Abstract
The first ς54 promoters in Chlamydia trachomatis L2 were mapped upstream of hypothetical proteins CT652.1 and CT683. Comparative genomics indicated that these ς54 promoters and potential upstream activation binding sites are conserved in orthologous C. trachomatis D, C. trachomatis mouse pneumonitis strain, and Chlamydia pneumoniae (CWL029 and AR39) genes.
Chlamydia is an organism of major medical and veterinary significance; however, its obligate intracellular existence makes genetic investigations a challenge. The unique developmental cycle of Chlamydia involves the interconversion between the infectious elementary body and the metabolically active reticulate body (23). Although the key morphological stages of chlamydial development are understood (19, 23) and the developmental expression of over 20 genes has been determined (12, 18), the elements which regulate this developmental gene expression are yet to be elucidated. Our recent investigations (18) identified temporal expression of the three C. trachomatis RNA polymerase sigma factors, ς66 (major ς70 homolog), ς28, and ς54. Several reports have characterized ς66 promoters (8, 17, 29), but to date, no promoters have been identified for the developmental-stage-specific sigma factors, ς28 and ς54. The present study utilized the complete Chlamydia trachomatis genome sequence (27) and a modified fluorescence-based primer extension (PE) assay to identify and map the first ς54 promoters for Chlamydia.
Transcription initiation from ς54 promoters is a multistep process involving the recognition of the promoter by ς54, binding of the core RNA polymerase to the ς54 to form a closed complex, and subsequent activation to an open complex following binding by an enhancer binding protein (EBP) (20, 21). In most cases the EBP binds an upstream activator sequence (UAS) located within 200 bp of the promoter (15, 21) and is brought into contact with the ς54-RNA polymerase complex by DNA looping, an event mediated by the integration host factor (IHF) or intrinsic DNA bends (9). In addition to the ς54 gene (rpoN), recently identified in the C. trachomatis genome, genes for the NtrC family EBP (ntrC) and IHF (ihfA) were also found to be present (27). More detailed analysis of the translated amino acid sequences identified that RpoN (ς54) contains a perfect RpoN box (ARRTVAKYR), which is responsible for recognition of the cognate promoter (30), and the chlamydial NtrC homolog has an exact match to the ς54-binding domain, GAFTGA (7). Furthermore, the chlamydial NtrC has six of seven conserved amino acids of the UAS binding domain, GESGCGK (7) (the underlined amino acid is nonconserved). We previously reported the late-stage-specific expression of rpoN (18) and subsequently confirmed that ntrC was transcribed by reverse transcription-PCR analysis (data not shown). These observations led us to hypothesize that some chlamydial genes would be regulated by NtrC-activated ς54-mediated transcription initiation. Of the cognate promoters for all eubacterial sigma factors, ς54 promoters are the most highly conserved (2) and hence lend themselves to a computational search of the full chlamydial genome. We therefore used the Findpatterns database searching program of the Australian National Genome Information Service to search the C. trachomatis D genome for sequences corresponding to the ς54 consensus promoter (TGGCAC-N5-TTGC), allowing up to two mismatches. We identified 427 potential matches and analyzed these for both orientation and proximity to C. trachomatis open reading frames (ORFs). Only nine putative ς54 promoters were identified within 400 bp upstream of the C. trachomatis ORFs, encoding ychF, yebL, pkn5, cysQ, and secA product homologs and hypothetical proteins CT620, CT652.1, CT683, and CT734. Transcription within each ORF was confirmed by reverse transcription-PCR analysis on C. trachomatis RNA (data not shown). While this study was being undertaken Studholme and Buck (28) reported the identification of a ς54 promoter upstream of C. trachomatis AAC68830 and Chlamydia pneumoniae AAD18864 which corresponds to our mapped promoter for CT652.1. Our search strategy identified more candidate promoters and mapped two ς54 promoters for Chlamydia.
