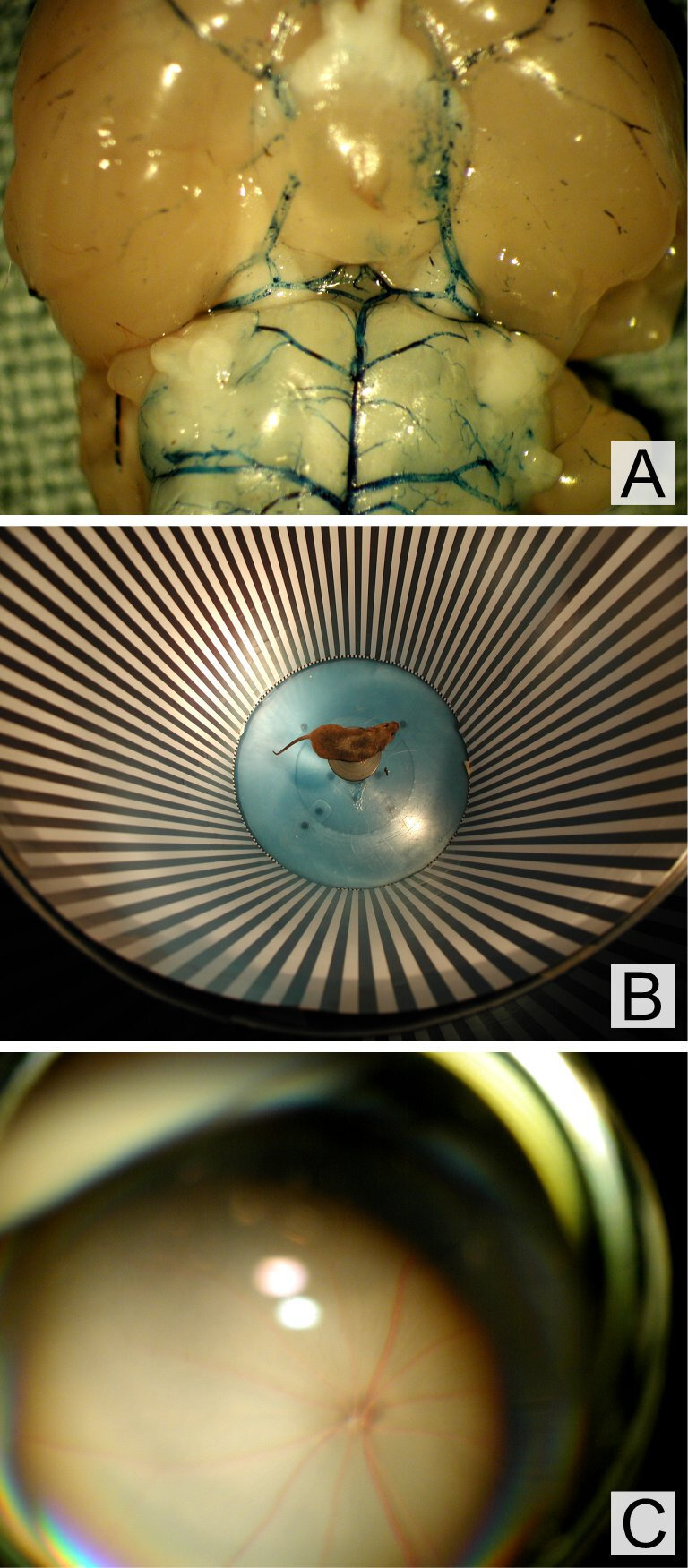
(A) Visualisation of the patent posterior communicating arteries within the circle of Willis, (B) assessment of visual function by optokinetic response to a rotating whole field stimulus, (C) fundus photo of a mouse during bicommon carotid artery occlusion. No signs for ischaemia, no bleedings were observed.
DBA/2J mice are commonly used in glaucoma research and they are accepted as a valid model for this purpose.18 The mice are genetically modified to develop glaucoma.19 Mutations in two genes (glycosylated protein nmb and tyrosinase-related protein 1) lead to changes in the iris structure. That results in iris pigment dispersion and iris pigment atrophy20 21 with raised IOP. Because of this high IOP, these mice then develop a secondary angle-closure glaucoma22 in a very variable time period starting at 8 months. In the majority of DBA/2J mice glaucoma appears after the age of 10.5 months.23
For this study, nine 6-week-old female SV-129 mice and 20 6-week-old female DBA/2J mice were used. Animals had access to laboratory chow and water ad libitum, including the day of surgery. They were maintained on a 12-hour light/dark cycle in a thermo-regulated environment (16°C–20°C). The mice were randomly divided into groups, each group living in a separate cage the entire duration of the study. Survival time was 10–12 weeks after bicommon carotid artery occlusion (BCCAO) or sham operation.
Bicommon carotid artery occlusion
The mice were randomly divided into four distinct groups, namely in BCCAO clamped SV-129 mice (n=5), sham operated SV-129 mice (n=4), BCCAO clamped DBA/2J mice (n=11) and non-clamped DBA/2J mice (n=9). In order to have glaucomatous ON damage in DBA/2J mice at the time of BCCAO, surgical intervention was done after 13 months23 in all BCCAO / sham operated animals. Under general inhalation anaesthesia with 2.5% isoflurane and in aseptic conditions, a ventral midline cervical incision was made exposing the left and right common carotid arteries (CCAs). Each CCA was carefully separated from the vagosympathetic trunk and occluded with a sterile microvascular clip (Fujita Medical Instruments, Tokyo, Japan). Body temperature was monitored using a heating blanket during surgery. After 40 min, the clip was removed and the incision was sutured with Vicryl 5–0. Sham operated controls underwent the same surgical manipulations without occlusion of the CCAs.
