Abstract
Some ingredients bind more acid in the stomach than others which can increase gastric pH in weaned pigs causing decreased protein digestion and allow pathogenic micro-organisms to proliferate. Our objective was to measure acid-binding capacity at a pH of 4 (ABC-4) of common nursery ingredients and determine additivity in diets. Ingredient categories included: cereal grains, vegetable proteins, animal proteins and milk, vitamin premixes and minerals, amino acids, and fiber sources. A 0.5-g sample of each ingredient was suspended in 50 mL of distilled deionized water and titrated with 0.1-N hydrochloric acid. Sample ABC-4 was calculated as the amount of acid in milliequivalents (meq) required to lower 1 kg to a pH of 4. Cereal grains were found to have lower ABC-4 compared to other ingredients. Vegetable proteins had higher ABC-4 with more variation than cereal grains. Soybean meal (SBM) had an ABC-4 of 602 ± 28.2 meq. Soy protein concentrate and enzymatically treated soybean meal (ESBM) had higher ABC-4 compared to SBM while fermented soybean meal (FSBM) was lower. Zinc oxide (ZnO) and calcium carbonate (CaCO3) had the highest ABC-4 among all ingredients with values of 21,863 ± 598.7 and 18,384 ± 769.7 meq, respectively. Following ingredient analysis, a series of diets were analyzed to determine additivity by comparing the differences between calculated and analyzed ABC-4 value. All diets analyzed had lower ABC-4 than calculated values; however, analyzed ABC-4 increased along with calculated values across diets. The first series of diets were arranged in a 2 × 5 factorial consisting of increasing CaCO3 with or without ZnO. There was a ZnO × CaCO3 interaction (P = 0.020) for difference between calculated and analyzed ABC-4. Within the interaction, differences between calculated and analyzed ABC-4 increased (linear, P < 0.001) as CaCO3 increased in diets without ZnO, but not in diets with ZnO. The second series of diets analyzed consisted of different levels of SBM with either FSBM or ESBM included at 5% of the diet. Differences between calculated and analyzed values were not different between treatments (P = 0.640). In conclusion, perfect ABC-4 additivity in diets was not found due to lower analyzed than calculated values; however, analyzed ABC-4 still increased as calculated values increased. This data suggests diet ABC-4 can be adjusted through selection of ingredients but more feeding trials are needed to determine its impact on pig performance.
Keywords: acid-binding capacity, diets, ingredients, nursery pig
Acid-binding capacity can be manipulated through careful selection of ingredients used in nursery pig diets.
INTRODUCTION
The postweaning period is a stressful and critical period of the young pig’s life and much interest has been directed towards positively influencing the gastrointestinal tract (GIT) to maximize lifetime production and health status (Pluske, 2016). Newly weaned pigs undergo a dramatic change in diet composition from a highly palatable and digestible milk diet to a dry cereal-based diet. Stomach acid secretion is low in the suckling pigs and the main source of acidity comes from bacterial fermentation of lactose to lactic acid (Cranwell et al., 1968). However, at weaning the amount of lactic acid in the stomach is reduced compared to a suckling pig (Yen, 2001). In North America, it is common for pigs to be weaned between 17 and 21 d with a relatively underdeveloped GIT (McGlone and Johnson, 2003). Pigs’ hydrochloric acid production is limited until the pig reaches 7 to 8 wk of age (Pluske, 2016; Pearlin et al., 2020). Switching pigs to a solid diet at weaning may lead to an increase in gastric pH as high as 5.0 for several days postweaning (Kidder and Manners, 1978). Low stomach pH is important for protein digestion and when stomach pH increases above 3.5, pepsin activity rapidly declines (Longland 1991; Zijlstra et al., 1996; Yen, 2001; Pluske, 2003). Increased gastric pH allows opportunistic pathogens to survive and compromise the digestive tract leading to clinical infection, disease, and possible death (Bolduan et al., 1988).
Poor acidification of the stomach can be attributed to the high acid-binding capacity (ABC) of feed ingredients (Batonon-Alavo et al., 2016). The concept of ABC involves manipulating the stomach’s acidity by incorporating low acid-binding ingredients (Lawlor et al., 2005). Therefore, the use of low acid-binding ingredients (avoidance of high acid-binding ingredients) in the diets of newly weaned pigs could be utilized to maintain an acidic environment in the stomach and improve feed utilization. Lawlor et al. (2005) evaluated ingredients common in early nursery swine diets and reported minerals had the highest ABC values, specifically calcium carbonate and zinc oxide (ZnO). A study by Warner et al. (2022) reported an improvement in daily weight gain and feed efficiency with no effect on feed intake as calcium carbonate percentage in the diet decreased postweaning. These data are an indication of potentially better feed utilization in diets with lower ABC; however, additional research is warranted to understand if the response was due to low ABC, changing calcium carbonate levels, or other factors. Lawlor et al. (2005) reported vegetable proteins, such as soybean-meal, were found to have a relatively high ABC when compared to cereal grains and make up a large percentage of early nursery pig diets, thus heavily influencing the ABC. Therefore, partially replacing soybean meal in the diets of nursery pigs with an ingredient low in ABC can not only reduce the instance of transient hypersensitivity but could also allow more efficient nutrient utilization from reduced ABC.
The ABC of complete diets can be predicted if each ingredient value in the diet is known. Batonon-Alvao et al. (2016) reported additivity of ABC from mixing wheat, soybean meal, and dicalcium phosphate and found calculated and analyzed values to be similar. However, it is common for early nursery diets to contain a variety of specialty ingredients, such as animal proteins and dairy products, to improve palatability and digestibility. Further investigation is warranted to determine additivity of ABC in complex early nursery diets and allow successful manipulation of ABC in complete diets to potentially improve utilization of nutrients and subsequent performance and GIT health of nursery pigs.
Therefore, the objective of this experiment was to evaluate the ABC-4 of common North American feed ingredients used in diets of nursery pigs and determine if ABC-4 values are additive when mixed to form complete diets.
MATERIALS AND METHODS
General
The experiment was conducted at the Kansas State University Swine Nutrition Laboratory in Manhattan, KS. Ingredients commonly used in early nursery diets were obtained from commercial sources.
