Abstract
The widespread accumulation of nanoplastics is a growing concern for the environmental and human health. However, studies on the mechanisms of nanoplastic-induced developmental toxicity are still limited. Here, we systematically investigated the potential biological roles of nanoplastic exposure in zebrafish during the early developmental stage. The zebrafish embryos were subjected to exposure to 100 nm polystyrene nanoplastics with different concentrations (0, 100, 200, and 400 mg/L). The results indicated that nanoplastic exposure could decrease the hatching and survival rates of zebrafish embryos. In addition, the developmental toxicity test indicated that nanoplastic exposure exhibits developmental toxicity via the inhibition of the heart rate and body length in zebrafish embryos. Besides, behavioral activity was also significantly suppressed after 96 h of nanoplastic exposure in zebrafish larvae. Further biochemical assays revealed that nanoplastic-induced activation of the oxidative stress responses, including reactive oxygen species accumulation and enhanced superoxide dismutase and catalase activities, might affect developmental toxicity in zebrafish embryos. Furthermore, a quantitative polymerase chain reaction assay demonstrated that the mRNA levels of the base excision repair (BER) pathway-related genes, including lig1, lig3, polb, parp1, pold, fen1, nthl1, apex, xrcc1, and ogg1, were altered in zebrafish embryos for 24 h after nanoplastic exposure, indicating that the activation of the BER pathway would be stimulated after nanoplastic exposure in zebrafish embryos. Therefore, our findings illustrated that nanoplastics could induce developmental toxicity through activation of the oxidative stress response and BER pathways in zebrafish.
1. Introduction
Microplastics and nanoplastics refer to all plastic particles less than 5 mm in diameter.1 In recent decades, the plastic pollution has been listed as the second largest environmental science problem in the world and is as famous as global threats such as ocean acidification, climate change, and ozone depletion.2,3 The main sources of plastic pollution are poor waste management practices, garbage dumping, improper disposal, or runoff in industrial and agricultural activities.4,5 Different from the environmental contamination by larger plastic pieces, microplastics and nanoplastics can be ingested due to their smaller sizes and may thus accumulate along the food chain6−9 and subsequently be introduced to animals and humans.10−16 After analyzing microplastics in food and water, studies revealed that up to 250 plastic microparticles per liter were present in mineral water for human consumption,17 and microplastics were also detected in sugar, salt, alcohol, and honey.18−20 Therefore, it is speculated that humans consume 80 g of microplastics per day by eating plants.21
Accumulated evidence illustrates that microplastics are harmful to the human body. Ingestion of microplastics often causes oxidative stress, inflammation, and DNA damage.22 It was found that microplastics containing heavy metals could cause lipid peroxidation and other oxidative damage in the hippocampus, leading to increased mortality.23 Mate and Schuelke’s study showed that microplastics exposure can increase the level of reactive oxygen species (ROS) in crabs, thus turning on the antioxidant defense mechanism to cope with oxidative stress.24 ROS are intracellular chemical species that contain oxygen (O2) and are reactive toward lipids, proteins, and DNA.25 Superoxide dismutase (SOD) and catalase (CAT) are important components of antioxidant enzymes in the biological system. The accumulation of polystyrene microplastics leads to lipid accumulation and liver inflammation in fish. In addition, antioxidant enzymes, including CAT and SOD, were significantly activated, indicating that microplastics are responsible for the recovery of oxidative stress.26
Notably, a recent study revealed that microplastics can be divided into smaller nanoplastics (less than 1 μm in diameter),27,28 which might more easily infiltrate or accumulate in animal or human organs because of their smaller size.29 Based on the literature review, the European Food Safety Agency (EFSA) concluded that particles less than 150 μm in diameter might cross the intestinal mucosal barrier, whereas particles less than 1.5 μm in diameter could be transported to deeper tissues. In addition, nanoplastics are generally difficult to isolate from the environment or organism, which would greatly affect human health. Thus, plastic waste in water might affect human health in a cumulative manner through particulate toxicity, chemical toxicity, and providing a substrate for microbial breeding habitats.3,30
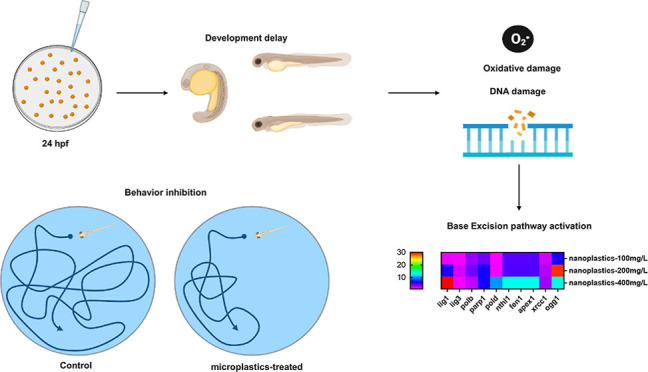
A previous report showed that the initial developmental disruption of individuals, such as morphological changes (i.e., circulatory changes, musculoskeletal diseases, and yolk sac changes),31 can significantly lead to losses in adulthood.32 Although environmental nanoplastics pollution has impaired human health, the biological toxicity effect and the underlying mechanism the effect of nanoplastic exposure on the development in organisms have still not been fully characterized. As an aquatic vertebrate animal, the zebrafish model is extensively utilized to study the toxicity of environmental pollutants, benefitting from their small size, easy reproduction, short life cycle, and lower maintenance cost.33 which encouraged us to illustrate the potential developmental toxicity of nanoplastic exposure in vivo. Herein, our study explored the effects and underlying mechanisms of nanoplastic exposure on developmental toxicity in zebrafish embryos. The analysis of developmental parameters showed that the exposure of zebrafish to nanoplastics can affect embryonic development. Further analyses showed that nanoplastics can boost ROS accumulation, increase CAT activity, affect SOD activity, induce apoptosis, and alter the base excision repair (BER) pathway-related gene expression at the mRNA level. Thus, our findings revealed the mechanism of nanoplastic exposure-induced developmental toxicity via the activation of the oxidative stress response and BER pathway in zebrafish embryos.
2. Results
2.1. Characterization of Polystyrene Nanoplastics
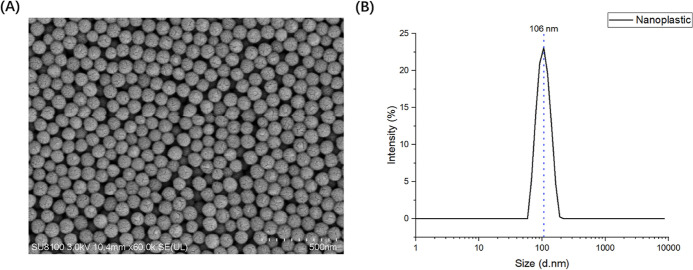
The polystyrene nanoplastic particles of average 106 nm diameter were detected by using scanning electron microscopy (SEM) analysis (Figure 1A) and dynamic light scattering (DLS) (Figure 1B), respectively. The particles were non-fluorescent and white in color. The aggregation effect of microplastics resulted in the larger hydration diameter.37 The average zeta potential was −22.3333 mV.
Figure 1.
Characterization of polystyrene nanoplastics. (A) Morphological characteristics of nanoplastics. (B) Size distribution of nanoplastics.
