Abstract
Virus-caused public health outbreaks represent a serious threat to humans all over the world. The rampant new 2019 coronavirus (SARS-CoV-2) has wreaked havoc on China and the rest of the world since December 2019. Now focus is on effective reduction of corona and other viral and bacterial infections in hospitals, public and private sectors, households, schools, etc. Metal and metal oxide nanoparticles, carbon nanotubes, heterostructures, patterned surfaces, and graphene-based materials have shown up to 99.9998% efficacy against bacteria, mold, and viruses. The stability, long shelf life, and robustness of inorganic nanoparticles make them desirable for antimicrobial nanofinishes. These inorganic antimicrobial agents are more stable than organic antibacterial compounds at high temperature and pressure. The high specific surface area-to-volume ratios and unique physicochemical characteristics of nanoparticles are largely responsible for their antibacterial actions. But their immobilization is a huge challenge. To address this issue, NPs were modified with (glycidoxypropyl) trimethoxysilane (GPTS) and applied on cotton fabric. The silane part of GPTS reacted with the NPs under acidic conditions while epoxy reacted with cotton under alkaline conditions. Treated cotton fabric showed good antiviral and antibacterial activity even after severe industrial washing.
Graphical abstract
Keywords: Antibacterial, Antiviral, Cotton, Immobilization of NPs, Medical textile, Ultraviolet protection
Introduction
As health awareness has increased, people have begun to focus on eliminating or reducing harmful pathogens from the surfaces or environment. Almost the current global crisis is over, efforts should be stepped up to prevent viral infections in the future. Therefore, a variety of materials are being developed as antimicrobial, antiviral, antifungal, or anti-yeast and investigated on textile substrates or products. Viruses pose a serious challenge to the pharmaceutical, medical and biotechnology fields as one of the leading causes of human disease and death. In recent years, nanotechnology has become one of the most significant and fascinating frontier topics in physics, chemistry, technology, and biology [1]. It has tremendous potential for multiple breakthroughs that, in the not-too-distant future, will change the path of technological progress in a wide range of applications. Various researches on manufacturing antimicrobial nanoparticles for reducing illnesses without causing bacterial resistance have been conducted [2].
Current advances in nanotechnology offer a platform for reducing the risk of transmission of infectious agents from anti-pollution equipment. Important advancement can be achieved by inserting the antiviral NPs in the woven and non-woven fabrics. For this purpose, metallic or non-metallic nanoparticles, inorganic and organic materials, and compounds can be used as active ingredients [3]. However different material has different chemical and physical nature and stability. The degree of antiviral functions will require different applications and preparation processes. From the last decade’s nanoparticle system, NPs are extensively studied for antibacterial [4, 5] and antiviral treatments [5–7]. Therefore, nanocoatings can be up to 99.998% effective against viruses and bacteria and are 1000 times more efficient than previous technologies existing in the market. They can work on multiple levels at the same time. Its uses enhanced antiviral and antibacterial action, which is environmentally friendly and non-toxic. Nanoparticles of various materials such as metal nanoparticles, carbon nanotubes, metal oxide nanoparticles, and graphene-based materials have demonstrated antimicrobial and antiviral activity [8].
The various antiviral and antimicrobial mechanisms of nanomaterials are largely attributed to their physicochemical properties and high surface-to-volume ratio [9]. There are at least two areas of study that can be identified. One of them is related to modification of nanoparticles with organic molecules [10]. When the chemical interaction occurs at surface of viruses among the molecules–receptors and functionalizers, it affects the viruses. Other one is related to antiviral activity of pure nanoparticles [11]. Microscopic studies have shown that the nanoparticles are attached to the surface of the virus, which leads to local changes in the surface, for example the accumulation of glycoproteins and prevent the penetration of virus [12]. A variety of nanoparticles (for example silver, silicone, titanium, magnesium, and zinc) are used as antiviral agents.
These nanoparticles work against various viruses like influenza virus [13, 14], herpes simplex virus [15], foot and mouth disease virus [16], HIV [17], poliovirus [18], rotavirus, etc. As previously stated, the transformation in functionality of NPs is due to their high surface energy and increased surface area, and due to presence of hydroxyl groups at the surface of NPs potential modification or grafting of versatile groups at their surface could be done for various durable functionalities. To adhere them to textile substrates, several cross-linkers and binders like trichloro (methyl) silane (TCMS), 3(trimethoxysilyl) propyl-N,N,N-dimethyloctadecyl ammonium chloride [5] tetraethoxysilane (TEOS), hexadecyltrimethoxysilane (C16TMS) [19] and 1,2-Bis(triethoxysilyl)ethane (BTSE) [20–30] have been used that affect the inherent properties of textile and functionality of inorganic NPs like silica, titanium, etc. [31–36].
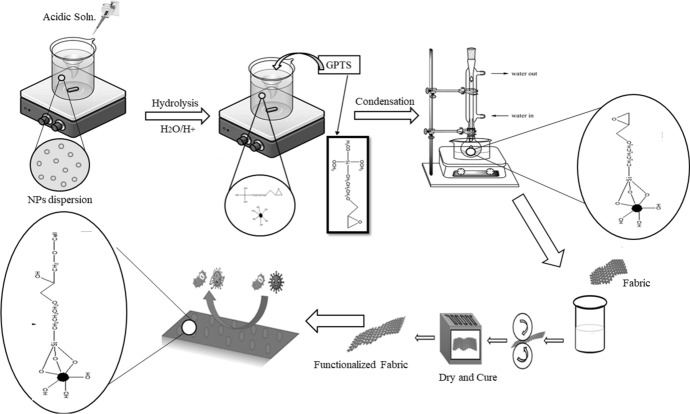
Keeping this advantage in mind and to solve this issue, the initial step was taken to investigate the endurance of NPs on cotton fabric using silane coupling agents (3-glycidoxypropyl)trimethoxysilane (GPTS) and after functionalized using silane coupling agents such as GPTS-modified ZNO NPs were applied on fabric by pad dry cure method. GPTS increased their binding with the cotton fabric with least negative impact on inherent properties of fabric [20]. SEM analysis was performed on unwashed and washed cotton fabrics treated with modified GPTS to confirm durability. Treated cotton fabric has shown excellent antibacterial as well as antiviral properties before and after washing. Such antibiotic textiles are potential candidate for different medical applications.
Experimental
Material
Zinc Oxide nanopowder (ZnO, 99 + 100%, 10–30 nm) was purchased from US Research Nanomaterials, Inc. 3-Glycidoxypropyltrimethoxysilane (> 97.0%) was purchased from TCI, Lab grade. NaOH was purchased from DAEJUNG, HCl by Riedel-de Haen Germany and Ethanol from Sigma Aldrich. Tryptone soya agar and Tryptone soya broth were purchased from OXOID Hants, UK. Bacterial strains (Escherichia coli, Staphylococcus aureus) were sourced from National University of Sciences & Technology (NUST) Islamabad, Pakistan. In this study, chemicals were used without further purification. Plain cotton fabric (areal density; 150 g/m2) was purchased from domestic textile industry.
