Abstract
Introduction
Patients with post-COVID-19 syndrome may present cognitive and emotional symptomatology. This study aims to analyse the results of an outpatient neuropsychological intervention programme for post-COVID-19 syndrome.
Method
In June 2020 Institut Guttmann started an outpatient post-COVID-19 neurorehabilitation programme, including respiratory therapy, physiotherapy, and neuropsychological rehabilitation. Before and after the programme, the cognitive-emotional state of all participants is assessed. Six months after treatment, a follow-up assessment is administered (which includes a collection of information on various aspects of daily life).
Results
The sample analysed consisted of 123 patients (mean age: 51 years, SD: 12.41). Seventy-four per cent (n = 91) had cognitive impairment and underwent cognitive treatment (experimental group); the remaining 26% (n = 32) constituted the control group. After the intervention, the experimental group improved in working memory, verbal memory (learning, recall and recognition), verbal fluency and anxious-depressive symptomatology. The control group showed changes in immediate memory, verbal memory (learning and recognition) and depressive symptomatology, although the effect size in the latter two was smaller than in the experimental group. Six months after treatment, 44.9% of the patients were unable to perform their pre-COVID-19 work activity, and 81.2% reported difficulties in their activities of daily living.
Conclusions
Neuropsychological rehabilitation is an effective tool to treat the cognitive-emotional deficits present in post-COVID-19 syndrome. However, months after the end of treatment, not all patients recover their pre-COVID-19 functional level.
Keywords: Post–COVID-19 syndrome, Neuropsychological rehabilitation, Cognition, Emotion, Longitudinal study
Abstract
Introducción
Las personas con síndrome post-COVID-19 pueden presentar sintomatología cognitiva y emocional. Este estudio tiene como objetivo analizar los resultados de un programa ambulatorio de intervención neuropsicológica dirigido a pacientes con síndrome post-COVID-19.
Método
En junio de 2020 Institut Guttmann inicia un programa ambulatorio de neurorrehabilitación post-COVID-19, que incluye terapia respiratoria, fisioterapia y rehabilitación neuropsicológica. Antes y después del programa se valora el estado cognitivo-emocional de todos los participantes. Seis meses después del tratamiento se administra una valoración de seguimiento (en la que se recoge información sobre diversos aspectos de la vida diaria).
Resultados
La muestra analizada estaba formada por 123 pacientes (edad media: 51 años, DS: 12,41). El 74% (n = 91) presentaba alteraciones cognitivas y realizó tratamiento cognitivo (grupo experimental); el 26% (n = 32) restante constituyó el grupo control. Tras la intervención, el grupo experimental mejoró en memoria de trabajo, memoria verbal (aprendizaje, recuerdo y reconocimiento), fluencia verbal y sintomatología ansioso-depresiva. El grupo control mostró cambios en memoria inmediata, memoria verbal (aprendizaje y reconocimiento) y sintomatología depresiva, si bien el tamaño del efecto en las dos últimas fue menor que en el grupo experimental. Seis meses después del tratamiento, el 44,9% de los pacientes no podía realizar la actividad laboral previa al COVID-19. El 81,2% refirió dificultades en sus actividades de la vida diaria.
Conclusiones
La rehabilitación neuropsicológica es una herramienta eficaz para tratar las alteraciones cognitivo-emocionales presentes en el síndrome post-COVID-19. Sin embargo, meses después de finalizar el tratamiento, no todos los pacientes recuperan el nivel funcional pre-COVID-19.
Palabras clave: Síndrome post-COVID-19, Rehabilitación neuropsicológica, Cognición, Emoción, Estudio longitudinal
Introduction
Since the official report of the first COVID-19 outbreak in December 2019, several research groups have analysed the neuropsychological impact of the disease. Published studies suggest that it causes attentional, concentration, memory, and executive function deficits, as well as language alterations manifesting as anomia.1, 2, 3, 4 These deficits may be present in the acute phase,1 and even persist for weeks or months after the SARS-CoV-2 infection.2, 3, 4 Together with these cognitive alterations, symptoms of anxiety and depression have also been observed. The review conducted by Vanderlind et al.5 reports considerable variability in the presence of anxiety (5%–55% of cases) and depression (10%–68.5% of cases). These authors suggest that the prevalence of symptoms of anxiety and depression is similar in hospitalised and non-hospitalised patients.5 Tomasoni et al.6 observed that anxiety and depression are associated with the persistence of several such symptoms as asthenia, dyspnoea, and cognitive deficits. Alemanno et al.7 and Wahlgren et al.8 place special emphasis on the need for cognitive and emotional aspects to be integrated into the assessment of patients with COVID-19.
Most patients with SARS-CoV-2 infection recover within days or weeks. However, approximately 10% continue to present symptoms months after infection, which is known as post–COVID-19 syndrome.9, 10 This syndrome is characterised by a series of symptoms that appear during or after SARS-CoV-2 infection, persist for longer than 12 weeks, and cannot be explained by other causes. It usually presents as a combination of symptoms that frequently overlap and may fluctuate and change over time, affecting multiple systems.11
Cognitive alterations are one of the symptoms described by patients with post-COVID-19 syndrome.12, 13, 14 These alterations affect 15% of patients,15 although their prevalence varies according to the method of assessment. The meta-analysis by Ceban et al.16 highlights that studies using objective measures (e.g., cognitive tests) detect greater prevalence of cognitive alterations than those using subjective measures (for example, questionnaires). Cognitive deficits may present in multiple domains, mainly affecting attention, concentration, and executive function.17, 18 Deficits in episodic memory17, 18 and information processing speed20, 22 have also been observed. Symptoms of anxiety and depression have also been observed in patients with post–COVID-19 syndrome several months after the acute phase of the disease. Huang et al.20 suggest that 23% of patients who required hospitalisation in the acute phase present symptoms of anxiety and depression 6 months after hospital discharge.