Protocol
All ingredient samples and diets were ground to achieve a consistent particle size of approximately 400 µm using a Rancilio Rocky Doserless Grinder (Rancilio Group; Villastanza, Italy). Approximately 100 g of each sample was then placed into a sample bag and stored at –20 °C until ABC-4 was determined using a modified procedure established by Lawlor et al. (2005). Modifications include beaker size, pH meter, and ending equilibrium criteria. Half a gram sample of each ingredient and diet was weighed on a weigh boat then suspended in 50 mL of distilled de-ionized water. All samples, apart from minerals, were placed in a 100 mL beaker with a magnetic stir bar. Minerals were placed in a 250 mL beaker with a magnetic stir bar because of a large amount of acid required to lower pH. The beaker was placed on a stir plate and samples were stirred until suspended in solution. Initial pH of each sample was recorded using a Mettler Toledo SevenCompact pH/Ion S220 meter. Each day of analysis, the pH meter was calibrated using a Fisher calibrated buffer solution. Titrations were performed by the addition of 0.1 N hydrochloric acid (HCl) in increments of 0.1 to 5.0 mL depending on ingredient and stage of titration. Once a stable pH of 4.00 ± 0.04 was reached, ABC-4 was calculated as the amount of acid in milliequivalents (meq) required to achieve pH of 4 for 1 kg of sample. Samples with an initial pH less than 4 were titrated using sodium hydroxide (NaOH) to raise the pH. Sample pH equilibrium was determined once the meter indicated stabilization.
Ingredient Analysis
Ingredients were obtained from various commercial feed mills in the United States or ingredient suppliers (Table 1). Ingredients were grouped into categories: cereal grains, vegetable proteins, animal proteins and milk, vitamin premixes and minerals, amino acids (AA), and fiber sources. Each sample was analyzed three separate times to observe any variation within source and ABC-4 was calculated as the average of the triplicate analysis. This value was then used in statistical analysis. The below sources of ingredients listed are an indication of manufacturing location and may not be representative of collection location.
Table 1.
Acid-binding capacity at pH 4 (ABC-4) of ingredients common in early nursery diets1
| Item: | N 2 | Average initial pH | Average ABC-4 (meq) |
|---|---|---|---|
| Cereal grains | |||
| Corn | 5 | 6.50 | 84 ± 18.6 |
| Sorghum | 2 | 7.04 | 110 ± 14.1 |
| Red winter wheat | 2 | 6.70 | 43 ± 4.7 |
| White wheat | 2 | 6.67 | 60 ± 0.0 |
| Barley | 2 | 5.49 | 77 ± 4.7 |
| Whole oats | 3 | 6.33 | 122 ± 23.4 |
| Oat groats | 2 | 6.44 | 107 ± 37.7 |
| Dried distillers grains with solubles | 3 | 4.85 | 147 ± 43.7 |
| Cereal Blend | 1 | 6.08 | 107 |
| Vegetable protein | |||
| Soybean-meal | 6 | 6.98 | 602 ± 28.2 |
| Expelled SBM | 1 | 7.13 | 567 |
| Soy protein concentrate3 | 2 | 7.36 | 737 ± 165.0 |
| Fermented SBM4 | 3 | 4.69 | 207 ± 100 |
| Fermented soy isolate5 | 1 | 3.95 | -13 |
| Enzymatically treated SBM6 | 1 | 6.29 | 753 |
| HPDDGs7 | 2 | 4.87 | 100 ± 84.9 |
| Animal proteins and milk | |||
| Fish meal8 | 2 | 6.32 | 1,380 ± 150.9 |
| Spray-dried bovine plasma9 | 1 | 6.99 | 713 |
| Poultry meal10 | 1 | 6.74 | 1,007 |
| Spray-dried whey powder | 1 | 6.29 | 440 |
| Whey permeate | 1 | 6.16 | 520 |
| Crystalline Lactose | 1 | 7.09 | 53 |
| Vitamins and minerals | |||
| Calcium carbonate | 3 | 9.62 | 18,384 ± 769.7 |
| Zinc oxide | 3 | 9.55 | 21,863 ± 598.7 |
| Monocalcium phosphate | 1 | 4.21 | 73 |
| Dicalcium phosphate | 3 | 5.54 | 2,693 ± 848.8 |
| Calcium propionate | 1 | 9.11 | 9,240 |
| Sodium chloride | 2 | 6.00 | 15 ± 5.0 |
| Vitamin premix | 2 | 6.99 | 10,767 ± 613.8 |
| Trace mineral premix | 2 | 5.37 | 7,867 ± 264.0 |
| Vitamin trace mineral premix | 1 | 4.67 | 1,727 |
| Manganese | 1 | 8.22 | 2,347 |
| Sodium metabisulfite | 1 | 4.00 | 0 |
| Choline chloride | 1 | 5.21 | 40 |
| Amino acids | |||
| L-Lys | 2 | 5.76 | 83 ± 4.7 |
| DL-Met | 2 | 5.54 | 137 ± 4.7 |
| L-Thr | 2 | 5.43 | 160 ± 0.0 |
| L-Trp | 1 | 5.11 | 120 |
| L-Val | 1 | 5.53 | 193 |
| L-Ile | 1 | 5.76 | 200 |
| Fiber source | |||
| Beet pulp | 3 | 5.57 | 151 ± 25.3 |
Acid-binding capacity-4 (ABC-4) was determined by the amount of 0.1 N HCl required to lower the initial pH of a sample to a stable pH of 4.00 ± 0.04 (Lawlor et al., 2005). Each sample was analyzed three times and value reported is average of sample means ± standard deviation of sample means.
Number of samples analyzed, with each sample analyzed in triplicate.
Xsoy, CJ Bio, Seoul, South Korea; SoyTide, CJ Bio, Seoul, South Korea.
MEpro, Prairie AquaTech, Brookings, SD; Fermex, Purina Animal Nutrition, Shoreview, MN; Proplex T, ADM Animal Nutrition, Quincy, IL.
AX3, TripleA, Hornsyld, Denmark.
HP 300, Hamlet Protein, Findlay, OH.
