Abstract
Objective
Diabetic retinopathy (DR) is the retinal consequence of chronic progressive diabetic microvascular leakage and occlusion. Non-proliferating diabetic retinopathy (NPDR) is the early stage of DR. It eventually occurs to some degree in all patients with diabetes mellitus. In recent years, many clinical trials have shown that Compound Danshen Dripping Pill (CDDP) may be associated with the improvement of NPDR symptoms. The aim of this study was to quantitatively summarize the association between CDDP and the therapeutic effects of NPDR.
Methods
It was conducted that a systematic literature search of PubMed, Web of Science, CNKI, VIP and Wanfang Data updated in June 2020 with the following search terms: “diabetic retinopathy” or “retinopathy” or “DR” or “NPDR”, in combination with “Compound Danshen Dripping Pill” or “Salvia miltiorrhiza” or “Danshen”. Risk ratio (RR) and weighted mean difference (WMD) with their 95% confidence interval (CI) was calculated between treatment and control groups. The sensitivity analyses were undertaken by removing each individual study when high heterogeneity appeared. Subgroup analysis, Meta-regression, and publication bias analysis were also conducted. The strength of evidence was evaluated with the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) method.
Results
Twenty-six RCTs involving 2047 subjects were included to conduct a Meta-analysis after screening the studies, extracting the data, and assessing the study quality. The Stata15.0 software was utilized for processing. Meta-analysis indicated that curative effects of treatment group with CDDP was significantly better than control [RR = 0.54, 95% CI (0.40, 0.73); moderate-quality evidence]. In addition, the results showed that CDDP was significantly associated with improving retinal hemorrhages [WMD = −0.62, 95% CI (−0.78, −0.46); low-quality evidence], the vision [WMD = 0.14, and 95% CI (0.09, 0.19), low-quality evidence], fundus fluorescence angiography [RR = 0.37 and 95% CI (0.23, 0.60); low-quality evidence], reduction of retinal microaneurysm [WMD = −3.74 and 95% CI (−4.38, −3.11); moderate-quality evidence], hemangioma volume [WMD = −3.15, 95%CI (−3.45, −2.85); moderate-quality evidence], macular thickness [WMD = −5.52, 95%CI = (−64.27, −48.78); low-quality evidence], mean defect [WMD = −1.65 and 95% CI (−1.95, −1.34); very low-quality evidence], fasting blooding glucose [WMD = −0.95, 95% CI (−1.19, −0.70); low-quality evidence), hemoglobin A1c [WMD = −0.62, 95% CI (−0.93, −0.30); low-quality evidence], high sensitive C reaction protein [WMD = −5.66, 95% CI (−8.01, −3.31); low-quality evidence]. Sensitivity, subgroup, and Meta-regression analyses were also assessed.
Conclusion
The study demonstrated that CDDP has beneficial clinical effects for treating NPDR and improve the vision. Moreover, it indicated that oral CDDP in NPDR patients led to significant regulation of serum level of fasting blooding glucose, hemoglobin A1c and high sensitive C reaction protein, which was associated with the pathogenesis of NPDR. However, high-quality and large randomized clinical trials will be needed to prove the consequence in future.
Keywords: Compound Danshen Dripping Pill, diabetic retinopathy, Meta-analysis, randomized controlled clinical trial, systemic review
1. Introduction
Diabetes mellitus (DM), one of the most common chronic metabolic diseases currently in the world, is characterized by hyperglycemia which results from the deficiency of insulin secretion or resistance to the action of insulin, or both (Amer Diabet, 2013). The International Diabetes Federation estimates that there are approximately 425 million adults (20–79 years) who were suffered from DM in 2017 with a projected increase of 592 million by the year 2035 (Guariguata et al., 2014). Numerous studies have demonstrated that DM is a risk factor for the development of microvascular complications, which can remarkably decrease the quality of life of diabetes patients with abnormal blood glucose.
Diabetic retinopathy (DR) is a common microvascular complication of diabetes, characterized by microaneurysm, lipid exudation, macular edema, capillary occlusion and neovascularization, vitreous hemorrhage. DR has become a significant public health problem that affects more than 90% of diabetic patients, which can cause different degrees of vision loss and ultimate blindness in working-aged individuals worldwide (Kowluru & Chan, 2007). DR has a complex process, which can be divided into two stages clinically: non-proliferating diabetic retinopathy (NPDR) and proliferating diabetic retinopathy (PDR) (Wilkinson et al., 2003). NPDR is the early stage of DR and is characterized by damaging retinal vasculature, increasing vascular permeability, thickening of the basement membrane, formation of the microangioma, loss of pericytes and neovascularization (Arroba & Valverde, 2017). The pathology of PDR is more severe than that of NPDR, which is defined by vitreous hemorrhage, retinal scars and detachment (Das, Stroud, Mehta, & Rangasamy, 2015).
It is gradually discovered that DR is a chronic inflammation and retinal neurodegeneration, which may be associated with local microvascular injury and microcirculation disorders (Altmann and Schmidt, 2018, Ji et al., 2019). For NPDR, surgical treatment and laser photocoagulation are the most commonly used therapeutic methods in clinical treatment (Abu El-Asrar, 2013, Sato et al., 2013), and increased physical activity is associated with less severe levels of DR as well (Praidou, Harris, Niakas, & Labiris, 2017). In patients with DR, several oral medications such as protein kinase C inhibitor (Aiello et al., 2011), Candesartan (Tillin, Orchard, Malm, Fuller, & Chaturvedi, 2011) and renin-angiotensin blockade (Wilkinson-Berka, Rana, Armani, & Agrotis, 2013) can effectively control the progression of DR and inhibit early lesions. However, these treatments cannot completely reverse retinal damage in patients with DR. Also, the visual acuity of patients could be severely damaged if NPDR is not successfully prevented and cured. Therefore, it is necessary to develop a new therapeutic strategy for DR progression at an early stage.
In traditional Chinese medicine (TCM) theory, DR is developed because of blood stasis, which causes hemorrhage and angiogenesis (Duan, Jin, Jie, & Ye, 2011). Compound Danshen Dripping Pill (CDDP, obtained from Tasly Pharmaceutical, Tianjin, China), the first traditional Chinese medicine to complete Phase III clinical trials by the US Food and Drug Administration (FDA), consists of Salvia miltiorrhiza Bunge, Panax notoginseng and Borneol. Animal experiment results showed that CDDP had retinal neuroprotective effects that were independent of blood glucose levels. These CDDP-induced neuroprotective effects may be mediated by retinal cell apoptosis inhibition and increased neuropeptide Y expression (Zhang et al., 2018). Furthermore, as Chinese herb medicine, CDDP can be used extensively to treat blood stasis and improve blood circulation including angina pectoris, hyperlipidemia and myocardial infarction (Chu et al., 2011, Yao et al., 2015).
In recent years, several reports demonstrate that CDDP has noticeable curative effect on early DR, including the control of microaneurysm and hemorrhage and the improvement of visual acuity and visual field. Based on the data from related randomized controlled trials, we conducted Meta-analysis to further confirm the efficacy of CDDP, which may be helpful in the management of NPDR.
2. Methods
2.1. Databases and search strategy
Relevant literatures were systemically retrieved from the database inception to June 2020 without language restriction, including English databases PubMed, Web of Science, the Chinese databases China National Knowledge Infrastructure (CNKI), the VIP information resource integration service platform (VIP), and Wanfang Data knowledge service platform (Wanfang Data). The search terms utilized were (“diabetic retinopathy” or “retinopathy” or “DR” or “NPDR”) and (“Compound Danshen Dripping Pill” or “S. miltiorrhiza” or “Danshen”). Furthermore, the references of eligible studies and previously systematic reviews were manually searched to identify further relevant studies.
2.2. Inclusion criteria
All studies selected were designed to be clinical randomized controlled trials (RCTs). Included studies were selected to meet the following criteria: (1) Patients were diagnosed and confirmed as NPDR; (2) CDDP was used as an intervention, and a placebo or conventional medication was used as a control; (3) The outcome included macular thickness, vision, mean defect (MD) of visual field, microaneurysms, hemangioma volume, hemorrhage improvements as well as fundus fluorescence angiography (FFA), fasting blooding glucose (FBG), hemoglobin A1c (HbA1c), high sensitive C reaction protein (hs-CRP); (4) Treatment lasted four weeks or more.