The post–COVID-19 syndrome may have a significant functional impact, especially in the workplace. In the survey conducted by David et al.,12 27.3% of individuals with symptoms at the time of the survey were working in the same conditions as before the infection. A reduction in working hours was required by 45.6%, and 23.3% had not returned to their jobs. The remaining 3.8% corresponds to individuals who worked as volunteers or did not mention their work.
Several neurorehabilitation programmes have been implemented with the aim of treating the sequelae of COVID-19.21, 22, 23, 24 Few studies into neuropsychological rehabilitation have been published.25, 26, 27 Although it is too early to determine its effectiveness, preliminary results suggest that neuropsychological rehabilitation is a potentially useful tool for treating cognitive/emotional deficits associated with post-COVID-19 syndrome.26 However, no information is currently available on the durability of symptoms after the treatment is completed. The objective of this study is to analyse the results of a neuropsychological rehabilitation programme aimed at patients with post–COVID-19 syndrome. The effects of treatment were evaluated by applying an evaluation protocol before and after the intervention, and at 6 months after treatment.
Material and methods
Participants
In June 2020, the Institut Guttmann neurorehabilitation hospital started an outpatient neurorehabilitation programme aimed at patients with post–COVID-19 sequelae. In our study, we selected patients meeting the following inclusion criteria: 1) age of 18 years or older at the time of the infection, 2) PCR-confirmed SARS-CoV-2 infection, and 3) meeting criteria for post–COVID-19 syndrome.9, 10 The British National Institute for Health and Care Excellence (NICE) established that post–COVID-19 syndrome is characterised by a series of signs and symptoms that develop during or after the SARS-CoV-2 infection and persist beyond 12 weeks after infection, which cannot be explained by other diagnoses. We excluded those patients who presented 1) neurological and/or psychiatric disease prior to SARS-CoV-2 infection, 2) cognitive impairment prior to COVID-19, and 3) severe neurological disease secondary to the virus (e.g., encephalopathy, stroke, myelopathy, or polyradiculoneuropathies).
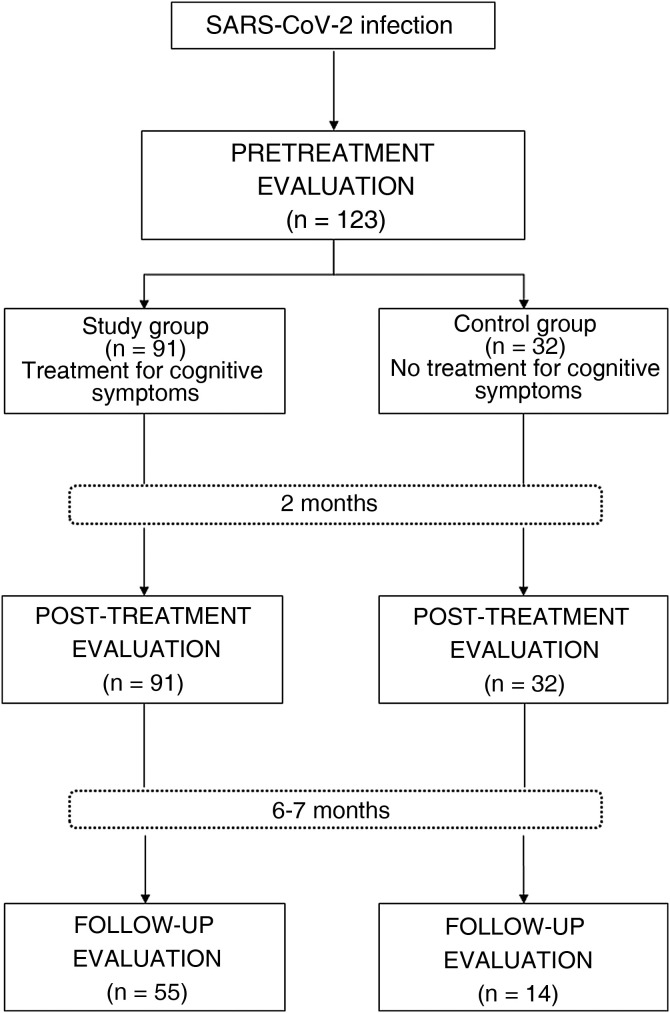
Between June 2020 and September 2021, 134 patients participated in our post–COVID-19 syndrome outpatient rehabilitation programme. A total of 123 met the study inclusion criteria. Table 1 provides a summary of the demographic and clinical data of the sample.
Table 1.
Clinical and demographic variables of the patients included in the study.
| Total sample (n = 123) |
Study group (n = 91) |
Control group (n = 32) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Standard deviation | Range | Mean | Standard deviation | Range | Mean | Standard deviation | Range | |
| Age | 51.02 | 12.40 | 20-81 | 49.7 | 12.06 | 20-77 | 54.69 | 12.85 | 28-81 |
| Time between infection and treatment onset (in months) | 7.7 | 3.45 | 3-17.1 | 7.4 | 3.1 | 3-16.6 | 8.5 | 4.03 | 3.13-17.1 |
| Frequency | Frequency | Frequency | |||||||
| Sex | |||||||||
| Men | 51 (41.5%) | 36 (39.6%) | 15 (46.9%) | ||||||
| Women | 72 (58.5%) | 55 (60.4%) | 17 (53.1%) | ||||||
| Level of educationa | |||||||||
| Primary education | 18 (14.6%) | 14 (15.4%) | 4 (12.5%) | ||||||
| Secondary education | 36 (29.3%) | 25 (27.5%) | 11 (34.4%) | ||||||
| Further education | 69 (56.1%) | 52 (57.1%) | 17 (53.1%) | ||||||
| Admitted to hospitalb | |||||||||
| No | 66 (53.7%) | 52 (57.1%) | 14 (43.8%) | ||||||
| Yes | 57 (46.3%) | 39 (42.9%) | 18 (56.3%) | ||||||
| Ward | 28 (49.12%) | 21 (53.85%) | 7 (38.9%) | ||||||
| ICU | 29 (50.88%) | 18 (46.15%) | 11 (61.1%) | ||||||
ICU: intensive care unit.
