Abstract
Considering the importance of acetylcholine esterase (AChE, BchE) and α-glucosidase in the treatment of Alzheimer’s disease and diabetes mellitus, the synthesis of novel azinane triazole-based derivatives as effective acetylcholinesterase (AchE), α-glucosidase, urease, lipoxygenase (LOX), and butyrylcholinesterase (BChE) inhibitors is described. Azinane analogue (2) was merged with 1,2,4-triazole to acquire 1-(4-toluenesulfonyl)-4-(3-mercapto-4-methyl-4H-1,2,4-triazol-5-yl) piperidine (8) through a list of intermediates including 1-(4-toluenesulfonyl)-4-(ethoxycarbonyl) piperidine (3), 1-(4-toluenesulfonyl)-4-(2-hydrazinocarbonyl)piperidine (5), and 1-(4-toluenesulfonyl)-4-[1-(methyl amino thiocarbonyl)-2-hydrazinocarbonyl]piperidine (7). The target molecules, 1-(4-toluenesulfonyl)-4-[3-(N-alkyl/phenyl/aryl-2-ethanamoyl thio)-4-methyl-4H-1,2,4-triazol-5-yl] piperidine (12a–o), were achieved through the reaction of 8 with N-alkyl/phenyl/aryl-2-bromo ethanamides (11a–o) as electrophiles. These electrophiles were accomplished by a benign reaction of alkyl/phenyl/aryl amines (9a–o) and 2-bromo ethanoyl bromide (10). The spectral study of IR, 1D-NMR, and EI-MS corroborated the synthesized compounds. Methyl phenyl and methyl phenyl-substituted derivatives 12d and 12m with IC50 = 0.73 ± 0.54; 36.74 ± 1.24; 19.35 ± 1.28; 0.017 ± 0.53; and 0.038 ± 0.50 μM are found to be the most potent AChE, α-glucosidase, urease, and BChE inhibitors. The high inhibition potential of synthesized molecules against AChE, α-glucosidase, urease, and BChEenzymes inferred their role in enzyme inhibition properties.
1. Introduction
The most important role in the field of synthesis of new bioactive molecules is played by heterocyclic chemistry. Medicinal chemistry that deals with the pharmaceutical and medical sciences is associated with the design and development of bioactive drug molecules. Such heterocyclic compounds comprising oxygen and nitrogen were shown to have the most active bioactivities. Numerous different chemicals have been developed, each with unique pharmacological properties.1 Among the most challenging tasks for a medicinal chemist is to explore a good agent. The formulation of heterocyclic structures containing a high nitrogen content has increased significantly in recent years due to their utility in various fields like explosives, pyrotechnics, propellants, and, most notably, chemotherapy. Because of their biological and synthetic importance, triazoles and their fused heterocyclic-derived products have attracted a lot of interest in past few years.2
Azolic derivatives like triazole, thiazole, thiadiazole, and oxadiazole are pharmacologically effective components that have been thoroughly examined for numerous bioactivities because of their appropriate use in medicinal chemistry.3 Triazole is a five-member heterocyclic ring with the chemical formula C2H3N3, that contains three nitrogen and two carbon atoms.4,5 It can also be identified in numerous isoforms, 1,2,4-triazole and 1,2,3-triazole, both of which are also regarded as pyrrodiazoles. Triazoles seem to be white-to-pale yellow crystalline with a faint aroma that is water and alcohol soluble or have melting points of 120 and 260 °C, respectively.6 The importance of triazole analogues has been well justified in materials science, nano-chemistry, biology, medicine, and agriculture.6 The medicinal importance of the triazole moiety was enough justified by its analogues showing antifungal,7 antiviral,8 anticancer, anticonvulsant, antibacterial, and anti-inflammatory activities.9−12 Among the different synthetic techniques, both conventional and microwave methods have been utilized.13,14
Acetylcholine has a key role in nervous system diseases. The cholinesterase enzymes hydrolyze it to choline, resulting in Alzheimer’s disease, a nervous system disorder. The acetylcholinesterase or butyrylcholinesterase inhibitors reverse this condition by blocking the enzymes.15,16 Polysaccharides are a good source of energy that metabolize into digestible monosaccharides. The disorder of the endocrine system impacts the function of the glucosidase enzyme, resulting in high sugar levels in the body. There are a lot of health problems associated with high sugar levels in the body.17
The different bio-regulatory molecules related to the lipoxygenase are produced in mammals. Therefore, several disorders like inflammation, asthma, tumor angiogenesis, and so forth have a link with this enzyme.18−20 The different digestive and urinary tract infections are due to the presence of high-level ammonia and carbamide. This high level is generated due to the activity of the urease on urea.21−23
Azinane triazole-based derivatives (12a–o) were designed to incorporate different bioactive functionalities into one unit to acquire high bioactivity potentials. The synthesis of a variety of triazole derivatives was aimed to evaluate their inhibition potential against cholinesterase and α-glucosidase enzymes, in search of new drug candidates for Alzheimer’s disease and diabetes mellitus, respectively. Structural analysis revealed that both BChE and AChE have two main substrate binding sites: (i) catalytic triad (CT) (ii) anionic site (AS). Therefore, the present derivatives interacting AS and CT could be novel compounds for the management of Alzheimer’s disease. The synthesized ethanamide derivatives (12a–o) were also tested against lipoxygenase and urease enzymes in search of new drug candidates for different diseases linked to these enzymes. The results declared a series of compounds to be active against AChE, BChE, and α-glucosidase enzymes with valuable inhibition potential to be employed as new drug candidates against Alzheimer’s disease and diabetes mellitus.
