Abstract
Objective
This study systematically reviewed the effect of melatonin (MLT) on quality of life (QoL) and symptoms among patients with cancer.
Design
Systematic review and meta-analysis.
Data sources
Cochrane Library, PubMed, Embase, Web of Science, Medline, CINAHL, Scopus, ClinicalTrials.gov, China Biology Medicine (CBM), ProQuest and Open Grey were searched from inception to November 2021.
Eligibility criteria
We included randomised controlled trials (RCTs) assessing the effects of MLT on QoL, sleep quality, fatigue, depression, pain, stomatitis rate and stomatitis severity in adult patients with cancer, without language restrictions. Studies that reported the effects of MLT along with other interventions and had incomplete or absent outcome data were excluded.
Data extraction and synthesis
Two independent reviewers extracted data, and another two reviewers assessed the risk of bias. The risk of bias for each eligible study was assessed using the Cochrane assessment tool. The mean difference or standard mean difference (SMD) with 95% CIs was used in the computation of continuous variables to synthesise data. The relative risk was used for dichotomous outcomes. Heterogeneity was assessed and quantified (I2 statistic).
Results
A total of 19 qualified studies that included 2101 patients with cancer (MLT: 1078, control: 1023) were included in the meta-analysis. The results indicated that MLT had no significant effect on QoL (SMD=−0.01, 95% CI (−0.14 to 0.11), p=0.83), sleep quality (SMD=−0.18, 95% CI (−0.62 to 0.26), p=0.42), fatigue (SMD=−0.34, 95% CI (−0.73 to 0.06), p=0.10), pain (SMD=−0.34, 95% CI (−0.7 to 0.02), p=0.06) or stomatitis severity (RR=0.78, 95% CI (0.47 to 1.30), p=0.35). MLT reduced stomatitis rate among patients with cancer (RR=0.47, 95% CI (0.26 to 0.88), p=0.02), except those with head and neck cancer (RR=1.09, 95% CI (0.92 to 1.29), p=0.35). MLT eased depression in patients who received administration for more than 14 days (SMD=−0.14, 95% CI (−0.27 to –0.01), p=0.03) and those who underwent surgery (SMD=−0.17, 95% CI (−0.32 to –0.03), p=0.02).
Conclusion
The findings showed that MLT did not improve the QoL, sleep quality, fatigue, pain or stomatitis severity among patients with cancer. It had a limited effect on decreasing the stomatitis rate and easing depression. Different treatments, durations and cancer types were the main sources of heterogeneity. Further large-scale RCTs are urgently needed. In addition, the effects of different combinations of MLT dosage and duration, administration types and joint measures are worthy of further study.
PROSPERO registration number
CRD42021292855.
Keywords: adult oncology, pharmacology, clinical pharmacology
Strengths and limitations of this study.
A strict search strategy was used in multiple databases.
Most of the studies were of high quality with a low risk of bias, which could further lend confidence to the current pooled results.
We widely explored the effectiveness of melatonin (MLT) in different populations, treatments, dosages and durations in subgroup analysis.
For every dimension of MLT, including quality of life, sleep, fatigue, depression, pain and stomatitis, the literature is limited, which limits the generality of the conclusion.
The main significant results were from subgroup analysis of the limited studies, and the results should be interpreted prudently.
Introduction
Melatonin (MLT) is an important endogenous indolamine that is synthesised and secreted into the systemic circulation and cerebrospinal fluid by the pineal gland and has recognised antiaging, anti-inflammatory and antioxidant properties.1 As a strong antifibrotic agent,2 MLT can be used as a desired preconditioning agent in cell-based therapy.3 4 It also has a substantial role in regulating the circadian rhythm and sleep during the night.5 6 Recent studies have proven the effect of MLT on limiting skeletal muscle frailty, prolonging physical performance7 and preventing bone loss.8 In the oncology field, MLT has significantly apoptotic, angiogenic, oncostatic and antiproliferative effects on various oncological cells.9 It was proven that low levels of MLT might be a risk factor for breast cancer.10 Meanwhile, MLT coadministration improves the sensitivity of cancers to inhibition by conventional drugs and reduces the toxic consequences of anticancer drugs while increasing their efficacy.11 There is major concern about the symptoms induced by cancer and cancer treatment that patients encounter, including physical symptoms and psychological/spiritual distress,12 leading to decreased quality of life (QoL). Equally, MLT plays an important role in enhancing QoL by improving survival and decreasing symptoms.13 The positive association between MLT and various health outcomes in patients with cancer has been shown in some studies.14 A recent meta-analysis revealed that MLT may benefit patients with cancer by improving survival and ameliorating the side effects of chemotherapy.15 Palmer et al showed the neuroprotective effect of MLT to counteract the adverse effects of chemotherapy on cognitive function, sleep quality and depressive symptoms in patients with breast cancer.16 A recent clinical trial concluded that MLT supply decreased the levels of fatigue in patients with breast cancer.17 However, some of the recent published findings suggest conflicting results18 19 that MLT intervention cannot improve the QoL, reduce the symptom burden or present the uncertain results.20 We are not aware of any systematic reviews and meta-analyses that have synthesised the evidence of the function of MLT in patients with cancer. The effect of MLT on health outcomes in the cancer group mains nonspecific and ambiguous. Thus, with accumulating evidence, we performed a systematic review and meta-analysis of randomised controlled trials (RCTs) to investigate the roles of MLT in improving QoL and symptoms in patients with cancer.
Materials and methods
Search strategy
A thorough search was conducted in the Cochrane Library, PubMed, Embase, Web of Science, Medline, CINAHL, Scopus, ClinicalTrials.gov and China Biology Medicine (CBM) from inception to November 2021 for RCTs without language restrictions. Sources of unpublished studies and grey literature were searched through ProQuest and Open Grey. We used medical subject headings and text words to search the studies. The search strategies are provided in online supplemental file 1.
bmjopen-2022-060912supp001.pdf (53.9KB, pdf)
Eligibility criteria
Participants
Studies including adult patients (≥18 years) who were diagnosed with cancer according to National Cancer Institute codes, regardless of cancer type, cancer stage (early, middle or advanced) and current treatment (such as radiation therapy, chemotherapy, surgery, targeted therapy, immunotherapy, combination of any of the above treatments or without any treatment), were eligible.
Interventions and controls
All trials that reported and evaluated the effects of MLT were included. Literature were excluded if they met the following criteria: (1) they were not RCTs; (2) studied the effects of MLT along with other interventions and (3) incomplete or absent outcome data.
Outcomes
The primary outcome was QoL. The scores of sleep quality, fatigue, depression and pain, stomatitis rate and stomatitis severity were the secondary outcomes.
Studies
Only RCTs were eligible.
Data extraction
Two independent researchers (SY, TW) extracted the data of eligible studies and performed double-checks. Any disagreements and differences were resolved by a third investigator (XB). The following data from the full text of selected studies were extracted: first author’s name, year of publication, the characteristics of the patients, the characteristics of the intervention and the control groups (study design, form of intervention, dose of MLT supplementation, duration), number of participants in each group and outcome data (means and SD for continuous data; number of incidents for dichotomous data).
Risk of bias assessment
Two reviewers (YT, HC) independently evaluated the risk of bias for each eligible study using the Cochrane assessment tool, which consists of the following seven domains: ‘random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias’. Each question can be rated as follows: yes (+), low risk of bias; unclear (?), unclear risk of bias; no (−), high risk of bias. Any disagreement between the reviewers was resolved by discussion until consensus was reached.
Data analysis
The meta-analysis was performed using Review Manager Software (V.5.3). The scores of QoL and symptoms was estimated by the mean difference (MD) when trials measured an outcome using the same measurement method or scale. We used the standard MD (SMD) when different instruments were used to measure the same outcome. For dichotomous outcomes (such as stomatitis rate and stomatitis severity), we used relative risk (RR). The effect size (ES) and the 95% CI were computed. ESs with scores of 0.2–0.5, 0.5–0.8 and >0.8 were considered as small, medium and large effects, respectively. Forest plots were used to display the pooled ES, 95% CI, weight in percentage. If variability was presented by measures other than the mean or SD, we used standard approaches for estimating data. If the studies did not report SD, we used the following formula to calculate missing SD: SD=√N×(upper 95% CI-lower 95% CI)/3.92. If a study provided medians and IQRs, we transformed the median and IQR to the mean and SD by a method for nonnormal data.21 I2 was used to measure the statistical heterogeneity among the trials in each analysis. If p>0.1 and I2 <50%, a fixed-effects model was adopted; if p<0.1 and I2 ≥50%, then a random-effects model was adopted. If heterogeneity was identified, subgroup analyses were conducted on different cancer types, treatments, dosages and durations. Sensitivity analysis was performed by removing studies with a high or unclear risk of bias. Reporting and publication bias were investigated by visually examining the degree of asymmetry of a funnel plot.