Method
Modification of ZnO nanopowder
ZnO nanopowder was modified by silane coupling agent. GPTS was used for the modification of ZnO nanopowder to develop the bioactive cotton fabric. 4 g dried ZnO nanopowder was dispersed in 100 ml ethanol at ambient conditions. For homogeneous dispersion formation, a probe-sonicator was used for 60 mints without using any dispersing agent. After homogenous dispersion formation, water was added (calculated stoichiometrically) for the hydrolysis of GPTS. For the hydrolysis of trimethoxysilane parts of GPTS, water is necessary under acidic conditions. One mole of GPTS requires three moles of water for complete hydrolysis. Please see below reference. After that pH was adjusted between 4.5 and 5.0 through mineral acid to accelerate the hydrolysis. Then, GPTS was added in different concentrations (as given in Table 1) on the weight of nanopowder which was then refluxed for about 5 h at 60 °C for complete reaction of GPTS with the ZnO NPs.
Table 1.
Optimization of GPTS concentration to functionalize the NPs
| Sr | ZnO NPs | Sample code | GPTS concentration (on the weight of NPs in % age) (%) |
|---|---|---|---|
| 1 | 2 g | A | 50 |
| 2 | B | 100 | |
| 3 | C | 150 |
Design of experiment
Different concentrations of GPTS were used to modify the NPs on the weight of NPs. The design of experiment for the study is given in Table 1.
Application of modified ZnO nanopowder on cotton fabric
As the NPs were modified with GPTS that contained epoxy group in its chemical structure. This epoxy group could possibly react with cellulose under slightly alkaline conditions [20]. Therefore, the cotton was causticized before application of modified NPs onto it by dipping in sodium hydroxide solution (10 g/L) and dried at 100 °C. Treated cotton fabric was dipped in dispersion and squeezed between two rollers of padder (VPM 250-A, Tsujii Dyeing Machine Manufacturing Co.) at pressure of 1.5 bar, followed by drying at 120 °C for 1 min. The dried fabric was then cured at 170 °C for 3 min and stored at ambient conditions.
Characterization of modified ZnO NPs
Zetasizer Analysis
Zetasizer was used to detect the particle size by dynamic light scattering method that has compliance with regulatory standard tests described in ISO 13322-2: 2021, and ISO 22412 [37]. The aqueous dispersion of NPs will be collected and measured by zetasizer. The data of average size and distribution were analyzed.
Fourier transfer infrared spectroscopy (FTIR)
Attenuated total reflectance Fourier transform-infrared spectroscopy (ATR-FTIR) equipped with Zinc selenide grid was used to examination the functional groups grafted at the surface after the modification of ZnO NPs with GPTS and analysis of chemical structure in the range 4500–400 cm−1 according to standard test method: ASTM E168 [38].
Characterization of treated cotton fabric
FTIR analysis
FTIR analysis was done for untreated and treated fabrics to confirm the functional groups present at the surface before and after treatment of cotton with GPTS-modified NPs and FTIR spectra were analyzed.
Surface morphology of treated fabric
The chemical composition and surface morphology of untreated and treated fabrics before and after industrial washing were observed by using Scanning Electron Microscopy (SEM) standard equipped with EDX.
Washing durability
The washing durability of cotton fabrics treated with modified ZnO NPs was confirmed. The ISO 105-C03 standard test method [39] for multiple washing cycles was used. The samples were placed in standard detergent solution containing certain number of steel balls at varied temperatures, and time conditions using liquor ratios for different washing cycles and test standard. The testing was performed in a laboratory-scale Laundrometer by Pyrotec S (Roaches dyeing machine, UK). Afterward, samples were analyzed under SEM to observe the distribution of NPs at the surface of cotton fabric after washing.
Antibacterial testing
Qualitative antibacterial testing was carried out by agar (disk) diffusion method. For that, gram-positive (E. coli ATCC 8739) and gram-negative bacterial (S. aureus ATCC 6538) and gram-positive (S. aureus) and gram-negative (E. coli) bacterial strains were used. While quantitative antibacterial activity of control and treated cotton fabric was investigated by ISO-20743 standard test method. Agar plates were prepared for bacterial growth using 2 × 106 CFU/ml dilution. Cut the sample 3.8 cm and autoclaved it (Temp. 121 °C, 15 min). The untreated and treated samples were the placed-on agar plates previously inoculated with 1 ml of bacterial suspension for 60 s and a weight of 200 g was applied on the surface of sample to press it down. After that, the fabric swatches were detached from the agar surface and immediately transferred to glass vials containing 30 ml of saline solution. The solution was then placed on vortex mixer for 2–3 min for proper shaking. Then, the solution was serially diluted up to 106 using broth media followed by plating of the dilutions on agar plates as described in norm. The plates were then incubated at standard incubation conditions (37 °C for 24 h) to get bacterial count at 0 h. For bacterial count at 24 h, the samples detached from the afar surface were immediately placed in empty petri plates with inverted position. The plates were incubated, and same procedure was repeated next day of the preparation and plating of dilutions. The number of inoculated bacterial colonies at 0 h and number of survived bacterial colonies after 24 h was calculated for both untreated and treated fabric. Following equation was employed for the calculation of percentage reduction in bacterial colonies,
Antiviral testing
Quantitative antiviral testing of control and treated cotton fabric was performed according to ISO 18184 method [40]. Cotton fabrics were used as control samples. An antiviral fabric was prepared by the immobilization of zinc oxide NPs by using silane coupling agent. Dengue virus and hepatitis C virus (HCV) were used as model viruses. To test the antiviral performance of the treated fabric, virus suspension was injected onto the product and then rinsed out of the product in order to test its antiviral effectiveness. The infectivity titer of the virus in the washed-out suspension was determined. Counting plaque forming units (number of virus capable of forming plaques per unit volume) was used to measure the viral infectivity titer of the test suspension [40].
TCID50 method and preparation of growth medium
The TCID50 method was preferred to determine the dilution of the viral suspension required to induce a cytopathic effect (structural variations in host cells that are due to viral attack) in 50% of cell units. 96-well microplates were chosen for this purpose, along with other essential sterilizing apparatus. The growing media was prepared according to ISO 18184 method. Defrosting of cryopreserved host cells, then a new cell culture flask was prepared (20 ml growth medium). Following that, host cells were transferred to flask. The flask was then placed in a CO2 incubator set to 37 °C for 24 h to cultivate the cells at the bottom. Subsequently, using the recipe and procedure indicated in ISO 18184, the grown cells were transformed to subcultures, 1 ml of this subculture was added to 20 ml of growing media in a container. Then, in each of the 96 wells of the microplates, 0.1 mL of solution was added. After that, the plates were placed in an incubator at 37 °C for 5 days. The replication of cells was verified using a microscope after 5 days. The surface of the cultured cells was rinsed according to ISO 6330–2012 [41]. This flask solution was labeled as “FLASK A.”
