Abstract
We report the case of a woman in her 60s with intravascular leiomyomatosis. She suffered from numerous non-specific symptoms including weight loss, anaemia and sudden swelling of the left lower extremity. CT imaging showed the presence of an enlarged left ovary and a thrombus extending from the left ovarian venous plexus intruding into the right atrium of the heart. Cancer antigen 125 was 20 U/mL. Pelvic transvaginal ultrasound examination identified two normal ovaries and a mass adjacent to the left ovary. A second opinion on the CT scan was requested at a oncogynaecological multidisciplinary team meeting, where the radiologist of the team identified an intervascular leiomyomatosis. After further investigation, surgical treatment was planned and completed in collaboration with the departments of cardiothoracic and vascular surgery. The patient recovered fully.
Keywords: Obstetrics and gynaecology, Gynecological cancer, Cardiothoracic surgery, Vascular surgery, Surgical oncology
Background
Uterine fibroids, also known as leiomyomas, are benign neoplasms in fertile women that originate from the smooth muscle cells in the uterine wall.1 The estimated cumulative incidence by the of age 50 is between 70% and 80%, depending on ethnicity and risk factors (table 1).2 3 In extremely rare cases these benign neoplasms can exhibit the intravascular quasimalignant growth patterns characteristic of intravascular leiomyomatosis (IVL).4 To date, this has been reported in approximately 300 cases, with cardiac involvement occurring in less than 100 cases.5 The conditions resemble each other clinically, and both will often remain asymptomatic for long periods of time.6 Previous case reports assert that surgical intervention is the preferred treatment of IVL, and to prevent relapse, complete tumour excision is necessary.1 5–10
Table 1.
Overview of factors affecting development of uterine leiomyomas
| Risk factors | Potential risk factors | Preventive factors |
Age25
|
Chlamydia infection26
|
Physical activity27 |
| Early age at menarche28 | Diabetes29
|
Fruit and vegetable consumption30 |
| Nulliparity31 | Pelvic inflammatory disease26
|
Menopause32 |
African decent25
|
Vitamin D deficiency33
|
Increased parity34 |
| Increased stress27 35 | Use of oral contraceptives36
|
Smoking37 |
| Hypertension38 | ||
| Obesity27 | ||
| Alcohol consumption25 | ||
| Red meat consumption30 | ||
Caffeine consumption30
|
||
| Thyroid disease39 |
The present case report describes the diagnostic challenge IVL represents and emphasises the importance of being aware of rare diseases when the clinical findings are contradictory to the suspected diagnosis. Our report also elucidates the value and benefits of requesting a second opinion in a multidisciplinary setting when diagnosis and treatment prove challenging.
Case presentation
A woman in her 60s suffered from non-specific symptoms including weight loss and anaemia. Previously, she had four uncomplicated pregnancies with vaginal deliveries. She was postmenopausal, had no history of uterine fibroids, had never received hormone replacement therapy and had no genetic predisposition to venous thrombosis. The patient had been successfully treated for breast cancer 10 years previously. She had a history of prolonged alcohol abuse, and ultrasonically verified hepatic steatosis had been identified.
The patient was referred twice to a diagnostic centre. At the first visit to the diagnostic centre, blood samples revealed liver injury and haemolysis, but the patient declined further investigation. Approximately 3 months later, the patient was rereferred to the diagnostic centre. In the meantime, she had undergone optional alcohol detoxification therapy. In the rehabilitation clinic, she experienced sudden generalised swelling of the left lower extremity. Blood samples revealed a further decrease in haemoglobin to 4.5 mmol/L. These findings led to a second referral to the diagnostic centre, and she now consented to further investigation.
CT imaging of the thorax, abdomen and pelvis showed a thrombus in the right atrium and inferior vena cava (IVC) with a single thrombotic spur in the left renal vein and a partially thrombosed left ovarian vein. Although difficult to distinguish from the surrounding tissue, the left ovary was estimated to be enlarged to 8.7 cm and was surrounded by multiple varicose veins. In conclusion, venous thrombosis and ovarian cancer were suspected.
Treatment with therapeutic low-molecular-weight heparin (LMWH) was initiated, and she was referred to cardiology and gynaecological investigation at a secondary treatment centre.
Transthoracic echocardiography confirmed the presence of a thrombus in the IVC with intrusion into the right atrium and attachment to part of the tricuspid valve.
Transvaginal ultrasound confirmed the presence of a mass adjacent to the left ovary. The uterus and the adnexa were identified and described as normal, and no ascites was detected. CA 125 was 20 U/mL (ref<35 U/mL). Thus, the clinical findings did not clearly support the suspected diagnosis of ovarian cancer.
However, the diagnostic centre still suspected ovarian cancer, and therefore, the examining gynaecologist requested a second opinion on the CT imaging at the oncogynaecological multidisciplinary meeting in the tertiary treatment centre of the region. The team radiologist unequivocally confirmed the presence of IVL based on two main findings:
The distribution pattern with origin in the left adnexa and dissemination through the venous system up to and including the tricuspid valve (figures 1–3).