2.3. Exclusion criteria
The criteria to exclude studies were as follows: (1) Study of the control groups with unclear treatment strategy or the treatment groups using interventions other than CDDP; (2) Duplicate publications, and literature on nonclinical studies such as medical record reviews, retrospective clinical studies and animal experiments; (3) Clinical studies with unclear statistical analysis; (4) Patients were taking DR medications, such as intravitreal anti-VEGF, statin, fibrates; (5) Patients were treated with photocoagulation; (6) Patients had liver and kidney dysfunction; (7) Patients were pregnant or lactating.
2.4. Data extraction and quality assessment
Two authors (Lei Hao and Yu-jing Sun) independently reviewed the retrieved studies to filter and extract information according to the inclusion and exclusion criteria, and any disagreements were resolved through group discussion and consensus with the third reviewer (Yuan-xue Liu). According to the Jadad scale, the studies were graded as low quality (scores of 0–2) or high quality (scores of 3–5). If there was a disagreement during cross-checking, it was further discussed with or dealt by a third-party researcher. The detailed scoring criteria were as follows: (1) application of a randomized method, (2) application of blinding method, and (3) application of withdrawal and loss to follow-up. The extracted data from each study included author, year of publication, study population, age, sex, number of participants in treatment and control groups, interventions, duration, macular thickness, vision, MD, microaneurysm number, hemangioma volume, hemorrhage improvements, FFA, FBG, HbA1c and hs-CRP. We assessed the quality of the evidence supporting study findings by using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (risk of bias, imprecision, inconsistency, indirectness and publication bias) (Gordon, Andrew, Regina, Gunn, & Holger, 2008). According to GRADE, RCTs were considered higher-quality evidence, and observational studies were of lower quality. The quality of the evidence for each outcome was rated high, moderate, low or very low.
2.5. Statistical analysis
The Stata15.0 was used to carry out the Meta-analysis. Heterogeneity among studies was assessed by the chi-square test, with the I2 statistic. If the I2 > 50% and P < 0.10, the heterogeneity among studies existed, and the random-effects model was used for data analysis. Otherwise, the fixed-effect model was adopted. The analyses of count data were presented as the risk ratios (RR) or values with 95% confidence interval (95%CI), and the continuous data were presented as weighted mean difference (WMD) and their 95% CI. The evidence of heterogeneity was necessitated further subgroup analysis and Meta-regression to determine the possible factors underlying the heterogeneity. Besides, sensitivity analyses were undertaken by removing each individual study from the Meta-analysis when high heterogeneity appeared (I2 ≥ 75%). The Peters, Egger test and the Funnel plot were used to test publication bias. Egger test (P < 0.05) was considered to be representative of statistically significant publication bias, and there was no publication bias if the P value was more than 0.05 in Peters test.
3. Results
3.1. Search results
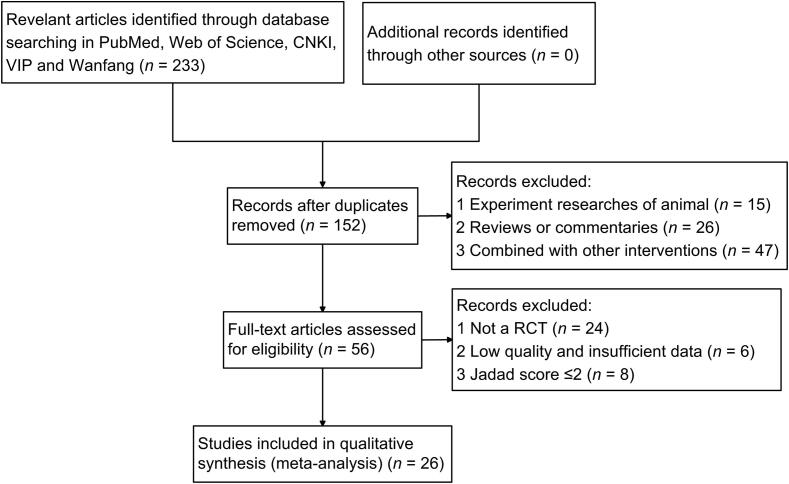
A total of 233 studies were identified from the five electronic bibliographic databases, among which 81 duplicate studies were removed. Based on the review of titles and abstracts, 16 non-RCTs, 15 animal trials, 26 reviews or commentaries, and 47 studies that combined with other interventions were excluded. Eventually, 26 eligible studies were identified as prospective randomized controlled trials for further Meta-analysis. Fig. 1 had depicted the detailed selection process for study identification.
Fig. 1.
Flowchart and strategy of Meta-analysis.
3.2. Characteristics of included studies
The characteristics of the included articles in this Meta-analysis were summarized in Table 1, including author and publication year, sample size, participant age, treatment duration, treatment and control group, outcome measures and Jadad score. In the 26 trials (Bai, 2017, Bao et al., 2006, Chen, 2019, Chen and Zhong, 2006, Ding and Chen, 2012, Fu et al., 2017, He and Zhen, 2013, Jin et al., 2009, Li, 2019, Li, 2017, Lian et al., 2015, Liu, 2018, Liu and Hao, 2011, Luo et al., 2015, Meng et al., 2011, Qi, Tan, Li, & Wang, 2007, Ruan et al., 2017, Shi, 2010, Wang et al., 2019, Wang et al., 2016, Xu, 2011, Xu, 2019, Yan and Yuan, 2014, Zhao, 2019, Zhou, 2008, Zhu, 2018) between 2006 and 2019, 2047 participants between the age of 32 and 78 years old were included: The treatment group of CDDP and the control group with calcium dobesilate (six studies); The treatment group of CDDP combined with calcium dobesilate and the control group with calcium dobesilate (10 studies); The treatment group of CDDP and the control group with placebo (six studies); The treatment group of CDDP and the control group with Vitamin B1 and/or Vitamin C and/or inosine tablets and/or Luding Tablets and/or Panshending Tablets (three studies); The treatment group of CDDP + Alprostadil and the control group with Alprostadil (one study). The course of treatment ranged from one month to six months.
Table 1.
Characteristic of included studies.