NexPro, Poet, Sioux Falls, SD; Protomax, ICM, Colwich, KS.
TASA, Lima, Peru; Omega Protein, Houston TX.
APC, Ankeny, IA.
AV-E Digest, XFE Products, Des Moines, IA.
Cereal grains included: corn (IA, KS, MI, MN, PA), soft red winter wheat (IN and MI), white wheat (MI), sorghum (KS), barley (OH, PA), whole oats (KS, MI), oat groats (KS), dried distillers grains with solubles (IA, KS, MN), and cereal blend (Quincy Farm Products, Quincy, IL). Vegetable proteins included: soybean meal (IA, KS, MI, MN, PA), expelled soybean meal (OH), soy protein concentrate (Xsoy, CJ Bio, Seoul, South Korea; SoyTide, CJ Bio, Seoul, South Korea), fermented soybean meal (MEpro, Prairie AquaTech, Brookings, SD; Fermex, Purina Animal Nutrition, Shoreview, MN; Proplex T, ADM Animal Nutrition, Quincy, IL), fermented soy isolate (AX3, TripleA, Hornsyld, Denmark), enzymatically treated soybean meal (HP 300, Hamlet Protein, Findlay, OH), and high protein dried distillers grains with solubles (NexPro, Poet, Sioux Falls, SD; Protomax, ICM, Colwich, KS). Animal proteins and milk included: fish meal (TASA, Lima, Peru; Omega Protein, Houston, TX), spray-dried bovine plasma (APC, Ankeny, IA), poultry meal (AV-E Digest, XFE Products, Des Moines, IA) spray-dried whey powder (KS), whey permeate (KS), and crystalline lactose (MN). Amino acids included: L-Lys (Ajinomoto, Eddyville, IA), DL-Met (Adisseo, Alpharetta, GA), L-Thr (Ajinomoto, Eddyville, IA), L-Trp (Ajinomoto, Eddyville, IA), L-Val (Ajinomoto, Eddyville, IA), L-Ile (Ajinomoto, Eddyville, IA). Vitamin premixes and minerals included: calcium carbonate (IA, KS, MI), ZnO (Zinc Nacional, Monterrey, Mexico), dicalcium phosphate (PCS, Northbrook, IL; MN), monocalcium phosphate (PCS, Northbrook, IL), calcium propionate (NutraBlend, Neosho, MO), vitamin premix (Swine Nutrition Guide, Kansas State University, Manhattan, KS; manufacturer: DSM, Exton, PA), trace mineral premix (Swine Nutrition Guide, Kansas State University, Manhattan, KS; manufacturer: Nutra Blend, Neosho, MO), a combined vitamin and trace mineral premix (Hubbard Feeds, Mankato, MN), manganese (MN), sodium chloride (KS), sodium metabisulfite (MI), and choline chloride (KS). Fiber source included: beet pulp shreds (KS, MI).
Early Nursery Diets
Two different early nursery diets were formulated to determine if ABC-4 values for ingredients are additive by comparing calculated ABC-4 with analyzed values. All diets were mixed in the Kansas State Applied Swine Nutrition Laboratory (Manhattan, KS). Ingredients used to formulate diets were from the same samples used in the ingredient analysis to reduce any variation in ABC from ingredient source. The same procedures as individual ingredients were used to determine analyzed ABC-4 of complete feeds.
The first series of diets analyzed were phase 1 nursery diets intended for pigs weighing 5 to 7 kg. A total of 10 diets were arranged in a 2 × 5 factorial with increasing levels of calcium carbonate with or without the inclusion of 3,000 mg/kg of Zn from ZnO (Table 2). Calcium carbonate and ZnO diets were selected because of their high ABC-4. Diets were formulated to contain 0, 0.45, 0.90, 1.35, and 1.80% calcium carbonate at the expense of corn. Diets without ZnO contained 110 mg/kg of Zn from the trace-mineral premix.
Table 2.
Calcium carbonate titration with or without ZnO diet composition1
| No ZnO | Added ZnO | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | CaCO3: | 0% | 0.45% | 0.90% | 1.35% | 1.80% | 0% | 0.45% | 0.90% | 1.35% | 1.80% |
| Corn | 52.50 | 52.05 | 51.60 | 51.15 | 50.70 | 52.10 | 51.65 | 51.20 | 50.75 | 50.30 | |
| Soybean meal | 15.60 | 15.60 | 15.60 | 15.60 | 15.60 | 15.60 | 15.60 | 15.60 | 15.60 | 15.60 | |
| Whey powder | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | 20.00 | |
| Enzymatically treated SBM | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | |
| Fish meal | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | |
| Bovine plasma | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | |
| Calcium carbonate | --- | 0.45 | 0.90 | 1.35 | 1.80 | --- | 0.45 | 0.90 | 1.35 | 1.80 | |
| Monocalcium phosphate | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | |
| L-Lys-HCl | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | |
| DL-Met | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 | |
| L-Thr | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | |
| L-Trp | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | |
| L-Val | 0.09 | 0.09 | 0.09 | 0.09 | 0.09 | 0.09 | 0.09 | 0.09 | 0.09 | 0.09 | |
| Vitamin premix with phytase2 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | |
| Trace mineral premix | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | |
| Zinc oxide | --- | --- | --- | --- | --- | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Calculated ABC-4, meq3 | 316 | 398 | 481 | 563 | 645 | 403 | 486 | 568 | 650 | 733 | |
A total of 10 diets were formulated to contain increasing levels of calcium carbonate with or without 3,000 ppm of Zn from ZnO. Diets without ZnO contained 110 ppm of Zn from the trace mineral premix. Calcium carbonate was added to the diet at the expense of corn. Diets were formulated for pigs weighing 5.0 to 7.0 kg of BW.
Ronozyme HiPhos 2700 (DSM, Parsippany, NJ) provided an estimated release of 0.13% STTD P with 750 FTU/kg.
Individual sample acid-binding capacity-4 (ABC-4) values were used for calculations and not ingredient mean values (Lawlor et al., 2005).