Contrast enhancement in the tumourous tissue in the arterial phase of the CT scan demonstrating vessel formations that are never present in a venous thrombus (figure 1).
Figure 1.
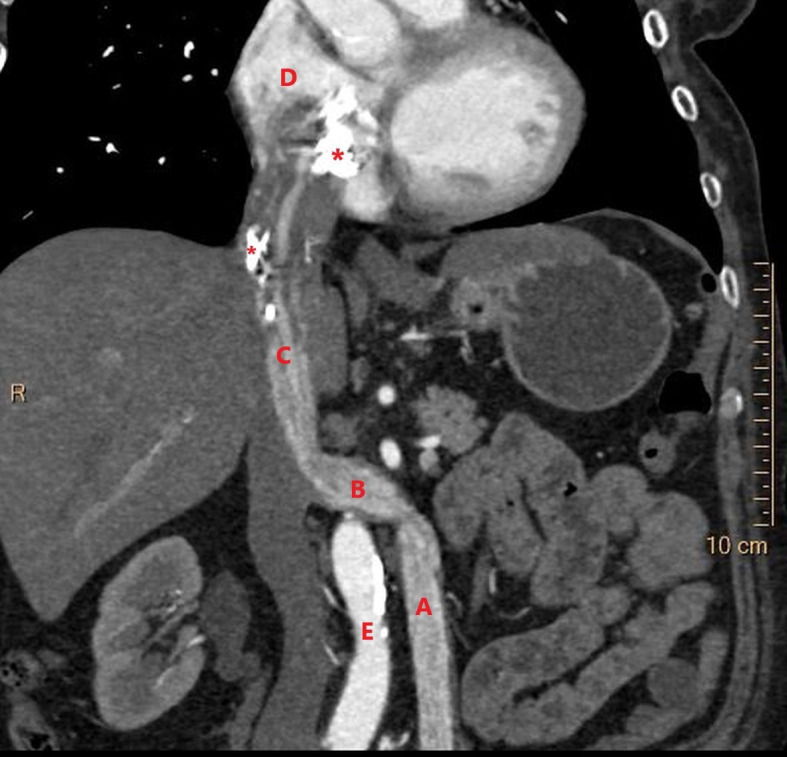
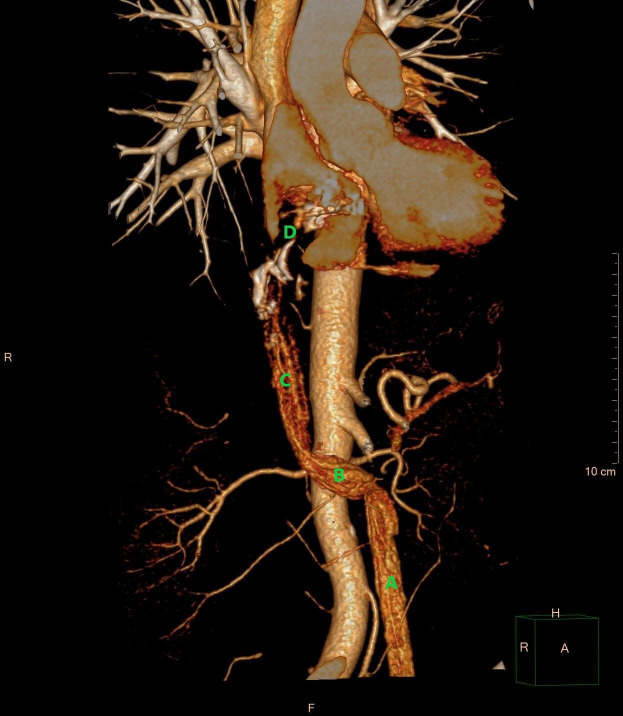
Coronal reconstruction of CT scan of the thorax and abdomen in the late arterial phase. The CT image demonstrates the intravascular leiomyomatosis in the left ovarian vein (A), extending through the left renal vein (B) into the inferior vena cava (C), and progressing into the right atrium of the heart (D). (E) aorta. The upper part of the tumour is seen with several areas of calcification (*). – Courtesy of Gratien Andersen, MD.
Figure 2.