| Included studies | Sample size (T/C) | Age /years | Duration /months | Treatment groups | Control groups | Outcomes | Jadad scores |
|---|---|---|---|---|---|---|---|
| Liu & Hao, 2011 | 52 (26/26) | 39–76 | 3 | CDDP | Vitamin B1 + Vitamin C + Inosine tablets | Hemorrhage, vision, MD, microaneurysm | 3 |
| He & Zhen, 2013 | 84 (42/42) | 32–70 | 2 | CDDP | Placebo | Hemorrhage, microaneurysm | 3 |
| Zhu, 2018 | 114 (57/57) | 52–73 52–74 |
2 | CDDP | Placebo | Efficacy, hemorrhage, vision, MD | 4 |
| Yan & Yuan, 2014 | 60 (40/20) | 65.60 68.80 |
6 | CDDP | Placebo | Hemorrhage, vision, MD, microaneurysm | 4 |
| Liu, 2018 | 178 (89/89) | 49–78 45–76 |
1 | CDDP | Placebo | FBG, HbA1c, hs-CRP | 3 |
| Xu, 2011 | 80 (40/40) | 52.30 54.50 |
3 | CDDP | Luding tablets + Vitamin C + Panshending tablets | Efficacy | 3 |
| Chen & Zhong, 2006 | 63 (31/32) | 54.60 ± 10.40 58.12 ± 9.31 |
3 | CDDP | Calcium dobesilate | Efficacy | 3 |
| Wang et al., 2016 | 90 (45/45) | 57.15 ± 6.68 57.06 ± 6.72 |
2 | CDDP | Calcium dobesilate | Efficacy, FFA | 3 |
| Qi, Tan, Li, & Wang, 2007 | 42 (23/19) | 36–72 | 3 | CDDP | Vitamin B1 + Luding tablets | Hemorrhage, vision, MD, microaneurysm | 3 |
| Li, 2019 | 120 (60/60) | 58.11 ± 3.43 58.00 ± 3.56 |
4 | CDDP | Calcium dobesilate | Efficacy, hemorrhage, vision, MD, microaneurysm | 4 |
| Jin et al., 2009 | 58 (30/28) | 62.78 ± 7.69 61.11 ± 7.27 |
3 | CDDP | Calcium dobesilate | Hemorrhage, vision, MD, microaneurysm | 3 |
| Bao et al., 2006 | 67 (37/30) | 47–70 48–69 |
6 | CDDP | Calcium dobesilate | HbA1c | 3 |
| Fu et al., 2017 | 62 (31/31) | 43.70 ± 4.50 44.30 ± 4.10 |
2 | CDDP + Alprostadil | Alprostadil | Efficacy, hemorrhage, MD, hemangioma volume, macular thickness, hs-CRP |
4 |
| Bai, 2017 | 76 (38/38) | 40–72 39–71 |
4 | CDDP + Calcium dobesilate | Calcium dobesilate | Efficacy, vision, MD, hemorrhage, hemangioma volume, macular thickness, hs-CRP |
4 |
| Zhou, 2008 | 46 (28/18) | 50.40 ± 8.70 50.50 ± 9.36 |
6 | CDDP + Calcium dobesilate | Calcium dobesilate | Efficacy | 4 |
| Chen, 2019 | 82 (41/41) | 36–74 35–72 |
2 | CDDP + Calcium dobesilate | Calcium dobesilate | Microaneurysm, FBG, HbA1c, hs-CRP | 4 |
| Xu, 2019 | 86 (43/43) | 53.11 ± 4.41 53.06 ± 4.39 |
4 | CDDP + Calcium dobesilate | Calcium dobesilate | Efficacy, MD, hemorrhage, hemangioma volume,macular thickness | 4 |
| Wang et al., 2019 | 48 (24/24) | 41–62 40–64 |
3 | CDDP + Calcium dobesilate | Calcium dobesilate | Hemorrhage, hemangioma volume,macular thickness | 4 |
| Shi, 2010 | 68 (35/33) | 38–76 | 3 | CDDP + Calcium dobesilate | Calcium dobesilate | Efficacy | 3 |
| Ruan, 2017 | 70 (35/35) | 52.50 ± 1.10 52.80 ± 1.70 |
4 | CDDP + Calcium dobesilate | Calcium dobesilate | Efficacy, hemorrhage, hemangioma volume, MD, macular thickness, FBG, HbA1c |
4 |
| Zhao, 2019 | 106 (53/53) | 38–75 40–76 |
2 | CDDP | Placebo | Efficacy, hemorrhage, MD, macular thickness, hemangioma volume, hs-CRP |
4 |
| Meng et al., 2011 | 58 (30/28) | 50.60 ± 8.70 51.20 ± 7.90 |
6 | CDDP + Calcium dobesilate | Calcium dobesilate | Efficacy | 4 |
| Li, 2017 | 178 (89/89) | 56.50 ± 7.20 55.80 ± 6.80 |
2 | CDDP + Calcium dobesilate | Calcium dobesilate | Efficacy, FBG, HbA1c | 3 |
| Ding et al., 2012 | 76 (38/38) | 45–66 42–60 |
2 | CDDP + Calcium dobesilate | Calcium dobesilate | Efficacy | 3 |
| Lian et al., 2015 | 112 (56/56) | 58.90 ± 8.10 58.90 ± 7.60 |
6 | CDDP | Placebo | Efficacy, hemorrhage, exudate, FFA | 4 |
| Luo et al., 2015 | 57 (28/29) | 59.54 ± 7.46 57.80 ± 10.03 |
3 | CDDP | Calcium dobesilate | Hemorrhage, vision, microaneurysm | 4 |
3.3. Primary outcome
3.3.1. Clinical therapeutic effectiveness
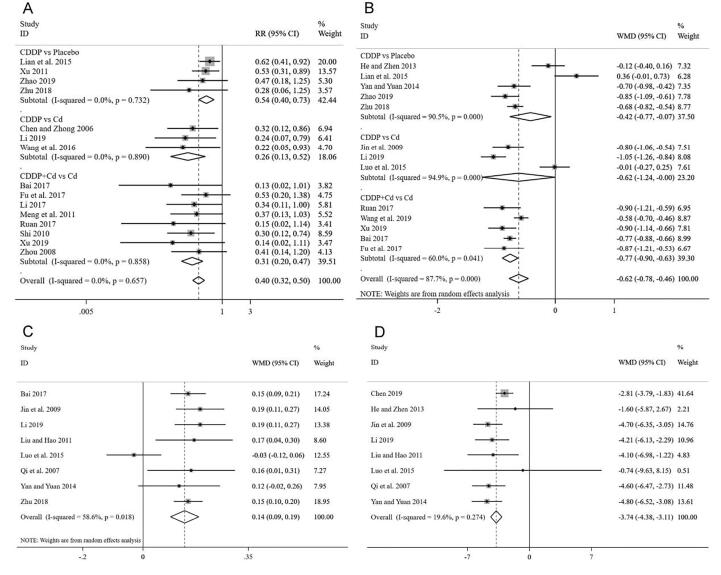
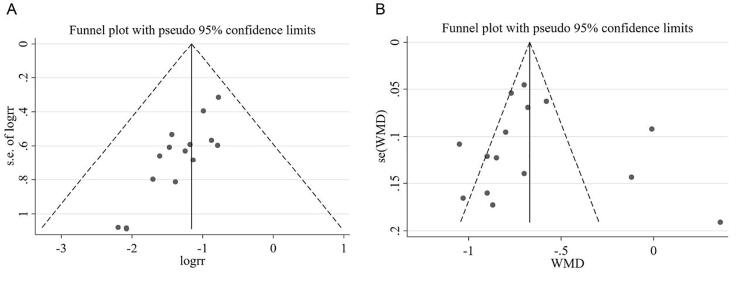
The efficacy evaluation criteria was based on guiding principles of clinical research on traditional Chinese medicine new drugs for diabetic retinopathy (Zheng, 2002). A Meta-analysis of the 15 articles (Bai, 2017, Chen and Zhong, 2006, Fu et al., 2017, Li, 2017, Li, 2019, Lian et al., 2015, Meng et al., 2011, Ruan et al., 2017, Shi, 2010, Wang et al., 2016, Xu, 2011, Xu, 2019, Zhao, 2019, Zhou, 2008, Zhu, 2018) showed that, for the total effective treatment rate, the homogeneity test results were I2 = 0% and P = 0.657, indicating no significant heterogeneity. Thus, the fixed-effect model was adopted and further subgroup analysis showed that the clinical effective rate of participants only with CDDP was significantly better than placebo [RR = 0.54, 95% CI (0.40, 0.73)], CDDP was also significantly better than Calcium dobesilate (Cd) [RR = 0.26, 95% CI (0.13, 0.52)], and CDDP combining with Cd was more effective than Cd alone [RR = 0.31, 95% CI (0.20, 0.47)], as shown in Fig. 2A. The publication bias was evaluated using funnel plot and Peters test. There was no publication bias analyzed by Peters test for the clinical efficacy rate of CDDP (P = 0.287), and the funnel plot was shown in Fig. 3A.
Fig. 2.
Forest plot of clinical therapeutic effectiveness (A), hemorrhage (B), vision (C), microaneurysms (D).
Fig. 3.
Funnel plot of clinical efficacy rate (A) and hemorrhage (B).
3.3.2. Hemorrhage
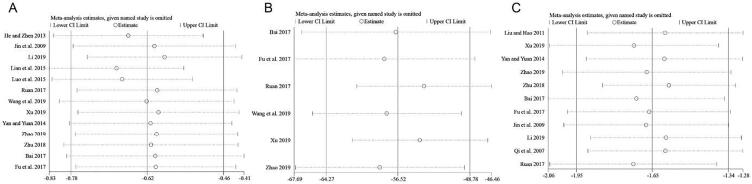
Thirteen studies (Bai, 2017, Fu et al., 2017, He and Zhen, 2013, Jin et al., 2009, Li, 2019, Lian et al., 2015, Luo et al., 2015, Ruan et al., 2017, Wang et al., 2019, Xu, 2019, Yan and Yuan, 2014, Zhao, 2019, Zhu, 2018) involving 1053 participants with 537 in the treatment group and 516 in control group were included in Meta-analysis for hemorrhage. The total results showed that the improvement of hemorrhage in treatment group was significantly better than controls [WMD = −0.62, 95% CI (−0.78, −0.46)] with significant heterogeneity (I2 = 87.7%), using a random-effects model (Fig. 2B). The results of subgroup analysis indicated that the hemorrhages area of participants only used CDDP was significantly decrease compared with placebo [WMD = −0.42, 95% CI (−0.77, −0.07)] and Cd [WMD = −0.62, 95% CI (−1.24, −0.00)], and combining CDDP with Cd was more effective than Cd [WMD = −0.77, 95% CI (−0.90–0.63)]. The sensitivity analysis further showed that there was no significant change between the total effect amount and the combined effect amount after excluding each certain study, suggesting a stable result from the Meta-analysis (Fig. 4A). The funnel plot and further Egger test were used to evaluate publication bias. As a result, though, the funnel plot showed a left–right asymmetry (Fig. 3B), but P = 0.423, suggesting that there was no sign of publication bias analyzed for the effect of CDDP on NPDR. Moreover, the individual associations between hemorrhage area and study-level variables were evaluated by univariable Meta-regression using restricted maximum likelihood method. The prespecified variables included gender (male vs female), number of participants, mean age and duration. According to the univariable Meta-regression, the results indicated that the heterogeneity could not be well explained by each of the prespecified variables (Table 2).