Optokinetic head response testing (head tracking)
Visual function was assessed non-invasively by optokinetic response (head tracking) to a rotating whole field stimulus (figure 1B).24 Optokinetic head response (OKHR) was assessed in SV-129 mice before BCCAO and before sacrifice as we previously published.25
Custom-made optokinetic apparatus consisted of a stationary platform (diameter 5 cm) for the placement of the unrestrained animal surrounded by a drum (diameter 30.5 cm, height 61 cm) with the inner surface lined with a pattern of alternating black and white vertical stripes. Patterns with spatial frequencies of 0.033 and of 0.066 cycle/degree were used. The drum with the visual pattern was rotated at stationary speeds ranging from 1°/s to 60°/s clockwise and counterclockwise by a motor (GM9413-4, Pittman, Harleysville, Pennsylvania, USA). The angular velocity of the drum was measured using a contact tachometer (High-Accuracy Digital Contact Tachometer, McMaster-Carr, Dayton, New Jersey, USA). A video camera was mounted on a stand above the platform (Olympus digital camera, C-3000, Olympus Optical, Tokyo, Japan) to record the OKHR. The contrast of the white and black stripes, calculated as a Michelson contrast ((Lmax – Lmin)/(Lmax+Lmin); L=luminance), measured by a luminance metre (LS-100, Konica Minolta Sensing, Osaka, Japan) averaged 0.77.
The animals were first tested using a pattern with a spatial frequency of 0.033 cycle/degree, and the angular velocity of the drum was increased stepwise. If the animal showed head tracking in all tested speeds at this spatial frequency, a pattern with a spatial frequency of 0.066 cycle/degree was used, and the angular velocity was increased in a stepwise fashion until the maximum angular velocity still inducing a head tracking response was reached. The product of the maximum angular velocity of the drum (degree/second) with the spatial frequency of the pattern (cycle/degree) was defined as the frequency of extinction (FE) (cycle/second).26 27 FE was confirmed using the video recordings in a masked manner.
Statistical analysis was performed using Tukey’s multiple comparison test. Statistical significance was assumed to be reached with p<0.05.
Fundus exams
Retinal fundus photographs were taken before BCCAO, during the hypoperfusion period (with the clips in situ) and before sacrifice (figure 1C). The mice were anaesthetised as described above and pupils were dilated with two drops of Cyclopentolate 1% (Mydriaticum Acon, Mississauga, Canada). The fundus was visualised using a VOLK superfield lens (magnification ×0.76, VOLK, Mentor, Ohio, USA) and operating microscope (OPMI 1, Zeiss, Jena, Germany). The photographs were taken with a digital camera (C-3000, Olympus Optical) mounted on an operating microscope with a steady mount (steady mount, VOLK Optical, Mentor, Ohio, USA).
IOP measurement
IOP was measured before BCCAO or sham operation, 7 weeks after operation and before sacrifice. For this purpose, animals were briefly anaesthetised with isoflurane. IOP was measured using TonoLab (Type TV02, Tiolat Oy, Helsinki, Finland).
Microscopic quantification (stereology) of the ON
Histologic preparation: 10–12 weeks after BCCAO or sham operation, all animals were sacrificed with carbon dioxide for histopathological analysis. The ON was then prepared and cut roughly between the eye and the optic chiasm extraconal 3 mm behind the globe. After 13 months the RGCs damage in DBA/2J glaucomatous mice included corresponding axonemal alterations23 28 making the analysis of the axons at this site representative of the degenerative process without need of deeper analysis of RGC perikarya. One ON of each animal was randomly chosen for assessment. The tissue was then prepared for transmission electron microscopy (TEM) following standard procedures.29 After fixing the samples they were embedded in Epon and ultrathin sections produced for observation in a Phillips CM 12 TEM.
Stereological assessment
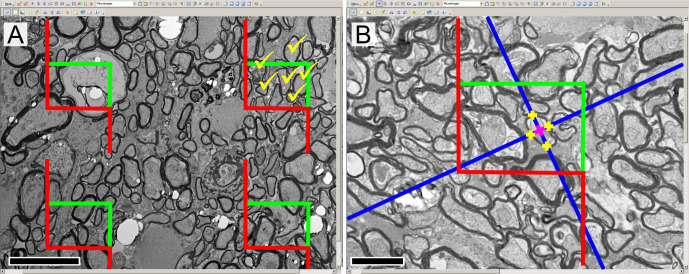
The numbers of axons in the ON as well as their mean diameter were assessed by applying unbiased design-based stereology30–33. Those estimation principles strictly follow an assumption-free systematic sampling procedure with computations that are rigorously mathematically sound and which cannot be validated with data34. Systematic uniform random sampling was performed on the entire ON cross section. Since all axons run more or less orthogonally through a section plane of the nerve, this pure 2D quantification is still unbiased. The axons were sampled by the Fractionator principle: the number of axons counted within an unbiased counting frame35 covering a defined area fraction was multiplied with its proportion in order to get the absolute number per ON (figure 2A). The Nucleator principle36 was used to get the mean cross sectional area on the same sampled axons (figure 2B). Stereological assessment was done with the NewCAST stereology programme (Visiopharm, Hørsholm. Denmark).
Figure 2.
(A) Description of the fractionator tool, applied to imaging (EM cross-section, optic nerve of DBA/2J). For each systematically sampled micrograph four unbiased counting frames are virtually applied with the NewCAST programme. Axons laying within the rectangular frames, not touching the red lines (‘exclusion lines’) are counted; exemplary estimation in the top right frame (yellow ticks). (B) Description of the nucleator tool. The NewCAST programme allows integrating the nucleator tool directly on the virtual counting frames. The blue colour lines are computed isotropically from an arbitrarily chosen point in the centre of the axon, and the intersections of these lines with the boundary are then marked (yellow crosses). The four different obtained lengths from the centre are then directly integrated and calculated in the programme. Scale bar for (A, B)=5 µm (magnification ×3400). EM, electron microscopy.