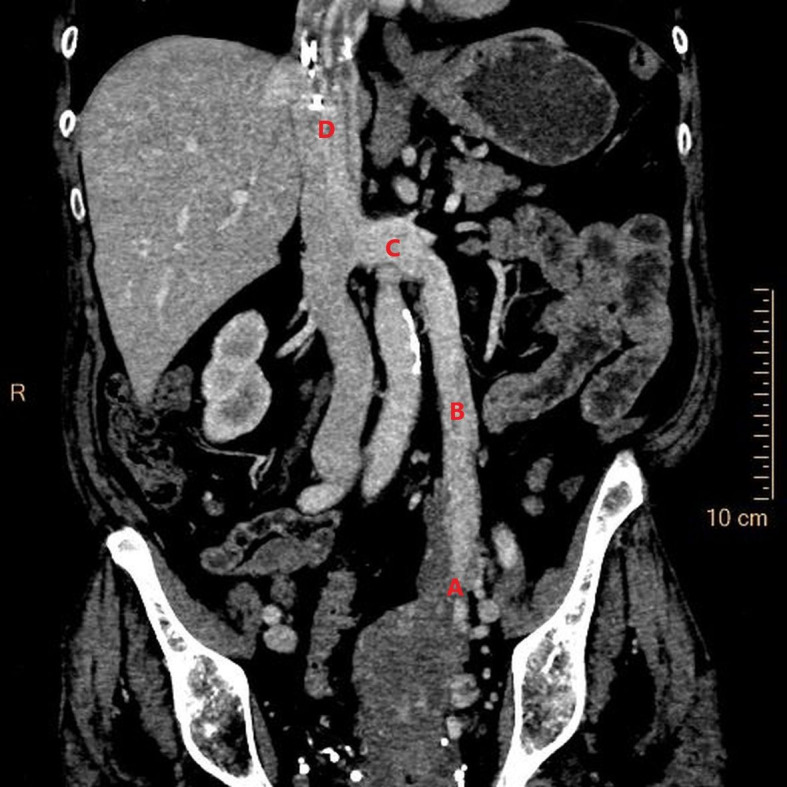
Coronal reconstruction of CT abdomen and pelvis in the venous phase. The CT image demonstrates intravascular leiomyomatosis originating from the left adnexa (A) and extending through the left ovarian vein (B) and into the left renal vein (C) from where it continues through the inferior vena cava (D). Courtesy of Gratien Andersen, MD.
Figure 3.
3D volume rendering demonstrating an intravascular tumour in the left ovarian vein (A), the left renal vein (B), the inferior vena cava (C), and the right atrium (D). The tumour in the right atrium is shown as a contrast filling defect (D). Courtesy of Gratien Andersen, MD. 3D, three dimensions.
The multidisciplinary conclusion was to offer surgical treatment in collaboration with the departments of cardiothoracic and vascular surgery.
Investigations
Preoperative investigations were as follows:
Blood samples: Normal liver function: (alkaline phosphatase: 104 U/L, bilirubin: 6 µmol/L, international normalised ratio: 0.9, gamma-glutamyl transferase: 83 U/L, albumin: 41 g/L and alanine aminotransferase: 24 U/L), normal kidney function (serum creatinine 99 µmol/L), haemoglobin 5.2 mmol/L. No biomarkers indicated the need for further medical investigation prior to surgery.
Spirometry: Normal lung function (forced expiratory volume in 1 s, FEV1: 2.58 L; 98% of expected, FVC: 3.12 L; 101% of expected, index FEV1/FVC: 83%).
Doppler transthoracic echocardiography: There was no dilatation or hypertrophy of the left atrium or the ventricles. Cardiac contraction was normal with an ejection fraction of 60%. The diameter of the ascending aorta was normal. The right atrium with the presumed tumour mass was slightly dilated. The tumour mass involved the tricuspid valve and extended far into the IVC. Tricuspid insufficiency in the direction of the interatrial septum was detected. No obstruction of the IVC was found. Thrombus formation was detected on the periphery of the tumour mass - an indication for continued treatment with therapeutic LMWH.
Cardiac CT angiography: The presence of tumour deposits were confirmed. There was no evidence of pericardial effusion. There was no further cardiac valve pathology. No coronary artery disease was detected.
The clinical and biochemical assessment showed that the patient had a good performance status (PS=0).11 The role of surgical intervention was discussed in multiple multidisciplinary meetings. No contraindications for surgery were noted. The patient was informed of the findings, need for surgery and risks of the procedure, and consented to proceed.
Differential diagnosis
Non-specific symptoms, detection of a mass in the pelvic region and the presence of venous thrombosis in a woman are findings consistent with ovarian cancer. CA 125 is elevated in most women with epithelial derived ovarian malignancy. In non-epithelial ovarian cancer relevant tumour markers are inhibin B and anti-Müllerian hormone, which indicate granulosa cell tumours, while increased human chorionic gonadotropin, lactate dehydrogenase and alpha fetoprotein are biomarkers of germ cell tumours. These tumour markers would have been the next step in the diagnostic investigation had IVL not been identified on CT imaging.
In rare cases, leiomyosarcomas can be difficult to differentiate from IVL using diagnostic imaging and only histological examination can eliminate this differential diagnosis.10 12
Pedunculated ovarian fibroid, clinically presenting as Meigs’s syndrome with the triad of benign ovarian tumour, pleural effusion and ascites, is a potential differential diagnosis. However, CT detection of severe venous tumourous formations make this diagnosis less likely.
The ovarian tumour could also be benign and unrelated to venous involvement, making primary ovarian leiomyoma a possible differential diagnosis.