2.2. Polystyrene Nanoplastics Affect the Survival and Hatching Rates of Zebrafish
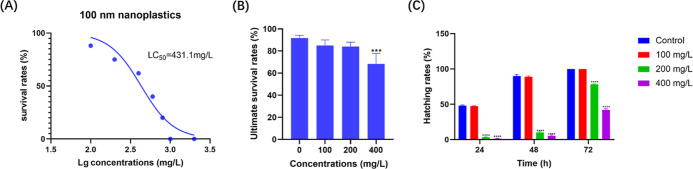
To gain insights into the effects of nanoplastics on zebrafish embryonic development, we first identified the median lethal concentration (LC50) of 100 nm nanoplastics in zebrafish embryos. The results indicated that the LC50 of nanoplastics was 431.1 mg/L after the treatment of 24 hpf zebrafish embryos for 96 h (n = 100 for each group) (Figure 2A). Therefore, the zebrafish embryos were treated to a range of nanoplastics from 100 to 400 mg/L in the following experiments.
Figure 2.
Effects of nanoplastic exposure on the survival and hatching rate of zebrafish embryos. (A) Graphical estimation of the LC50 of 96 h nanoplastic exposure in zebrafish embryos. (B) Survival rates of nanoplastic-treated zebrafish embryos at different concentrations (n = 100 for each group). (C) Hatching rates of different groups of nanoplastic-treated zebrafish embryos at different time points (n = 100 for each group). Data are shown as the mean ± SD. ***P < 0.001 and ****P < 0.0001 compared with the control.
To further study the effects of nanoplastics on the survival and hatching rates in zebrafish, we determined the survival rates of 24 hpf zebrafish embryos treated with nanoplastics for 96 h (Figure 2B). In the higher concentration group (400 mg/L), we noticed that the survival rate of zebrafish embryos was significantly decreased to 68.33% (P = 0.0003) after nanoplastic treatment for 96 h. In addition, we found that 100 mg/L nanoplastic treatment was almost insufficient to affect the hatching rates, whereas 200 and 400 mg/L nanoplastic exposure resulted in obviously delayed hatching rates in zebrafish embryos (P < 0.0001) (Figure 2C). These results suggested that nanoplastic exposure, especially at higher concentrations, significantly decreased the survival and hatching rates of zebrafish embryos.
2.3. Nanoplastic Exposure Impairs the Heart Rate, Body Length, and Behavioral Activity of Zebrafish
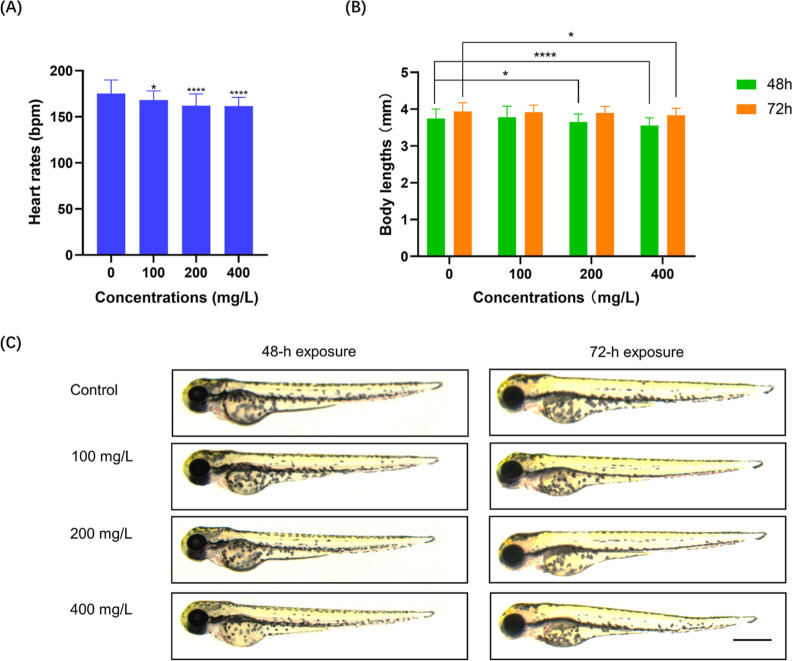
To determine the developmental toxicity of nanoplastic exposure in zebrafish, we next determined the effects of exposing 24 hpf zebrafish embryos to nanoplastics for 48 and 72 h on the heart rate and body length. The results indicated that the heart rate was significantly decreased after 72 h of nanoplastic exposure (P < 0.0001) (Figure 3A), suggesting that nanoplastic exposure may impair the development of zebrafish embryos. In addition, we noticed that a decreased zebrafish larvae length was detected in 200 mg/L (P < 0.05) and 400 mg/L groups (P < 0.0001) after 48 h of nanoplastic exposure, while for only the 400 mg/L group compared with the control group, the body length decreased significantly (P < 0.05) after 72 h of nanoplastic exposure (Figure 3B,C).
Figure 3.
Nanoplastic exposure inhibits the heart rate and decreases body length in zebrafish embryos. (A) Heart rates of 24 hpf zebrafish embryos treated with nanoplastics for 72 h (n = 20 for each group). (B) Quantification of the body length of zebrafish embryos with or without nanoplastic treatment at different concentrations after 48 or 72 h of exposure. (C) Representative images of zebrafish embryos with or without nanoplastic treatment at different concentrations after 48 or 72 h of exposure. Scale bar, 0.5 mm. Data are shown as the mean ± SD. *P < 0.05, **P < 0.01, and ****P < 0.0001 compared with the control. bpm, beats per min.
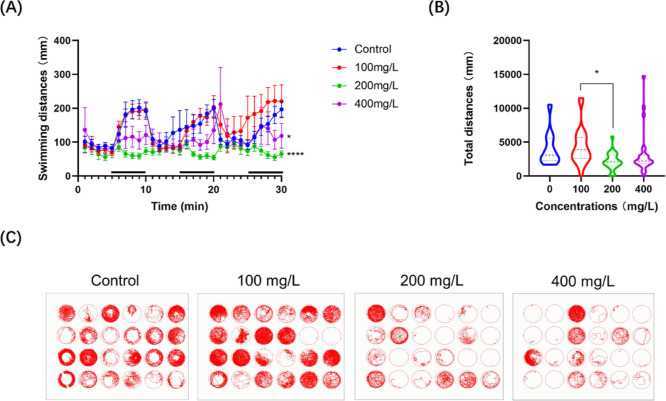
Further investigations revealed that the 96 h nanoplastic treatment significantly (P < 0.05) affects the locomotor activity of zebrafish larvae at 5 days post-fertilization (dpf). Notably, the behavioral analysis of zebrafish larvae treated with different concentrations demonstrated significant differences in the distance travelled and trajectories. The weaker the swimming ability of larvae in each hole, the more the vacancy left in the well. In this context, exposure to a lower concentration (less than 100 mg/L) of nanoplastics seems to be insufficient to inhibit the behavioral activity, whereas the distances swam by zebrafish larvae in the higher concentration (more than 200 mg/L) groups were markedly decreased (P < 0.05) after nanoplastic exposure (Figure 4A–C), suggesting that nanoplastic exposure concentrations might be positively correlated with developmental disruption in zebrafish embryos.
Figure 4.
Nanoplastic exposure impairs the behavioral ability of zebrafish larvae. (A) Average distance traveled by larvae treated with nanoplastics in 1 min under 5 min light and dark (black bars on the x-axis) conditions for 30 min. (n = 24 for each group) (B) Total distances of zebrafish swimming. (C) Trajectory graph of zebrafish larvae with or without nanoplastic treatment at different concentrations after 96 h exposure. Data are shown as the mean ± SD. *P < 0.05 and ****P < 0.0001 compared with the control.