Patient and public involvement
No patients were involved.
Results
Literature search
The initial search identified 1670 publications through PubMed, Embase, Medline, Scopus, SinoMed, Web of Science, Cochrane and Clinical Trials. After excluding 501 duplicates, a total of 1161 studies were retrieved for title and abstract screening. After screening the titles and abstracts, 1111 articles were excluded, and 50 papers were retrieved for full text review. Out of 50 retrieved papers, 1 article was excluded due to wrong languages,22 6 articles were excluded without complete data, 14 articles were excluded without full text, 9 articles were excluded without target outcome and 1 article was excluded due to a non-RCT study.23 Therefore, a total of 19 articles were included in the final meta-analysis.24–42 The flow chart of the literature search is shown in figure 1.
Figure 1.
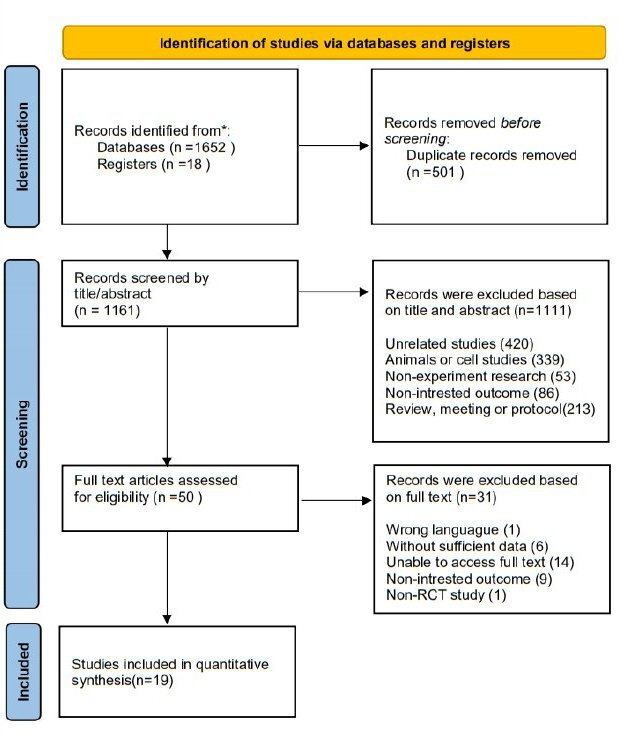
Study flow diagram. RCT, randomised controlled trial. *The number of records identified from each database and register searched were: pubmed (16), embase (38), medline (289), scopus (508), sinomed (21), web of science (633), cochrane (136), clinical trial (18).
Quality assessment
We used the Cochrane scoring system to assess the quality of the included studies. Two reviewers had different opinions on bias in one article in ‘incomplete outcome data’, and one article in ‘selective reporting’. Through the discussion, final consensus was achieved. The overall risk of bias as shown was moderate (figure 2). Nearly all studies reported appropriate random sequence generation. Most studies reported completed data and had low risk of bias on the item ‘selective reporting’. Almost one-third of the studies did not report blindness in the outcome assessment. The individual risk of bias for each study is presented in figure 3.
Figure 2.
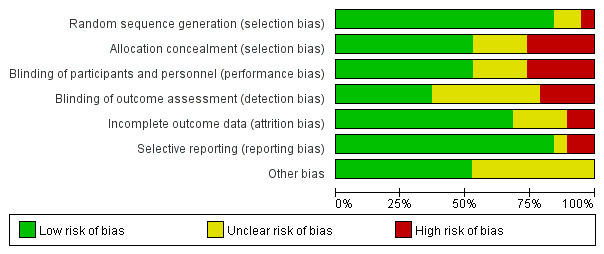
The overall risk of bias.
Figure 3.
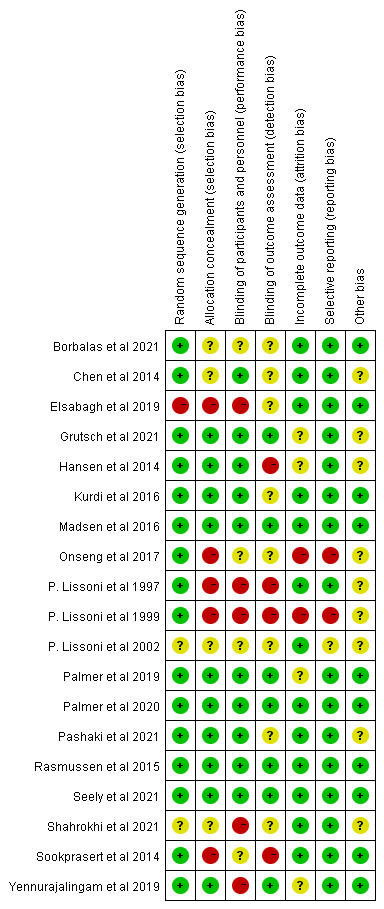
The individual risk of bias for each study.
Literature characteristics
The characteristics of the patients, interventions, controls and measures are shown in table 1.
Table 1.
The characteristics of the literature
| Author | Year | Population | Mean age (intervention/ control) |
Study design | Time of duration | Administration time | Intervention group | Control group | Outcome | Intervention (N) |
Control (N) |
| Lissoni et al31 | 1997 | Patients with metastatic solid tumours under chemotherapy | 61/58 | RCTs | Every day without a break until disease progression | Every night | 20 mg oral MLT | Placebo | Stomatitis rate | 39 | 40 |
| Lissoni et al30 | 1999 | Patients with metastatic solid tumour under chemotherapy | 53/56 | RCTs | 7 days prior to chemotherapy, continued after chemotherapy interruption, until disease progression | Every night | 20 mg oral MLT | Placebo | Stomatitis rate | 124 | 126 |
| Lissoni et al29 | 2002 | Untreated patients with metastatic solid tumours under chemotherapy | 66/65 | RCTs | At least 2 months | Every night | 20 mg oral MLT plus support care | Placebo plus support care | Stomatitis rate | 98 | 102 |
| Hansen et al27 | 2014 | Patients undergoing breast cancer surgery | 51/60 | RCTs | 10days: 2 days preoperatively till 8 days postoperatively | One hour before bedtime | 6 mg oral MLT | Placebo | Depression (MDI), Sleepiness (KSS, VAS), Fatigue (VAS), Pain (VAS) | 28 | 26 |
| Chen et al24 | 2014 | Breast cancer survivors | 59/59 | RCTs | 4 months | Each night at nine pm | 3 mg oral MLT | Placebo | Sleep (PSQI), depression (CES-D) | 48 | 47 |
| Sookprasert et al41 | 2014 | Patients with non-small cell lung cancer receiving chemotherapy | 56.8/55.6 | RCTs | 2 months: during chemotherapy | At night after 21:00 | 10 mg or 20 mg oral MLT | Placebo | QOL(FACT-L), Mucositis rate | 88 | 38 |
| Lund Rasmussen et al33 | 2015 | Patients with advanced cancer who reported significantly tired in palliative care unit | 64/65 | RCTs | 7 days | Every night | 20 mg oral MLT | Placebo | Fatigue (MFI-20), QOL (EORTC QLQ-C15-PAL), Insomnia (EORTC QLQ-C15-PAL) | 21 | 23 |
| Madsen et al34 | 2016 | Patients undergoing breast cancer surgery | 51/59 | RCTs | 2 weeks: 3 days preoperatively until 2 weeks postoperatively | One hour before bedtime | 6 mg oral MLT | Placebo | Sleep (VAS, KSS), pain (VAS) | 27 | 21 |
| Kurdi et al28 | 2016 | Patients with cancer with Insomnia | 55.2/49.64 | RCTs | 14 days | At 19:00 hours | 3 mg oral MLT | Placebo | Sleep (AIS) | 25 | 25 |
| Onseng et al35 | 2017 | Patients with head and neck cancer receiving concurrent chemoradiation | 47.3/49.6 | RCTs | 35days: 5 days a week throughout the 7 weeks of chemoradiation | At night after 21:00 | 10 mL of a 0.2% MLT niosome oral gargle plus 20 mg oral dosage | placebo | QOL (FACT—H&N), Mucositis rate, Mucositis severity (WHO-G) | 19 | 20 |
| Elsabagh et al25 | 2019 | Patients with head and neck cancer undergoing radiotherapy | 57.8/55.9 | RCTs | Six weeks | 30 min before sleeping | 20 mg oral MLT | Placebo | Stomatitis severity (WHO-G), Stomatitis rate, Pain (NRS) | 20 | 20 |
| Palmer et al36 | 2019 | Patients with breast cancer receiving chemotherapy | 54.24/54.11 | RCTs | 10 days during treatment. | One hour before bedtime | 20 mg oral MLT | Placebo | Pain (NRS), Sleep (PSQI), Depression (BDI) | 18 | 18 |
| Yennurajalingam et al42 | 2019 | Patients with advanced cancer with poor sleep quality | Not clearly | RCTs | 14 days | At bedtime | 20 mg oral MLT plus bright white light therapy | Bright white light therapy alone | Sleep (PSQI), insomnia (ISI), fatigue (FACIT-F), depression (HADS), QOL(FACT) | 6 | 8 |
| Palmer et al37 | 2020 | Patients with breast cancer undergoing chemotherapy after lumpectomy or mastectomy | 54.24/54.11 | RCTs | 10 days: 3 days prior to chemotherapy and seven following days | One hour before bedtime | 20 mg oral MLT | Placebo | Depressive symptoms (BDI-II), Sleep quality (PSQI), QOL (EORTC QLQ-C30) | 18 | 18 |
| Sedighi Pashaki et al38 | 2021 | Patients with breast cancer during adjuvant chemotherapy and radiotherapy | 50.47/46.05 | RCTs | 8 weeks: from 1 week before until 1 month after the adjuvant radiotherapy | One hour before bedtime | 18 mg oral MLT | Placebo | Fatigue (BFI) | 38 | 36 |
