Abstract
The PI3K-Akt-mechanistic (formerly mammalian) target of the rapamycin (mTOR) signaling pathway is important in a variety of biological activities, including cellular proliferation, survival, metabolism, autophagy, and immunity. Abnormal PI3K-Akt-mTOR signalling activation can promote transformation by creating a cellular environment conducive to it. Deregulation of such a system in terms of genetic mutations and amplification has been related to several human cancers. Consequently, mTOR has been recognized as a key target for the treatment of cancer, especially for treating cancers with elevated mTOR signaling due to genetic or metabolic disorders. In vitro and in vivo, rapamycin which is an immunosuppressant agent actively suppresses the activity of mTOR and reduces cancer cell growth. As a result, various sirolimus-derived compounds have now been established as therapies for cancer, and now these medications are being investigated in clinical studies. In this updated review, we discuss the usage of sirolimus-derived compounds and other drugs in several preclinical or clinical studies as well as explain some of the challenges involved in targeting mTOR for treating various human cancers.
Keywords: Cancer, Rapamycin, mTOR pathway, mTORC1, mTORC2, mTOR inhibitors, Targeted therapy
Introduction
Cancer refers to abnormal cell growth, which often proliferates uncontrollably and is likely to metastasize and invade neighbouring cells or tissues [1, 91, 93]. A variety of factors are associated with cancer development, including DNA mutation, accumulation of cellular stress, genetic predisposition, abnormal cellular metabolism and signalling, infections, environmental pollution, and an unhealthy lifestyle [5, 41, 68, 78, 94, 104]. Inherited genetic defects, for instance, mutations in certain tumor suppressor genes can increase the risk of cancer development [9]. Some of the inheritably received genetic flaws (such as mutations in BRCA1 or BRCA2) and infectious diseases may raise the risks of cancer. Environmental pollution, irradiation or poor lifestyle, for example, smoking can enhance DNA damage and thus can lead to cancer [4, 49, 51, 94, 104]. Damaged DNA can be repaired by cellular DNA repair machinery, and in case of severe DNA insult, if the repair mechanism fails, cells are led to death by apoptosis [24, 29, 47, 92]. When the damaged cells evade the DNA repair mechanisms and apoptosis, they grow in an uncontrolled manner and become cancerous [45, 52, 104, 123].
The PI3K (phosphatidylinositol 3 kinase) signaling pathway has a very important role in carcinogenesis [117]. Activating mutations in the PIK3CA (phosphatidylinositol-4,5-bisphosphonate 3-kinase, catalytic subunit alpha polypeptide) gene—through the PI3K/AKT/mTOR signaling pathway—induce the synthesis of cyclooxygenase 2, which in turn establishes the formation of prostaglandins. Cyclooxygenase 2 and prostaglandin E2 have a strong angiogenic, antiapoptotic effect favoring the growth and survival of tumor cells [30]. PIK3CA mutations are detected in approximately 40% of estrogen receptor (HR+) and epidermal growth factor receptor 2 (HER2-) breast cancers [6]. This mutation induces excessive activation of the alpha isoform of the enzyme PI3K (phosphatidyl inositol 3 kinases) [28]. This is part of an intracellular signaling pathway involved in the development of tumors and the emergence of resistance to oncological treatments. This PIK3CA mutation found in patients with breast cancer has a much lower response to tyrosine kinase inhibitors, such as lapatinib and trastuzumab. Also, the mutation has a predictive value on the response to adjuvant hormone therapy [28].
A large body of studies has reported that dysregulations in PI3K/mTOR are associated with the development of various types of cancer in humans [16, 17, 57, 83, 98, 109]. Because of the strong association in cancer, studies are being carried out to develop the inhibitors of PI3K/mTOR to treat different types of cancer. The mTOR (mechanistic/mammalian target of rapamycin) pathway was first discovered in late 1970 after the isolation of the mTOR inhibitor, rapamycin [42, 77, 107]. The mTOR inhibitors are a family of compounds that are being used for treating several human diseases such as cancer, autoimmune diseases and neurodegeneration. mTOR is a threonine/serine kinase which belongs to the family of phosphoinositide 3-kinase-related kinase (PI3K). Dysregulation of mTOR signaling has been reported to be associated not only with cancer but also with autoimmune disease, obesity, neurodegeneration, infectious diseases, and ageing [26, 83, 98, 109]. Arresting mTOR signaling with specific inhibitors, for instance, rapamycin and rapalogues are being studied extensively in both clinical and preclinical settings for better treatment of these different diseases.
Recent phase II clinical studies with rapamycin for the treatment of multiple sclerosis have revealed promising outcomes [12]. To limit the potential undesired side effects of current mTOR inhibitors, it is important to identify more potent novel targets. ATP competitive inhibitors of mTOR, for example, OSI-027 and its analogues are promising anticancer drugs [74]. Furthermore, recently revealed crystal structures of the mTOR complex would provide new insights for the advancement of more powerful and efficient mTOR inhibitors in future. The clear-cut efficacy of rapamycin and rapalogues in multiple therapeutic settings has propelled interest to discover new types of inhibitors that may be more potent and eventually with fewer side effects than rapamycin and rapalogues that include ATP competitive mTOR inhibitors. The current review summarizes the use of sirolimus and its derivatives and addresses potential limitations in targeting mTOR signaling for the treatment of cancer.
Methodology
This comprehensive and up-to-date analysis highlights pharmacological uses as potential cytostatic agents of sirolimus and its derivatives in controlling mTOR signaling for cytostatic therapy. The data were obtained by analyzing databases in the electronic scientific literature, including online databases for medicine: Pubmed/Medline, Web of Science, TRIP Database, Scopus, Google Scholar, SciFinder, Clinicaltrials.gov, using the next MeSH terms: “Antineoplastic Agents/pharmacology”, “Antineoplastic Agents/therapeutic use”, “Drug Resistance”, “Neoplasm”, “Humans”, “Molecular Targeted Therapy/methods”, “Neoplasms/drug therapy”, “Neoplasm Proteins/antagonists & inhibitors”, “Neoplasms/metabolism”, “Protein Kinase Inhibitors/pharmacology,” “Protein Kinase Inhibitors/therapeutic use”, “Signal Transduction/ drug effects”, “TOR Serine-Threonine Kinases/antagonists & inhibitors”, “Serine-Threonine Kinases/metabolism”. The most important pharmacological data have been summarized in tables and figures.
mTOR inhibitors: chemistry and mechanistic perspectives on cancer
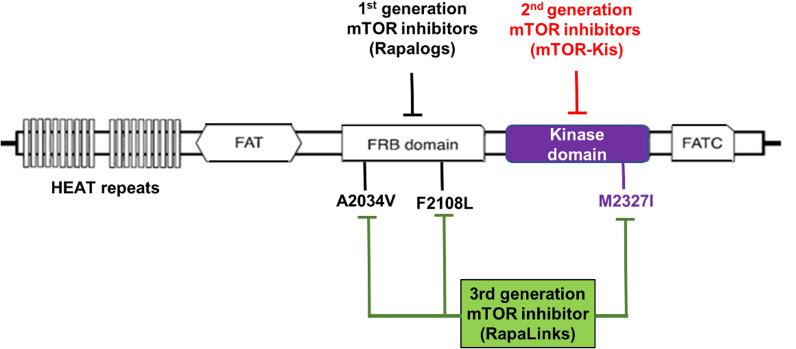
The pharmacological target of FK506-binding protein 12-rapamycin-associated protein 1 (mTOR) is made up of 2549 amino acids with many structural domains (Fig. 1. HEAT (presence of anti-parallel helices in the elongation factor 3, Huntingtin, TOR1, and PP2A) repeats, FAT (for FRAP, ATM, TRAP), FATC (for C-terminal FAT) domains, kinase, and FRB are examples. As shown in Fig. 1, HEAT repeats are positioned at the N-terminal of mTOR and are necessary for mTOR multimerization. mTOR binds to FRB-FK506 binding protein 12 (FKBP12) and rapamycin via FRB-FK506 binding protein 12 (FKBP12)-rapamycin binding-domain. FAT, FATC domains and kinase are all necessary for PIKK activity in phosphatidylinositol 3-kinase-related kinases (PIKKs) [71].
Fig. 1.
Schematic representation of different domains of mTOR and the inhibitors where those binds
To suppress mTOR activity, mTOR inhibitors of the first-generation interact with FKBP12, which further binds to the FRB domain of mTOR. Second-generation mTOR inhibitors work as ATP-competitors by competing with ATP molecules for attaching to the mTOR kinase domain. The third generation of mTOR inhibitors is a more recent family of inhibitors that are developed to be active against drug resistance in cancer cells with mTOR FRB/kinase domain mutations [75].
The first generation mTOR inhibitors
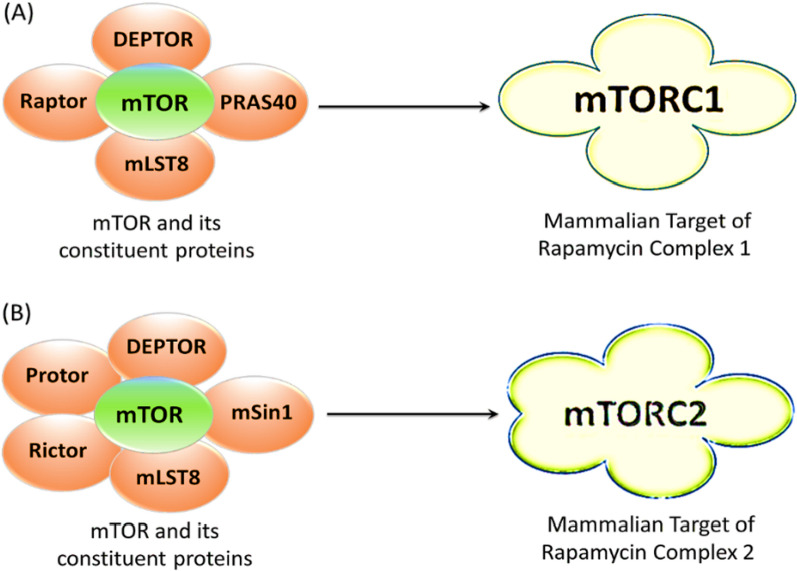
Rapamycin, the prototype mTOR inhibitor, was originally used for over two decades as an immunosuppressant, preventing T-cell activation. Rapamycin has a selective immunosuppressive action by inhibiting the stimulation of T cells induced by some stimuli, blocking the intracellular signaling, dependent and independent of calcium. The research results have shown that the immunosuppressive mechanisms of rapamycin are other than the mechanisms of action of ciclosporin, tacrolimus and other immunosuppressive agents. Preclinical pharmacological studies suggest that rapamycin binds to the specific cytosolic protein FKPB-12, and the FKPB-12/rapamycin complex blocks activation of mTOR, a kinase critical for cell cycle progression. By blocking mTOR, specific pathways of intracellular signal transduction are inhibited. The final effect is to stop the activation of lymphocyte cells, which generates immunosuppression. In vivo, rapamycin has a direct effect on immune-mediated responses to suppress T- and B-cell activation, such as allograft rejection [67]. Rapamycin, on the other hand, does not directly block mTOR kinase activity. Instead, it binds to mTORC1, in a domain close to the active site of the kinase, but not to mTORC2 [13, 19]. As a result, it only inhibits certain of mTORC1's actions. The main components of mTORC1 and mTORC2 are shown in Fig. 2 [40, 80].