Preparation of virus suspensions
The cryopreserved dengue virus was defrosted in the same manner as the host cells were. The defrosted dengue virus was transferred to a new test tube and dilution with maintenance medium was initiated. As a result, the viral concentration was adjusted to TCID 50/ml. A suspension (from the concentration of a 103 PFU/ ml) of dengue virus (1.0 ml) was put onto the surface of the cells in the “FLASK A” and spread evenly across the surface. The flask was then placed in a CO2 incubator at 34 °C for one hour to allow the virus to adsorb on the cells. After 1 h, 1.5 ppm trypsin, the flask was placed into incubator for two days at 34 °C to multiply the dengue virus. The multiplied viral suspension was centrifuged for 15 min at 4 °C. The centrifugation tube's supernatant was then removed. The viral suspension produced had a concentration of around 108 PFU/ml. Then, the virus suspension was diluted tenfold using EMEM or filtered water as a diluent, yielding a dengue virus test suspension with a concentration of approximately 1 × 107 PFU/ml. The virus suspension diluted with EMEM is now known as the 1/1 EMEM virus suspension, whereas the virus suspension diluted with filtered water is known as the 1/10 EMEM virus suspension. Same process was repeated to prepare hepatitis C virus (HCV) suspension.
Principle
Cut the control and treated unwashed and washed samples 20 × 20 mm (0.40 g) and placed in 30 ml vial and autoclaved it for 15 min at 121 °C for sterilization. According to ISO 18184, plaque counting method was performed to measure the virus infectivity titer. Control and treated unwashed and washed samples were inoculated with 0.2 ml viral suspension (1 × 107 PFU/ml) to examine the stability of virus and left for contact time. To determine the virus's initial titer, control samples were used. After 60 min, the fabric was recovered in 20 mL of cell culture fluid and counted on a suitable cell line. After the required incubation time, the TCID50 was determined. Antiviral activity was determined by comparing the antiviral test material to that recovered from the control fabric.
Results and discussion
Reaction Mechanism
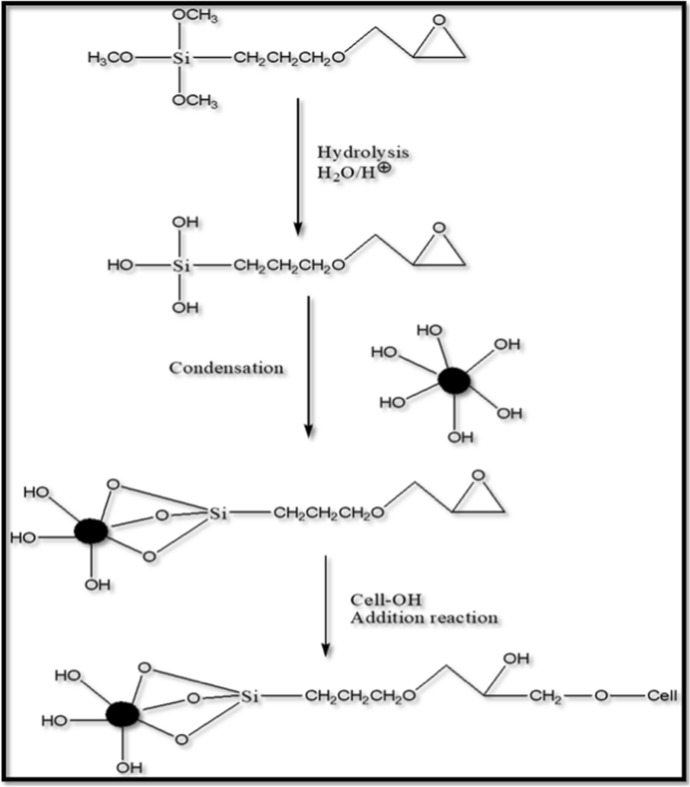
One organic, and three hydrolyzable substituents are present in GPTS. The alkoxy groups were hydrolyzed to generate silanol-containing species in the majority of surface treatment applications. The three labile groups hydrolyzed in acidic conditions during the reaction of these silanes at first. These silanes form hydrogen bonds with one or more −OH groups on the surface of one ZnO NP or three ZnO NPs after hydrolysis. After the condensation reaction, which removes the small molecule, a covalent connection is created with the NPs. as the reaction proceeds, illustrated in the reaction scheme. Under slightly alkaline conditions, the epoxy part. Of the silane reacts with. Cellulose, as shown in Fig. 1, which depicts the entire crosslinking. Reaction of ZnO nanoparticles with GPTS and cotton Fabric.
Fig. 1.
Schematic of molecular level attachment of modified ZnO NPs to cotton fabric
Characterization of ZnO NPs
Zetasize of unmodified and modified Zn NPs
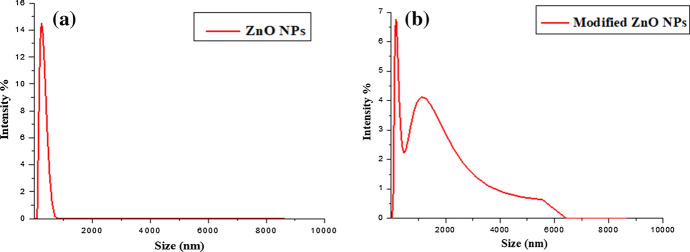
Size of ZnO nanoparticles and functionalized ZnO nanoparticles was determined by zetasizer analysis. The size of functionalized particles is slightly greater than simple nanoparticles. Size of zinc oxide nanoparticles is in range of 257 nm while modified zinc oxide nanoparticles are in range of 282 nm as shown in Fig. 2. The nanoparticles used in this work are of size 10–30 nm. However, the size determined by zetasizer is of agglomerates which are formed due to aggregation of nanoparticles. During modification, the multiple silanol groups can react more than one nanoparticle leading to formation of further agglomeration. Modified NPs were slightly larger in size which confirm the presence of silane group on their surface.
Fig. 2.