2.4. Nanoplastic Exposure Induces an Oxidative Stress Response and Apoptosis in Zebrafish Embryos
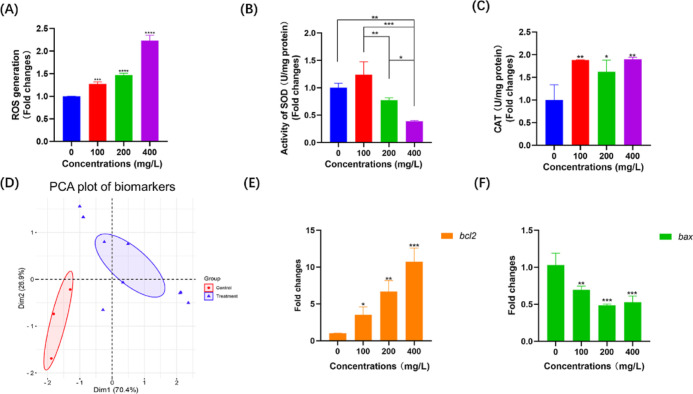
Since developmental toxicity is usually accompanied by an oxidative stress response and apoptosis,34−36 we then evaluated the effects of nanoplastic exposure on ROS accumulation and the regulation of CAT and SOD activities after treatment with nanoplastics at different concentrations in zebrafish embryos. As expected, the nanoplastic-treated zebrafish embryos displayed significantly enhanced ROS accumulation (Figure 5A). After 24 h of 100, 200, and 400 mg/L nanoplastic treatment, the levels of ROS were increased by 1.27- (P < 0.001), 1.47- (P < 0.0001), and 2.23-fold (P < 0.0001), respectively, compared with that of the nontreated control group (Figure 5A). In addition, the results indicated that CAT activity was increased after nanoplastic exposure at different concentrations in all three groups of zebrafish embryos (Figure 5B), suggesting that the CAT synthesis pathway might be activated by nanoplastic treatment in zebrafish. Intriguingly, we found that the SOD activity was increased in the lower concentration group (less than 100 mg/L) but decreased in the higher concentration groups (more than 200 mg/L) after the exposure of 24 hpf zebrafish embryos to nanoplastics for 24 h (Figure 5C). Considering that multiple biomarkers were evaluated in this process, a principal component analysis (PCA) was performed for determining the differences between experimental groups after nanoplastic exposure in zebrafish embryos (Figure 5D). The PCA plot showed that the experimental groups were significantly distant from the control groups, indicating that nanoplastic exposure could result in significant alterations in various biochemical factors in zebrafish embryos.
Figure 5.
Nanoplastic exposure induces the oxidative stress response and apoptosis in zebrafish embryos. (A-C) Determinations of ROS accumulation (A) and SOD (B) and CAT (C) activities in 24 hpf zebrafish embryos after 24 h nanoplastic treatment (n = 30 for each group). (D) PCA plot of biomarkers (ROS, SOD, and CAT) in nanoplastic-treated and control groups of zebrafish embryos. (E,F) mRNA levels of bcl2 (D) and bax (E) in 24 h nanoplastic-treated zebrafish embryos (n = 30 for each group). Data are shown as the mean ± SD. *P < 0.05, ***P < 0.001, and ****P < 0.0001 compared with the control.
To further confirm whether apoptosis occurred during the early developmental stage in nanoplastic-treated zebrafish embryos, we then determined the expression levels of apoptosis-related genes after treating 24 hpf zebrafish embryos to nanoplastics for 24 h. Quantitative polymerase chain reaction (qPCR) assays indicated that the mRNA level of bcl2, which is known as a key apoptotic regulator, was significantly upregulated (P < 0.05) in nanoplastic-treated zebrafish embryos (Figure 5E). In contrast, we found that the expression of antiapoptotic genes was decreased in zebrafish after nanoplastic exposure for 24 h (Figure 5F). Therefore, these results demonstrated that apoptosis is associated with the developmental toxicity of microplastic exposure in zebrafish embryos.
2.5. Activation of the BER Pathway Is Involved in Nanoplastic-Induced Developmental Toxicity
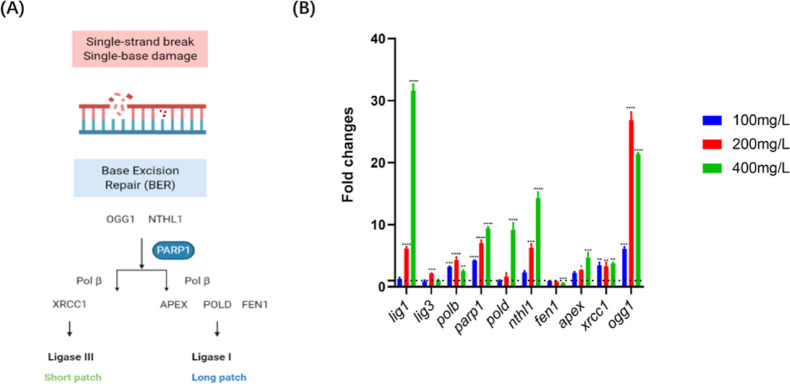
Previous studies indicated that the BER pathway performs an important part in oxidative stress-related DNA damage37−39 and subsequently prevents developmental abnormalities.40 In this context, we therefore attempted to measure the regulation of potential key genes of the BER pathway in zebrafish embryos after nanoplastic exposure. BER is the primary DNA repair pathway that corrects base lesions induced by oxidation, alkylation, and deamination.41 In this process, BER facilitates the repair of damaged DNA via two general pathways, including short- and long-patch.42 Importantly, several key regulators, such as endonuclease III-like (NTHL1), 8-oxoguanine DNA glycosylase (OGG1), apyrimidinic endonuclease (APEX), flap endonuclease 1 (FEN1), DNA polymerases (POLB and POLD), X-ray repair cross complementing 1 (XRCC1), DNA ligases 1 and 3 (LIG1 and LIG3), and poly (ADP-ribose) polymerase 1 (PARP1), were found to be essential for the BER pathway (Figure 6A).42,43
Figure 6.
Regulation of mRNA levels of BER pathway-related genes in nanoplastic-treated zebrafish embryos. (A) Schematic illustration of the BER pathway in eukaryotes. (B) mRNA levels of key genes in the BER pathway in zebrafish embryos after 24 h of nanoplastic exposure at different concentrations (n = 30 for each group). Data are shown as the mean ± SD. *P < 0.05, ***P < 0.001, and ****P < 0.0001 compared with the control.
In nanoplastic-treated zebrafish embryos, the expression levels of several genes, including lig1, pold, nthl1, parp1, apex, and xrrc1, were statistically increased (P < 0.05), whereas only the fen1 expression was decreased (P < 0.05) after microplastic exposure in zebrafish embryos. Notably, we found that the expressions of lig3, polb, and ogg1 were increased in the lower concentration groups (less than 200 mg/L) and slightly decreased in the 400 mg/L microplastic exposure group in zebrafish (Figure 6B). Thus, our results demonstrated that the activation of the BER pathway may be a stress response for the oxidative DNA damage induced in nanoplastic-treated zebrafish embryos.
3. Discussion
In a realistic aquatic ecosystem, the nanoplastics usually persist over long-term periods (months to years) with lower concentrations. Previous reports indicated that the typical nanoplastic concentrations were 150–2400 particles/m3, whereas in a harbor adjacent to a plastic production facility, the concentration was 102,000/m3.44 We have tested the toxicological effects of nanoplastics using a median lethal concentration (LC50) assay,45 followed by for a series of concentrations lower than LC50 for subsequent experiments. However, for investigating the toxic effects and mechanisms of nanoplastics on aquatic organisms, the complicated environmental factors might disturb the effects of nanoplastics in organisms. Therefore, the laboratory condition usually focuses on one or two organisms and lasts for shorter periods (hours to days), and the outcomes from simplified and individual studies in laboratorial conditions with a higher concentration might accurately evaluate the risk for human health and partially reflect the toxicity effects of nanoplastics in realistic environmental ecosystems.