Primary education (≤ 8 years of formal schooling); secondary education (9-12 years of formal schooling); further education (≥ 13 years of formal schooling).
Patients requiring hospital admission in the acute phase COVID-19.
Of our sample, 74% of patients (n = 91) showed cognitive alterations (detected by neuropsychological examination) and underwent treatment for cognitive symptoms as part of the post–COVID-19 syndrome outpatient rehabilitation programme. This group of patients was considered the study group. The remaining 26% (n = 32) presented normal results in the neuropsychological examination and therefore did not receive treatment for cognitive symptoms; these were treated as the control group (Fig. 1 ). All participants presenting symptoms of anxiety and depression underwent emotional intervention, regardless of the group in which they were included.
Figure 1.
Flow chart of the study.
Instruments
All patients included in the post–COVID-19 syndrome outpatient rehabilitation programme were assessed using a brief neuropsychological battery. Due to the pandemic (years 2020 and 2021), the battery was designed to be administered both at the consultation and remotely (e.g., by telephone or videoconference).
The battery tested the following cognitive domains: orientation to person, space, and time, assessed using the corresponding subtest of the Barcelona Test28; attention span and working memory, tested with the forward and backward digit span of the Wechsler Adult Intelligence Scale III29, 30; learning and long-term memory (verbal memory, delayed free recall and recognition), evaluated with the Rey Auditory Verbal Learning Test (RAVLT)31, 32; and verbal fluency, assessed with the phonemic verbal fluency test of the Spanish-language neuropsychological battery.33
In addition to these cognitive tests, we administered the Hospital Anxiety and Depression Scale (HADS) with the aim of detecting the possible presence of symptoms of anxiety and depression.34, 35 Lastly, during follow-up, we conducted a brief semi-structured interview to collect information on the impact of the condition on several aspects of daily living. The interview addressed the following issues: 1) employment status (is the patient currently working?, has the patient returned to work as normal?, has the patient experienced difficulties performing everyday workplace tasks?); 2) study (is the patient currently studying?, has the patient noticed any difficulties in following classes or studying?); and 3) presence of difficulties in managing everyday personal issues. Based on the answers obtained, we created the categories included in Table 2 .
Table 2.
Semi-structured interview and response categories.
| Area | Question | Category 1 | Category 2 |
|---|---|---|---|
| Employment status | Is the patient currently working? | No | On leave |
| Retired/pensioner | |||
| Unemployed | |||
| Yes | |||
| Only if the previous question is answered “Yes”. | |||
| Has the patient returned to work as normal? | Yes | Does the patient work in the same conditions as before COVID-19? | |
| No | Works with adaptations | ||
| Works with a reduction in working hours | |||
| Has the patient noticed difficulties in performing daily tasks? | No | ||
| Yes | Attention-focus | ||
| Cognitive slowing | |||
| Memory-retention capacity | |||
| Tiredness-physical fatigue | |||
| Education | Is the patient currently studying? | No | |
| Yes | |||
| Only if the previous question is answered “Yes”. | |||
| Has the patient noticed difficulties in following classes or studying? | No | ||
| Yes | Attention-focus | ||
| Cognitive slowing | |||
| Memory-retention capacity | |||
| Tiredness-physical fatigue | |||
| Management of everyday affairs | Has the patient noticed difficulties in managing everyday affairs? | No | |
| Yes | Frequent forgetfulness | ||
| Executive functions (organisation, multiple tasks) | |||
| Disorientation in space | |||
| Use of external memory aids (diary) | |||
Procedure
The post–COVID-19 syndrome outpatient rehabilitation programme, lasting 8 weeks, included respiratory therapy, physiotherapy, and neuropsychological rehabilitation. The latter included treatment for cognitive symptoms, training in compensatory strategies (to minimise the functional repercussions of cognitive deficits), and an emotional intervention.
Patients underwent treatment for cognitive symptoms from their homes using the telerehabilitation platform Guttmann NeuroPersonalTrainer® (GNPT®).36 Based on the results of the tests included in the pretreatment evaluation, GNPT®’s Intelligent Therapy Assistant (based on artificial intelligence algorithms) created a personalised plan according to the patient’s cognitive profile. This plan included cognitive tasks (“packaged” into 1-hour therapeutic sessions). The catalogue of tasks in the GNPT® includes several cognitive domains: attention (selective, sustained, and divided), memory (verbal, visual, and working), executive functions (planning, inhibition, flexibility, sequencing, and categorisation), and mental calculation. In accordance with the patient’s cognitive profile, the Intelligent Therapy Assistant selects the most suitable tasks and adjusts their difficulty through a combination of parameters (e.g., number of stimuli, type of stimulus, speed of presentation, task duration). The patients included in the study completed a mean of 4.1 sessions of treatment for cognitive symptoms per week. The training in compensatory strategies included the following aspects: 1) promoting the use of preserved cognitive resources, 2) promoting the use of strategies for specific situations (e.g., simplification of activities, time organisation, control of interferences), and 3) training in the use of external aids (e.g., diaries, mobile phone, etc.). Every patient obtaining a score suggestive of symptoms of anxiety or depression on the HADS scale (anxiety ≥ 8 or depression ≥ 8) underwent an emotional intervention. The aims of the intervention were: 1) to reduce the symptoms of anxiety and/or depression, and 2) to provide tools for managing the unease associated with a disease of uncertain prognosis.
We conducted 3 neuropsychological evaluations: one before starting the intervention (pretreatment evaluation), another during the last week of the intervention (post-treatment evaluation), and a third at 6-7 months after treatment (follow-up evaluation). Pretreatment and follow-up evaluations were performed remotely (by telephone). The post-treatment evaluation was performed at the consultation or remotely. Evaluations lasted approximately one hour.
Statistical analysis
We performed a descriptive analysis of the sociodemographic and clinical variables of the total sample, as well as for the study and control groups. We calculated measures of central tendency and dispersion with the aim of determining the characteristics of the variables under study.