Fig. 4.
Sensitivity analysis result of hemorrhage area (A), macular thickness (B) and MD (C).
Table 2.
Results of Meta-regression analysis of hemorrhage.
| ES | Exp(b) | Std. Err. | t | P>|t| | 95% Conf. Interval | |
|---|---|---|---|---|---|---|
| Gender | 1.084278 | 0.3182035 | 0.28 | 0.788 | 0.5683549 | 2.06853 |
| Number | 0.999757 | 0.0051021 | −0.05 | 0.963 | 0.9885902 | 1.01105 |
| Age | 1.01234 | 0.0210653 | 0.59 | 0.568 | 0.9670209 | 1.059782 |
| Duration | 1.057154 | 0.0934905 | 0.63 | 0.543 | 0.8701703 | 1.284316 |
3.3.3. Vision
Eight studies (Bai, 2017, Jin et al., 2009, Li, 2019, Liu and Hao, 2011, Luo et al., 2015, Qi, Tan, Li, & Wang, 2007, Yan and Yuan, 2014, Zhu, 2018) with a total of 579 participants were included to investigate the difference of visual acuity between two groups. Moderate heterogeneity was found in these articles (P = 0.018, I2 = 58.6%), and the evaluation by the random-effects model revealed that there were significant differences among these studies. The pooled WMD estimate showed a much higher value of visual acuity in the treatment group taking CDDP compared with control group [WMD = 0.14, and 95% CI (0.09, 0.19)] (Fig. 2C).
3.3.4. Microaneurysm number
Eight studies (Chen, 2019, He and Zhen, 2013, Jin et al., 2009, Li, 2019, Liu and Hao, 2011, Luo et al., 2015, Qi, Tan, Li, & Wang, 2007, Yan and Yuan, 2014) reported microaneurysm number involving 555 participants with 290 in the treatment group and 265 in control group, and significant heterogeneity difference did not exist (P = 0.274, I2 = 19.6%). Thus, the fixed-effect model was used to statistical analysis, and the result in Fig. 2D showed that microaneurysm number significantly decreased in the treatment group compared with controls [WMD = −3.74 and 95% CI (−4.38, −3.11)].
3.3.5. FFa
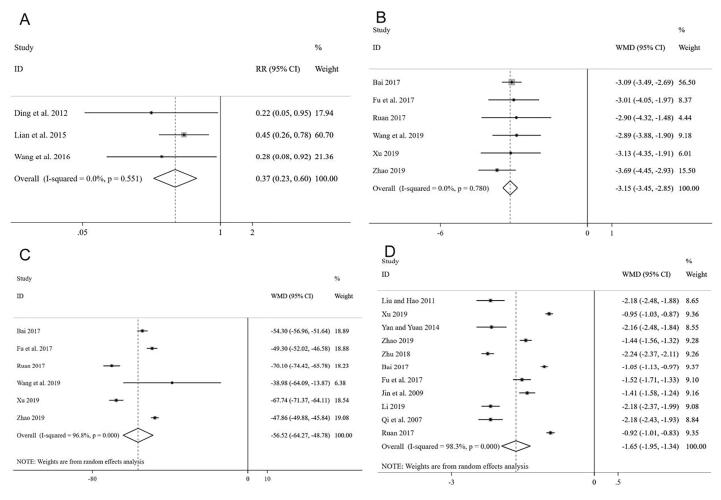
The heterogeneity analysis based on the three trials (Ding and Chen, 2012, Lian et al., 2015, Wang et al., 2016) reporting relevant data showed that there was no heterogeneity among these studies (P = 0.551, I2 = 0%), and the fixed effect model was adopted to analyze data. The results suggested that the patients taking CDDP had a noticeable advantage in changing the FFA of diabetic retinopathy compared with those who do not take CDDP [RR = 0.37 and 95% CI (0.23, 0.60)] (Fig. 5A).
Fig. 5.
Forest plot of FFA (A), hemangioma volume (B), macular thickness (C) and MD (D).
3.3.6. Hemangioma volume
In this Meta-analysis, six studies (Bai, 2017, Fu et al., 2017, Ruan et al., 2017, Wang et al., 2019, Xu, 2019, Zhao, 2019) involving 448 participants reported the hemangioma volume. A fixed-effects model was used to analyze the data due to no heterogeneity (P = 0.78, I2 = 0%). The results indicated that the volume of the hemangioma has dramatically improved after taking CDDP in the treatment group compared with controls [WMD = −3.15, 95%CI (−3.45, −2.85)] (Fig. 5B).
3.3.7. Macular thickness
Six RCTs (Bai, 2017, Fu et al., 2017, Ruan et al., 2017, Wang et al., 2019, Xu, 2019, Zhao, 2019) with a total of 448 participants were included to investigate the changes of macular thickness between the treatment group and controls. Significant heterogeneity was found (P = 0.000, I2 = 96.8%) among the trials, and the random-effects model revealed that the macular thickness changed significantly after taking CDDP [WMD = −56.52, 95% CI = (−64.27, −48.78)] (Fig. 5C), suggesting that CDDP showed favorable effects in decreasing macular thickness of NPDR. Although substantial heterogeneity was observed in the magnitude of the effect amount, the conclusion was not affected after sequential exclusion of any specific study (Fig. 4B).
3.3.8. MD
Eleven studies (Bai, 2017, Fu et al., 2017, Jin et al., 2009, Li, 2019, Liu and Hao, 2011, Qi, Tan, Li, & Wang, 2007, Ruan et al., 2017, Xu, 2019, Yan and Yuan, 2014, Zhao, 2019, Zhu, 2018) with a total of 845 participants provide data to observe the difference of MD between treatment and control groups, and they did not show homogeneity (I2 = 98.3%). A random-effects model showed that there were remarkable differences among these studies. Compared with those who do not take CDDP in the control group, the MD value of the visual field notably decreased in the treatment group [WMD = −1.65 and 95% CI (−1.95, −1.34)] (Fig. 5D). The results of sensitivity analysis did not show any significant differences after excluding any study from this Meta-analysis (Fig. 4C). Furthermore, a Meta-regression analysis were conducted to explore heterogeneity based on gender, number of participants, mean age, duration and allocation. Overall, gender (P = 0.181), number (P = 0.937), mean age (P = 0.055), duration (P = 0.393) and allocation (P = 0.693) did not significantly affect the relationship between MD and NPDR (Table 3).
Table 3.
Results of Meta-regression analysis of MD.
| ES | Exp(b) | Std. Err. | t | P>|t| | 95% Conf. Interval | |
|---|---|---|---|---|---|---|
| Gender | 0.588972 | 0.2152843 | −1.45 | 0.181 | 0.2576243 | 1.346488 |
| Number | 0.999438 | 0.0069255 | −0.08 | 0.937 | 0.9838937 | 1.015228 |
| Age | 1.053794 | 0.0250609 | 2.20 | 0.055 | 0.9986002 | 1.112038 |
| Duration | 1.137683 | 0.1636272 | 0.90 | 0.393 | 0.8217155 | 1.575146 |
| Allocation | 0.221476 | 0.5390795 | 0.41 | 0.693 | −1.053244 | 1.496196 |
3.3.9. FBg
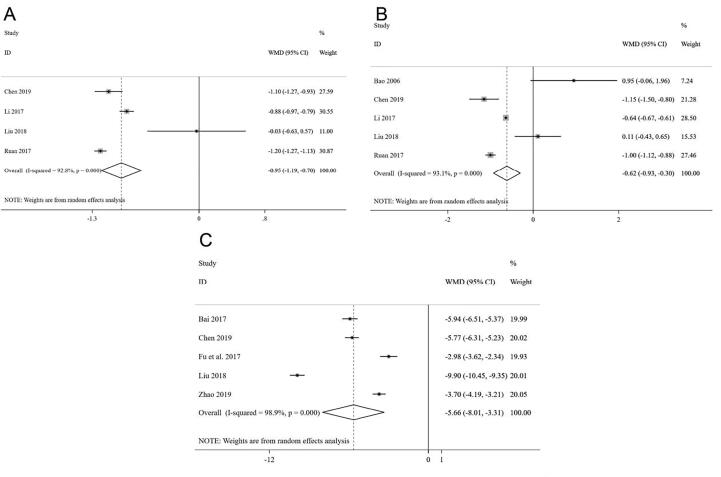
Four studies (Chen, 2019, Li, 2017, Liu, 2018, Ruan et al., 2017) among 508 participants reported on changes in fasting blooding glucose (mmol/L), and the pooled standardized mean difference showed a statistically significant reduction in fasting blooding glucose in CDDP group (WMD = −0.95, 95% CI (−1.19, −0.70)) compared with the control group, with a high heterogeneity (I2 = 92.8%) (Fig. 6A).