The chorion of zebrafish embryos can effectively block polystyrene nanoparticles with a diameter of 100 nm.46 The adsorption of nanoplastics on the outer surface of the chorion changes the mechanical properties of the chorion, which may lead to an anoxic microenvironment that subsequently extends the incubation period of zebrafish embryos.46 A previous study indicated that hypoxia caused by microplastics is likely to result in the death of zebrafish embryos and therefore reduce the survival rate.47 Microplastics or nanoplastics can also combine with other chemicals, such as heavy metals, influencing their bioavailability and toxicity in the organisms.48 In this study, we used nanoplastics alone, and Lee’s work has indicated that nanoplastics synergistically accelerated the inhibition of hatching.49 In view of the large number of marine pollutants, the adsorption of microplastics means that they can combine with a variety of toxic compounds to deepen the toxicity.
It is noted that the survival of vertebrates under hypoxia includes reducing various processes, such as heart function and cell cycle processes, to match the energy supply with the energy demand. Nanoplastic exposure also creates a hypoxic environment for zebrafish, which slows the heart rate. We found that the body length was decreased after the exposure of zebrafish embryos to nanoplastics, which might be initially caused by delayed hatching and then limited by poor nutrient absorption in later development, for the nanoparticles initially accumulate in the yolk sac and the head and later in other regions, such as the liver, pancreas, gall bladder, pericardium, and GI tract.50 Zebrafish embryos obtain nutrients from their yolk sac until 5 dpf, which can eliminate the differences caused by nutrients.51 However, the results indicated that the growth rates of zebrafish embryos in the 400 mg/L nanoplastic treatment group were inhibited within the first 72 h developmental stage, implying that nanoplastic exposure might permanently impair the development of zebrafish embryos.
A previous study indicated that hypoxia leads to heart damage and reduced ATP synthesis, resulting in decreased behavioral ability,52 which is consistent with our results that the behavioral ability of zebrafish larvae is inhibited after microplastic exposure (Figure 4A–C). In addition, Chen et al. concluded that oxidative damage is one of the main reasons for the behavior inhibition in zebrafish larvae.53 It may also be that nanoplastics affect the neural development of zebrafish larvae, resulting in a reduction in their behavioral ability. In addition, a recent report demonstrated that the accumulation of polyethylene microplastics could trigger a behavioral disorder and subsequently cause an impact on the anxiety behavior and defensive anti-predatory response in mice through the food chain,15 indicating that microplastics might induce neurotoxicity in mice. In a realistic environment, microplastics and nanoplastics often work in synergy with other chemicals in nature. Together with other natural compounds, they reduce the secretion of acetylcholine and induce hypoactivity and a disorganized swimming pattern in zebrafish larvae.54
It is known that excessive oxygen radicals are the main culprit of oxidative stress in vivo.55 SOD and CAT are regarded as important components of antioxidant enzymes in the oxidative stress response. The dysfunction of ROS and oxidative stress in the cell would lead to severe disorders and diseases.56 Our results indicated that SOD first increased and then decreased with the increasing microplastic concentration (Figure 5B), which may be because lower nanoplastic concentrations cause oxidative damage to the body, resulting in an increase in the SOD concentration. When the nanoplastic concentration exceeds the self-regulated concentration of the zebrafish body, the synthesis of SOD will be affected, resulting in a decrease in the SOD concentration.57 As discovered, SOD can catalytically convert the superoxide radical or singlet oxygen radical generated in tissues through the metabolism or reactions in cells to hydrogen peroxide and molecular oxygen.58 CAT decomposes hydrogen peroxide into water and oxygen. We therefore speculated that the oxidative damage to cells caused by nanoplastics is mostly decomposed by CAT.
Several previous studies indicated that microplastics and nanoplastics could induce the ROS metabolism and oxidative stress through regulating antioxidants, including SOD and CAT, in zebrafish. After microplastic or nanoplastic exposure, significant induction was determined in the activities of SOD and CAT.26,59 In contrast, other reports showed no significant change in CAT activity after microplastic exposure.60,61 Our results indicated that SOD activity were increased in the lower concentration group but decreased in higher concentration groups in zebrafish embryos. The upregulation of CAT activity was also observed in zebrafish embryos exposed to nanoplastics. We expected that these differences might be caused by the sizes of microplastics or nanoplastics, exposure times, exposure concentrations, and different developmental stages of zebrafish embryos. Furthermore, regulation of bcl2 and bax expressions demonstrated the activation of oxidative stress-induced apoptosis in nanoplastic-treated zebrafish embryos (Figure 5D,E), suggesting that nanoplastic exposure probably causes apoptosis by boosting ROS accumulation and/or affecting SOD and CAT activity.62,63
As an important pathway for DNA damage repair, the regulation of BER pathway-related gene expression levels suggested that the BER pathway was related to nanoplastic-induced DNA oxidative damage in zebrafish embryos (Figure 6B). In this context, OGG1 and NTHL1, as complex glycosylases, are able to recognize and detach damaged bases and create an AP site in DNA.64 Then, APE acts on this site to continue the BER pathway repair.65 The expression levels of ogg1, nthl1, and apex generally display an upward trend after nanoplastic exposure in zebrafish embryos. Additionally, DNA polymerases, POLB and POLD, were mainly associated with the gap-fill work. FEN1 participates in the long patch BER pathway to complete the repair process.66 Moreover, as a central scaffolding protein in the BER pathway, XRCC1 can interact with LIG3 and PARP and undertake important tasks in sealing the DNA ends.65 During the early embryonic developmental stage, the embryos develop rapidly, and the mechanism by which the embryos face pressure in the early stage is imperfect, which may make the embryos more sensitive to harmful compounds.67 Our results indicated that the mRNA levels of most of the BER pathway-related genes were upregulated after nanoplastic exposure, except fen1, suggesting that the BER pathway might be a protective mechanism triggered at higher concentrations in nanoplastic-treated zebrafish embryos.
4. Conclusions
The microplastic and nanoplastic pollution has been an emerging threat to human health.68 They have entered the human food chain either by inhalation or by ingestion, particularly of shellfish and crustaceans.69 In addition, nanoplastics are potentially more hazardous than microplastics because they might easily permeate biological membranes.29 Zebrafish have been increasingly used to investigate the toxicity of microplastics and nanoplastics due to their low cost, optical clarity, high fecundity, and short life cycle.70 Herein, we discovered that polystyrene nanoplastic exposure could lead to developmental toxicity by promoting ROS accumulation in zebrafish embryos. Interestingly, further investigations revealed that apoptosis was also involved in nanoplastic-induced development toxicity in zebrafish, suggesting that nanoplastic exposure might trigger oxidative stress-mediated apoptosis in this process.71 In addition, the results indicated that the expression levels of several BER pathway-related genes, including lig1, pold, nthl1, apex, xrrc1, lig3, polb, parp1, and ogg1, in zebrafish embryos were significantly changed after microplastic treatment, suggesting that DNA damage was probably caused by the activation of oxidative stress and inflammation induced by nanoplastic exposure in zebrafish embryos.72 Therefore, these findings highlight that nanoplastic exposure could induce an oxidative stress response and activate the BER pathway to defend against oxidative DNA damage, which distributes the potential risks that come along with nanoplastic exposure.
5. Materials and Methods
5.1. Ethics Statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of Sichuan University. All experiments were performed according to the regulations and guidelines established by the Ministry of Science and Technology of the People’s Republic of China (Approval number: 2006-398).
