Abstract
Objective
Trifolium pratense has many healing properties, including fewer complications of menopause, cancer cell suppression, reducing blood glucose and lipids, as well as cardiovascular beneficial effects. The purpose of this study was to identify the phytochemical and mineral composition of T. pratense.
Methods
Plant aerial parts were harvested and dried, and then hydroalcoholic and alcoholic extracts were prepared. Gas chromatography–mass spectrometry (GC–MS) analytical method was used to identify volatile compounds then liquid chromatography-electrospray ionization mass spectrometry (LC-ESI-MS) was used to identify polyphenols and the mineral elements were identify by inductively coupled plasma atomic emission spectrometer/ICP-AES and scanning electron microscope-energy-dispersive X-ray spectroscopy (SEM-EDS) methods. Total phenolic content (TPC) was determined based on colorimetric method, and total flavonoid content (TFC) was established based on the folin-chiocalteau reagent. Furthermore, two assays (DPPH and FRAP) were used to measure the antioxidant capacity of T. pratense ethanolic extract.
Results
A total of 37 polyphenols and 107 peaks were identified by LC-ESI-MS analysis, and the GC/MS method also detected 21 volatile compounds, the most important of which were methylcyclopentane, dimethylpentanal and hexadecanol. A total of 18 mineral elements, including K, Mg, Al, Si, Zn, Ni, Cu, Se, Co, Fe, Mn, and Ca in the plant, were identified ICP-AES and SEM-EDS analysis.
Conclusion
T. pratense has many therapeutic compounds such as polyphenol (isoflavone and flavonoids), volatile compounds, and essential mineral elements, which can be formulated purely and used in the pharmaceutical and traditional medicine industries.
Keywords: flavonoid, GC–MS, isoflavones, LC-ESI-MS, red clover, SEM-ED X-ray spectroscopy, Trifolium pratense L.
1. Introduction
Red clover (Trifolium pratense L.) is one of the most important leguminous forage plants in temperate and humid areas, which is very valuable in livestock feeds with a height of 10–15 cm and oval, three-leafed leaves (Fig. 1). There are about 300 species of clover in the world, of which 25 species are important for agriculture, and only nine species are commercially grown (Kroyer, 2004). T. pratense is considered as an herb with a variety of therapeutic properties in human studies and animal models. This plant can be used to treat common diseases such as diabetes, high blood pressure and blood lipids (Khazaei & Pazhouhi, 2018). Nowadays, researches have another dimension due to the identification of various compounds such as polyphenol and isflavonoids with anti-tumor properties and supporting the immune system and treating diseases such as Alzheimer's (Occhiuto, Zangla, Samperi, Palumbo, Pino, & De Pasquale, 2008).
Fig. 1.

T. pratense cultivated in research field of Kermanshah University of Medical Sciences.
Natural polyphenols (e.g., phenolic acids, flavonoids, and tannins etc.) are produced by plants and are involved in their defense mechanisms against biotic and abiotic stresses. They have the scavenger capacity for oxidative free radicals, such as those derived from lipids and nucleic acids that underlie their utility in reducing the risk of certain age‐related degeneration's diseases (Tyagi, Singh, Sharma, & Aggarwal, 2010). Phytoestrogens, a large group of compounds containing a number of phenolic hydroxyl groups, are the major components of soybean, and have been shown to be protective of several diseases, particularly cancers (Shahrokhi, Ghanimatdan, Bazm, & Karimi, 2014).
Isoflavone compounds such as formononetin, biochanin A, genistein, and daidzein presented in T. pratense have estrogenic properties in the form of glucoside and malonate, which are hydrolyzed by flora and intestinal mucosal cells (Setchell, Brown, Zimmer-Nechemias, Brashear, Wolfe, & Kirschner, 2002). LC-MS analysis identified about 10 malonates in T. pratense flowers and other LC-ESI-MS study revealed the existence of > 30 flavonoids, including about 20 malonates (Lin, He, Lindenmaier, Yang, Cleary, & Qiu, 2000). LC-ESI-MS study of ethanolic extract of T. pratense revealed the existence of daidzin, glycetin, calycosin 7-O-α-D-glucoside, luteolin 7-O-α-D-glucoside, genistin, hyperoside, isoquercitrin, ononin, apigenin, 7-O-α-D-glucoside, and biochanin A (Klejdus, Vitamvásová-Štěrbová, & Kubáň, 2001).
Numerous analytical procedures (TLC, LC-DAD, LC-FLD, LC-MS, GC and CE) have been developed for the quantification of phenolic and isoflavone compounds in herbs. Liquid chromatography-mass spectrometry (LC-MS) is extensively used for the analysis of phenolic compounds and mineral elements of the herbs or medicinal plants and tea leaves from all over the world (Akbari, Khazaei, Khazaei, & Naseri, 2019). Different techniques, such as flame- or electrothermal- atomic absorption spectrometry (FAAS, ETAAS), inductively coupled plasma-optical emission spectrometry (ICP-OES) or mass spectrometry (ICP-MS), and scanning electron microscope (SEM) are used to identify the mineral elements in solvent extracts (Rezić, 2011).
The combination ofSEM and energy dispersive spectroscopy (EDS) allows for an elemental analysis performed on different microscopic sections of the various samples such as stone, herbal extracts, and synthetic nanoparticles with uncertain elemental composition. EDS can detect major (higher than 10% wt) and minor (between 1 and 10% wt) elements concentrations (Kabata-Pendias, 2000, Ramamurthy and Kannan, 2009). EDS technique is capable of producing elemental distribution maps. SEM-EDS method is used to identify the elements in solid samples, while ICP-MS used for solvent samples. ICP-MS is used to identify the elements in industrial solutions, drinking water, herbal extracts, drugs and radioactive specimens. In ICP-AES the extract is transformed into aerosols by nebulizer and sprayed. The hot argon gas in the machine after the drying of the extract causes its particles to atomize (Maher et al., 2001). The present study describes the development of an LC-ESI-MS/MS method for analyzing the phytochemical content, GC/MS to identify volatile compounds with low molecular weight, and SEM-EDS/ICP-AES method for analyzing the mineral elements in the different parts of T. pratense.
2. Materials and methods
2.1. Plant sample preparation
Plant seeds were obtained from Karaj Seed and Plant Improvement Institute and cultivated at a research farm. In spring, the leaf and stem of the plant were collected and immediately frozen in liquid nitrogen (samples can be stored at − 70 °C until used). All samples were surface sterilized with 0.1% sodium hypochlorite (20 min) and rinsed with double-deionized water (four times). Lyophilize frozen samples for 2 to 3 d (dried samples can be stored at − 20 °C until use). After drying, cut samples into small pieces and grind them into fine powder.
For LC-ESI/MS analyzing, 2 g of the powder was mixed with 2 mL of 0.1 mol/L HCl and 10 mL of acetonitrile (ACN) in a 125 mL screw-top flask, stirred (2 h at room temperature) and filtered (Whatman No. 41 UK). The filtrate was dried in a vacuum evaporator and then dissolved in 10 mL of 80% methanol. The sample was filtered (0.45 μm filter unit, Cameo syringe-filter, nylon), transferred to 1 mL vials. Analytical standards of coumaroylquinic acid (≥98%), cinnamic acid (≥97%), quercetin-3,7-diglucoside (≥98%), myricetin-3-O-rhamnoside (≥97%), coumaric acid-O-pentoside (≥97%), myricetin-3-O-acetyl rhamnoside (≥97%), genistein (≥98%), daidzein 7-O-β-D-glucoside (≥98%), formononetin (≥98%), apigenin 6,8-diglucoside (≥98%), apigenin-7-O-glucoside (≥98%), ellagic acetyl rhaminoside (≥98%) and biochanin A (≥98%) were purchased from Sigma-Aldrich. Standards were dissolved in methanol–water (1:1, volume percentage). Due to low solubility of standard compounds, solutions were filtered (0.20 µm filter) (Naseri, Khazaei, Ghanbari, & Bazm, 2019).