The second series of diets analyzed were phase 2 nursery diets intended for pigs weighing 7 to 12 kg. A total of three diets were formed with different levels of soybean meal, feed-grade AA, and specialty soy proteins products (Table 3). The two soy products included fermented soybean meal (MEpro, Prairie AquaTech, Brookings, SD) and enzymatically treated soybean meal (HP 300, Hamlet Protein, Findlay, OH) because of their low and high ABC-4, respectively. The first diet consisted of low soybean meal and high feed-grade AA with 5% fermented soybean meal. The second diet consisted of low soybean meal and high feed-grade AA with 5% enzymatically treated soybean meal. The third diet consisted of high soybean meal and low feed-grade AA with 5% enzymatically treated soybean meal. These diets were selected because of the large difference in ABC-4 for different protein sources.
Table 3.
Soybean meal and specialty protein diets1
| Item | Diet: | Low SBM, Fermented SBM | Low SBM, Enzymatically treated SBM | High SBM, Enzymatically treated SBM |
|---|---|---|---|---|
| Corn | 60.69 | 58.48 | 52.43 | |
| Soybean meal | 20.42 | 22.78 | 29.49 | |
| Whey powder | 10.00 | 10.00 | 10.00 | |
| Fermented SBM2 | 5.00 | --- | --- | |
| Enzymatically treated SBM3 | --- | 5.00 | 5.00 | |
| Calcium carbonate | 0.75 | 0.80 | 0.78 | |
| Monocalcium phosphate | 1.05 | 0.90 | 0.80 | |
| Salt | 0.58 | 0.55 | 0.55 | |
| L-Lys-HCl | 0.51 | 0.51 | 0.30 | |
| DL-Met | 0.21 | 0.20 | 0.15 | |
| L-Thr | 0.20 | 0.20 | 0.10 | |
| L-Trp | 0.04 | 0.03 | --- | |
| L-Val | 0.15 | 0.15 | --- | |
| Vitamin premix with phytase4 | 0.25 | 0.25 | 0.25 | |
| Trace mineral premix | 0.15 | 0.15 | 0.15 | |
| Total | 100 | 100 | 100 | |
| Calculated ABC-4, meq5 | 352 | 408 | 443 | |
A total of three diets were formulated to contain different levels of soybean meal and synthetic amino acids. Two different soy products were included in the diet based on their analyzed ABC-4 values. The diets were formulated for pigs weighing 7.0 to 12.0 kg of BW.
MEpro, Prairie AquaTech, Brookings, SD.
HP 300, Hamlet Protein, Findlay, OH.
Ronozyme HiPhos 2700 (DSM, Parsippany, NJ) provided an estimated release of 0.13% STTD P with 1,250 FTU/kg.
Individual sample acid-binding capacity-4 (ABC-4) values were used for calculations and not ingredient mean values (Lawlor et al., 2005).
Statistical Analysis
All data analysis was performed using the RStudio environment (Version 1.3.1093, RStudio, Inc., Boston, MA) using R programming language [Version 4.0.2 (2020-06-22), R Core Team, R Foundation for Statistical Computing, Vienna, Austria]. For ingredient analysis, descriptive statistics were generated using the summary function with value reported as average of sample means ± standard deviation of sample means. For calcium carbonate and ZnO diet analysis, data were analyzed as a completely randomized design using the lm function with difference between calculated and analyzed ABC-4 as the experimental unit. The main effects of ZnO and calcium carbonate, as well as their interactions, were tested. For soybean meal and specialty protein analysis, data were analyzed as a completely randomized design using the lm function with difference between calculated and analyzed ABC-4 as the experimental unit. The main effects of diet were tested. For testing of all hypotheses, differences were considered significant at P ≤ 0.05 and marginally significant at 0.05 < P ≤ 0.10.
RESULTS
Ingredient Analysis
The mean ABC-4 values of each ingredient are reported as the average of sample means ± standard deviation of sample means (Table 1). Cereal grains with respect to other ingredients were found to have a low ABC-4. Corn was found to have an ABC-4 of 84 ± 18.6 meq, with barley having a similar value to corn. Dried distillers grains with solubles (DDGS), cereal blend, oat products, and sorghum were among the highest of ABC-4 within cereal grains and wheat products were found to have the lowest values.
Vegetable proteins were among two ingredient categories with the most variation between ingredients and sources. Soybean meal was found to have an ABC-4 of 602 ± 28.2 meq. Other soy products had a wide range of ABC-4 values. Enzymatically treated soybean meal and soy protein concentrate had higher ABC-4 than soybean meal, whereas fermented soybean meal had a lower value. However, there was a great variation between soy protein concentrate and fermented soybean meal with standard deviations of 165.0 and 100.0 meq, respectively. Fermented soy isolate had an ABC-4 of -13 meq that had to be titrated with NaOH. Finally, high protein dried distillers grains (HPDDGs) had a low ABC-4 relative to other vegetable proteins.
Animal proteins and milk collectively, with the exception of crystalline lactose, were higher in ABC-4 than vegetable proteins. Fish meal had the highest ABC-4 of 1,380 ± 150.9 meq most likely due to its high Ca content. Spray-dried bovine plasma, poultry meal, spray-dried whey powder, and whey permeate had lower values than fish meal, but still relatively high values compared to other ingredients tested. Crystalline lactose had the lowest ABC-4 of all animal proteins and milk with a value of 53 meq.
Vitamin premixes and minerals had the highest ABC-4 between categories and had the most variation, as expressed by the standard deviations. Calcium carbonate and ZnO had the highest ABC-4 of 18,384 ± 769.7 and 21,863 ± 598.7 meq, respectively. These were also the two ingredients with the highest ABC-4 overall. The vitamin premix, trace-mineral premix, vitamin trace mineral premix, manganese, calcium propionate, and dicalcium phosphate also had high ABC-4, but did not reach the level of calcium carbonate and ZnO. Monocalcium phosphate, sodium chloride, choline chloride, and sodium metabisulfite had low values among vitamin premixes and minerals.
Amino acids were more consistent compared to other ingredient categories. All feed-grade AA analyzed had an ABC-4 between 83 and 200 meq, with L-Lys and L-Ile having the lowest and highest value, respectively.
The fiber source tested was another ingredient low in ABC-4 compared to the other categories. Beet pulp shreds were found to have an ABC-4 of 151 ± 25.3 meq.