| Seely et al39 | 2021 | Patients with cancer following lung cancer resection | 67.2/67.2 | RCTs | One year after surgery | One hour before bedtime | 20 mg oral MLT | Placebo | Fatigue (MFI-20), QOL(QLQ-LC13), Sleep (MOS), Depression (BDI 2), Pain (BPI) | 356 | 353 |
| Shahrokhi et al40 | 2021 | Patients with colorectal cancer undergoing chemotherapy with sleep disorder | 63.63/64.11 | RCTs | 4 weeks of treatment | At bedtime | 6 mg oral MLT | 10 mg zolpidem | Sleep (GSQS, PSQI), Depression (HRSD) | 45 | 45 |
| Grutsch et al26 | 2021 | NSCLC patients under chemotherapy | 60.3/63 | RCTs | From intervention to death | At 8:00 hours or at 20:00 hours | 20 mg oral MLT | Placebo | QOL(QLQ-C30), Fatigue (QLQ-C30), Pain (QLQ-C30), Sleep (PSQI) | 20 | 18 |
| A. Lozano et al 32 | 2021 | Patients with head and neck cancer undergoing radiation therapy and chemical treatment | 59/56 | RCTs | 5 days a week, lasting 7 weeks | Not clearly | 3% oral MLT gel plus standard symptomatic treatment for stomatitis | Placebo plus standard symptomatic treatment for stomatitis | Stomatitis severity (WHO-G), Stomatitis rate | 40 | 39 |
AIS, Athens insomnia scale; BDI 2, Beck Depression Inventory 2; BDI-II, Beck Depression Inventory; BFI, Brief Fatigue Inventory; BPI, Brief Pain Inventory; CES-D, Center for Epidemiologic Studies-Depression; EORTC QLQ-C30, European Organisation for Cancer Research and Treatment of Cancer Quality of Life Questionnaire; EORTC QLQ-C15-PAL, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 15 Palliative Version; FACIT-F, Functional Assessment of Cancer Illness Therapy-Fatigue subscale; FACT, Functional Assessment of Cancer Illness Therapy; FACT-H&N, Functional Assessment of Cancer Therapy-H&N Version 4; FACT-L, Functional Assessment of Cancer Therapy-Lung; GSQS, Sleep Quality Scale; HADS, Hospital Anxiety and Depression Scale; HRSD, Hamilton Rating Scale for Depression; ISI, Insomnia Severity Index; KSS, Karolinska Sleepiness Scale; MDI, Major Depression Inventory; MFI-20, Multidimensional Fatigue Inventory; MLT, Melatonin; MOS, Medical Outcomes Study Sleep Survey; N, number; NRS, Numeric Rating Scales; PSQI, Pittsburgh Sleep Quality Index; QLQ-LC13, Lung Cancer-13 modules; RCTs, randomised controlled trials; VAS, Visual Analogue Scale; WHO-G, WHO grading system.
Participants
The publication dates ranged from 1997 to 2021. Among the 19 studies included in the systematic review, the mean age of the participants ranged from 46.05 to 67.2. The sample size ranged from 14 to 709 participants. Regarding treatment trajectory, 12 studies were conducted in patients with cancer with adjuvant chemotherapy and/or radiotherapy,25 26 29–32 35–38 40 41 1 was in patients with advanced cancer with fatigue,33 1 was in breast cancer survivors,24 3 were in patients with cancer with surgery,27 34 39 and 2 were in patients with advanced cancer with poor sleep quality.28 42 Regarding cancer diagnosis, six studies were in breast cancer,24 27 34 36–38 two were in non-small-cell lung cancer,26 39 three were in head and neck cancer,25 32 35 one was in colorectal cancer40 and seven studies were not restricted to cancer type but were mostly in patients with advanced cancer.28–31 33 41 42
Intervention
The follow-up period ranged from 7 days to 1 year. The MLT dosage varied between 3 and 20 mg. Regarding the types of MLT administration, 17 involved oral MLT,24–31 33 34 36–42 1 involved MLT oral gargle32 and 1 was combined.35 Nearly all studies gave the MLT at night, except one which compared dosage given both in the morning and at night.26
Instruments
All studies used standardised and validated tools. QoL was measured by four validated tools: the European Organisation for Cancer Research,26 37 Treatment validated for the Brazilian population (QLQ-BR 23),37 Functional Assessment of Cancer Therapy,41 42 Ferrans and Powers Quality of Life Index,26 European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 15 Palliative Version (EORTC QLQ-C15-PAL)33 and Lung Cancer-13.39 Sleep quality was measured by the Pittsburgh Sleep Quality Index,24 26 36 37 40 42 Visual Analogue Scale (VAS),27 34 Karolinska Sleepiness Scale,27 34 Sleep Quality Scale,40 Athens insomnia scale28 and Medical Outcomes Study Sleep Survey.39 Depression was measured by the Beck Depression Inventory,37 39 Beck Depression Inventory,36 Center for Epidemiologic Studies-Depressio,24 Major Depression Inventory,27 Hamilton Rating Scale for Depression,40 Hospital Anxiety and Depression Scale.42 Fatigue was measured by the Multidimensional Fatigue Inventory,33 EORTC QLQ-C15-PAL (fatigue domain),33 Brief Fatigue Inventory,38 VAS,27 Multidimensional Fatigue Index 20 questionnaire,39 QLQ-C30 (fatigue domain)26 and Functional Assessment of Cancer Illness Therapy-Fatigue subscale.42 Pain was measured by VAS,27 34 Brief Pain Inventory,39 QLQ-C30 (pain domain)26 and Numeric Rating Scales.25 36 The incidence of stomatitis was calculated by the ratio of the occurrence number to the total number.25 29–32 35 41
Meta-analysis
Effect of MLT on QoL
Overall, six clinical trials evaluated the effect of MLT on QOL. The results showed that there was no statistically significant difference between the intervention and control groups (SMD=−0.01, 95% CI (−0.14 to 0.11), p=0.83) with no heterogeneity (I2=0%, p=0.42) (figure 4). All six studies used a 20 mg MLT dosage. Subgroup analysis based on study durations (p=0.65–0.92) and treatment types(p=0.45–0.6) showed no significant differences.
Figure 4.
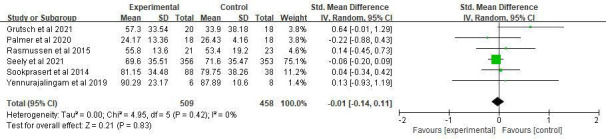
Forest plot of the effect of MLT on QoL among patients with cancer. MLT, melatonin; QoL, quality of life.
Effect of MLT on sleep quality
Nine clinical trials evaluated the effect of MLT on sleep quality. Pooled ES from the random effect model showed that there was no significant effect on sleep quality (SMD=−0.18, 95% CI (−0.62 to 0.26), p=0.42) (figure 5). There was significant heterogeneity between studies (I2=87%, p<0.001). We deleted a study40 with obvious heterogeneity and I2 decreased to 79% (SMD=−0.35, 95% CI (−0.73 to 0.03), p=0.07). Subgroup analysis based on dosage, study durations, treatments, different combinations of dosage and duration showed no significant differences between subgroups (table 2).