Fig. 2.
Constituent proteins of mTORC1 and mTORC2. A a central signaling molecule that is the mammalian target of rapamycin (mTOR) serves as a core constituent to form a distinctive complex with other molecules DEPTOR, PRAS40, mLST8 and Raptor, resulting in the formation of the mTORC1 complex, while the B mTOR with other five proteins DEPTOR, mSin1, Mlst8, Rictor and Protor forms the mTORC2 complex. Both the distinct protein complexes regulate several cellular mechanisms
According to a recent study, the binding of rapamycin-FKBP12 and mTOR does not disrupt the mTORC1 dimer, but it does restrict access to the active site cleft from 20 to 10, showing that the FRB domain works as a barrier to the binding site of an active substrate [11]. Although rapamycin is extremely selective for mTOR, it does not effectively block all mTORC1 [101] actions and may inhibit mTORC2 in certain cell types when therapy is continued for a long time [81]. Though rapamycin does not directly interact with mTORC2, attaching with mTOR in a complex form with FKBP12, it can indirectly affect mTORC2 [114]. The prototype rapamycin's pharmacokinetic properties are not ideal. This prompted the additional study, which resulted in the discovery of rapamycin analogues (also known as rapalogs) with superior 'drug-like' effects. Several such compounds have been published in the literature, demonstrating their efficacy in the treatment of disorders such as cancer. These include RAD001 (everolimus, created by Novartis) [86, 87], CCI-779 (temsirolimus, developed by Wyeth-Ayerst/Pfizer), and AP23573 (ridaforolimus, developed by Merck/Ariad). Novartis recently demonstrated several semi-synthetic rapamycin analogues [2], that are mTORC1 inhibitors which have the potential to treat a variety of illnesses and disorders, including cancer, transplant rejection, neurological disorders, inflammation, autoimmune diseases, age-related disorders, fungal infections, and many more. These semi-synthetic rapamycin analogues have typically been derivatized at different skeletal carbons of the macrolide ring (such as C16, C32, and C40) to improve aqueous solubility, oral bioavailability, and other pharmacokinetic features [2].
Temsirolimus was authorized by the US Food and Drug Administration in 2007 for treating advanced-stage renal cell cancer. Temsirolimus is a selective mTOR inhibitor, it binds to the intracellular protein FKBP-12, and the complex FKBP-12/temsirolimus binds to mTOR, which controls the division of cancer cells, thus inhibiting its activity. In vitro experimental studies, showed that at high concentrations, temsirolimus binds to mTOR inhibiting its activity in the absence of FKBP-12. Also, the results of the studies showed a biphasic, dose-dependent response for cell growth inhibition. High concentrations resulted in complete inhibition of cell growth in vitro, whereas inhibition mediated only by the FKBP-12/temsirolimus complex led to a decrease of approximately 50% in cancer cell proliferation. Therefore, inhibition of mTOR activity causes (i) growth delay in the G1 stage at nanomolar concentrations; (ii) growth interruption at micromolar concentrations in the treated tumor cells, as a result of the selective interruption of the protein translation process cell cycle regulators such as D-type cyclins, c-myc and ornithine decarboxylase. When mTOR activity is inhibited, its ability to phosphorylate is blocked, and it implicitly controls the activity of the protein translation factors 4E-BP1 and S6K and the PI3 kinase/AKT metabolic pathway that controls cell division. In addition, mTOR also regulates the translation of inducible factors by hypoxia, HIF-1 and HIF-2 alpha. These transcription factors regulate the tumor's ability to adapt to hypoxic microclimates and to produce vascular endothelial growth factor (VEGF), with an angiogenic role. Therefore, the antitumor effect of temsirolimus can be attributed, in part, to its ability to decrease HIF and VEGF values in the tumor or the tumor microclimate, thus affecting tumor vascular development.
Everolimus (RAD001) has since been utilized as a single chemotherapeutic drug as well as in combination for different malignancies, including HER2-positive breast cancer and neuroendocrine tumors [54]. Everolimus is also a selective mTOR inhibitor which binds to the intracellular protein FKBP-12 and forms a complex that inhibits the activity of the mTOR-1 complex (mTORC1). Inhibition of the mTORC1 signaling pathway interacts with ribosomal protein translation and synthesis by decreasing activity of protein kinase S6 at the level of ribosomes (S6K1) and the protein-binding eukaryotic elongation factor 4E (4EBP-1) that regulate proteins involved in the cell cycle, angiogenesis and glycolysis. S6K1 phosphorylates estrogen receptor activator function domain 1, which is responsible for ligand-independent receptor activation. In vitro and in vivo studies have shown that Everolimus reduces the levels of VEGF involved in angiogenesis in cancer cells. Also, it is an important inhibitor of the growth and proliferation of tumor cells, endothelial cells, fibroblasts, vascular smooth muscle cells and reduces glycolysis in solid tumors.
Despite their powerful activity, rapamycin and rapalogs have not been used to their full therapeutic potential. The limitations of therapy with rapalogs derive from possible interactions with some CYP3A4 inhibitors that can decrease their metabolism, increasing their blood levels. For example: antifungals (clotrimazole, fluconazole, voriconazole), antibiotics (clarithromycin), protease inhibitors (ritonavir, telaprevir). Adverse effects of immunosuppressants such as infections, nervous system, cardiac or gastrointestinal disorders may also occur.
Second-generation mTOR inhibitors
Because rapamycin has a limited ability to regulate all actions of mTORC1, and thus its application in cancer treatment, a great deal of research has gone into developing compounds that can block the catalytic activity of mTOR. These can block all phosphorylation processes mediated by mTORC1, but they will also affect mTORC2. On average, half of the maximal inhibitory concentration (IC50) of these inhibitors against mTOR function is significantly lower than that of PI3K. Because suppression of mTORC1 and mTORC2 may result in stronger effectiveness than mTORC1 inhibition, this class of inhibitors might be a better alternative to rapalogues for cancer therapy.
A group of researchers studied in vitro the mTOR inhibitors PP242 and PP30, which have a central pyrazolo[3,4-d]pyrimidine ring with a C4 amino group, two different heterocyclic substituents, and an N1 isopropyl substituent on C3 [7]. With IC50 values of 8 nM and 80 nM, correspondingly, these drugs demonstrated remarkable selectivity for mTOR among a panel of 219 kinases. Both inhibited mTORC1 and mTORC2 in an ATP-competitive manner and had greater impacts on cell cycle, cell growth and proliferation, and cap-dependent translation rather than the prototype inhibitor rapamycin [7]. Following a high-throughput screen and subsequent lead optimization campaign, Pfizer-Wyeth researchers identified WYE-354, WAY-600, and WYE-687 as effective ATP-competitive mTOR inhibitors with identical pyrazolo[3,4-d] pyrimidine moiety as core functional moiety [119]. These compounds have a 4-piperidinyl-1-substituted moiety in N1 and a C4 morpholino substituent, and in enzymatic studies, they suppress mTOR with IC50 values in a range of 5–9 nM, with great selectivity (greater than 100-fold) against PI3Ks. In contrast to rapalogs, in vitro, they reduced phosphorylation of mTORC2 and mTORC1 substrates in response to amino acids, growth factors as well as PI3K/Akt overexpression. Structure–activity relationships exploration of the lead molecules, particularly modification on piperidine ring and functionalization of carbamate and urea groups on C6 phenyl, resulted in the revelation of highly potent and selective mTOR inhibitors, for example, compounds 6–9 with an IC50 value of 0.5 nM against mTOR as determined by an enzymatic assay [119].
Using the X-ray crystal structured pyrazolopyrimidine inhibitor interacts with PI3K and molecular docking studies with the mTOR homology model, it was discovered that 3 hydrogen bonds may be created between the pyrazolopyrimidine inhibitor and the mTOR ATP-binding pocket [106]. According to Fig. 3, there could be two H-bonds between Asp2195 and urea NHs and, one between Lys2187 and the carbonyl of the urea.
Fig. 3.
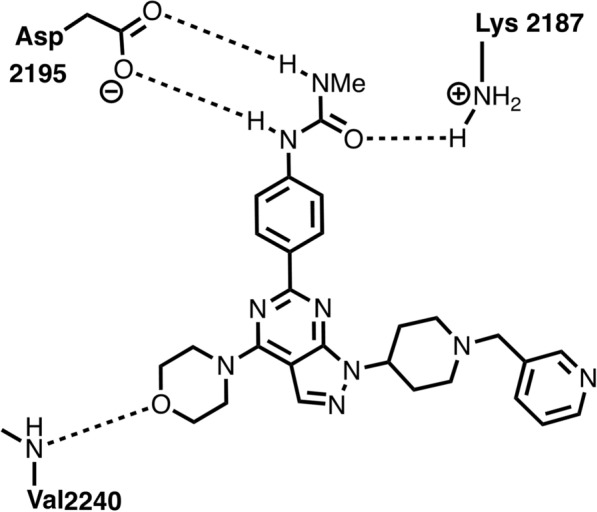
Proposed origin of potency and selectivity of pyrazolopyrimidine analogs for mTOR
Following the identification of the pharmacophore responsible for mTOR inhibition, tremendous effort was expanded in the quest for selective mTOR inhibitors [50, 120]. The central pyrazolopyrimidines structures were extended to thienopyrimidines and triazines core structures, and multiple publications on strong mTOR inhibitors based on these structural motifs were reported.
Torin 1 was first disclosed by Nathanael Gray's group [100] and subsequently developed by AstraZeneca. This drug has a low nanomolar IC50 against mTOR and a 100-fold selectivity over other kinases in vitro.