Complete Diets
Calcium carbonate and ZnO.
Calculated and analyzed ABC-4 increased with the amount of calcium carbonate and ZnO added in the diet. The difference between calculated and analyzed ABC-4 was used to test ZnO and limestone’s additivity when mixed with other ingredients to form complete diets.
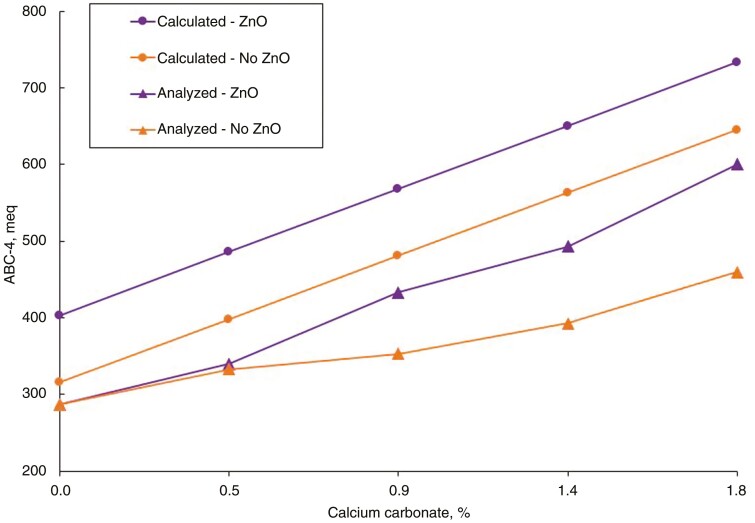
There was a ZnO × calcium carbonate interaction observed (P = 0.015) for difference between calculated and analyzed ABC-4 (Table 4; Figure 1). Within the interaction, the difference between calculated and analyzed ABC-4 increased (linear, P < 0.001) as calcium carbonate levels increased in diets without ZnO. The difference between analyzed and calculated ABC-4 at 0% calcium carbonate was 29 meq without ZnO. This difference increased along with the addition of calcium carbonate with the largest difference of 185 meq observed at 1.80% calcium carbonate. However, the difference between calculated and analyzed ABC-4 did not significantly change (P > 0.05) as calcium carbonate increased in the diet when ZnO was present. The lowest and highest differences were observed at 0% and 1.35% calcium carbonate with the difference between analyzed and calculated ABC-4 being 116 and 157 meq, respectively.
Table 4.
Evaluation of calculated vs. analyzed acid-binding capacity-4 on increasing calcium carbonate with or without the presence of ZnO1
| No ZnO | Added ZnO | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Calcium carbonate: | 0% | 0.45% | 0.90% | 1.35% | 1.80% | 0% | 0.45% | 0.90% | 1.35% | 1.80% |
| Calculated ABC-4 (meq) | 316 | 398 | 481 | 563 | 645 | 403 | 486 | 568 | 650 | 733 |
| Analyzed ABC-4 (meq)2 | 287 ± 11.5 | 333 ± 23.1 | 353 ± 23.1 | 393 ± 11.5 | 460 ± 20.0 | 287 ± 11.5 | 340 ± 20.0 | 433 ± 11.5 | 493 ± 11.5 | 600 ± 20.0 |
| Difference3 | 29c | 65c | 128b | 170a | 185a | 116b | 146ab | 135b | 157ab | 133b |
A total of 10 diets were analyzed in a 2 × 5 factorial design with increasing levels of calcium carbonate at the expense of corn with or without 3,000 ppm of Zn from ZnO. Each diet was sampled and analyzed three times.
Analyzed acid-binding capacity-4 (ABC-4) values are reported as the diet average from three samples ± standard deviation within diet (Lawlor et al., 2005).
ZnO × calcium carbonate interaction (P = 0.015). Dose effect of calcium carbonate when ZnO not included at pharmacological levels, linear P < 0.001. Dose effect of calcium carbonate when ZnO included at pharmacological levels, linear P = 0.175. Main effect of calcium carbonate (linear, P < 0.001). Main effect of ZnO (P = 0.009). SEM = 9.9.
Figure 1.
Line graph of calculated vs. analyzed acid-binding capacity-4 (ABC-4) on increasing calcium carbonate with or without the presence of ZnO.
Soybean meal and specialty protein.
The inclusion of enzymatically treated soybean meal and high levels of soybean meal increased the calculated and analyzed ABC-4 of complete diets compared to a diet with fermented soybean meal and low levels of soybean meal (Table 5). Similar to the first series of diets analyzed, all diets had lower analyzed values than calculated values. All three diets had similar differences between calculated and analyzed ABC-4 values. As a result, the differences between calculated and analyzed ABC-4 of all three diets was not significant (P = 0.640). The average difference between calculated and analyzed ABC-4 was 107 meq.
Table 5.
Evaluation of calculated vs. analyzed acid-binding capacity-4 of diets formulated with different protein sources and levels of soybean meal1
| Item | Low SBM, fermented SBM2 | Low SBM, enzymatically treated SBM3 | High SBM, enzymatically treated SBM3 | SEM | P = |
|---|---|---|---|---|---|
| Calculated ABC-4 (meq) | 403 | 457 | 487 | --- | --- |
| Analyzed ABC-4 (meq)4 | 300 ± 20.0 | 353 ± 11.5 | 373 ± 11.5 | --- | --- |
| Difference | 103 | 104 | 114 | 8.6 | 0.637 |
A total of three diets were analyzed three times with different levels of soybean meal and synthetic amino acids. Two different soy products were used based on their analyzed acid-binding capacity-4 (ABC-4; Lawlor et al., 2005). Each diet was sampled and analyzed three times.
MEpro; Prairie Aquatech; Brookings, SD.
HP 300; Hamlet Protein; Findlay, OH.
Analyzed ABC-4values are reported as the diet average from three titration ± standard deviation within diet.