For GC–MS analyzing, 10 g of the powdered leaves was soaked in 99.6% ethyl alcohol (72 h) then incubated overnight and filtered along with sodium sulfate to remove the sediments and traces of water in the filter paper (Whatman No. 41 UK). The paper was wet with 95% ethanol and sodium sulfate. The filtrate is then concentrated to 1 mL by bubbling nitrogen gas into the solution. The extract contained both polar and non-polar phytocomponents of the plant material. Then, 2 μL of the extract was used for injection in the device GC–MS for analysis. To condense the solution, a vacuum distillation unit (Heidolph Collegiate, LABOROTA 4000) at 55 °C was used. (Khazaei & Pazhouhi, 2019).
2.2. LC-ESI/MS analysis
MS analysis was carried out on an ESI (negative mode) using the Agilent Zorbax Eclipse Atlantis T3 C18 Mass Spectrometer (3 µm, 2.1 × 150 mm) column coupled to a HCT ultra ion trap MS detector fitted with an ESI ion source (Shimadzu LC/MS device 2010 A). For chromatographic separation, the same column and conditions as described above were used. The standard analysis of the machine was carried out according to the following conditions: injection volumes: 10 μL; dwell time: 500 ms; flow rate: 0.2 mL/min; detection gain: 1.8 kV; fragmentor:135; operation mode: negative. Mobile phase: A, acetonitrile with 0.1% formic acid; B, H2O with 0.1% formic acid; column temperature:25 °C; capillary voltage: 3.5 kV; dry gas flow (N2-grade 5): 1.2 L/min; nebulizer pressure: 15 psi; and capillary temperature: 285 °C.
Mass spectra were recorded between mass ranges 130–1100 amu. Gradient condition due to the percentage of solvent flow in the column is according to the time and flow velocity, respectively: Time (min) 0, 15, 20, 25, and 30; phase B (%) 95, 10, 5, 5, and stop; phase A (%) 5, 10, 95, 95 and stop; flow rate: 0.3 mL/min in all times (source conditions on a set of polyphenolic compounds standards include flavonoid aglycones and glycosylated flavonoids). Quality control (QC) samples were then prepared by diluting separate analyte stock solutions with diluent with the same amount of known internal standard (Akbari et al., 2019, Namera et al., 2015).
2.3. GC–MS analysis
GC–MS analysis of the ethanol extract of T. pratense was performed using a GC Clarius 500 Perkin Elmer comprising an AOC-20i auto-sampler and a GC–MS equipped with an Elite-1 (100% dimethyl poly siloxane) column (30 × 0.25 mm 1 μm df) fused a capillary column (30 × 0.25 μm ID × 0.25 μm df). Helium (99.999%) (5 nines) was used as the carrier gas at a flow rate of 1 mL/min in the split mode (10:1). For GC–MS detection, an electron ionization system was operated in electron impact mode with ionization energy of 70 eV.
An aliquot of 2 μL of ethanol solution of the sample was injected into the column with the injector temperature at 200 °C. The ion-source temperature was 200 °C, the GC oven temperature started at 110 °C and holding for 2 min, raised to 200 °C with an increase of 10 °C/min, then 5 °C/min to 280 °C, ending with a 9 min at 280 °C. Ion source temperature was maintained at 200 °C. Mass spectra were taken at 70 eV. The solvent delay was 0 to 2 min, and the total GC/MS running time was 48 min. The relative percentage amount of each component was calculated by comparing its average peak area to the total areas. A scan interval of 0.5 s and fragments from 45 to 450 Da was maintained. The total running time was 36 min. The mass-detector used in this analysis was Turbo-Mass Gold-Perkin-Elmer, and the software adopted to handle mass spectra and chromatograms was a Turbo-Mass ver-5.2 (Ezhilan & Neelamegam, 2012; Akbari Bazm, Goodarzi, Shahrokhi, & Khazaei, 2020).
2.4. SEM-EDS analysis
One gram of T. pratense alcoholic extract (99.6% ethyl alcohol) was prepared and coated with gold, placed in a special stage and placed in the microscope's (Seron Technology's high competitive normal SEM, AIS2300C, Korea) sample chamber with an energy dispersive x-ray spectrometer (EDS). The analysis conditions of the device for identifying the elements in the extract were as follows: Accelerating Voltage: 12 kV, Beam Shift & Rotation: 250 µm (X, Y), Image Rotation (360°), Vacuum: ~10-4 Torr (Starlin, Ragavendran, Raj, Perumal, & Gopalakrishnan, 2012).
2.5. ICP analysis
Fifteen gram of T. pratense powdered was soaked in 99.6% ethyl alcohol for 8 h by soxhlet extraction, then incubated on water bath at 60 °C and filtered (Whatman No. 41 UK). The dark greenish brown thick solution was stored in a glass vial at 4 °C. One g of the extract was placed in a 55% nitric acid solution in a warm water bath at 85 °C for 10 min. After cooling, 50 mL of distilled water was poured on it. Finally, the solution was analyzed under standard conditions by inductively coupled plasma atomic emission spectrometer.
The analysis was performed using a standard instrument, and the software interface version 5 was evaluated. The analysis was performed using an ARCOS model instrument from M/s Spectro, Germany and the software interface Smart Analyzer Vision 5.01.0921 was evaluated. The analysis conditions of the device for identifying the elements in the extract were as follows: Nebulizer flow: 0.801 L/min, Auxiliary flow: 1.009 L/min, Coolant flow: 11.04 L/min Pump speed, 34 rpm, and Plasma power: 1500 W, R.F generator: 1.6 KW (Bazm et al., 2018; Senila, Drolc, Pintar, Senila, & Levei, 2014; Goodarzi, & Akbari, 2016).
2.6. Total phenolic compounds
Thephenolic content of the extracts was measured using the modified Folin-Ciocalteu assay (Slinkard and Singleton method) by placing gallic acid as standard. First, to determine the calibration curve, 50 μL of 0.024, 0.075, 0.105, 0.3, and 0.4 mg/mL gallic acid with methanol was mixed. Samples were introduced into 0.8 mL of sodium carbonate (Na2CO3) (7.5%) and 1.0 mL Folin-Ciocalteu’s reagent (diluted 10-fold) and were mixed. 50 μL of the ethanolic extract was added to above described solution. All samples were read after 2 h of incubation at 30 °C with a wavelength of 765 nm (Shimadzu UV–Vis spectrophotometer). Results were expressed as milligrams of gallic acid equivalent per gram of fresh plant (mg GAE/g) (Slinkard & Singleton, 1977).
2.7. Total flavonoid content
Total flavonoid content was determined using a modified method (Miliauskas et al., 2004). A total of 10 mg of ethanolic extract was dissolved in 5 mL of methanol, and then 1 mL of the resulting solution was added to 1 mL of methanol dissolved in aluminum trichloride (AlCl3), 0.1 mL of potassium acetate (1 mol/L), 2.8 mL of water and the volume was increased to 25 mL with methanol. Then the absorbance of the resulting solution was read after incubation at 25 °C for 30 min at 415 nm. Total flavonols were expressed as milligrams of rutin equivalent (RE) per 1 g of extract (mg RUE/g).
2.8. DPPH radical scavenging activity
DPPH was determined using a modified method (Brand-Williams, Cuvelier, & Berset, 1995). Aliquots (25–170 μL) of the T. pratense extracts were placed in a cuvette, and 3 mL of 0.06 mmol/L methanol DPPH• radical solution was added. Absorbance measurements commenced immediately. The DPPH radical scavenging effect, after 3 h for all samples measured in absorbance at 516 nm during in the dark condition. Then, according to the Trolox calibration curve, The DPPH radical scavenging effect of the extracts was expressed as μmol Trolox equivalent per 100 g of dried T. pratense (μmol eq. Trolox/10 g plant).