Ku-0063794 from KuDOS Pharmaceuticals which is now a part of AstraZeneca is another example of an ATP-competitive mTOR inhibitor with strong anti-proliferative activity against cancer cells in vitro [37]. AstraZeneca researchers later used Ku-0063794 to produce AZD8055, an orally accessible version of the former having antiproliferative action and an IC50 of 50 nM [27, 95].
XL388 is another selective small-molecule ATP-competitive mTOR inhibitor having 8 nM and 166 nM IC50 respectively, that inhibits mTORC1 and mTORC2 in vitro [61]. In MCF-7 cells, this candidate effectively inhibits mTORC1 phosphorylation of p70S6K (Thr389) and mTORC2 phosphorylation of Akt (Ser473). It was found to be particularly effective in solid as well as hematopoietic cell lines of tumor in combination with paclitaxel/carboplatin and doxorubicin or as a single drug.
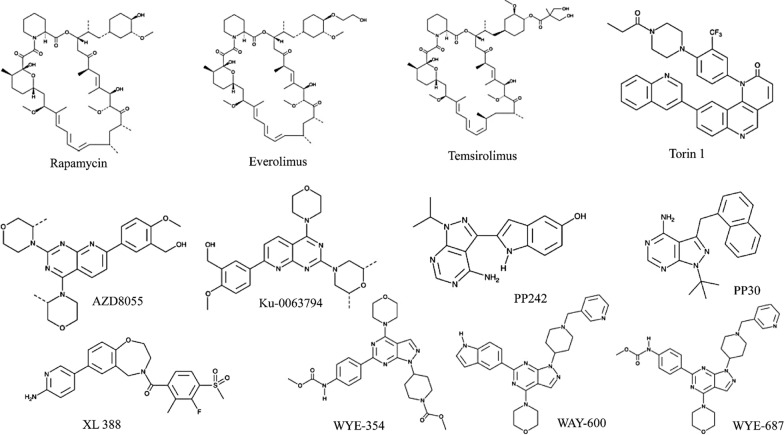
The chemical structures of the most representative mTOR inhibitors are summarized in Fig. 4.
Fig. 4.
Chemical structures of mTOR inhibitors
mTOR inhibitors in clinical studies
Because of sirolimus's efficacy in preclinical studies, sirolimus-derived compounds have now been proposed for use in several clinical studies as anti-cancer medicines (see Table 1; Fig. 5 for a summary).
Table 1.
The inhibitors for mTORC1/2 complexes that are being tested alone or in combination with other therapeutics in different phases of clinical trials for several known malignancies
| mTOR inhibitor |
Combinational therapy | Type of cancer/diseases | Clinical status | References/ClinicalTrials ID |
|---|---|---|---|---|
| AZD2014 | N/A | Glioblastoma Multiforme | Phase 1 | NCT02619864 |
| AZD2014 | Anastrozole | Hormone Receptor-Positive endometrial carcinoma | Phase 1 & 2 | NCT02730923 |
| AZD2014 | Olaparib and AZD5363 |
Breast Cancer Malignant Female Reproductive System Neoplasm |
Phase 1 & 2 | NCT02208375 |
| Everolimus (RAD001) | N/A | Prostate Cancer Patients with Detectable PSA Following Prostatectomy | Phase 1 | NCT01548807 |
| AZD2014 | N/A | NF2 Patients with Progressive or Symptomatic Meningiomas | Phase 2 | NCT02831257 |
| Vistusertib (AZD2014) | N/A | Recurrent Grade II-III Meningiomas | Phase 2 | NCT03071874 |
| Everolimus | Levonorgestrel-Releasing Intrauterine System | Atypical Hyperplasia or Stage IA Grade 1 Endometrial Cancer | Phase 2 | NCT02397083 |
| AZD2014 | Rituximab | Relapsed/Refractory Diffuse Large B Cell Lymphoma | Phase 2 | NCT02752204 |
| MLN0128 | MLN1117 oral inhibitor of the PI3K (alpha) isoform | Advanced Nonhematologic Malignancies | Phase 1 | NCT01899053 |
|
Milled MLN0128 API |
Unmilled MLN0128 API and Paclitaxel | Advanced Nonhematologic Malignancies | Phase 1 | NCT02412722 |
| MLN2480 | MLN0128 or Alisertib, or Paclitaxel, or Cetuximab, or Irinotecan | Advanced Nonhematologic Malignancies | Phase 1B | NCT02327169 |
| AZD2014 | Paclitaxel |
Ovarian cancer Squamous cell lung cancer |
Phase 1 | NCT02193633 |
| TAK228 | Paclitaxel |
advanced/Recurrent Epithelial Ovarian, Fallopian Tube Primary Peritoneal Cancer |
Phase 2 | NCT03648489 |
| Sirolimus | N/A | Cardiovascular Abnormalities/Vascular Malformations | Phase 3 | NCT01811667 |
| AP23573 (Ridaforolimu) | N/A | Advanced Sarcoma | Phase 2 | NCT00093080 |
| Rapamycin | Placebo | Aging and associated complications | Phase 2 | NCT02874924 |
| Everolimus | Imatinib mesylate | Metastatic or Unresectable Kidney Cancer | Phase 2 | NCT00331409 |
| MLN0128 | Paclitaxel; Trastuzumab | Advanced Solid Malignancies Hematologic Malignancies | Phase 1 | NCT01351350 |
| WXFL10030390 | N/A |
Advanced Solid Tumors Lymphoma |
Phase 1 | NCT03730142 |
| Metformin | N/A | Well-differentiated Neuroendocrine Tumors | Phase 2 | NCT02279758 |
| SF1126 | N/A | Advanced or Metastatic Solid Tumors | Phase 1 | NCT00907205 |
| Everolimus | N/A | Chronic Allograft Dysfunction in Renal Transplantation | Phase 4 | NCT01046045 |
| Sirolimus | N/A | Congenital Vascular Malformations | Phase 3 | NCT03987152 |
| Sirolimus | N/A | Peutz-Jeghers Syndrome | Phase 4 | NCT03781050 |
| RAD001 (Everolimus) | N/A | Tuberous Sclerosis Lymphangioleiomyomatosis | Phase 1 & 2 | NCT00457964 |
| RAD001 (Everolimus) | N/A |
Subependymal Giant Cell Astrocytoma Tuberous Sclerosis |
Phase 1 & 2 | NCT00411619 |
| RAD001 (Everolimus) | N/A |
Epilepsy Tuberous Sclerosis Complex |
Phase 1 & 2 | NCT01070316 |
| Sirolimus | Placebo |
Polycystic Kidney, Type 1 & Type 2 Autosomal Dominant Disease |
Phase 3 | NCT02055079 |
| Sirolimus | N/A |
Blue Rubber Bleb Nevus Syndrome Hereditary Sporadic Venous Malformation |
Phase 4 | NCT03767660 |
| Gedatolisib | Palbociclib/Letrozole Or Palbociclib/Fulvestrant | Metastatic Breast Cancer | Phase 1B | NCT02684032 |
|
Arm 1 Everolimus/ tacrolimus |
Calcineurin inhibitors | Renal Transplant and associated complications | Phase 4 | NCT01935128; [80] |
| RAD001 (Everolimus) | Placebo | Tuberous Sclerosis Complex (TSC) Lymphangioleiomyomatosis (LAM) | Phase 3 | NCT00790400; [80] |
| CCI-779 (Temsirolimus) | N/A | Breast and Renal cancer | Phase 2 | [73] |
ClinicalTrials ID has been taken from https://clinicaltrials.gov
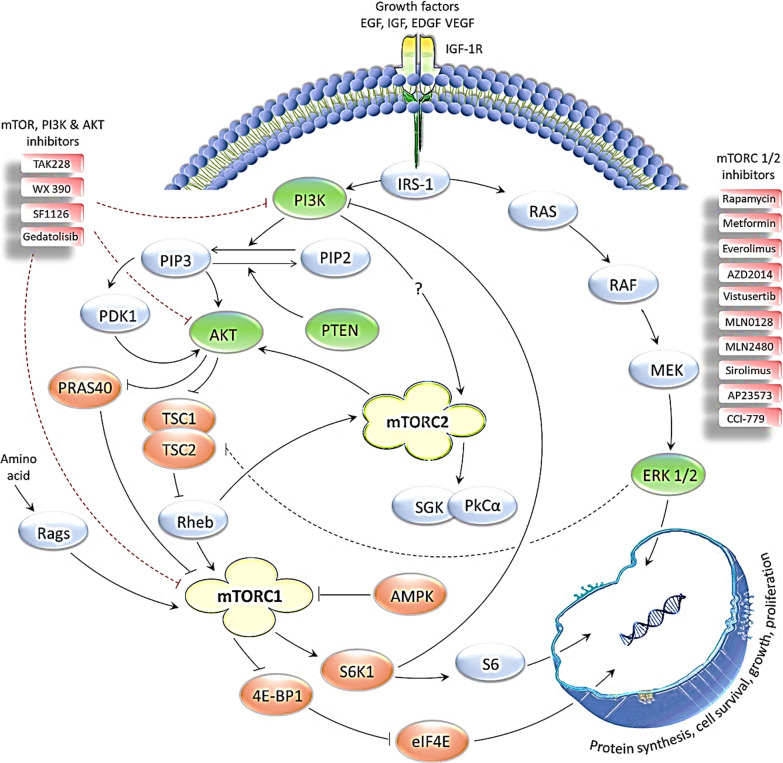
Fig. 5.
The mTORC1/2 signaling pathway and its inhibitors in clinical trials
Temsirolimus (CCI-779; Wyeth-Ayerst), deforolimus (AP23573; Ariad Pharmaceuticals), and everolimus (RAD001; Novartis) are three analogues currently being studied [3, 34, 69]. All three drugs, like sirolimus, work by creating complexes with FKBP12, that link to and further suppress mTOR. They've been restructured to improve water solubility and stability. The C40 hydroxyl of sirolimus is typically substituted with esters or ethers in synthetic modifications [32]. Temsirolimus is an ester derivative of sirolimus that can be given intravenously or orally, whereas everolimus is a hydroxyethyl ether derivative that can be given orally. Deforolimus is a phosphonate replacement that can be given intravenously or orally [32, 103]. When taken according to the right cancer treatment plan, sirolimus analogues do not cause immunosuppression.
Temsirolimus was originally tested in patients with solid tumors for example breast, lung, and kidney malignancies in phase I clinical studies. Temsirolimus was delivered intravenously once daily for 5 days every 2 weeks or once weekly, according to two different dosing regimes [46, 73]. During these two investigations, 87 patients were given temsirolimus. Three partial responses (PRs are well-defined as at least a 50% decrease in overall tumor size) were found, one for kidney, one for breast, and one for lung tumors. Two patients with renal cancer achieved minor responses (reductions of tumor of 34% and 39%, respectively), and two patients had disease stability for longer than 6 months. As a result of these findings, a couple of clinical phase II studies for temsirolimus was started.