DISCUSSION
Early nursery diets typically contain a variety of ingredients to improve palatability and digestibility. Ingredients that have a high ABC-4 will bind more acid in the stomach of the newly weaned pig (Lawlor et al., 2005). The binding of acid in the stomach of newly weaned pigs can raise the stomach pH which is detrimental to the overall performance and health (Bouldan et al., 1988; Yen, 2001). A high gastric pH inhibits protein digestion and is associated with proliferation of harmful micro-organisms that can result in severe cases of postweaning diarrhea leading to diminished performance or death (Kidder and Manners, 1978; Longland, 1991; Partanen and Mroz 1999).
The present study aimed to determine the ABC-4 of common early nursery ingredients utilized in North America. Buffering capacity is also a way to measure an ingredient’s ability to resist a change in pH. Buffering capacity is calculated by diving the ABC-4 by the total change in pH, therefore buffering capacity can also be calculated from the values reported in Table 1. This study also aimed to determine additivity of ingredient values for successful diet formulation regarding ABC-4. Overall, analyzed ingredient ABC-4 were generally similar to values reported by Lawlor et al. (2005) with the differences present most likely due to processing procedures and geographical location. Specifically, plant-based ingredient’s ABC-4 can be influenced by season, soil fertility, rate of fertilization, and stage of maturity which affects their ion concentration (Jasaitis et al., 1987).
In addition to ABC-4, Lawlor et al. (2005) also measured acid-binding capacity at a pH of 3. Acid-binding capacity-3 and ABC-4 follow the same procedure previously described with the difference being an ending pH of 3 and 4, respectively. However, the current study focuses on ABC-4 because a pH of 4 is considered to be the limit at which protein digestion begins to decrease and pathogenic organisms proliferate in the GIT of pigs (Prohaszka and Baron, 1980; Ravindran and Kornegay, 1993).
Calcium carbonate and ZnO had the highest ABC-4 of all ingredients analyzed. Lawlor et al. (2005) also reported high ABC-4 for calcium carbonate and ZnO, but the authors values were approximately 5,452 and 5,542 meq lower than the current study, respectively. Zinc oxide’s high ABC-4 can raise a complete diet’s value even though it is included at low percentages. Although a high ABC-4 is thought to be detrimental to the newly weaned pigs, it is likely the beneficial antimicrobial properties of ZnO outweigh the potential negative effects of increased ABC-4 of the diet. Zinc ions prevent attachment and translocation of pathogenic bacteria, but there are growing concerns of its use at pharmacological levels (Huang et al., 1999; Starke et al., 2014). Low ABC-4 diets may improve gut health of pigs to provide some of the benefit of Zn at pharmacological levels. Calcium carbonate had the second highest ABC-4 of all ingredients analyzed. Bouldan et al. (1988) suggested limiting the calcium (Ca) content in the diets of early nursery pigs to improve health but was unsure of the growth response to the limited mineral content. However, Warner et al. (2022) reported low calcium carbonate levels in the diets of early nursery pigs improved gain with no effect on intake indication better feed utilization. Lawlor et al. (2006) reported the addition of fumaric acid and reduction of minerals to lower the ABC-4 of diets was able to improve nursery pig performance in the first two-weeks post-weaning predominantly when diarrhea was present. The results of these studies suggest low ABC-4 diets can improve nutrient digestion and inhibit pathogenic organisms in early nursery pigs.
Soybean meal is generally used as the main protein source in diets for pigs in North America. Similar to the current study, Lawlor et al. (2005) reported soybean meal to have an ABC-4 of 642 ± 51.1 meq and Batonon-Alavo et al. (2016) reported a value of 610 ± 6.8 meq. Soybean meal was found to have a high ABC-4 relative to corn and these are the two main ingredients in many swine diets in the United States. Therefore, the diets containing higher soybean meal levels will have higher ABC-4 values. Soybean meal can also vary in concentrations of Ca depending on the source (Huang et al., 2017). The ABC-4 of the diet may be affected more so if the soybean meal source is high in Ca. The inclusion of feed-grade amino acids can lower the soybean meal inclusion and thus decrease ABC-4 of the diet. Amino acid values for the current study were all less than the reported values from Lawlor et al. (2005), but only by an average of 53 meq for Lys, Met, Thr, and Trp. Specialty protein sources can also be utilized to partially replace soybean meal and decrease ABC-4. However, specialty protein products have varying ABC-4 values. Soy protein concentrate and enzymatically treated soybean meal had higher ABC-4 than conventional soybean meal, whereas fermented soybean meal had lower values. Based on vegetable protein analysis, it appears fermentation of soybean meal reduces ABC-4. The sample of fermented soy isolate was found to have a negative ABC-4. This product not only has a low ABC-4 from the fermentation process but is also acid washed further reducing its value. Therefore, a specialty protein source that has a low ABC-4, such as fermented soybean meal or fermented soy isolate, could be utilized to decrease the ABC-4 of the diet.
All cereal grains in the current study had ABC-4 values similar to the values reported by Lawlor et al. (2005), although the mean ABC-4 with the exception of oat products were slightly lower in the current study. Cereal grains, such as corn, were lower in ABC-4 compared to other ingredients analyzed. Wheat was found to have the lowest ABC-4 of all cereal grains. Wheat-based diets can lower ABC-4 from 40 to 150 meq depending on its dietary inclusion compared to corn-based diets.
Animal proteins and milk had higher ABC-4 compared to other ingredients with the exception of crystalline lactose. The high ABC-4 is most likely related to the Ca and protein contents in these ingredients (Jasaitis et al., 1987; Bouldan et al., 1988; Lawlor et al., 2005). Even though these ingredients would appear to raise ABC-4, their effects on the diet ABC-4 are not as great because of the ingredients they often replace. Inclusion of fish meal in the diet reduces the need for calcium carbonate and soybean meal which also contribute to ABC-4 (Rojas and Stein, 2012). However, fish meal was an ingredient with a high standard deviation for ABC-4. Fish meal from the current study had a much higher ABC-4 than the value reported by Lawlor et al. (2005) of 738 ± 219.3 meq. As a result, the source and composition of fish meal should be considered when evaluating ABC-4 of a diet. Spray-dried bovine plasma, whey powder, and whey permeate are highly digestible protein sources for young pigs and their ABC-4 are similar to soybean meal (Kim et al., 2012; Rojas and Stein, 2012). Therefore, the ingredients will not raise, or lower ABC-4 drastically compared to using soybean meal. However, using crystalline lactose as opposed to whey powder or whey permeate as the lactose source could lower the overall diet ABC-4.