Statistical analysis was based on the non-parametric Kruskal-Wallis test and the two-stage step-up post hoc test for multiple comparison. Statistical significance was assumed to be reached with p<0.05.
Results
Five SV/129 mice were analysed after sham operation and first compared with four animals that underwent BCCAO operation. These subjects were compared with eight animals having DBA/2J glaucoma alone and eleven animals having DBA/2J glaucoma and BCCAO operation. One animal of the BCCAO group alone and one animal of the DBA/2J glaucoma and BCCAO group died after surgery and was excluded from the analysis.
Fundus exams
No obvious hypoperfusion of the fundus was seen during BCCAO. The width of the blood columns of the retinal vessels remained unchanged. No haemorrhages, no cotton-wool-spots, no dilated veins and no retinal oedema were seen during or after BCCAO. Comparing the ON heads before BCCAO and before sacrifice, no change of the ONH appearance was noted.
IOP measurements
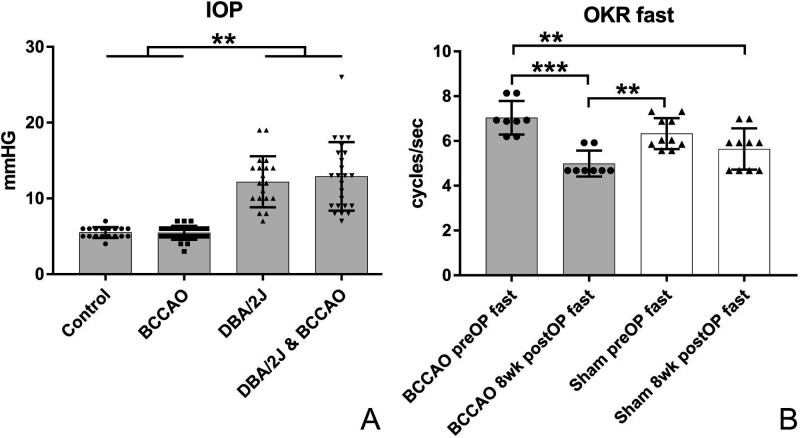
In SV-129 mice, IOP was not significantly different between animals with BCCAO and sham operated mice. In DBA/2J mice, IOP measured before BCCAO or sham operation and after 7 weeks and before sacrifice showed no significant difference between BCCAO or sham operated groups (figure 3A).
Figure 3.
(A) Intraocular pressure (IOP) of the four study groups. There was no difference in IOP levels between non glaucoma mice (SV-129) with or without (sham operated) bicommon carotid artery occlusion (BCCAO). There was no significant difference in IOP between DBA/2J mice with and without BCCAO as well. The graph shows the situation at 14 months of life. ** indicates p<0.001. (B) optokinetic head response at 0.066 cycle per degree as frequency of extinction (FE) (cycle/second). FE was significantly reduced in experimental animals 8 weeks after BCCAO at a spatial frequency of 0.066 cycle/degree. ** indicates p < 0.01, *** indicates p < 0.001.
OKHR testing and visual threshold
All SV-129 mice showed a consistent baseline visual threshold before intervention (BCCAO or sham operation). In the experimental group, FE was significantly reduced 8 weeks after intervention compared with preoperative (figure 3B) at a spatial frequency of 0.066 cycle/degree (7.04 cycle/s=±0.75 vs 4.99 cycle/s±0.56, p=0.01). No significant difference was found in the sham operated group comparing FE before and 8 weeks after intervention (6.33 cycle/s±0.69 vs 5.64 cycle/s±0.92, p=0.54).
FE was not significantly different between groups at lower spatial frequency (0.033 cycle/degree). In our hands DBA/2J mice did not show a consistent OKHR at baseline which is in accordance with the literature.37 These results were excluded from further analysis.
Morphometric analysis of the ON
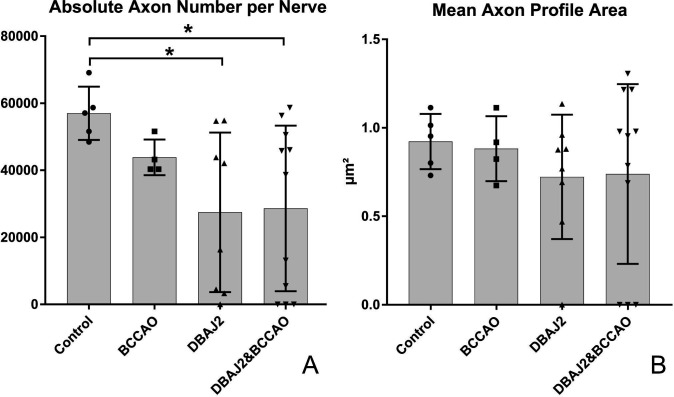
Quantitative data are summarised in table 1. The most striking result was a decrease in total axon number per ON between controls and all experimental groups. In DBA/2J and combined DBA/2J & BCCAO mice the total axon number decreased by roughly 50%, while the 23% axon reduction in the BCCAO without glaucoma group only was significant using the direct t-test. The difference in axon loss between BCCAO and both DBA/2J mice was not statistically significant despite the nearly 30% decrease. This lack of significance was influenced by the high variation of the values in both DBA/2J groups (figure 4A).
Table 1.