2. Results and Discussion
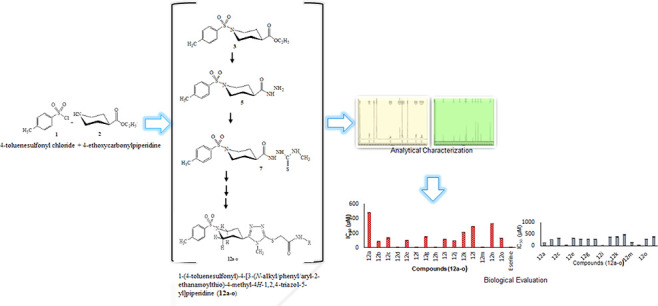
Ethanamide derivatives (12a–o) of the 1,2,4-triazole bearing azinane moiety have been synthesized through consecutive six steps. First four steps depicted the synthesis of the 1,2,4-triazole nucleus as 1-(4-toluenesulfonyl)-4-(3-mercapto-4-methyl-4H-1,2,4-triazol-5-yl) piperidine (8) through the formation of 1-(4-toluenesulfonyl)-4-(ethoxycarbonyl)piperidine (3), 1-(4-toluenesulfonyl)-4-(2-hydrazino carbonyl)piperidine (5), and 1-(4-toluenesulfonyl)-4-[1-(methylaminothiocarbonyl)-2-hydrazinocarbonyl]piperidine (7). The step five explained the formation of N-alkyl/phenyl/aryl-2-bromoethanamides (11a–o) by the reaction of different alkyl/phenyl/aryl amines (9a–o) and 2-bromoethanoyl bromide (10) in an aqueous basic medium. These electrophiles were made to react with equimolar compound 8 to acquire 1-(4-toluenesulfonyl)-4-[3-(N-alkyl/phenyl/aryl-2-ethanamoylthio)-4-methyl-4H-1,2,4-triazol-5-yl] piperidine (12a–o). Ice cold water helped to develop the precipitates of the target molecules. The protocol of synthesis is given in Scheme 1, and the different varying groups are depicted in Table 1. Furthermore, the synthesized compounds were subjected to the evaluation of their enzyme inhibition potential against AChE, α-glucosidase, urease, LOX, and BChE enzymes.
Scheme 1. Synthesis of 1-(4-Toluenesulfonyl)-4-[3-(N-alkyl/phenyl/aryl-2-ethanamoylthio)-4-methyl-4H-1,2,4-triazol-5-yl] Piperidine (12a–o).
Table 1. Different Synthesized Analogues (12a–o).
2.1. Chemistry
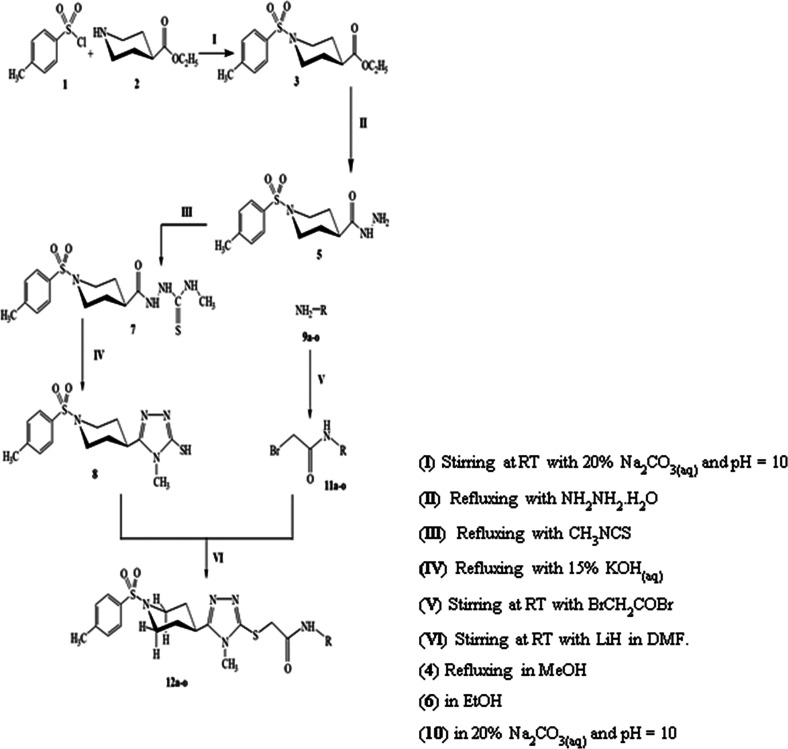
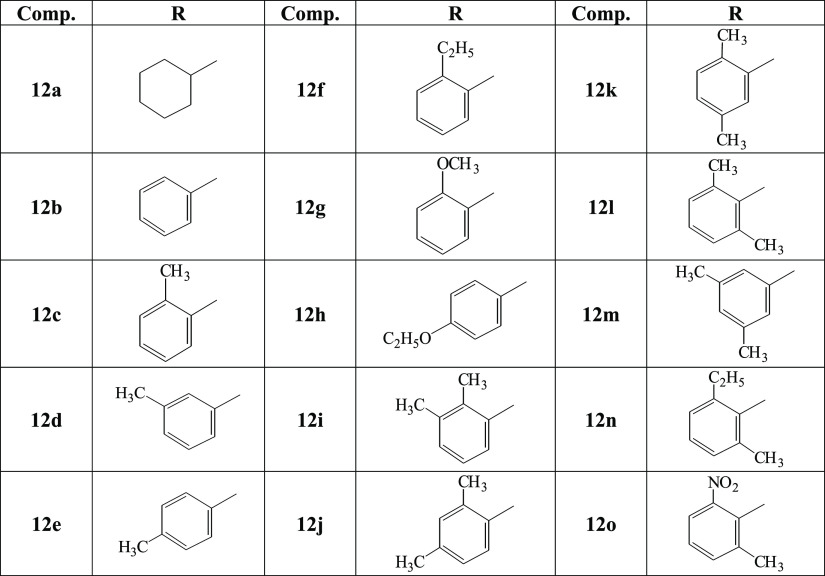
The compound 12m has been explicated as a single compound discussion from this acetamide series (12a–o). The compounds 12a–l, n, and o have been structurally elucidated similarly. The off-white amorphous solid, 12m (C25H31N5O3S2, 513 g mol–1, justified through te mass spectrum), possessed a melting point of 174–176 °C with 85% yield. The IR data was known to possess the following stretching frequencies for prominent functionalities, 3235 (N–H), 3045 (Ar C–H), 2970 (C–H), 1652 (C=O), 1581 (Ar C=C), 1429 (S=O), and 1139 (C–N). The 4-Methylphenylsulfonyl moiety was justified through three signals in the 1H NMR spectrum (Figure 1a) at δ 7.64 (d, J = 8.2 Hz, 2H, H-2″ & H-6″), 7.45 (d, J = 8.0 Hz, 2H, H-3″ & H-5″), and 2.41 (s, 3H, CH3-7″); and five signals in the 13C NMR spectrum (Figure 1b) at δ 143.4 (C-1″), 138.5 (C-4″), 129.8 (C-3″ & C-5″), 127.5 (C-2″ & C-6″), and 20.9 (C-7″). The 3,5-Dimethylphenyl moiety was justified through three signals in the 1H NMR spectrum (Figure 1a) at δ 7.14 (s, 2H, H-2‴′’ & H-6‴′), 6.70 (s, 1H, H-4‴′), and 2.21 (s, 