In one phase II trial of 109 patients with primary advanced or metastatic breast cancer, 10 had PRs (a 9.2% response rate) [25]. Temsirolimus treatment resulted in one complete response and seven PRs in 111 individuals with renal cell cancer (7% response rate). [10]. Temsirolimus has also shown promising results in treating endometrial cancer. In another phase II study of individuals with recurrent or metastatic endometrial carcinoma, 26 percent (5 of 19 patients) had PRs, while 60 percent had stable disease (12 of 19 patients). Furthermore, in phase II trials, temsirolimus showed a remarkable potential for mantle cell lymphoma [112]. In addition, temsirolimus is the first sirolimus-derived compound to go through phase III clinical trials for effective renal cancer therapy. Patients who received temsirolimus as a single intravenous agent had a significantly higher median survival of 10.9 months than patients who received the standard cancer therapy of interferon- [IFN] (7.3 months) [79]. In May 2007, the US FDA authorized temsirolimus for treating advanced renal cell carcinoma based on its efficacy in this phase III trial [3, 31].
In phase, I investigations of everolimus for treating solid tumors, oral dosages of 20–30 mg on weekly basis were devised, and further suppression of S6K in peripheral mononuclear cells of blood was found as an alternate measure for therapeutic action [22]. Phase II clinical studies of Everolimus in individuals with renal and endometrial cancer has completed recently and a hematological malignancy phase I/II study has been done [8, 97]). Everolimus was given orally to 27 individuals with a range of hematological malignancies (such as mantle cell lymphoma, acute myelogenous leukemia, and B-chronic lymphocytic leukemia). Two patients had favorable hematological responses, while mTOR signaling inhibition was tested in 9 patients, with 6 showing a reduction in S6K and/or 4E-BP1 phosphorylation [118]).
A phase III clinical study of Everolimus in people with advanced metastatic carcinoma of the renal cell was recently investigated [64]. Throughout the research, 272 participants were given a single oral dose of everolimus daily (10 mg). In general, everolimus therapy was usually well tolerated, with 63 percent of patients (171 out of 272) demonstrating disease stability (a disorder that stayed constant for a minimum of 56 days), demonstrating that everolimus is an efficient treatment choice for advanced carcinoma of the renal cell.
Clinical testing for Deforolimus is now in its early phases, while several phase I and phase II clinical trials have already been over. Deforolimus was administered intravenously daily in a phase I trial for 5 days in every 2 weeks in individuals with resistant or advanced solid tumors [62]. mTOR suppression, as evidenced within 4 h after deforolimus treatment, as revealed by dephosphorylation of 4E-BP1 in 12.5 percent of patients (four of 32 patients) [62]. Furthermore, in phase II trials of deforolimus, it has been studied in patients with advanced-stage sarcomas as well as resistant hematological malignancies (through intravenous administration); nevertheless, preliminary data show poor objective response rates [97]. Deforolimus decreased mTOR signaling in patients with high-grade sarcomas, as evidenced by a reduction in the amounts of the ribosomal protein S6 which is being phosphorylated in tumor sections [31, 48].
The upstream signaling molecules that play a crucial role in mTORC1/2 signaling are the PI3K, PTEN and AKT. Both of the mTORC1/2 complexes are important in cellular growth, survival, proliferation, motility, protein synthesis and autophagy. All of the inhibitors depicted here are currently being tested in different phases of clinical trials for several disorders, majorly for different types of cancers. TAK228, an oral inhibitor has been developed to inhibit PI3K/AKT/mTOR, while WX390, SF1126 and Gedatolisib are reported to target PI3K and mTOR. All of the remaining inhibitors are being evaluated in clinical trials for mTORC1/2 inhibition. The figure is adapted from [40, 76, 80].
Therapeutic perspectives, limitations and challenges associated with targeting mTOR
Cancer treatment has evolved rapidly over the last decade, toward a personalized approach [47, 89]. Modern technologies today allow a molecular characterization that outlines a unique picture for each patient. Based on tumor genomic changes, new treatment targets have been discovered, some of which can be acted upon directly through personalized therapies. Tumor genomic testing has evolved, from several biomarkers to extensive panels—allowing the analysis of all mutations that can be acted upon by targeted therapies, and even more, biomarkers that allow patients to be included in clinical trials for treatments not yet approved. Regarding the therapeutic targets in cancer, research in this field has led to the approval of many drugs in recent years and there are many other molecules still in clinical or preclinical studies, so we can expect complex changes in therapeutic standards in cancer in the near future [72].
Although their initial success, ATP-competitive mTOR inhibitors have yet to reach their therapeutic potential for a variety of reasons such as:
-
i.
Inhibiting mTOR activates a variety of feedback loops targeting upstream signaling pathways, which boost cancerous cell survival and further metastasis when activated [102].
-
ii.
Because mTOR signaling is essential for normal cell function, the total blockage is extremely harmful to healthy tissues [124].
-
iii.
mTORC1 inhibits autophagy, and treatment with an mTOR inhibitor may induce autophagy, thus promoting cancer cell survival, as seen with AZD8055[99].
-
iv.
Any clinically relevant mTOR mutations that increase mTOR's catalytic activity could drastically diminish the efficiency of such inhibitors in cancer cells [44].
To solve this issue, Rodrik-Outmezguine has effectively linked the rapamycin and INK-128 binding sites leading to the generation of a bifunctional mTOR inhibitor called RapaLink [75]. This hybrid molecule now contains both rapamycin and an mTOR kinase inhibitor, which are linked via a non-perturbing, strain-free crosslinker of optimal length. The linker permits the chemical to connect with the FRB domain of mTOR by interacting with FKBP12, along with reaching the kinase domain of mTOR, allowing it to serve as an ATP-competitive inhibitor (Fig. 6). Both RapaLinks (1 & 2) inhibited mTORC1 and mTORC 2 with IC50 of 10 nM, and mice xenografts of MCF-7 cells were shown to be more sensitive to RapaLink-1 than parent rapamycin and INK-128. Furthermore, after 9 months of treatment, RapaLink-treated cells did not acquire chemotherapeutic drug resistance, but considerable resistance was identified after 3 months of treatment with first- or second-generation mTOR inhibitors. This discovery opened the way for developing a new generation of mTOR inhibitors.
Fig. 6.
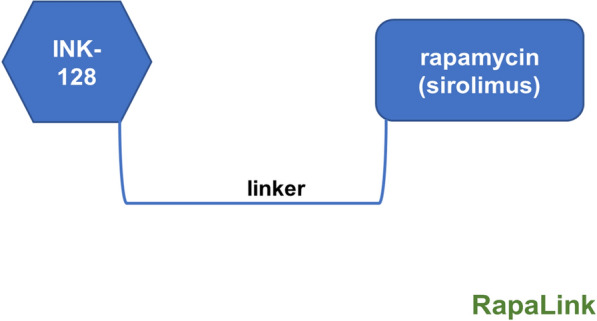
Generation of RapaLinks. Linking an mTOR kinase inhibitor INK-128 (or MLN0128) to rapamycin led to RapaLinks which exhibited improved efficacy in tumor-bearing mice than each of the constituents alone
The clinical use of mTOR inhibitors has also shown other effects. For example, some studies conducted on mice showed that Sirolimus extended their life almost three times [21]. Rapalogs were approved by the FDA in the early 2000s and some research has suggested them as potential antiaging drugs [88]. If more than ten years ago Sirolimus was used as an immunomodulator, in high doses it was observed that it can act as an immunostimulator, especially in elderly patients with oncological diseases [33]. In addition, some studies have indicated that in patients with cardiovascular diseases to whom stents were applied, the administration of Rapamycin reduces the restenosis rate of the stents [59]. Other research has shown that mTOR inhibitors can induce some metabolic and stress responses that promote longevity, although exactly how this happens is still not clear [21].
Despite the efficacy of sirolimus derivatives in preclinical research as anti-cancer drugs, it is crucial to remember that inhibitors of mTOR have not shown to be as efficient as predicted. Based on this, sirolimus as a wide-ranging monotherapy for treating cancer may be unsuccessful. As a result, determining which patients might benefit the most from sirolimus medication is crucial and exploring sirolimus as part of combination medicines for cancer treatment [31]. In addition to the compounds mentioned above, recently evolving compounds that regulate/inhibit mTORC signalling, and its associated components, and might be useful for the treatment of various types of cancers are summarized in Table 2.
Table 2.