Numeric results of the stereological assessment
| Animal no | Axon no | Diff to CO | Axon profile | Diff to CO | ||||
| n | n per nerve | µm² | ||||||
| Control (CO) | 5 | Mean | 56 991 | 0.923 | ||||
| SD | 7938 | 0.156 | ||||||
| CV | 13.9% | 16.9% | ||||||
| BCCAO | 4 | Mean | 43 851 | −23.1% | (*) | 0.882 | −4.4% | ns |
| SD | 5330 | 0.184 | ||||||
| CV | 12.2% | 20.8% | ||||||
| DBA/2J | 8 | Mean | 27 442 | −51.8% | * | 0.723 | −21.7% | ns |
| SD | 23 795 | 0.351 | ||||||
| CV | 86.7% | 48.6% | ||||||
| DBA/2J and BCCAO | 11 | Mean | 28 625 | −49.8% | * | 0.739 | −19.9% | ns |
| SD | 24 677 | 0.508 | ||||||
| CV | 86.2% | 68.8% |
(*), p<0.05 in t-test only; *, p<0.05; BCCAO, bicommon carotid artery occlusion; CV, Coefficient of variation; ns, not significant.
Figure 4.
(A) Absolute number of axons per entire optic nerve (ON) cross section. Bar indicates group mean, whiskers±1 SD and symbols indicate individual animal values. Symbols at the baseline indicate nerves with complete atrophy. Asterisk indicates significant differences with p<0.05, (B) Mean axon profile area on the ON cross sections. Bar indicates group mean, whiskers±1 SD and symbols indicate individual animal values. Symbols at the baseline indicate nerves with complete axonemal atrophy. BCCAO, bicommon carotid artery occlusion.
The mean axon profile area was only slightly different among all groups and was not significant. The variation within DBA/2J- and DBA/2J & BCCAO groups compared with controls or BCCAO alone mice (figure 4B) was striking.
The statistical comparison between DBA/2J and DBA/2J & BCCAO mice and indirectly versus control and BCCAO-only groups was difficult due to high variation within the glaucomatous groups. The massive variability was caused by a highly inconstant severity of glaucomatous damage in DBA/2J mice. Some DBA/2J mice showed ON atrophy, where axons were still identifiable and measurable, while others showed total atrophy with no detectable axons. In the DBA/2J alone group, one mouse showed such total ON atrophy. In the DBS/2J & BCCAO combined group, three animals showed total ON atrophy. Even by a strong increase in the number of animals the somewhat dramatic variations of quantitative results in both glaucomatous groups would not yield a statistically more homozygous data field.
Discussion
Primary open-angle glaucoma (POAG) is commonly divided into two subgroups on the basis of IOP. Patients with IOP ≤21 mm Hg are described as having a normal tension glaucoma (NTG) and patients having IOP >21 mm Hg as having a high tension glaucoma. High IOP alone is thought to provoke a glaucomatous optic neuropathy with typical changes of ON and visual field. Lowering IOP has been shown to slow the progression of glaucomatous damage.38 Numerous studies have been performed showing a neuroprotective effect of memantine, an NMDA receptor antagonist, also diminishing the negative effect of IOP to the ON in animals such as DBA/2J mice39 40 or monkeys.41 Nevertheless, no evidentiary effect has been shown so far in humans.42 In NTG lowering IOP can also reduce glaucoma progression,43 but additional risk factors have been postulated such as local or systemic vascular abnormalities.7 8 In particular damage due to hypoperfusion and reperfusion to the ON and the visual structures in the brain has been postulated causing reactive oxygen species44 and thus impairing the integrity of the visual pathways. Blood pressure instability, especially nocturnal overdipping, sleep apnoea, vascular dysregulation (Flammer syndrome),45 altered neurovascular coupling, altered vascular perfusion of ON and surrounding retina measured by optic coherence angiography have all been described in glaucoma. As a consequence, in recent years, it has been postulated that POAG might be a continuum from a primarily IOP dependent to a primary altered perfusion dependent disease.
To our knowledge, this is the first study using unbiased stereological quantification to estimate the loss in axon number in the ON in a glaucoma model, combined with hypoperfusion and reperfusion. ON atrophy and loss in ON axons are reliable indicators of RCG loss in the context of glaucomatous alterations.23 28 The stereological axon number estimation in our SV-129 controls (57 991±7938), is sound with values published by Williams et al (63 772±4339)46 taking into account certain methodological differences.
We examined first the effect of single episode mild hypoperfusion–reperfusion damage to the mouse ON and consecutively the effect to the visual function.
In order to induce perfusion, reperfusion damage to the central visual structures without causing pronounced primary damage to the eye itself, an incomplete global cerebral ischaemic mouse model was chosen.47–49 In this model, both CCAs are temporally ligated (BCCAO) whereas the patent posterior cerebral arteries stemming from the basilar artery directly and via the circle of Willis continue to supply blood flow in the remainder of the central nervous system.50 51 It has been shown that the perfusion of the eyes and ON is sufficient such that no histological damage can be observed directly after 45 min of BCCAO, and electroretinography shows only a transient and reversible reduction of the b-wave.47
In contrast, our results indicate that mild transient forebrain ischaemia followed by reperfusion has a late effect on the mouse ON. The decrease in axon number in animals after BCCAO is significant in direct comparison to the sham group. In accordance, the visual function evaluated by OKHR to a rotating whole field stimulus is significantly reduced in small spatial frequencies. The OKHR is processed via an alternate pathway.52 It is a compensatory eye and head movement to stabilise the image on the retina. The visual input is processed by a specific population of motion-sensitive and direction-sensitive RGCs distributed across the retina.52 These RGCs project to the accessory optic system and the nucleus of the optic tract. Damage to the retina, the ON or the accessory optic system alters the optokinetic reflex, and this visomotor reflex indeed has been used to detect visual dysfunction.53–56
After showing that 40 min of BCCAO leads to ON damage, we investigated the effect of a combination of high IOP and BCCAO.