For the inferential analysis, we used non-parametric tests as the data did not follow a normal distribution. Intergroup comparisons used the Mann–Whitney U test for 2 independent samples, whereas intragroup comparisons used the Wilcoxon signed-rank test. Values of P < .05 were considered statistically significant in both cases.
The relevance of the differences observed (effect size) was estimated using the Pearson correlation coefficient. As a guideline, we considered the effect size to be small when the r value was between 0.1 and 0.3, moderate when it was between 0.3 and 0.5, and large when it was higher than 0.5.37, 38
Results
Significant differences were observed in sex (P < .001) and age (P < .001) between hospitalised and non-hospitalised patients. Of the hospitalised patients, 64.9% (n = 37) were men; of the non-hospitalised patients, 78.8% (n = 52) were women. Regarding age, hospitalised patients were older than non-hospitalised patients (mean age [SD]: 57 years [12.22] vs 45.8 years [10.04]).
Of all patients, 96.7% presented SARS-CoV-2 infection during the year 2020. Specifically, 62.6% (n = 77) in March, 13% (n = 16) in April, and 21.1% (n = 26) between May and December 2020. The 4 remaining participants (3.3%) presented the infection in 2021 (2 in January, 1 in February, and 1 in April).
We observed no statistically significant differences between groups in terms of clinical and demographic characteristics.
In the pretreatment evaluation, we observed statistically significant differences between groups for the following variables: RAVLT-learning, RAVLT-recall, RAVLT-recognition, and verbal fluency. The control group obtained better scores on these variables than the study group. We did not observe intergroup differences in the post-treatment or follow-up evaluations (Table 3 ).
Table 3.
Intergroup comparisons of the pretreatment, post-treatment, and follow-up evaluations. Direct scores (mean [standard deviation]), level of significance, and effect size.
| Pretreatment evaluation |
Post-treatment evaluation |
Follow-up evaluation |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SG | CG | p | r | SG | CG | p | r | SG | CG | p | r | |
| Orientation to person | 7 (0) | 7 (0) | 1 | 0 | 7 (0) | 7 (0) | 1 | 0 | 7 (0) | 7 (0) | 1 | 0 |
| Orientation to space | 5 (0) | 5 (0) | 1 | 0 | 5 (0) | 5 (0) | 1 | 0 | 5 (0) | 5 (0) | 1 | 0 |
| Orientation to time | 22.98 (0.14) | 22.97 (0.17) | .771 | 0.03 | 23 (0) | 23 (0) | 1 | 0 | 22.84 (0.83) | 22.86 (0.36) | .427 | 0.09 |
| Forward digit span | 5.79 (1.06) | 5.75 (1.19) | .559 | 0.05 | 6.13 (1.18) | 5.91 (1.14) | .296 | 0.09 | 5.71 (1.24) | 6.00 (1.24) | .528 | 0.08 |
| Backward digit span | 4.31 (1.05) | 4.34 (0.86) | .726 | 0.03 | 4.77 (1.13) | 4.37 (0.90) | .122 | 0.14 | 4.55 (1.21) | 4.64 (1.21) | .564 | 0.07 |
| RAVLT-learning | 41.29 (10.11) | 45 (8.06) | .046 | 0.18 | 49.30 (9.38) | 46.19 (7.71) | .092 | 0.14 | 51.35 (12.10) | 51.57 (9.81) | .765 | 0.03 |
| RAVLT-recall | 7.69 (2.59) | 9.22 (2.74) | .004 | 0.26 | 10.18 (2.73) | 9.56 (2.53) | .235 | 0.11 | 10.71 (3.17) | 10.5 (3.52) | .816 | 0.03 |
| RAVLT-recognition | 11.69 (2.79) | 12.5 (3.19) | .047 | 0.18 | 13.21 (2.62) | 13.13 (2.79) | .782 | 0.02 | 12.05 (3.19) | 13.21 (2.22) | .291 | 0.13 |
| Verbal fluency | 35.86 (11.35) | 41.59 (14.65) | .033 | 0.19 | 42.86 (12.65) | 42.78 (14.70) | .708 | 0.03 | 39.93 (13.30) | 44.64 (11.67) | .135 | 0.18 |
| HADS-anxiety | 9.28 (4.33) | 8.66 (5.72) | .453 | 0.07 | 7.84 (4.24) | 8.03 (5.88) | .958 | 0 | 7.91 (5.09) | 7.57 (4.60) | .905 | 0.01 |
| HADS-depression | 8.72 (3.78) | 8.19 (5) | .593 | 0.05 | 6.8 (4.48) | 7.1 (5.37) | .958 | 0 | 7.15 (4.64) | 5.64 (4.39) | .341 | 0.01 |
CG: control group; HADS: Hospital Anxiety and Depression Scale; RAVLT: Rey Auditory Verbal Learning Test; SG: study group.
P-values < .05 are shown in bold.
Direct scores -mean (standard deviation), significance level (P) and effect size (r).
