Abstract
This study focused on the co-occurrence of aflatoxins (AFs) and ochratoxin A (OTA) in traditionally and industrially dried red pepper flakes (DRPFs) and isot pepper flakes (IPFs). Following the multitoxin immunoaffinity column (IAC) clean-up, high-performance liquid chromatography coupled with fluorescence detection (HPLC-FLD) was used to quantify AFs and OTA. The limit of detection (LOD) and limit of quantification (LOQ) values were 0.11 and 0.18 μg kg–1 (AFB1), 0.04 and 0.08 μg kg–1 (AFB2), 0.13 and 0.18 μg kg–1 (AFG1), 0.04 and 0.11 μg kg–1 (AFG2), and 0.10 and 0.21 μg kg–1 (OTA), respectively. AFB1, AFB2, AFG1, and OTA were found to be positive in 93, 74, 17, and 94% of all samples, respectively. The contamination levels in positive samples ranged from 0.23 to 38.69, 0.04 to 2.14, 0.13 to 0.88, and 0.18 to 52.19 μg kg–1 for AFB1, AFB2, AFG1, and OTA, respectively, while no AFG2 was found above the detection limit (0.04 μg kg–1). None of the industrial isot peppers exceeded the European Union limits, while the levels of AFB1 (5 μg kg–1), total AFs (10 μg kg–1), and OTA (20 μg kg–1) of the traditional peppers were above the limit by 30% (16/54), 26% (14/54), and 4% (2/54), respectively. Co-occurrence of AFB1-AFB2-OTA was the most frequent, accounting for 54% (29/54) of all samples. At the upper bound (UB), estimated average exposure to AFB1, total AFs, and OTA was determined to be 0.175, 0.189, and 0.124 ng kg–1 bw day–1 in all samples, respectively. The margin of exposure (MoE) value of AFB1 and total AFs was found to be 977 and 909, indicating high health concerns compared to OTA (MoE >10,000). AFB1 and total AFs may result in 0.0058 and 0.0062 liver cancer cases/100,000 person/year at UB, respectively, and weekly OTA exposure was 0.868 ng kg–1 bw, well below the provisional tolerable weekly intake, hence not of health concern. AFs exposure could endanger health, whereas OTA posed no toxicological concerns through dried red pepper consumption
1. Introduction
Mycotoxigenic fungi (mostly Aspergillus, Fusarium, and Penicillium) can produce mycotoxins as secondary metabolites.1 Almost a quarter of crops were contaminated with mycotoxins annually, exposing nearly 4.5 billion people to mycotoxins through their daily diets.2 Mycotoxins have numerous health impacts that can be acute or mostly chronic, including cancer.3 Moreover, aflatoxins (AFs) were categorized as Group I by the International Agency for Research on Cancer (IARC) due to their highly toxic and carcinogenic effects on living organisms. Depending on the fluorescence color response under UV light, aflatoxins are divided into, B (blue; AFB1, AFB2) and G (green; AFG1, AFG2), with AFB1 being the most carcinogenic. Second, OTA was classified into Group 2B (possibly carcinogenic), a causative agent of the Balkan endemic nephropathy.4
Red peppers were categorized as paprika (nonpungent, sweet) and chili (pungent, hot) and represent any form of Capsicum spp.5 Red pepper (Capsicum annuum L. Solanaceae) was consumed freshly and mostly in dried form.6,7 Chili and pepper cultivation reached 1,990,926 ha worldwide, with a production value of 38,027,164 tons. Turkey ranked third after China and Mexico with 2,625,669 tons in 2019.8 Accordingly, the production of dried chili and pepper was 242,276 tons in Turkey.9
Dried red pepper flakes (DRPFs) could be produced in two ways: using traditional techniques as sun-dried or industrially as oven-dried.10 The majority of red peppers grown in the southeastern part of Turkey are classified as isot pepper flakes (IPFs), the traditional deep-red pepper. Isot, known as the traditional “Şanlıurfa pepper”, has also been geographically indicated.9Isot is darker in color, hotter in taste than ordinary dried red peppers, and turns black after sun-drying. To maintain its dark color, traditional isot production involves storing red peppers in plastic bags without direct exposure to sunlight.6,10
Aspergillus section Flavi and Nigri were found more frequently than Penicillium and Fusarium species in Capsicum spp.7,11 Mycotoxins can contaminate red peppers, particularly during harvesting, drying, processing, storage, and transportation.7,12 Many mycotoxins, including AFs (total AFs),13,14 OTA,14 zearalenone,15 fumonisins,16 deoxynivalenol,17 trichothecenes,17 citrinin,15 sterigmatocystin, and roquefortine C,18 were detected in red peppers. Furthermore, red pepper is a suitable substrate for a variety of mycotoxins; particularly, AFs and OTA are prevalent in red peppers.13,14,17 In this regard, the natural occurrence of AFs and OTA in red peppers was documented across the world, including Brazil,14,19 Chile,13 Greece,20 Indonesia,21 Lebanon,16 India,22 Italy,17,23 Nigeria,24 and South Africa.18 Therefore, research into mycotoxins in red peppers is critical for both domestic and international trade along with public health.
RASSF (Rapid Alert System for Food and Feed) has been ensuring food safety across Europe since 1979.25 During the 2020–2021 period, RASSF received 27 notifications on mycotoxins in chili, paprika, and capsicum peppers. Notably, 67% (18/27) of these were contaminated with AFs at unacceptable levels, representing 77% (14/18) of the chilies, placing them in the border rejection category. It is noteworthy that half of these chilies (9/18) originated from India. On the other hand, OTA (>20 μg kg–1) was found in 33% (5 chilies, 4 paprikas) of the peppers in the alert section, indicating a serious health risk. The presence of multiple mycotoxins in red peppers is still waiting to be addressed, and climate change may trigger the proliferation of mycotoxigenic fungi and subsequent multimycotoxin contamination.26 Given Turkey’s importance as a dried red pepper exporter,8 monitoring red pepper-related multiple mycotoxin exposure is critical. Furthermore, co-exposure to different combinations of mycotoxins could increase the cumulative risk more than exposure to a single type of mycotoxin alone.27
In this study, the lack of regulations on the co-occurrence of mycotoxins in Capsicum spp.28,29 led us to examine the co-occurrence rate of AFs-OTA in red peppers. Besides, IACs containing monoclonal antibodies were mainly employed in red peppers to detect mycotoxins individually.7,30 On the other hand, multimycotoxin IACs were used in several studies to extract AFs and OTA from red peppers.14,31−33 However, there are no reports on the use of multimycotoxin IACs to evaluate AFs and OTA in red peppers from Turkey. Instead, AFs and OTA extraction in red peppers was thoroughly investigated using monoclonal antibody-containing IACs.30,34,35 The use of multimycotoxin IAC can speed up the analysis and lower costs.31 Thus, the objectives of this research were: (i) to extract AFs and OTA from DRPFs and IPFs using multimycotoxin IAC with simultaneous detection, (ii) to compare current levels of AFs-OTA with acceptable limits, and (iii) to estimate dietary exposure and risk characterization through red pepper consumption in Turkey.