2.9. Ferric reducing antioxidant power (FRAP) assay
FRAP method based on the pH‐dependent (pH 3.6) color change where the yellow Fe3+-TPTZ complex is reduced to the Fe2+-TPTZ complex. FRAP method was adjusted according to Benzie and Strain modified method (Benzie & Strain, 1996). In this method, the extract was dissolved in methanol solution (1 mg/mL). Then added 20 µL of the resulting solution to 180 µL of distilled water and 2 µL of FRAP solution. Finally, the absorbance of the solution and methanol as blank was read at 593 nm and expressed as mmol Fe2+ sulfate heptahydrate per mg of plant (mmol Fe2+/mg plant).
3. Results
3.1. LC-ESI/MS analysis
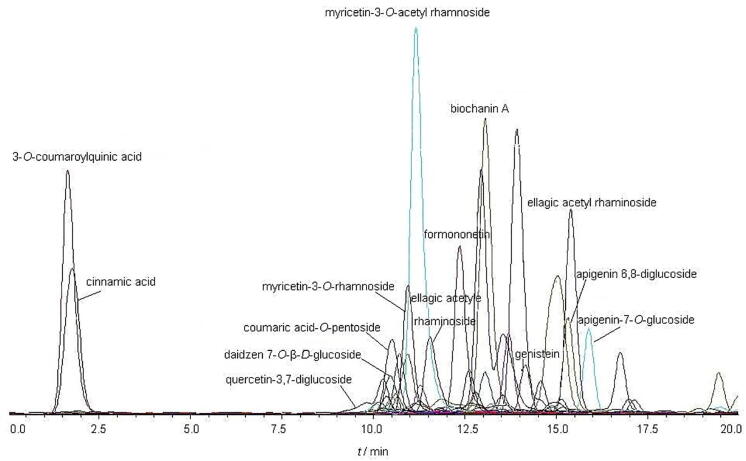
Liquid chromatographic gradient conditions were optimized. The optimized gradient allowed good peak resolution with total runtime lower than 20 min (Fig. 2).
Fig. 2.
Representative chromatogram of standard solution containing 3-O-coumaroylquinic acid, cinnamic acid, quercetin-3,7-diglucoside, myricetin-3-O-rhamnoside, coumaric acid-O-pentoside, myricetin-3-O-acetyl rhamnoside, daidzein 7-O-β-D-glucoside, formononetin, apigenin 6,8-diglucoside, apigenin-7-O-glucoside, genistein, ellagic acetyl rhaminoside and biochanin A.
To obtain an acceptable separation of major isoflavonoids and other interference compounds (flavonoids, saponins, etc.) a 20-min chromatographic run was used. LC-ESI/MS analysis was then applied for the full characterization of the different compounds in the extracts of T. pratense. After chromatograms were prepared in the negative ion mode, 46 compounds and 104 peak (intensity > 5 × 104) were identified. The chromatograms obtained are displayed in Fig. 2 and they demonstrated the hydroalcoholic extract of T. pratense. A full MS scan, in the form of a total ion current chromatogram (TIC), was initially acquired, following which reconstructed ion chromatograms (RICs) were generated for each of the expected m/z values based on the molecular weights of the possible constituents. The extracts are separated from each other according to the molecular weight [M−H]− of the expected compounds and acquisition time, Counts (Mass-to-charge) × 104 and counts listed in Table 1.
Table 1.
Qualitative analyses by LC-ESI/MS and LC-ESI/MS of T. pratense.
| Compounds |
m/z [M−H]- |
MS/MS fragments | Acquisition time | Counts (Mass-to-charge) × 104 |
|---|---|---|---|---|
| Cinnamic acid (1 4 9) 3-O- (Z)-p-Coumaroylquinic acid (3 3 7) Epigallocatechin (3 0 6) Caffeic acid (1 7 9) |
149 | 149 337 306 179 |
1.72 | 4.8 |
| Coumaric acid-O-pentoside (2 9 5) Kaempferol galloylglucoside (5 9 9) Quercetin-3,7-diglucoside (6 2 5) |
295 | 295 599 625 |
10.3 | 23.4 |
| Kaempferol-3,7-di-O-glucoside (6 0 9) | 609 | 609 | 10.7 | 49.2 |
| Myricetin-3-O-rhamnoside (4 6 3) Quercetin-3-O-galactoside (4 6 4) |
463 | 463 464 |
10.92 | 234.6 |
| Myricetin-3-O-acetyl rhamnoside (5 0 5) Fraxidin (2 2 3) |
505 | 505 223 |
11.3 | 441.9 |
| Ellagic acetyl rhaminoside (4 8 9) Myricetin-3-O-acetyl rhamnoside (5 0 5) Rhamnetin-3-O-(6-O“-acetylglucoside) (5 1 9) |
489 | 489 505 519 |
11.5 | 121.6 |
| Formononetin (2 6 7) Genistein (2 6 8) |
267 | 267 268 |
12.3 | 8.6 |
| Biochanin A-7-glucoside (4 4 5) Formononetin (2 6 7) Genistein (2 6 8) Daidzein 7-O-β-D-glucoside (4 1 7) |
445 | 445 267 268 417 |
12.6 | 6.8 |
| α-Tocopherol (5 0 1) Formononetin (2 6 7) Daidzein 7-O-β-D-glucoside (4 1 7) Biochanin A-7-glucoside (4 4 5) |
501 | 501 267 417 445 |
12.7 | 7.8 |
| Biochanin A (2 8 3) Ophiopogonanone A (3 2 7) Daidzein 7-O-β-D-glucoside (4 1 7) Epimedoside A (6 6 3) |
283 | 283 327 417 663 |
12.91 | 23.4 |
| Apigenin-7-O-glucoside (4 3 1) Hesperetin (3 0 1) Biochanin A (2 8 3) 9,12,13-trihydroxy-10,15-octadecadienoic acid (3 2 7) |
431 | 431 265 283 301 327 |
13.5 | 11.22 |
| Nosylicariside II (6 6 1) Biochanin A (2 8 3) Hesperetin (3 0 1) 9,12,13-Trihydroxy-10,15-octadecadienoic acid (3 2 7) Vitexin-O-(maloyl)rhamnoside (6 9 3) |
661 | 661 283 301 327 693 |
13.7 | 6.91 |
| Formononetin (2 6 7) | 267 | 267 | 14.1 | 37.2 |
| Malvidin (3 3 1) Ethyl gallate (1 9 7) Gallocatechin (3 0 7) Apigenin 6,8-diglucoside (5 9 3) |
331 | 331 197 307 593 |
14.6 | 4.06 |
| Apigenin 6,8-diglucoside (5 9 3) Galloyl-HHDP-hexoside (6 3 3) |
593 | 593 633 |
14.8 | 3.58 |
| Daidzin 4′-O-glucuronide (5 9 2) Galloyl-HHDP-hexoside (6 3 3) Kaempferol-3-O-sophoroside-7-O-glucoside (7 7 7) Coumaric acid-O-rhamnoside (3 0 9) |
592 | 592 309 633 777 |
15 | 5.36 |
| Epigallocatechin (3 0 5) Apigenin 6,8-diglucoside (5 9 3) Galloyl-HHDP-hexoside (6 3 3) Hydroxy-octadecatrienoic acid (2 9 3) |
305 | 305 593 633 293 |
15.1 | 3.31 |
| Biochanin A (2 8 3) | 283 | 283 | 15.3 | 648.6 |
| Apigenin 7-O-glucoside (4 3 5) Ethyl gallate (1 9 7) Biochanin A (2 8 3) |
435 | 435 197 283 |
15.8 | 6.21 |
| Ganoderic acid AM1 (5 1 3) Ethyl gallate (1 9 7) Hydroxy-octadecatrienoic acid (2 9 3) 6-C-Pentosyl-8-C-hexosyl apigenin (5 6 4) Ferulic acid-O-hexoside derivative (6 8 9) |
513 | 513 197 293 564 689 |
16.75 | 3.52 |
| Paeoniflorin (4 8 0) Genistein 8-C-glucoside-xyloside (3 1 1) Quercetin-3-O-pentoside (4 3 3) Kaempferol galloylglucoside (5 9 9) |
480 | 480 311 433 599 |
17 | 6.52 |
| Chlorogenic acid derivative (4 5 1) Ethyl gallate (1 9 7) 8-Prenylnaringenin (3 3 9) Apigenin 7-O-glucoside (4 3 5) Oxypaeoniflorin isomer /Mono galloyl hexoside (4 9 4) |
451 | 451 197 339 435 494 |
19.44 | 2.38 |
Compounds characterized for the first time by LC-ESI-MS (intensity > 5 × 104). The identification was confirmed by direct comparison with standard compound, respectively.