Figure 5.
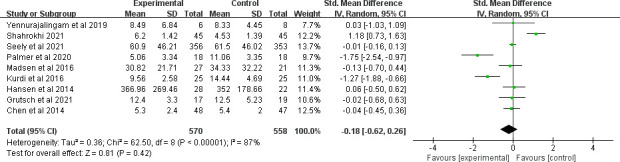
Forest plot of the effect of MLT on sleep quality among patients with cancer. MLT, melatonin.
Table 2.
Subgroup analyses of melatonin supplementation on sleep quality
| N | WMD (95% CI) | Heterogeneity I2 (p value) | P within group | |
| Overall effect | 8 | −0.35 (−0.73 to 0.03) | 79% (p<0.0001) | 0.07 |
| Dosage | ||||
| <10 mg | 4 | −0.32 (−0.88 to 0.23) | 77% (p=0.005) | 0.25 |
| ≥10 mg | 4 | −0.42 (−1.16 to 0.32) | 84% (p=0.0004) | 0.27 |
| Duration | ||||
| <2 weeks | 2 | −0.01 (−0.33 to 0.32) | 0% (p=0.76) | 0.96 |
| ≥2 weeks | 6 | −0.51 (−1.07 to 0.05) | 85% (p<0.00001) | 0.08 |
| Combination | ||||
| <10 mg + ≥2 weeks | 3 | −0.46 (−1.18 to 0.27) | 83% (p=0.003) | 0.22 |
| >10 mg + ≥2 weeks | 3 | −0.01 (−0.16 to 0.13) | 0% (p=1.00) | 0.86 |
| >10 mg + <2 weeks | 1 | −1.75 (−2.54 to 0.97) | – | <0.01 |
| <10 mg + <2 weeks | 1 | 0.06 (−0.5 to 0.62) | – | 0.83 |
| Combined treatment | ||||
| Under chemotherapy | 2 | −0.87 (−2.57 to 0.82) | 91% (p=0.0009) | 0.31 |
| Under surgery | 3 | −0.02 (−0.15 to 0.12) | 0% (p=0.89) | 0.83 |
| With insomnia | ||||
| Yes | 2 | −0.7 (−1.96 to 0.57) | 77% (p=0.04) | 0.28 |
| No | 6 | −0.23 (−0.58 to 0.13) | 73% (p=0.002) | 0.21 |
N, number of the literatures.
Effect of MLT on fatigue
The overall ES of MLT for fatigue alleviation was medium (SMD=−0.34, 95% CI (−0.73 to 0.06), p=0.10) with high heterogeneity (p=0.002, I2=74%). However, there was no significant difference. The study of Sedighi Pashaki et al38 showed great heterogeneity because only this one proved a significantly lower level of fatigue in the intervention group. We removed it, and the heterogeneity decreased to 0% (figure 6).
Figure 6.
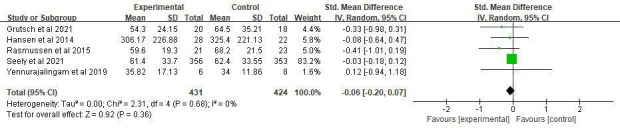
Forest plot of the effect of MLT on fatigue among patients with cancer. MLT, melatonin.
Effect of MLT on depression
Six clinical trials evaluated the effect of MLT on depression. Only Palmer et al showed a significant effect on depression.37 The overall treatment effect on depression showed that there was no statistically significant difference between the intervention and control groups (SMD=−0.24, 95% CI (−0.53 to 0.05), p=0.10) with high heterogeneity (p=0.03, I2=60%). A sensitivity analysis was performed by removing one study from the analysis (figure 7).37 Regarding subgroup analysis, a significant difference was observed for different study durations and treatments, although both showed a slight ES. Patients who received an intervention duration greater than 14 days had significantly lower depression (SMD=−0.14, 95% CI (−0.27 to –0.01), p=0.03) with low heterogeneity (p=0.4, I2=0%) (figure 8). Meanwhile, MLT alleviated depression in patients with cancer who underwent surgery (SMD=−0.17, 95% CI (−0.32 to –0.03), p=0.02) with low heterogeneity (p=0.35, I2=0%) compared with those received chemotherapy (figure 9). No significant difference was observed among studies on the different dosages (p=0.27–0.43), cancer diagnosis (p=0.20) and combined chemotherapy (p=0.13–0.42).
Figure 7.
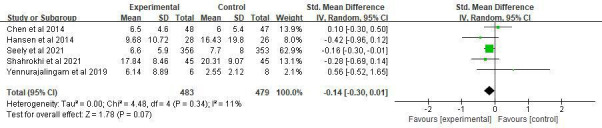
Forest plot of the effect of MLT on depression among patients with cancer. MLT, melatonin.
Figure 8.
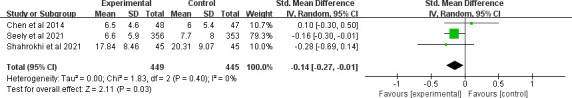
Forest plot of longer MLT duration on depression among patients with cancer. MLT, melatonin.
Figure 9.
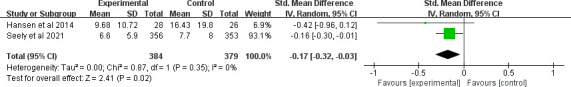
Forest plot of MLT on depression among patients with cancer underwent surgery. MLT, melatonin.
Effect of MLT on pain
Five clinical trials evaluated the effect of MLT on pain (SMD=−0.34, 95% CI (−0.7 to 0.02), p=0.06) with high heterogeneity among studies (p=0.03, I2=62%). No significant difference was observed among studies on the cancer diagnosis (p=0.27–0.47), combined treatments (p=0.37), durations (p=0.11) and dosages (p=0.16–0.27). Sensitivity analysis was performed by removing one study,25 and the heterogeneity decreased to 0% (figure 10).
Figure 10.
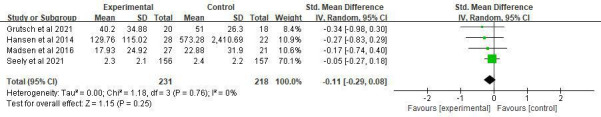
Forest plot of the effect of MLT on pain among patients with cancer. MLT, melatonin.
Effect of MLT on stomatitis
Regarding stomatitis, seven clinical trials evaluated the effect of MLT on the incidence of stomatitis. The finding showed that there was no significant ES (RR=0.71, 95% CI (0.45 to 1.13), p=0.15) (figure 11), with high heterogeneity (p<0.001, I2=86%). All of the study durations were more than 2 weeks, and all patients accepted chemotherapy or radiotherapy. In addition, nearly all these clinical trials gave an MLT of 20 mg, except one that used a 3% MLT oral gel.32 However, removing it or not caused little change to heterogeneity and ES. Further subgroup analysis showed that the cancer type might be the main source of heterogeneity. MLT did not reduce the incidence of stomatitis among patients with head and neck cancer under adjuvant chemotherapy or radiotherapy (RR=1.09, 95% CI (0.92 to 1.29), p=0.35), with low heterogeneity (p=0.64, I2=0%). However, it had significant value in patients with other kinds of tumours except head and neck cancer (RR=0.47, 95% CI (0.26 to 0.88), p=0.02) with high heterogeneity (p=0.03, I2=66%) (figure 12).
Figure 11.
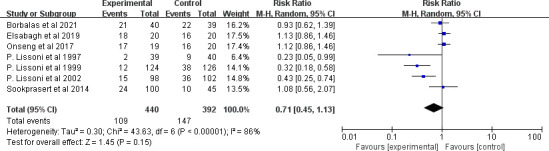
Forest plot of the effect of MLT on stomatitis incidence among patients with cancer. MLT, melatonin.
Figure 12.
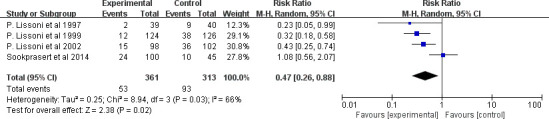
Forest plot of the effect of MLT on stomatitis incidence among patients with cancer except head and neck cancer. MLT, melatonin.
For stomatitis severity, three clinical trials evaluated the effect of MLT on reducing 3–4 grade (severe) stomatitis according to the WHO grading system.43 The overall treatment effect showed that the intervention had no statistically significant difference between the intervention and control groups (RR=0.78, 95% CI (0.47 to 1.30), p=0.35) with low heterogeneity (p=0.22, I2=35%) (figure 13).
Figure 13.