Zetasizer analysis of (a) ZnO NPs and (b) modified ZnO NPs
FTIR of unmodified and modified ZnO NPs
The FTIR spectra of unmodified and modified ZnO NPs are shown in Fig. 3. The analysis of unmodified NPs shows that the broad absorption spectrum between 3350 cm−1 and 2953 cm−1 is due to the stretching of hydroxyl groups (−OH) on the surface of NPs and absorbed moisture content. Another narrow peak may be seen at 1404 cm−1 that indicates the bending vibration of the −OH group caused by inorganic crystalline structures combining large and fine bands of some hydrated substances. The metal–oxygen (ZnO stretching vibrations in between 400 and 700 cm−1) absorption peak at 612 cm−1 [42]. In analysis of modified NPs −OH group stretching vibrations are responsible for the wide intensity peak at 3426 cm–1. Around 2925 cm−1 and 2856 cm−1, the C–H stretching vibrations occur. The peaks at 1601 cm−1 revealed that there is still a minor quantity of −OH groups on the surface of the zinc NPs. The CH2 vibrations of the propyl chain and the GPTS group are at 1422 cm−1 [43]. The condensation reaction of NPs (−OH group) with the silanol group is indicated by a peak around 1129 cm−1. Si–O–Si vibrations cause the peak at 881 cm−1, while peak at 613 cm−1 is of ZnO stretching.
Fig. 3.
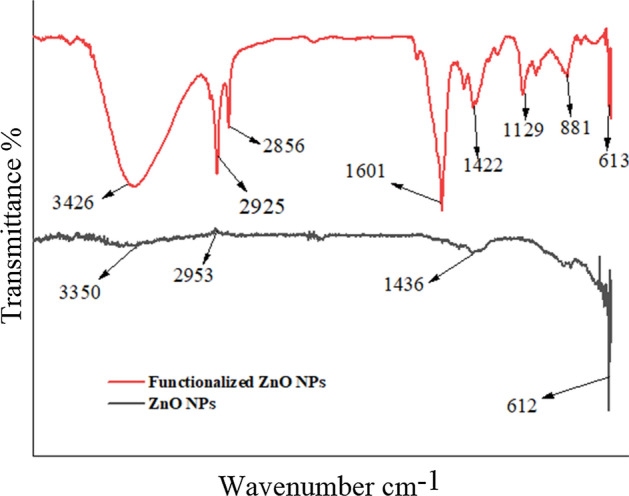
FTIR spectra of ZnO NPS and functionalized ZnO NPs
Characterization of treated fabric
Surface morphology of treated fabric
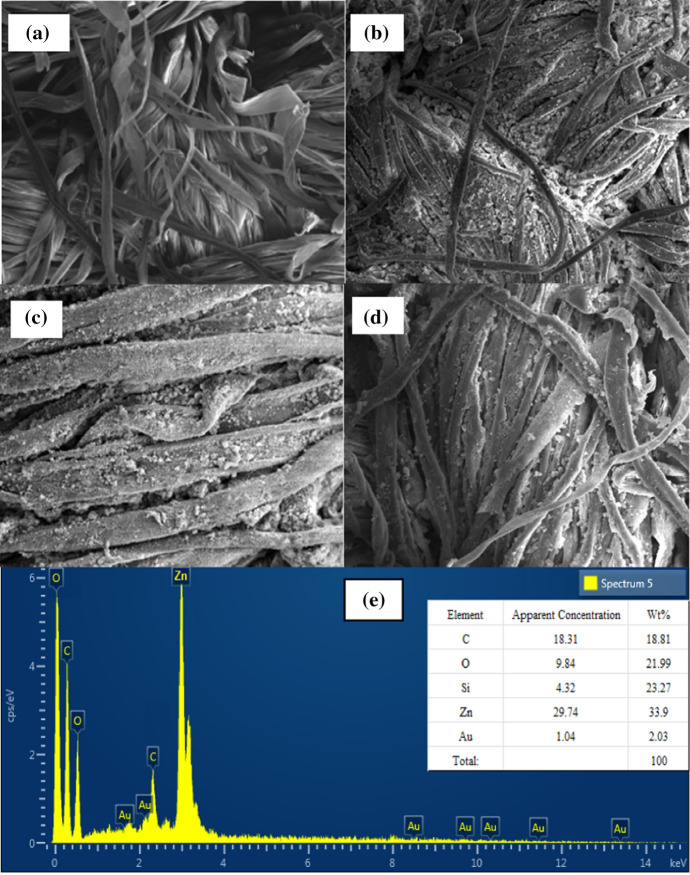
The scanning electron microscope (SEM) analysis was performed on untreated (Figs. 4 and 5), treated unwashed, and washed cotton fabrics treated with 100% GPTS o.w.n modified ZnO NPs. Figure 6 depicts that the distribution of NPs on the surface of cotton fabric. Figure 6b shows that the greatest number of NPs are bonded to the substrate as scattered thin film. To examine the durability of ZnO NPs at the surface, treated cotton fabrics were washed and examined again through SEM analysis. Figure 6d clearly shows that even after 30 washing cycles, a significant quantity of NPs remains attached to the surface despite intense laundering action under harsh conditions. The chemical bonding of NPs with the substrate could be possibly the major reason for their adhesion. The epoxy group in the silane coupling agent attached with the modified NPs reacted with cellulose as illustrated in the reaction mechanism (Fig. 1). As a result, GPTS cross-linked the NPs with cotton fabric, making them durable. Before and after washing, the amount of GPTS-treated NPs attachment was similar. Further to confirm the presence of elements and their percentage at surface of treated fabric, EDX analysis was done, and the results are shown in Fig. 6e. The highest percentage of Zn is followed by C, O, and Si. The small amount of Au was also present that could be due to golden plating done to make surface conductive before SEM analysis.
Fig. 4.
SEM micrograph of cotton fabric treated with GPTS-modified ZnO NPs: (a) untreated fabric, (b) treated unwashed, (c) 10 times washed, (d) 30 times washed, and (e) EDX spectrum and elemental analysis at the cotton fabric surface
Fig. 5.
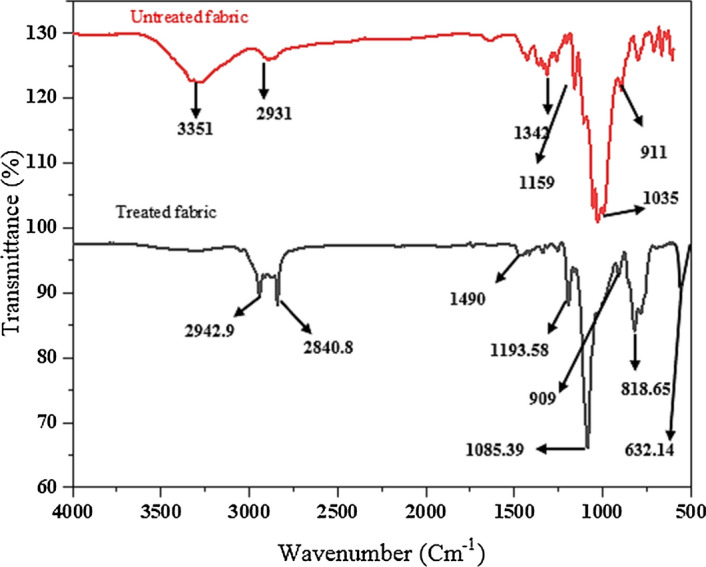
FTIR spectra of untreated and ZNO-treated fabrics
Fig. 6.