2. Results and Discussion
2.1. Method Performance
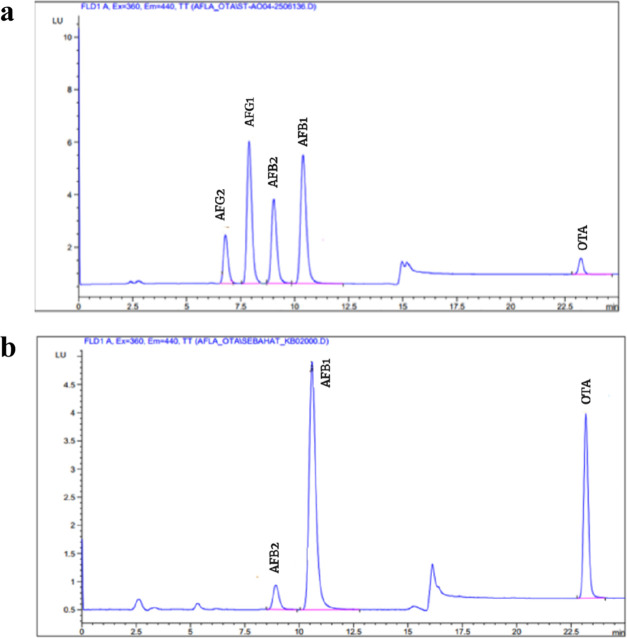
AFG2, AFG1, AFB2, AFB1, and OTA were eluted in that sequence, with retention times of 6.96, 8.15, 9.11, 10.6, and 23.2 min, respectively. When the analytes were processed, no interference peaks appeared during the retention time, and the chromatograms showed good resolution. Figure 1a illustrates the chromatogram of standard solutions for AFs and OTA, while Figure 1b shows their natural occurrence in traditional DRPFs. All analytes had correlation coefficients greater than 0.98, indicating good linearity. The LODs and LOQs of the method are presented in Table 1, together with recoveries and repeatability values. Mean recoveries of AFs and OTA ranged from 63.8 to 89.8 and 75.8 to 87.0%, respectively. With good precision, RSD values for AFs and OTA ranged from 1.0 to 24.4 and 3.1 to 13.9%, respectively. Based on Commission Regulation (EC) No. 401/2006, the recoveries are adequate (between 50 and 120% for <1 μg kg–1 AFs, OTA with RSD <40% and between 70 and 110% for 1–10 μg kg–1 AFs, OTA with RSD <20%), except for AFG2 at 3 μg kg–1, which was less than 70%.36 Stroka et al.37 suggested that the recovery of AFG2 could not be less than 60% for a solution of 5 ng mL–1 toxin. Recently, when employing AflaOchra coloumn, Iha et al.14 found the lowest recovery rate for AFG2 in paprika, which was lower than the present findings (at the upper spike level of 5 ng g–1, recovery for AFG2 was determined to be 51%). Similarly, Hernandez Hierro et al.33 documented that at the 5 μg kg–1 spike level, recoveries for aflatoxins B (>97.8%) were higher than G types (>70.7%) and OTA (75.7%) in paprika samples extracted with the AflaOchra column. However, Brera et al.23 compared the performance of single- (AflaPrep and OchraPrep) and multimycotoxin (AflaOchraPrep) IACs in the recovery of paprika samples, which yielded the same (85, 84, and 70% for AFB1, AFB2, and AFG2) or even higher (from 75 to 78% for AFG1 and from 90 to 96% for OTA, respectively). In some cases, monoclonal IAC extraction of AFs resulted in lower recovery for AFG2 (<70%).19 Aligning with the current results, Trucksess et al.38 stated that IACs containing AFs-specific monoclonal antibodies had higher recovery rates than those containing both AFs-OTA-specific antibodies. In general, multimycotoxin IAC resulted in lower recoveries for AFG2.14,31,33,38 For example, Brera et al.23 extracted paprika samples with (0.1 and 10%) and without (only PBS) Tween 20 and concluded that PBS solution achieved the best results. Recently, Iha et al.14 recommended using PBS containing 0.5 and 1% of Tween 20 in the mycotoxin extraction from paprika and spices, respectively, resulting in the reduction of interfering peaks. Similarly, Palma et al.13 proposed adjusting the concentration of Tween 20–10% for improved AFs recovery. In the present work, 1% of Tween 20 in PBS was used as an extraction solvent, which needs to be optimized for the higher recovery of AFG2. Fortunately, the method’s performance was unaffected by the low recovery of AFG2.4
Figure 1.
HPLC-FLD chromatograms. (a) AFs working standard solution 1.2 μg AFG2 L–1, 4 μg AFG1 L–1, 1.2 μg AFB2 L–1, 4 μg AFB1 L–1, and OTA (4 μg OTA L–1). (b) Naturally contaminated traditional dried red pepper flakes (DRPFs) with 1.52 μg AFB2 kg–1, 29.92 μg AFB1 kg–1, and 52.19 μg OTA kg–1.
Table 1. Performance of the Analytical Method for isot and Dried Red Pepper Flakesa,b,c.
| recovery values |
|||||
|---|---|---|---|---|---|
| mycotoxins | LOD (μg kg–1) | LOQ (μg kg–1) | spiking level (μg kg–1) | recovery ± SD (%) | RSD (%) |
| AFB1 | 0.11 | 0.18 | 1 | 80.5 ± 17.3 | 21.4 |
| 2 | 81.8 ± 1.4 | 1.7 | |||
| 10 | 89.8 ± 4.3 | 4.8 | |||
| AFB2 | 0.04 | 0.08 | 0.3 | 80.3 ± 17.8 | 22.2 |
| 0.6 | 79.1 ± 3.0 | 3.8 | |||
| 3 | 83 ± 2.8 | 3.4 | |||
| AFG1 | 0.13 | 0.18 | 1 | 75.7 ± 18.5 | 24.4 |
| 2 | 73.4 ± 2.8 | 3.9 | |||
| 10 | 72.6 ± 1.1 | 1.5 | |||
| AFG2 | 0.04 | 0.11 | 0.3 | 63.8 ± 1.1 | 1.7 |
| 0.6 | 64.0 ± 0.9 | 1.4 | |||
| 3 | 64.7 ± 0.6 | 1.0 | |||
| OTA | 0.10 | 0.21 | 0.5 | 75.8 ± 2.4 | 3.1 |
| 1 | 87.0 ± 12.1 | 13.9 | |||
| 3 | 85.3 ± 9.6 | 11.3 | |||
SD = Standard deviation.
RSD = Relative standard deviation = SD / mean × 100.
LOD = Limit of detection, LOQ = Limit of quantification.
2.2. Presence of AFs and OTA in Red Pepper Samples
AFB1, AFB2, AFG1, and OTA were found to be positive in 93, 74, 17, and 94% of 54 samples tested, with mean contamination levels of 6.36, 0.30, 0.35, and 4.52 μg kg–1, and ranges of 0.23–38.69, 0.04–2.14, 0.13–0.88, and 0.18–52.19 μg kg–1, respectively, while AFG2 was not detected above the LOD of 0.04 μg kg–1. AFs and OTA were found in DRPFs and IPFs, mostly in higher frequencies but at lower concentrations (Figures S1a–c). Compared with their industrially processed counterparts, traditionally processed red peppers had higher mean levels for AFB1 (9.17 and 4.30 μg kg–1), AFs (9.77 and 4.62 μg kg–1), and OTA (6.95 and 1.69 μg kg–1), respectively (Table 2). Throughout industrial processing, peppers were dried at controlled temperature, air velocity, and humidity, resulting in minimal fungal growth and contamination.10 Moreover, adding salt increases sodium levels by suppressing fungal growth.39 In traditional processing, microenterprises dry the pepper as a whole pepper,40 resulting in a closed and high-humidity environment inside the pepper that favors fungal growth and increases mycotoxin levels..41 Another issue is food adulteration, which occurs when red peppers are sprinkled with water, resulting in a fungal infestation. Since elevated humidity facilitates water absorption and promotes the synthesis of mycotoxins, regular monitoring of humidity and refrigeration parameters was thought to be critical as a preventive measure.42 To prevent the occurrence of mycotoxins, unwounded chilies need to be carefully washed and dried following harvesting. When it comes to packaging, hermetic sealing helps to create an unfavorable environment for fungal proliferation.40
Table 2. Incidence and Concentration Levels of AFs and OTA in Traditional and Industrial isot Pepper Flakes (IPFs) or Dried Red Pepper Flakes (DRPFs).
| IPFs | DRPFs | all samples | ||||||
|---|---|---|---|---|---|---|---|---|
| mycotoxins | parameter | all IPFs (n = 13) | traditional (n = 11) | industrial (n = 2) | all DRPFs (n = 41) | traditional (n = 31) | industrial (n = 10) | n = 54 |
| AFB1 | positive samples,an (%) | 10 (76.9) | 10 (90.9) | 0 (0) | 40 (97.5) | 31 (100) | 9 (90) | 50 (93) |
| range (μg kg–1) | 0.23–38.69 | 0.23–38.69 | n.d.c | 0.20–37.95 | 0.28–37.95 | 0.2–2.52 | 0.23–38.69 | |
| mean value (μg kg–1) | 3.65 | 4.30 | n.d. | 7.22 | 9.17 | 1.17 | 6.36 | |
| median value (μg kg–1) | 0.56 | 0.64 | n.d. | 1.92 | 6.09 | 1.11 | 1.43 | |
| no. samples >EU MLd (%) | 1 (7.7) | 1 (9) | 0 (0) | 15 (36.5) | 15 (48.3) | 0 (0) | 16 (29.6) | |
| AFB2 | positive samples, n (%) | 8 (61.5) | 8 (72.2) | 0 (0) | 32 (78) | 28 (90.3) | 4 (40) | 40 (74) |
| range (μg kg–1) | 0.04–1.13 | 0.04–1.13 | n.d. | 0.04–2.14 | 0.04–2.14 | 0.04–0.07 | 0.04–2.14 | |
| mean value (μg kg–1) | 0.15 | 0.17 | n.d. | 0.35 | 0.45 | 0.03 | 0.30 | |
| median value (μg kg–1) | 0.04 | 0.06 | n.d. | 0.07 | 0.28 | 0.02 | 0.07 | |
| AFG1 | positive samples, n (%) | 1 (7.7) | 1 (9.1) | 0 (0) | 8 (19.5) | 7 (22.6) | 1 (10) | 9 (17) |
| range (μg kg–1) | n.d.–0.77 | n.d.–0.77 | n.d. | n.d.–0.88 | 0.13–0.88 | n.d.–0.17 | 0.13–0.88 | |
| mean value (μg kg–1) | 0.12 | 0.13 | n.d. | 0.11 | 0.12 | 0.08 | 0.35 | |
| median value (μg kg–1) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.07 | |
| AFG2 | positive samples, n (%) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0 (0) |
| total AFb | positive samples, n (%) | 10 (76.9) | 10 (90.9) | 0 (0) | 40 (97.5) | 31 (100) | 9 (90) | 50 (93) |
| range (μg kg–1) | n.d.–40.61 | 0.33–40.61 | n.d. | 0.30–39.26 | 0.40–39.26 | 0.30–2.77 | 0.28–40.61 | |
| mean value (μg kg–1) | 3.93 | 4.62 | n.d. | 7.7 | 9.77 | 1.3 | 7.31 | |
| median value (μg kg–1) | 0.7 | 0.83 | n.d. | 2.06 | 6.49 | 1.22 | 6.8 | |
| no. samples >EU ML (%) | 1 (7.7) | 1 (9) | 0 (0) | 13 (31.7) | 13 (41.9) | 0 (0) | 14 (25.9) | |
| OTA | positive samples, n (%) | 11 (84.6) | 9 (81.8) | 2 (100) | 40 (97.5) | 31 (100) | 9 (90) | 51 (94) |
| range (μg kg–1) | 0.05–12.62 | 0.05–12.62 | 0.19–0.34 | 0.35–52.19 | 0.18–52.19 | 0.35–2.43 | 0.18–52.19 | |
| mean value (μg kg–1) | 1.47 | 1.69 | 0.27 | 5.48 | 6.95 | 0.91 | 4.52 | |
| median value (μg kg–1) | 0.5 | 0.66 | 0.27 | 2.57 | 3.21 | 0.69 | 1.95 | |
| no. samples >EU ML (%) | 0 (0) | 0 (0) | 0 (0) | 2 (4.9) | 2 (6.5) | 0 (0) | 2 (3.7) | |
| average aw | 0.43 | 0.39 | 0.66 | 0.54 | 0.54 | 0.58 | 0.52 |
Positive samples, n (%): mycotoxin level ≥ LOD.