In our case, alcohol-related liver disease was a potential differential diagnosis but was excluded because of normal liver enzymes and no stigmata of chronic liver disease such as jaundice, spider naevi, enlargement of the liver, palmar erythema, caput medusa or ascites. A surgical procedure is potentially life-threatening in a patient with liver insufficiency, emphasising the importance of this differential diagnostic consideration in our case.
Treatment
Treatment with LMWH was paused, with the last dose being administered 3 days before surgery.
This decision was carefully evaluated in the surgical preparation with consideration of regional guidelines for perioperative anticoagulant treatment, bleeding risk associated with the procedure and the risk of major bleeding complications that LMWH treatment is known to increase.13 The procedure was assessed to have a significant risk of major bleeding complications, hence an anticoagulant treatment strategy that would provide the patient with close to normal homeostasis during the procedure was chosen.
The surgical procedure was a two-step procedure aimed at complete surgical resection.
IVL is usually loosely attached to and rarely invades the vessel wall, enabling uncomplicated removal of intracardiac and intracaval lesions by gentle downward traction of the involved veins.1 9
(1) Cardiothoracic surgery: The first step of the procedure included the excision of pathological tissue in the right atrium of the heart.
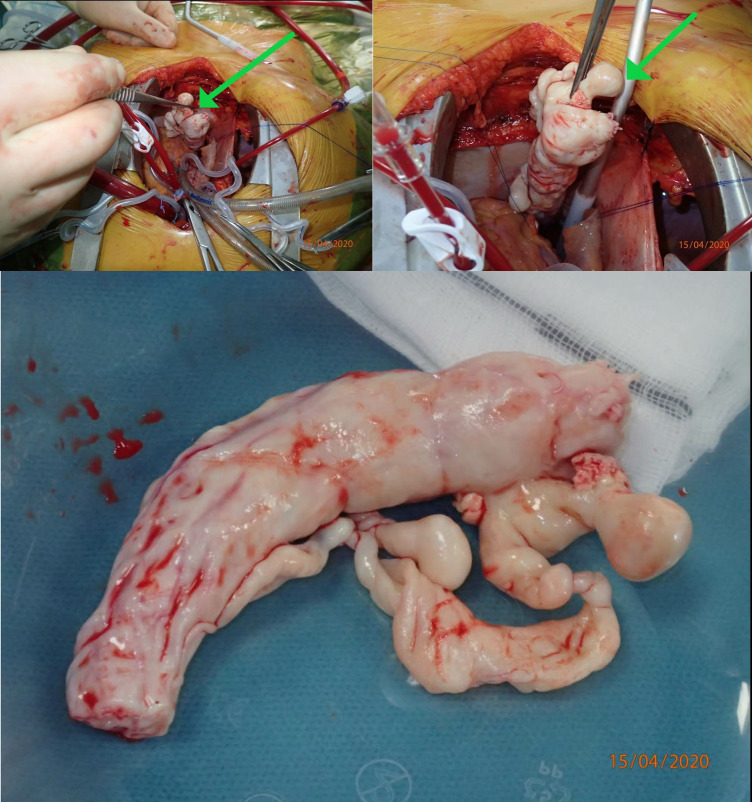
Initially, median sternotomy was performed. A syringe was placed in the aorta, and catheters were inserted directly into the superior vena cava and right atrium for extracorporeal circulation. Hypothermic perfusion was initiated, and the patient was cooled to 18°C. The aorta was clamped, and cardiac arrest induced with blood cardioplegia followed by circulatory arrest. A right atriotomy was performed, and a long white tumour mass with a diameter of 3 cm was grasped using a-traumatic forceps and gently retracted from the atrium and IVC (figure 4). The atriotomy was closed, perfusion restarted and the aortic clamp was removed. Circulatory arrest was necessary for 9 min. The patient remained intubated on a ventilator and sedated with remifentanil and propofol between the two-step procedure.
Figure 4.
Perioperative pictures demonstrating the resection of the tumour mass (arrows) from the right atrium and inferior vena cava. The resected tumour mass is shown in the bottom picture. Courtesy of Kaj Erik Klaaborg, MD.
Five hours after the first procedure, the patient returned to the operating theatre because 800 mL of blood was detected in the mediastinal drain. The bleeding sites were identified and ligated at the sternal edge and the catheter injection site in the superior vena cava.
Two days later, the second step of the procedure was performed to remove the remaining tumour and the site of origin.