PE analysis to determine the transcription start site (TSS) of chlamydial transcripts is difficult for genes expressed at low levels. Since fluorescence detection has greater sensitivity than conventional radioactive methods, we modified recent methodology (1, 24) to perform PE analysis on total RNA isolated from Chlamydia-infected cells. C. trachomatis L2/434/Bu was propagated in 109 HEp2 cells for 30 h before RNA was extracted as previously described (18). Twenty micrograms of total RNA was annealed to 10 pmol of fluorescently end-labeled primer (either 5′6-FAM or 5′TET, synthesized by Pacific Oligos, Lismore, Australia) in a 10-μl volume for 10 min at 75°C in a thermal cycler. The reagents for cDNA synthesis (10 mM dithiothreitol, 1 mM each deoxynucleoside triphosphate, 20 U of RNase inhibitor, and 40 U of Expand reverse transcriptase in buffer [supplied by Rosche]) were added on ice, and the reaction mixture was incubated for 90 min at 42°C. The cDNA was precipitated in 0.3 M sodium acetate with 2.5 volumes of ethanol following RNase incubation using 25 ng of DNase-free RNase (Rosche) for 30 min at 37°C. The PE products were resuspended in 8 μl of 95% (vol/vol) formamide–10 mM EDTA (pH 9.0) and run on a 6 M urea–4.5% polyacrylamide TBE (45 mM Tris-borate, 1 mM EDTA, pH 8.0) gel using an ABI 337 instrument, with GeneScan (Applied Biosystems) analysis using GS 500Rox molecular size markers (PE Biosystems) to determine the size of the 5′6-FAM- or 5′TET-labeled single-stranded DNA.
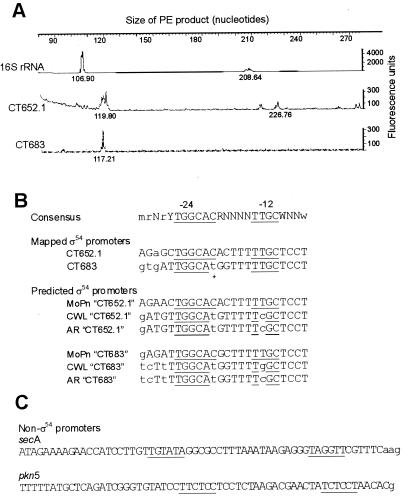
The 16S rRNA gene was used to establish the PE assay on C. trachomatis serovar L2 RNA using primer 16S.PE (5′6-FAM-GAACCAAGATCAAATTCTCAG). We identified two transcripts as seen by fluorescent peaks for PE products at 107 and 209, respectively (Fig. 1A). These correspond to the previously determined TSS for C. trachomatis mouse pneumonitis (MoPn) strain, where two promoters were defined (10). PE analysis for the C. trachomatis pkn5, secA, cysQ, ychF, CT652.1, and CT863 genes was undertaken using primers pkn5.PE (5′6-FAM-CGAGAAGAGTGCTCATCCACACC), secA.PE (5′6-FAM-TATTCTCTCTTGGGAGGATCCG), cysQ.PE (5′TET-GCATCAGTGACAGCATAGCCTGC), ychF.PE (5′6-FAM-CTACTATTCCACACTCTGTTTGTC), CT652/1.PE (5′6-FAM-CATGGATGTACGCTCTTTCCGAC), and CT683.PE (5′TET-CTGCTTGCTCGTACTCACCACTC), respectively. Definite fluorescent peaks were generated for the pkn5, secA, CT652.1, and CT683 genes, whereas only nonspecific fluorescent peaks were obtained for ychF and cysQ PE reactions. The CT652.1 and CT683 PE products were 120 and 117 nucleotides (Fig. 1A), respectively, which map the TSS in the correct position to the predicted ς54 promoter and Shine-Dalgarno sequences (Fig. 2). The CT652.1 PE reaction also generated some lower-intensity peaks (with one peak of over 100 fluorescence units at 227 nucleotides) which could be the result of nonspecific priming, since no obvious promoter sequences were identified in the corresponding nontranscribed sequence of CT652.1 (data not shown). The TSSs mapped against the noncoding sequences upstream of pkn5 and secA allowed alternative promoters to be proposed based on homology to the ς70-like consensus promoters (Fig. 1C). The inability to map TSSs for ychF and cysQ maybe due to either insufficient transcript, failure to induce transcription from these genes under the growth conditions used, or the genes being part of an operon and the promoter not being within 400 bp of the ORF.
FIG. 1.