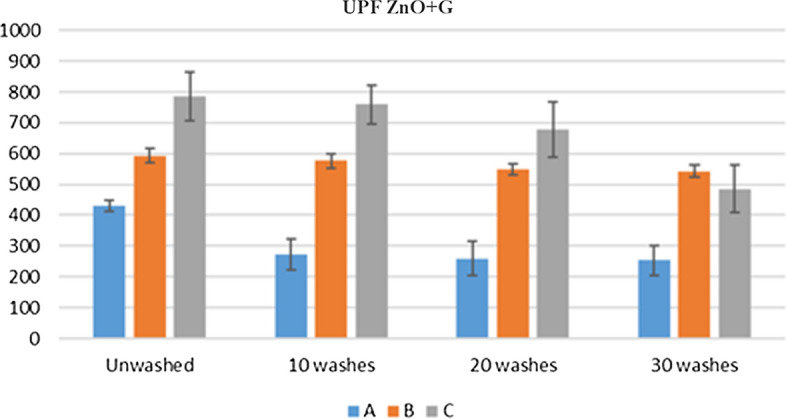
UPF values before and after wash for fabric with ZnO immobilized by silane
FTIR of untreated and treated fabric
FTIR spectrum of untreated and ZnO-treated fabric are shown in Fig. 4. Absorption peak in untreated fabric at 3351 cm−1 is due to presence of OH group which suppresses in FTIR of treated fabric which confirms the crosslinking of modified NPs. Peaks at 2931 cm−1, 2893 cm−1 are due to C–H stretching. Absorption peak in the range of 1604 cm−1 is due to moisture content. Peaks at 1109 cm−1 and 1035 cm−1 indicate the C–O stretching. Peaks at 1342 cm−1, 1159 cm−1, and 1035 cm−1 are the characteristic peaks of cotton [44].
After treatment with modified ZnO NPs, the cotton fabric showed a characteristic peak at 818.65 cm-1 that is due to epoxy group of GPTS. While the disappearance of peak at 3351 cm−1 was due to the consumption of −OH groups as in reaction scheme (Fig. 1) the whole reaction process has been shown. The epoxy ring attached with ZnO NPs opened and reacted with −OH groups at the surface of cotton fabric due to which the broad peak (3351 cm-1) was disappeared showing the attachment of NPs at the surface of fabric through GPTS [4]. FTIR spectra are also clearly showing that ZnO absorption band near 632 cm−1 that appeared at the cotton fabric surface after treatment with ZnO NPs.
Ultraviolet protection of fabric before and after treatment
The application of modified ZnO NPs dispersion on cotton fabric was examined for UPF. Textiles with a UPF of greater than 40 are said to protect the wearer from the sun's damaging rays. The UPF Values of treated cotton increased as the concentration, of silane coupling agent was increased showing a greater UV protection. The UPF values of samples after 10, 20, and 30 washing cycles are shown in Fig. 5. There was only a slight decrease between the values of unwashed, 10, 20, and 30 washes which show that removal of NPs was very slight under the vigorous washing action, indicating that the NPs had a strong adhesion to the cotton fabric. As already mentioned in reaction scheme (Fig. 1) that the NPs were bonded with the surface of fabric through GPTS. The whole reaction scheme has shown how the strong adhesion was made possible. The UPF results are also supported by SEM analysis that after various washing cycles the huge number of NPs were present at the surface of cotton fabric demonstrating the highly durable character of NPs on the cotton fabric.
Functional properties of treated fabric
Antibacterial Activity of untreated and treated fabrics
Qualitative evaluation: The antibacterial activity of ZnO–silane immobilized fabric (treated with highest concentration of GPTS) was assessed by using qualitative testing method to investigate the efficacy under different conditions. After successful immobilization, antibacterial activity testing was performed on a sample with the optimum washing durability. E. coli and S. aureus were used for this purpose.
From Fig. 7, it can be observed that greater number of bacterial colonies were present at the surface of fabric and around the edges of fabric, but a clear zone can be seen around the surface of treated fabric and no colony was seen at the surface of fabric. This could be possibly due to reactive oxygen species (O2−, H2O2, and OH−) generated due to photocatalytic activity ZnO NPs and also this could be possibly due to release of zinc ions from ZnO NPs [45]. The inhibitory effect was observed more against S. aureus with a zone of inhibition of 16.5 mm diameter as compared to E. coli with a zone of inhibition of 13.5 mm diameter as shown in Fig. 7. The experiment was run in duplicate, and a mean value was calculated and is given in Table 2.
Fig. 7.
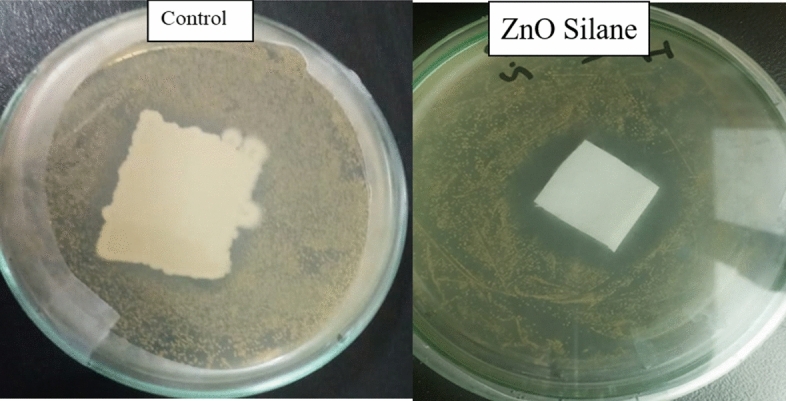
Disk diffusion method, showing antibacterial activity of GPTS–silane-modified ZnO deposited fabric through zone of inhibition
Table 2.
Antibacterial assay by qualitative measurement of zone of inhibition
| Fabrics treated | Organisms | Diameter | Mean | St. Dev. | |
|---|---|---|---|---|---|
| 1 | 2 | ||||
| ZnO–silane | S. aureus | 16 mm | 17 mm | 16.5 | 0.5 |
| E. coli | 14 mm | 13 mm | 13.5 | 0.5 | |
| Control | S. aureus | Zero | Zero | Zero | Zero |
| E. coli | Zero | Zero | Zero | Zero | |
Quantitative Evaluation
To carry out the quantitative evaluation of antibacterial activity ISO 20743 was followed. Gram-negative bacteria E. coli and gram-positive bacteria S. aureus were used for this purpose.