Total AF: sum of AFB1, AFB2, AFG1, and AFG2..
n.d.: not detected, <LOD. The LODs for AFB1, AFB2, AFG1, AFG2, and OTA are 0.11, 0.04, 0.13, 0.04, and 0.1 μg kg–1, respectively.
EU ML: European Union maximum level (5 μg kg–1 for AFB1; 10 μg kg–1 for total AFs; 20 μg kg–1 for OTA).
The Turkish Food Codex adheres to European Commission Regulation No. 165/2010.43 In European Commission Regulation No. 165/2010,28 maximum permitted limits are only available for AFs and OTA in Capsicum spp. products (dried fruits thereof, whole or ground, including chilies, chili powder, cayenne, and paprika) with 5 μg kg–1 for AFB1 and 10 μg kg–1 for total AFs (sum of AFB1, AFB2, AFG1, and AFG2). The European Commission Regulation No. 2015/113729 raised the maximum limit of OTA from 15 μg kg–1 to 20 μg kg–1. However, the regulations for foods containing multiple mycotoxins are not yet covered by European Union (EU) legislation. Furthermore, 30% (16/54), 26% (14/54), and 4% (2/54) of the samples surpassed the EU limit for AFB1, total AFs, and OTA, respectively. It should be highlighted that only traditional peppers surpassed the limits for total AFs (13/31 of DRPFs and 1/11 of IPFs), AFB1 (15/31 of DRPFs and 1/11 of IPFs), and OTA (2/31 of DRPFs). Furthermore, the present data revealed that aflatoxin B types were more frequently detected than their G counterparts. This could be explained by Aspergillus flavus and Aspergillus parasiticus contamination of dried chili.21 More recently, Ezekiel et al.24 reported that chili peppers were mostly associated with aflatoxigenic A. flavus. Likewise, Ozbey and Kabak30 reported that A. flavus was the most common fungi in red peppers, producing more aflatoxin B than A. parasiticus. Another study from Korea showed that 90% (9/10) of the A. flavus strains could produce AFs (146.88–909.53 μg kg–1) in ground red peppers.44
Over the last three decades, various studies have been undertaken to determine the levels of AFs and OTA in dried red peppers. Tosun and Ozden35 and Set and Erkmen45 focused on the levels of OTA and AFs in unpacked and packed dried red peppers from Turkey. The results pointed out that all unpacked peppers were contaminated with OTA between 1.1 and 31.7 μg kg–1, while packed counterparts had lower contamination within the range of 0.6–1.0 μg kg–1.35 Similarly, only unpacked peppers surpassed the limits for AFs and AFB1 by 17.1 and 23.1%, respectively, with values ranging from 0.087 to 97.4 and 0.067 to 89.99 μg kg–1, respectively.45 In this context, Bircan46 noted that all bazaar-sourced peppers surpassed the legal limits for AFs compared to supermarket-sourced equivalents in Turkey. The study indicated that paprika samples (27/30) had AFB1 (0.5–116.4 μg kg–1) and AFs (0.5–124.6 μg kg–1), while all chili powders (15/15) contaminated with AFB1 (1.6–80.4 μg kg–1) and AFs (1.8–85.9 μg kg–1), respectively. The results were found to be consistent with the existing data since unpacked dried red peppers were mostly sold in an open form on the streets and bazaars and were commonly produced by traditional techniques under uncontrolled conditions on the ground with sunlight.35 A similar situation was documented in Malaysia; Jalili and Jinap47 noted that 65 and 81.3% of 80 chilies contained AFs (0.2–79.7 μg kg–1) and OTA (0.2–101.2 μg kg–1), respectively, emphasizing that peppers from the open market had higher contamination levels than those from the supermarket. Accordingly, 25% of chili peppers from local and farmer’s markets in Nigeria exceeded the limit of AFs.24 In Korea, Ahn et al.12 detected lower OTA levels in mechanically dried paprikas than in sun-dried counterparts (0.82 and 3.83 μg kg–1, respectively). It should be highlighted that only traditional DRPFs and IPFs red peppers surpassed the acceptable limits by 42% (13/31) and 9% (1/11) for total AFs, and 48% (15/31) and 9% (1/11) for AFB1, respectively, while 6.5% (2/31) in traditional DRPFs exceeded the limits for OTA and by 48% (15/31) and 9% (1/11) in traditional DRPFs and IPFs for AFB1, respectively. Furthermore, the present data revealed that aflatoxin B types were more frequently detected than their G counterparts. This could be explained by A. flavus and A. parasiticus contamination of dried chili.21 More recently, Ezekiel et al.24 reported that chili peppers were mostly associated with aflatoxigenic A. flavus. Likewise, Ozbey and Kabak30 reported that A. flavus was the most common fungi in red peppers, producing more aflatoxin B than A. parasiticus. Another study from Korea showed that 90% (9/10) of the A. flavus strains could produce AFs (146.88-909.53 μg kg–1) in ground red peppers.44
2.3. Simultaneous Occurrence of AFs and OTA in Red Pepper Samples
AFB1-AFB2-OTA combination was detected in 23 out of 41 (56%) DRPFs and 6 out of 13 (46.2%) traditional IPFs. In other words, 27 out of 42 (64%) co-occurrences were from all traditional peppers (DRPFs + IPFs), while 17% (2/12) of them came from industrial DRPFs, accounting for 54% (29/54) of all samples. Importantly, neither co-occurrence nor exceedance of EU levels was detected in industrial IPFs. In addition, 17, 17, and 4% of 54 samples showed co-contamination patterns with AFB1-AFB2-AFG1-OTA, AFB1-OTA, and AFB1-AFB2, respectively (Figure S2). AFs-OTA contamination was found to be lower in IPFs than in DRPFs, especially in industrial types, which could be explained by the water activity,48 the type of peppers,32,49 drying temperature, and climate.50 Climate conditions in Turkey’s southeast area, which are warm and humid, may enhance fungus development in soil and air, placing red peppers at danger of mycotoxin accumulation while sun-drying on the ground.45 Water activity (aw) values ranged from 0.266 to 0.686 (Table 2), and IPFs had comparatively lower water activity values than DRPFs, which may help to reduce elevated mycotoxin levels. Supporting this evidence, Marín et al.48 stated that aw values below 0.85 limit the growth of aflatoxigenic A. flavus in chili by 50%. The co-occurrence of AFs and OTA in red-scaled peppers has been documented across the world. In line with the current findings, Özbey and Kabak30 detected that 62.5% (15/24) of Turkey-based chili flakes and 41% (9/22) of chili powder samples had AFs-OTA co-occurrence, representing 52% (24/46) of all chilies. Exceedances of the limits for AFB1, AFs, and OTA in chili flakes were 16.7, 12.5, and 16.7%, while in chili powder, it was 13.6%, 4.5%, and 13.6%, respectively. They also noted that the red chili flakes and chili powder peppers with the highest OTA contamination (53.4 and 98.24 μg kg–1, respectively) also had the highest concentrations of AFB1 (11.45 and 35.77 μg kg–1, respectively). The present findings called attention that excessive OTA levels (25.86 and 52.19 μg kg–1, respectively) had the highest levels of AFB1 (37.95 and 29.92 μg kg–1, respectively).