Analysis of chlamydial promoters. (A) PE analysis of C. trachomatis L2 RNA with fluorescent primers against the 16S rRNA (6-FAM labeled), CT652.1 (6-FAM labeled) and CT683 (TET labeled) using GeneScan software. Primer sequences are given in the text, and the sizes of PE products greater than 100 fluorescence units are shown under the traces. Similar traces were obtained in a repeat PE experiment. (B) Consensus ς54 promoter (defined in reference 2) in alignment with the mapped C. trachomatis L2 ς54 promoters for CT652.1 and CT683. Uppercase and lowercase nucleotides in Consensus represent greater than 80% and 60 to 80% conservation to the consensus in ς54 mapped promoters, respectively. Uppercase nucleotides in the chlamydial sequences (CT, MoPn, CWL029, and AR39) correspond to homology with the consensus, and lowercase nucleotides represent lack of homology. Underlined nucleotides represent greater than 95% homology to the consensus of all ς54 promoters where the TSS has been mapped (2). The CT683 t highlighted with an asterisk is replaced by the consensus C in C. trachomatis serovar D. CT, C. trachomatis L2; MoPn, C. trachomatis MoPn; CWL and AR, C. pneumoniae CWL029 and AR39, respectively. (C) Non-ς54 promoters (underlined) were predicted on the basis of homology to the ς70 consensus (TTGACA-[N15-20]-TATAAT) for C. trachomatis L2 pkn5 and secA where TSSs (lowercase) failed to map to the ς54 consensus promoter identified in this study.
FIG. 2.
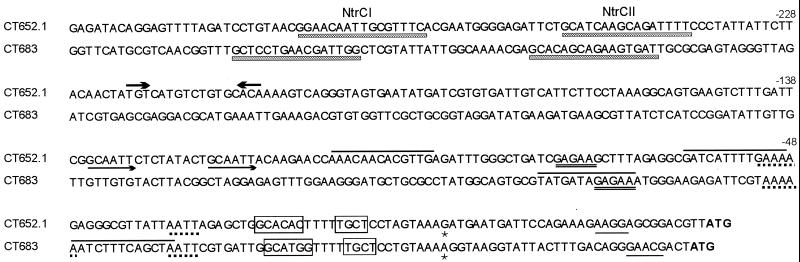
Analysis of the upstream sequences of C. trachomatis CT652.1 and CT683. The upstream sequences from −317 to the start codon (ATG) (boldface) of C. trachomatis L2 CT652.1 and CT683 are aligned with respect to the TSS (asterisk). The numbers represent nucleotides upstream of the TSS. The patterns below and above the sequence are represented as follows: underline, predicted Shine-Dalgarno sequence; box, predicted ς54 promoter; shaded underbox, predicted NtrC binding sites (NtrCI and NtrCII); overline, predicted IHF binding sites conserved in C. trachomatis L2, D, and MoPn; double underline, conservation of purine rich sequence in similar positions between CT652.1 and CT683 in C. trachomatis (L2, D, and MoPn) and C. pneumoniae (CWL029 and AR39); unidirectional arrows, direct sequence repeats conserved in C. trachomatis L2, D, and MoPn; bidirectional arrows, putative NifA binding site conserved in C. trachomatis L2, D, and MoPn (TGT-N10-ATA in MoPn). Variations from the C. trachomatis L2 NtrCI and NtrCII for C. trachomatis D and MoPn are GCAACAATTGCGTTTC (D, CT652.1 NtrCI), CGAACAGCTGCATTTC (MoPn, CT652.1 NtrCI), GCGTAAGGAGATTTC (MoPn, CT652.1 NtrCII), GCTCCTGAACAACTGT (MoPn, CT683 NtrCI), and GTACCGCTGATGTAAT (MoPn, CT683 NtrCI), where the underlined nucleotides represent changes to the sequence (shaded underbox).
Our genomic ς54 promoter searching analysis was done against the C. trachomatis D genome; however, the TSSs were experimentally determined for C. trachomatis L2, and thus the equivalent CT652.1 and CT683 sequences were retrieved from the C. trachomatis L2 genome (http://violet.berkeley.edu:4231). When the predicted ς54 promoter sequences are compared to the extended consensus proposed by Barrios and colleagues (2), the CT652.1 promoter exactly matches the “uppercase” consensus, and the CT683 promoter is a perfect match with serovar D and has one mismatch with serovar L2 (Fig. 1B). The genome sequences for C. trachomatis MoPn (25), C. pneumoniae CWL029 (14), and C. pneumoniae AR39 (25) were searched for CT652.1 and CT683 homologs, in order to confirm if the chlamydial ς54 promoters are conserved between different chlamydial strains and species. Homologs to both CT652.1 and CT683 were found in C. trachomatis MoPn (TC0022 and TC0055, respectively), C. pneumoniae CWL029 (Cpn0725 and Cpn0693, respectively) and C. pneumoniae AR39 (CP0021 and CP0053, respectively). The overall conservation of the predicted ς54 promoters (Fig. 1B) indicates that the ς54 promoters may be utilized for the equivalent genes in C. trachomatis MoPn and C. pneumoniae CWL029 and AR39.