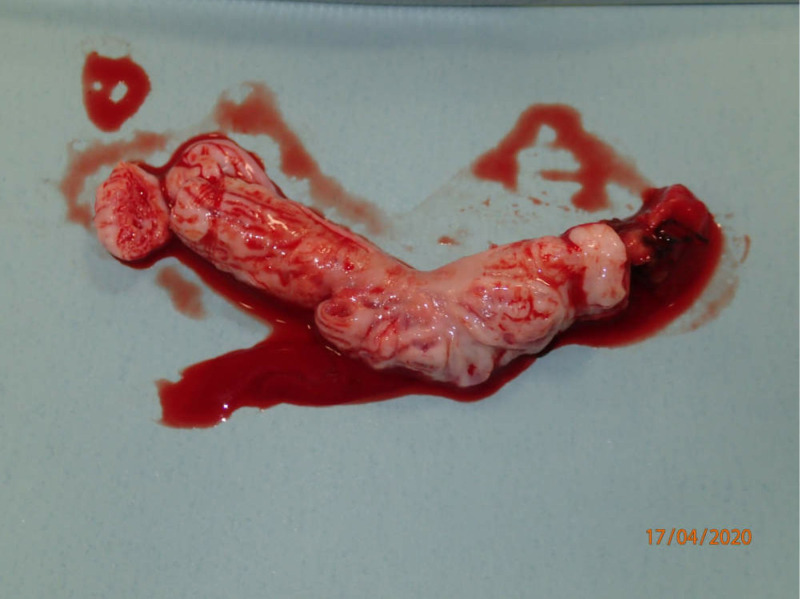
Vascular surgery: A midline laparotomy was performed, and complete tumour resection from the IVC and left renal vein was achieved with the use of direct ultrasound and palpation. A tumour mass was removed from the left ovarian vein inlet into the renal vein, and the vein was closed (figure 5).
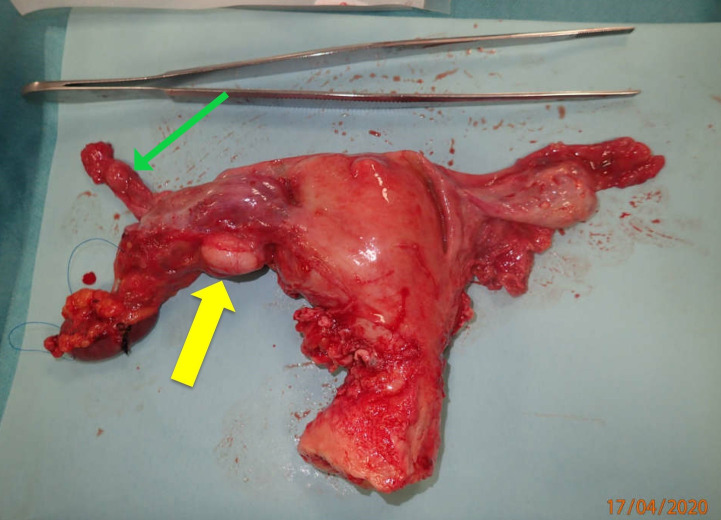
Gynaecological surgery: Using the same laparotomy access, total hysterectomy and bilateral salpingo-oophorectomy was performed (figure 6).
Figure 5.
Tumourmass resected from the left ovarian vein during the vascular surgery component of the second step of the two-step procedure. Courtesy of Kaj Erik Klaaborg, MD.
Figure 6.
Uterus with adnexa and presence of a leiomyoma (yellow arrow marking the leiomyoma adjacent to the left ovary) (green arrow marking the left ovarian vein). Courtesy of Kaj Erik Klaaborg, MD.
All resected material was sent for histopathological examination.
Postoperative complications included an episode of atrial flutter and transient renal insufficiency. The patient was discharged 14 days after admission.
Outcome and follow-up
Histopathology examination confirmed intravascular leiomyoma originating from the left ovarian vein—confirming the diagnosis of IVL. No signs of malignancy were detected. No further treatment was needed.
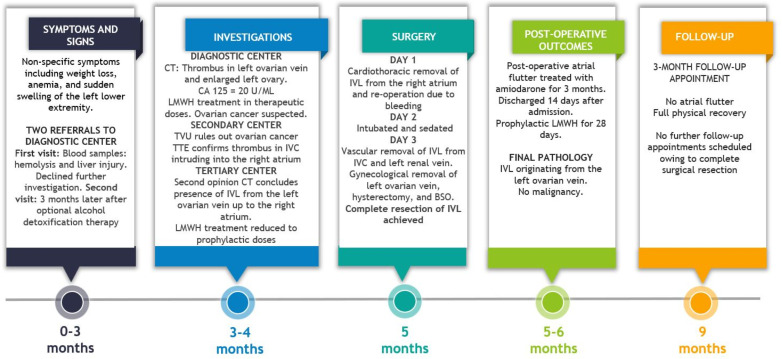
Continuation of LMWH treatment was re-evaluated. Overall, the patient received LMWH for 1 month but in therapeutic doses for only 1 week. Atrial flutter was present only during the surgical procedure, and amiodarone treatment was discontinued at 3 months follow-up. At this point, the patient was fully recovered and no further follow-up was indicated. An overview of case events is provided in figure 7.
Figure 7.