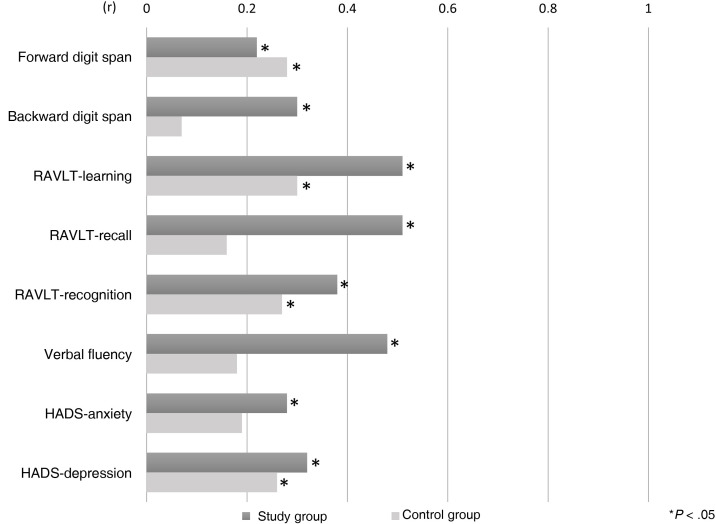
In the study group, we observed statistically significant differences between scores in the pre- and post-treatment evaluations for the forward digit span, backward digit span, RAVLT-learning, RAVLT-recall, RAVLT-recognition, verbal fluency, HADS-anxiety, and HADS-depression. The control group also presented statistically significant differences between scores in the pre- and post-treatment evaluations. Specifically, we found differences in the forward digit span, RAVLT-learning, RAVLT-recognition, and HADS-depression (Table 4 ). The study group presented larger effect sizes than the control group for the backward digit span (0.30 vs 0.07), RAVLT-learning (0.51 vs 0.30), RAVLT-recall (0.51 vs 0.16); RAVLT-recognition (0.38 vs 0.27), verbal fluency (0.48 vs 0.19), HADS-anxiety (0.28 vs 0.19), and HADS-depression (0.32 vs 0.26). The control group only presented a larger effect size than the study group in the forward digit span (0.22 vs 0.28) (Fig. 2 ).
Table 4.
Intragroup comparisons of the pretreatment and post-treatment evaluations. Direct scores (mean [standard deviation]), level of significance, and effect size.
| Study group |
Control group |
|||||||
|---|---|---|---|---|---|---|---|---|
| Pretreatment | Post-treatment | p | r | Pretreatment | Post-treatment | p | r | |
| Orientation to person | 7 (0) | 7 (0) | 1 | 0 | 7 (0) | 7 (0) | 1 | 0 |
| Orientation to space | 5 (0) | 5 (0) | 1 | 0 | 5 (0) | 5 (0) | 1 | 0 |
| Orientation to time | 22.98 (0.14) | 23 (0) | .157 | 0.10 | 22.97 (0.17) | 23 (0) | .317 | 0.12 |
| Forward digit span | 5.79 (1.06) | 6.13 (1.18) | .003 | 0.22 | 5.75 (1.19) | 5.91 (1.14) | .025 | 0.28 |
| Backward digit span | 4.31 (1.05) | 4.77 (1.13) | < .001 | 0.30 | 4.34 (0.86) | 4.37 (0.90) | .564 | 0.07 |
| RAVLT-learning | 41.29 (10.11) | 49.30 (9.38) | < .001 | 0.51 | 45 (8.06) | 46.19 (7.71) | .017 | 0.30 |
| RAVLT-recall | 7.69 (2.59) | 10.18 (2.73) | < .001 | 0.51 | 9.22 (2.74) | 9.56 (2.53) | .200 | 0.16 |
| RAVLT-recognition | 11.69 (2.79) | 13.21 (2.62) | < .001 | 0.38 | 12.5 (3.19) | 13.13 (2.79) | .032 | 0.27 |
| Verbal fluency | 35.86 (11.35) | 42.86 (12.65) | < .001 | 0.48 | 41.59 (14.65) | 42.78 (14.70) | .280 | 0.19 |
| HADS-anxiety | 9.28 (4.33) | 7.84 (4.24) | < .001 | 0.28 | 8.66 (5.72) | 8.03 (5.88) | .137 | 0.19 |
| HADS-depression | 8.72 (3.78) | 6.8 (4.48) | < .001 | 0.32 | 8.19 (5) | 7.1 (5.37) | .040 | 0.26 |
HADS: Hospital Anxiety and Depression Scale; RAVLT: Rey Auditory Verbal Learning Test.
P-values < .05 are shown in bold.
Direct scores -mean (standard deviation)-, significance level (p) and effect size (r).
Figure 2.
Effect size of the differences between pretreatment and post-treatment evaluations in each group.
Regarding the follow-up evaluation (performed 6-7 months after completing the rehabilitation programme), the study group showed statistically significant differences between scores on the post-treatment evaluation and the follow-up evaluation in the forward digit span (z = –2.987; P = .003; r = 0.25) and the RAVLT-recognition (z = –2.985; P = .003; r = 0.25). In both cases, performance was poorer in the follow-up evaluation than in the post-treatment evaluation. The control group showed no differences between results in the post-treatment and follow-up evaluations. In the comparisons between the pretreatment and the follow-up evaluation, the study group showed statistically significant differences in RAVLT-learning (z = –4.746; P < .00; r = 0.39), RAVLT-recall (z = –5.094; P < .00; r = 0.42), and verbal fluency (z = –2.570; P = .10; r = 0.01). Controls showed statistically significant differences in the RAVLT-learning (z = –2.418; P = .16; r = 0.36), RAVLT-recall (z = –2.068; P = .03; r = 0.30), RAVLT-recognition (z = –2.056; P = .04; r = 0.30), and HADS-depression (z = –2.571; P = .010; r = 0.38). The effect sizes were larger in the study group than in the control group for the RAVLT-learning (0.39 vs 0.36), and RAVLT-recall (0.42 vs 0.30). The effect sizes were larger in the control group than in the study group for the RAVLT-learning (0 vs 0.30) and HADS-depression (0.16 vs 0.38).
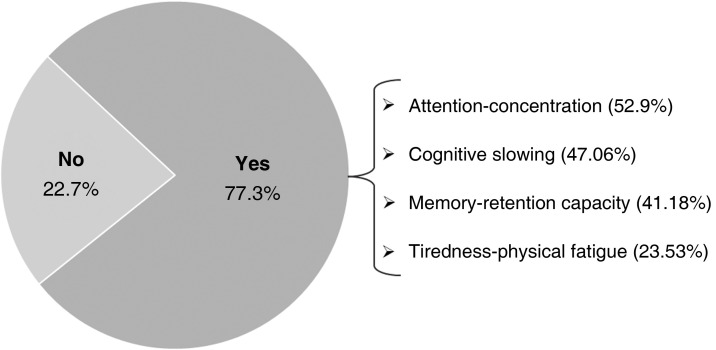
In the follow-up evaluation, 33.3% (n = 23) of participants had returned to work, whereas 44.9% (n = 31) were temporarily unable to work. The remaining 21.8% were not working, either because they were receiving a pension (n = 11) or because they were unemployed (n = 4). Of the patients who returned to work, 77.3% (n = 17) reported difficulties performing their professional tasks (Fig. 3 ). In the study group, 34.5% (n = 19) were working and 43.6% (n = 24) were temporarily unable to work. In the control group, 28.6% (n = 4) were working and 50% (n = 7) were temporarily unable to work.