Having mapped these first two ς54 promoters in Chlamydia, we analyzed the sequences upstream of the TSSs and the equivalent sequences in C. trachomatis (D and MoPn) and C. pneumoniae (CWL029 and AR39) for the presence of NtrC binding sites and other, perhaps Chlamydia-specific, elements. Although published NtrC binding sites show limited consensus across eubacteria and often appear in unmatched pairs (the NtrC binds as a dimer), we searched the upstream sequences for patterns resembling the NtrC UAS (5, 6, 16, 26, 31) and found two potential sites in the upstream sequences of both CT652.1 and CT683 (NtrCI and NtrCII in Fig. 2). The sequences had an overall consensus between C. trachomatis L2, D, and MoPn, and alternative NtrC binding sites could be identified in the equivalent C. pneumoniae upstream sequences.
Our search for common elements between the upstream sequences of both C. trachomatis L2 CT652.1 and CT683 revealed three sequence patterns, GAGAA, (A/G)AAAA, and TAAT, located in similar positions within 100 bp upstream of the TSS (Fig. 2). When we searched for intra- and interspecies conservation, we found that (A/G)AAAA is completely conserved and GAGAA was replaced by alternative purine-rich sequences in C. trachomatis (D and MoPn) and C. pneumoniae (CWL029 and AR39) sequences. The TAAT element is conserved in the same position relative to the predicted ς54 promoters across all CT652.1 and CT683 upstream sequences investigated, except that C. pneumoniae CT683 has TAGT. Two extra putative regulatory elements for CT652.1 were identified by comparison of different chlamydial strains and species (Fig. 2). First, the UAS, TGT-N10/11-ACA (22), for the NtrC-like EBP, NifA (15), was conserved in the C. trachomatis and C. pneumoniae sequences. Second, a perfect direct repeat, GC(A/T)AT separated by 9 bp is conserved between the C. trachomatis L2, D, and MoPn sequences. The strong conservation of sequence patterns in noncoding regions of the genome, both between genes and between species, supports a role in regulation.
Since IHF binding sites are often found between the UAS and ς54 promoter, we examined the sequence between the ς54 promoter and putative UAS for IHF binding sites by searching for the consensus WATCAA-N4-WTR (13, 32). We identified two putative IHF binding sites in the upstream sequences of CT652.1 and CT683 (Fig. 2). Without a transformation system for Chlamydia, it is difficult to obtain functional data to support the involvement of the above-mentioned sequence patterns in the two-component ς54 regulatory system.
These first two chlamydial ς54 promoters have been mapped upstream of hypothetical proteins. The ORF for CT652.1 shows no homology to any other known proteins by BLAST analysis, and the CT683 ORF contains the ubiquitous tetratricopeptide repeat module involved in protein-protein interaction (3). The chlamydial CT683 has been classified as both an O-linked GlcNAc transferase (27) and a type III secretion chaperone (25), proteins which play a direct role in signal transduction (4, 11). Since CT652.1 and CT683 are probably regulated by ς54-mediated transcription initiation, we propose that they might be required for reticulate body-to-elementary body conversion, since our earlier investigations identified rpoN expression during mid- to late-stage-specific chlamydial development (18). Further analysis to determine the temporal expression and function of CT652.1 and CT683 will be required to elucidate their role in chlamydial development and pathogenesis. It is hard to imagine that Chlamydia has rpoN and ntrC genes for regulating only two genes; thus, different search strategies are required to identify other ς54 promoters in Chlamydia. Since ς66-regulated promoters often show limited homology to the major ς factor consensus (8, 12, 17, 29), it is possible that a unique class of ς54 promoters exist in Chlamydia.
Acknowledgments
This work was supported by National Health and Medical Research Council grant 981383, and S.A.M. is the recipient of an Australian Research Council postdoctoral fellowship.