Phosphorus is important for energy metabolism, nucleic acid synthesis, bone formation, and mineralization of early nursery pigs (Berndt and Kumar, 2009). Dicalcium phosphate contains one anion per molecule whereas monocalcium phosphate contains two (Lima et al., 1991). As a result, monocalcium phosphate contains more negative charges that lower its ABC-4 relative to dicalcium phosphate. Therefore, depending on the P source used, ABC-4 can change. Dicalcium phosphate was found to have a much higher ABC-4 than monocalcium phosphate as expected. Utilizing monocalcium phosphate as the P source can reduce the ABC-4 in the diet.
Premixes are added to swine diets to ensure the pig is receiving adequate vitamin and mineral levels. Trace mineral premixes had a higher ABC-4 relative to other ingredients because of the cations that are associated with the minerals present. Vitamin premixes analyzed had an ABC-4 even greater than the trace mineral premixes. The vitamins present in a vitamin premix do not significantly contribute to its ABC-4, compared with the carrier, namely calcium carbonate. Therefore, ABC-4 of vitamin premixes can change depending on the source and level of carrier used.
Feed additives often represent a small percentage of the total diet; however, their composition is important to understand how it will affect the ABC-4 of the diet. Feed additives that contain high amounts of minerals, and more specifically Ca, can increase the diet ABC-4 even at small inclusions. Therefore, feed additives should not be overlooked when formulating a diet to a low ABC-4 to potentially improve weaned pig performance.
In both diet sets analyzed, the analyzed ABC-4 was less than the calculated values using the ingredient analysis. Therefore, it cannot be concluded that ingredients are perfectly additive in complete diets. It is unclear why analyzed values do not match calculated values, but the inclusion of calcium carbonate and presence of ZnO appear to contribute to this discrepancy. Calcium carbonate’s ABC-4 contribution in complete diets may be overestimated when using individual ingredient values. Further investigation revealed once calcium carbonate is less than 2% of a diet, its ABC-4 contribution is decreased. The results indicate an ABC-4 of about 8,000 meq would appear to be more appropriate for calcium carbonate when ZnO is not present in the diets to have calculated values align more closely with analyzed values. However, this assumes that all other ingredient ABC-4 values in the diet are perfectly additive. The variation of ABC-4 in ingredients and additivity of other ingredients used should be considered as well. Complete diet analysis also indicates ZnO is not directly additive in complete diets and its contribution to ABC-4 appears to depend on the calcium carbonate level. However, to our knowledge there is no mechanism between ZnO and calcium carbonate that would drive this interaction. It is also possible that these ingredients are interacting with an additional ingredient or ingredients within the diet resulting in the discrepancy. Therefore, further investigation is warranted to understand additivity of calcium carbonate and ZnO and any potential interactions that may be occurring to influence ABC-4.
Acid-binding capacity-4 in complete diets were able to be successfully manipulated by including different levels of soybean meal, feed-grade AA, and specialty protein sources. When soybean meal was high in the diet, the ABC-4 increased. The inclusion of enzymatically treated soybean meal also increased ABC-4 compared to diets containing fermented soybean meal. However, analyzed ABC-4 were all lower than calculated values, similar to the results of the calcium carbonate and ZnO diets. As previously stated, the reason for the discrepancy between calculated and analyzed ABC-4 requires further investigation.
In conclusion, complete additivity of ingredients can not be concluded and warrants further investigation. Reduced ABC-4 of the diet can still be successfully manipulated through careful selection of ingredients. Feeding strategies such as low calcium carbonate levels, low soybean meal inclusion (high feed-grade AA), and use of low ABC-4 ingredients can be used to target a low ABC-4 in complete diets. Targeting a low ABC-4 in the diets of nursery pigs immediately post-weaning may alleviate problems in the early nursery stage such as poor growth, impaired GIT health, and mortality. However, further investigation is warranted to determine the optimal ABC-4 of early nursery pigs of a specific weight range to optimize performance.
ACKNOWLEDGMENTS
Contribution no. 22-288-J from the Kansas Agricultural Experiment Station, Manhattan, KS, 66506.
Conflict of interest statement. The authors declare no conflict of interest.
Contributor Information
Ethan B Stas, Department of Animal Sciences and Industry, Kansas State University, Manhattan, KS 66506-0201, USA.
Mike D Tokach, Department of Animal Sciences and Industry, Kansas State University, Manhattan, KS 66506-0201, USA.
Joel M DeRouchey, Department of Animal Sciences and Industry, Kansas State University, Manhattan, KS 66506-0201, USA.
Robert D Goodband, Department of Animal Sciences and Industry, Kansas State University, Manhattan, KS 66506-0201, USA.
Jason C Woodworth, Department of Animal Sciences and Industry, Kansas State University, Manhattan, KS 66506-0201, USA.
Jordan T Gebhardt, Department of Diagnostic Medicine/Pathobiology, College of Veterinary Medicine, Kansas State University, Manhattan, KS 66506-0201, USA.