Santos et al.7 reported that all Capsicum samples (17 paprika, 11 chilies, and 4 smoked paprika) contained AFs (1.8–83.7 μg kg–1) and OTA (4.3–474.7 μg kg–1) in peppers, along with deoxynivalenol (2 chilies and 1 paprika), zearalenone (4 chilies and 4 paprikas), and trichothecenes (2 chilies). Previously, these researchers found that 75% (48/64) of paprika and 65% (23/35) of chilies contained at least one type of mycotoxin, and the presence of OTA was associated with AFs.5
Recently, Iqbal et al.42 reported that 75.4 and 71% of 252 chili sauces tested positive for AFs and OTA, respectively, exceeding the limits of AFB1, total AFs, and OTA by 44.8, 42.1, and 23%, respectively, demonstrating the importance of monitoring mycotoxins in chili-derived products. Similarly, Motloung et al.18 detected AFB1 in 4/7 of paprika, 2/14 of coarse chili, and 4/4 of ground chili samples with concentrations ranging from 3 to 19, 8 to 11, and 7 to 8 μg kg–1, respectively. Moreover, the frequency of OTA was less than our results for 1/7, 1/14, and 3/4 of the corresponding samples. Finally, the simultaneous occurrence of AFB1 and OTA were determined in 1/4 of the ground chili.
The simultaneous presence of AFs-OTA in dried red peppers might be due to contamination with distinct fungi or one type of fungus that produces different mycotoxins.18 Furthermore, when one type of mycotoxin is detected, it is quite common for other types to occur in the same substrate.51 Mycotoxin cocktails can have synergistic or additive effects on living organisms.52 According to Sedmíková et al.,53 OTA may boost its mutagenic properties when AFB1 and OTA coexist in the same substrate.
2.4. Dietary Exposure Assessment and Risk Characterization
The exposure estimates for AFB1, total AFs, and OTA (0.174, 0.187, and 0.124 ng kg–1 bw day–1, respectively, at mean MB) are presented in Table 3. The higher the MoE value, the lower the exposure, especially when it exceeds the cutoff value of 10,000.54 It must be highlighted that no levels of exposure to AFB1 and OTA were judged safe, but the MoE was used to prioritize the risk management process.27 The MoE estimations for AFB1 and total AFs were 977 and 909, respectively This situation demonstrates a potential health risk associated with the consumption of dried red pepper, as well as the need to take precautions. Unlike, the MoE value for OTA was far higher than the threshold level of 10,000 in red peppers, indicating that it was not of health concern. The estimated weekly OTA exposure was derived by multiplying the daily exposure (0.124 ng kg–1 bw day–1) by 7, yielding 0.868 ng kg–1 bw week–1, which was dramatically lower than the PTWI of 100 ng kg–1 bw55 and 120 ng kg–1 bw,56 representing 0.87 and 0.73% of the corresponding PTWI values (Table 3). As a result, long-term co-exposure to AFs and OTA through dried red peppers may result in serious health effects.
Table 3. Dietary Exposure of AFB1, Total AFs, and OTA and Risk Assessment.
| concentration (μg kg–1) |
exposure (ng kg–1 bw day–1)e |
liver cancer risk (case/100000 persons)f |
||||
|---|---|---|---|---|---|---|
| MBb | LBa–UBc | MBb | LBa–UBc | MoEd | MBb | LBa–UBc |
| AFB1 | ||||||
| 6.363 | 6.359–6.367 | 0.174 | 0.174–0.175 | 977 | 0.0057 | 0.0057–0.0058 |
| total AFs | ||||||
| 6.792 | 6.711–6.887 | 0.187 | 0.184–0.189 | 909 | 0.0061 | 0.0060–0.0062 |
| OTA | ||||||
| 4.515 | 4.512–4.521 | 0.124 | 0.123–0.124 | >10000 | ||
LB (lower bound): results below the LOD were replaced with 0, and unquantified values (between LOD and LOQ) were replaced by LOD.
MB (middle bound): results below the LOD were replaced with the value of LOD/2, and unquantified values (between LOD and LOQ) were replaced by LOQ/2.
UB (upper bound): results below the LOD were replaced with LOD, and unquantified values (between LOD and LOQ) were replaced by LOQ.
MOE, Margin of exposure, the ratio of benchmark dose, and the estimated intake of AFB1 and total AFs (170 ng kg–1 b.w day–1) or OTA (21.0 μg kg–1 b.w day–1) to MB of exposure.
The average body weight for the Turkish adult population was estimated to be 72.8 kg, and all values were expressed in μg kg–1.
Liver cancer risk, liver cancer cases/100,000 population/year.
Although there have been numerous studies on red pepper mycotoxins, little information is known on their dietary exposure and MoE. For example, in Sri Lanka, the MoE level of AFB1 in red chili (45-78) was far lower than current findings (977), showing that chilies are a major public health concern.57 A study on traditional Turkish sürk cheese (which contains various spices, including chili peppers) indicated that the MoE and EDI levels of AFB1 were 2982 and 0.057 ng kg–1 bw day–1, respectively. Since cheese has little amount of chili peppers which posed lower risks compared to present findings, while the EDI for OTA was 0.205 ng kg–1 bw day–1 with a PTWI value of 1.44, posing a higher risk (0.124 ng kg–1 bw day–1).58 In Pakistan, Iqbal et al.42 calculated the dietary exposure by employing the lowest and the maximum mean levels of mycotoxins instead of the substitution method. At the lowest concentration, the dietary exposure to AFB1, total AFs, and OTA in chili sauce samples were found to be 0.127, 0.337, and 0.417 ng kg–1 bw day–1, respectively, being higher than our exposure data except for AFB1. In another study, AFB1 exposure from Turkey-based dried red peppers was estimated to be 1.5 ng kg–1 bw day for a 20 g day–1 red pepper consumption,59 which was 9-fold higher than our findings. On the other hand, Tosun and Ozden35 observed that OTA exposure through dried red peppers was 0.3 ng kg–1 bw day–1 for 0.6 g day–1 consumption. More recently, Koutsias et al.20 found that AFB1 exposure in red pepper flakes was 0.02 ng kg–1 bw day.
According to Külahi and Kabak,60 OTA contamination occurred throughout the pre- and postharvest stages, at 0.181 μg kg–1 (MB estimate) in 34% (17/50) of chilies. Further, OTA exposure was detected to be 0.011 ng kg–1 bw week–1 (MB estimate), serving 0.01% of EFSA PTWI of 120 ng kg–1 bw and contributing to 0.4% of total OTA exposure. Apart from red peppers, the MoE values for AFB1 and AFs exposure in Turkey-based dried figs were detected to be 34,000 and 18,889, respectively, with 0.0002–0.0003 liver cancer cases/100,000 persons/year, showing negligible health concern (Oktay Basegmez, 2019). Dietary exposure and MoE values for AFB1 (0.04–0.12 ng kg–1 bw day–1, 1417–4250) and OTA (0.03–0.07 ng kg–1 bw day–1, >10,000) were estimated in Iran-based dried fruits (including mulberry, date, fig, and apricot), revealing that AFs but not OTA could raise health issues.62
In the present study, quantitative liver cancer risk results showed that AFB1 and total AFs were linked to 0.0057 and 0.0060 liver cancer cases/100,000 persons/year at LB estimate, respectively, which was far lower than that reported by Yogendrarajah et al.57 (0.046 and 0.028 cases/100,000 persons/year for AFB1 in North and South of Sri Lankan chilies, respectively). Due to differences in mycotoxin concentrations, consumption rates, and different food commodities, estimates of liver cancer risk can vary by country and region; for example, Huong et al.63 reported that rice and its products had the highest AFB1 (22 ng kg–1 bw day–1, 1.5 cases/100,000 persons, MoE = 8) and OTA (7.9 ng kg–1 bw day–1, MoE = 2674) exposure at MB estimate. The dietary exposure levels of AFB1 and AFs in hazelnuts were calculated as 0.016 and 0.023 ng kg–1 bw day–1, and for dried figs with 0.003 and 0.005 ng kg–1 bw day–1 (UB estimate), respectively, since the dietary contribution of hazelnuts was higher than that of dried figs.64 Similarly, Bol et al.65 investigated pasta and bakery products and observed the MoE (24.6) and EDI (6.9 ng kg–1 bw day–1) values of AFB1, indicating a substantial health risk, while OTA exposure was 4.88 ng kg–1 bw day–1, serving 30.5% of PTWI with negligible risk. In particular, nearly half of infants and children were exposed to at least two mycotoxins at elevated concentrations because of their low body weight.66 Accordingly, the populations with lower body weight were much more susceptible to mycotoxins, e.g., the average body weight in Vietnam was 50 kg.63 Furthermore, dietary exposure and risk assessments may vary by country or even region, which are also proportional to mycotoxin levels and consumption rates..57,59 Likewise, the results may differ among studies due to methodological differences, such as how the LOD and LOQ are determined and how left-censored data are processed (<LOD or <LOQ).61
3. Conclusions
The simultaneous detection of AFs and OTA in DRPFs and IPFs using multitoxin IAC clean-up was evaluated along with the dietary exposure risk assessment. With the exception of AFG2, the method’s performance in detecting AFs and OTA in dried red peppers was satisfactory. All industrially processed red peppers met the regulatory limits for AFB1, total AFs, or OTA. In contrast, traditional peppers exceeded the EU limits by 30% (16/54), 26% (14/54), and 4% (2/54), respectively, indicating the need for improved storage and drying settings in conjunction with good agricultural and manufacturing practices. Since traditionally processed DRPFs contained a higher frequency of AFs than OTA, their long-term consumption may raise the risk for health; thus, red peppers should be routinely controlled for mycotoxin levels to minimize consumer exposure. Among the co-occurrence patterns, AFB1-AFB2-OTA was the most frequent (54%) in all samples. Exposure to AFs and AFB1 (977 and 909, respectively) through red pepper consumption appeared to be of greater concern than OTA (MoE >10,000) for the adult population in Turkey. Risk assessments revealed that exposure to AFB1 and total AFs was associated with 0.0058 and 0.0062 liver cancer cases/100,000 persons/year (UB estimate). Weekly OTA exposure was 0.868 ng kg–1 bw week–1, which was far below the PTWI, hence not of health concern, accounting for 0.87 and 0.73% of the JECFA and EFSA PTWI values of 100 and 120 ng kg–1 bw, respectively. A thorough toxicological investigation is needed to establish limits on the concurrent exposure to multiple mycotoxins in dried red peppers and other foods.