Evolving compounds and reagents regulating or inhibiting mTOR signaling, and its associated components, in various cancers
| Tested compounds | Preclinical study/mechanisms | Refs. |
|---|---|---|
| RMC-4627 |
In vitro models of B-cell acute lymphoblastic leukemia RMC-4627 BCR-ABL ↓4E-BP1 phosphorylation ↓ cancer cells progression ↓viability ↓ cancer cell survival |
[55] |
|
1,4-O-diferuloylsecoisolariciresinol (IM-1) |
In vitro mice embryonic fibroblast cells ↑nuclear translocation ↑S6K kinase ↑4E-BP1 ↑cytotoxicity ↑apoptosis |
[116] |
| Pierreione B (IM-2) | ||
| DL001 |
In vitro PC3 cells MEFs mice embryonic fibroblasts ↓hyperactive mTORC1 In vivo C57BL/6J mice ↓side effects of rapalogs |
[85] |
| DHM25 |
In vitro triple-negative breast cancer cells ↓Akt phosphorylation |
[35] |
| 3HOI-BA-01 |
In vitro non-small cell lung cancer cells ↓mTOR kinase In vivo mice ↓tumor growth |
[113] |
|
PF-5212384 PD-901 |
In vitro 14 HNSCC cell lines ↑ cells in G0-/G1 phase ↓PI3K/mTOR ↓NF-κB, ↓AP-1, ↓IL8 ↓cells proliferation, ↓apoptosis, ↓angiogenesis |
[63] |
| P529 |
In vitro GBM cells ↓AKT (Ser-473),↓NDRG1 (Thr-346) ↓PKCα (Ser-657) ↓cancer cells growth, ↓invasiveness In vivo Mice GBM xenograft ↓Tumor groth |
[18] |
| JR-AB2-011 (Palomid 529) |
In vitro LLC-PK1, LLC-Mdr1a, LLC-MDR1 ↓ cancer cells’ growth In vivo WT and KO mice with gliomas ↑ blood brain passage |
[56] |
| W922 |
In vitro HCT116, MCF-7, A549 ↓cancer cells viability In vivo mice xenograft model ↑ cell cycle arrest in G0-G1 phase ↑ apoptosis |
[111] |
The existence of the sirolimus-resistant mTOR signaling complex, mTORC2, must be considered when evaluating the usage of sirolimus derivatives for treatment. Sirolimus is considered to impair the interaction between mTOR and raptor by targeting the mTORC1 complex; however, sirolimus therapy does not affect the mTORC2 complex. As a result, in the presence of sirolimus, mTORC2 is free to signal. mTORC2 regulates the cytoskeleton, but more significantly, it is the kinase that phosphorylates Akt [15, 82]. Akt activation requires phosphorylation of Ser473 within the hydrophobic motif, as well as Thr308 phosphorylation in the activation loop [39]. Although PDK1 has long been known to phosphorylate Akt at Thr308, this has been only just exposed that mTORC2 is an enzyme that phosphorylates Ser473 in Akt [15, 82]. Rictor phosphorylation was reduced, and mTORC2 enabled Akt phosphorylation in vitro at Ser473, proving mTORC2 as the secondary kinase involved in Akt regulation, known as PDK2 [15, 82]. Because sirolimus only inhibits mTORC1 and the discovery of mTORC2 as PDK2 highlights some remarkable questions about the usage of sirolimus derivatives in cancer therapy. Because Akt is involved in numerous pro-survival and growth-promoting pathways, the continuous stimulation of Akt by mTORC2 with the combination of sirolimus in the setting of cancer is quite significant. However, some recent evidence suggests that extended sirolimus treatment inhibits mTORC2 [81]. It was postulated that after continuous sirolimus treatment, the cell compensates for mTORC1 inactivation by creating additional mTORC1 complexes, reducing the accessibility of mTOR to support the development of mTORC2. As a result, long-term sirolimus therapy blocks any beneficial signaling action relayed to Akt by mTORC2, strengthening the anti-cancer effects of mTOR inhibitors.
The signaling pathway of PI3K/Akt/mTOR is a significant regulatory mechanism that regulates a wide range of cellular processes. As a result, targeting this pathway for cancer treatment impacts key cellular processes in unpredictable ways, which might lead to mTOR inhibition resistance or perhaps a worsening of tumor development. The suppression of a negative feedback loop controlled by S6K by sirolimus is a good example. mTOR activates S6K in the presence of nutrients and growth factors. By blocking the insulin receptor substrate-1 (IRS-1) protein, S6K creates a negative feedback loop [60]. IRS-1 phosphorylation by S6K identifies it for breakdown or inhibition, resulting in decreased PI3K and Akt signaling [38, 43, 90]. However, S6K is no longer stimulated by mTOR in the presence of sirolimus or its derivatives, resulting in increased IRS-1-mediated signaling, reduced IRS-1 degradation, and elevated PI3K and Akt activity [66].
An analogous control mechanism has been reported for the platelet-derived growth factor receptor (PDGFR), in which S6K generated signal regulates PDGFR expression [121]. Disrupting these negative feedback systems has significant effects for the efficacy of sirolimus analogs in cancer therapy. For example, sirolimus treatment of cancer cells increased Akt phosphorylation (Ser473) and activation [23, 66]. Inhibition of mTOR and S6K may increase PI3K/Akt signaling, which may enhance carcinogenesis and alter tumor susceptibility to some other chemotherapeutic treatments. In a current study, O'Reilly et al. found that sirolimus therapy increased Akt phosphorylation (Ser473) and activation in cancer cells [66]. Akt phosphorylation was also shown to be higher in tumors from patients receiving everolimus medication. Skeen et al., on the other hand, revealed that despite disrupting the S6K-IRS-1 negative feedback loop, sirolimus therapy still prevented carcinogenesis [96].
Recent findings back up the usage of sirolimus in conjunction with other anti-cancer medicines. Trials utilizing the inhibitors of IGF-I/insulin signaling are already in progress [122]. In the future, it is critical to establish indicators of sirolimus sensitivity or resistance so that individuals can receive the right treatment and avoid developing chemoresistance. For establishing the utmost successful combination therapy for patients who do not have any effectiveness of conventional therapeutic procedures, more study into the probable synergism between sirolimus and standard-of-care drugs is needed [53]. A variety of difficulties concerning mTOR signaling and sirolimus action must be addressed in the preclinical context. The mTORC2 complex, for example, is poorly understood. Understanding the relevance of this signaling complex and clarifying its possible role in cancer will also need the identification of downstream targets of mTORC2 [36].
The quest for new mTOR inhibitors that aren't based on sirolimus will be a priority. Affecting the kinase domain of mTOR with novel small molecules to suppress both mTORC1 and mTORC2 activity might improve the efficacy of mTOR suppression in cancer therapy [70]. Compound 401, for example, is a novel drug that inhibits both the TORC1 and TORC2 actions of mTOR [14]. This chemical, however, is not selective for mTOR and further focuses on DNA-dependent protein kinase. It is also critical to make use of available medications that can block mTOR, like as AICAR and metformin, to get a better understanding of mTOR signaling in both normal and altered cells. More research is needed to investigate the importance and consequences of mTORC2 inhibition in tumor development. The toxicity of these compounds could be one of the most important consequences of mTOR inhibitors. For example, data from recent studies showed that the compound NVP-BEZ235 (dactolisib) has anticancer efficacy on cell lines in vitro, but on in vivo models with orthotopic glioblastoma xenograft mice, adverse effects such as alopecia, hyperglycemia, liver cytolysis [65]. Therefore, safety and toxicity studies of these compounds should be carried out in the future. The development of mTORC2 inhibitors and the potential synergism of cancer therapeutic with sirolimus derivatives is a field of research that needs to be also investigated further [31].
Also, as future perspectives, mTOR inhibitors should be considered immunosuppressive drugs that reduce or suppress the activity of the immune system [58]. They can be prescribed for the treatment of autoimmune diseases (systemic lupus erythematosus, psoriasis, rheumatoid arthritis, inflammatory bowel disease) or the prevention of graft rejection in organ transplants (liver, kidney, heart) [115]. The main advantage of immunosuppressive therapy is the improvement of the patient's quality of life [110]. Adverse reactions such as nephrotoxicity, and increased risk of malignancy or infections require careful monitoring of treatment [108]. By binding to FKBP12, sirolimus forms a complex that binds to the enzyme mTOR, which it inhibits [105]. Thus, the progression of the cell cycle from the G1 phase to the S phase is blocked. Sirolimus is combined with tacrolimus or glucocorticoids and is used to prevent organ transplant rejection. Hyperlipidemia, thrombocytopenia, anemia, oral ulcers, diarrhea, and infertility are side effects that may occur during treatments [84].
Concluding remarks
mTOR, a serine/threonine-protein kinase, is a major regulator of several fundamental cellular functions, including development, multiplication, mRNA translation, and cytoskeletal architecture. mTOR signaling dysfunction promotes cellular development and proliferation and has been linked to a variety of human malignancies. Increased mTOR signaling is particularly related to human malignancies defined by the loss or mutation of critical tumor suppressors including STK11, TSC1/2, and PTEN which seem to be important for regulating the PI3K/Akt pathway [20]. As a result, mTOR has become a crucial cancer therapeutic target. Sirolimus and its variants are powerful and selective mTOR inhibitors that have gotten a lot of interest as possible anti-cancer drugs. For treating cancer patients, sirolimus analogues are now being studied in phase II and phase III clinical trials. So far, clinical studies show that sirolimus's performance as a single agent as a broad-range anti-cancer therapy may be rather restricted; nonetheless, certain cancers, such as endometrial carcinoma, renal cell carcinoma, and mantle cell lymphoma, respond well to sirolimus. Notably, Temisrolimus has already been approved by the FDA for treating advanced renal cell cancer.
Since the development of sirolimus occurred more than 30 years ago, much has been learnt about the significance of mTOR in cellular process coordination and its relevance in cancer. Despite recent improvements in the research of mTOR signaling in cells, notably its development as a therapeutic target for cancer treatment, more work must be done to completely comprehend the relevance of mTOR and its functions in cell biology and illness. Consequently, upcoming research will specify the insights to comprehending mTOR and its significance in medical health.
Author contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas—that is, revising or critically reviewing the article; giving final approval of the version to be published; agreeing on the journal to which the article has been submitted; and confirming to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Funding
Not Applicable.
Availability of data and materials
Not Applicable.
Declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
Authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Eunus S. Ali, Email: eunus2ali@yahoo.com
Kangkana Mitra, Email: kangkana.mitra.kms@gmail.com.
Shamima Akter, Email: shamima1606@gmail.com.
Sarker Ramproshad, Email: ramproshad131135@gmail.com.
Banani Mondal, Email: banani091110@gmail.com.
Ishaq N. Khan, Email: ishaqkhan.ibms@kmu.edu.pk
Muhammad Torequl Islam, Email: mti031124@gmail.com.
Javad Sharifi-Rad, Email: javad.sharifirad@gmail.com.
Daniela Calina, Email: calinadaniela@gmail.com.