6H, CH3-7‴′ & CH3-8‴′); and five signals in the 13C NMR spectrum (Figure 1b) at δ 137.7 (C-3‴′ & C-5‴′), 132.3 (C-1‴′), 125.0 (C-4‴′), 116.8 (C-2‴′ & C-6‴′), and 21.0 (C-7‴′ & C-8‴′). The 3,5-Disubstituted-6-methyl-1,2,4-triazole moiety was justified through one signal in the 1H NMR spectrum at δ 3.45 (s, 3H, CH3-6); and three signals in the 13C NMR spectrum (Figures 2a and 1b) at δ 157.8 (C-5), 148.7 (C-3), and 29.8 (C-6). The piperidine moiety was justified through five signals in the 1H NMR spectrum (Figure 2a) at δ 3.63–3.61 (m, 2H, He-2′ & He-6′), 2.85–2.83 (m, 1H, H-4′), 2.42–2.39 (m, 2H, Ha-2′ & Ha-6′), 1.92–1.89 (m, 2H, He-3′ & He-5′), and 1.72–1.68 (m, 2H, Ha-3′ & Ha-5′); and three signals in the 13C NMR spectrum (Figure 2b) at δ 45.4 (C-2′ & C-6′), 37.6 (C-4′), and 28.8 (C-3′ & C-5′).
Figure 1.
(a) 1H NMR and (b) 13C NMR spectra of the 12m compound (aromatic).
Figure 2.
(a) 1H NMR and (b) 13C NMR spectra of compound 12m (aliphatic).
2.2. Biological Activities
Azinane-triazole-based compounds (12a–o) have been further utilized for bioactivity analysis against AChE, α-glucosidase, urease, LOX, and BChE enzymes (Tables 2, 3 and 4). The results have been given as % inhibition and IC50 values. The literature review has suggested the bioactivity potential of such analogues against the said enzymes including AChE, α-glucosidase, and urease.24,25
Table 2. AChE and α-Glucosidase Inhibition Activity of 12a–oa.
| AChE
inhibition |
α-glucosidase inhibition |
|||
|---|---|---|---|---|
| compound | inhibition (%) at 0.5 mM | IC50 (μM) | inhibition (%) at 0.5 mM | IC50 (μM) |
| 12a | 52.37 ± 0.78 | 476.32 ± 0.57 | 87.25 ± 1.65 | 134.85 ± 1.39 |
| 12b | 89.15 ± 0.68 | 85.46 ± 0.45 | 76.23 ± 1.58 | 264.27 ± 1.24 |
| 12c | 82.36 ± 0.67 | 134.61 ± 0.53 | 72.45 ± 1.75 | 327.82 ± 1.57 |
| 12d | 98.78 ± 0.78 | 0.73 ± 0.54 | 91.52 ± 1.43 | 36.74 ± 1.24 |
| 12e | 87.43 ± 0.77 | 97.86 ± 0.65 | 72.75 ± 1.58 | 314.58 ± 1.24 |
| 12f | 39.62 ± 0.55 | 74.95 ± 1.74 | 275.82 ± 1.37 | |
| 12g | 79.24 ± 0.89 | 149.85 ± 0.67 | 71.45 ± 1.73 | 267.42 ± 1.52 |
| 12h | 37.41 ± 0.62 | 73.12 ± 1.58 | 286.56 ± 1.25 | |
| 12i | 82.51 ± 0.85 | 117.35 ± 0.64 | 31.53 ± 1.37 | |
| 12j | 88.36 ± 0.79 | 94.63 ± 0.57 | 72.25 ± 1.85 | 365.95 ± 1.42 |
| 12k | 65.27 ± 0.74 | 212.86 ± 0.59 | 71.16 ± 1.64 | 385.56 ± 1.32 |
| 12l | 62.25 ± 0.86 | 287.69 ± 0.63 | 53.67 ± 1.67 | 463.26 ± 1.36 |
| 12m | 92.73 ± 0.87 | 0.42 ± 0.67 | 87.21 ± 1.46 | 142.73 ± 1.23 |
| 12n | 61.53 ± 0.65 | 328.14 ± 0.45 | 92.65 ± 1.64 | 29.37 ± 1.32 |
| 12o | 81.53 ± 0.96 | 129.63 ± 0.73 | 72.23 ± 1.51 | 285.64 ± 1.23 |
| standard | 91.27±1.17a | 0.04±0.001a | 65.73±1.93b | 375.82±1.76b |
Note: a = Eserine, b = Acarbose.