Fig. 6.
Forest plot of FBG (A), HbA1c (B), and hs-CRP (C).
3.3.10. HbA1c
Among all enrolled studies, five trials (Bao et al., 2006, Chen, 2019, Li, 2017, Liu, 2018, Ruan et al., 2017) involving 575 participants with 291 in the treatment group and 284 in control group had evaluated the HbA1c. Compared with the control group, the result showed a weak trend toward in HbA1c in treatment group taking CDDP [WMD = −0.62, 95% CI (−0.93, −0.30)], with a high level of heterogeneity (I2 = 93.1%) (Fig. 6B).
3.3.11. hs-CRP
Five of the enrolled articles (Bai, 2017, Chen, 2019, Fu et al., 2017, Liu, 2018, Zhao, 2019) including 504 participants had assessed the hs-CRP, and the pooled data demonstrated that the treatment group showed a statistically significant reduction in hs-CRP [WMD = −5.66, 95% CI (−8.01, −3.31)] compared with patients without taking CDDP in control group, and high statistical heterogeneity could be detected (I2 = 98.9%) (Fig. 6C).
3.4. Grading quality of evidence
GRADE was used to evaluate the level of evidence in the Meta-analysis. Table 4 presented the results of all outcomes in GRADE summary-of-finding tables. The quality of the evidence for the clinical therapeutic effectiveness, microaneurysm number, and hemangioma volume was moderate because of high risk of bias. Only the MD was judged as very low-quality evidence because of high risk of bias, inconsistency and publication bias, and the others were judged as low-quality evidence due to high risk of bias, inconsistency or publication bias.
Table 4.
Assessment of quality of evidence.
| Certainty assessment |
Summary of findings |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (studies) Follow-up | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall certainty of evidence | Study event rates /% |
Relative effect (95% CI) | Anticipated absolute effects |
||
| With control | With treatment | Risk with control | Risk difference with treatment (95% CI) | ||||||||
| Efficacy (Critical outcome) | |||||||||||
| 1380 (15 RCTs) range 2–6 months |
serious1 | not serious | not serious | not serious | undetected | ⨁⨁⨁◯ MODERATE1 due to risk of bias |
446/683 (65.3%) |
616/697 (88.4%) |
RR 1.35(1.28 to 1.43) | 653 per 1,000 |
229 more per 1000 (from 183 more to281 more) |
| Hemorrhage (Better indicated by lower values) | |||||||||||
| 1053 (13 RCTs) range 2–6 months |
serious1 | serious2 | not serious | not serious | undetected | ⨁⨁◯◯ LOW1,2 due to risk of bias, inconsistency |
516 | 537 | – | MD 0.65 lower (0.8 lower to 0.5 lower) | |
| Vision (Better indicated by higher values) | |||||||||||
| 579 (eight RCTs) range 2–6 months |
serious1 | serious2 | not serious | not serious | undetected | ⨁⨁◯◯ LOW1,2 due to risk of bias, inconsistency |
277 | 302 | – | MD 0.14 higher (0.1 higher to 0.18 higher) | |
| Microaneurysm number (Better indicated by lower values) | |||||||||||
| 555 (eight RCTs) range 2–6 months |
serious1 | not serious | not serious | not serious | undetected | ⨁⨁⨁◯ MODERATE1 due to risk of bias |
265 | 290 | – | MD 3.74 lower (4.38 lower to 3.11 lower) | |
| FFA (Better indicated by lower values) | |||||||||||
| 278 (three RCTs) range 2–6 months |
serious1 | not serious | not serious | not serious | publication bias strongly suspected3 | ⨁⨁◯◯ LOW due to risk of bias, publication bias1,2 |
64/139 (46.0%) |
18/139 (12.9%) |
RR 0.28(0.18 to 0.44) | 460 per 1000 |
332 fewer per 1000 (from 378 fewer to 258 fewer) |
| Hemangioma volume (Better indicated by lower values) | |||||||||||
| 448 (six RCTs) range 2–4 months |
serious1 | not serious | not serious | not serious | undetected | ⨁⨁⨁◯ MODERATE1 due to risk of bias |
224 | 224 | – | MD 3.15 lower (3.45 lower to 2.85 lower) | |
| Macular thickness (Better indicated by lower values) | |||||||||||
| 448 (six RCTs) range 2–4 months |
serious1 | serious2 | not serious | not serious | undetected | ⨁⨁◯◯ LOW1,2 due to risk of bias, inconsistency |
224 | 224 | – | MD 56.52 lower (64.27 lower to 48.78 lower) | |
| MD (Better indicated by lower values) | |||||||||||
| 845 (11 RCTs) range 2–6 months |
serious1 | serious2 | not serious | not serious | publication bias strongly suspected3 | ⨁◯◯◯ VERY LOW due to risk of bias, inconsistency, publication bias1,2,3 |
409 | 436 | – | MD 1.64 lower (1.95 lower to 1.34 lower) | |
| FBG (Better indicated by lower values) | |||||||||||
| 508 (four RCTs) range 1–4 months |
serious1 | serious2 | not serious | not serious | undetected | ⨁⨁◯◯ LOW1,2 due to risk of bias, inconsistency |
254 | 254 | – | MD 0.95 lower (1.19 lower to 0.7 lower) | |
| Hb A1c (Better indicated by lower values) | |||||||||||
| 575 (five RCTs) range 1–4 months |
serious1 | serious2 | not serious | not serious | undetected | ⨁⨁◯◯ LOW1,2 due to risk of bias, inconsistency |
284 | 291 | – | MD 0.62 lower (0.93 lower to 0.3 lower) | |
| hs-CRP (Better indicated by lower values) | |||||||||||
| 504 (five RCTs) range 1–6 months |
serious1 | serious2 | not serious | not serious | undetected | ⨁⨁◯◯ LOW1,2 due to risk of bias, inconsistency |
252 | 252 | – | MD 5.66 lower (8.01 lower to 3.31 lower) | |
The quality assessment is shown both graphically by the number of + signs and as high, moderate, low, or very low quality.
Risk of bias: (a) lack of allocation concealment; (b) lack of blinding of participants and personnel (c) lack of blinding of outcome assessment; (d) Incomplete accounting of patients and outcome events; (e) Selective outcome reporting; (f) including non-randomized controlled trial. Lack of allocation concealment and inadequate blinding caused risk of bias.
The inconsistency was considered serious because significant heterogeneity.
The publication bias was assessed via the Eggers test, Peters test and the funnel plot.
4. Discussion
DR is a leading cause of visual impairment in people of working age. The prevalence rate of DR for all adults with DM is estimated to be 34.6%, and an estimate of the prevalence rate for vision-threatening diabetic retinopathy is 10.2% (Kempen et al., 2004, Zhang et al., 2010). Based on both clinical trials and epidemiologic studies, it is generally believed that duration of diabetes and severity of hyperglycemia are the major risk factors for developing retinopathy, and excessive oxidative stress reaction will undergo in retinal cells under the high glucose concentration, and this series of reactions will lead to damage and loss of peripheral cell, and even damage to retinal endothelial cells and promote apoptosis finally (Wong et al., 2008). Clinically, the therapy of western medicine is considered as the conventional treatment for diabetic retinopathy. Most patients are usually treated by calcium dobesilate, anti-VEGF therapy, retinal laser photocoagulation, and even vitreous retinal surgery, but noninvasive treatment is not very effective.