William C. Cho, Email: chocs@ha.org.hk
References
- 1.Global Burden of Disease Study. Global, regional, and national burden of colorectal cancer and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol; 2022. [DOI] [PMC free article] [PubMed]
- 2.Abdel-Magid AF. Rapalogs potential as practical alternatives to rapamycin. ACS Publications; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abraham RT, Eng CH. Mammalian target of rapamycin as a therapeutic target in oncology. Expert Opin Ther Targets. 2008;12:209–222. doi: 10.1517/14728222.12.2.209. [DOI] [PubMed] [Google Scholar]
- 4.Ali ES, Lipońska A, O'Hara BP, Amici DR, Torno MD, Gao P, Asara JM, Yap M-NF, Mendillo ML, Ben-Sahra I. The mTORC1-SLC4A7 axis stimulates bicarbonate import to enhance de novo nucleotide synthesis. Mol Cell. 2022;82:3284. doi: 10.1016/j.molcel.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali ES, Sahu U, Villa E, O’hara BP, Gao P, Beaudet C, Wood AW, Asara JM, Ben-Sahra I. ERK2 phosphorylates PFAS to mediate posttranslational control of de novo purine synthesis. Mol Cell. 2020;78:1178–1191.e6. doi: 10.1016/j.molcel.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson EJ, Mollon LE, Dean JL, Warholak TL, Aizer A, Platt EA, Tang DH, Davis LE. A systematic review of the prevalence and diagnostic workup of PIK3CA mutations in HR+/HER2-metastatic breast cancer. Int J Breast Cancer. 2020;2020:3759179. doi: 10.1155/2020/3759179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apsel B, Blair JA, Gonzalez B, Nazif TM, Feldman ME, Aizenstein B, Hoffman R, Williams RL, Shokat KM, Knight ZA. Targeted polypharmacology: discovery of dual inhibitors of tyrosine and phosphoinositide kinases. Nat Chem Biol. 2008;4:691–699. doi: 10.1038/nchembio.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong AJ, Halabi S, Eisen T, Broderick S, Stadler WM, Jones RJ, Garcia JA, Vaishampayan UN, Picus J, Hawkins RE, Hainsworth JD, Kollmannsberger CK, Logan TF, Puzanov I, Pickering LM, Ryan CW, Protheroe A, Lusk CM, Oberg S, George DJ. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. Lancet Oncol. 2016;17:378–388. doi: 10.1016/S1470-2045(15)00515-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asgharian P, Tazekand AP, Hosseini K, Forouhandeh H, Ghasemnejad T, Ranjbar M, Hasan M, Kumar M, Beirami SM, Tarhriz V, Soofiyani SR, Kozhamzharova L, Sharifi-Rad J, Calina D, Cho WC. Potential mechanisms of quercetin in cancer prevention: focus on cellular and molecular targets. Cancer Cell Int. 2022;22:257. doi: 10.1186/s12935-022-02677-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atkins MB, Hidalgo M, Stadler WM, Logan TF, Dutcher JP, Hudes GR, Park Y, Liou S-H, Marshall B, Boni JP. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 11.Aylett CH, Sauer E, Imseng S, Boehringer D, Hall MN, Ban N, Maier T. Architecture of human mTOR complex 1. Science. 2016;351:48–52. doi: 10.1126/science.aaa3870. [DOI] [PubMed] [Google Scholar]
- 12.Bagherpour B, Salehi M, Jafari R, Bagheri A, Kiani-Esfahani A, Edalati M, Kardi MT, Shaygannejad V. Promising effect of rapamycin on multiple sclerosis. Mult Scler Relat Disord. 2018;26:40–45. doi: 10.1016/j.msard.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Ballou LM, Lin RZ. Rapamycin and mTOR kinase inhibitors. J Chem Biol. 2008;1:27–36. doi: 10.1007/s12154-008-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballou LM, Selinger ES, Choi JY, Drueckhammer DG, Lin RZ. Inhibition of mammalian target of rapamycin signaling by 2-(morpholin-1-yl) pyrimido [2, 1-α] isoquinolin-4-one. J Biol Chem. 2007;282:24463–24470. doi: 10.1074/jbc.M704741200. [DOI] [PubMed] [Google Scholar]
- 15.Bayascas JR, Alessi DR. Regulation of Akt/PKB Ser473 phosphorylation. Mol Cell. 2005;18:143–145. doi: 10.1016/j.molcel.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Sahra I, Howell JJ, Asara JM, Manning BD. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science. 2013;339:1323–1328. doi: 10.1126/science.1228792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ben-Sahra I, Hoxhaj G, Ricoult SJH, Asara JM, Manning BD. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science. 2016;351:728–733. doi: 10.1126/science.aad0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benavides-Serrato A, Lee J, Holmes B, Landon KA, Bashir T, Jung ME, Lichtenstein A, Gera J. Specific blockade of Rictor-mTOR association inhibits mTORC2 activity and is cytotoxic in glioblastoma. PLoS ONE. 2017;12:e0176599. doi: 10.1371/journal.pone.0176599. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10:868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 20.Bjornsti M-A, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 21.Blagosklonny MV. Rapamycin for longevity: opinion article. Aging (Albany NY) 2019;11:8048–8067. doi: 10.18632/aging.102355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boulay A, Zumstein-Mecker S, Stephan C, Beuvink I, Zilbermann F, Haller R, Tobler S, Heusser C, O'reilly T, Stolz B, Marti A, Thomas G, Lane HA. Antitumor efficacy of intermittent treatment schedules with the rapamycin derivative RAD001 correlates with prolonged inactivation of ribosomal protein S6 kinase 1 in peripheral blood mononuclear cells. Cancer Res. 2004;64:252–261. doi: 10.1158/0008-5472.CAN-3554-2. [DOI] [PubMed] [Google Scholar]
- 23.Buck E, Eyzaguirre A, Brown E, Petti F, Mccormack S, Haley JD, Iwata KK, Gibson NW, Griffin G. Rapamycin synergizes with the epidermal growth factor receptor inhibitor erlotinib in non–small-cell lung, pancreatic, colon, and breast tumors. Mol Cancer Ther. 2006;5:2676–2684. doi: 10.1158/1535-7163.MCT-06-0166. [DOI] [PubMed] [Google Scholar]
- 24.Buga AM, Docea AO, Albu C, Malin RD, Branisteanu DE, Ianosi G, Ianosi SL, Iordache A, Calina D. Molecular and cellular stratagem of brain metastases associated with melanoma. Oncol Lett. 2019;17:4170–4175. doi: 10.3892/ol.2019.9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan S, Scheulen ME, Johnston S, Mross K, Cardoso F, Dittrich C, Eiermann W, Hess D, Morant R, Semiglazov V. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2005;23:5314–5322. doi: 10.1200/JCO.2005.66.130. [DOI] [PubMed] [Google Scholar]
- 26.Chiarini F, Evangelisti C, Mccubrey JA, Martelli AM. Current treatment strategies for inhibiting mTOR in cancer. Trends Pharmacol Sci. 2015;36:124–135. doi: 10.1016/j.tips.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, Vincent JP, Ellston R, Jones D, Sini P. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Can Res. 2010;70:288–298. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 28.De Mattos-Arruda L. PIK3CA mutation inhibition in hormone receptor-positive breast cancer: time has come. ESMO Open 2020; 5. [DOI] [PMC free article] [PubMed]
- 29.Docea AO, Mitrut P, Grigore D, Pirici D, Calina DC, Gofita E. Immunohistochemical expression of TGF beta (TGF-beta), TGF beta receptor 1 (TGFBR1), and Ki67 in intestinal variant of gastric adenocarcinomas. Rom J Morphol Embryol. 2012;53:683–692. [PubMed] [Google Scholar]
- 30.Dong C, Wu J, Chen Y, Nie J, Chen C. Activation of PI3K/AKT/mTOR pathway causes drug resistance in breast cancer. Front Pharmacol. 2021;12:628690. doi: 10.3389/fphar.2021.628690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dowling RJ, Pollak M, Sonenberg N. Current status and challenges associated with targeting mTOR for cancer therapy. BioDrugs. 2009;23:77–91. doi: 10.2165/00063030-200923020-00002. [DOI] [PubMed] [Google Scholar]
- 32.Easton J, Houghton P. mTOR and cancer therapy. Oncogene. 2006;25:6436–6446. doi: 10.1038/sj.onc.1209886. [DOI] [PubMed] [Google Scholar]
- 33.El Hage A, Dormond O. Combining mTOR inhibitors and T cell-based immunotherapies in cancer treatment. Cancers (Basel) 2021;13:1359. doi: 10.3390/cancers13061359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 35.Fouqué A, Delalande O, Jean M, Castellano R, Josselin E, Malleter M, Shoji KF, Hung MD, Rampanarivo H, Collette Y, Van De Weghe P, Legembre P. A novel covalent mTOR inhibitor, DHM25, shows in vivo antitumor activity against triple-negative breast cancer cells. J Med Chem. 2015;58:6559–6573. doi: 10.1021/acs.jmedchem.5b00991. [DOI] [PubMed] [Google Scholar]
- 36.Fu W, Hall MN. Regulation of mTORC2 signaling. Genes. 2020;11:1045. doi: 10.3390/genes11091045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.García-Martínez JM, Moran J, Clarke RG, Gray A, Cosulich SC, Chresta CM, Alessi DR. Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR) Biochem J. 2009;421:29–42. doi: 10.1042/BJ20090489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greene MW, Sakaue H, Wang L, Alessi DR, Roth RA. Modulation of insulin-stimulated degradation of human insulin receptor substrate-1 by Serine 312 phosphorylation. J Biol Chem. 2003;278:8199–8211. doi: 10.1074/jbc.M209153200. [DOI] [PubMed] [Google Scholar]
- 39.Guertin DA, Sabatini DM. An expanding role for mTOR in cancer. Trends Mol Med. 2005;11:353–361. doi: 10.1016/j.molmed.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 41.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Hare SH, Harvey AJ. mTOR function and therapeutic targeting in breast cancer. Am J Cancer Res. 2017;7:383–404. [PMC free article] [PubMed] [Google Scholar]
- 43.Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR. The TSC1-2 tumor suppressor controls insulin–PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hassan B, Akcakanat A, Sangai T, Evans KW, Adkins F, Eterovic AK, Zhao H, Chen K, Chen H, Do KA, Xie SM, Holder AM, Naing A, Mills GB, Meric-Bernstam F. Catalytic mTOR inhibitors can overcome intrinsic and acquired resistance to allosteric mTOR inhibitors. Oncotarget. 2014;5:8544–8557. doi: 10.18632/oncotarget.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- 46.Hidalgo M, Buckner JC, Erlichman C, Pollack MS, Boni JP, Dukart G, Marshall B, Speicher L, Moore L, Rowinsky EK. A phase I and pharmacokinetic study of temsirolimus (CCI-779) administered intravenously daily for 5 days every 2 weeks to patients with advanced cancer. Clin Cancer Res. 2006;12:5755–5763. doi: 10.1158/1078-0432.CCR-06-0118. [DOI] [PubMed] [Google Scholar]