5.2. Characterization of Nanoplastics
Nanoplastics were obtained from Huge Biotechnology Co., Ltd. (DS100, density of 1.05 g/cm3, CV % = 3, Shanghai, China). The microplastic morphology was photographed by SEM (SU8100, Hitachi, Japan). The DLS spectra and zeta potential were determined using a Zetasizer Nano ZS (Malvern Instruments, Worcestershire, UK). The sample was dispersed in deionized water.
5.3. Zebrafish Breeding, Husbandry, and Exposure Test
Wild-type strain (AB) zebrafish were purchased from the China Zebrafish Resource Center (Wuhan, China). Zebrafish were raised at 28 °C with a 14:10 h light/dark cycle and fed freshly hatched brine shrimp (Artemia nauplii) at 9 am and 6 pm per day. For breeding, three male and three female adult zebrafish were matched to produce embryos in each breeding tank. The zebrafish embryos were collected by siphoning the bottom of the tank the next day and maintained in an E3 embryonic medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, and 0.33 mM MgSO4 in 1 L of distilled water). At 24 hpf, zebrafish embryos were assigned to 100 mm Petri dishes (100 embryos per dish) and treated with nanoplastics with different concentrations. The potential toxicity of chemical substances was determined using an LC50 assay that exposed 24 hpf zebrafish embryos to nanoplastics for 96 h. Briefly, taking the common logarithm of concentration as the abscissa and the probability unit of mortality as the ordinate, the LC50 values of 96 h and 95% confidence limit were obtained through a regression equation. In this study, the concentrations of 100 nm diameter nanoplastics used for experiments were 100, 200, and 400 mg/L. Untreated zebrafish embryos were defined as the negative control. All experiments were performed independently in three replicates.
5.4. Developmental Toxicity Test
The zebrafish embryos with or without the nanoplastic treatment were characterized for developmental toxicity at different exposure time points. The survival rates were measured as the percentages of surviving zebrafish embryos at different concentrations for 72 h (n = 100 for each group). The hatching rates were recorded every 24 h after microplastic exposure in 24 hpf zebrafish embryos (n = 100 for each group). In addition, the heart rates (n = 20 for each group) and body lengths (n = 50 for each group) of zebrafish embryos were measured under a Stereo microscope (Leica M205FA, Leica microsystems, Germany) at 48 and/or 72 h after nanoplastic exposure.
5.5. Behavioral Test
At the 96 h nanoplastic exposure time point, the motor capabilities of the nanoplastic-treated zebrafish larvae were assessed by determining swimming activities,73 which was slightly modified from the previously reported protocol.74,75 Briefly, the 5 dpf zebrafish larvae of the different groups (n = 24 for each group) were assigned to a 24-well plate (one larva in each well). The swimming trajectory and total distance travelled by each larva were measured using a Zebralab Video-Track system (ViewPoint Life Science, France) through the 5 min cycle light-to-dark photoperiod and the following 30 s cycle sound and vibration stimulus. All experiments were performed independently in three replicates.
5.6. Biochemical Assay
To determine the activation of the oxidative stress response in nanoplastic-treated zebrafish embryos, 24 hpf embryos (n = 30 for each group) treated with or without nanoplastics at different concentrations (0, 100, 200, and 400 mg/L) for 24 h were collected for ROS, SOD, or CAT assays. All experiments were performed independently in three replicates.
5.6.1. ROS Assay
ROS levels in nanoplastic-exposed zebrafish embryos were detected using a reactive oxygen species assay kit (S0033S, Beyotime, Shanghai, China) according to the standard procedure. In brief, the zebrafish embryos (n = 30 for each group) in different groups were collected and homogenized in ice-cold lysis buffer. All the samples were centrifuged at 15,000g at 4 °C for 20 min. The 24 μL supernatants were then transferred to a 96-well plate and incubated at room temperature for 5 min. According to the experimental protocol, 1 × PBS (PH 7.4) and a 10 μM DCF-DA solution were added, and the plates were incubated at 37 °C for 30 min. The fluorescence intensities of each sample were determined using a microplate reader (BioTek Synergy H1, USA) with excitation at 485 nm and emission at 530 nm. The protein concentrations were detected using a BCA protein quantification kit (E112-02, Vazyme, China). The ROS generation was in relation to protein quantity. All experiments were performed independently at least three times.
5.6.2. Measurement of SOD and CAT Activities
The homogenized samples from nanoplastic-treated zebrafish embryos (n = 30 for each group) were centrifuged and harvested before evaluating SOD and CAT activities. Thereafter, the SOD and CAT activities of the samples from different groups were measured by using an SOD activity examination kit (BC0175, Solarbio, Beijing, China) and a CAT activity examination kit (D799598, Sangon Biotech, Shanghai, China), respectively, according to the manufacturer’s instructions. The CAT and SOD contents were in relation of protein quantity. All samples were analyzed in three independent replicates.
5.7. Total RNA Extraction and qPCR Assay
After exposing 24 hpf zebrafish embryos to nanoplastics for 24 h, the total RNA from the different groups (n = 30 for each group) was extracted by using TRIzol (Invitrogen, Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s instructions. Thereafter, the purity and quality of the RNA were detected using a spectrophotometer (Thermo Fisher Scientific). The RNA was reverse-transcribed by using a qPCR assay using a reverse transcription PCR system on the CFX Maestro System (Bio-Rad Laboratories, Inc., CA). The PCR protocol was started with a denaturation step at 95 °C for 5 min, followed by 36 cycles at 95 °C for 10 s, 60–62 °C for 30 s, and 72 °C for 30 s. Three replicate samples were performed for different groups. The sequences of the primers for the qPCR assay are provided in Table 1.
Table 1. Primer Sequences Used to Target BER Pathway Genes in Zebrafish Embryos.
| primer sequences (5′–3′) | length (bp) | annealing temperatures (°C) | |
|---|---|---|---|
| β-actin | F: CTACAATGAGCTCCGTGTTGC; R: CCTCATAGATGGGCACGGTG | 239 | 62 |
| bcl-2 | F: TTCTAACCGTGGCCGAAGAG; R: GCCCGTTCAGGTAGTCAGTC | 162 | 62 |
| bax | F: TACTTTGCCTGTCGCCTTGT; R: CAGCGAGGAAAACTCCGACT | 193 | 62 |
| pold | F: CTCCTCCCACTTCGTTCCAC; R: CATATACGCCGCAACTCCCT | 466 | 62 |
| fen1 | F: TCACGGACTTGCAAAGCTCA; R: TCCTGTCGAACCGCAATCAA | 138 | 60 |
| lig3 | F: AGCAAAGGCTGCGAAAACAG; R: CCTAGCGTGTGTGTGGCTAA | 300 | 62 |
| polb | F: TCCCTGAACGAAGGAATCAC; R: ATCTTTGCACCGACTCCATC | 179 | 60 |
| xrcc1 | F: AACCCTTTTCGTGCGGATCT; R: ACCCTTATCTGCGCTTTGCT | 489 | 60 |
| nthl1 | F: TCAAGCCCTCCTGTCACCTA; R: TCGCCAATCATAAGGCTCCC | 100 | 60 |
| apex1 | F: AATAAAGTGTTGGGTGTACGTG; R: CAGGAGGTGATCTTCATATTGG | 250 | 60 |
| lig1 | F: ATGGACAGGCCAAGGGAAAG; R: CTAGGATTGCTCGACTGCGT | 117 | 60 |
| parp1 | F: CTTCAGAGGCGAAGACGGTT; R: TCAAAGCATTCCCTTCATTCAGAT | 246 | 60 |
| ogg1 | F: CAAGATCTTACAGACCCTTGTG; R: CAAACTTGTCCAGTGACATCAG | 232 | 60 |
5.8. Quantification and Statistical Analysis
Statistical significance was accepted at P < 0.05, and values were presented as means ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to determine the significant differences between mean values, and the Dunnett’s test was used to determine the significant difference (P < 0.05) between microplastic-treated and control groups. The ANOVA results and the figures were obtained and plotted using Graphpad Prism 8 (GraphPad Software, San Diego, CA). A PCA was performed by Bioinformatics (http://www.bioinformatics.com.cn) for visualization to see the group differences after nanoplastic exposure.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (32072706 and U1901206) and the Science and Technique Foundation of Guangdong Province (210728156901639).