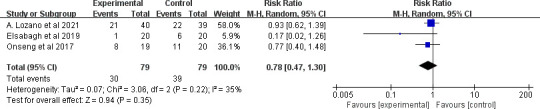
Forest plot of the effect of MLT on stomatitis severity among patients with cancer. MLT, melatonin.
Discussion
To the best of our knowledge, this study is the first meta-analysis to investigate the effect of MLT on QoL, sleep quality and other symptoms, such as fatigue, depression, pain and stomatitis, in patients with cancer. Unfortunately, in this study, we did not prove the beneficial effect of MLT on QoL, sleep quality, fatigue or pain. However, it has the potential to improve depression and reduce the incidence of stomatitis with small ESs.
Most of the suffering that patients with cancer now face comes from disturbing symptoms, such as poor sleep, fatigue, depression, pain and so on. Effective symptom control greatly improves QoL. Thus, the effect of MLT on QoL might be achieved through relieving symptoms. Innominato et al revealed that bedtime MLT was associated with a significant improvement in sleep quality, fatigue severity, QoL, and social and cognitive function in patients with advanced breast cancer.44 However, contradictory conclusions revealed that MLT did not improve appetite, weight, or QoL in cachectic patients with advanced cancer.45 In addition, a previous study reported beneficial short-term effects of MLT on sleep but not QoL.46 Our review included six trials that regarded the QoL of patients with cancer as a health outcome. None of them proved a significant improvement in QoL in the intervention group, although Grutsch et al26 and Sookprasert et al41 provided a trend for better QoL compared with baseline. For such invalid effectiveness, one of the possible interpretations might be the differences due to the study population, interventions and measurements. Another explanation might be the multidimensional properties of QoL, which contained domains of physical, psychology, spirit and social. Thus, the mere elimination of symptoms played a limited role in improving the QoL, especially for patients with cancer who were faced with many other disturbing aspects.
Due to the important role in regulating the circadian rhythm and sleep, many studies have been conducted to verify the value of MLT on sleep. MLT may be preferable to traditional hypnotics in the management of insomnia.47 A network meta-analysis supports the effectiveness of MLT in improving sleep-onset difficulties.48 A review of the influence of dietary sources of MLT on sleep quality indicated that the sources of MLT consumption of milk and sour cherries may improve sleep quality.49 There are many conflicting studies regarding different populations, dosages and durations. Fatemeh et al found the significant effects of MLT on sleep quality in patients with respiratory diseases, metabolic disorders and sleep disorders but not in mental disorders, neurodegenerative diseases and breast cancer.50 Under the condition of using the Pittsburgh Sleep Quality Index as a unified measurement tool, 20 mg MLT for 10 days in patients with breast cancer under chemotherapy showed a positive sleeping improvement,37 while the same dosage for at least 28 days revealed a meaningless result in patients with lung cancer.51 Meanwhile, the optimal combination of dosage and duration remains unknown. Innominato et al found that 5 mg for 2 months has a positive effect on sleep quality and QoL in patients with advanced breast cancer.44 Similarly, in patients with advanced cancer, the combination of 14 days 20 mg MLT plus bright white light therapy did not improve sleep quality.42 Under fewer doses, 14 days 3 mg MLT actually improved sleep in patients with cancer with insomnia.28 Our review revealed that MLT could not improve sleep. The subgroup analysis did not find a significant difference in different MLT durations, dosages, or combinations of dosage and duration. The optimal combination of dosage and duration in improving sleep for patients warrants further exploration. The administration type is another factor. It was found that a 2 mg prolonged release MLT formulation for 14 days resulted in significant and clinically meaningful improvements in sleep quality, morning alertness and sleep-onset latency in primary insomnia patients52 and in Parkinson’s disease patients with a poor sleep quality.53 However, most of the studies we included used oral MLT. How the administration type affects effect on sleep in patients with cancer remains to be studied. The effectiveness of the combination of bright light and MLT remains controversial. Yennurajalingam et al proved that it could not work in patients with advanced cancer with insomnia,42 while it could improve subjective daytime sleepiness in patients with delayed sleep phase disorder.54
MLT may be an effective treatment for patients with chronic fatigue syndrome.55 Nevertheless, in this study, none of the studies showed any improvement in fatigue in patients with cancer. Only a high-quality trial proved a significant effect of MLT on patients with reast cancer undergoing adjuvant chemotherapy and radiotherapy,38 with MLT doses of 18 mg a day from 1 week before until 1 month after adjuvant radiotherapy. The evidence supporting the usage of MLT for cancer-related fatigue (CRF) is limited. Short-term use of dexamethasone or methylprednisolone is recommended for the control of CRF in patients with metastatic cancer according to European Society for Medical Oncology guidelines,56 while the use of eszopiclone, megestrol acetate and MLT is not recommended for the control of CRF. However, the preventive effect of MLT on CRF is still under study. Nonpharmaceutical interventions were also recommended,56 such as relaxation exercise, massage, cognitive–behavioural therapy and physical activity, which were demonstrated to have moderate-to-large ESs.57 Multimodal therapy, qigong, aerobic exercise and cognitive–behavioural therapy might be the best choices for CRF.58
MLT seems to be able to ease pain. However, the results have varied in different studies. Lee et al found that the prophylactic administration of MLT conferred significant clinical benefits in reducing postoperative pain and opioid use, as well as improved sensory recovery following orthognathic surgery.59 Tunay et al found that preoperative oral MLT led to a reduction in pain scores, total morphine consumption and supplemental analgesic requirements after surgery.60 MLT could also improve pain in females with primary dysmenorrhea.61 However, the evidence was limited in critically ill patients in the ICU and patients after total knee arthroplasty.62 63 For patients with cancer, the evidence is also restricted. Our review revealed that MLT had no effect on pain relief. Only Elsabagh et al found a beneficial effect of MLT on alleviating pain in patients with head and neck cancer undergoing radiotherapy, with a dosage of 20 mg for 6 weeks.25 At the same time, Palme et al found a drop in pain scores from baseline in the intervention group.36 The minor role of MLT on pain in patients with cancer could be explained by the fact that cancer-related pain is one of the most common and troublesome symptoms affecting patients with cancer with high severity.64 For such severe pain, effective analgesics, such as opioids, are more helpful. In addition, despite the availability of effective treatments, cancer-related pain may be inadequately controlled in up to 50% of patients. Thus, multidisciplinary interventions are required,65 and single MLT seems too weak for cancer pain.
Circadian rhythm disruption underlies the pathophysiology of psychiatric disorders, especially depression.6 MLT is a pleiotropic regulator molecule, and its analogues have been observed to resynchronise the circadian rhythm and to alleviate depressive symptoms.66 However, duration and treatment might affect the antidepressant effects of MLT, and both showed a slight ES. We found that MLT supplementation had a significant effect in patients who received more than 14 days of treatments and those who underwent surgery. Our assumption is that patients under operation tend to be in the early stages of the disease with lighter disease load and slight depression. The antidepressant effect of a long MLT duration in patients with less serious disease was shown in some studies. For example, MLT for 12 weeks had beneficial effects on decreasing depression in women with polycystic ovary syndrome,67 patients with Parkinson’s disease68 and diabetic haemodialysis patients.69 Nevertheless, it had no prophylactic antidepressant effect on acute coronary syndrome70 or patients with acute mania.71
Oral mucositis refers to inflammation and ulceration of the oral mucosa, which is a frequent side effect of cancer therapy.72 Stomatitis, especially the grade 3 or 4 mucositis,73 can hamper oral nutrition, resulting in malnutrition, reduce QoL and introduce the need for dose reductions and interruption of chemotherapy.74 MLT has potential direct antitumour activity and has been proven to modulate the effects of cancer chemotherapy by enhancing therapeutic efficacy and reducing toxicity.75 Our review showed that MLT had no effect on mucositis. Further subgroup analysis showed that the cancer type was the major source of heterogeneity. MLT could not reduce the stomatitis rate among patients with head and neck cancer, while it had a slightly significant effect in patients with other tumours. Among the studies conducted in patients with head and neck cancer, Borbalas et al35 found that oral MLT gel demonstrated a consistent trend in lowering incidence and shortening mucositis duration.32 Onseng et al revealed that adjuvant MLT delayed the onset of oral mucositis. Elsabagh et al found that MLT reduced severe oral mucositis development.25 None of them proved that MLT could reduce the incidence of stomatitis. The possible interpretation was the significant toxicity of systemic high doses of chemotherapy and radiotherapy for head and neck cancer.76 Compared with other patients with cancer who only received chemotherapy or radiotherapy, most patients with head and neck cancer received the combined chemoradiotherapy. In addition, radiation in the head and neck increases the odds of stomatitis occurrence. We also found that in the MLT group, the reported incidence of stomatitis was higher in patients with head and neck cancer (52.5%–90%) than in other cancer populations (5.12%–24%). Moreover, our review revealed that MLT could not reduce the severity of stomatitis. A meta-analysis showed that probiotics might reduce the incidence and mitigate the severity of cancer therapy-induced mucositis.77 Additionally, photobiomodulation was recommended for the prevention of mucositis.78 79 However, how they affect patients with head and neck cancer under chemoradiotherapy is still unknown.