- 47.Ianoși SL, Batani A, Ilie MA, Tampa M, Georgescu SR, Zurac S, Boda D, Ianosi NG, Neagoe D, Calina D, Tutunaru C, Constantin C. Non-invasive imaging techniques for the in vivo diagnosis of Bowen's disease: three case reports. Oncol Lett. 2019;17:4094–4101. doi: 10.3892/ol.2019.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iwenofu OH, Lackman RD, Staddon AP, Goodwin DG, Haupt HM, Brooks JSJ. Phospho-S6 ribosomal protein: a potential new predictive sarcoma marker for targeted mTOR therapy. Mod Pathol. 2008;21:231–237. doi: 10.1038/modpathol.3800995. [DOI] [PubMed] [Google Scholar]
- 49.Jain D, Chaudhary P, Varshney N, Bin Razzak KS, Verma D, Zahra TRK, Janmeda P, Sharifi-Rad J, Dastan SD, Mahmud S, Docea AO, Calina D. Tobacco smoking and liver cancer risk: potential avenues for carcinogenesis. J Oncol 2021. [DOI] [PMC free article] [PubMed]
- 50.Kaplan J, Verheijen JC, Brooijmans N, Toral-Barza L, Hollander I, Yu K, Zask A. Discovery of 3, 6-dihydro-2H-pyran as a morpholine replacement in 6-aryl-1H-pyrazolo [3, 4-d] pyrimidines and 2-arylthieno [3, 2-d] pyrimidines: ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR) Bioorg Med Chem Lett. 2010;20:640–643. doi: 10.1016/j.bmcl.2009.11.050. [DOI] [PubMed] [Google Scholar]
- 51.Kholodov AS, Tarasenko IA, Zinkova EA, Teodoro M, Docea AO, Calina D, Tsatsakis A, Golokhvast KS. The study of airborne particulate matter in Dalnegorsk town. Int J Environ Res Public Health. 2021;18:9234. doi: 10.3390/ijerph18179234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kiwerska K, Szyfter K. DNA repair in cancer initiation, progression, and therapy-a double-edged sword. J Appl Genet. 2019;60:329–334. doi: 10.1007/s13353-019-00516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuroshima K, Yoshino H, Okamura S, Tsuruda M, Osako Y, Sakaguchi T, Sugita S, Tatarano S, Nakagawa M, Enokida H. Potential new therapy of Rapalink-1, a new generation mammalian target of rapamycin inhibitor, against sunitinib-resistant renal cell carcinoma. Cancer Sci. 2020;111:1607–1618. doi: 10.1111/cas.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kwitkowski VE, Prowell TM, Ibrahim A, Farrell AT, Justice R, Mitchell SS, Sridhara R, Pazdur R. FDA approval summary: temsirolimus as treatment for advanced renal cell carcinoma. Oncologist. 2010;15:428–435. doi: 10.1634/theoncologist.2009-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee BJ, Mallya S, Dinglasan N, Fung A, Nguyen T, Herzog L-O, Thao J, Lorenzana EG, Wildes D, Singh M, Smith JAM, Fruman DA. Efficacy of a novel bi-steric mTORC1 inhibitor in models of B-cell acute lymphoblastic leukemia. Front Oncol. 2021;11:673213. doi: 10.3389/fonc.2021.673213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin F, Buil L, Sherris D, Beijnen JH, Van Tellingen O. Dual mTORC1 and mTORC2 inhibitor Palomid 529 penetrates the blood–brain barrier without restriction by ABCB1 and ABCG2. Int J Cancer. 2013;133:1222–1233. doi: 10.1002/ijc.28126. [DOI] [PubMed] [Google Scholar]
- 57.Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21:183–203. doi: 10.1038/s41580-019-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lutz M, Mielke S. New perspectives on the use of mTOR inhibitors in allogeneic haematopoietic stem cell transplantation and graft-versus-host disease. Br J Clin Pharmacol. 2016;82:1171–1179. doi: 10.1111/bcp.13022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma G, Song L, Ma N, Shuai J, Wu W, Wan J, Zhao Z, Li G, Yin S, Ding S, Li J, Jia B, Tong X, Mo D, Gao F, Sun X, Deng Y, Huo X, Li W, Chen K, Miao Z. Safety and efficacy of rapamycin-eluting vertebral stents in patients with symptomatic extracranial vertebral artery stenosis. Front Neurol. 2021;12:2147. doi: 10.3389/fneur.2021.649426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol. 2004;167:399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller N. Abstract B146: XL388: a novel, selective, orally bioavailable mTORC1 and mTORC2 inhibitor that demonstrates pharmacodynamic and antitumor activity in multiple human cancer xenograft models. AACR.2009
- 62.Mita MM, Mita AC, Chu QS, Rowinsky EK, Fetterly GJ, Goldston M, Patnaik A, Mathews L, Ricart AD, Mays T, Knowles H, Rivera VM, Kreisberg J, Bedrosian CL, Tolcher AW. Phase I trial of the novel mammalian target of rapamycin inhibitor deforolimus (AP23573; MK-8669) administered intravenously daily for 5 days every 2 weeks to patients with advanced malignancies. J Clin Oncol. 2008;26:361–367. doi: 10.1200/JCO.2007.12.0345. [DOI] [PubMed] [Google Scholar]
- 63.Mohan S, Vander Broek R, Shah S, Eytan DF, Pierce ML, Carlson SG, Coupar JF, Zhang J, Cheng H, Chen Z. MEK inhibitor PD-0325901 overcomes resistance to PI3K/mTOR inhibitor PF-5212384 and potentiates antitumor effects in human head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:3946–3956. doi: 10.1158/1078-0432.CCR-14-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA, Hollaender N, Urbanowitz G, Berg WJ, Kay A, Lebwohl D, Ravaud A. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 65.Netland IA, Førde HE, Sleire L, Leiss L, Rahman MA, Skeie BS, Gjerde CH, Enger P, Goplen D. Dactolisib (NVP-BEZ235) toxicity in murine brain tumour models. BMC Cancer. 2016;16:657. doi: 10.1186/s12885-016-2712-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O'reilly KE, Rojo F, She Q-B, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Can Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ohm B, Jungraithmayr W. B cell immunity in lung transplant rejection - effector mechanisms and therapeutic implications. Front Immunol. 2022;13:845867. doi: 10.3389/fimmu.2022.845867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pavlova NN, Zhu J, Thompson CB. The hallmarks of cancer metabolism: still emerging. Cell Metab. 2022;34:355–377. doi: 10.1016/j.cmet.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petroulakis E, Mamane Y, Le Bacquer O, Shahbazian D, Sonenberg N. mTOR signaling: implications for cancer and anticancer therapy. Br J Cancer. 2006;94:195–199. doi: 10.1038/sj.bjc.6602902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Popova NV, Jücker M. The role of mTOR signaling as a therapeutic target in cancer. Int J Mol Sci. 2021;22:1743. doi: 10.3390/ijms22041743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886–1918. doi: 10.3390/ijms13021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quetglas-Llabrés MM, Quispe C, Herrera-Bravo J, Catarino MD, Pereira OR, Cardoso SM, Dua K, Chellappan DK, Pabreja K, Satija S, Mehta M, Sureda A, Martorell M, Satmbekova D, Yeskaliyeva B, Sharifi-Rad J, Rasool N, Butnariu M, Bagiu IC, Bagiu RV, Calina D, Cho WC. Pharmacological properties of bergapten: mechanistic and therapeutic aspects. Oxid Med Cell Longev. 2022;2022:8615242. doi: 10.1155/2022/8615242. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.Raymond E, Alexandre J, Faivre S, Vera K, Materman E, Boni J, Leister C, Korth-Bradley J, Hanauske A, Armand J-P. Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol. 2004;22:2336–2347. doi: 10.1200/JCO.2004.08.116. [DOI] [PubMed] [Google Scholar]
- 74.Rehan M. An anti-cancer drug candidate OSI-027 and its analog as inhibitors of mTOR: computational insights into the inhibitory mechanisms. J Cell Biochem. 2017;118:4558–4567. doi: 10.1002/jcb.26117. [DOI] [PubMed] [Google Scholar]
- 75.Rodrik-Outmezguine VS, Okaniwa M, Yao Z, Novotny CJ, Mcwhirter C, Banaji A, Won H, Wong W, Berger M, De Stanchina E, Barratt DG, Cosulich S, Klinowska T, Rosen N, Shokat KM. Overcoming mTOR resistance mutations with a new-generation mTOR inhibitor. Nature. 2016;534:272–276. doi: 10.1038/nature17963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 77.Sabatini DM. Twenty-five years of mTOR: uncovering the link from nutrients to growth. Proc Natl Acad Sci. 2017;114:11818–11825. doi: 10.1073/pnas.1716173114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sani TA, Mohammadpour E, Mohammadi A, Memariani T, Yazdi MV, Rezaee R, Calina D, Docea AO, Goumenou M, Etemad L, Shahsavand S. Cytotoxic and apoptogenic properties of Dracocephalum kotschyi aerial part different fractions on calu-6 and mehr-80 lung cancer cell lines. Farmacia. 2017;65:189–199. [Google Scholar]
- 79.Santoni M, Massari F. Chapter 25—mTOR pathway in renal cell carcinoma. In: Maiese K, editor. Molecules to medicine with mTOR. Boston: Academic Press; 2016. [Google Scholar]
- 80.Saran U, Foti M, Dufour JF. Cellular and molecular effects of the mTOR inhibitor everolimus. Clin Sci (Lond) 2015;129:895–914. doi: 10.1042/CS20150149. [DOI] [PubMed] [Google Scholar]
- 81.Sarbassov DD, Ali SM, Sengupta S, Sheen J-H, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 82.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the Rictor-mTOR complex. Science. 2005;307:1098. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 83.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scalea JR, Levi ST, Ally W, Brayman KL. Tacrolimus for the prevention and treatment of rejection of solid organ transplants. Expert Rev Clin Immunol. 2016;12:333–342. doi: 10.1586/1744666X.2016.1123093. [DOI] [PubMed] [Google Scholar]
- 85.Schreiber KH, Arriola Apelo SI, Yu D, Brinkman JA, Velarde MC, Syed FA, Liao C-Y, Baar EL, Carbajal KA, Sherman DS, Ortiz D, Brunauer R, Yang SE, Tzannis ST, Kennedy BK, Lamming DW. A novel rapamycin analog is highly selective for mTORC1 in vivo. Nat Commun. 2019;10:3194. doi: 10.1038/s41467-019-11174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schuler W, Sedrani R, Cottens S, Häberlin B, Schulz M, Schuurman H-J, Zenke G, Zerwes H-G, Schreier MH. SDZ RAD, a new rapamycin derivative: pharmacological properties in vitro and in vivo. Transplantation. 1997;64:36–42. doi: 10.1097/00007890-199707150-00008. [DOI] [PubMed] [Google Scholar]