Graphical timeline of events with symptoms, investigations, treatment and outcome. Courtesy of Emil Kolstad, MS and Katrine Fuglsang, MD. CA 125, cancer antigen 125; BSO, bilateral salpingo-oophorectomy; IVC, inferior vena cava; IVL, intravascular leiomyomatosis; LMWH, low-molecular-weight heparin; TTE, transthoracic echocardiography; TVU, transvaginal ultrasound.
Discussion
Patients with IVL are typically women in their mid-40s who may be suffering from menorrhagia and pelvic pain. The symptoms of IVL are diverse and non-specific.10 The gross and microscopic benign looking smooth muscle cell tumour, which constitutes IVL does not metastasise in distant organs but exhibits intravenous proliferation.6 7 Clinical manifestations depend on tumour size and the degree of vessel extension. The condition can be potentially fatal. Patients are often asymptomatic, even at disease stages where tumour masses have reached the IVC. Symptoms develop when atrial extension induces cardiac insufficiency.5
Furthermore, the close resemblance between IVL and uterine fibroids makes it an often overlooked, misdiagnosed or inadequately treated condition, explaining why many published cases report discovery of the disease at advanced stages where cardiac involvement has occurred.6 14 In our case, the diagnosis was suspected because the clinical and biochemical findings did not clearly indicate ovarian cancer, and specific findings on the CT scan confirmed the suspicion of IVL. The most useful modalities for detection of IVL are CT and MRI, both modalities being described as favourable compared with other modalities.5 12 15 However, radiologists must be aware of specific imaging findings, and currently, no evidence distinguishes the two modalities in terms of diagnostic accuracy.10 12 15
The exact aetiology of IVL remains unclear. Two theories have been proposed: Either smooth muscle cells originate in the vessel wall or direct vascular invasion occurs as an extension of a primary tumour arising in a uterine leiomyoma. At present, the second theory is favoured4 5 16; however, in the case presented here the IVL originated in the ovarian vein.
The recurrence rate of IVL is unknown.10 Previous studies describe the probability of recurrence as being close to zero when complete tumour resection is achieved.17 18 In several case reports follow-up (between 4 months and 5 years) is recorded in patients after treatment for IVL, but to our knowledge, there are no reports of recurrence in patients in whom complete tumour resection was achieved.1 5 7 9 10 15 19 20
Based on this inadequate evidence, the patient in the present case attended only a single follow-up appointment 3 months after the surgery. No further follow-up was indicated because complete tumour resection was achieved.
In incompletely resected or unresectable IVL, long-term follow-up should be recommended because previous studies estimate a 5-year recurrence rate between 16.6% and 30%.12 14 21
As mentioned, current studies on IVL agree that complete tumour resection is crucial in the management of the disease.1 5–10 Surgical strategies differ between one-stage or two-stage procedures. If complete resection can be achieved through one-stage surgery, this is preferred. However, this approach must be carefully evaluated, as it can be a prolonged and extensive procedure (the patient must have a good PS), and all venous attachment sites must be assessed.22 23 In frail IVL patients or those with significant co-morbidities, a two-stage procedure is the optimal choice.22 The first stage of this procedure is intravenous and intracardiac leiomyomectomy, and the second stage is pelvic resection and hysterectomy including bilateral salpingo-oophorectomy.24 In our case, a two-stage procedure was chosen due to disease extension and comorbidities. Furthermore, vascular surgeons performed part of the venous leiomyomectomy in the second stage to ensure complete venous resection.
Patient’s perspective.
My symptoms up to the time of diagnosis were a mixture of dizziness, shortness of breath, and general discomfort. At this time, I also had an excessive alcohol intake that complicated my condition. It became clear that something else was wrong when I developed severe oedema of my feet, ankles, and legs. Because my daughter, who is a doctor, insisted, I was referred for further examinations. At this time, I did not understand what was going on around me. But my daughter continuously insisted that my symptoms were not just because of the alcohol abuse, and insisted on further examinations. Without her, I probably would not be here today.
From the time I was informed of the results of the first CT scan until the operation, I do not remember much. I do, however, recall the last surgical consultation before surgery. The cardiac surgeon told me that I would have to go through a massive surgery, with the risk of losing my life. Without it, I would have 2 years left to live at the most. It was not until then that I realised how ill I was.
I went through surgery shortly after that, and all I remember was thinking about how much I wanted to see my grandchildren graduate from high school.
It was a massive surgical procedure, but I have literally no physical complaints from it today. Mentally however, it has taken its toll, and I still find myself being hit by a feeling of great depression from time to time when it all comes back to me.
Learning points.
Second opinions and a multidisciplinary team approach are valuable in cases of unresolved and challenging diagnosis and treatment.
Surgery is the preferred treatment of intravascular leiomyomatosis (IVL), and complete resection is key to avoiding recurrence of the disease.
Joint venture surgery with gynaecologists, vascular and cardiothoracic surgeons is necessary to achieve complete resection in cases of advanced disease and maximises positive outcome.
There is no evidence base of long-term outcome data after complete resection of IVL.