4. Materials and Methods
4.1. Samples
Fifty-four red pepper flakes were collected, including 41 dried red pepper flakes (DRPFs, 31 traditional and 10 industrial) and 13 red-black isot pepper flakes (IPFs, 11 traditional and 2 industrial). DRPFs and IPFs were produced both industrially (mechanically dried, under controlled conditions) and traditionally (sun-dried, based on traditional techniques). Unfortunately, since IPFs were mostly produced by traditional methods, the source of industrially processed IPFs was limited. Samples were obtained randomly from various retail markets, herbal shops, bazaars, and local producers in various cities (Adana, Gaziantep, Kahramanmaraş, Şanlıurfa, Istanbul, and Ankara) throughout Turkey. Sampling was done following EC 401/2006, which states that the total sample weight for spices should be at least 500 g.36 For unpackaged samples (sold by weight), random sampling was performed to gather samples from diverse areas of a batch, and all pepper flakes were ground to a flake size of roughly 1–3 mm. Water activity (aw) was measured by a hygrometer (Novasina, LabTouch-aw, Lachen, Switzerland) at 25 °C ± 1. Further, samples were kept at −18 °C and analyzed at room temperature.
4.2. Chemicals and Materials
The aflatoxin standard solution was supplied from Supelco (Bellefonte, PA; Aflatoxin B and G mix, Cat. No. 46304-U). In 1 mL of methanol, each standard mix contains 0.3 μg of AFG2, AFB2, and 1 μg of AFG1, AFB1. Aflatoxin standards (0.2–10.8 ng mL–1 for AFB1 and AFG1, and 0.06–3.24 ng mL–1 for AFB2 and AFG2) were dissolved in methanol:water (20:30, v/v). The OTA standard was purchased from Sigma-Aldrich (Steinheim, Germany), and working standard sets (0.1–5 ng OTA mL–1) were prepared in acetonitrile/water/acetic acid (99:99:2, v/v/v). The AflaOchraTest immunoaffinity column (Vicam, Watertown, MA) was employed in clean-up and preconcentration steps. All analytical and high-performance liquid chromatography (HPLC)-grade chemicals and reagents were supplied from Merck (Darmstadt, Germany). Phosphate-buffered saline (PBS) and phosphate buffer (PB) were prepared according to Trucksess et al.67
4.3. Aflatoxin and Ochratoxin A analysis
4.3.1. Sample Extraction and IAC Clean-Up
Based on Trucksess et al. (2008) and Stroka et al. (2000), red peppers were extracted and cleaned up with slight modifications. According to combined methods, relevant mycotoxins were extracted and followed by AflaOchraTest IAC clean-up and HPLC-FLD with postcolumn derivatization. Samples (5 g) were mixed with 25 mL of methanol–0.5% sodium hydrogen carbonate (NaHCO3) (7:3, v/v) and 1 g of sodium chloride (NaCl) and shaken (Janke and Kunkel, IKA-Labortechnik KS 250) at 400 rpm for 10 min. Following centrifugation for 10 min at 8228g (Eppendorf Centrifuge 5804R, Hamburg, Germany), 7 mL of the upper phase was transferred to another tube. PB (28 mL) containing 1% Tween 20 was added to the tube and vortexed. Following glass microfiber filtration, AflaOchraTest was employed for 25 mL of the filtrate with 1–2 drops/s. Then, 5 mL of 10 mM PBS and pure water were used to wash the column; finally, 3 mL of air was passed through the column for the remaining drops. After 1 mL of methanol was used to elute bounded AFs and OTA from the column, 1 mL of pure water was added before analysis.
4.3.2. HPLC-FLD Analysis
Agilent Technologies 1100 series with a quaternary pump, solvent distribution system, fluorescence detector (FLD), degasser, and Rheodyne injector with a 100 L loop was used for HPLC analysis. The Chemstation 3D solution program was used to process samples and collect data. In chromatographic separations, a 250 mm×4.6mm ODS-Hypersil C18 column (5 μm particle size; Hichrome Ltd., Reading, U.K.) was utilized. For postcolumn derivatization, CoBrA-cell (Vicam, Watertown) was employed, maintaining the column at 30 °C with a flow rate of 1 mL min–1. Mobile phase A (water:acetonitrile:methanol, 6:2:3, v/v/v with 350 μL of 4 M nitric acid, HNO3, and 120 mg of potassium bromide, KBr) and mobile phase B (acetonitrile:water:acetic acid, 99:99:2, v/v/v) were used for AFs and OTA under gradient conditions, respectively, at a flow rate of 1 mL min–1. Elution was conducted as follows: 100% mobile phase A for 12 min and 100% mobile phase B for an isocratic duration of up to 25 min. The wavelength of excitation (λEx) and emission (λEm) was set to 360 and 440 nm for AFs (B1, B2, G1, and G2) until 12 min; 333 and 477 nm for OTA, respectively.33 For injection, the volume of samples and standards was set to 100 μL.
4.4. Analytical Quality Parameters
Linearity, sensitivity, recovery, and precision were assessed as a part of the method validation plan to determine AFB1, AFB2, AFG1, AFG2, and OTA in dried red pepper samples. Linearity was determined using seven-point calibration ranging from 0.2 to 10.8 ng mL–1 for AFB1 and AFG1, 0.06 to 3.24 ng mL–1 for AFB2 and AFG2, and 0.1 to 5 ng mL–1 for OTA, in triplicate. Linearity was calculated using peak area and analyte concentration. Linear regression analysis was performed to calculate the method’s linearity, which was represented as a correlation coefficient (R2).
Method’s sensitivity was expressed as detection (LOD) and quantification (LOQ) limits were derived according to recovery data,68 based on signal-to-noise (S/N) ratios of 3 and 10, respectively, with the following equations
where “B” is the mean of blank samples and “S” is the sample standard deviation, with 10 injections.
Spiking was conducted using toxin-free blank red pepper samples containing certain concentrations of AFs (1, 2, and 10 μg kg–1 of AFB1 and AFG1; 0.3, 0.6, and 3 μg kg–1 of AFB2 and AFG2) and OTA (0.5, 1, and 3 μg kg–1) used for method’s accuracy. All samples were brought to room temperature overnight before extraction. Spiking was conducted in six replicates. Following HPLC-FLD quantification, the final mycotoxin content in a spiked sample (as explained in Sections 4.3.1 and 4.3.2) was compared to the known initial concentration to estimate recoveries. The precision was calculated at three different concentrations through a six-replicated analysis of spiked samples on the same day and expressed as the percent relative standard deviation (RSD) of the replicate measurements.
4.5. Dietary Exposure and Risk Assessment
4.5.1. Margin of Exposure (MoE) Approach
For the calculation of dietary exposure, the margin of exposure (MoE) approach was used, which was a fraction of the BMDL10 (benchmark dose lower confidence limit 10) and estimated daily intake (EDI) of AFB1 or OTA, where BMDL10 is the lowest dose to cause a 10% increase in cancer incidence in rodents.69 Considering the risks of liver and renal cancer, the BMDL10 value for AFB1 and OTA was estimated to be 170 ng kg–1 bw day–1,54 and 21 μg kg–1 bw day–1,56 respectively. EDI level was calculated using average toxin concentrations via the substitution method.70 Thus, the values under LOD were substituted with 0, LOD/2, and LOQ to calculate lower (LB), middle (MB), and upper bound (UB) levels, respectively.61−63 Per capita consumption of dried red peppers was considered 2 g day–1, and the average body weight (bw) for Turkish adults was approximately 72.8 kg.9 The dietary exposure was determined by multiplying the contamination level per body weight with the dietary intake.62 EDI value was calculated as shown in eq 1
| 1 |
4.5.2. Risk Assessment
Given that the MoE score indicates the degree of concern for mycotoxin exposure, an additional risk assessment is required to detect the risk of liver cancer.69 People who tested positive (PHBsAg+) and negative (PHBsAg–) for hepatitis B surface antigen (eq 2) were included in calculations, and consuming AFB1-contaminated foods was linked with a 30-fold increase in the risk of getting liver cancer.3 Hepatitis B virus infection was classified as intermediate endemicity (2–8%) or high (> 8%) by the World Health Organization,71 and HbsAg prevalence was reported to be 6.2 and 8.2% in the urban and rural areas, respectively, in Turkey’s southeastern region.61,72 Therefore, the risk of liver cancer was computed under the worst-case scenario, with an assumed population value of 8%. The risk of AFB1-related liver cancer was calculated by multiplying the EDI and the average carcinogenic potency (Pcancer) (eq 3).63
| 2 |
| 3 |
PHBsAg+ = 0.3 cancers/year/100,000 population ng–1 AFB1 kg–1 bw day–1; PHBsAg– = 0.01 cancers/year/100,000 population ng–1 AFB1 kg–1 bw day–1; Pop. PHBsAg+ = proportion of population positive with Hepatitis B (0.08); Pop. PHBsAg– = proportion of population negative with Hepatitis B (0.92); Pcancer = 0.3 × 0.08 + 0.01 × 0.92 = 0.033.