Author Contributions
M.F. and J.L. contributed equally to this work. J.L., C.L., X.Y., and Y.W. established the research ideas and designed methods; M.F., J.L., Y.W., C.L., J.Z., M.W., L.D., and X.C. conducted the experimental operations; M.F. and J.L. analyzed the data; M.F., J.L., X.Y., and Y.W. wrote and revised the manuscript. J.L. and Y.W. supervised the research. All authors read and approved the manuscript for publication.
The authors declare no competing financial interest.
References
- Frias J. P. G. L.; Nash R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. 10.1016/j.marpolbul.2018.11.022. [DOI] [PubMed] [Google Scholar]
- Amaral-Zettler L. A.; Zettler E. R.; Slikas B.; Boyd G. D.; Melvin D. W.; Morrall C. E.; Proskurowski G.; Mincer T. J. The biogeography of the Plastisphere: implications for policy. Front. Ecol. Environ. 2015, 13, 541–546. 10.1890/150017. [DOI] [Google Scholar]
- Vethaak A. D.; Leslie H. A. Plastic Debris Is a Human Health Issue. Environ. Sci. Technol. 2016, 50, 6825–6826. 10.1021/acs.est.6b02569. [DOI] [PubMed] [Google Scholar]
- Leslie H. A.; Brandsma S. H.; van Velzen M. J. M.; Vethaak A. D. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142. 10.1016/j.envint.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Mahon A. M.; O’Connell B.; Healy M. G.; O’Connor I.; Officer R.; Nash R.; Morrison L. Microplastics in Sewage Sludge: Effects of Treatment. Environ. Sci. Technol. 2017, 51, 810–818. 10.1021/acs.est.6b04048. [DOI] [PubMed] [Google Scholar]
- do Sul J. A. I.; Costa M. F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352–364. 10.1016/j.envpol.2013.10.036. [DOI] [PubMed] [Google Scholar]
- Boerger C. M.; Lattin G. L.; Moore S. L.; Moore C. J. Plastic ingestion by planktivorous fishes in the North Pacific Central Gyre. Mar. Pollut. Bull. 2010, 60, 2275–2278. 10.1016/j.marpolbul.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Cedervall T.; Hansson L. A.; Lard M.; Frohm B.; Linse S. Food Chain Transport of Nanoparticles Affects Behaviour and Fat Metabolism in Fish. PLoS One 2012, 7, e32254 10.1371/journal.pone.0032254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P.; Nelson K. Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.). Environ. Pollut. 2013, 177, 1–3. 10.1016/j.envpol.2013.01.046. [DOI] [PubMed] [Google Scholar]
- de Sá L. C.; Oliveira M.; Ribeiro F.; Rocha T. L.; Futter M. N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future?. Sci. Total Environ. 2018, 645, 1029–1039. 10.1016/j.scitotenv.2018.07.207. [DOI] [PubMed] [Google Scholar]
- Santana M. F. M.; Moreira F. T.; Turra A. Trophic transference of microplastics under a low exposure scenario: Insights on the likelihood of particle cascading along marine food-webs. Mar. Pollut. Bull. 2017, 121, 154–159. 10.1016/j.marpolbul.2017.05.061. [DOI] [PubMed] [Google Scholar]
- Silva-Cavalcanti J. S.; Silva J. D. B.; França E. J.; Araújo M. C. B.; Gusmão F. Microplastics ingestion by a common tropical freshwater fishing resource. Environ. Pollut. 2017, 221, 218–226. 10.1016/j.envpol.2016.11.068. [DOI] [PubMed] [Google Scholar]
- Smith M.; Love D. C.; Rochman C. M.; Neff R. A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. 10.1007/s40572-018-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint B.; Raffael B.; Angers-Loustau A.; Gilliland D.; Kestens V.; Petrillo M.; Rio-Echevarria I. M.; Van den Eede G. Review of micro- and nanoplastic contamination in the food chain. Food Addit. Contam., Part A 2019, 36, 639–673. 10.1080/19440049.2019.1583381. [DOI] [PubMed] [Google Scholar]
- da Costa Araújo A. P.; Malafaia G. Microplastic ingestion induces behavioral disorders in mice: A preliminary study on the trophic transfer effects via tadpoles and fish. J. Hazard. Mater. 2021, 401, 123263. 10.1016/j.jhazmat.2020.123263. [DOI] [PubMed] [Google Scholar]
- da Costa Araújo A. P.; de Andrade Vieira J. E.; Malafaia G. Toxicity and trophic transfer of polyethylene microplastics from Poecilia reticulata to Danio rerio. Sci. Total Environ. 2020, 742, 140217. 10.1016/j.scitotenv.2020.140217. [DOI] [PubMed] [Google Scholar]
- Schymanski D.; Goldbeck C.; Humpf H. U.; Fürst P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018, 129, 154–162. 10.1016/j.watres.2017.11.011. [DOI] [PubMed] [Google Scholar]
- Cox K. D.; Covernton G. A.; Davies H. L.; Dower J. F.; Juanes F.; Dudas S. E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019, 53, 7068–7074. 10.1021/acs.est.9b01517. [DOI] [PubMed] [Google Scholar]
- Mühlschlegel P.; Hauk A.; Walter U.; Sieber R. Lack of evidence for microplastic contamination in honey. Food Addit. Contam., Part A 2017, 34, 1982–1989. 10.1080/19440049.2017.1347281. [DOI] [PubMed] [Google Scholar]
- Afrin S.; Rahman M.; Hossain N.; Uddin K.; Malafaia G. Are there plastic particles in my sugar? A pioneering study on the characterization of microplastics in commercial sugars and risk assessment. Sci. Total Environ. 2022, 837, 155849. 10.1016/j.scitotenv.2022.155849. [DOI] [PubMed] [Google Scholar]
- Ebere E. C.; Wirnkor V. A.; Ngozi V. E. Uptake of Microplastics by Plant: a Reason to Worry or to be Happy?. World Sci. News 2019, 131, 256–267. [Google Scholar]
- Rochman C. M.; Hoh E. H.; Kurobe T.; Teh S. J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. 10.1038/srep03263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinhui J.; Sudong S.; Yan Y.; Xia X.; Jiahao J.; Yongjian Y.; Jinhui S. Effects of microplastics and attached heavy metals on growth, immunity, and heavy metal accumulation in the yellow seahorse, Hippocampus kuda Bleeker. Mar. Pollut. Bull. 2019, 149, 110510. 10.1016/j.marpolbul.2019.110510. [DOI] [PubMed] [Google Scholar]
- Matés J. M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000, 153, 83–104. 10.1016/s0300-483x(00)00306-1. [DOI] [PubMed] [Google Scholar]
- Glasauer A.; Chandel N. S. Ros. Curr. Biol. 2013, 23, R100–R102. 10.1016/j.cub.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Lu Y.; Zhang Y.; Deng Y.; Jiang W.; Zhao Y.; Geng J.; Ding L.; Ren H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. 10.1021/acs.est.6b00183. [DOI] [PubMed] [Google Scholar]