This combination is interesting since an impact of disturbed blood supply to the ON has been widely postulated in some glaucoma patients.7 It has been shown that cardiovascular disease history and low blood pressure are risk factors for glaucoma progression.57 A possible theory is that a high and/or fluctuating IOP might lead to ON damage. Independently, cardiovascular diseases or low blood pressure, alone or in combination with disturbed autoregulation of the ON perfusion, might lead to a perfusion, reperfusion damage to the visual system and the ON as well. In some cases, the combination of the two mechanisms might cause the progression of glaucomatous ON damage.
In this study, DBA/2J mice were chosen because the model is well described in the literature, without the necessity of manipulations of the mouse eye. Our stereological results clearly showed a drastic reduction in axon number within 13 months. On the base on the axon numbers estimated by Williams et al in younger mice (63 351, mean age 85 days, not published specifically),46 a loss of approximately 55% is theoretically calculable, considering our two DBA/2J groups, however, with a huge variability of the damage up to total nerve atrophy. In addition, DBA/2J mice have been shown to have a patent posterior communicating artery within the circle of Willis to allow the blood supply of the brain via the basilar artery. For the same reason, SV-129 mice were chosen as controls due to a comparable arterial situs.50
Various other techniques were developed to raise IOP in experimental animals. These include episcleral vein cauterisation, injection of indocyanine green dye into the anterior chamber and diode laser treatment or cauterisation of the limbus with a laser.58–61 A possible disadvantage of these techniques could be the direct or indirect collateral damage to structures of the mouse eye such as the blood supply among others due to the small size of the mouse eye which could cause a large skewing of the results.
The first result of the analysis of high IOP DBA/2J mice compared with ones with a high IOP-BCCAO combination is that the effect of IOP to the ON is by far predominant. ON in some animals with high IOP developed total atrophy whereas mice with BCCAO alone showed only mild damage to the ON. Second, the study shows large interindividual variation of ON atrophy in the DBA/2J mouse model,23 62 obscuring a possible additional effect of perfusion damage. A postulated multiplying effect of the two impairing mechanisms could not be shown. The only hint towards an addition effect of BCCAO in combination with high IOP might be the higher rate of mice with total ON atrophy within the combined group (n=3 vs n=1), although IOP measurements were not significantly different in both groups.
RGC loss in DBA/2J mice starts between 8 and 9 months of age and is prevalent in the majority of mice older than 10.5 months.23 One might argue that an additional effect of BCCAO might be visible in an earlier stage of ON atrophy. The rationale behind the idea to choose old DBA/2J mice was that disturbed perfusion has been shown in humans mostly in progressive advanced glaucoma. Therefore, the time point of sacrifice has been chosen where a significant RGC loss can be observed in nearly all mice.
The findings of a missing or only small worsening effect of BCCAO on pressure-related ON damage in DBA/2J mice can be seen analogously in the clinical situation of glaucoma patients. Patients with high IOP show faster progression rates compared with patients with NTG63 indicating a larger impact of IOP on the ON compared with a suspected vascular effect.
A limitation of our study is that the reperfusion damage was an irrevocable one-time event. In order to evoke a glaucoma like situation, a repeated hypoperfusion, reperfusion damage would better reflect the disease condition. This might be a major limitation considering possibly mild hypoperfusion, reperfusion events in humans over years to decades. Additional in humans with progressive NTG, a disturbed autoregulation in small blood vessels has been described,64 making the subject more sensitive to mild fluctuations in IOP and/or blood pressure, which can’t be imitated in an animal model. The same applies to the microenvironment of the ON. In humans with glaucoma, the physiological surrounding of the ON might be different compared with healthy subjects or animals. For example, an increased retinal venous pressure has been described in glaucoma patients,65 which might affect the perfusion of the ON directly.
Another limitation of this study is, as previously mentioned, the large interindividual variation of ON damage in the DBA/2J glaucoma model. In the present experimental setting the IOP DBA/2J effect on the ON structure was significantly greater than the one of BCCAO which made it practically impossible to discriminate the much smaller additional effect of the latter. Even a much higher number of animals would not have clarified the results regarding the tremendous variability within groups. A dedicated investigation would be appropriate to illuminate the high variability under the given experimental context. The use of ERG in this model would have been helpful to see early and possibly additional changes of BCCAO and IOP in the period before sacrifice. In order to simulate the situation in humans, a longer ischaemia time was not attempted as a stroke was not the aim of the study. Finally, while the DBA/2J mouse model is well described, the glaucoma pathway in humans might be different from this model and the extrapolation to humans might be limited.
In conclusion, this study, based on an unbiased stereology, shows that a mild hypoperfusion, reperfusion damage to the mouse forebrain leads to axon atrophy within the ON. The DBA/2J mouse model of elevated IOP leads to a striking morphological and unbiased morphometrical damage of the ON structure, with large interindividual variation. The effect of a combination of hypoperfusion, reperfusion damage with high IOP is obscured by this large variation as well as by the much higher impact of IOP.
Further studies are needed to investigate the effect of possibly repeated perfusion/reperfusion damages to the glaucoma mouse ON at different time points.
Acknowledgments
We would like to gratefully thank Beat Haenni for the histological and electron microscopic preparation of ON material and Dr Hilary Grabe for proofreading the manuscript. This study was partially performed on instruments of the Microscopy Imaging Center (MIC) of the University of Bern.