AFB1 exposure (even at relatively low levels, ≤1 ng kg–1 bw day–1 in developed countries) can contribute to liver cancer.73,74 AFB1 has no safe level because of its severe carcinogenic and genotoxic effects; hence, it should be “as low as reasonably achievable (ALARA)” to protect public health.75 The average potency for liver cancer from eq 3 was calculated as 0.033, which was utilized in the present risk estimations (Table 3). However, Kuiper-Goodman76 calculated the provisional maximum tolerable daily intake (PMTDI) for AFB1 (1 ng kg–1 bw day–1). Therefore, for non-European countries, exposing 1 ng kg–1 bw day–1 of AFB1 would cause liver cancer by 0.083 cases/100,000 persons/year.3 As a result, the risk of liver cancer in Turkey was recalculated using the value 0.083 (Risk of liver cancer = 0.083 × EDI of AFB1), yielding 0.0144,0.0145, and 0.0146 cases/100,000 persons/year for MB, LB, and UB exposure levels, respectively, which were higher than the current estimates. However, for OTA, provisional tolerable weekly intake (PTWI) was set as 100 ng kg–1 bw55 and 120 ng kg–1 bw,56 respectively. Further, estimated OTA exposure was compared to the PTWI value to determine the risk assessment; exceeding the PTWI posed a risk.
Acknowledgments
This research was supported by the Scientific Research Council of Istanbul Technical University (grant no. 37077). The authors thank Dr. Harika Çankaya (Harran University, Department of Food Engineering) for kindly donating isot pepper flakes.
Glossary
Abbreviations Used
- AFs
aflatoxins
- AFB1
aflatoxin B1
- AFB2
aflatoxin B2
- AFG1
aflatoxin G1
- AFG2
aflatoxin G2
- DRPFs
dried red pepper flakes
- EDI
estimated daily intake
- EFSA
European Food Safety Authority
- FAO
Food and Agricultural Organization of the United Nations
- HPLC-FLD
high-performance liquid chromatography-fluorescence detector
- IARC
International Agency for Research on Cancer
- IAC
immunoaffinity column
- IPFs
isot pepper flakes
- LB
lower bound
- LOD
limit of detection
- LOQ
limit of quantification
- MB
middle bound
- MoE
margin of exposure
- OTA
ochratoxin A
- PTWI
provisional tolerable weekly intake
- RASSF
rapid alert system for food and feed
- RSD
relative standard deviation
- TWI
tolerable weekly intake
- UB
upper bound
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c02236.
Experimental details; contamination ranges of the AFs and OTA, and co-occurrence rates of their combinations in red pepper samples (PDF)
Author Contributions
S.O. conducted the experiments and wrote the original draft. F.K.G. designed and supervised the research project along with the manuscript correction.
The authors declare no competing financial interest.
Supplementary Material
References
- Ostry V.; Malir F.; Toman J.; Grosse Y. Mycotoxins as Human Carcinogens—the IARC Monographs Classification. Mycotoxin Res. 2017, 33, 65–73. 10.1007/s12550-016-0265-7. [DOI] [PubMed] [Google Scholar]
- Eskola M.; Kos G.; Elliott C. T.; Hajšlová J.; Mayar S.; Krska R. Worldwide Contamination of Food-Crops with Mycotoxins: Validity of the Widely Cited ‘FAO Estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. 10.1080/10408398.2019.1658570. [DOI] [PubMed] [Google Scholar]
- FAO/WHO. World Health Organization & Food and Agriculture Organization of the United Nations. Evaluation of Certain Food Additives and Contaminants: Forty-Ninth Report of the Joint FAO/WHO Expert Committee on Food Additives. World Heal. Organ., 1999. https://apps.who.int/iris/handle/10665/42142. [PubMed]
- IARC. International Agency for Research on Cancer Monographs on the Evaluation of Carcinogenic Risks to Humans. Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins, Vol. 56; World Heal. Organ., 1993.
- Santos L.; Marín S.; Sanchis V.; Ramos A. J. Co-Occurrence of Aflatoxins, Ochratoxin A and Zearalenone in Capsicum Powder Samples Available on the Spanish Market. Food Chem. 2010, 122, 826–830. 10.1016/j.foodchem.2010.03.070. [DOI] [Google Scholar]
- Hayoglu I.; Didin M.; Turkoglu H.; Fenercioglu H. The Effects of Processing Methods on Some Properties of Hot Red and Red-Blackish Ground Peppers. Pakistan J. Biol. Sci. 2005, 8, 1420–1423. 10.3923/pjbs.2005.1420.1423. [DOI] [Google Scholar]
- Santos L.; Marín S.; Mateo E. M.; Gil-Serna J.; Valle-Algarra F. M.; Patiño B.; Ramos A. J. Mycobiota and Co-Occurrence of Mycotoxins in Capsicum Powder. Int. J. Food Microbiol. 2011, 151, 270–276. 10.1016/j.ijfoodmicro.2011.09.011. [DOI] [PubMed] [Google Scholar]
- FAO. Food and Agricultural Organization of the United Nations. FAO Statistical Databases and Data Sets. Food Agric. Organ., 2020. http://www.faostat.fao.org/.
- TSI. Turkish Statistical Institute. The Summary of Agricultural Statistics. Turkish Stat. Inst., 2020. http://tuik.gov.tr.
- Korkmaz A.; Hayaloglu A. A.; Atasoy A. F. Evaluation of the Volatile Compounds of Fresh Ripened Capsicum annuum and Its Spice Pepper (Dried Red Pepper Flakes and Isot). LWT 2017, 84, 842–850. 10.1016/j.lwt.2017.06.058. [DOI] [Google Scholar]
- Chuaysrinule C.; Maneeboon T.; Roopkham C.; Mahakarnchanakul W. Occurrence of Aflatoxin- and Ochratoxin A-Producing Aspergillus Species in Thai Dried Chilli. J. Agric. Food Res. 2020, 2, 100054 10.1016/j.jafr.2020.100054. [DOI] [Google Scholar]
- Ahn J.; Kim D.; Jang H.; Kim Y.; Shim W.-B.; Chung D.-H. Occurrence of Ochratoxin A in Korean Red Paprika and Factors to Be Considered in Prevention Strategy. Mycotoxin Res. 2010, 26, 279–286. 10.1007/s12550-010-0067-2. [DOI] [PubMed] [Google Scholar]
- Palma P.; Godoy M.; Vidal M.; Rivera A.; Calderón R. Adaptation, Optimization, and Validation of a Sensitive and Robust Method for the Quantification of Total Aflatoxins (B1, B2, G1, and G2) in the Spice Merkén by HPLC-FLD with Post-Column Derivatization. Microchem. J. 2022, 178, 107342 10.1016/j.microc.2022.107342. [DOI] [Google Scholar]
- Iha M. H.; Rodrigues M. L.; Trucksess M. W. Multitoxin Immunoaffinity Analysis of Aflatoxins and Ochratoxin A in Spices. J. Food Saf. 2021, 41, e12921 10.1111/jfs.12921. [DOI] [Google Scholar]
- Li M.; Tong Z.; Gao X.; Zhang L.; Li S. Simultaneous Detection of Zearalenone, Citrinin, and Ochratoxin A in Pepper by Capillary Zone Electrophoresis. Food Addit. Contam. Part A 2020, 37, 1388–1398. 10.1080/19440049.2020.1769197. [DOI] [PubMed] [Google Scholar]
- El Darra N.; Gambacorta L.; Solfrizzo M.; Gambacorta L.; Solfrizzo M. Multimycotoxins Occurrence in Spices and Herbs Commercialized in Lebanon. Food Control 2019, 95, 63–70. 10.1016/j.foodcont.2018.07.033. [DOI] [Google Scholar]
- Gambacorta L.; Magistà D.; Perrone G.; Murgolo S.; Logrieco A. F.; Solfrizzo M. Co-Occurrence of Toxigenic Moulds, Aflatoxins, Ochratoxin A, Fusarium and Alternaria Mycotoxins in Fresh Sweet Peppers (Capsicum annuum) and Their Processed Products. World Mycotoxin J. 2018, 11, 159–173. 10.3920/WMJ2017.2271. [DOI] [Google Scholar]
- Motloung L.; De Saeger S.; De Boevre M.; Detavernier C.; Audenaert K.; Adebo O. A.; Njobeh P. B. Study on Mycotoxin Contamination in South African Food Spices. World Mycotoxin J. 2018, 11, 401–409. 10.3920/WMJ2017.2191. [DOI] [Google Scholar]
- Iha M. H.; Rodrigues M. L.; de Cássia Briganti R. Survey of Aflatoxins and Ochratoxin A in Spices from Brazilian Market. Braz. Arch. Biol. Technol. 2021, 64, 0–8. 10.1590/1678-4324-2021210244. [DOI] [Google Scholar]
- Koutsias I.; Kollia E.; Makri K.; Markaki P.; Proestos C. Occurrence and Risk Assessment of Aflatoxin B1 in Spices Marketed in Greece. Anal. Lett. 2021, 54, 1995–2008. 10.1080/00032719.2020.1832509. [DOI] [Google Scholar]
- Wikandari R.; Mayningsih I. C.; Sari M. D. P.; Purwandari F. A.; Setyaningsih W.; Rahayu E. S.; Taherzadeh M. J. Assessment of Microbiological Quality and Mycotoxin in Dried Chili by Morphological Identification, Molecular Detection, and Chromatography Analysis. Int. J. Environ. Res. Public Health 2020, 17, 1847. 10.3390/ijerph17061847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadil mon V.; John Kennedy Z.; Paranitharan V.; Karthikeyan S. Mycotic Contamination and Aflatoxin Potential of Molds in Capsicum annum (Chili), and Chili Powder Commercialized in South Indian Markets. Toxicon 2022, 210, 109–114. 10.1016/j.toxicon.2022.02.016. [DOI] [PubMed] [Google Scholar]
- Brera C.; Debegnach F.; De Santis B.; Pannunzi E.; Berdini C.; Prantera E.; Gregori E.; Miraglia M. Simultaneous Determination of Aflatoxins and Ochratoxin A in Baby Foods and Paprika by HPLC with Fluorescence Detection: A Single-Laboratory Validation Study. Talanta 2011, 83, 1442–1446. 10.1016/j.talanta.2010.11.031. [DOI] [PubMed] [Google Scholar]
- Ezekiel C. N.; Ortega-Beltran A.; Oyedeji E. O.; Atehnkeng J.; Kössler P.; Tairu F.; Hoeschle-Zeledon I.; Karlovsky P.; Cotty P. J.; Bandyopadhyay R. Aflatoxin in Chili Peppers in Nigeria: Extent of Contamination and Control Using Atoxigenic Aspergillus flavus Genotypes as Biocontrol Agents. Toxins 2019, 11, 429 10.3390/toxins11070429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RASSF. The Rapid Alert System for Food and Feed, European Commission. Eur. Comm. 2021. https://webgate.ec.europa.eu/rasff-window/screen/search.