We are grateful to Stephen Myers for technical assistance and to Kelly Ewen (Australian Genome Research Facility, Melbourne, Australia) for the GeneScan analysis.
REFERENCES
- 1.Altermann E, Jurgen R, Klein K, Henrich B. Synthesis and automated detection of fluorescently labeled primer extension products. BioTechniques. 1999;26:96–101. doi: 10.2144/99261st03. [DOI] [PubMed] [Google Scholar]
- 2.Barrios H, Valderrama B, Morett E. Compilation and analysis of ς54-dependent promoter sequences. Nucleic Acids Res. 1999;27:4305–4313. doi: 10.1093/nar/27.22.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blatch G L, Lassle M. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessays. 1999;21:932–939. doi: 10.1002/(SICI)1521-1878(199911)21:11<932::AID-BIES5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 4.Brummel J H, Steele-Mortimer O, Finlay B B. Bacterial invasion: force feeding by Salmonella. Curr Biol. 1999;9:R277–R280. doi: 10.1016/s0960-9822(99)80178-x. [DOI] [PubMed] [Google Scholar]
- 5.Cheema A K, Choudhury N R, Das H K. A- and T-tract-mediated intrinisic curvature in native DNA between the binding site of the upstream activator NtrC and the nifLA promoter of Klebsiella pneumoniae facilitates transcription. J Bacteriol. 1999;181:5296–5302. doi: 10.1128/jb.181.17.5296-5302.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cullen P J, Bowman W C, Hartnett D-F, Reilly S C, Kranz R G. Translational activation by an NtrC enhancer-binding protein. J Mol Biol. 1998;278:903–914. doi: 10.1006/jmbi.1998.1745. [DOI] [PubMed] [Google Scholar]
- 7.Dischert W, Vignais P M, Colbeau A. The synthesis of Rhodobacter capsulatus HupSL hydrogenase is regulated by the two-component HupT/HupR system. Mol Microbiol. 1999;34:995–1006. doi: 10.1046/j.1365-2958.1999.01660.x. [DOI] [PubMed] [Google Scholar]
- 8.Douglas A L, Hatch T P. Mutagenesis of the P2 promoter of the major outer membrane protein gene of Chlamydia trachomatis. J Bacteriol. 1996;178:5573–5578. doi: 10.1128/jb.178.19.5573-5578.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dworkin J, Jovanovic G, Model P. Role of upstream activation sequences and integration host factor in transcriptional activation by the constitutively active prokaryotic enhancer-binding protein PspF. J Mol Biol. 1997;273:377–388. doi: 10.1006/jmbi.1997.1317. [DOI] [PubMed] [Google Scholar]
- 10.Engel J N, Ganem D. Chlamydial rRNA operons: gene organization and identification of putative tandem promoters. J Bacteriol. 1987;169:5678–5685. doi: 10.1128/jb.169.12.5678-5685.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart G W. Dynamic O-linked glycosylation of nuclear cytoskeletal proteins. Annu Rev Biochem. 1997;66:315–335. doi: 10.1146/annurev.biochem.66.1.315. [DOI] [PubMed] [Google Scholar]
- 12.Hatch T P. Developmental biology. In: Stephens R S, editor. Chlamydia: intracellular biology, pathogenesis, and immunity. Washington, D.C.: ASM Press; 1999. pp. 29–67. [Google Scholar]
- 13.Hoover T R, Santero E, Porter S, Kustu S. The integration host factor stimulates interaction of RNA polymerase with NIFA, the transcriptional activator for nitrogen fixation operons. Cell. 1990;63:11–22. doi: 10.1016/0092-8674(90)90284-l. [DOI] [PubMed] [Google Scholar]
- 14.Kalman S, Mitchell W, Marathe R, Lammel C, Fan J, Hyman R W, Olinger L, Grimwood J, Davis R W, Stephens R S. Comparative genomes of C. pneumoniae and C. trachomatis. Nat Genet. 1999;21:385–389. doi: 10.1038/7716. [DOI] [PubMed] [Google Scholar]