- Andrady A. L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Lambert S.; Wagner M. Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere 2016, 145, 265–268. 10.1016/j.chemosphere.2015.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi S.; Behzadi S.; Laurent S.; Laird Forrest M. L.; Stroeve P.; Mahmoudi M. Toxicity of nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. 10.1039/c1cs15188f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanale C.; Massarelli C.; Savino I.; Locaputo V.; Uricchio V. F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Publ. Health 2020, 17, 1212. 10.3390/ijerph17041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira A. C.; Gomes T.; Ferreira Machado M. R. F.; Rocha T. L. The zebrafish embryotoxicity test (ZET) for nanotoxicity assessment: from morphological to molecular approach. Environ. Pollut. 2019, 252, 1841–1853. 10.1016/j.envpol.2019.06.100. [DOI] [PubMed] [Google Scholar]
- Dimitriadi A.; Papaefthimiou C.; Genizegkini E.; Sampsonidis I.; Kalogiannis S.; Feidantsis K.; Bobori D. C.; Kastrinaki G.; Koumoundouros G.; Lambropoulou D. A.; Kyzas G. Z.; Bikiaris D. N. Adverse effects polystyrene microplastics exert on zebrafish heart-Molecular to individual level. J. Hazard. Mater. 2021, 416, 125969. 10.1016/j.jhazmat.2021.125969. [DOI] [PubMed] [Google Scholar]
- Malafaia G.; de Souza A. M.; Pereira A. C.; Gonçalves S.; da Costa Araújo A. P.; Ribeiro T. L.; Rocha T. L. Developmental toxicity in zebrafish exposed to polyethylene microplastics under static and semi-static aquatic systems. Sci. Total Environ. 2020, 700, 134867. 10.1016/j.scitotenv.2019.134867. [DOI] [PubMed] [Google Scholar]
- Kupsco A.; Schlenk D. Oxidative Stress, Unfolded Protein Response, and Apoptosis in Developmental Toxicity. Int. Rev. Cell Mol. Biol. 2015, 317, 1–66. 10.1016/bs.ircmb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Wang H.; Duah P. A.; Retyunskiy V.; Liu Y.; Chen G. Zinc pyrithione (ZPT) -induced embryonic toxicogenomic responses reveal involvement of oxidative damage, apoptosis, endoplasmic reticulum (ER) stress and autophagy. Aquat. Toxicol. 2022, 248, 106159. 10.1016/j.aquatox.2022.106195. [DOI] [PubMed] [Google Scholar]
- Lite C.; Guru A.; Juliet M.; Arockiaraj J. Embryonic exposure to butylparaben and propylparaben induced developmental toxicity and triggered anxiety-like neurobehavioral response associated with oxidative stress and apoptosis in the head of zebrafish larvae. Environ. Toxicol. 2022, 37, 1988. 10.1002/tox.23545. [DOI] [PubMed] [Google Scholar]
- Wang R.; Li C. S.; Qiao P.; Xue Y. Y.; Zheng X.; Chen H. Y.; Zeng X. L.; Liu W. G.; Boldogh I.; Ba X. Q. OGG1-initiated base excision repair exacerbates oxidative stress-induced parthanatos. Cell Death Dis. 2018, 9, 628. 10.1038/s41419-018-0680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. L.; Chen W. Y.; Mukda S.; Yang Y. R.; Sun S. F.; Chen S. D. Oxidative DNA damage is concurrently repaired by base excision repair (BER) and apyrimidinic endonuclease 1 (APE1)-initiated nonhomologous end joining (NHEJ) in cortical neurons. Neuropathol. Appl. Neurobiol. 2020, 46, 375–390. 10.1111/nan.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y.; Karbaschi M.; Cooke M. S. Mycoplasma infection of cultured cells induces oxidative stress and attenuates cellular base excision repair activity. Mutat. Res., Genet. Toxicol. Environ. Mutagen. 2019, 845, 403054. 10.1016/j.mrgentox.2019.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C.; Luo J. J.; Liu Y.; Yang X. J. The oxidative stress responses caused by phthalate acid esters increases mRNA abundance of base excision repair (BER) genes in vivo and in vitro. Ecotoxicol. Environ. Saf. 2021, 208, 111525. 10.1016/j.ecoenv.2020.111525. [DOI] [PubMed] [Google Scholar]
- Robertson A. B.; Klungland A.; Rognes T.; Leiros I. DNA Repair in Mammalian Cells. Cell. Mol. Life Sci. 2009, 66, 981–993. 10.1007/s00018-009-8736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindi N. N.; Elsakrmy N.; Ramotar D. The base excision repair process: comparison between higher and lower eukaryotes. Cell. Mol. Life Sci. 2021, 78, 7943–7965. 10.1007/s00018-021-03990-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright G.; Gassman N. R. Transcriptional dysregulation of base excision repair proteins in breast cancer. DNA Repair 2020, 93, 102922. 10.1016/j.dnarep.2020.102922. [DOI] [PubMed] [Google Scholar]
- Cole M.; Lindeque P.; Halsband C.; Galloway T. S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. 10.1016/j.marpolbul.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Oksel C.; Ma C. Y.; Liu J. J.; Wilkins T.; Wang X. Z. Literature Review of (Q)SAR Modelling of Nanomaterial Toxicity. Adv. Exp. Med. Biol. 2017, 947, 103–142. 10.1007/978-3-319-47754-1_5. [DOI] [PubMed] [Google Scholar]
- Duan Z. H.; Duan X. Y.; Zhao S.; Wang X. L.; Wang J.; Liu Y. B.; Peng Y. W.; Gong Z. Y.; Wang L. Barrier function of zebrafish embryonic chorions against microplastics and nanoplastics and its impact on embryo development. J. Hazard. Mater. 2020, 395, 122621. 10.1016/j.jhazmat.2020.122621. [DOI] [PubMed] [Google Scholar]
- Padilla P. A.; Roth M. B. Oxygen deprivation causes suspended animation in the zebrafish embryo. Proc. Natl. Acad. Sci. U.S.A. 2001, 98, 7331–7335. 10.1073/pnas.131213198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos D.; Félix L.; Luzio A.; Parra S.; Bellas J.; Monteiro S. M. Single and combined acute and subchronic toxic effects of microplastics and copper in zebrafish (Danio rerio) early life stages. Chemosphere 2021, 277, 130262. 10.1016/j.chemosphere.2021.130262. [DOI] [PubMed] [Google Scholar]
- Lee W. S.; Cho H. J.; Kim E.; Huh Y. H.; Kim H. J.; Kim B.; Kang T.; Lee J. S.; Jeong J. Bioaccumulation of polystyrene nanoplastics and their effect on the toxicity of Au ions in zebrafish embryos. Nanoscale 2019, 11, 3173–3185. 10.1039/c8nr09321k. [DOI] [PubMed] [Google Scholar]
- Pitt J. A.; Kozal J. S.; Jayasundara N.; Massarsky A.; Trevisan R.; Geitner N.; Wiesner M.; Levin E. D.; Di Giulio R. T. Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio). Aquat. Toxicol. 2018, 194, 185–194. 10.1016/j.aquatox.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tshering G.; Plengsuriyakarn T.; Na-Bangchang K.; Pimtong W. Embryotoxicity evaluation of atractylodin and β-eudesmol using the zebrafish model. Comp. Biochem. Physiol., Part C: Pharmacol., Toxicol. Endocrinol. 2021, 239, 108869. 10.1016/j.cbpc.2020.108869. [DOI] [PubMed] [Google Scholar]