According to the theory of traditional Chinese medicine, the pathogenesis of DR is a deficiency of qi and yin and qi failing to circulate blood. So the principle of treatment is to nourish qi and yin and promote blood circulation to resolve blood stasis (Ou, Yang, & Peng, 2019). The previous studies of the CDDP method for NPDR have been conducted in clinical. However, due to variations in the sample size and methodological quality of these researches, the efficacy of CDDP for NPDR is still not fully understood. Therefore, a systematic review and Meta-analysis were conducted to compare the clinical efficacy of patients who were treated with CDDP and combined with conventional western drugs, placebo, and other medicine. This Meta-analysis included 26 randomized clinical studies, and the results showed that, compared with the control group, CDDP has obvious clinical curative effect in visual function recovery by decreasing macular thickness, retinal microaneurysm number and hemangioma volume, promoting the control of hemorrhage, improving FFA, visual acuity and visual field, FBG, HbA1c and hs-CRP. The results suggested that CDDP may delay retinopathy progression at the early stage of DR and enhance the vision and quality of life through the microcirculation improvement further. In addition, no serious adverse drug reactions were observed during all the studies, which suggested that CDDP was safe for the treatment of NPDR. What is different from the previous research (Huang, Bao, Jin, & Lian, 2017) is that more new articles are included in this study, and sensitivity analyses are undertaken to explore the influence on the overall results by each individual study. Furthermore, Meta-regression are performed to search for the potential source of heterogeneity and we did not observe evidence that any prespecified variables represented the cause of the observed heterogeneity.
CDDP, a modern Chinese medicine preparation listed in the Chinese Pharmacopeia, is now widely applied for the clinical prevention and treatment of coronary heart disease, angina pectoris and other cardiovascular conditions. CDDP is composed of the mixed extracts of S. miltiorrhiza, P. notoginseng and Borneol. Among them, S. miltiorrhiza possesses remarkable antioxidant properties owing to its ability to upregulate endogenous antioxidant enzymes and induce free radical scavenging (Lin & Hsieh, 2010). Salvianolic acid A, a chemical constituent with antioxidant properties in S. miltiorrhiza, has been shown to inhibit phosphorylation of Src and Akt directly, thereby prevent endothelial cell proliferation and angiogenesis during proliferative diabetic retinopathy (Yang et al., 2012). Rosmarinic acid, another phytoconstituent of S. miltiorrhiza, inhibits retinal neovascularization by causing cell cycle arrest at G2 and M phase (Kim et al., 2009). Since oxidative stress-induced retinal neurodegeneration is one of the critical pathological events involved in the pathogenesis of DR, S. miltiorrhiza may be utilized for preventing apoptosis of retinal neuron. In addition, S. miltiorrhiza can be used to treat blood stasis and improve blood circulation due to its positive effects on myocardial energy metabolism improvement, antiplatelet aggregation and neuroprotective effects (Pills, 2017; Zhang et al., 2018).
P. notoginseng extracts have an effect of anti-coagulation, anti-inflammation, antioxidant, and inhibition of lipid deposition and platelet aggregation, owing to several kinds of saponin, such as ginsenoside Re 14, ginsenoside Rk1, ginsenoside Rg1, ginsenoside Rb1 and notoginsenoside R1 (Bermudez et al., 2010). Retinopathy macular edema is a common cause of vision impairment in DR patients with the presence of hard exudates. VEGF is one of the most potent pro-angiogenic agents, which can induce the proliferation, migration and angiogenesis of endothelial cells, and the inhibition of its release attenuates retinal neovascularization in proliferative diabetic retinopathy (Godoy, Betts-Obregon, & Tsin, 2014). Recent studies have shown that oxidative stress and inflammation were involved in the pathogenesis of DR (Gao et al., 2013), these beneficial effects of saponins can be utilized in the treatment of DR. According to the report, ginsenoside Rk1 showed significant inhibition in VEGF-induced and AGE induced retinal endothelial permeability, thereby reducing retinal edema, which indicating that P. notoginseng extracts could attenuate symptoms associated with diabetic retinopathy (Maeng et al., 2013). Borneol has the effect of promoting coronary‑dilating and analgesic, as an adjuvant and guiding drug, contributes to the entry of active ingredients of S. miltiorrhiza and P. notoginseng into the body tissues and organs (Zhang, Gao, Shang, Zhao, & Wang, 2003). More broadly, these Chinese herbs can be used for the treatment of diabetic retinopathy owing to the active ingredients by which they alleviate pathological occurrences in retina caused by hyperglycaemia. Consequently, the CDDP treatment may be used as one of the effective and safe therapy for DR.
An increasing number of epidemiological evidences had identified several factors associated with the DR, such as blood glucose level, duration of diabetes and obesity (Atchison & Barkmeier, 2016). It has been reported that the elevated FBG level and higher HbA1c concentration were associated with the risk factors for DR (Corcóstegui et al., 2017). In the Diabetes Control and Complications Trial (DCCT) study of patients with type 1 diabetes, intensive therapy to maintain normal glucose blood and HbA1c levels reduced the risk for the development of retinopathy and the progression of retinopathy (Nathan et al., 1993). It also has been confirmed that hs-CRP is one of the inflammation markers for consideration as a predictor of cardiovascular risk (Rifai & Ridker, 2001). With population-based studies, the inflammation assessed by the measurement of serum hs-CRP is associated with the development of diabetes (Tan et al., 2003) Furthermore, it also has been reported that DR is negatively associated with serum hs-CRP level with the mechanism of this phenomenon is that some humoral factors related to DR might negatively modulate the CRP production (Keiko et al., 2005). All this suggested that CDDP might have positive effect on glycemic control, serum inflammatory factors reduction and inflammation inhibition to retard the progression of DR and improve of the symptom of patients. Notably, significant heterogeneity was observed in the Meta-analysis of FBG, HbA1c and hs-CRP. There are several sources of heterogeneity, including differences in the treatment, the treated population, the study design, or the data analysis method. We could not be able to estimate heterogeneity precisely because of the small sample size Meta-analysis provides the little information about heterogeneity (Paul, 2015)
Nevertheless, there are several limitations in this study. First, in view of the fact that CDDP is a kind of TCM preparation, all literatures for this Meta-analysis were from China, among which two were in English and the rest were in Chinese. Therefore, lacking relevant foreign experiments, the systematic review and Meta-analysis of this study were regional and may be cause selection bias. Second, most studies only mentioned the random trials, but the specific method of random sequence generation used, RCTs of allocation concealment and blinding of outcome assessment is unknown. Furthermore, the methodological quality of many of the included RCTs was generally low and might have a high risk of bias. Third, most of the articles were related to positive results, which would likely lead to the overestimation of actual treatment effects. Fourth, the heterogeneity was still very high though we considered different methods of administration in the subgroup analysis. Furthermore, the source of heterogeneity could not be detected by Meta-regression, and the high heterogeneity might be due to the study designs and methods and methodological qualities as well. We also found that the association between CDDP and the severity level of NPDR was not analyzed because of a lack of enough data. Therefore, more multicenter RCTs with large sample and high quality are needed to confirm the beneficial effects of CDDP or CDDP combined with conventional treatments on NPDR treatment.
5. Conclusion
In summary, this systemic review demonstrated the therapeutic effects of CDDP treatment in NPDR patients, which was involved decreasing macular thickness, retinal microaneurysm number and hemangioma volume, promoting the control of hemorrhage, improving FFA, visual acuity and visual field. Moreover, our findings conclusively demonstrated that oral CDDP in NPDR patients led to significant regulation of serum level of fasting blooding glucose, hemoglobin A1c and high sensitive C reaction protein, which was associated with the pathogenesis of NPDR. According to GRADE, the quality of the evidence for each outcome was moderate to very low. Taking account of the limits in this study, to investigate the effect of CDDP on NPDR for providing some guidance suggestions in the clinical practice, more rigorous RCTs of the long-term with a large sample size and high methodological standards are needed to enhance the strength of evidence.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Science and Technology Major Project for Significant New Drugs Development (2017ZX09301005).