Table 3. Urease and LOX Inhibition Activity of 12a–oa.
| Compound | urease
inhibition |
LOX
inhibition |
||
|---|---|---|---|---|
| inhibition (%) at 0.25 mM | IC50 (μM) | inhibition (%) at 0.25 mM | IC50 (μM) | |
| 12a | 25.34 ± 1.42 | 39.47 ± 0.82 | ||
| 12b | 31.23 ± 1.42 | 35.25 ± 0.42 | ||
| 12c | 75.45 ± 1.72 | 163.82 ± 1.45 | 36.47 ± 0.76 | |
| 12d | 98.22 ± 1.65 | 19.35 ± 1.28 | 68.34 ± 0.82 | 32.57 ± 0.42 |
| 12e | 39.26 ± 1.43 | 48.36 ± 0.43 | ||
| 12f | 36.35 ± 1.78 | 62.38 ± 0.75 | 96.32 ± 0.37 | |
| 12g | 42.87 ± 1.51 | 41.55 ± 0.51 | ||
| 12h | 42.63 ± 1.52 | 63.53 ± 0.52 | 92.45 ± 0.26 | |
| 12i | 75.82 ± 1.79 | 142.62 ± 1.52 | 24.12 ± 0.59 | |
| 12j | 73.52 ± 1.65 | 167.25 ± 1.34 | 23.54 ± 0.85 | |
| 12k | 76.15 ± 1.52 | 165.37 ± 1.27 | 29.45 ± 0.72 | |
| 12l | 46.19 ± 1.76 | 45.29 ± 0.56 | ||
| 12m | 98.65 ± 1.67 | 14.29 ± 1.27 | 37.45 ± 0.37 | |
| 12n | 32.85 ± 1.39 | 35.65 ± 0.49 | ||
| 12o | 56.35 ± 1.67 | 241.76 ± 0.42 | 64.28 ± 0.73 | 93.81 ± 0.34 |
| standard | 98.28±1.73a | 21.37±1.36a | 89.25±0.62b | 2.34±0.35b |
Thiourea.
Quercetin.
Table 4. BChE Inhibition Activity of 12a–o.
| BChE
inhibition |
||
|---|---|---|
| compound | inhibition (%) at 0.5 mM | IC50 (μM) |
| 12a | 21.93 ± 0.62 | |
| 12b | 63.42 ± 0.75 | 279.42 ± 0.59 |
| 12c | 37.83 ± 0.42 | |
| 12d | 95.29 ± 0.74 | 0.017 ± 0.53 |
| 12e | 96.47 ± 0.76 | 0.053 ± 0.51 |
| 12f | 39.78 ± 0.57 | |
| 12g | 24.72 ± 0.56 | |
| 12h | 16.51 ± 0.35 | |
| 12i | 36.47 ± 0.75 | |
| 12j | 37.62 ± 0.87 | |
| 12k | 49.23 ± 0.94 | |
| 12l | 41.87 ± 0.63 | |
| 12m | 91.25 ± 0.45 | 0.038 ± 0.50 |
| 12n | 36.24 ± 0.59 | |
| 12o | 39.65 ± 0.42 | |
| Standard | 91.27±1.17a | 0.04±0.001a |
Note: a = Eserine.
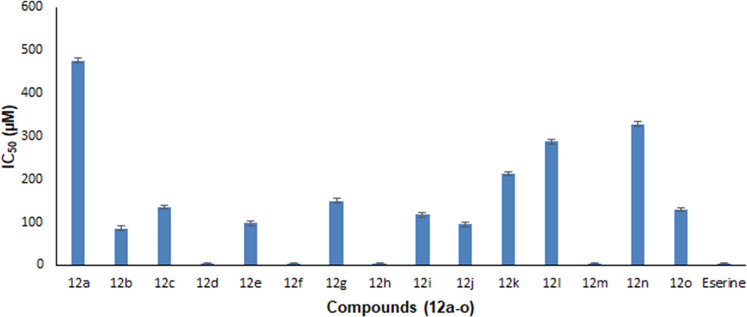
The whole series of molecules remained active against the AChE enzyme (Table 2, Figure 3). The excellent activity was possessed by five compounds, 12b (bearing phenyl moiety), 12d (bearing 3-methyl phenyl moiety), 12e (bearing 4-methyl phenyl moiety), 12j (bearing 2,4-dimethyl phenyl moiety), and 12m (bearing 3,5-dimethyl phenyl moiety). Derivatives 12d and 12m are 1.01-fold more potent than the standard. It looks that the presence of uni or dimethyl phenyl groups offers a suitable spatial arrangement to fit in the active site and consequently improved inhibitory activity against AChE. Among the compounds bearing cyclohexyl and phenyl groups, the compound bearing the aromatic system was more active. Among the methyl phenyl substituents, meta- and para-substituted remained the most active ones. The ortho-substituted compounds bearing methyl, methoxy, or ethyl groups remained the least active ones.