Various isoflavone were identified by LC-ESI/MS. The retention time, m/z, [M−H]- and typical fragment ions for individual peaks confirmed the presence of isoflavones in T. pratense leaves and stem. By comparing the retention time and mass spectrum with corresponding standards, the followings were present in the plant, respectively: the cinnamic acid (m/z 149 [M−H]-), 3-O-(Z)-p-coumaroylquinic acid (m/z 337 [M−H]-), epigallocatechin(m/z 306 [M−H]-), caffeic acid (m/z 179 [M−H]-), coumaric acid-O-pentoside (m/z 295 [M−H]-), kaempferol galloylglucoside (m/z 599 [M−H]-), quercetin-3,7-diglucoside (m/z 625 [M−H]-), kaempferol-3,7-di-O-glucoside (m/z 609 [M−H]-), myricetin-3-O-rhamnoside (m/z 463 [M−H]-), quercetin-3-O-galactoside (m/z 464 [M−H]-), myricetin-3-O-acetyl rhamnoside (m/z 505 [M−H]-), fraxidin (m/z 223 [M−H]-), ellagic acetyl rhaminoside (m/z 489 [M−H]-), vitexin-O-(maloyl)-rhamnoside (m/z 693 [M−H]-), myricetin-3-O-acetyl rhamnoside (m/z 505 [M−H]-), rhamnetin-3-O-(6-O“-acetylglucoside) (m/z 519 [M−H]-), formononetin (m/z 267 [M−H]-), genistein (m/z 268 [M−H]-), biochanin A-7-glucoside (m/z 445 [M−H]-), daidzein 7-O-β-D-glucoside (m/z 417 [M−H]-), α-tocopherol (m/z 501 [M−H]-), ferulic acid-O-hexoside derivative (m/z 689 [M−H]-), biochanin A (m/z 283 [M−H]-), coumaric acid-O-rhamnoside (m/z 309 [M−H]-), ophiopogonanone A (m/z 327 [M−H]-), epimedoside A (m/z 663 [M−H]-), apigenin-7-O-glucoside (m/z 431 [M−H]-), hesperetin (m/z 301 [M−H]-), 9,12,13-trihydroxy-10,15-octadecadienoic acid (m/z 327 [M−H]-), nosylicariside II (m/z 661 [M−H]-), malvidin (m/z 331 [M−H]-), ethyl gallate (m/z 197 [M−H]-), gallocatechin (m/z 307 [M−H]-), apigenin 6,8-diglucoside (m/z 593 [M−H]-), galloyl-HHDP-hexoside (m/z 633 [M−H]-), daidzin 4′-O-glucuronide (m/z 592 [M−H]-), kaempferol-3-O-sophoroside-7-O-glucoside (m/z 777 [M−H]-), ganoderic acid AM1 (m/z 513 [M−H]-), 6-C-pentosyl-8-C-hexosyl apigenin (m/z 564 [M−H]-), paeoniflorin (m/z 480 [M−H]-), genistein 8-C-glucoside-xyloside (m/z 311 [M−H]-), quercetin-3-O-pentoside (m/z 433 [M−H]-), kaempferol galloylglucoside (m/z 599 [M−H]-599), 8-prenylnaringenin (m/z 339 [M−H]-339),chlorogenic acid derivative (m/z 451 [M−H]-) and oxypaeoniflorin isomer/ mono galloyl hexoside (m/z 494 [M−H]-) (Table 1). Further LC-ESI/MS analysis were carried out on-line using the dependent full scanning mode in which the MS software selected ions of certain intensity for further fragmentation experiments on the basis of a set of parameters predetermined by the operator.
3.2. GC–MS analysis
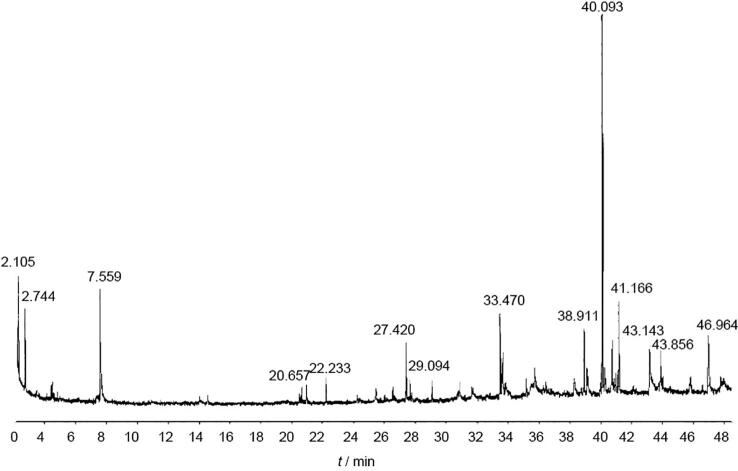
GC–MS chromatogram analysis of the ethanolic extract of T. pratensewas shown in Fig. 3, Fig. 4. Comparison of the mass spectra of the constituents with the NIST library, showed 21 peaks which indicating the presence of 21 phytochemical constituents (Table 2).
Fig. 3.
GC–MS Chromatogram of ethanolic extract of T. pratense.
Fig. 4.
Mass spectrum and structure of phytocomponents identified by GC–MS in ethanolic extracts of T. pratense.
Table 2.
Phytocomponents identified in methanolic leaf extract of T. pratense by GC–MS.
| Peak | RT/min | Compounds | Molecular formula | MW/(g·mol−1) | Area sum/% |
|---|---|---|---|---|---|
| 1 | 2.066 | Hexane | C6H14 | 86.18 | 2.96 |
| 2 | 2.105 | 2,3-dimethylpentanal | C7H14O | 114.19 | 7.07 |
| 3 | 2.299 | Methylcyclopentane | C6H12 | 84.162 | 7.18 |
| 4 | 2.744 | Cyclohexane | C6H12 | 84.16 | 5.91 |
| 5 | 7.559 | 4-Hydroxy-4-methyl-2-pentanone | C6H12O2 | 116.16 | 5.8 |
| 6 | 20.657 | 3-Trifluoroacetoxypentadecane | C17H31F3O2 | 324.428 | 1.05 |
| 7 | 22.233 | Hexylcyclohexane | C12H24 | 1.68 | |
| 8 | 27.42 | 7-Tetradecene | C14H28 | 168.324 | 3.65 |
| 9 | 27.42 | n-Tridecanol | C13H28O | 200.366 | 2.9 |
| 10 | 29.094 | Octylcyclohexane | C14H28 | 196.378 | 1.54 |
| 11 | 33.47 | 1-Hexadecanol | C16H34O | 242.44 | 6.22 |
| 12 | 38.911 | 1-Nonadecene | C19H38 | 266.513 | 4.63 |
| 13 | 38.911 | 10-Heneicosene | C21H42 | 294.567 | 1.5 |
| 14 | 40.098 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | C20H40O | 296.539 | 25.03 |
| 15 | 40.098 | 3-Eicosyne | C20H38 | 278.524 | 4.05 |
| 16 | 41.166 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | C20H40O | 296.539 | 5.05 |
| 17 | 41.166 | 9-Eicosyne | C20H38 | 278.524 | 1.96 |
| 18 | 43.148 | n-Hexadecanoic acid | C16H32O2 | 256.4 | 2.29 |
| 19 | 43.856 | 2-Methyl-1-hexadecanol | C17H36O | 256.474 | 2.04 |
| 20 | 43.856 | n-Tetracosanol-1 | C24H50O | 354.65 | 4.14 |
| 21 | 46.964 | Phytol | C20H40O | 128.1705 | 3.36 |
This analysis showed that according to the amount of compounds, respectively 3,7,11,15-tetramethyl-2-hexadecen-1-ol (25.03%), methylcyclopentane (7.18%), 2,3-dimethylpentanal (7.07%), 1-hexadecanol (6.22%), cyclohexane (5.991%), 4-hydroxy-4-methyl-2-pentanone (5.8%), 3,7,11,15-tetramethyl-2-hexadecen-1-ol (5.05%), 1-nonadecene (4.63%), n-tetracosanol-1 (4.14%), 3-eicosyne (4.05%), 7-tetradecene (3.65%), phytol (3.36%), hexane (2.96%), n-tridecanol (2.9%), n-hexadecanoic acid (2.29%), 2-methyl-1-hexadecanol (2.04%), 9-eicosyne (1.96%), hexylcyclohexane (1.68%), octylcyclohexane (1.54%), 10-heneicosene (1.5%), and 3-trifluoroacetoxypentadecane (1.05%) is present in this plant.