Footnotes
Contributors: SAF: designed the study, performed the animal experiments and wrote the manuscript, including data interpretation. Responsible for the overall content. QS: performed the stereological microscopy (quantification), data analysis and writing the manuscript. YY: initial design of the study, helped with data interpretation and writing the manuscript. NG: initial design of the study, helped with data interpretation and writing the manuscript. VVW: performed animal experiments. BEF: helped with data interpretation and writing the manuscript. SAT: helped designing the study and designed the microscopic quantification (stereology), as well as interpreting the data. Helped considerably with manuscript writing and created the figures and table. Supervised the stereology and finalised the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.GBD 2019 Blindness and Vision Impairment Collaborators, Vision Loss Expert Group of the Global Burden of Disease Study . Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to vision 2020: the right to sight: an analysis for the global burden of disease study. Lancet Glob Health 2021;9:e144–60. 10.1016/S2214-109X(20)30489-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jonas JB, Aung T, Bourne RR, et al. Glaucoma. Lancet 2017;390:2183–93. 10.1016/S0140-6736(17)31469-1 [DOI] [PubMed] [Google Scholar]
- 3.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet 2004;363:1711–20. 10.1016/S0140-6736(04)16257-0 [DOI] [PubMed] [Google Scholar]
- 4.Hoyng PFJ, Kitazawa Y. Medical treatment of normal tension glaucoma. Surv Ophthalmol 2002;47 Suppl 1:S116–24. 10.1016/s0039-6257(02)00322-3 [DOI] [PubMed] [Google Scholar]
- 5.Chauhan BC, Drance SM. The relationship between intraocular pressure and visual field progression in glaucoma. Graefes Arch Clin Exp Ophthalmol 1992;230:521–6. 10.1007/BF00181772 [DOI] [PubMed] [Google Scholar]
- 6.Friedman DS, Wilson MR, Liebmann JM, et al. An evidence-based assessment of risk factors for the progression of ocular hypertension and glaucoma. Am J Ophthalmol 2004;138:S19–31. 10.1016/j.ajo.2004.04.058 [DOI] [PubMed] [Google Scholar]
- 7.Flammer J, Orgül S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res 2002;21:359–93. 10.1016/s1350-9462(02)00008-3 [DOI] [PubMed] [Google Scholar]
- 8.Harris A, Siesky B, Zarfati D, et al. Relationship of cerebral blood flow and central visual function in primary open-angle glaucoma. J Glaucoma 2007;16:159–63. 10.1097/01.ijg.0000212290.08540.93 [DOI] [PubMed] [Google Scholar]
- 9.Ong K, Farinelli A, Billson F, et al. Comparative study of brain magnetic resonance imaging findings in patients with low-tension glaucoma and control subjects. Ophthalmology 1995;102:1632–8. 10.1016/s0161-6420(95)30816-0 [DOI] [PubMed] [Google Scholar]
- 10.Gupta N, Ang L-C, Noël de Tilly L, et al. Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br J Ophthalmol 2006;90:674–8. 10.1136/bjo.2005.086769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yücel YH, Gupta N. Paying attention to the cerebrovascular system in glaucoma. Can J Ophthalmol 2008;43:342–6. 10.3129/i08-059 [DOI] [PubMed] [Google Scholar]
- 12.Yücel YH, Zhang Q, Gupta N, et al. Loss of neurons in magnocellular and parvocellular layers of the lateral geniculate nucleus in glaucoma. Arch Ophthalmol 2000;118:378–84. 10.1001/archopht.118.3.378 [DOI] [PubMed] [Google Scholar]
- 13.Yücel YH, Zhang Q, Weinreb RN, et al. Atrophy of relay neurons in magno- and parvocellular layers in the lateral geniculate nucleus in experimental glaucoma. Invest Ophthalmol Vis Sci 2001;42:3216–22. [PubMed] [Google Scholar]
- 14.Yücel YH, Zhang Q, Weinreb RN, et al. Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog Retin Eye Res 2003;22:465–81. 10.1016/s1350-9462(03)00026-0 [DOI] [PubMed] [Google Scholar]
- 15.Yücel YH, Gupta N, Zhang Q, et al. Memantine protects neurons from shrinkage in the lateral geniculate nucleus in experimental glaucoma. Arch Ophthalmol 2006;124:217–25. 10.1001/archopht.124.2.217 [DOI] [PubMed] [Google Scholar]
- 16.Johnson H, Cowey A. Transneuronal retrograde degeneration of retinal ganglion cells following restricted lesions of striate cortex in the monkey. Exp Brain Res 2000;132:269–75. 10.1007/s002210000384 [DOI] [PubMed] [Google Scholar]
- 17.Yang G, Kitagawa K, Matsushita K, et al. C57Bl/6 strain is most susceptible to cerebral ischemia following bilateral common carotid occlusion among seven mouse strains: selective neuronal death in the murine transient forebrain ischemia. Brain Res 1997;752:209–18. 10.1016/s0006-8993(96)01453-9 [DOI] [PubMed] [Google Scholar]
- 18.Libby RT, Anderson MG, Pang I-H, et al. Inherited glaucoma in DBA/2J mice: pertinent disease features for studying the neurodegeneration. Vis Neurosci 2005;22:637–48. 10.1017/S0952523805225130 [DOI] [PubMed] [Google Scholar]