- Perrone G.; Ferrara M.; Medina A.; Pascale M.; Magan N. Toxigenic Fungi and Mycotoxins in a Climate Change Scenario: Ecology, Genomics, Distribution, Prediction and Prevention of the Risk. Microorganisms 2020, 8, 1496. 10.3390/microorganisms8101496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q.; Jiang K.; Tang Z.; Fan K.; Meng J.; Nie D.; Zhao Z.; Wu Y.; Han Z. Exposure Assessment of Multiple Mycotoxins and Cumulative Health Risk Assessment: A Biomonitoring-Based Study in the Yangtze River Delta, China. Toxins 2021, 13, 103 10.3390/toxins13020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EC. European Commission European Commission Regulation (EU) No 165/ 2010 of 26 February 2006 Amending Regulation (EC) No. 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs as Regards Aflatoxins Off. J. Eur. Union L, 2010. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:050:0008:0012:EN:PDF.
- EC. European Commission Commission Regulation (EU) 2015/1137 of 13 July 2015 Amending Regulation (EC) No 1881-2006 as Regards the Maximum Level of Ochratoxin A in Capsicum spp. Spices. Off. J. Eur. Union L, 2015. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015R1137&from=DE.
- Özbey F.; Kabak B. Natural Co-Occurrence of Aflatoxins and Ochratoxin A in Spices. Food Control 2012, 28, 354–361. 10.1016/j.foodcont.2012.05.039. [DOI] [Google Scholar]
- Wan Ainiza W. M.; Jinap S.; Sanny M. Simultaneous Determination of Aflatoxins and Ochratoxin A in Single and Mixed Spices. Food Control 2015, 50, 913–918. 10.1016/j.foodcont.2014.10.051. [DOI] [Google Scholar]
- Casquete R.; Rodríguez A.; Hernández A.; Martín A.; Bartolomé T.; Córdoba J. J.; Córdoba M. G. Occurrence of Toxigenic Fungi and Mycotoxins during Smoked Paprika Production. J. Food Prot. 2017, 80, 2068–2077. 10.4315/0362-028X.JFP-17-164. [DOI] [PubMed] [Google Scholar]
- Hernández Hierro J. M.; Garcia-Villanova R. J.; Torrero P. R.; Fonseca I. M. T. Aflatoxins and Ochratoxin a in Red Paprika for Retail Sale in Spain: Occurrence and Evaluation of a Simultaneous Analytical Method. J. Agric. Food Chem. 2008, 56, 751–756. 10.1021/jf073002c. [DOI] [PubMed] [Google Scholar]
- Set E.; Erkmen O. Occurrence of Aflatoxins in Ground Red Chili Pepper and Pistachio Nut. Int. J. Food Prop. 2014, 17, 2322–2331. 10.1080/10942912.2013.800985. [DOI] [Google Scholar]
- Tosun A.; Ozden S. Ochratoxin A in Red Pepper Flakes Commercialised in Turkey. Food Addit. Contam. Part B Surveill. 2016, 9, 46–50. 10.1080/19393210.2015.1121929. [DOI] [PubMed] [Google Scholar]
- EC. European Commission. European Commission Regulation (EC) No 401/2006 of 23 February 2006 Laying down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs Off. J. Eur. Union 2006L 7012–34.https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R0401&from=EN.
- Stroka J.; Anklam E.; Jörissen U.; Gilbert J. Immunoaffinity Column Cleanup with Liquid Chromatography Using Post-Column Broamination for Determination of Aflatoxins in Peanut Butter, Pistachio Paste, Fig Paste, and Paprika Powder: Collaborative Study. J. AOAC Int. 2000, 83, 320–340. 10.1093/jaoac/83.2.320. [DOI] [PubMed] [Google Scholar]
- Trucksess M.; Weaver C.; Oles C.; D’Ovidio K.; Rader J. Determination of Aflatoxins and Ochratoxin A in Ginseng and Other Botanical Roots by Immunoaffinity Column Cleanup and Liquid Chromatography. J AOAC Int. 2006, 89, 624–630. 10.1093/jaoac/89.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J.; Rodríguez R.; Garcia-Cela E.; Medina A.; Magan N.; Lima N.; Battilani P.; Santos C. Overview of Fungi and Mycotoxin Contamination in Capsicum Pepper and in Its Derivatives. Toxins 2019, 11, 27. 10.3390/toxins11010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman A. D. Storage of Red Chili Pepper under Hermetically Sealed or Vacuum Conditions for Preservation of Its Quality and Prevention of Mycotoxin Occurrence. J. Stored Prod. Res. 2010, 46, 155–160. 10.1016/j.jspr.2010.02.002. [DOI] [Google Scholar]
- Atasoy A. F.; Hayoğlu İ.; Korkmaz A.; Kara E.; Yildirim A. Geleneksel Ev Isot Baharatının Aflatoksin Içeriğinin Belirlenmesi Üzerine Bir Araştırma. Harran Tarım ve Gıda Bilim. Derg. 2017, 21, 35–40. 10.29050/harranziraat.303132. [DOI] [Google Scholar]
- Iqbal S. Z.; Mumtaz A.; Mahmood Z.; Waqas M.; Ghaffar A.; Ismail A.; Pervaiz W. Assessment of Aflatoxins and Ochratoxin a in Chili Sauce Samples and Estimation of Dietary Intake. Food Control 2021, 121, 107621 10.1016/j.foodcont.2020.107621. [DOI] [Google Scholar]
- TFC. Regulation No. 2011/28157 the Maximum Allowed Level of Food Contaminants; Official Gazette of Publication: Ankara, Turkey, 2011, Vol. 28157. http://www.resmigazete.gov.tr. [Google Scholar]
- Ham H.; Kim S.; Kim M.-H.; Lee S.; Hong S. K.; Ryu J.; Lee T. Mycobiota of Ground Red Pepper and Their Aflatoxigenic Potential. J. Microbiol. 2016, 54, 832–837. 10.1007/s12275-016-6480-2. [DOI] [PubMed] [Google Scholar]
- Set E.; Erkmen O. The Aflatoxin Contamination of Ground Red Pepper and Pistachio Nuts Sold in Turkey. Food Chem. Toxicol. 2010, 48, 2532–2537. 10.1016/j.fct.2010.06.027. [DOI] [PubMed] [Google Scholar]
- Bircan C. The Determination of Aflatoxins in Spices by Immunoaffinity Column Extraction Using HPLC. Int. J. Food Sci. Technol. 2005, 40, 929–934. 10.1111/j.1365-2621.2005.01025.x. [DOI] [Google Scholar]
- Jalili M.; Jinap S. Natural Occurrence of Aflatoxins and Ochratoxin A in Commercial Dried Chili. Food Control 2012, 24, 160–164. 10.1016/j.foodcont.2011.09.020. [DOI] [Google Scholar]
- Marín S.; Colom C.; Sanchis V.; Ramos A. J. Modelling of Growth of Aflatoxigenic A. flavus Isolates from Red Chilli Powder as a Function of Water Availability. Int. J. Food Microbiol. 2009, 128, 491–496. 10.1016/j.ijfoodmicro.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Erdogan A. The Aflatoxin Contamination of Some Pepper Types Sold in Turkey. Chemosphere 2004, 56, 321–325. 10.1016/j.chemosphere.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Cho S. H.; Lee C. H.; Jang M. R.; Son Y. W.; Lee S. M.; Choi I. S.; Kim S. H.; Kim D. B. Aflatoxins Contamination in Spices and Processed Spice Products Commercialized in Korea. Food Chem. 2008, 107, 1283–1288. 10.1016/j.foodchem.2007.08.049. [DOI] [Google Scholar]
- Huff W. E.; Kubena L. F.; Harvey R. B.; Doerr J. A. Mycotoxin Interactions in Poultry and Swine. J. Anim. Sci. 1988, 66, 2351–2355. 10.2527/jas1988.6692351x. [DOI] [PubMed] [Google Scholar]
- Smith M.-C. C.; Madec S. S. S.; Coton E.; Hymery N. Natural Co-Occurrence of Mycotoxins in Foods and Feeds and Their in Vitro Combined Toxicological Effects. Toxins 2016, 8, 94 10.3390/toxins8040094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmíková M.; Reisnerová H.; Dufková Z.; Bárta I.; Jílek F. Potential Hazard of Simultaneous Occurrence of Aflatoxin B1 and Ochratoxin A. Vet. Med. 2001, 46, 169–171. 10.17221/7876-VETMED. [DOI] [Google Scholar]
- EFSA. European Food Safety Authority. Risk Assessment of Aflatoxins in Food 2020, 18 (3), 6040.https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2020.6040. [DOI] [PMC free article] [PubMed]
- JECFA . The Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants: Sixty-Eighth Report of the Joint FAO/WHO Expert Committee on Food Additive. WHO Technical Report Series No 947; WHO: Geneva, Switzerland, 2007. pp 169-180.