- 15.Kustu S, Santero E, Keener J, Popham D, Weiss D. Expression of sigma 54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol Rev. 1989;53:367–376. doi: 10.1128/mr.53.3.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu C-D, Abdelal A T. Role of ArgR in activation of the ast operon, encoding enzymes of the arginine succinyltransferase pathway in Salmonella typhimurium. J Bacteriol. 1999;181:1934–1938. doi: 10.1128/jb.181.6.1934-1938.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathews S A, Sriprakash K S. The RNA polymerase of Chlamydia trachomatis has a flexible sequence requirement at the −10 and −35 boxes of its promoters. J Bacteriol. 1994;176:3765–3789. doi: 10.1128/jb.176.12.3785-3789.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathews S A, Volp K M, Timms P. Development of a quantitative gene expression assay for Chlamydia trachomatis identified temporal expression of ς factors. FEBS Lett. 1999;458:354–358. doi: 10.1016/s0014-5793(99)01182-5. [DOI] [PubMed] [Google Scholar]
- 19.McClarty G. Chlamydiae and the biochemistry of intracellular parasitism. Trends Microbiol. 1994;2:157–164. doi: 10.1016/0966-842x(94)90665-3. [DOI] [PubMed] [Google Scholar]
- 20.Merrick M J. In a class of its own—the RNA polymerase sigma factor ς54 (ςN) Mol Microbiol. 1993;10:903–909. doi: 10.1111/j.1365-2958.1993.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 21.Morett E, Segovia L. The sigma-54 bacterial enhancer-binding protein family: mechanism of action and phylogenetic relationship of their functional domains. J Bacteriol. 1993;175:6067–6074. doi: 10.1128/jb.175.19.6067-6074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morett E, Buck M. In vivo studies on the interaction of the RNA polymerase-ς54 with the Klebsiella pneumoniae and Rhizobium meliloti nifH promoters. J Mol Biol. 1989;210:65–77. doi: 10.1016/0022-2836(89)90291-x. [DOI] [PubMed] [Google Scholar]
- 23.Moulder J W. Interaction of chlamydiae and host cells in vivo. Microbiol Rev. 1991;55:143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peltonen T, Mantsala P. Isolation and characterization of a purC(orf)QLF operon from Lactobacillus lactis MG1614. Mol Gen Genet. 1999;261:31–41. doi: 10.1007/s004380050938. [DOI] [PubMed] [Google Scholar]
- 25.Read T D, Brunham R C, Shen C, Gill S R, Heidelberg J F, White O, Hickey E K, Peterson J, Utterback T, Berry K, Bass S, Linher K, Weidman J, Khouri H, Craven B, Bowman C, Dodson R, Gwinn M, Nelson W, DeBoy R, Kolonay J, McClarty G, Salzberg S L, Eisen J, Fraser C M. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Res. 2000;28:1397–406. doi: 10.1093/nar/28.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reitzer L J, Magasanik B. Transcription of glnA in E. coli is stimulated by activator bound to sites far from the promoter. Cell. 1986;45:785–792. doi: 10.1016/0092-8674(86)90553-2. [DOI] [PubMed] [Google Scholar]
- 27.Stephens R S, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, Mitchell W, Olinger L, Tatusov R L, Zhao Q, Koonin E V, Davis R W. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 28.Studholme D J, Buck M. New roles of ςN in small genomes. Microbiology. 2000;146:4–5. doi: 10.1099/00221287-146-1-4. [DOI] [PubMed] [Google Scholar]
- 29.Tan M, Gaal T, Gourse R L, Engel J N. Mutational analysis of the Chlamydia trachomatis rRNA P1 promoter defines four regions important for transcription in vitro. J Bacteriol. 1998;180:2359–2366. doi: 10.1128/jb.180.9.2359-2366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor M, Butler R, Chambers S, Casimiro M, Badii F, Merrick M. The RpoN-box motif of the RNA polymerase sigma factor ςN plays a role in promoter recognition. Mol Microbiol. 1996;22:1045–1054. doi: 10.1046/j.1365-2958.1996.01547.x. [DOI] [PubMed] [Google Scholar]
- 31.Wassem R, de Souza E M, Yates M G, Pedrosa F de O, Buck M. Two roles for integration host factor at an enhancer dependent nifA promoter. Mol Microbiol. 2000;35:756–764. doi: 10.1046/j.1365-2958.2000.01746.x. [DOI] [PubMed] [Google Scholar]
- 32.Weiner L, Brissette J L, Ramani N, Model P. Analysis of the proteins and cis-acting elements regulating the stress-induced phage shock protein operon. Nucleic Acids Res. 1995;23:2030–2036. doi: 10.1093/nar/23.11.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]