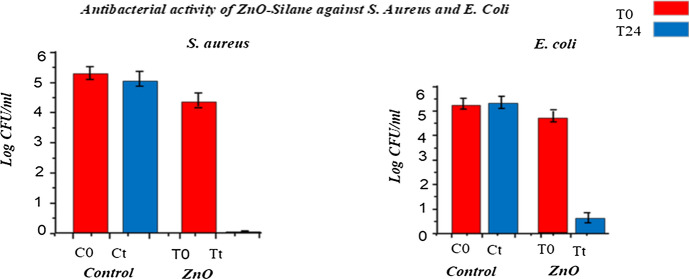
Figure 8 and Table 3 show the number of gram-positive and gram-negative bacterial population in logarithmic value of colony forming unit per ml (2 × 108 CFU/ml) after inoculating at 0 h and 24 h. From the figure and table values, it was observed that treated sample has shown 99.99% bacterial growth inhibition and the activity was durable even up to 30 industrial washing cycles. This strong antibacterial potential of samples treated with ZnO nanoparticles could be ascribed to reactive oxygen species (like O2−, H2O2, and OH−) produced from ZnO NPs which causes bacterial cell wall damage resulting in the inactivation of several important mechanisms such as mitochondrial functioning, intracellular outflow eventually causing bacterial cell death. Furthermore, the remarkable durability of antibacterial potential to washing could be due to strong cross-linking between fabric and ZnO NPs due to GPTS cross-linker.
Fig. 8.
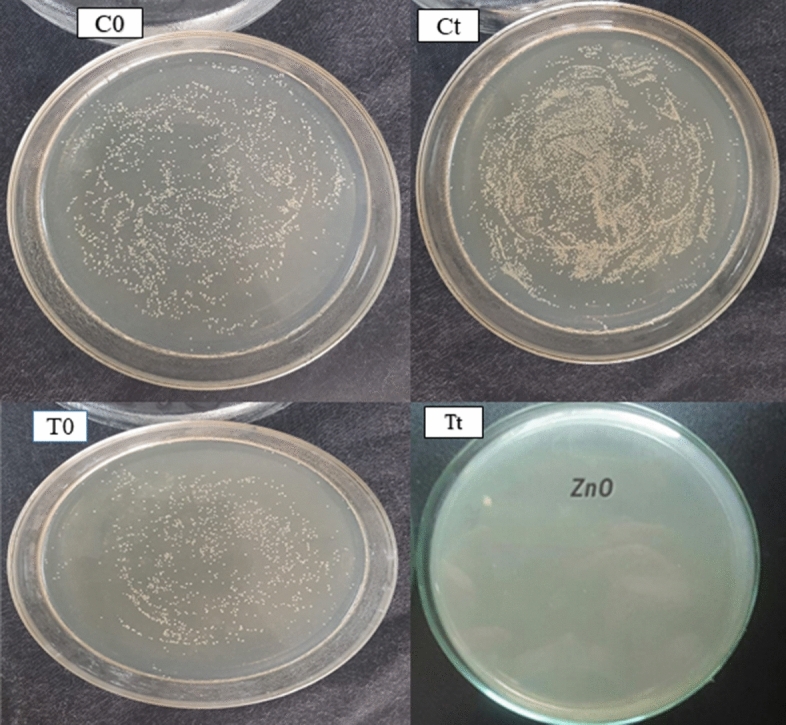
Quantitative antibacterial activity of control and treated samples
Table 3.
Percent reduction of bacteria for ZnO–silane-treated sample
| Bacteria | S. aureus | % Reduction | E. coli | % Reduction | ||
|---|---|---|---|---|---|---|
| Bacterial count of control sample | 0 h (C0) | 272 | – | 0 h (C0) | 275 | – |
| 24 h (Ct) | 287 | – | 24 h (Ct) | 290 | – | |
| Bacterial count of treated unwashed sample | 0 h (T0) | 267 | 99.99 | 0 h (T0) | 270 | 95 |
| 24 h (Tt) | 1 | 24 h (Tt) | 2 | |||
| Bacterial count of treated after 30 washes | 0 h (T0) | 269 | 99.99 | 0 h (T0) | 273 | 95 |
| 24 h (Tt) | 1 | 24 h (Tt) | 2 | |||
In Fig. 9, the left side shows the antibacterial activity of untreated sample and treated sample against S. aureus at 0 h and 24 h, respectively. At right side untreated sample and treated sample tested against E. coli at 0 h and 24 h, respectively.
Fig. 9.
Antibacterial activity untreated (control) and ZnO-treated sample
Antiviral activity of untreated and treated fabrics
Quantitative evaluation
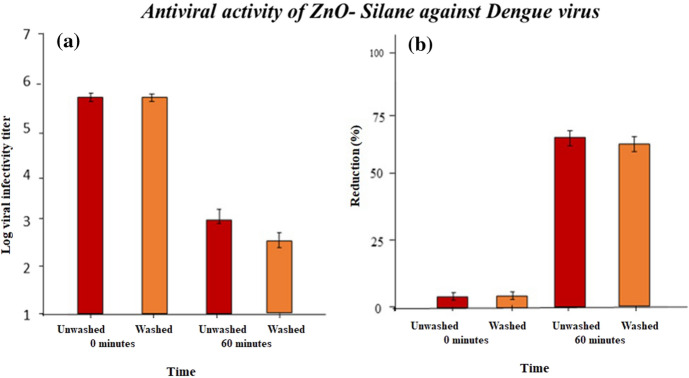
To investigate the antiviral efficacy quantitatively ISO 18184 standard method was used. Control samples and zinc oxide NPs’ immobilized samples with 100% GPTS concentration were selected for antiviral testing. From the literature, it was confirmed that zinc oxide NPs are effective against herpes simplex virus and Influenza virus. Various theories explained the antiviral activity of nanoparticles as ZnO NPs release Zn2+ ions as well as a variety of reactive oxygen species, which can damage proteins, lipid membranes, and nucleic acids and diffuse into the host cells resulting in inactivation and cell death of viruses, even though the mechanism of action has not been thoroughly investigated. There was no antiviral activity observed with cotton alone, indicating that the antiviral activity was caused by the zinc-coated fabric. Fabric treated with zinc oxide NPs was effective against dengue virus and hepatitis C virus (HCV). After 60 min of exposure, reduction % of unwashed treated fabric was 70% and after 30 washes it was 67% effective against dengue virus as shown in Fig. 10.
Fig. 10.