3.3. SEM analysis
Elemental analysis using a SEM-EDS spectrum has revealed the presence in order of C (534.40) > O (56.47) > K (53.17) > Cl (30.18) > Ge (7.89) > Mg (6.08) > Al (4.27) > Si (2.45) > Zn (1.47) > Ni (1.40) > Cu (1.26) > Co (1.08) > Fe (0.91) > Pb (0.87) > Mn (0.63) > Ca (0.52). The results show higher concentration of carbon (Fig. 5, Table 3).
Fig. 5.
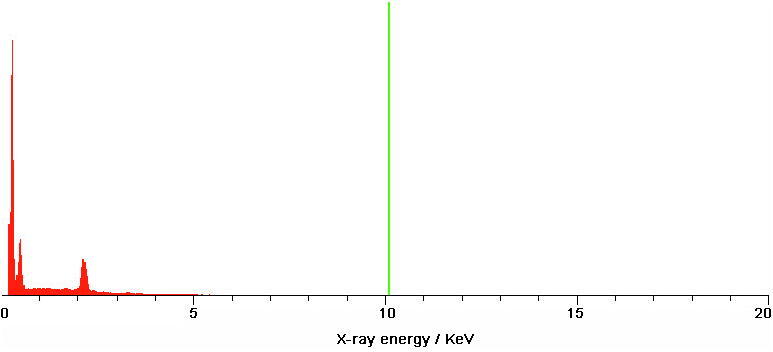
Elements concentration (wt %) and intensity (c/s) in T. pratense plant using a SEM-EDS spectrum that include the characteristic X-ray peaks of the element (0–20 keV X-ray energy/2K X-ray intensity).
Table 3.
Elements concentration (wt %) and intensity (c/s) in T. pratense plant using a SEM-EDS (n = 3).
| Element | Intensity (C/S) | Concentrations (Wt. %, Mean ± SD)* |
|---|---|---|
| C | 534.4 | 70.127 ± 5.21 |
| O | 56.47 | 22.197 ± 2.20 |
| Mg | 6.08 | 0.321 ± 0.011 |
| Al | 4.27 | 0.186 ± 0.0062 |
| Si | 2.45 | 0.093 ± 0.0051 |
| Cl | 30.18 | 1.131 ± 0.094 |
| K | 53.17 | 2.17 ± 0.26 |
| Ca | 0.52 | 0.022 ± 0.0016 |
| Mn | 0.63 | 0.045 ± 0.0069 |
| Fe | 0.91 | 0.072 ± 0.0091 |
| Co | 1.08 | 0.099 ± 0.011 |
| Ni | 1.40 | 0.144 ± 0.029 |
| Cu | 1.26 | 0.160 ± 0.042 |
| Zn | 1.47 | 0.222 ± 0.063 |
| Ge | 7.89 | 2.091 ± 0.92 |
| Pb | 0.87 | 0.091 ± 0.0081 |
3.4. ICP-AES analysis
Elemental analysis has revealed the presence in order of C > O > K > Cl > Ge > Mg > Al > Si > Zn > Ni > Cu > Se > Co > Fe > Pb > Hg > Mn > I > Ca. The results showed higher concentration of carbon (Table 4).
Table 4.
Concentration of elements found in alcholic soxhlet leaf extracts of T. pratense plants using ICP-AES technique (mean ± SD, n = 3).
| Elements | Wavelength / nm | Concentrations / (µg/mL) |
|---|---|---|
| C | 127.72 | 780.124 ± 12.12 |
| O | 130.22 | 214.215 ± 9.812 |
| Mg | 279.079 | 44.120 ± 0.912 |
| Al | 176.640 | 31.089 ± 0.814 |
| N | 131.05 | ND |
| Si | 251.612 | 29.099 ± 0.614 |
| Cl | 134.724 | 50.140 ± 1.124 |
| K | 766.490 | 110.161 ± 4.112 |
| Ba | 455.404 | ND |
| Se | 196.10 | 1.123 ± 0.061 |
| Ca | 422.673 | 0.371 ± 0.044 |
| Li | 670.780 | ND |
| Mn | 257.611 | 0.410 ± 0.012 |
| Fe | 259.940 | 0.981 ± 0.023 |
| I | 178.262 | 0.382 ± 0.00 |
| P | 178.276 | ND |
| S | 180.731 | ND |
| Co | 230.786 | 1.061 ± 0.018 |
| Ni | 231.604 | 3.910 ± 0.16 |
| Cd | 214.438 | ND |
| Cu | 324.754 | 2.044 ± 0.23 |
| Zn | 213.856 | 6.090 ± 0.17 |
| Na | 589.592 | ND |
| As | 189.042 | ND |
| Ge | 164.919 | 42.092 ± 2.06 |
| Pb | 220.353 | 0.713 ± 0.94 |
| Sc | 361.384 | ND |
| Hg | 184.95 | 0.642 ± 0.073 |
ND = Not detected means less than 0.01 µg/mL.
Elements such as Sc, N, Ba, Li, S, P, Cd, and Na were not detected. Elements such as iron, copper, zinc, manganese, and selenium etc. have immunomodulatory functions and thus the extract of this plant can be used to treat various diseases, including cancer, because it has multimineral properties. Of course, the amount of elements in the plant extracts depends on the climatic conditions (including rainfall, soil, type of crop and type of fertilizers used for plant growth) and its cultivation and harvesting season.
Feed supplements with selenium content (0.2–0.3 µg/mL) are added to diets to prevent deficiency and resultant diseases such as white muscle disease in cattle and sheep and acute myopathy in horses (Edmonson, Norman, & Suther, 1993). Selenium supplementation (10 µg/mL for 30 d) might modulate the lipid profile, mainly reducing the level of TC, non-HDL-C, and atherogenic index in animal models (Sartori, Pinton, da Rocha, Gai, & Nogueira, 2016). Furthermore, 1 µg/mL selenium supplementation could restore the reduced T3 and T4 hormones in the serum of high-fat diet-fed rabbits and same dose in drinking water could reduce aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) in diabetic rats (Kang, Bansal, & Mehta, 2000).
The results of this study show that due to the high levels of selenium in this plant (1.123 ppm), it can be used as a plant source to compensate for this deficiency. However, the Selenium in the diet at > 5 µg/mL may produce mild clinical effects after prolonged exposure of ≥ 30 d (Edmonson, Norman, & Suther, 1993). The target supplementary dietary levels of copper and zinc are 5–15 and 20–80 µg/mL, respectively, which more or less over time can cause symptoms of deficiency of these minerals (Olukosi, Van Kuijk, & Han, 2018). Due to the amount of copper (2.044 µg/mL) and zinc (6.09 µg/mL) present in this plant, it could be used as a suitable source of therapeutic dose in people with a deficiency of these minerals.