- 87.Schuurman H-J, Cottens S, Fuchs S, Joergensen J, Meerloo T, Sedrani R, Tanner M, Zenke G, Schuler W. SDZ RAD, a new rapamycin derivative: synergism with cyclosporine. Transplantation. 1997;64:32–35. doi: 10.1097/00007890-199707150-00007. [DOI] [PubMed] [Google Scholar]
- 88.Selvarani R, Mohammed S, Richardson A. Effect of rapamycin on aging and age-related diseases—past and future. GeroScience. 2021;43:1135–1158. doi: 10.1007/s11357-020-00274-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Semwal P, Painuli S, Abu-Izneid T, Rauf A, Sharma A, Daştan SD, Kumar M, Alshehri MM, Taheri Y, Das R, Mitra S, Emran TB, Sharifi-Rad J, Calina D, Cho WC. Diosgenin: an updated pharmacological review and therapeutic perspectives. Oxid Med Cell Longev. 2022;2022:1035441. doi: 10.1155/2022/1035441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol. 2004;14:1650–1656. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 91.Sharifi-Rad J, Quispe C, Butnariu M, Rotariu LS, Sytar O, Sestito S, Rapposelli S, Akram M, Iqbal M, Krishna A, Kumar NVA, Braga SS, Cardoso SM, Jafernik K, Ekiert H, Cruz-Martins N, Szopa A, Villagran M, Mardones L, Martorell M, Docea AO, Calina D. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 2021;21:318–318. doi: 10.1186/s12935-021-02025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharifi-Rad J, Quispe C, Imran M, Rauf A, Nadeem M, Gondal TA, Ahmad B, Atif M, Mubarak MS, Sytar O, Zhilina OM, Garsiya ER, Smeriglio A, Trombetta D, Pons DG, Martorell M, Cardoso SM, Razis AFA, Sunusi U, Kamal RM, Rotariu LS, Butnariu M, Docea AO, Calina D. Genistein: an integrative overview of its mode of action, pharmacological properties, and health benefits. Oxid Med Cell Longev. 2021;2021:3268136. doi: 10.1155/2021/3268136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharifi-Rad J, Quispe C, Patra JK, Singh YD, Panda MK, Das G, Adetunji CO, Michael OS, Sytar O, Polito L, Živković J, Cruz-Martins N, Klimek-Szczykutowicz M, Ekiert H, Choudhary MI, Ayatollahi SA, Tynybekov B, Kobarfard F, Muntean AC, Grozea I, Daştan SD, Butnariu M, Szopa A, Calina D. Paclitaxel: application in modern oncology and nanomedicine-based cancer therapy. Oxid Med Cell Longev. 2021;2021:3687700. doi: 10.1155/2021/3687700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharifi-Rad M, Kumar NVA, Zucca P, Varoni EM, Dini L, Panzarini E, Rajkovic J, Fokou PVT, Azzini E, Peluso I, Mishra AP, Nigam M, El Rayess Y, El Beyrouthy M, Polito L, Iriti M, Martins N, Martorell M, Docea AO, Setzer WN, Calina D, Cho WC, Sharifi-Rad J. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front Physiol. 2020;11:21. doi: 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sini P, James D, Chresta C, Guichard S. Simultaneous inhibition of mTORC1 and mTORC2 by mTOR kinase inhibitor AZD8055 induces autophagy and cell death in cancer cells. Autophagy. 2010;6:553–554. doi: 10.4161/auto.6.4.11671. [DOI] [PubMed] [Google Scholar]
- 96.Skeen JE, Bhaskar PT, Chen C-C, Chen WS, Peng X-D, Nogueira V, Hahn-Windgassen A, Kiyokawa H, Hay N. Akt deficiency impairs normal cell proliferation and suppresses oncogenesis in a p53-independent and mTORC1-dependent manner. Cancer Cell. 2006;10:269–280. doi: 10.1016/j.ccr.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 97.Smolewski P. Recent developments in targeting the mammalian target of rapamycin (mTOR) kinase pathway. Anticancer Drugs. 2006;17:487–494. doi: 10.1097/00001813-200606000-00001. [DOI] [PubMed] [Google Scholar]
- 98.Takei N, Nawa H. mTOR signaling and its roles in normal and abnormal brain development. Front Mol Neurosci. 2014;7:28. doi: 10.3389/fnmol.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tao Z, Li T, Ma H, Yang Y, Zhang C, Hai L, Liu P, Yuan F, Li J, Yi L, Tong L, Wang Y, Xie Y, Ming H, Yu S, Yang X. Autophagy suppresses self-renewal ability and tumorigenicity of glioma-initiating cells and promotes Notch1 degradation. Cell Death Dis. 2018;9:1063. doi: 10.1038/s41419-018-0957-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thoreen CC, Sabatini DM. Rapamycin inhibits mTORC1, but not completely. Autophagy. 2009;5:725–726. doi: 10.4161/auto.5.5.8504. [DOI] [PubMed] [Google Scholar]
- 102.Tian T, Li X, Zhang J. mTOR signaling in cancer and mTOR inhibitors in solid tumor targeting therapy. Int J Mol Sci. 2019;20:755. doi: 10.3390/ijms20030755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tsang CK, Qi H, Liu LF, Zheng XS. Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Discov Today. 2007;12:112–124. doi: 10.1016/j.drudis.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 104.Turgeon M-O, Perry NJS, Poulogiannis G. DNA damage, repair, and cancer metabolism. Front Oncol. 2018;8:15. doi: 10.3389/fonc.2018.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vellanki S, Garcia AE, Lee SC. Interactions of FK506 and rapamycin with FK506 binding protein 12 in opportunistic human fungal pathogens. Front Mol Biosci. 2020;7:588913. doi: 10.3389/fmolb.2020.588913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Verheijen JC, Richard DJ, Curran K, Kaplan J, Lefever M, Nowak P, Malwitz DJ, Brooijmans N, Toral-Barza L, Zhang W-G. Discovery of 4-morpholino-6-aryl-1 H-pyrazolo [3, 4-d] pyrimidines as highly potent and selective ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR): optimization of the 6-aryl substituent. J Med Chem. 2009;52:8010–8024. doi: 10.1021/jm9013828. [DOI] [PubMed] [Google Scholar]
- 107.Vezina C, Kudelski A, Sehgal S. Rapamycin (AY-22, 989), a new antifungal antibiotic I. taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot. 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 108.Viana SD, Reis F, Alves R. Therapeutic use of mTOR inhibitors in renal diseases: advances, drawbacks, and challenges. Oxid Med Cell Longev. 2018;2018:3693625. doi: 10.1155/2018/3693625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Villa E, Ali ES, Sahu U, Ben-Sahra I. Cancer cells tune the signaling pathways to empower de novo synthesis of nucleotides. Cancers (Basel) 2019;11(5):688. doi: 10.3390/cancers11050688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Waldner M, Fantus D, Solari M, Thomson AW. New perspectives on mTOR inhibitors (rapamycin, rapalogs and TORKinibs) in transplantation. Br J Clin Pharmacol. 2016;82:1158–1170. doi: 10.1111/bcp.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang J, Liang D, Zhang XP, He CF, Cao L, Zhang SQ, Xiao X, Li SJ, Cao YX. Novel PI3K/Akt/mTOR signaling inhibitor, W922, prevents colorectal cancer growth via the regulation of autophagy. Int J Oncol. 2021;58:70–82. doi: 10.3892/ijo.2020.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Witzig TE, Geyer SM, Ghobrial I, Inwards DJ, Fonseca R, Kurtin P, Ansell SM, Luyun R, Flynn PJ, Morton RF. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23:5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 113.Xie H, Lee M-H, Zhu F, Reddy K, Huang Z, Kim DJ, Li Y, Peng C, Lim DY, Kang S, Jung SK, Li X, Li H, Ma W, Lubet RA, Ding J, Bode AM, Dong Z. Discovery of the novel mTOR inhibitor and its antitumor activities in vitro and in vivo. Mol Cancer Ther. 2013;12:950–958. doi: 10.1158/1535-7163.MCT-12-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xie J, Wang X, Proud CG. mTOR inhibitors in cancer therapy. F1000Res 2016; 5. [DOI] [PMC free article] [PubMed]
- 115.Xu Z, Chu M. Advances in immunosuppressive agents based on signal pathway. Front Pharmacol. 2022;13:917162. doi: 10.3389/fphar.2022.917162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yan J, Zhou H, Kong L, Zhang J, Zhao Q, Li Y. Identification of two novel inhibitors of mTOR signaling pathway based on high content screening. Cancer Chemother Pharmacol. 2013;72:799–808. doi: 10.1007/s00280-013-2255-1. [DOI] [PubMed] [Google Scholar]
- 117.Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18:26. doi: 10.1186/s12943-019-0954-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yee KW, Zeng Z, Konopleva M, Verstovsek S, Ravandi F, Ferrajoli A, Thomas D, Wierda W, Apostolidou E, Albitar M, O’brien S, Andreeff M, Giles FJ. Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (RAD001) in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2006;12:5165–5173. doi: 10.1158/1078-0432.CCR-06-0764. [DOI] [PubMed] [Google Scholar]
- 119.Yu K, Toral-Barza L, Shi C, Zhang WG, Lucas J, Shor B, Kim J, Verheijen J, Curran K, Malwitz DJ, Cole DC, Ellingboe J, Ayral-Kaloustian S, Mansour TS, Gibbons JJ, Abraham RT, Nowak P, Zask A. Biochemical, cellular, and in vivo activity of novel ATP-competitive and selective inhibitors of the mammalian target of rapamycin. Cancer Res. 2009;69:6232–6240. doi: 10.1158/0008-5472.CAN-09-0299. [DOI] [PubMed] [Google Scholar]
- 120.Zask A, Kaplan J, Verheijen JC, Richard DJ, Curran K, Brooijmans N, Bennett EM, Toral-Barza L, Hollander I, Ayral-Kaloustian S. Morpholine derivatives greatly enhance the selectivity of mammalian target of rapamycin (mTOR) inhibitors. J Med Chem. 2009;52:7942–7945. doi: 10.1021/jm901415x. [DOI] [PubMed] [Google Scholar]
- 121.Zhang H, Bajraszewski N, Wu E, Wang H, Moseman AP, Dabora SL, Griffin JD, Kwiatkowski DJ. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J Clin Investig. 2007;117:730–738. doi: 10.1172/JCI28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Z, Zhou L, Xie N, Nice EC, Zhang T, Cui Y, Huang C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct Target Ther. 2020;5:113–113. doi: 10.1038/s41392-020-00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zlatian OM, Comanescu MV, Rosu AF, Rosu L, Cruce M, Gaman AE, Calina CD, Sfredel V. Histochemical and immunohistochemical evidence of tumor heterogeneity in colorectal cancer. Rom J Morphol Embryol. 2015;56:175–181. [PubMed] [Google Scholar]
- 124.Zou Z, Tao T, Li H, Zhu X. mTOR signaling pathway and mTOR inhibitors in cancer: progress and challenges. Cell Biosci. 2020;10:31. doi: 10.1186/s13578-020-00396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not Applicable.