Figure 3.
AChE inhibition of synthesized compounds (12a–o).
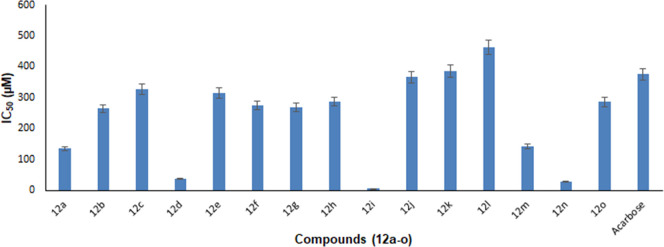
The whole series of molecules remained active against the α-glucosidase enzyme (Table 2, Figure 4). All derivatives have shown more inhibition activities of the α-glucosidase enzyme than that of acarbose (the reference standard). The excellent activity was possessed by two compounds, 12d (bearing the 3-methyl phenyl moiety) and 12n (bearing the 2-ethyl-6-methyl phenyl moiety). The most active compound was 12n. Among the compounds bearing cyclohexyl and phenyl groups, the compound bearing the aliphatic system was more active. Among the methyl phenyl substituents, meta-substituted remained the most active one. Among the ortho-substituted compounds bearing methyl, methoxy, or ethyl groups, methyl-substituted remained the least active one. Among the dimethyl-substituted compounds, the one compound bearing the 3,5-dimethyl phenyl group remained the most active one. The results obtained from the α-glucosidase enzyme inhibition assay exhibited a 1.4-fold increase in inhibition by azinane-triazole-based compounds (12a–o) than that of acarbose (375.82 ± 1.76 μM).
Figure 4.
α-Glucosidase inhibition of synthesized compounds (12a–o).
The series of molecules remained less active against urease, LOX, and BChE enzymes. Only six compounds (12c, d, i, j, k, and o) remained active against urease, four compounds (12d, f, h, and o) against LOX, and four compounds (12b, d, e and m) against BChE. Therefore, these results depict the selective activity of molecules against AChE, BChE, and α-Glucosidase enzymes.
3. Conclusions
In the present paper, synthesis and in vitro AChE, α-glucosidase, urease, BChE, and LOX inhibition of 15 azinane-triazole-based compounds are presented. All prepared derivatives are structurally elaborated through 1H NMR, 13C NMR, IR, and EI-MS techniques. Among all synthesized compounds, 12d, 12m, and 12n are found to be reasonably active against AChE, urease, and BChE, while all synthesized compounds (12a–o) are active against α-glucosidase. The most bioactive target compounds may be subjected to in vivo analysis to find their position regarding new drug candidates.
4. Experimental Section
The analytical grade solvents were used without further purification. A Bruker spectrometer recorded C13 NMR & 1H NMR spectra at 150 and 600 MHz in deuterated DMSO, respectively. The Jasco-320A spectrometer recorded IR spectra by the potassium bromide pellet method. The JMS-HX-110 spectrometer recorded EI-MS spectra. The TLC plates were viewed in UV light at 254 nm or iodine vapors using n-hexane and ethyl acetate. Aluminum plates were pre-coated by silica gel. Melting points were computed through the Griffin and George apparatus and were uncorrected. All the chemicals were obtained from Alfa Aesar, Sigma-Aldrich, and Merck.
4.1. Synthesis of Ethanamide Derivatives (12a–o)
4.1.1. Procedure for 1-(4-toluenesulfonyl)-4-(ethoxycarbonyl) Piperidine (3)
Equimolar 4-ethoxycarbonylpiperidine (2; 0.05 mol) and 4-toenesulfonyl chloride (1; 0.05 mol) were dispersed in 20% Na2CO3(aq) in a round-bottom (RB) flask on stirring respectively at room temperature (RT). The pH was adjusted and maintained at 10 by Na2CO3(aq). The reaction was monitored by TLC. The pH was adjusted to 6 by dilute HCl(aq), and precipitates were collected, washed, and dried.
4.1.2. Procedure for 1-(4-toluenesulfonyl)-4-(2-hydrazinocarbonyl) Piperidine (5)
Equimolar compound 3 and hydrazine monohydrate (4) were refluxed with methanol (MeOH; 150 mL) in a RB flask for 4 h at room temperature. The reaction was monitored through TLC. MeOH was distilled off, and the precipitates were collected, washed with n-hexane (50 mL), and dried in air.
4.1.3. Procedure for 1-(4-toluenesulfonyl)-4-[1-(methylaminothiocarbonyl)-2-hydrazinocarbonyl] Piperidine (7)
Equimolar compound 5 and methyl isothiocyanate (6; 0.045 mol) were refluxed with ethanol (EtOH; 150 mL) in a RB flask for 2 h. The reaction was monitored by TLC. EtOH was distilled off, and the precipitates were collected, washed with distilled water, and dried in air. (For detailed synthesis, see Supporting Information S8 to S12).