References
- Abu El-Asrar A.M. Evolving strategies in the management of diabetic retinopathy. Middle East African Journal of Ophthalmology. 2013;20(4):273–282. doi: 10.4103/0974-9233.119993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello L.P., Vignati L., Sheetz M.J., Zhi X., Girach A., Davis M.D.…Milton R.C. Oral protein kinase c beta inhibition using ruboxistaurin efficacy, safety, and causes of vision loss among 813 patients (1,392 eyes) with diabetic retinopathy in the protein kinase C beta inhibitor-diabetic retinopathy study and the protein kinase C beta inhibitor-diabetic retinopathy study 2. Retina-the Journal of Retinal and Vitreous Diseases. 2011;31(10):2084–2094. doi: 10.1097/IAE.0b013e3182111669. [DOI] [PubMed] [Google Scholar]
- Altmann C., Schmidt M.H.H. The role of microglia in diabetic retinopathy: Inflammation, microvasculature defects and neurodegeneration. International Journal of Molecular Sciences. 2018;19(1):110. doi: 10.3390/ijms19010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer Diabet A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36:S67–S74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroba A.I., Valverde Á.M. Modulation of microglia in the retina: New insights into diabetic retinopathy. Acta Diabetologica. 2017;54(6):527–533. doi: 10.1007/s00592-017-0984-z. [DOI] [PubMed] [Google Scholar]
- Atchison E., Barkmeier A. The role of systemic risk factors in diabetic retinopathy. Current Ophthalmology Reports. 2016;4(2):84–89. doi: 10.1007/s40135-016-0098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y.X. Efficacy of calcium dobesilate combined with Compound Danshen Dripping Pills in the treatment of diabetic retinopathy and its effect on serum inflammatory factors. Journal of Qiqihar University of Medicine. 2017;38(22):2641–2643. [Google Scholar]
- Bao L.Z., Yang G., Wang H.B. Clinical observation of complex Salvia miltiorrhiza drill and calcicum dobesilate on diabetic retinopathy. Hebei Journal of Traditional Chinese Medicine. 2006;28:304–305. [Google Scholar]
- Bermudez V., Finol F., Parra N., Parra M., Perez A., Penaranda L.…Velasco M. PPAR- gamma agonists and their role in type 2 diabetes mellitus management. American Journal of Therapeutics. 2010;17(3):274–283. doi: 10.1097/MJT.0b013e3181c08081. [DOI] [PubMed] [Google Scholar]
- Chen W.F. Analysis of the effect of Compound Danshen Dripping Pills combined with alprostadil on early diabetic retinopathy. Journal of Bethune Medical Science. 2019;17:464–465. [Google Scholar]
- Chen Y., Zhong J.Y. Clinical study of Compound Danshen Dripping Pills treating simple diabetic retinopathy. Journal of Chinese Community Doctor. 2006;23:75. [Google Scholar]
- Chu Y., Zhang L., Wang X.Y., Guo J.H., Guo Z.X., Ma X.H. The effect of Compound Danshen Dripping Pills, a Chinese herb medicine, on the pharmacokinetics and pharmacodynamics of warfarin in rats. Journal of Ethnopharmacology. 2011;137(3):1457–1461. doi: 10.1016/j.jep.2011.08.035. [DOI] [PubMed] [Google Scholar]
- Corcóstegui B., Durán S., González-Albarrán M.O., Hernández C., Ruiz-Moreno J.M., Salvador J.…Simó R. Update on diagnosis and treatment of diabetic retinopathy: A consensus guideline of the working group of ocular health (Spanish Society of Diabetes and Spanish Vitreous and Retina Society) Journal of Ophthalmology. 2017;2017:1–10. doi: 10.1155/2017/8234186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Stroud S., Mehta A., Rangasamy S. New treatments for diabetic retinopathy. Diabetes Obesity & Metabolism. 2015;17(3):219–230. doi: 10.1111/dom.12384. [DOI] [PubMed] [Google Scholar]
- Ding K., Chen H. Effect of Compound Danshen Dripping Pills combined with calcium dobesilate on diabetic retinopathy. Shaanxi Journal of Traditional Chinese Medicine. 2012;33(12):1595–1596. [Google Scholar]
- Duan J.G., Jin M., Jie C.H., Ye H.H. Standard of TCM diagnosis and treatment of diabetic retinal lesions. World Journal of Integrated Traditional and Western Medicine. 2011;6:632–637. [Google Scholar]
- Fu T.T., Ruan H., Liu B. Effect of Compound Danshen Dripping Pills combined with Alprostadil injection on early diabetic retinopathy and effects on TNF-α, IL-2, IL-10 and VEGF. Journal of Liaoning University of TCM. 2017;19(9):186–189. [Google Scholar]
- Gao D., Guo Y., Li X., Li X., Li Z., Xue M.…Yang S. An aqueous extract of Radix astragali, Angelica sinensis, and Panax notoginseng is effective in preventing diabetic retinopathy. Evidence-Based Complementary and Alternative Medicine. 2013;2013:1–11. doi: 10.1155/2013/578165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy D.L., Betts-Obregon B.S., Tsin A.T. Ginsenoside-Rb1 inhibition of VEGF release–structure and activity relations (sar) perspective. Medical Hypothesis, Discovery & Innovation Ophthalmology Journal. 2014;3(2):38–39. [PMC free article] [PubMed] [Google Scholar]
- Gordon H.G., Andrew D.O., Regina K., Gunn E.V., Holger J.S. What is quality of evidence and why is it important to clinicians? British Medical Journal. 2008;336(7651):995–998. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guariguata L., Whiting D.R., Hambleton I., Beagley J., Linnenkamp U., Shaw J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Research and Clinical Practice. 2014;103(2):137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- He G.Y., Zhen D.H. Clinical observation of Compound Danshen Dripping Pills treating of nonproliferative diabetic retinopathy. Journal of Lanzhou University. 2013;39(4):76–78. [Google Scholar]
- Huang W., Bao Q.I., Jin D.E., Lian F.M. Compound Danshen Dripping Pill for treating nonproliferative diabetic retinopathy: A Meta-analysis of 13 randomized controlled trials. Evidence-Based Complementary and Alternative Medicine. 2017;2017:1–10. doi: 10.1155/2017/4848076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji S.F., Zhang J., Fan X.D., Wang X.Q., Ning X.N., Zhang B.B.…Yan H. The relationship between mean platelet volume and diabetic retinopathy: A systematic review and Meta-analysis. Diabetology & Metabolic Syndrome. 2019;11:25. doi: 10.1186/s13098-019-0420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M., Deng H., Yuan W., Yang G. Clinical observation of Compound Danshen Dripping Pills treating of early diabetic retinopathy. Journal of Chinese Community Doctor. 2009;25(16):32–33. [Google Scholar]
- Keiko T., Mikio A., Mariko Y., Minoru U., Machi F., Takayuki N.…Tokio S. Retinopathy and hypertension affect serum high-sensitivity C-reactive protein levels in Type 2 diabetic patients. Journal of Diabetes and its Complications. 2005;19(3):123–127. doi: 10.1016/j.jdiacomp.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kempen J.H., O'Colmam B.J., Leske C., Haffner S.M., Klein R., Moss S.E.…Friedman D.S. The prevalence of diabetic retinopathy among adults in the United States. Archives of Ophthalmology. 2004;122(4):552–563. doi: 10.1001/archopht.122.4.552. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Lee B.J., Kim J.H., Yu Y.S., Kim M.Y., Kim K.W. Rosmarinic acid suppresses retinal neovascularization via cell cycle arrest with increase of p21 (WAF1) expression. European Journal of Pharmacology. 2009;615(1-3):150–154. doi: 10.1016/j.ejphar.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Kowluru R.A., Chan P.S. Oxidative stress and diabetic retinopathy. Experimental Diabetes Research. 2007;2007:1–12. doi: 10.1155/2007/43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. Evaluation of the clinical efficacy of calcium dobesilate capsules and Compound Danshen Drip Pills in the treatment of diabetic retinopathy. Aerospace Medicine. 2017;28(10):1229–1231. [Google Scholar]
- Li L. Effectiveness of Compound Danshen Dripping Pills in the treatment of patients with early diabetic retinopathy. World Latest Medicne Information (Electronic Version) 2019;19(55):352–354. [Google Scholar]
- Lian F., Wu L., Tian J., Jin M., Zhou S., Zhao M.…Tong X. The effectiveness and safety of a danshen-containing Chinese herbal medicine for diabetic retinopathy: A randomized, double-blind, placebo-controlled multicenter clinical trial. Journal of Ethnopharmacology. 2015;164:71–77. doi: 10.1016/j.jep.2015.01.048. [DOI] [PubMed] [Google Scholar]