Figure 3.
Difficulties performing workplace tasks.
Of the participants surveyed, 26.1% (n = 18) were studying some course before completing treatment. Of these, 77.8% (n = 14) reported difficulties following classes or studying. Lastly, 81.2% (n = 56) reported difficulties in activities of daily living, with 73.9% (n = 51) mentioning that they needed external memory aids (diaries, calendars, notes).
Discussion
In this study, we analysed the results of a neuropsychological rehabilitation programme aimed at patients with post–COVID-19 syndrome. In the pretreatment evaluation, the study group (the group receiving treatment for cognitive symptoms) showed poorer performance in RAVLT-learning, RAVLT-recall, RAVLT-recognition, and verbal fluency than the control group (no cognitive treatment). After treatment, both groups showed similar performance in the post-treatment evaluation. In intragroup comparisons of pre- and post-treatment performance, both groups showed improvements in cognitive performance. However, the study group presented larger effect sizes in the backward digit span, RAVLT (learning, recall, and recognition), and verbal fluency. Furthermore, the study group showed significant improvements in the symptoms of anxiety and depression in the post-treatment evaluations, with the effect size being larger than in the control group. In the follow-up evaluation, the control group presented similar results to those recorded in the post-treatment evaluation for cognitive performance and prevalence of symptoms of anxiety and depression. The study group showed poorer performance in the forward digit span and RAVLT-recognition than in the post-treatment evaluation. However, in the remaining tests administered, performance was similar to that obtained in the post-treatment evaluation. We cannot explain this poorer performance.
Some authors recommend cognitive rehabilitation for patients with acquired brain injury,39, 40 as well as for other conditions.41, 42, 43 Based on the assumption that a wide range of patients with cognitive alterations may benefit from this type of therapeutic approach, we considered it appropriate to integrate cognitive rehabilitation into the post-COVID-19 syndrome outpatient rehabilitation programme. To this end, we implemented a cognitive rehabilitation programme similar to that used in patients attended at our centre due to acquired brain injury. The results obtained in this study suggest that, regardless of the underlying condition, cognitive rehabilitation is a useful tool for treating alterations affecting cognitive function.
We observed that post–COVID-19 syndrome has a significant impact on daily life, months after completing the rehabilitation programme. Of the surveyed patients, 44.9% were temporarily unable to work, whereas 77.3% of those who had returned to work described difficulties performing workplace activities. These results are consistent with those described in the meta-analysis by Ceban et al.16 These authors suggest that 29%-47.4% of patients did not return to work, and 8%-38.9% reported alterations in their working capacity. Beyond the professional sphere, 81.2% of the patients surveyed in our study reported difficulties with activities of daily living, with 73.9% needing to use external memory aids. The literature also includes reports of difficulties with activities of daily living, although with a great variability (1%-68.4% of cases).16 It is therefore important to highlight that, in line with the conclusions of Cicerone et al.,40 the cognitive/emotional improvements observed at the psychometric level do not necessarily represent a recovery of pre–COVID-19 functional status.
Our study does have some limitations. Only a limited number of psychometric tests were administered, which has conditioned the amount of information available on patients’ cognitive status. This probably also prevented the detection of some cognitive alterations described in post–COVID-19 syndrome, such as difficulty concentrating17, 18 or cognitive slowing.17, 19 Furthermore, we are aware that remote administration, with the subsequent lack of on-site meetings, leads to a loss of qualitative/semiological information. Future studies should seek to perform more extensive neuropsychological evaluations with the aim of improving the treatment offered to these patients.
Regarding the neuropsychological rehabilitation programme, it is plausible that the post-treatment improvements observed in the study group may be explained by a practice effect (learning due to repeated exposure to the tests administered). However, this seems not to be the case. A previous study performed by our team, whose sample included some of the patients included in this study, corrected the raw scores to try to control for a possible practice effect in the post-treatment evaluation.23 After applying the correction factor, researchers continued to observe statistically significant differences between the results obtained in the pre- and post-treatment evaluations, suggesting that changes are not explained by a practice effect. In the control group, we cannot rule out a practice effect that may explain the variations observed between the different evaluations.
In terms of sample characteristics, we may highlight 3 relevant issues. Firstly, practically all patients (96.7% [n = 119]) were infected with SARS-CoV-2 in 2020. Considering the variants affecting Spain by that time, our patients were infected with some of the earliest variants of the virus, such as 19A, 19B, 20A, 20B, 20C, 20D, 20E (EU1), 20 G, 20H (Beta, V2), 20I (Alpha, V1), and 20J (Gamma, V3).44 The emergence of the alpha variant (which infected the majority of patients in the first half of 2021) and the delta variant (the predominant form in the second half of that year),45 together with mass vaccination in the same year, makes it difficult to determine how these SARS-CoV-2 variants may affect cognitive performance. From the previously described working hypothesis, it is plausible that, once more, neuropsychological evaluation is a useful tool in those cases presenting cognitive-emotional alterations. Secondly, our sample included older adults (≥ 60 years). The cognitive alterations detected in these patients may be explained by the presence of other neurodegenerative processes, and not only by post–COVID-19 syndrome. Therefore, neuropsychological rehabilitation may be less effective in older adults due to the ageing process (whether normal or pathological). Lastly, we collected no information on patients’ physical and respiratory status. The copresence of cognitive/emotional alterations and physical or respiratory difficulties probably explains why some patients did not return to their work, as well as the presence of difficulties with activities of daily living.