4.2. Acetylcholinesterase Inhibition Assay
Ellman’s method was utilized to evaluate the cholinesterase (AChE and BChE) inhibition activity26−28 with minor modifications to enhance the accuracy of the results. The first enzyme (10 μL) was incubated with the test compounds (0.05 mM; 10 to 100 mM) in phosphate buffer (0.05 mM; pH 7.7; 60 μL). Then, acetyl thiocholine iodide (for AChE) or butyryl thiocholine chloride (for BChE) and DTNB (0.05 mM; 10 μL) were added to the previous mixture, incubated, and the optical density (OD) was monitored using 96-well plate readers (Synergy HT, BioTek, USA). Eserine was used as a positive control. Triplicate readings were noted. The percentage inhibition (%) was calculated by the following formulae in eq 1. The IC50 values were calculated through Ez-Fit software (Perrella Scientific Inc. Amherst, USA) or computing data in Graph Pad Prism 5 software.
| 1 |
4.3. α-Glucosidase Assay
The reported method was utilized to assess the inhibition of the α-glucosidase enzyme.29 The test compound (10 to 100 μL; 0.05 mM) and enzyme (10 μL) were incubated in phosphate buffers (70 μL; pH 6.8; 0.05 M). The substrate p-nitrophenyl glucopyranoside (0.5 mM; 10 μL) was added, followed by post-incubation. Triplicate readings were noted with Acarbose as a reference standard. The % inhibition and IC50 values were calculated as described above.
4.4. Urease Inhibition Assay
The reported method of Virk et al. was utilized to assess inhibition of urease.30 Enzyme (10 μL) and test compounds (10 μL) were pre-incubated in phosphate buffer with pH 6.8 (0.5 M) at 37 °C for 10 min. The substrate (urea; 0.5 mM; 500 μL) was added, and the mixture was incubated and pre-read. The phenol hypochlorite reagent was added, followed by incubation for 10 min, and the optical density (OD) was measured by a microplate reader. Triplicate readings were noted with thiourea as a reference standard. The % inhibition and IC50 values were calculated as described above.
4.5. Lipoxygenase Assay
The reported method was utilized to assess inhibition of LOX.18 The test compound (10 μL) and enzyme were incubated in phosphate buffer (pH 8; 0.1 M; 55 μL) at 25 °C for 10 min. The substrate (25 μL) was added, incubated for 6 min, and post-read at 234 nm (Synergy HT, BioTeke, USA). Triplicate readings were noted using positive and negative controls with Baicalein as a reference standard. The % inhibition and IC50 values were calculated as described above.
4.6. Statistical Analysis
The triplicate values were subjected to statistical analysis in Microsoft Excel. Results are presented as mean ± SEM. The CL of results varied 85–90%.
Acknowledgments
The authors are thankful to HEC for HEC project no. 9512/Punjab/NRPU/R&D/HEC/2017 and PHEC for research project no. PHEC/ARA/PIRCA/20183/6.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c03779.
Detailed synthesis of S8 to S12o that includes (a) procedure for 1-(4-toluenesulfonyl)-4-(3-mercapto-4-methyl-4H-1,2,4-triazol-5-yl) piperidine (8); (b) procedure for N-alkyl/phenyl/aryl-2-bromoethanamides (11a–o); (c) procedure for 1-(4-toluenesulfonyl)-4-[3-(N-alkyl/phenyl/aryl-2-ethanamoylthio)-4-methyl-4H-1,2,4-triazol-5-yl]piperidine (12a–o), (d) 1-(4-toluenesulfonyl)-4-[3-(N-cyclohexyl-2-ethanamoylthio)-4-methyl-4H-1,2,4-triazol-5-yl] piperidine (12a), (e) 1-(4-toluenesulfonyl)-4-[3-(N-phenyl-2-ethanamoylthio)-4-methyl-4H-1,2,4-triazol-5-yl] piperidine (12b), (f) 1-(4-toluenesulfonyl)-4-{3-[N-(2-methylphenyl)-2-ethanamoylthio]-4-methyl-4H-1,2,4-triazol-5-yl} piperidine (12c), (g) 1-(4-Toluenesulfonyl)-4-{3-[N-(3-methylphenyl)-2-ethanamoylthio]-4-methyl-4H-1,2,4-triazol-5-yl}{Kumari, 2021 #63} piperidine (12d), (h) 1-(4-toluenesulfonyl)-4-{3-[N-(4-methylphenyl)-2-ethanamoylthio]-4-methyl-4H-1,2,4-triazol-5-yl} piperidine (12e), (i) 1-(4-toluenesulfonyl)-4-{3-[N-(2-ethylphenyl)-2-ethanamoylthio]-4-methyl-4H-1,2,4-triazol-5-yl} piperidine (12f), (j) 1-(4-toluenesulfonyl)-4-{3-[N-(2-methoxyphenyl)-2-ethanamoylthio]-4-methyl-4H-1,2,4-triazol-5-yl} piperidine (12g), (k) 1-(4-toluenesulfonyl)-4-{3-[N-(4-ethoxyphenyl)-2-ethanamoylthio]-4-methyl-4H-1,2,4-triazol-5-yl} piperidine (12h), (l) 1-(4-toluenesulfonyl)-4-{3-[N-(2,3-dimethylphenyl)-2-ethanamoylthio]-4-methyl-4H-1,2,4-triazol-5-yl} piperidine (12i), (m) 1-(4-toluenesulfonyl)-4-{3-[N-(2,4-dimethylphenyl)-2-ethanamoylthio]-4-methyl-4H-1,2,4-triazol-5-yl} piperidine (12j), (n) 1-(4-toluenesulfonyl)-4-{3-[N-(2,5-dimethylphenyl)-2-ethanamoylthio]-4-methyl-4H-1,2,4-triazol-5-yl} piperidine (12k), (o) 1-(4-toluenesulfonyl)-4-{3-[N-(2,6-dimethylphenyl)-2-ethanamoylthio]-4-methyl-4H-1,2,4-triazol-5-yl} piperidine (12l), (p) 1-(4-toluenesulfonyl)-4-{3-[N-(3,5-dimethylphenyl)-2-ethanamoylthio]-4-methyl-4H-1,2,4-triazol-5-yl} piperidine (12m), (q) 1-(4-toluenesulfonyl)-4-{3-[N-(2-ethyl-6-methylphenyl)-2-ethanamoylthio]-4-methyl-4H-1,2,4-triazol-5-yl} piperidine (12n), and (r) 1-(4-toluenesulfonyl)-4-{3-[N-(2-methyl-6-nitrophenyl)-2-ethanamoylthio]-4-methyl-4H-1,2,4-triazol-5-yl} piperidine (12o)(PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Pagniez F.; Lebouvier N.; Na Y. M.; Ourliac-Garnier I.; Picot C.; Le Borgne M.; Le Pape P. Biological exploration of a novel 1, 2, 4-triazole-indole hybrid molecule as antifungal agent. J. Enzyme Inhib. Med. Chem. 2020, 35, 398–403. 10.1080/14756366.2019.1705292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozorov K.; Zhao J.; Aisa H. A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. 10.1016/j.bmc.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkó M.; El Haimer M.; Kormányos Z.; Fülöp F. Synthesis of Novel N-Heterocyclic Compounds Containing 1, 2, 3-Triazole Ring System via Domino,“Click” and RDA Reactions. Molecules 2019, 24, 772. 10.3390/molecules24040772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts K. The Chemistry of 1, 2, 4-Triazoles. Chem. Rev. 1961, 61, 87–127. 10.1021/cr60210a001. [DOI] [Google Scholar]
- Matin M. M.; Matin P.; Rahman M. R.; Ben Hadda T.; Almalki F. A.; Mahmud S.; Ghoneim M. M.; Alruwaily M.; Alshehri S. Triazoles and their derivatives: Chemistry, synthesis, and therapeutic applications. Front. Mol. Biosci. 2022, 9, 864286. 10.3389/fmolb.2022.864286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloora S.; Shabaraya R.; Adhikari A. V. Facile synthesis of new imidazo [1, 2-a] pyridines carrying 1, 2, 3-triazoles via click chemistry and their antiepileptic studies. Bioorg. Med. Chem. Lett. 2013, 23, 3368–3372. 10.1016/j.bmcl.2013.03.086. [DOI] [PubMed] [Google Scholar]
- Ye N.; Chen H.; Wold E. A.; Shi P.-Y.; Zhou J. Therapeutic potential of spirooxindoles as antiviral agents. ACS Infect. Dis. 2016, 2, 382–392. 10.1021/acsinfecdis.6b00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musad E. A.; Mohamed R.; Ali Saeed B. A.; Vishwanath B. S.; Lokanatha Rai K. L. Synthesis and evaluation of antioxidant and antibacterial activities of new substituted bis (1, 3, 4-oxadiazoles), 3, 5-bis (substituted) pyrazoles and isoxazoles. Bioorg. Med. Chem. Lett. 2011, 21, 3536–3540. 10.1016/j.bmcl.2011.04.142. [DOI] [PubMed] [Google Scholar]
- Jha K. K.; Samad A.; Kumar Y.; Shaharyar M.; Khosa R. L.; Jain J.; Kumar V.; Singh P. Design, synthesis and biological evaluation of 1, 3, 4-oxadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 4963–4967. 10.1016/j.ejmech.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Ramaprasad G.; Kalluraya B.; Kumar B. S.; Hunnur R. K. Synthesis and biological property of some novel 1, 3, 4-oxadiazoles. Eur. J. Med. Chem. 2010, 45, 4587–4593. 10.1016/j.ejmech.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Rashid M.; Husain A.; Mishra R. Synthesis of benzimidazoles bearing oxadiazole nucleus as anticancer agents. Eur. J. Med. Chem. 2012, 54, 855–866. 10.1016/j.ejmech.2012.04.027. [DOI] [PubMed] [Google Scholar]
- Virk N. A.; Rehman M. A.; Abbasi S. Z.; Siddiqui U.; Rashid J.; Iqbal M.; Saleem M.; Ashraf W.; Shahid W.; Shah S. A. A. Conventional versus microwave assisted synthesis, molecular docking and enzyme inhibitory activities of new 3, 4, 5-trisubstituted-1, 2, 4-triazole analogues. Pak. J. Pharm. Sci. 2018, 31, 1501–1510. [PubMed] [Google Scholar]
- Kantar G. K.; Baltaş N.; Menteşe E.; Şaşmaz S. Microwave-assisted synthesis and investigation of xanthine oxidase inhibition of new phthalonitrile and phthalocyanines containing morpholino substituted 1, 2, 4-triazole-3-one. J. Organomet. Chem. 2015, 787, 8–13. 10.1016/j.jorganchem.2015.03.033. [DOI] [Google Scholar]
- Zhao T.; Ding K.-m.; Zhang L.; Cheng X.-m.; Wang C.-h.; Wang Z.-t. Acetylcholinesterase and butyrylcholinesterase inhibitory activities of β-carboline and quinoline alkaloids derivatives from the plants of genus Peganum. J. Chem. 2013, 2013, 717232. 10.1155/2013/717232. [DOI] [Google Scholar]