Acknowledgments
We thank the patient for providing a relevant perspective to the case report. Futhermore, we thank Kim Allan Terp, MD for surgical advice in preparation of the manuscript. We thank the multidiciplinary clinical team for their efforts.
Footnotes
Twitter: @EmilKolle
Contributors: In preparation of the case report, the following contributions were made: EMMK, SØ and KF contributed to the case reports conception and design. EMMK and KF planned the work process of the project. SØ prepared parts of the manuscripts first draft. EMMK wrote and prepared the main manuscript. EMMK, SØ and KF contributed to acquisition of data and relevant information. EMMK and KF analysed and interpretated all information and data in relation to the case report. EMMK and KF were responsible for correction and editing of the manuscript. EMMK and GA were responsible for the preparation and interpretation of the radiology images and image legends. GA provided the radiology images. EMMK made all graphical designs. EMMK and KF contributed to preparation and interpretation of the surgical and specimen images and image legends. All authors contributed to the manuscript and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Lou Y-F, Shi X-P, Song Z-Z. Intravenous leiomyomatosis of the uterus with extension to the right heart. Cardiovasc Ultrasound 2011;9:25. 10.1186/1476-7120-9-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med 2017;35:473–80. 10.1055/s-0037-1607264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188:100–7. 10.1067/mob.2003.99 [DOI] [PubMed] [Google Scholar]
- 4.Ordulu Z, Nucci MR, Dal Cin P, et al. Intravenous leiomyomatosis: an unusual intermediate between benign and malignant uterine smooth muscle tumors. Mod Pathol 2016;29:500–10. 10.1038/modpathol.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrone G, Crinò F, Morsolini M, et al. Multidisciplinary approach in the management of uterine intravenous leiomyomatosis with intracardiac extension: case report and review of literature. J Radiol Case Rep 2019;13:1–13. 10.3941/jrcr.v13i7.3607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valdés Devesa V, Conley CR, Stone WM, et al. Update on intravenous leiomyomatosis: report of five patients and literature review. Eur J Obstet Gynecol Reprod Biol 2013;171:209–13. 10.1016/j.ejogrb.2013.09.031 [DOI] [PubMed] [Google Scholar]
- 7.Low H-Y, Zhao Y, Huang K-S, et al. Intravenous leiomyomatosis of the uterus: a clinicopathological analysis of nine cases and literature review. Taiwan J Obstet Gynecol 2017;56:362–5. 10.1016/j.tjog.2017.04.017 [DOI] [PubMed] [Google Scholar]
- 8.Yu X, Fu J, Cao T, et al. Clinicopathologic features and clinical outcomes of intravenous leiomyomatosis of the uterus: a case series. Medicine 2021;100:e24228. 10.1097/MD.0000000000024228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu B, Liu C, Guan H, et al. Intravenous leiomyomatosis with inferior vena cava and heart extension. J Vasc Surg 2009;50:897–902. 10.1016/j.jvs.2009.04.037 [DOI] [PubMed] [Google Scholar]
- 10.Correia P, Castro A, Rocha A, et al. Pelvic intravenous leiomyomatosis - case report. Rev Bras Ginecol Obstet 2016;38:412–5. 10.1055/s-0036-1588002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the eastern cooperative Oncology Group. Am J Clin Oncol 1982;5:649–56. 10.1097/00000421-198212000-00014 [DOI] [PubMed] [Google Scholar]
- 12.Fasih N, Prasad Shanbhogue AK, Macdonald DB, et al. Leiomyomas beyond the uterus: unusual locations, rare manifestations. Radiographics 2008;28:1931–48. 10.1148/rg.287085095 [DOI] [PubMed] [Google Scholar]
- 13.Wagner J, Lock JF, Kastner C, et al. Perioperative management of anticoagulant therapy. Innov Surg Sci 2019;4:144–51. 10.1515/iss-2019-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du J, Zhao X, Guo D, et al. Intravenous leiomyomatosis of the uterus: a clinicopathologic study of 18 cases, with emphasis on early diagnosis and appropriate treatment strategies. Hum Pathol 2011;42:1240–6. 10.1016/j.humpath.2010.10.015 [DOI] [PubMed] [Google Scholar]
- 15.Bender LC, Mitsumori LM, Lloyd KA, et al. AIRP best cases in radiologic-pathologic correlation: intravenous leiomyomatosis. Radiographics 2011;31:1053–8. 10.1148/rg.314115013 [DOI] [PubMed] [Google Scholar]
- 16.Konrad P, Mellblom L. Intravenous leiomyomatosis. Acta Obstet Gynecol Scand 1989;68:371–6. 10.3109/00016348909028675 [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Wu L, Xu R, et al. Identification of the molecular relationship between intravenous leiomyomatosis and uterine myoma using RNA sequencing. Sci Rep 2019;9:1442. 10.1038/s41598-018-37452-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed M, Zangos S, Bechstein WO, et al. Intravenous leiomyomatosis. Eur Radiol 2004;14:1316–7. 10.1007/s00330-003-2186-z [DOI] [PubMed] [Google Scholar]