Iron deficiency is the most common cause of anemia, because it is essential for hemoglobin formation. Of about 15 mg/d of dietary iron, adults absorb only 1 mg, which is the approximate amount lost daily by cell destruction. An adult needs 60 µg/mL of iron per day in food, which is higher in women and growing children (0.5 mg/day than the other people) (Saljoughian, 2007). Although it is not high in iron (0.981 µg/mL), it could help iron deficiency in postmenopausal women (due to the presence of isoflavonoids). A safe reference dose (RfD) for mercury (5.5 × 10-3 mg/d to 1.0 × 10-3 mg/d for a person weighing between 120 and 220 lb) was determined based on methyl mercury poisonings and deaths that occurred in Japan (1953–1960, contaminated fish) from, and Iraq (1971–1972, contaminated grain).
Furthermore, the U.S. Food and Drug Administration (FDA) in 2002 set a limit of 1 µg/mL of mercury in foods and 0.3 ppm in fish for human consumption (Echols, Meadows, & Orazio, 2009). FDA at 2006 established an RfD for lead (Pb) at 0.5 µg/mL. But in some food products, at least this has been changed, for example, in dairy products, especially products containing a maximum detectable maximum chocolate content, which should not exceed 0.1 µg/mL (US Food and Drug Administration, 2006). However, the amount of leads and mercury in red clover are 0.713 ppm and 0.642 µg/mL respectively, which should be taken into account when consuming.
3.5. Total phenolic content (TPC), total flavonoid content (TFC) and antioxidant capacities (DPPH and FRAP assay)
The TPC of T. pratense (58.12 ± 6.21 mg GAE/g plant) and TFC (39.21 ± 4.26 mg RUE/g plant) was determined. Antioxidant compounds can reduce free radicals primarily by two mechanisms: hydrogen atom transfer (HAT) and single electron transfer (ET). The antioxidant capacity or radical scavenging capacity (RSC) of the ethanol extract of the flowers from leaves and stem of T .pratense was measured by DPPH assays. In the DPPH assay the ethanol extract gave a value at (149 ± 11.61) μmol eq. Trolox/10 g plant. In the FRAP method under acidic conditions, the blue ferrous complex from the yellow ferric‐tripyridyltriazine complex is formed by electron donors. T. pratense is already confirmed as being a good antioxidant capacity, and it was also a fine source of compounds with reducing capacity as it can be concluded by the FRAP value at 6620.15 ± 43.26 mmol Fe2+/mg that reducing free radicals (Table 5).
Table 5.
TPC, DPPH,TFC and FRAP assays results for ethanolic extracts from leaves and stems of T. pratense (mean ± SD, n = 3).
| Methods | Parameter value/Unit |
|---|---|
| Total phenolic content | 58.12 ± 6.21 (mg GAE/ g dried plant) |
| Total flavonoid content | 39.21 ± 4.26 (mg RUE/ g dried plant) |
| DPPH | 149 ± 11.61 (μmol eq. Trolox/10 g dried plant) |
| FRAP | 6620.15 ± 43.26 (mmol Fe(II)/mg dried plant) |
4. Discussion
In this study, for the first time, the introduction of SED-EDS for the identification of minerals in plant extracts as well as the analysis of volatile and polyphenolic compounds (flavonoids and isoflavones) of red clover species in Iran for therapeutic applications was investigated. The flavonoids are a large family of polyphenols including the flavanones, flavones, isoflavones, flavonols, and flavanonols which are derived from various phenylproponoid biosynthetic pathways. After LC-ESI/MS analysis, it was determined that the red clover contains formononetin, daidzein 7-O-β-D-glucoside, daidzin 4′-O-glucuronide, genistein, Genistein 8-C-glucoside-xyloside and biochanin A, biochanin A-7-glucoside isoflavones. In the LC-APCI-MS analysis of de Rijke et al., it was found that the plant contains daidzin, daidzein, genistin, genistein, ononin, sissotrin and biochanin A isoflavones compounds de (de Rijke, Zafra-Gómez, Ariese, Udo, and Gooijer, 2001).
In another LC-ESI/MS analysis in the aqueous methanolic extract of the flowers of T. pratense, the isoflavones and flavonoids, including genistin, genistin 6′′-O-malonate, genistein, formononetin, formononetin 7-O-α-D-glucoside 6′′-O-malonate, prunetin, biochanin a, ononin, irilone 4′-O-α-D-glucoside 6′′-O-malonate, trans-clovamide, caffeic acid derivative, pratensein 7-O-α-D-glucoside, isoquercitrin, isoquercitrin 6′′-O-malonate and afrormosin 7-O-α-D-glucoside were identified (Lin, He, Lindenmaier, Yang, Cleary, & Qiu, 2000).
Isoflavone compounds such as formononetin, biochanin A, genistein and daidzein, presents in this plant have estrogenic properties. These compounds also affect cognitive and mood performance by binding to β-estrogen receptor and changing dopaminergic, serotonergic, and cholinergic systems and reduce bone loss and aging of the skin due to menopause. Due to the presence of isoflavones in this plant, the extract of this plant or its compounds could be used purely for the treatment of various diseases, especially the reduction of menopausal symptoms (Han, Soares, Haidar, De Lima, & Baracat, 2002).
This is while the polyphenol compounds identified in our study included cinnamic acid, 3-O-(Z)-p-coumaroylquinic acid, epigallocatechin, caffeic acid, coumaric acid-O-pentoside, kaempferol galloylglucoside, quercetin-3,7-diglucoside, kaempferol-3,7-di-O-glucoside, myricetin-3-O-rhamnoside, quercetin-3-O-galactoside, myricetin-3-O-acetyl rhamnoside, myricetin-3-O-acetyl rhamnoside, ferulic acid-O-hexoside derivative, apigenin-7-O-glucoside, apigenin 6,8-diglucoside, gallocatechin, quercetin-3-O-pentoside and kaempferol galloylglucoside. Saviranta et al. after HPLC-MS analyzing in red clover leaves, flavonoids, including quercetin-galactoside, quercetin-glucoside, quercetin, kaempferol, pseudobaptigenin and maackiainhave been identified (Saviranta, Julkunen-Tiitto, Oksanen, & Karjalainen, 2010).
Accumulation of mineral elements in plants depends on soil properties, cultivation, total and plant-available amount of elements, and fertilization system, climate, as well as plant properties (Bengtsson, Öborn, Jonsson, Nilsson, & Andersson, 2003). In our study, all the elements in this plant were identified so that by using two methods (SEM and ICP), the amount of each elements were measured. However, few of the elements were identified in similar studies on mineral elements in this plant. Our SEM and ICP analysis showed that the red clover contains important minerals such as K, Mg, Al, Si, Zn, Ni, Cu, Se, Co, Fe, Mn, and Ca. Also, the presence of very low toxic compounds, including Lead (Pb) and mercury (Hg) in this plant was proven.
Meanwhile, Whittaker et al. identified the Al, Mg, Co, Ni, Ca, Ba, S, and K elements in the plant using path analysis (Whittaker, Vazzana, Vecchio, & Benedettelli, 2009). Another study showed that the plant contains elements such as Ca, P, K, Mg, Na, Cu, Zn, Mn, Fe and C. Reay et al. showed that the plant contains Fe, Zn, Mn, Cu, Ca, Mg, K, Na, P, and N elements and N (3670 µmol/g DW) and K (790 µmol/g DW) is the most common elements in this plant (Reay and Marsh, 1976, Shokri and Maadi, 2009). In our study, the amounts of C, O, K, Cl, Ge and Mg in this plant were higher than the rest of the elements in this plant. Some of the important elements in this plant, including selenium and phosphorus are in the other clover family (Trifolium alexandrium L. and T. repens L.) (Singh & Malhotra, 1976).