- Javid J.; Aziz-ur-Rehman M. A.; Abbasi S. Z.; Siddiqui J.; Iqbal N. A.; Virk S.; Rasool H. A.; Ali M.; Ashraf W.; Shahid S.; Hussain S.; Ali Shah S. A. Comparative conventional and microwave assisted synthesis of heterocyclic oxadiazole analogues having enzymatic inhibition potential. J. Heterocycl. Chem. 2021, 58, 93–110. 10.1002/jhet.4150. [DOI] [Google Scholar]
- Qaisar M. N.; Chaudhary B. A.; Sajid M. U.; Hussain N. Evaluation of α-glucosidase inhibitory activity of dichloromethane and methanol extracts of Croton bonplandianum Baill. Trop. J. Pharm. Res. 2014, 13, 1833–1836. 10.4314/tjpr.v13i11.9. [DOI] [Google Scholar]
- Abbasi M. A.; Ahmad V. U.; Zubair M. M.; Rashid A.; Farooq U.; Nawaz S. A.; Lodhi M. A.; Makhmoor T.; Choudhary M. I.; Rehman A.-u. Benzoylsalireposide an antioxidant, lipoxygenase and chymotrypsin inhibitor. Proc. Pak. Acad. Sci. 2005, 42, 121. [Google Scholar]
- Baylac S.; Racine P. Inhibition of 5-lipoxygenase by essential oils and other natural fragrant extracts. Int. J. Aromather. 2003, 13, 138–142. 10.1016/s0962-4562(03)00083-3. [DOI] [Google Scholar]
- Xu S.; Mueser T. C.; Marnett L. J.; Funk M. O. Jr Crystal structure of 12-lipoxygenase catalytic-domain-inhibitor complex identifies a substrate-binding channel for catalysis. Structure 2012, 20, 1490–1497. 10.1016/j.str.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. W.; Jan S.; Parveen S.; Khan R. A.; Saeed A.; Tanveer A. J.; Shad A. A. Phytochemical analysis and enzyme inhibition assay of Aerva javanica for ulcer. Chem. Cent. J. 2012, 6, 76. 10.1186/1752-153X-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lateef M.; Iqbal L.; Fatima N.; Siddiqui K.; Afza N.; Zia-ul-Haq M.; Ahmad M. Evaluation of antioxidant and urease inhibition activities of roots of Glycyrrhiza glabra. Pak. J. Pharm. Sci. 2012, 25, 99–102. [PubMed] [Google Scholar]
- Xiao Z.-P.; Peng Z.-Y.; Dong J.-J.; Deng R.-C.; Wang X.-D.; Ouyang H.; Yang P.; He J.; Wang Y.-F.; Zhu M.; Peng X.-C.; Peng W.-X.; Zhu H.-L. Synthesis, molecular docking and kinetic properties of β-hydroxy-β-phenylpropionyl-hydroxamic acids as Helicobacter pylori urease inhibitors. Eur. J. Med. Chem. 2013, 68, 212–221. 10.1016/j.ejmech.2013.07.047. [DOI] [PubMed] [Google Scholar]
- Ellman G. L.; Courtney K. D.; Andres V. Jr; Featherstone R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Iqbal J.; Rehman A.-u.; Abbasi M. A.; Siddiqui S. Z.; Rasool S.; Ashraf M.; Iqbal A.; Hamid S.; Chohan T. A.; Khalid H. Biological activity of synthesized 5-{1-[(4-chlorophenyl) sulfonyl] piperidin-4-yl}-2-mercapto-1, 3, 4-oxadiazole derivatives demonstrated by in silico and BSA binding studies. Braz. J. Pharm. Sci. 2020, 56, e18092 10.1590/s2175-97902020000118092. [DOI] [Google Scholar]
- Iqbal J.; Rehman A.; Abbasi M. A.; Siddiqui S. Z.; Khalid H.; Laulloo S. J.; Chohan T. A.; Rasool S.; Shah S. A. A. BSA Binding, molecular docking and in vitro biological screening of some new 1, 2, 4-triazole heterocycles bearing azinane nucleus. Pak. J. Pharm. Sci. 2020, 33, 149–160. [PubMed] [Google Scholar]
- Chapdelaine P.; Tremblay R. R.; Dubé J. P-Nitrophenol-alpha-D-glucopyranoside as substrate for measurement of maltase activity in human semen. Clin. Chem. 1978, 24, 208–211. 10.1093/clinchem/24.2.208. [DOI] [PubMed] [Google Scholar]
- Pohanka M.; Hrabinova M.; Kuca K.; Simonato J.-P. Assessment of acetylcholinesterase activity using indoxylacetate and comparison with the standard Ellman’s method. Int. J. Mol. Sci. 2011, 12, 2631–2640. 10.3390/ijms12042631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohanka M.; Holas O. Evaluation of 2, 6-dichlorophenolindophenol acetate as a substrate for acetylcholinesterase activity assay. J. Enzyme Inhib. Med. Chem. 2015, 30, 796–799. 10.3109/14756366.2014.976564. [DOI] [PubMed] [Google Scholar]
- Lodhi M. A.; Abbasi M. A.; Choudhary M. I.; Ahmad V. U. Kinetics studies on triacontanyl palmitate: a urease inhibitor. Nat. Prod. Res. 2007, 21, 721–725. 10.1080/14786410600906913. [DOI] [PubMed] [Google Scholar]
- Virk N. A.; Rehman A. u.; Abbasi M. A.; Siddiqui S. Z.; Iqbal J.; Rasool S.; Khan S. U.; Htar T. T.; Khalid H.; Laulloo S. J.; Ali Shah S. A. Microwave-assisted synthesis of triazole derivatives conjugated with piperidine as new anti-enzymatic agents. J. Heterocycl. Chem. 2020, 57, 1387–1402. 10.1002/jhet.3875. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.