- 19.Su Q, Zhang X, Zhang H, et al. Intravenous leiomyomatosis of the uterus: a retrospective single-center study in 14 cases. Biomed Res Int 2020;2020:1–14. 10.1155/2020/9758302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yano M, Katoh T, Nakajima Y, et al. Uterine intravenous leiomyomatosis with an isolated large metastasis to the right atrium: a case report. Diagn Pathol 2020;15:4. 10.1186/s13000-019-0913-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li B, Chen X, Chu Y-D, et al. Intracardiac leiomyomatosis: a comprehensive analysis of 194 cases. Interact Cardiovasc Thorac Surg 2013;17:132–8. 10.1093/icvts/ivt117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alizade K, Maddah G, Jafarian AH, et al. Management of intravenous leiomyomatosis of uterus with extension to heart. Arch Iran Med 2016;19:147–9. doi:0161902/AIM.0014 [PubMed] [Google Scholar]
- 23.Harris LM, Karakousis CP. Intravenous leiomyomatosis with cardiac extension: tumor thrombectomy through an abdominal approach. J Vasc Surg 2000;31:1046–51. 10.1067/mva.2000.104601 [DOI] [PubMed] [Google Scholar]
- 24.Gan H-L, Zhang J-Q, Bo P. The classification and surgical strategy of intracardiac leiomyomatosis. Asian J Surg 2009;32:129–36. 10.1016/S1015-9584(09)60383-3 [DOI] [PubMed] [Google Scholar]
- 25.Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol 1997;90:967–73. 10.1016/S0029-7844(97)00534-6 [DOI] [PubMed] [Google Scholar]
- 26.Moore KR, Cole SR, Dittmer DP, et al. Self-reported reproductive tract infections and ultrasound diagnosed uterine fibroids in African-American women. J Womens Health 2015;24:489–95. 10.1089/jwh.2014.5051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol 2016;59:2–24. 10.1097/GRF.0000000000000164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall LM, Spiegelman D, Goldman MB, et al. A prospective study of reproductive factors and oral contraceptive use in relation to the risk of uterine leiomyomata. Fertil Steril 1998;70:432–9. 10.1016/S0015-0282(98)00208-8 [DOI] [PubMed] [Google Scholar]
- 29.Baird DD, Travlos G, Wilson R, et al. Uterine leiomyomata in relation to insulin-like growth factor-I, insulin, and diabetes. Epidemiology 2009;20:604–10. 10.1097/EDE.0b013e31819d8d3f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiaffarino F, Parazzini F, La Vecchia C, et al. Diet and uterine myomas. Obstet Gynecol 1999;94:395–8. 10.1016/s0029-7844(99)00305-1 [DOI] [PubMed] [Google Scholar]
- 31.Velez Edwards DR, Baird DD, Hartmann KE. Association of age at menarche with increasing number of fibroids in a cohort of women who underwent standardized ultrasound assessment. Am J Epidemiol 2013;178:426–33. 10.1093/aje/kws585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Templeman C, Marshall SF, Clarke CA, et al. Risk factors for surgically removed fibroids in a large cohort of teachers. Fertil Steril 2009;92:1436–46. 10.1016/j.fertnstert.2008.08.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brakta S, Diamond JS, Al-Hendy A, et al. Role of vitamin D in uterine fibroid biology. Fertil Steril 2015;104:698–706. 10.1016/j.fertnstert.2015.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen CR, Buck GM, Courey NG, et al. Risk factors for uterine fibroids among women undergoing tubal sterilization. Am J Epidemiol 2001;153:20–6. 10.1093/aje/153.1.20 [DOI] [PubMed] [Google Scholar]
- 35.Vines AI, Ta M, Esserman DA. The association between self-reported major life events and the presence of uterine fibroids. Womens Health Issues 2010;20:294–8. 10.1016/j.whi.2010.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pavone D, Clemenza S, Sorbi F, et al. Epidemiology and risk factors of uterine fibroids. Best Pract Res Clin Obstet Gynaecol 2018;46:3–11. 10.1016/j.bpobgyn.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 37.Baron JA. Beneficial effects of nicotine and cigarette smoking: the real, the possible and the spurious. Br Med Bull 1996;52:58–73. 10.1093/oxfordjournals.bmb.a011533 [DOI] [PubMed] [Google Scholar]
- 38.Boynton-Jarrett R, Rich-Edwards J, Malspeis S, et al. A prospective study of hypertension and risk of uterine leiomyomata. Am J Epidemiol 2005;161:628–38. 10.1093/aje/kwi072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ott J, Kurz C, Braun R, et al. Overt hypothyroidism is associated with the presence of uterine leiomyoma: a retrospective analysis. Eur J Obstet Gynecol Reprod Biol 2014;177:19–22. 10.1016/j.ejogrb.2014.03.003 [DOI] [PubMed] [Google Scholar]