In conclusion, neuropsychological rehabilitation is an effective tool to treat cognitive/emotional –alterations derived from postCOVID-19 syndrome. In any case, 6 months after finishing treatment, not all patients had recovered their pre–COVID-19 functional status.
Conflicts of interest
Guttmann NeuroPersonalTrainer® is partly owned by Institut Guttmann. García-Molina, Sánchez-Carrión, and Rodríguez-Rajo have participated in its development.
Sources of funding
This study has received no specific funding from any public, commercial, or non-profit organisation.
References
- 1.Jaywant A., Vanderlind W.M., Alexopoulos G.S., Fridman C.B., Perlis R.H., Gunning F.M. Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology. 2021;46:2235–2240. doi: 10.1038/s41386-021-00978-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeria M., Cejudo J.C., Sotoca J., Deus J., Krupinski J. Cognitive profile following COVID-19 infection: clinical predictors leading to neuropsychological impairment. Brain Behav Immun Health. 2020;9 doi: 10.1016/j.bbih.2020.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woo M.S., Malsy J., Pöttgen J., Seddiq Zai S., Ufer F., Hadjilaou A., et al. Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun. 2020;2:fcaa205. doi: 10.1093/braincomms/fcaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou H., Lu S., Chen J., Wei N., Wang D., Lyu H., et al. The landscape of cognitive function in recovered COVID-19 patients. J Psychiatr Res. 2020;129:98–102. doi: 10.1016/j.jpsychires.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanderlind W.M., Rabinovitz B.B., Miao I.Y., Oberlin L.E., Bueno-Castellano C., Fridman C., et al. A systematic review of neuropsychological and psychiatric sequalae of COVID-19: implications for treatment. Curr Opin Psychiatry. 2021;34:420–433. doi: 10.1097/YCO.0000000000000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomasoni D., Bai F., Castoldi R., Barbanotti D., Falcinella C., Mulè G., et al. Anxiety and depression symptoms after virological clearance of COVID-19: a cross-sectional study in Milan, Italy. J Med Virol. 2021;93:1175–1179. doi: 10.1002/jmv.26459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alemanno F., Houdayer E., Parma A., Spina A., Del Forno A., Scatolini A., Angelone S., Brugliera L., Tettamanti A., Beretta L., Iannaccone S. COVID-19 cognitive deficits after respiratory assistance in the subacute phase: a COVID-rehabilitation unit experience. PLoS One. 2021;16:e0246590. doi: 10.1371/journal.pone.0246590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wahlgren C., Divanoglou A., Larsson M., Nilsson E., Östholm Balkhed Å, Niward K., Birberg Thornberg U., Lilliecreutz Gudmundsson E., Levi R. Rehabilitation needs following COVID-19: five-month post-discharge clinical follow-up of individuals with concerning self-reported symptoms. EClinicalMedicine. 2022;43 doi: 10.1016/j.eclinm.2021.101219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenhalgh T., Knight M., A’Court C., Buxton M., Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 10.Organización Mundial de la Salud . 2021. Enfermedad por coronavirus (COVID-19): afección posterior a la COVID-19.https://www.who.int/es/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-post-covid-19-condition [Accessed May 2022] Available from: [Google Scholar]
- 11.National Institute for Health and Care Excellence (NICE) 2022. COVID-19 rapid guideline: managing the long-term effects of COVID-19.https://www.nice.org.uk/guidance/ng188/ [Accessed 28 May 2022] Available from: [PubMed] [Google Scholar]
- 12.Davis H.E., Assaf G.S., McCorkell L., Wei H., Low R.J., Re’em Y., et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrigues E., Janvier P., Kherabi Y., Le Bot A., Hamon A., Gouze H., et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81:e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanichkachorn G., Newcomb R., Cowl C.T., Murad M.H., Breeher L., Miller S., et al. Post-COVID-19 syndrome (long Haul syndrome): description of a multidisciplinary clinic at mayo clinic and characteristics of the initial patient cohort. Mayo Clin Proc. 2021;96:1782–1791. doi: 10.1016/j.mayocp.2021.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jennings G., Monaghan A., Xue F., Mockler D., Romero-Ortuño R. A systematic review of persistent symptoms and residual abnormal functioning following acute COVID-19: ongoing symptomatic phase vs. post-COVID-19 syndrome. J Clin Med. 2021;16:5913. doi: 10.3390/jcm10245913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceban F., Ling S., Lui L.M.W., Lee Y., Gill H., Teopiz K.M., et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2022;101:93–135. doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delgado-Alonso C., Valles-Salgado M., Delgado-Álvarez A., Yus M., Gómez-Ruiz N., Jorquera M., et al. Cognitive dysfunction associated with COVID-19: a comprehensive neuropsychological study. J Psychiatr Res. 2022;150:40–46. doi: 10.1016/j.jpsychires.2022.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García-Sánchez C., Calabria M., Grunden N., Pons C., Arroyo J.A., Gómez-Anson B., et al. Neuropsychological deficits in patients with cognitive complaints after COVID-19. Brain Behav. 2022;12:e2508. doi: 10.1002/brb3.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrucci R., Dini M., Groppo E., Rosci C., Reitano M.R., Bai F., et al. Long-lasting cognitive abnormalities after COVID-19. Brain Sci. 2021;11:235. doi: 10.3390/brainsci11020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albu S., Rivas Zozaya N., Murillo N., García-Molina A., Figueroa Chacón C.A., Kumru H. Multidisciplinary outpatient rehabilitation of physical and neurological sequelae and persistent symptoms of covid-19: a prospective, observational cohort study. Disabil Rehabil. 