(a) Dengue residual viral load determined by TCID50 on nanocoated fabrics after a contact time of 0 h, 60 min, and (b) reduction % of dengue on nanoparticle-coated fabrics as calculated from log viral infectivity titer
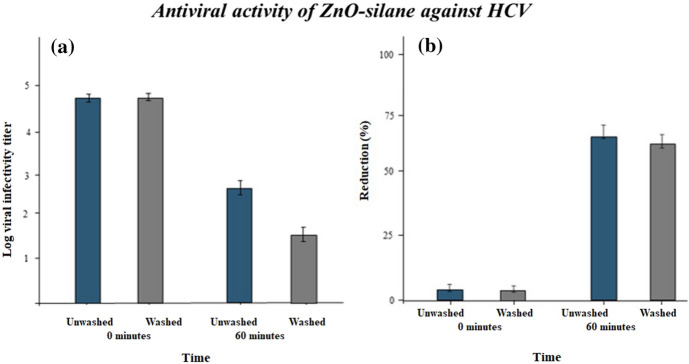
Like these results the antiviral activity against hepatitis C virus is given in Fig. 11. The percentage reduction was 72% before washing and after 30 washes it was 69%. From both results, it was confirmed that the treated fabric is effective against viruses before and after 30 industrial washing cycles. The durable antiviral activity is a need of day to develop personal protective equipment and avoid viral transfer in hospitals, clinics, and other medical institutions.
Fig. 11.
(a) HCV residual viral load determined by TCID50 on nanocoated fabrics after a contact time of 0 h, 60 min, and (b) reduction % of HCV on NPs-coated fabrics as calculated from log viral infectivity titer
Conclusion
Antimicrobial coatings with a broad spectrum of activity will also hinder the growth of bacteria and viruses that assist in the spread of disease. This procedure will be a novel treatment alternative in the event of future pandemic revival and emergence, which the world may face in the future. This research work was about the immobilization of metal oxide NPs, i.e., ZnO NPs on cotton fabric for durable multifunctional properties without affecting the comfort properties of fabric. Functionalized cotton fabric was effective against bacteria and viruses. The excellent durability of functional properties after several industrial washing cycles makes this fabric an ideal candidate for bioactive textiles used in medical field.
Acknowledgements
The conducted research work was supported by Higher Education Commission for funding this work through Rapid Research Program under Grant Number 74 and National Textile Research Centre, National Textile University, Pakistan.
Declarations
Conflict of interest
There is no conflict of interest among the authors regarding the publication of this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ngô C, Van de Voorde M. Nanotechnology in a nutshell. Nanotechnol a Nutshell. 2014 doi: 10.2991/978-94-6239-012-6. [DOI] [Google Scholar]
- 2.Yetisen AK, Qu H, Manbachi A, et al. Nanotechnology in textiles. ACS Nano. 2016;10:3042–3068. doi: 10.1021/acsnano.5b08176. [DOI] [PubMed] [Google Scholar]
- 3.Rivero PJ, Urrutia A, Goicoechea J, Arregui FJ. Nanomaterials for Functional Textiles and Fibers. Nanoscale Res Lett. 2015;10:1–22. doi: 10.1186/s11671-015-1195-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riaz S, Ashraf M, Hussain T, et al. Fabrication of robust multifaceted textiles by application of functionalized TiO2 Nanoparticles. Colloi Surf A Physi Eng Asp. 2019;581:123799. doi: 10.1016/j.colsurfa.2019.123799. [DOI] [Google Scholar]
- 5.Riaz S, Ashraf M, Hussain T, Hussain MT. Modification of silica nanoparticles to develop highly durable superhydrophobic and antibacterial cotton fabrics. Cellulose. 2019;26:5159–5175. doi: 10.1007/s10570-019-02440-x. [DOI] [Google Scholar]
- 6.Shagufta R. Fabrication of robust multifaceted textiles by application of functionalized TiO2 nanoparticles. Colloi Surf A Physi Eng Asp. 2019;581:123799. doi: 10.1016/J.COLSURFA.2019.123799. [DOI] [Google Scholar]
- 7.Szunerits S, Barras A, Khanal M, et al. Nanostructures for the inhibition of viral infections. Molecules. 2015;20:14051–14081. doi: 10.3390/molecules200814051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morais DS, Guedes RM, Lopes MA. Antimicrobial approaches for textiles: from research to market. Materials (Basel) 2016;9:498. doi: 10.3390/ma9060498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogdan J, Zarzyńska J, Pławińska-Czarnak J. Comparison of infectious agents susceptibility to photocatalytic effects of nanosized titanium and zinc oxides: a practical approach. Nanoscale Res Lett. 2015 doi: 10.1186/s11671-015-1023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westendorf Astrid Maria, Buer Jan, Überla Klaus, Epple Matthias. The potential of nanoparticles for the immunization against viral infections. J Mater Chem B. 2015;3(24):4767–4779. doi: 10.1039/C5TB00618J. [DOI] [PubMed] [Google Scholar]
- 11.Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnol. 2010;8:1. doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elechiguerra Jose Luis, Burt Justin L, Morones Jose R, Camacho-Bragado Alejandra, Gao Xiaoxia, Lara Humberto H, Yacaman Miguel Jose. Interaction of silver nanoparticles with HIV-1. J Nanobiotechnol. 2005 doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar R, Nayak M, Sahoo GC, et al. Iron oxide nanoparticles based antiviral activity of H1N1 influenza a virus. J Infect Chemother. 2019;25:325–329. doi: 10.1016/j.jiac.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Lin Z, Guo M, et al. Inhibition of H1N1 influenza virus-induced apoptosis by functionalized selenium nanoparticles with amantadine through ROS-mediated AKT signaling pathways. Int J Nanomedicine. 2018;13:2005–2016. doi: 10.2147/IJN.S155994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sametband M, Kalt I, Gedanken A, Sarid R. Herpes simplex virus type-1 attachment inhibition by functionalized graphene oxide. ACS Appl Mater Interfaces. 2014;6:1228–1235. doi: 10.1021/am405040z. [DOI] [PubMed] [Google Scholar]
- 16.Ardakani MR, Madadgar O. In vitro anti-foot-and-mouth disease virus activity of magnesium oxide. Nanoparticles. 2015;9:247–251. doi: 10.1049/iet-nbt.2014.0028. [DOI] [PubMed] [Google Scholar]
- 17.De Souza E, Silva JM, Hanchuk TDM, Santos MI, et al. Viral Inhibition Mechanism Mediated by Surface-Modified Silica Nanoparticles. ACS Appl Mater Interfaces. 2016;8:16564–16572. doi: 10.1021/acsami.6b03342. [DOI] [PubMed] [Google Scholar]
- 18.Heaselgrave Wayne, Kilvington Simon. The efficacy of simulated solar disinfection (SODIS) against coxsackievirus, poliovirus and hepatitis A virus. Journal of Water and Health. 2012;10(4):531–538. doi: 10.2166/wh.2012.128. [DOI] [PubMed] [Google Scholar]