Strengths and limitations
To the best of our knowledge, this study is the first meta-analysis to investigate the effect of MLT on QoL and symptoms in patients with cancer. Eleven databases were widely searched for eligible studies. Risk of bias analysis was conducted independently by two reviewers using the validated Cochrane assessment tool. The trial quality was generally moderate, with most studies having a low risk of bias, which could further lend confidence to the current pooled results. In the subgroup analysis, we also widely explored the effectiveness of MLT in different populations, treatments, dosages and durations. There are some limitations. The first is the insufficient literature. We reviewed many aspects of MLT, such as QoL, sleep, fatigue, depression, pain and stomatitis. A total of 19 articles were included in the final meta-analysis. However, for every dimension, the literature is limited, from only 5–9. This is mostly the result of the lack of RCTs of MLT in patients with cancer. Thus, insufficient data were used for synthesis. There were 14 excluded articles without full text, which limits the generality of the conclusion. Meanwhile, the assessment of publication bias was not allowed because no dimension had more than 10 references. Furthermore, the main significant results were from subgroup analysis, and the results should be interpreted prudently.
Conclusion
Due to its nontoxic property and beneficial effects,80 81 MLT is increasingly used as an adjuvant medicine in anticancer treatment. We included a moderate number of trials with varied populations and examined the effectiveness of MLT on patients with cancer to provide evidence-based findings on using MLT in a real clinical setting. Our review showed that MLT did not improve QoL, sleep quality, fatigue or pain among patients with cancer. MLT has positive effects on decreasing the stomatitis incidence and depression, which may make it a reasonable option for stomatitis and depression prevention in the clinic. Even so, there are still many restrictions. Further large-scale RCTs are urgently needed. In addition, the effects of different combinations of MLT dosage and durations, administration types and joint measures are worthy of further study.
Supplementary Material
Footnotes
Contributors: All authors had contributed to this study. XL and RF conceived and designed the original study protocol. RF and XB performed literature search and literature screening. SY and TW took responsibility for the integrity of the data and the data analysis. XL interpreted the results. YT and HC assessed the risk of bias of the studies. RF was responsible for writing the first draft of the paper and revision of the manuscript. XL is responsible for the overall content as guarantor. All authors critically reviewed and approved the final manuscript.
Funding: This study was funded by Special Science Popularization for Construction of Innovative Hunan Province (2019ZK4029).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This is a meta-analysis. The Hunan Cancer Hospital Research Ethics Committee has confirmed that no ethical approval is required.
References
- 1.Stacchiotti A, Favero G, Rodella LF. Impact of melatonin on skeletal muscle and exercise. Cells 2020;9:288. 10.3390/cells9020288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mortezaee K, Majidpoor J, Daneshi E, et al. Post-treatment of melatonin with CCl4 better reduces fibrogenic and oxidative changes in liver than melatonin co-treatment. J Cell Biochem 2018;119:1716–25. 10.1002/jcb.26331 [DOI] [PubMed] [Google Scholar]
- 3.Mortezaee K, Pasbakhsh P, Ragerdi Kashani I, et al. Melatonin pretreatment enhances the homing of bone marrow-derived mesenchymal stem cells following transplantation in a rat model of liver fibrosis. Iran Biomed J 2016;20:207–16. 10.7508/ibj.2016.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mortezaee K, Khanlarkhani N, Sabbaghziarani F, et al. Preconditioning with melatonin improves therapeutic outcomes of bone marrow-derived mesenchymal stem cells in targeting liver fibrosis induced by CCl4. Cell Tissue Res 2017;369:303–12. 10.1007/s00441-017-2604-1 [DOI] [PubMed] [Google Scholar]
- 5.Xie Z, Chen F, Li WA, et al. A review of sleep disorders and melatonin. Neurol Res 2017;39:559–65. 10.1080/01616412.2017.1315864 [DOI] [PubMed] [Google Scholar]
- 6.Satyanarayanan SK, Su H, Lin Y-W, et al. Circadian rhythm and melatonin in the treatment of depression. Curr Pharm Des 2018;24:2549–55. 10.2174/1381612824666180803112304 [DOI] [PubMed] [Google Scholar]
- 7.Maria S, Swanson MH, Enderby LT, et al. Melatonin-micronutrients osteopenia treatment study (MOTS): a translational study assessing melatonin, strontium (citrate), vitamin D3 and vitamin K2 (MK7) on bone density, bone marker turnover and health related quality of life in postmenopausal osteopenic women following a one-year double-blind RCT and on osteoblast-osteoclast co-cultures. Aging 2017;9:256–85. 10.18632/aging.101158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maria S, Samsonraj RM, Munmun F, et al. Biological effects of melatonin on osteoblast/osteoclast cocultures, bone, and quality of life: implications of a role for MT2 melatonin receptors, MEK1/2, and MEK5 in melatonin-mediated osteoblastogenesis. J Pineal Res 2018;64:12465. 10.1111/jpi.12465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhattacharya S, Patel KK, Dehari D, et al. Melatonin and its ubiquitous anticancer effects. Mol Cell Biochem 2019;462:133–55. 10.1007/s11010-019-03617-5 [DOI] [PubMed] [Google Scholar]
- 10.Veiga ECdeA, Simões R, Valenti VE, et al. Repercussions of melatonin on the risk of breast cancer: a systematic review and meta-analysis. Rev Assoc Med Bras 2019;65:699–705. 10.1590/1806-9282.65.5.699 [DOI] [PubMed] [Google Scholar]
- 11.Reiter R, Rosales-Corral S, Tan D-X, et al. Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. Int J Mol Sci 2017;18:843. 10.3390/ijms18040843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C, Li M, Meng H, et al. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci China Life Sci 2019;62:640–7. 10.1007/s11427-018-9461-5 [DOI] [PubMed] [Google Scholar]
- 13.Ginzac A, Dubois S, Hager M-O, et al. Quality of life for older patients with cancer: a review of the evidence supporting melatonin use. Aging Clin Exp Res 2020;32:2459–68. 10.1007/s40520-020-01532-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Posadzki PP, Bajpai R, Kyaw BM, et al. Melatonin and health: an umbrella review of health outcomes and biological mechanisms of action. BMC Med 2018;16:18. 10.1186/s12916-017-1000-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seely D, Wu P, Fritz H, et al. Melatonin as adjuvant cancer care with and without chemotherapy: a systematic review and meta-analysis of randomized trials. Integr Cancer Ther 2012;11:293–303. 10.1177/1534735411425484 [DOI] [PubMed] [Google Scholar]