2021:1–8. doi: 10.1080/09638288.2021.1977398. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Gerez J.J., Saavedra-Hernandez M., Anarte-Lazo E., Bernal-Utrera C., Perez-Ale M., Rodriguez-Blanco C. Short-term effects of a respiratory telerehabilitation program in confined COVID-19 patients in the acute phase: a pilot study. Int J Environ Res Public Health. 2021;18:7511. doi: 10.3390/ijerph18147511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stavrou V.T., Tourlakopoulos K.N., Vavougios G.D., Papayianni E., Kiribesi K., Maggoutas S., et al. Eight weeks unsupervised pulmonary rehabilitation in previously hospitalized of SARS-CoV-2 infection. J Pers Med. 2021;11:806. doi: 10.3390/jpm11080806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Udina C., Ars J., Morandi A., Vilaró J., Cáceres C., Inzitari M. Rehabilitation in adult post-COVID-19 patients in post-acute care with therapeutic exercise. J Frailty Aging. 2021;10:297–300. doi: 10.14283/jfa.2021.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel R., Savrides I., Cahalan C., Doulatani G., O’Dell M.W., Toglia J., et al. Cognitive impairment and functional change in COVID-19 patients undergoing inpatient rehabilitation. Int J Rehabil Res. 2021;44:285–288. doi: 10.1097/MRR.0000000000000483. [DOI] [PubMed] [Google Scholar]
- 26.García-Molina A., Espiña-Bou M., Rodríguez-Rajo P., Sánchez-Carrión R., Enseñat-Cantallops A. Programa de rehabilitación neuropsicológica en pacientes con síndrome post-COVID-19: una experiencia clínica [Neuropsychological rehabilitation program for patients with post-COVID-19 syndrome: a clinical experience] Neurologia. 2021;36:565–566. doi: 10.1016/j.nrl.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonizzato S., Ghiggia A., Ferraro F., Galante E. Cognitive, behavioral, and psychological manifestations of COVID-19 in post-acute rehabilitation setting: preliminary data of an observational study. Neurol Sci. 2022;43:51–58. doi: 10.1007/s10072-021-05653-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peña-Casanova J. Test Barcelona; Masson, Barcelona: 1991. Programa Integrado de Exploración Neuropsicológica. [Google Scholar]
- 29.Wechsler D. Psychological Corporation; San Antonio, TX: 1997. WAIS-III. [Google Scholar]
- 30.Wechsler D. TEA; Madrid: 1999. WAIS-III. Escala de inteligencia de Wechsler para adultos-III. [Google Scholar]
- 31.Rey A. Presses Universitaires de France; Paris: 1964. L ‘examen clinique en psychologie [Clinical tests in psychology] [Google Scholar]
- 32.Miranda J., Valencia R. English and Spanish versions of a memory test: word-length effects versus spoken-duration effects. HJB. 1997;19:171–181. doi: 10.1177/07399863970192005. [DOI] [Google Scholar]
- 33.Artiola-Fortuny L., Hermosillo-Romo D., Heaton R.K., Pardee R.E. Manual de Normas y Procedimientos para la Batería Neuropsicológica en Español. Tucson. 1999 [Google Scholar]
- 34.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 35.Terol M.C., López-Roig S., Rodríguez-Marín J., Martín-Aragón M., Pastor M.A., Reig M.T. Propiedades psicométricas de la Escala Hospitalaria de Ansiedad y Estrés (HAD) en población española. Ansiedad y Estrés. 2007;13:163–176. [Google Scholar]
- 36.Solana J., Cáceres C., García-Molina A., Opisso E., Roig T., Tormos J.M., et al. Improving brain injury cognitive rehabilitation by personalized telerehabilitation services: Guttmann neuropersonal trainer. IEEE J Biomed Health Inform. 2015;19:124–131. doi: 10.1109/JBHI.2014.2354537. [DOI] [PubMed] [Google Scholar]
- 37.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 38.Dominguez-Lara S. Magnitud del efecto, una guía rápida. EducMed. 2018;19:251–254. doi: 10.1016/j.edumed.2017.07.002. [DOI] [Google Scholar]
- 39.Rogers J.M., Foord R., Stolwyk R.J., Wong D., Wilson P.H. General and domain-specific effectiveness of cognitive remediation after stroke: systematic literature review and meta-analysis. Neuropsychol Rev. 2018;28:285–309. doi: 10.1007/s11065-018-9378-4. [DOI] [PubMed] [Google Scholar]
- 40.Cicerone K.D., Goldin Y., Ganci K., Rosenbaum A., Wethe J.V., Langenbahn D.M., et al. Evidence-based cognitive rehabilitation: systematic review of the literature from 2009 through 2014. Arch Phys Med Rehabil. 2019;100:1515–1533. doi: 10.1016/j.apmr.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez-Luengos I., Balboa-Bandeira Y., Lucas-Jiménez O., Ojeda N., Peña J., Ibarretxe-Bilbao N. Effectiveness of cognitive rehabilitation in Parkinson’s disease: a systematic review and meta-analysis. J Pers Med. 2021;11:429. doi: 10.3390/jpm11050429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thérond A., Pezzoli P., Abbas M., Howard A., Bowie C.R., Guimond S. The efficacy of cognitive remediation in depression: a systematic literature review and meta-analysis. J Affect Disord. 2021;284:238–246. doi: 10.1016/j.jad.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Vita A., Barlati S., Ceraso A., Nibbio G., Ariu C., Deste G., et al. Effectiveness, core elements, and moderators of response of cognitive remediation for schizophrenia: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2021;78:848–858. doi: 10.1001/jamapsychiatry.2021.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ministerio de Sanidad, Consumo y Bienestar Social . 2022. Información científica-técnica: Información microbiológica y variantes.https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov//documentos.htm [Accessed 28 May 2022] Available from: [Google Scholar]
- 45.Ministerio de Sanidad, Consumo y Bienestar Social . 2022. Evaluación rápida de riesgo: Variantes de SARS-CoV-2 en España: linaje BA.2 de Ómicron.https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/variantes.htm [Accessed 28 May 2022] Available from: [Google Scholar]