- 19.Petcu C, Purcar V, Spătaru CI, et al. The influence of new hydrophobic silica nanoparticles on the surface properties of the films obtained from bilayer hybrids. Nanomaterials. 2017;7:1–10. doi: 10.3390/nano7020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riaz S, Ashraf M, Hussain T, et al. Selection and Optimization of Silane Coupling Agents to Develop Durable Functional Cotton Fabrics Using TiO2 Nanoparticles. Fibers Polym. 2021;22:109–122. doi: 10.1007/s12221-021-9245-4. [DOI] [Google Scholar]
- 21.Yadav A, Prasad V, Kathe AA, et al. Functional finishing in cotton fabrics using zinc oxide nanoparticles. Bull Mater Sci. 2006;29:641–645. doi: 10.1007/s12034-006-0017-y. [DOI] [Google Scholar]
- 22.Kim HW, Kim BR, Rhee YH. Imparting durable antimicrobial properties to cotton fabrics using alginate–quaternary ammonium complex nanoparticles. Carbohydr Polym. 2010;79:1057–1062. doi: 10.1016/J.CARBPOL.2009.10.047. [DOI] [Google Scholar]
- 23.Mihailović D, Šaponjić Z, Radoičić M, et al. Functionalization of polyester fabrics with alginates and TiO2 nanoparticles. Carbohydr Polym. 2010;79:526–532. doi: 10.1016/J.CARBPOL.2009.08.036. [DOI] [Google Scholar]
- 24.Vigneshwaran Nadanathangam, Sampath Kumar AA, Kathe PV, Varadarajan Virendra Prasad. Functional finishing of cotton fabrics using zinc oxide–soluble starch nanocomposites. Nanotechnology. 2006;17(20):5087–5095. doi: 10.1088/0957-4484/17/20/008. [DOI] [Google Scholar]
- 25.Vigneshwaran N, Nachane RP, Balasubramanya RH, Varadarajan PV. A novel one-pot ‘green’ synthesis of stable silver nanoparticles using soluble starch. Carbohydr Res. 2006;341:2012–2018. doi: 10.1016/J.CARRES.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 26.Kan CW, Lam YL. Low stress mechanical properties of plasma-treated cotton fabric subjected to zinc oxide-anti-microbial treatment. Materials (Basel) 2013;6:314–333. doi: 10.3390/ma6010314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perera S, Bhushan B, Bandara R, et al. Morphological, antimicrobial, durability, and physical properties of untreated and treated textiles using silver-nanoparticles. Colloids Surfaces A Physicochem Eng Asp. 2013;436:975–989. doi: 10.1016/j.colsurfa.2013.08.038. [DOI] [Google Scholar]
- 28.Huang H, Yang X. Synthesis of polysaccharide-stabilized gold and silver nanoparticles: A green method. Carbohydr Res. 2004;339:2627–2631. doi: 10.1016/j.carres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Dastjerdi R, Montazer M, Shahsavan S. A novel technique for producing durable multifunctional textiles using nanocomposite coating. Colloids Surf B Biointerfaces. 2010;81:32–41. doi: 10.1016/j.colsurfb.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 30.Nazari A, Montazer M, Moghadam MB, Anary-Abbasinejad M. Self-cleaning properties of bleached and cationized cotton using nanoTiO2: A statistical approach. Carbohydr Polym. 2011;83:1119–1127. doi: 10.1016/j.carbpol.2010.09.015. [DOI] [Google Scholar]
- 31.Jalan V, Butola BS. Influence of Binder Type on Color Characteristics of Cotton Fabric Colored with a Photochromic Colorant. J Nat Fibers. 2018;15:229–238. doi: 10.1080/15440478.2017.1325425. [DOI] [Google Scholar]
- 32.Vigneshwaran N, Kumar S, Kathe, , et al. Functional finishing of cotton fabrics using zinc oxide–soluble starch nanocomposites. Nanotechnology. 2006;17:5087–5095. doi: 10.1088/0957-4484/17/20/008. [DOI] [Google Scholar]
- 33.Schindler WD, Hauser PJ. Chemical Finishing of Textiles. London: Springer; 2010. Hand building finishes; pp. 43–50. [Google Scholar]
- 34.McKeen LW, McKeen LW. Binders Fluorinated Coatings Finish Handb. Sawston: Elsevier; 2006. pp. 45–58. [Google Scholar]
- 35.Vanneste M. Functional Finishes for Textiles: Improving Comfort. Sawston: Performance and Protection. Elsevier; 2015. Easy care finishes for textiles; pp. 227–256. [Google Scholar]
- 36.Gupta D, Gulrajani ML. Functional Finishes for Textiles: Improving Comfort. Sawston: Performance and Protection. Elsevier; 2015. Self cleaning finishes for textiles; pp. 257–281. [Google Scholar]
- 37.(2020) ISO / DIS 13322–2 Particle size analysis — Image analysis methods — Part 2 : Dynamic image analysis methods. https://webstore.ansi.org/Standards/ISO/ISO133222021. Accessed (22 Jun 2022)
- 38.ASTM International (2021) Fourier transform infrared spectrometry ASTM E168, ASTM E1252. In: ASTM Int. https://www.intertek.com/polymers/testlopedia/ftir/. Accessed (22 Jun 2022)
- 39.ISO - ISO 105-C03:1989 - Textiles — Tests for colour fastness — Part C03: Colour fastness to washing: Test 3. https://www.iso.org/standard/3805.html. Accessed (22 Jun 2022)
- 40.Imoto Y, Seino S, Nakagawa T, Yamamoto TA. Quantitative Methods for Testing Antiviral Activities of Textile Fabrics. J Antimicrob Agents. 2017 doi: 10.4172/2472-1212.1000146. [DOI] [PubMed] [Google Scholar]
- 41.Standardization FOR, Normalisation DE (1987) International Standard Iso. 1987:
- 42.Srithar A, Kannan JC, Senthil TS. Preparation and characterization of Ag doped ZnO nanoparticles and its antibacterial applications academic discipline and sub-disciplines nano technology type. Method/App. 2016;13:6273–6279. [Google Scholar]
- 43.Yang M, Liu W, Jiang C, et al. Robust fabrication of superhydrophobic and photocatalytic self-cleaning cotton textile based on TiO2 and fluoroalkylsilane. J Mater Sci. 2019;54:2079–2092. doi: 10.1007/s10853-018-3001-1. [DOI] [Google Scholar]
- 44.Xu QB, Xie LJ, Diao H, et al. Antibacterial cotton fabric with enhanced durability prepared using silver nanoparticles and carboxymethyl chitosan. Carbohydr Polym. 2017;177:187–193. doi: 10.1016/j.carbpol.2017.08.129. [DOI] [PubMed] [Google Scholar]
- 45.Doakhan S, Montazer M, Rashidi A, et al. Influence of sericin/TiO2 nanocomposite on cotton fabric: Part 1 Enhanced antibacterial effect. Carbohydr Polym. 2013;94:737–748. doi: 10.1016/j.carbpol.2013.01.023. [DOI] [PubMed] [Google Scholar]