- 19.Libby RT, Li Y, Savinova OV, et al. Susceptibility to neurodegeneration in a glaucoma is modified by Bax gene dosage. PLoS Genet 2005;1:17–26. 10.1371/journal.pgen.0010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson MG, Smith RS, Hawes NL, et al. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet 2002;30:81–5. 10.1038/ng794 [DOI] [PubMed] [Google Scholar]
- 21.Howell GR, Libby RT, Marchant JK, et al. Absence of glaucoma in DBA/2J mice homozygous for wild-type versions of Gpnmb and TYRP1. BMC Genet 2007;8:45. 10.1186/1471-2156-8-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.John SW, Smith RS, Savinova OV, et al. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci 1998;39:951–62. [PubMed] [Google Scholar]
- 23.Schlamp CL, Li Y, Dietz JA, et al. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neurosci 2006;7:66. 10.1186/1471-2202-7-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas BB, Seiler MJ, Sadda SR, et al. Optokinetic test to evaluate visual acuity of each eye independently. J Neurosci Methods 2004;138:7–13. 10.1016/j.jneumeth.2004.03.007 [DOI] [PubMed] [Google Scholar]
- 25.Francis BM, Kim J, Barakat ME, et al. Object recognition memory and BDNF expression are reduced in young TgCRND8 mice. Neurobiol Aging 2012;33:555–63. 10.1016/j.neurobiolaging.2010.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonaventure N, Wioland N, Bigenwald J. Involvement of GABAergic mechanisms in the optokinetic nystagmus of the frog. Exp Brain Res 1983;50:433–41. 10.1007/BF00239210 [DOI] [PubMed] [Google Scholar]
- 27.Yücel YH, Jardon B, Kim MS, et al. Directional asymmetry of the horizontal monocular head and eye optokinetic nystagmus: effects of picrotoxin. Vision Res 1990;30:549–55. 10.1016/0042-6989(90)90067-u [DOI] [PubMed] [Google Scholar]
- 28.Strom RC, Williams RW. Cell production and cell death in the generation of variation in neuron number. J Neurosci 1998;18:9948–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mouton WG, Wagner MO, Haenni B, et al. The influence of age on valve disease in patients with varicose veins analysed by transmission electron microscopy and stereology. Vasa 2018;47:1–7. 10.1024/0301-1526/a000714 [DOI] [PubMed] [Google Scholar]
- 30.Mayhew TM, Sharma AK. Sampling schemes for estimating nerve fibre size. I. methods for nerve trunks of mixed fascicularity. J Anat 1984;139:45–58. [PMC free article] [PubMed] [Google Scholar]
- 31.Larsen JO. Stereology of nerve cross sections. J Neurosci Methods 1998;85:107–18. 10.1016/s0165-0270(98)00129-0 [DOI] [PubMed] [Google Scholar]
- 32.Geuna S, Tos P, Battiston B, et al. Verification of the two-dimensional disector, a method for the unbiased estimation of density and number of myelinated nerve fibers in peripheral nerves. Ann Anat 2000;182:23–34. 10.1016/S0940-9602(00)80117-X [DOI] [PubMed] [Google Scholar]
- 33.Tschanz S, Schneider JP, Knudsen L. Design-based stereology: planning, volumetry and sampling are crucial steps for a successful study. Ann Anat 2014;196:3–11. 10.1016/j.aanat.2013.04.011 [DOI] [PubMed] [Google Scholar]
- 34.Cruz-Orive LM, Weibel ER. Sampling designs for stereology. J Microsc 1981;122:235–57. 10.1111/j.1365-2818.1981.tb01265.x [DOI] [PubMed] [Google Scholar]
- 35.Gundersen HJ. Estimators of the number of objects per area unbiased by edge effects. Microsc Acta 1978;81:107–17. [PubMed] [Google Scholar]
- 36.Gundersen HJ. The nucleator. J Microsc 1988;151:3–21. 10.1111/j.1365-2818.1988.tb04609.x [DOI] [PubMed] [Google Scholar]
- 37.Barabas P, Gorusupudi A, Bernstein PS, et al. Mouse models of Stargardt 3 dominant macular degeneration. Adv Exp Med Biol 2016;854:137–43. 10.1007/978-3-319-17121-0_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the early manifest glaucoma trial. Arch Ophthalmol 2002;120:1268–79. 10.1001/archopht.120.10.1268 [DOI] [PubMed] [Google Scholar]
- 39.Atorf J, Scholz M, Garreis F, et al. Functional protective effects of long-term memantine treatment in the DBA/2J mouse. Doc Ophthalmol 2013;126:221–32. 10.1007/s10633-013-9380-3 [DOI] [PubMed] [Google Scholar]
- 40.Schuettauf F, Quinto K, Naskar R, et al. Effects of anti-glaucoma medications on ganglion cell survival: the DBA/2J mouse model. Vision Res 2002;42:2333–7. 10.1016/s0042-6989(02)00188-8 [DOI] [PubMed] [Google Scholar]
- 41.Hare W, WoldeMussie E, Lai R, et al. Efficacy and safety of memantine, an NMDA-type open-channel blocker, for reduction of retinal injury associated with experimental glaucoma in rat and monkey. Surv Ophthalmol 2001;45 Suppl 3:discussion S95-6:S284–9. 10.1016/S0039-6257(01)00200-4 [DOI] [PubMed] [Google Scholar]
- 42.Weinreb RN, Liebmann JM, Cioffi GA, et al. Oral memantine for the treatment of glaucoma: design and results of 2 randomized, placebo-controlled, phase 3 studies. Ophthalmology 2018;125:1874–85. 10.1016/j.ophtha.2018.06.017 [DOI] [PubMed] [Google Scholar]
- 43.Collaborative Normal-Tension Glaucoma Study Group . The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol 1998;126:498–505. 10.1016/S0002-9394(98)00272-4 [DOI] [PubMed] [Google Scholar]