- Qiang L. Y.; Cheng J. P. Exposure to microplastics decreases swimming competence in larval zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2019, 176, 226–233. 10.1016/j.ecoenv.2019.03.088. [DOI] [PubMed] [Google Scholar]
- Chen Q. Q.; Gundlach M.; Yang S. Y.; Jiang J.; Velki M.; Yin D. Q.; Hollert H. Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci. Total Environ. 2017, 584-585, 1022–1031. 10.1016/j.scitotenv.2017.01.156. [DOI] [PubMed] [Google Scholar]
- Santos D.; Luzio A.; Matos C.; Bellas J.; Monteiro S. M.; Félix L. Microplastics alone or co-exposed with copper induce neurotoxicity and behavioral alterations on zebrafish larvae after a subchronic exposure. Aquat. Toxicol. 2021, 235, 105814. 10.1016/j.aquatox.2021.105814. [DOI] [PubMed] [Google Scholar]
- Zhao F.; Li H.; Cao F. J.; Chen X. G.; Liang Y.; Qiu L. H. Short-term developmental toxicity and potential mechanisms of the herbicide metamifop to zebrafish (Danio rerio) embryos. Chemosphere 2019, 236, 124590. 10.1016/j.chemosphere.2019.124590. [DOI] [PubMed] [Google Scholar]
- Suzuki N.; Mittler R. Reactive oxygen species-dependent wound responses in animals and plants. Free Radic. Biol. Med. 2012, 53, 2269–2276. 10.1016/j.freeradbiomed.2012.10.538. [DOI] [PubMed] [Google Scholar]
- Jiang X. F.; Chang Y. Q.; Zhang T.; Qiao Y.; Klobucar G.; Li M. Toxicological effects of polystyrene microplastics on earthworm (Eisenia fetida). Environ. Pollut. 2020, 259, 113896. 10.1016/j.envpol.2019.113896. [DOI] [PubMed] [Google Scholar]
- Akinloye O. M.; Akinloye O. A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018, 54, 287–293. 10.1016/j.ajme.2017.09.001. [DOI] [Google Scholar]
- Qiao R.; Sheng C.; Lu Y.; Zhang Y.; Ren H.; Lemos B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. 10.1016/j.scitotenv.2019.01.245. [DOI] [PubMed] [Google Scholar]
- Parenti C. C.; Ghilardi A.; Della Torre C. D.; Magni S.; Del Giacco L. D.; Binelli A. Evaluation of the infiltration of polystyrene nanobeads in zebrafish embryo tissues after short-term exposure and the related biochemical and behavioural effects. Environ. Pollut. 2019, 254, 112947. 10.1016/j.envpol.2019.07.115. [DOI] [PubMed] [Google Scholar]
- Pitt J. A.; Trevisan R.; Massarsky A.; Kozal J. S.; Levin E. D.; Di Giulio R. T. D. Maternal transfer of nanoplastics to offspring in zebrafish (Danio rerio): a case study with nanopolystyrene. Sci. Total Environ. 2018, 643, 324–334. 10.1016/j.scitotenv.2018.06.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadi A.; Papaefthimiou C.; Genizegkini E.; Sampsonidis I.; Kalogiannis S.; Feidantsis K.; Bobori D. C.; Kastrinaki G.; Koumoundouros G.; Lambropoulou D. A.; Kyzas G. Z.; Bikiaris D. N. Adverse effects polystyrene microplastics exert on zebrafish heart-Molecular to individual level. J. Hazard. Mater. 2021, 416, 125969. 10.1016/j.jhazmat.2021.125969. [DOI] [PubMed] [Google Scholar]
- Koehler A.; Marx U.; Broeg K.; Bahns S.; Bressling J. Effects of nanoparticles in Mytilus edulis gills and hepatopancreas - A new threat to marine life?. Mar. Environ. Res. 2008, 66, 12–14. 10.1016/j.marenvres.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Malfatti M. C.; Antoniali G.; Codrich M.; Burra S.; Mangiapane G.; Dalla E.; Tell G. New perspectives in cancer biology from a study of canonical and non-canonical functions of base excision repair proteins with a focus on early steps. Mutagenesis 2020, 35, 129–149. 10.1093/mutage/gez051. [DOI] [PubMed] [Google Scholar]
- Luo M.; Delaplane S.; Jiang A.; et al. role of the multifunctional dna repair and redox signaling protein ape1/ref-1 in cancer and endothelial cells: small-molecule inhibition of the redox function of ape1. Antioxid. Redox Signaling 2008, 10, 1853. 10.1089/ars.2008.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illuzzi J. L.; Wilson D. M. Base Excision Repair: Contribution to Tumorigenesis and Target in Anticancer Treatment Paradigms. Curr. Med. Chem. 2012, 19, 3922–3936. 10.2174/092986712802002581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teraoka H.; Dong W.; Hiraga T. Zebrafish as a novel experimental model for developmental toxicology. Congenital Anomalies 2003, 43, 123–132. 10.1111/j.1741-4520.2003.tb01036.x. [DOI] [PubMed] [Google Scholar]
- Prata J. C.; da Costa J. P.; Lopes I.; Duarte A. C.; Rocha-Santos T. Environmental exposure to microplastics: an overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. 10.1016/j.scitotenv.2019.134455. [DOI] [PubMed] [Google Scholar]
- Waring R. H.; Harris R. M.; Mitchell S. C. Plastic contamination of the food chain: a threat to human health?. Maturitas 2018, 115, 64–68. 10.1016/j.maturitas.2018.06.010. [DOI] [PubMed] [Google Scholar]
- Bhagat J.; Zang L.; Nishimura N.; Shimada Y. Zebrafish: an emerging model to study microplastic and nanoplastic toxicity. Sci. Total Environ. 2020, 728, 138707. 10.1016/j.scitotenv.2020.138707. [DOI] [PubMed] [Google Scholar]
- Song Z.; Zhang Y.; Zhang H.; Rajendran R. S.; Wang R.; Hsiao C.; Li J.; Xia Q.; Liu K. Isoliquiritigenin triggers developmental toxicity and oxidative stress-mediated apoptosis in zebrafish embryos/larvae via Nrf2-HO1/JNK-ERK/mitochondrion pathway. Chemosphere 2020, 246, 125727. 10.1016/j.chemosphere.2019.125727. [DOI] [PubMed] [Google Scholar]
- Czarny P.; Wigner P.; Galecki P.; Sliwinski T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2018, 80, 309–321. 10.1016/j.pnpbp.2017.06.036. [DOI] [PubMed] [Google Scholar]
- Yuan L.; Qian L.; Qian Y.; Liu J.; Yang K.; Huang Y.; Wang C.; Li Y.; Mu X. Bisphenol F-induced neurotoxicity toward zebrafish embryos. Environ. Sci. Technol. 2019, 53, 14638–14648. 10.1021/acs.est.9b04097. [DOI] [PubMed] [Google Scholar]
- de Oliveira J. P. J.; Estrela F. N.; de Lima Rodrigues A. S.; Guimarães A. T. B.; Rocha T. L.; Malafaia G. Behavioral and biochemical consequences of Danio rerio larvae exposure to polylactic acid bioplastic. J. Hazard. Mater. 2021, 404, 124152. 10.1016/j.jhazmat.2020.124152. [DOI] [PubMed] [Google Scholar]
- Ahmad F.; Richardson M. K. Exploratory behaviour in the open field test adapted for larval zebrafish: impact of environmental complexity. Behav. Process. 2013, 92, 88–98. 10.1016/j.beproc.2012.10.014. [DOI] [PubMed] [Google Scholar]