LITERATURE CITED
- Batonon-Alavo, D. I., Bouza B., Cholet J. C. G., and Mercier Y.. . 2016. A method for determination of the acidifying value of organic acids used in pigs diets in the acid binding capacity at pH 4 (ABC-4) system. Anim. Feed Sci. 216:197–203. doi: 10.1016/j.anifeedsci.2016.03.021 [DOI] [Google Scholar]
- Berndt, T., and Kumar R.. . 2009. Novel mechanisms in the regulation of phosphorus homeostasis. Physiology (Bethesda) 24:17–25. doi: 10.1152/physiol.00034.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduan, G., Jung H., Schneider R., Block J., and Klenke B.. . 1988. Influence of fumaric-acid and propandiol-formiat on piglets. J. Anim. Physiol. Anim. Nutr. 59:72–78. doi: 10.111/j.1439-0396.1988.tb00057.x [DOI] [Google Scholar]
- Cranwell, P. D., Noakes D. E., and Hill K. J.. . 1968. Observations on the stomach content of the suckling pig. Proc. Nutr. Soc. 27:2–6A. [Google Scholar]
- Huang, S. X., McFall M., Cegielski A. C., and Kirkwood R. N.. . 1999. Effect of dietary zinc supplementation on Escherichia coli septicemia in weaned pigs. J. Swine Health Prod. 7:109–111. [Google Scholar]
- Huang, C. F., Stein H. H., Zhang L. Y., Defa L., and Lai C. H.. . 2017. Concentrations of minerals in pig feed ingredients commonly used in China. Transl. Anim. Sci 2017:126–136. doi: 10.2527/tas2017.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasaitis, D. K., Wohlt J. E., and Evans J. L.. . 1987. Influence of feed-ion content on buffering capacity of ruminant feedstuffs in vitro. J. Dairy Sci. 70:1391–1403. doi: 10.3168/jds.S0022-0302(87)80161-3 [DOI] [Google Scholar]
- Kidder, D. E., and Manners M. J.. . 1978. Digestion in the pig. Bristol: Scientechnica. [Google Scholar]
- Kim, B. G., Lee J. W., and Stein H. H.. . 2012. Energy concentration and phosphorus digestibility in whey powder, whey permeate, and low-ash whey permeate fed to weanling pigs. J. Anim. Sci. 90:289–295. doi: 10.2527/jas.2011-4145 [DOI] [PubMed] [Google Scholar]
- Lawlor, P. G., Lynch P. B., and Caffrey P. J.. . 2006. Effect of fumaric acid, calcium formate, and mineral levels in diets on the intake and growth performance of newly weaned pigs. Ir. J. Agric. Food Res. 45:61–71. [Google Scholar]
- Lawlor, P. G., Lynch P. B., Caffrey P. J., O’Reilly J. J., and O’Connell M. K.. . 2005. Measurements of the acid-binding capacity of ingredients used in pig diets. Ir. Vet. J 58:447–452. doi: 10.1186/2046-0481-58-8-447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima, F. R., Mendonҫa C. X., Alvarez J. C., Ratti G., Lenharo S. L. R., Kahn H., and Garzillo J. M. F.. . 1991. Chemical and physical evaluation of commercial dicalcium phosphates as sources of phosphorus in animal nutrition. Poult. Sci. 74:1659–1670. doi: 10.3382/ps.0741659 [DOI] [PubMed] [Google Scholar]
- Longland, A. C. 1991. Digestive enzyme activities in pigs and poultry. In: Fuller M. F., editor. In vitro digestion for pigs and poultry. Wallingford (UK): CAB International; p. 3–18. [Google Scholar]
- McGlone, J. J., and Johnson A. K.. . 2003. Welfare of the neonatal pig. In: Perspectives in pig science. Nottingham (UK): Nottingham University Press; p. 169–197. [Google Scholar]
- Partanen, K. H., and Mroz Z.. . 1999. Organic acids for performance enhancement in pig diets. Nutr. Res. Rev. 12:117–145. doi: 10.1079/095442299108728884 [DOI] [PubMed] [Google Scholar]
- Pearlin, B. V., Muthuvel S., Govidasamy P., Villavan M., Alagawany M., Farag M. R., Dhama K., and Gopi M.. . 2020. Role of acidifiers in livestock nutrition and health: A review. J. Anim. Physiol. Anim. Nutr 104:558–569. doi: 10.3390/ani10060952 [DOI] [PubMed] [Google Scholar]
- Pluske, J. R. 2016. Invited review: Aspects of gastrointestinal tract growth and maturation in the pre- and postweaning period of pigs. J. Anim. Sci. 94:399–411. doi: 10.2527/jas2015-9767 [DOI] [Google Scholar]
- Pluske, J. R., Kerton D. J., Cranwell P. D., Campbell R. G., Mullan B. P., King R. H., Power G. N., Pierzynowski S. G., Westrom B., Rippe C., . et al. 2003. Age, sex, and weight at weaning influence organ weight and gastrointestinal development of weanling pigs. Aust. J. Agric. Res. 54:515–527. doi: 10.1071/AR02156 [DOI] [Google Scholar]
- Prohaszka, L., and Baron F.. . 1980. The predisposing role of high dietary protein supplies in enteropathogenic E. coli infections of weaned pigs. Zent. Vet. B. 27:222–232. doi: 10.1111/j.1439-0450.1980.tb01908x [DOI] [PubMed] [Google Scholar]
- Ravindran, V., and Kornegay E. T.. . 1993. Acidification of weaner pig diets: a review. J. Sci. Food Agric. 62:313–322. doi: 10.1002/jsfa.2740620402 [DOI] [Google Scholar]
- Rojas, O. J., and Stein H. H.. . 2012. Nutritional value of animal proteins fed to pigs.University of Illinois, Urbana. [accessed February 1, 2022]. nutrition.ansci.illinois.edu/sites/default/files/2012ProcMidwestSwineNutrConf.9-24.pdf [Google Scholar]
- Starke, I. C., Pieper R., Neumann K., Zentek J., and Vahjen W.. . 2014. The impact of high dietary zinc oxide on the development of the intestinal microbiota in weaned pigs. FEMS Microbiol. Ecol. 87:416–427. doi: 10.1111/1574-6941.12233 [DOI] [PubMed] [Google Scholar]
- Warner, A. J., DeRouchey J. M., Tokach M. D., Woodworth J. C., Goodband R. D., and Gebhardt J. T.. . 2022. Effects of calcium carbonate level on weanling pig growth performance and fecal dry matter. J. Anim. Sci. 100:62. doi: 10.1093/jas/skac064.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen, J. T. 2001. Anatomy of the digestive system and nutritional physiology, In: Lewis, A. J., Southern, L. L., editors. Swine nutrition. Boca Raton (FL): CRC Press; p. 21–64. [Google Scholar]
- Zijlstra, R. T., Whang K. Y., Easter R. A., and Odle J.. . 1996. Effect of feeding a milk replacer to early weaned pigs on growth, body composition, and small intestinal morphology, compared with suckled littermates. J. Anim. Sci. 74:2948–2959. doi: 10.2527/1996.74122948x [DOI] [PubMed] [Google Scholar]