- 44.Luthra A, Gupta N, Kaufman PL, et al. Oxidative injury by peroxynitrite in neural and vascular tissue of the lateral geniculate nucleus in experimental glaucoma. Exp Eye Res 2005;80:43–9. 10.1016/j.exer.2004.08.016 [DOI] [PubMed] [Google Scholar]
- 45.Flammer J, Konieczka K. The discovery of the Flammer syndrome: a historical and personal perspective. Epma J 2017;8:75–97. 10.1007/s13167-017-0090-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams RW, Strom RC, Rice DS, et al. Genetic and environmental control of variation in retinal ganglion cell number in mice. J Neurosci 1996;16:7193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnett NL, Osborne NN. Prolonged bilateral carotid artery occlusion induces electrophysiological and immunohistochemical changes to the rat retina without causing histological damage. Exp Eye Res 1995;61:83–90. 10.1016/s0014-4835(95)80061-1 [DOI] [PubMed] [Google Scholar]
- 48.Tsuchiya M, Sako K, Yura S, et al. Cerebral blood flow and histopathological changes following permanent bilateral carotid artery ligation in Wistar rats. Exp Brain Res 1992;89:87–92. 10.1007/BF00229004 [DOI] [PubMed] [Google Scholar]
- 49.Ohtaki H, Mori S, Nakamachi T, et al. Evaluation of neuronal cell death after a new global ischemia model in infant mice. Acta Neurochir Suppl 2003;86:97–100. 10.1007/978-3-7091-0651-8_22 [DOI] [PubMed] [Google Scholar]
- 50.Fujii M, Hara H, Meng W, et al. Strain-related differences in susceptibility to transient forebrain ischemia in SV-129 and C57black/6 mice. Stroke 1997;28:discussion 11:1805–11. 10.1161/01.STR.28.9.1805 [DOI] [PubMed] [Google Scholar]
- 51.Barone FC, Knudsen DJ, Nelson AH, et al. Mouse strain differences in susceptibility to cerebral ischemia are related to cerebral vascular anatomy. J Cereb Blood Flow Metab 1993;13:683–92. 10.1038/jcbfm.1993.87 [DOI] [PubMed] [Google Scholar]
- 52.Simpson JI. The accessory optic system. Annu Rev Neurosci 1984;7:13–41. 10.1146/annurev.ne.07.030184.000305 [DOI] [PubMed] [Google Scholar]
- 53.Balkema GW, Mangini NJ, Pinto LH, et al. Visually evoked eye movements in mouse mutants and inbred strains. A screening report. Invest Ophthalmol Vis Sci 1984;25:795–800. [PubMed] [Google Scholar]
- 54.Cahill H, Nathans J. The optokinetic reflex as a tool for quantitative analyses of nervous system function in mice: application to genetic and drug-induced variation. PLoS One 2008;3:e2055. 10.1371/journal.pone.0002055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grüsser-Cornehls U, Böhm P. Horizontal optokinetic ocular nystagmus in wildtype (B6CBA+/+) and weaver mutant mice. Exp Brain Res 1988;72:29–36. 10.1007/BF00248497 [DOI] [PubMed] [Google Scholar]
- 56.Iwakabe H, Katsuura G, Ishibashi C, et al. Impairment of pupillary responses and optokinetic nystagmus in the mGluR6-deficient mouse. Neuropharmacology 1997;36:135–43. 10.1016/s0028-3908(96)00167-0 [DOI] [PubMed] [Google Scholar]
- 57.Bengtsson B, Leske MC, Hyman L, et al. Fluctuation of intraocular pressure and glaucoma progression in the early manifest glaucoma trial. Ophthalmology 2007;114:205–9. 10.1016/j.ophtha.2006.07.060 [DOI] [PubMed] [Google Scholar]
- 58.Grozdanic SD, Betts DM, Sakaguchi DS, et al. Laser-induced mouse model of chronic ocular hypertension. Invest Ophthalmol Vis Sci 2003;44:4337–46. 10.1167/iovs.03-0015 [DOI] [PubMed] [Google Scholar]
- 59.Aihara M, Lindsey JD, Weinreb RN. Experimental mouse ocular hypertension: establishment of the model. Invest Ophthalmol Vis Sci 2003;44:4314–20. 10.1167/iovs.03-0137 [DOI] [PubMed] [Google Scholar]
- 60.Mabuchi F, Aihara M, Mackey MR, et al. Optic nerve damage in experimental mouse ocular hypertension. Invest Ophthalmol Vis Sci 2003;44:4321–30. 10.1167/iovs.03-0138 [DOI] [PubMed] [Google Scholar]
- 61.Ruiz-Ederra J, Verkman AS. Mouse model of sustained elevation in intraocular pressure produced by episcleral vein occlusion. Exp Eye Res 2006;82:879–84. 10.1016/j.exer.2005.10.019 [DOI] [PubMed] [Google Scholar]
- 62.Della Santina L, Inman DM, Lupien CB, et al. Differential progression of structural and functional alterations in distinct retinal ganglion cell types in a mouse model of glaucoma. J Neurosci 2013;33:17444–57. 10.1523/JNEUROSCI.5461-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heijl A, Bengtsson B, Hyman L, et al. Natural history of open-angle glaucoma. Ophthalmology 2009;116:2271–6. 10.1016/j.ophtha.2009.06.042 [DOI] [PubMed] [Google Scholar]
- 64.Flammer J, Pache M, Resink T. Vasospasm, its role in the pathogenesis of diseases with particular reference to the eye. Prog Retin Eye Res 2001;20:319–49. 10.1016/S1350-9462(00)00028-8 [DOI] [PubMed] [Google Scholar]
- 65.Kim KE, Kim DM, Flammer J, et al. Central retinal venous pressure in eyes of normal-tension glaucoma patients with optic disc hemorrhage. PLoS One 2015;10:e0127920. 10.1371/journal.pone.0127920 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.