- 16.Palmer ACS, Zortea M, Souza A, et al. Clinical impact of melatonin on breast cancer patients undergoing chemotherapy; effects on cognition, sleep and depressive symptoms: a randomized, double-blind, placebo-controlled trial. PLoS One 2020;15:e0231379. 10.1371/journal.pone.0231379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sedighi Pashaki A, Mohammadian K, Afshar S, et al. A randomized, controlled, parallel-group, trial on the effects of melatonin on fatigue associated with breast cancer and its adjuvant treatments. Integr Cancer Ther 2021;20:1534735420988343. 10.1177/1534735420988343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semiglazova TY, Osipov M, Krivorotko P, et al. Melatonin and metformin in neoadjuvant chemotherapy in locally advanced breast cancer. Annals of Oncology 2019;30:v100. 10.1093/annonc/mdz241.003 [DOI] [Google Scholar]
- 19.Lissoni P, Mandalà M, Brivio F. Abrogation of the negative influence of opioids on IL-2 immunotherapy of renal cell cancer by melatonin. Eur Urol 2000;38:115–8. 10.1159/000020263 [DOI] [PubMed] [Google Scholar]
- 20.Farshchian N, Shirzadi M, Farshchian F. Evaluation of the melatonin effect on sleep quality in cancer patients. Tehran University Med J 2020;78:38–42. [Google Scholar]
- 21.McGrath S, Zhao X, Steele R, et al. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res 2020962280219889080. 10.1177/0962280219889080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sedighi Pashaki A, Afshar S, Mohamadian K, et al. Effects of melatonin on IL-6 serum level changes and fatigue caused by adjuvant chemoradiotherapy in breast cancer women: a randomized controlled trial. Ijbd 2021;13:23–32. 10.30699/ijbd.13.4.23 [DOI] [Google Scholar]
- 23.Innominato PF, Lim AS, Palesh O, et al. The effect of melatonin on sleep and quality of life in patients with advanced breast cancer. Supportive Care in Cancer 2016;24:1097–105. 10.1007/s00520-015-2883-6 [DOI] [PubMed] [Google Scholar]
- 24.Chen WY, Giobbie-Hurder A, Gantman K, et al. A randomized, placebo-controlled trial of melatonin on breast cancer survivors: impact on sleep, mood, and hot flashes. Breast Cancer Res Treat 2014;145:381–8. 10.1007/s10549-014-2944-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elsabagh HH, Moussa E, Mahmoud SA, et al. Efficacy of melatonin in prevention of radiation-induced oral mucositis: a randomized clinical trial. Oral Dis 2020;26:566–72. 10.1111/odi.13265 [DOI] [PubMed] [Google Scholar]
- 26.Grutsch J, Hrushesky W, Lis C. Daily evening melatonin prolongs survival among patients with advanced non-small-cell lung cancer. Biological Rhythm Research 2021. [Google Scholar]
- 27.Hansen MV, Andersen LT, Madsen MT, et al. Effect of melatonin on depressive symptoms and anxiety in patients undergoing breast cancer surgery: a randomized, double-blind, placebo-controlled trial. Breast Cancer Res Treat 2014;145:683–95. 10.1007/s10549-014-2962-2 [DOI] [PubMed] [Google Scholar]
- 28.Kurdi MS, Muthukalai SP. The efficacy of oral melatonin in improving sleep in cancer patients with insomnia: a randomized double-blind placebo-controlled study. Indian J Palliat Care 2016;22:295–300. 10.4103/0973-1075.185039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lissoni P. Is there a role for melatonin in supportive care? Support Care Cancer 2002;10:110–6. 10.1007/s005200100281 [DOI] [PubMed] [Google Scholar]
- 30.Lissoni P, Barni S, Mandalà M, et al. Decreased toxicity and increased efficacy of cancer chemotherapy using the pineal hormone melatonin in metastatic solid tumour patients with poor clinical status. Eur J Cancer 1999;35:1688–92. 10.1016/s0959-8049(99)00159-8 [DOI] [PubMed] [Google Scholar]
- 31.Lissoni P, Tancini G, Barni S, et al. Treatment of cancer chemotherapy-induced toxicity with the pineal hormone melatonin. Support Care Cancer 1997;5:126–9. 10.1007/BF01262569 [DOI] [PubMed] [Google Scholar]
- 32.Lozano A, Marruecos J, Rubió J, et al. Randomized placebo-controlled phase II trial of high-dose melatonin mucoadhesive oral gel for the prevention and treatment of oral mucositis in patients with head and neck cancer undergoing radiation therapy concurrent with systemic treatment. Clin Transl Oncol 2021;23:1801–10. 10.1007/s12094-021-02586-w [DOI] [PubMed] [Google Scholar]
- 33.Lund Rasmussen C, Klee Olsen M, Thit Johnsen A, et al. Effects of melatonin on physical fatigue and other symptoms in patients with advanced cancer receiving palliative care: a double-blind placebo-controlled crossover trial. Cancer 2015;121:3727–36. 10.1002/cncr.29563 [DOI] [PubMed] [Google Scholar]
- 34.Madsen MT, Hansen MV, Andersen LT, et al. Effect of melatonin on sleep in the perioperative period after breast cancer surgery: a randomized, double-blind, placebo-controlled trial. J Clin Sleep Med 2016;12:225–33. 10.5664/jcsm.5490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onseng K, Johns NP, Khuayjarernpanishk T, et al. Beneficial effects of adjuvant melatonin in minimizing oral mucositis complications in head and neck cancer patients receiving concurrent chemoradiation. J Altern Complement Med 2017;23:957–63. 10.1089/acm.2017.0081 [DOI] [PubMed] [Google Scholar]
- 36.Palmer ACS, Souza A, Dos Santos VS, et al. The effects of melatonin on the descending pain inhibitory system and neural plasticity markers in breast cancer patients receiving chemotherapy: randomized, double-blinded, placebo-controlled trial. Front Pharmacol 2019;10:1382. 10.3389/fphar.2019.01382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer ACS, Zortea M, Souza A, et al. Clinical impact of melatonin on breast cancer patients undergoing chemotherapy; effects on cognition, sleep and depressive symptoms: a randomized, double-blind, placebo-controlled trial. PLoS One 2020;15:e0231379. 10.1371/journal.pone.0231379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sedighi Pashaki A, Mohammadian K, Afshar S, et al. A randomized, controlled, parallel-group, trial on the effects of melatonin on fatigue associated with breast cancer and its adjuvant treatments. Integr Cancer Ther 2021;20:1534735420988343. 10.1177/1534735420988343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seely D, Legacy M, Auer RC, et al. Adjuvant melatonin for the prevention of recurrence and mortality following lung cancer resection (AMPLCaRe): a randomized placebo controlled clinical trial. EClinicalMedicine 2021;33:100763. 10.1016/j.eclinm.2021.100763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shahrokhi M, Ghaeli P, Arya P, et al. Comparing the effects of melatonin and zolpidem on sleep quality, depression, and anxiety in PatientsWithColorectalCancerUndergoingChemotherapy. Basic Clin Neurosci 2021;12:105–14. 10.32598/bcn.12.1.1650.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sookprasert A, Johns NP, Phunmanee A, et al. Melatonin in patients with cancer receiving chemotherapy: a randomized, double-blind, placebo-controlled trial. Anticancer Res 2014;34:7327–37. [PubMed] [Google Scholar]
- 42.Yennurajalingam S, Carmack C, Balachandran D, et al. Sleep disturbance in patients with cancer: a feasibility study of multimodal therapy. BMJ Support Palliat Care 2021;11:170–9. 10.1136/bmjspcare-2019-001877 [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization . The world health organization has developed a grading system for mucositis based on clinical appearance and functional status, 2018. Available: http://www.gelclair.nl/Institutional.aspx?Pagina=239&SM=230&Lingua=EN [Accessed 29 Mar 2022].