In the study of volatile compounds of this plant, it was found that it contains fugitive compounds such as 3, 7, 11, 15-tetramethyl-2-hexadecen-1-ol, methylcyclopentane, 2, 3-dimethylpentanal, 1-hexadecanol, cyclohexane, 4-hydroxy-4-methyl-2-pentanone and 3, 7, 11, 15-tetramethyl-2-hexadecen-1-ol (Reay & Marsh, 1976). Using a GLC-MS analysis, showed that the plant contains volatile compounds, including 2-methylbutanol, hexanol, 3-hexenol, 2, 3-dihydroxybutane, β-ocimene, 3-hexenyl acetate, acetic acid, methyl salicylate and 1-phenylethano.Another study, using GC/MS techniques, also showed other volatile compounds, including 3-methyl-1-butanol, ethyl butanoate, 3,7,11,15-tetramethyl-2-hexadecen-1-ol, hexadecanoic acid, 3-methylbutanoic acid, dimethylpyridine, methyl hexanoate, benzaldehyde and 1-heptanol (Rodrigues & do Céu Costa, 2007).
An analysis of GC/MS has shown in the studies of the presence of volatile compounds, including hexane (Higgins & Smith, 1972), methylcyclopentane (Fukui & Doskey, 2000), cyclohexane (Yoshihara, Yoshikawa, Kunimatsu, Sakamura, & Sakuma, 1977), 4-hydroxy-4-methyl-2-pentanone (Coote, Wayne, Regtop, & Biffin, 2004), 7-tetradecene, 1-nonadecen (Parekh & Felice, 2014), 10-heneicosene (Kami, 1978) and n-tetracosanol-1 (Houx, Garrett, & McGraw, 2008) which were identified in our analysis of these compounds in this plant.In addition, at the end of our study, new volatile compounds were identified that were not reported in previous studies of the plant, which are 3-trifluoroacetoxypentadecane, n-tridecanol, octylcyclohexane, 1-hexadecanol, 3-eicosyne, 3,7,11,15-tetramethyl-2-hexadecen-1-ol, 9-eicosyne and 2-methyl-1-hexadecanol.
Some of these compounds have therapeutic properties, including 3,7,11,15-Tetramethyl-2-hexadecen-1-ol (R/T 40.98) can be an antioxidant, antimicrobial and anti-inflammatory (Jananie, Priya, & Vijayalakshmi, 2011), n-hexadecanoic acid (R/T 43.148) can be an antifungal, antioxidant, hypocholesterolemic, nematicide, anti-androgenic flavor, hemolytic, 5α-reductase inhibitor, potent antimicrobial agent, antimalarial and antifungal (Akpuaka, Ekwenchi, Dashak, & Dildar, 2013), 4-hydroxy-4-methyl-2-pentanone (R/T 7.559)9-eicosane (R/T 41.166), 1-hexadecanol (R/T 33.47) and 10-heneicosene (R/T 38.911) can be an antioxidant and potent antimicrobial agent (Neelamma, Rao, & Anuradha, 2011). Due to flavonoids and isoflavones and essential minerals identified for this study, the clover extracts or its purified compounds can be used for various treatments.
A study showed that the value of total phenolics of T. pratense leaves was 62.65 and flowers 58.53 mg GAE/gdried plant. It was in line with our result of the value of total phenolics of Iranian red clover (58.12 mg GAE/g dried plant) (Tava, Pecio, Lo Scalzo, Stochmal, & Pecetti, 2019). However, in another study on the total phenolics and total flavonoid of the T. pratense (aerial parts) methanolic extract, the amount of total phenolics 31.94 mg GAE/g was reported. In the same study, the amount of total flavonoid was reported at 13.03 mg RUE/g (Khorasani, Mat, Mohajer, & Banisalam, 2015), while our study showed that the leaf and stem extracts contained 39.21 mg RUE/g. Also, in the present study, the antioxidant capacity was 149 ± 11.61 (μmol eq. Trolox/10 g dried plant) using DPPH solution and 6620.15 ± 43.26 (mmol Fe (II)/mg dried plant) in FRAP solution.
5. Conclusion
Isoflavone compounds such as formononetin, biochanin A, genistein and daidzein, as well as flavonoid compounds including apigenin, kaempferol, ferulic acid, quercetin, caffeic acid, coumaric acid and myricetin, which have many medicinal properties that can be purified and used in the pharmaceutical industry. Volatile compounds such as 3, 7, 11, 15-tetramethyl-2-hexadecen-1-ol, methylcyclopentane, 2, 3-dimethylpentanal,1-hexadecanol, cyclohexane and 4-hydroxy-4-methyl-2-pentanone have the highest concentrations (5%<) in this plant. Using two different methods (ICP and SEM), 18 mineral elements were identified in this plant that some essential mineral elements, including Se, Cu, Fe, Co, and Zn, along with therapeutic and medicinal properties play a role in the metabolism of plant cells and the production of their compounds. In addition to mineral elements with therapeutic properties, two toxic elements (Pb and Hg) were detected in this plant, which had lower concentrations than other elements. In future studies, a comparison between the effect of season, plant genus and geographical area on the amount of minerals and polyphenolic compounds in clover will be examined.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Kermanshah University of Medical Science (grant number: 980292) for Ph.D. thesis.
References
- Akbari, B. M., Khazaei, M., Khazaei, F., Naseri L. (2019). Nasturtium officinale L. hydroalcoholic extract improved oxymetholone‐induced oxidative injury in mouse testis and sperm parameters. Andrologia, e13294. [DOI] [PubMed]
- Akbari Bazm M., Goodarzi N., Shahrokhi S.R, Khazaei M. The effects of hydroalcoholic extract of Vaccinium arctostaphylos L. on sperm parameters, oxidative injury and apoptotic changes in oxymetholone‐induced testicular toxicity in mouse. Andrologia. 2020;3(52):e13522. doi: 10.1111/and.13522. [DOI] [PubMed] [Google Scholar]
- Akpuaka A., Ekwenchi M.M., Dashak D.A., Dildar A. Biological activities of characterized isolates of n-hexane extract of Azadirachta indica A. Juss (Neem) leaves. Nature and Science. 2013;11:141–147. [Google Scholar]
- Bazm M.A, Khazaei M., Ghanbari E., Naseri L. Protective effect of Vaccinium arctostaphylos L. fruit extract on gentamicin-induced nephrotoxicity in rats. Comparative Clinical Pathology. 2018;5(27):1327–1334. [Google Scholar]
- Bengtsson H., Öborn I., Jonsson S., Nilsson I., Andersson A. Field balances of some mineral nutrients and trace elements in organic and conventional dairy farming—a case study at Öjebyn, Sweden. European Journal of Agronomy. 2003;20:101–116. [Google Scholar]
- Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C. Antioxidative activity of phenolic composition of commercial extracts of sage and rosemary. LWT-Food Science and Technology. 1995;28:25–30. [Google Scholar]
- Coote, J., Wayne, M., Regtop, H., Biffin, J. (2004). Inventors; JUPITAR Pty Ltd, assignee. Emulsion and dispersion formulations and method. United States patent application US 10/478,155.