- EFSA. Opinion of the Scientific Panel on Contaminants in the Food Chain on a Request from the Commission Related to Ochratoxin A in Food EFSA J 20063651–56.https://www.efsa.europa.eu/en/efsajournal/pub/365.
- Yogendrarajah P.; Jacxsens L.; Lachat C.; Walpita C. N.; Kolsteren P.; De Saeger S.; De Meulenaer B. Public Health Risk Associated with the Co-Occurrence of Mycotoxins in Spices Consumed in Sri Lanka. Food Chem. Toxicol. 2014, 74, 240–248. 10.1016/j.fct.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Sakin F.; Tekeli İO.; Yipel M.; Kürekci C. Occurrence and Health Risk Assessment of Aflatoxins and Ochratoxin a in Sürk, a Turkish Dairy Food, as Studied by HPLC. Food Control 2018, 90, 317–323. 10.1016/j.foodcont.2018.03.012. [DOI] [Google Scholar]
- Kilic S.; Cam I. B.; Tongur T.; Kilic M. Health Risk Assessment of Exposure to Heavy Metals and Aflatoxins via Dietary Intake of Dried Red Pepper from Marketplaces in Antalya, Southern Turkey. J. Food Sci. 2018, 83, 2675–2681. 10.1111/1750-3841.14322. [DOI] [PubMed] [Google Scholar]
- Kulahi A.; Kabak B. A Preliminary Assessment of Dietary Exposure of Ochratoxin A in Central Anatolia Region, Turkey. Mycotoxin Res. 2020, 36, 327–337. 10.1007/s12550-020-00397-6. [DOI] [PubMed] [Google Scholar]
- Oktay Basegmez H. I. Dietary Exposure Assessment of Aflatoxin from Dried Figs in Turkey. Hittite J. Sci. Eng. 2019, 6, 173–177. 10.17350/hjse19030000144. [DOI] [Google Scholar]
- Heshmati A.; Zohrevand T.; Khaneghah A. M.; Mozaffari Nejad A. S.; Sant’Ana A. S. Co-Occurrence of Aflatoxins and Ochratoxin A in Dried Fruits in Iran: Dietary Exposure Risk Assessment. Food Chem. Toxicol. 2017, 106, 202–208. 10.1016/j.fct.2017.05.046. [DOI] [PubMed] [Google Scholar]
- Huong B. T. M.; Tuyen L. D.; Tuan D. H.; Brimer L.; Dalsgaard A. Dietary Exposure to Aflatoxin B1, Ochratoxin A and Fuminisins of Adults in Lao Cai Province, Viet Nam: A Total Dietary Study Approach. Food Chem. Toxicol. 2016, 98, 127–133. 10.1016/j.fct.2016.10.012. [DOI] [PubMed] [Google Scholar]
- Kabak B. Aflatoxins in Hazelnuts and Dried Figs: Occurrence and Exposure Assessment. Food Chem. 2016, 211, 8–16. 10.1016/j.foodchem.2016.04.141. [DOI] [PubMed] [Google Scholar]
- Bol E. K.; Araujo L.; Veras F. F.; Welke J. E. Estimated Exposure to Zearalenone, Ochratoxin A and Aflatoxin B1 through the Consume of Bakery Products and Pasta Considering Effects of Food Processing. Food Chem. Toxicol. 2016, 89, 85–91. 10.1016/j.fct.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Ojuri O. T.; Ezekiel C. N.; Eskola M. K.; Šarkanj B.; Babalola A. D.; Sulyok M.; Hajšlová J.; Elliott C. T.; Krska R. Mycotoxin Co-Exposures in Infants and Young Children Consuming Household- and Industrially-Processed Complementary Foods in Nigeria and Risk Management Advice. Food Control 2019, 98, 312–322. 10.1016/j.foodcont.2018.11.049. [DOI] [Google Scholar]
- Trucksess M. W.; Weaver C. M.; Oles C. J.; Fry F. S.; Noonan G. O.; Betz J. M.; Rader J. I. Determination of Aflatoxins B1, B2, G1, and G2 and Ochratoxin A in Ginseng and Ginger by Multitoxin Immunoaffinity Column Cleanup and Liquid Chromatographic Quantitation: Collaborative Study. J. AOAC Int. 2008, 91, 511–523. 10.1093/jaoac/91.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eurachem. The Fitness for the Purpose of Analytical Methods. A Laboratory Guide to Method Validation and Related Topics. Middlesex, TW110LY, United Kingdom: Eurachem Working Group, 2014. https://www.eurachem.org/images/stories/Guides/pdf/MV_guide_2nd_ed_EN.pdf.
- EFSA. European Food Safety Authority. Opinion of the Scientific Committee on a Request from EFSA Related to a Harmonized Approach for Risk Assessment of Substances Which Are Both Genotoxic and Carcinogenic EFSA J 20052821–31.https://www.efsa.europa.eu/en/efsajournal/pub/282.
- Scientific Report of EFSA: Management of Left-Censored Data in Dietary Exposure Assessment of Chemical Substances. EFSA J. 2010, 8, 1–96. 10.2903/j.efsa.2010.1557. [DOI] [Google Scholar]
- WHO. World Health Organization. Prevention of Mother-to-Child Transmission of Hepatitis B Virus: Guidelines on Antiviral Prophylaxis in Pregnancy: Web Annex A: Systematic Review of the Efficacy and Safety of Antiviral Therapy during Pregnancy. 2020. https://apps.who.int/iris/bitstream/handle/10665/333391/9789240002708-eng.pdf. [PubMed]
- Mehmet D.; Meliksah E.; Serif Y.; Gunay S.; Tuncer Ö.; Zeynep S. Prevalence of Hepatitis B Infection in the Southeastern Region of Turkey: Comparison of Risk Factors for HBV Infection in Rural and Urban Areas. Jpn. J. Infect. Dis. 2005, 58, 15–19. [PubMed] [Google Scholar]
- JECFA. The Joint FAO/WHO Expert Committee on Food Additives . Eighty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Contaminants in Food,. WHO Technical Report Series, 2017, pp 1-166.
- SCF . The European Union Scientific Committee for Food. European Commission DG XXIV Unit B3, Thirty-Fifth Report. Opinion on Aflatoxins B1, B2, G1, G2, M1, and Patulin, 1994.
- JECFA. The Joint FAO/WHO Expert Committee on Food Additives . Evaluation of Certain Food Additives and Contaminants: Fifty-Fifth Report of the Joint FAO/WHO Expert Committee on Food Additives, WHO Technical Report Series No 901, WHO: Geneva, Switzerland; 2001.
- Kuiper-Goodman T.Food Safety: Mycotoxins and Phycotoxins in Perspective. In Mycotoxins and Phycotoxins: Developments in Chemistry, Toxicology and Food Safety.Alakens, Fort Collins; Miraglia M.; van Egmond H. P.; Brera C.; Gilbert J., Eds.; 1998; pp 25–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.