- 44.Innominato PF, Lim AS, Palesh O, et al. The effect of melatonin on sleep and quality of life in patients with advanced breast cancer. Support Care Cancer 2016;24:1097–105. 10.1007/s00520-015-2883-6 [DOI] [PubMed] [Google Scholar]
- 45.Del Fabbro E, Dev R, Hui D, et al. Effects of melatonin on appetite and other symptoms in patients with advanced cancer and cachexia: a double-blind placebo-controlled trial. J Clin Oncol 2013;31:1271–6. 10.1200/JCO.2012.43.6766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russcher M, Koch BCP, Nagtegaal JE, et al. Long-Term effects of melatonin on quality of life and sleep in haemodialysis patients (melody study): a randomized controlled trial. Br J Clin Pharmacol 2013;76:668–79. 10.1111/bcp.12093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sumsuzzman DM, Choi J, Jin Y, et al. Neurocognitive effects of melatonin treatment in healthy adults and individuals with Alzheimer's disease and insomnia: a systematic review and meta-analysis of randomized controlled trials. Neurosci Biobehav Rev 2021;127:459–73. 10.1016/j.neubiorev.2021.04.034 [DOI] [PubMed] [Google Scholar]
- 48.Baglioni C, Bostanova Z, Bacaro V, et al. A systematic review and network meta-analysis of randomized controlled trials evaluating the evidence base of melatonin, light exposure, exercise, and complementary and alternative medicine for patients with insomnia disorder. J Clin Med 2020;9:9061949:1949. 10.3390/jcm9061949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pereira N, Naufel MF, Ribeiro EB, et al. Influence of dietary sources of melatonin on sleep quality: a review. J Food Sci 2020;85:5–13. 10.1111/1750-3841.14952 [DOI] [PubMed] [Google Scholar]
- 50.Fatemeh G, Sajjad M, Niloufar R, et al. Effect of melatonin supplementation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. J Neurol 2022;269:205–16. 10.1007/s00415-020-10381-w [DOI] [PubMed] [Google Scholar]
- 51.Hrushesky WJM, Lis CG, Levin RD. Daily evening melatonin prolongs survival among patients with advanced non-small-cell lung cancer. Biological Rhythm Research 2021:1–15. [Google Scholar]
- 52.Wade AG, Ford I, Crawford G, et al. Efficacy of prolonged release melatonin in insomnia patients aged 55-80 years: quality of sleep and next-day alertness outcomes. Curr Med Res Opin 2007;23:2597–605. 10.1185/030079907X233098 [DOI] [PubMed] [Google Scholar]
- 53.Ahn JH, Kim M, Park S, et al. Prolonged-release melatonin in Parkinson's disease patients with a poor sleep quality: a randomized trial. Parkinsonism Relat Disord 2020;75:50–4. 10.1016/j.parkreldis.2020.03.029 [DOI] [PubMed] [Google Scholar]
- 54.Wilhelmsen-Langeland A, Saxvig IW, Pallesen S, et al. A randomized controlled trial with bright light and melatonin for the treatment of delayed sleep phase disorder: effects on subjective and objective sleepiness and cognitive function. J Biol Rhythms 2013;28:306–21. 10.1177/0748730413500126 [DOI] [PubMed] [Google Scholar]
- 55.van Heukelom RO, Prins JB, Smits MG, et al. Influence of melatonin on fatigue severity in patients with chronic fatigue syndrome and late melatonin secretion. Eur J Neurol 2006;13:55–60. 10.1111/j.1468-1331.2006.01132.x [DOI] [PubMed] [Google Scholar]
- 56.Fabi A, Bhargava R, Fatigoni S, et al. Cancer-Related fatigue: ESMO clinical practice guidelines for diagnosis and treatment. Ann Oncol 2020;31:713–23. 10.1016/j.annonc.2020.02.016 [DOI] [PubMed] [Google Scholar]
- 57.Hilfiker R, Meichtry A, Eicher M, et al. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: a systematic review incorporating an indirect-comparisons meta-analysis. Br J Sports Med 2018;52:651–8. 10.1136/bjsports-2016-096422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu C, Zheng Y, Duan Y, et al. Nonpharmacological interventions for cancer-related fatigue: a systematic review and Bayesian network meta-analysis. Worldviews Evid Based Nurs 2019;16:102–10. 10.1111/wvn.12352 [DOI] [PubMed] [Google Scholar]
- 59.Lee TYC, Curtin JP. The effects of melatonin prophylaxis on sensory recovery and postoperative pain following orthognathic surgery: a triple-blind randomized controlled trial and biochemical analysis. Int J Oral Maxillofac Surg 2020;49:446–53. 10.1016/j.ijom.2019.07.006 [DOI] [PubMed] [Google Scholar]
- 60.Laflı Tunay D, Türkeün Ilgınel M, Ünlügenç H, et al. Comparison of the effects of preoperative melatonin or vitamin C administration on postoperative analgesia. Bosn J Basic Med Sci 2020;20:117–24. 10.17305/bjbms.2019.4379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keshavarzi F, Mahmoudzadeh F, Brand S, et al. Both melatonin and meloxicam improved sleep and pain in females with primary dysmenorrhea-results from a double-blind cross-over intervention pilot study. Arch Womens Ment Health 2018;21:601–9. 10.1007/s00737-018-0838-x [DOI] [PubMed] [Google Scholar]
- 62.Gandolfi JV, Di Bernardo APA, Chanes DAV, et al. The effects of melatonin supplementation on sleep quality and assessment of the serum melatonin in ICU patients: a randomized controlled trial. Crit Care Med 2020;48:e1286–93. 10.1097/CCM.0000000000004690 [DOI] [PubMed] [Google Scholar]
- 63.Kirksey MA, Yoo D, Danninger T, et al. Impact of melatonin on sleep and pain after total knee arthroplasty under regional anesthesia with sedation: a double-blind, randomized, placebo-controlled pilot study. J Arthroplasty 2015;30:2370–5. 10.1016/j.arth.2015.06.034 [DOI] [PubMed] [Google Scholar]
- 64.Neufeld NJ, Elnahal SM, Alvarez RH. Cancer pain: a review of epidemiology, clinical quality and value impact. Future Oncol 2017;13:833–41. 10.2217/fon-2016-0423 [DOI] [PubMed] [Google Scholar]
- 65.Yang J, Wahner-Roedler DL, Zhou X, et al. Acupuncture for palliative cancer pain management: systematic review. BMJ Support Palliat Care 2021;11:264–70. 10.1136/bmjspcare-2020-002638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ali T, Rahman SU, Hao Q, et al. Melatonin prevents neuroinflammation and relieves depression by attenuating autophagy impairment through FOXO3a regulation. J Pineal Res 2020;69:e12667. 10.1111/jpi.12667 [DOI] [PubMed] [Google Scholar]
- 67.Shabani A, Foroozanfard F, Kavossian E, et al. Effects of melatonin administration on mental health parameters, metabolic and genetic profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. J Affect Disord 2019;250:51–6. 10.1016/j.jad.2019.02.066 [DOI] [PubMed] [Google Scholar]
- 68.Daneshvar Kakhaki R, Ostadmohammadi V, Kouchaki E, et al. Melatonin supplementation and the effects on clinical and metabolic status in Parkinson's disease: a randomized, double-blind, placebo-controlled trial. Clin Neurol Neurosurg 2020;195:105878. 10.1016/j.clineuro.2020.105878 [DOI] [PubMed] [Google Scholar]
- 69.Ostadmohammadi V, Soleimani A, Bahmani F, et al. The effects of melatonin supplementation on parameters of mental health, glycemic control, markers of cardiometabolic risk, and oxidative stress in diabetic hemodialysis patients: a randomized, double-blind, placebo-controlled trial. J Ren Nutr 2020;30:242–50. 10.1053/j.jrn.2019.08.003 [DOI] [PubMed] [Google Scholar]
- 70.Madsen MT, Zahid JA, Hansen CH, et al. The effect of melatonin on depressive symptoms and anxiety in patients after acute coronary syndrome: the MEDACIS randomized clinical trial. J Psychiatr Res 2019;119:84–94. 10.1016/j.jpsychires.2019.09.014 [DOI] [PubMed] [Google Scholar]
- 71.Quested DJ, Gibson JC, Sharpley AL, et al. Melatonin in acute mania investigation (MIAMI-UK). A randomized controlled trial of add-on melatonin in bipolar disorder. Bipolar Disord 2021;23:176–85. 10.1111/bdi.12944 [DOI] [PubMed] [Google Scholar]
- 72.Anderson PM, Lalla RV. Glutamine for amelioration of radiation and chemotherapy associated mucositis during cancer therapy. Nutrients 2020;12:12061675. 10.3390/nu12061675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akagi S, Fujiwara T, Nishida M, et al. The effectiveness of rebamipide mouthwash therapy for radiotherapy and chemoradiotherapy-induced oral mucositis in patients with head and neck cancer: a systematic review and meta-analysis. J Pharm Health Care Sci 2019;5:16. 10.1186/s40780-019-0146-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuba S, Yamanouchi K, Matsumoto M, et al. Study protocol for efficacy and safety of steroid-containing mouthwash to prevent chemotherapy-induced stomatitis in women with breast cancer: a multicentre, open-label, randomised phase 2 study. BMJ Open 2020;10:e033446. 10.1136/bmjopen-2019-033446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lissoni P, Chilelli M, Villa S, et al. Five years survival in metastatic non-small cell lung cancer patients treated with chemotherapy alone or chemotherapy and melatonin: a randomized trial. J Pineal Res 2003;35:12–15. 10.1034/j.1600-079x.2003.00032.x [DOI] [PubMed] [Google Scholar]
- 76.Correa MEP, Cheng KKF, Chiang K, et al. Systematic review of oral cryotherapy for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2020;28:2449–56. 10.1007/s00520-019-05217-x [DOI] [PubMed] [Google Scholar]
- 77.Shu Z, Li P, Yu B, et al. The effectiveness of probiotics in prevention and treatment of cancer therapy-induced oral mucositis: a systematic review and meta-analysis. Oral Oncol 2020;102:104559. 10.1016/j.oraloncology.2019.104559 [DOI] [PubMed] [Google Scholar]
- 78.Zadik Y, Arany PR, Fregnani ER, et al. Systematic review of photobiomodulation for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer 2019;27:3969–83. 10.1007/s00520-019-04890-2 [DOI] [PubMed] [Google Scholar]
- 79.Cronshaw M, Parker S, Anagnostaki E, et al. Photobiomodulation and oral mucositis: a systematic review. Dent J 2020;8:87. 10.3390/dj8030087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gurunathan S, Qasim M, Kang M-H, et al. Role and therapeutic potential of melatonin in various type of cancers. Onco Targets Ther 2021;14:2019–52. 10.2147/OTT.S298512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Y, Li S, Zhou Y, et al. Melatonin for the prevention and treatment of cancer. Oncotarget 2017;8:39896–921. 10.18632/oncotarget.16379 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-060912supp001.pdf (53.9KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.