- Lin T.H., Hsieh C.L. Pharmacological effects of Salvia miltiorrhiza (Danshen) on cerebral infarction. Chinese Medicine. 2010;5(1):22. doi: 10.1186/1749-8546-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. The effectiveness of Compound Danshen Dripping Pills on vascular endothelial function in patients with early diabetic retinopathy. Psychiatrist Magazine. 2018;36:118–119. [Google Scholar]
- Liu M.Y., Hao J. Clinical observation of Compound Danshen Dripping Pills treating of early diabetic retinopathy. Journal of Chinese and Foreign Medical Treatment. 2011;10:9–11. [Google Scholar]
- Luo D., Qin Y., Yuan W., Deng H., Zhang Y., Jin M. Compound Danshen Dripping Pill for treating early diabetic retinopathy: A randomized, double-dummy, double-blind study. Evidence-Based Complementary and Alternative Medicine. 2015;2015:1–7. doi: 10.1155/2015/539185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng Y.S., Maharjan S., Kim J.H., Park J.H., Suk Yu Y., Kim Y.M.…Torrens C. Rk1, a ginsenoside, is a new blocker of vascular leakage acting through actin structure remodeling. Plos One. 2013;8(7):e68659. doi: 10.1371/journal.pone.0068659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X.M., Zhang S.S., Duan Y.C. Clinical observation of Compound Danshen Dripping Pills combined with calcium dobesilate treating diabetic retinopathy. Journal of Medical Forum. 2011;32(13):137–138. [Google Scholar]
- Nathan D.M., Genuth S., Lachin J., Cleary P., Crofford O., Davis M.…Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The New England Journal of Medicine. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Ou C., Yang Y.J., Peng Q.H. Yiqi Yangyin Huoxue Method in treating diabetic retinopathy: A systematic review and Meta-analysis. Evidence-Based Complementary and Alternative Medicine. 2019;2019(02):1–7. doi: 10.1155/2019/6020846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul H. The heterogeneity statistic I(2) can be biased in small Meta-analyses. BMC Medical Research Methodology. 2015;15:35. doi: 10.1186/s12874-015-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praidou A., Harris M., Niakas D., Labiris G. Physical activity and its correlation to diabetic retinopathy. Journal of Diabetes and Its Complications. 2017;31(2):456–461. doi: 10.1016/j.jdiacomp.2016.06.027. [DOI] [PubMed] [Google Scholar]
- Qi C.X., Tan X.H., Li Q.G., Wang X.L. Clinical study of diabetic retinopathy treated by Compound Danshen Dripping Pills treating diabetic retinopathy. Journal of Chinese Medicinal Materials. 2007;30(3):375–377. [PubMed] [Google Scholar]
- Rifai N., Ridker P.M. High-sensitivity C-reactive protein a novel and promising marker of coronary heart disease. Clinical Chemistry. 2001;47:403–411. [PubMed] [Google Scholar]
- Ruan Y.X., Chen M., Liu Z.Q., Wang Y.L., Sun N., Huang X., Gan H. Clinical study on the treatment of diabetic retinopathy by oral Compound Danshen Dripping Pill combined with calcium dobesilate. Medical Science Journal of Central South China. 2017;45(1):18–23. [Google Scholar]
- Sato T., Morita S.I., Bando H., Sato S., Ikeda T., Emi K. Early vitreous hemorrhage after vitrectomy with preoperative intravitreal bevacizumab for proliferative diabetic retinopathy. Middle East African Journal of Ophthalmology. 2013;20(1):51–55. doi: 10.4103/0974-9233.106387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H.B. Experience in the treatment of diabetic retinopathy. Journal of Medical Forum. 2010;31(4):100–102. [Google Scholar]
- Tan K.C.B., Wat N.M.S., Tam S.C.F., Janus E.D., Lam T.H., Lam K.S.L. C-reactive protein predicts the deterioration of glycemia in Chinese subjects with impaired glucose tolerance. Diabetes Care. 2003;26(8):2323–2328. doi: 10.2337/diacare.26.8.2323. [DOI] [PubMed] [Google Scholar]
- Tillin T., Orchard T., Malm A., Fuller J., Chaturvedi N. The role of antihypertensive therapy in reducing vascular complications of type 2 diabetes. Findings from the Diabetic Retinopathy Candesartan Trials-Protect 2 study. Journal of Hypertension. 2011;29(7):1457–1462. doi: 10.1097/HJH.0b013e3283480db9. [DOI] [PubMed] [Google Scholar]
- Wang D.Q., Guan R.J., Wang Q.H., Li L., Wan L. Clinical study on Salvia miltiorrhiza+calcium dobesilate as the treatment of early retinopathy of type 2 diabetes mellitus. Journal of Hainan Medical University. 2019;25(18):1421–1431. [Google Scholar]
- Wang H.M., Tian D.Z., Han X.Y. Observation of the effect of Compound Danshen Dripping Pills treating non proliferative diabetic retinopathy. Journal of Clinical Rational Drug Use. 2016;9(8):49–50. [Google Scholar]
- Wilkinson C.P., Ferris F.L., Klein R.E., Lee P.P., Agardh C.D., Davis M.…Verdaguer J.T. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- Wilkinson-Berka J.L., Rana I., Armani R., Agrotis A. Reactive oxygen species, Nox and angiotensin II in angiogenesis: Implications for retinopathy. Clinical Science. 2013;124(9–10):597–615. doi: 10.1042/CS20120212. [DOI] [PubMed] [Google Scholar]
- Wong T.Y., Liew G., Tapp R.J., Schmidt M.I., Wang J.J., Mitchell P.…Shaw J. Relation between fasting glucose and retinopathy for diagnosis of diabetes: Three population-based cross-sectional studies. Lancet. 2008;371(9614):736–743. doi: 10.1016/S0140-6736(08)60343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H. Observation of therapeutic effect of Compound Danshen Dripping Pills treating diabetic retinopathy. Journal of China Practical Medicine. 2011;6(35):142–143. [Google Scholar]
- Xu H.T. Effect of Compound Danshen Dripping Pills combined with calcium dobesilate on diabetic retinopathy. Chinese Journal of Convalescent Medicine. 2019;28(8):884–886. [Google Scholar]
- Yan X.Q., Yuan M.R. Observation of curative effect of Compound Danshen Dripping Pills on 60 elderly patients with diabetic retinopathy. Lishizhen Medicine and Materia Medica Research. 2014;25(9):2187–2188. [Google Scholar]
- Yang L.L., Li D.Y., Zhang Y.B., Zhu M.Y., Chen D., Xu T.D. Salvianolic acid A inhibits angiotensin II-induced proliferation of human umbilical vein endothelial cells by attenuating the production of ROS. Acta Pharmacologica Sinica. 2012;33(1):41–48. doi: 10.1038/aps.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Feng Y., Lin W. Systematic review and Meta-analysis of randomized controlled trials comparing Compound Danshen Dripping Pills and isosorbide dinitrate in treating angina pectoris. International Journal of Cardiology. 2015;182:46–47. doi: 10.1016/j.ijcard.2014.12.112. [DOI] [PubMed] [Google Scholar]
- Zhang B.L., Gao X.M., Shang H.C., Zhao Y.J., Wang Y.Y. Study on pharmaceutical matters and functional mechanisms of complex prescriptions of Radix Salvial Milti Orrhizal. Modernization of Traditional Chinese Medicine and Materia Medica-World Science and Technology. 2003;5:14–17. [Google Scholar]
- Zhang X., Saaddine J.B., Chou C.F., Cotch M.F., Cheng Y.J., Geiss L.S.…Klein R. Prevalence of diabetic retinopathy in the United States, 2005–2008. Jama-Journal of the American Medical Association. 2010;304(6):649–656. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Xiao X.H., Zheng J., Li M., Yu M., Ping F.…Wang X. Compound Danshen Dripping Pill inhibits retina cell apoptosis in diabetic rats. Frontiers in Physiology. 2018;9:1501. doi: 10.3389/fphys.2018.01501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C.Q. Clinical effect of Compound Danshen (Salvia miltiorrhiza) Pills in the treatment of diabetic retinopathy. Journal of Clinical Research. 2019;36:4–7. [Google Scholar]
- Zheng X.Y. Guiding principles of clinical research on Chinese traditional medicine new drugs. China Medical Science and Technology Press. 2002:312–316. [Google Scholar]
- Zhou L.J. Clinical observation of Compound Danshen Dripping Pills treating diabetic retinopathy. Journal of Chinese Medicine Guides. 2008;5(1):77. [Google Scholar]
- Zhu J.H. The effect of Compound Danshen Dripping Pills on early diabetic retinopathy. Health Horizon. 2018;10:99–100. [Google Scholar]