- de Rijke E., Zafra-Gómez A., Ariese F., Udo A., Gooijer C. Determination of isoflavone glucoside malonates in Trifolium pratense L. (red clover) extracts: Quantification and stability studies. Journal of Chromatography A. 2001;932:55–64. doi: 10.1016/s0021-9673(01)01231-6. [DOI] [PubMed] [Google Scholar]
- Echols K.R., Meadows J.C., Orazio C.E. Pollution of aquatic ecosystems II: Hydrocarbons, synthetic organics, radionuclides, heavy metals, acids, and thermal pollution. Encyclopedia of Inland Waters. 2009:120–128. [Google Scholar]
- Edmonson A.J., Norman B.B., Suther D. Survey of state veterinarians and state veterinary diagnostic laboratories for selenium deficiency and toxicosis in animals. Journal of the American Veterinary Medical Association. 1993;202(6):865–872. [PubMed] [Google Scholar]
- Ezhilan B.P., Neelamegam R. GC-MS analysis of phytocomponents in the ethanol extract of Polygonum chinense L. Pharmacognosy Research. 2012;4:11. doi: 10.4103/0974-8490.91028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Doskey P.V. Identification of nonmethane organic compound emissions from grassland vegetation. Atmospheric Environment. 2000;34:2947–2956. [Google Scholar]
- Goodarzi Nader, Akbari Mohsen. Renal structure and its concentrating ability in the Persian squirrel (Sciurus anomalus) Comparative Clinical Pathology. 2016;25(2):445–448. [Google Scholar]
- Han K.K., Soares J.J., Haidar M.A., De Lima G.R., Baracat E.C. Benefits of soy isoflavone therapeutic regimen on menopausal symptoms. Obstetrics & Gynecology. 2002;99:389–394. doi: 10.1016/s0029-7844(01)01744-6. [DOI] [PubMed] [Google Scholar]
- Higgins V.J., Smith D.G. Separation and identification of two pterocarpanoid phytoalexins produced by red clover leaves. Phytopathology. 1972;62:235–238. [Google Scholar]
- Houx J.H., Garrett H.E., McGraw R.L. Applications of black walnut husks can improve orchardgrass and red clover yields in silvopasture and alley cropping plantings. Agroforestry Systems. 2008;73:181–187. [Google Scholar]
- Jananie R.K., Priya V., Vijayalakshmi K. Determination of bioactive components of Cynodon dactylon by GC-MS analysis. New York Science Journal. 2011;4:1–5. [Google Scholar]
- Kabata-Pendias A. CRC Press; 2000. Trace elements in soils and plants; p. 24. [Google Scholar]
- Kami T. Aromatic constituents of forage crops. 3. Qualitative and quantitative analyses of the essential oils of red and ladino white clovers. Journal of Agricultural and Food Chemistry. 1978;26:1194–1197. [Google Scholar]
- Kang B.P.S., Bansal M.P., Mehta U. Hyperlipidemia and type I 5′-monodeiodinase activity. Biological Trace Element Research. 2000;77(3):231–239. doi: 10.1385/BTER:77:3:231. [DOI] [PubMed] [Google Scholar]
- Khazaei, M., Pazhouhi, M. (2018). Protective effect of hydroalcoholic extracts of Trifolium pratense L. on pancreatic β cell line (RIN-5F) against cytotoxicty of streptozotocin. 2018. Research in Pharmaceutical Sciences, 13: 324. [DOI] [PMC free article] [PubMed]
- Khazaei M., Pazhouhi M. Antiproliferative effect of Trifolium pratens L. extract in human breast cancer cells. Nutrition and Cancer. 2019;71:128–140. doi: 10.1080/01635581.2018.1521443. [DOI] [PubMed] [Google Scholar]
- Khorasani, E. A., Mat, T. R., Mohajer, S., Banisalam, B. (2015). Antioxidant activity and total phenolic and flavonoid content of various solvent extracts from in vivo and in vitro grown Trifolium pratense L. (Red Clover). Biomedical Research International, 2015, 643285. [DOI] [PMC free article] [PubMed]
- Klejdus B., Vitamvásová-Štěrbová D., Kubáň V. Identification of isoflavone conjugates in red clover (Trifolium pratense) by liquid chromatography–mass spectrometry after two-dimensional solid-phase extraction. Analytica Chimica Acta. 2001;450:81–97. [Google Scholar]
- Kroyer G.T. Red clover extract as antioxidant active and functional food ingredient. Innovative Food Science & Emerging Technologies. 2004;5:101–105. [Google Scholar]
- Lin L.Z., He X.G., Lindenmaier M., Yang J., Cleary M., Qiu S.X., et al. LC-ESI-MS study of the flavonoid glycoside malonates of red clover (Trifolium pratense) Journal of Agricultural and Food Chemistry. 2000;48:354–365. doi: 10.1021/jf991002+. [DOI] [PubMed] [Google Scholar]
- Maher W., Forster S., Krikowa F., Snitch P., Chapple G., Craig P. Measurement of trace elements and phosphorus in marine animal and plant tissues by low-volume microwave digestion and ICP-MS. Atomic Spectroscopy-Norwalk Connecticut. 2001;22:361–370. [Google Scholar]
- Miliauskas G., Venskutonis P.R., Van Beek T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chemistry. 2004;85:231–237. [Google Scholar]
- Naseri L., Khazaei M., Ghanbari E., Bazm M.A. Rumex alveollatus hydroalcoholic extract protects CCl4-induced hepatotoxicity in mice. Comparative Clinical Pathology. 2019;28:557–565. [Google Scholar]
- Neelamma M., Rao P.V., Anuradha G.H. Synthesis and structural studies on transition metal complexes derived from 4-hydroxy-4-methyl–2-pentanone-1H-benzimidazol-2-yl-hydrazone. Journal of Chemistry. 2011;8:29–36. [Google Scholar]
- Namera A., Kawamura M., Nakamoto A., Saito T., Nagao M. Comprehensive review of the detection methods for synthetic cannabinoids and cathinones. Forensic Toxicology. 2015;33:175–194. doi: 10.1007/s11419-015-0270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Occhiuto F., Zangla G., Samperi S., Palumbo D.R., Pino A., De Pasquale R., et al. The phytoestrogenic isoflavones from Trifolium pratense L. (Red clover) protects human cortical neurons from glutamate toxicity. Phytomedicine. 2008;15:676–682. doi: 10.1016/j.phymed.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Olukosi O.A., Van Kuijk S., Han Y. Copper and zinc sources and levels of zinc inclusion influence growth performance, tissue trace mineral content, and carcass yield of broiler chickens. Poultry Science. 2018;97(11):3891–3898. doi: 10.3382/ps/pey247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh, S., Felice, C. P. (2014). Inventors; Sweetwater Energy Inc, Assignee. Preservation of Biomass for Pretreatment. United States patent application, US 13/931,303.
- Ramamurthy N., Kannan S. SEM-EDS analysis of soil and plant (Calotropis gigantea Linn) collected from an industrial village, Cuddalore Dt, Tamil Nadu, India. Biophysical Journal. 2009;19:219–226. [Google Scholar]
- Reay P.F., Marsh B. Element composition of ryegrass and red clover leaves during a growing season. New Zealand Journal of Agricultural Research. 1976;19:469–472. [Google Scholar]
- Rezić I. Determination of engineered nanoparticles on textiles and in textile wastewaters. Trac Trends in Analytical Chemistry. 2011;30:1159–1167. [Google Scholar]
- Rodrigues Ana, do Céu Costa Maria. Volatile composition of red clover (Trifolium pratense L.) forages in Portugal: The influence of ripening stage and ensilage. Food Chemistry. 2007;104(4):1445–1453. [Google Scholar]
- Saljoughian M. Iron deficiency anemia: A closer look. US Pharmacists. 2007;32(8):26–37. [Google Scholar]
- Sartori O.C.E., Pinton S., da Rocha J.T., Gai B.M., Nogueira C.W. The hypolipidemic action of a diet supplemented with p, p’-methoxyl-diphenyl diselenide is not directly related to its antioxidant property. Canadian Journal of Physiology and Pharmacology. 2016;94(6):662–668. doi: 10.1139/cjpp-2015-0411. [DOI] [PubMed] [Google Scholar]
- Saviranta N.M., Julkunen-Tiitto R., Oksanen E., Karjalainen R.O. Leaf phenolic compounds in red clover (Trifolium pratense L.) induced by exposure to moderately elevated ozone. Environmental Pollution. 2010;158:440–446. doi: 10.1016/j.envpol.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Setchell K.D., Brown N.M., Zimmer-Nechemias L., Brashear W.T., Wolfe B.E., Kirschner A.S., et al. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. American Journal of Clinical Nutrition. 2002;76:447–453. doi: 10.1093/ajcn/76.2.447. [DOI] [PubMed] [Google Scholar]
- Senila M., Drolc A., Pintar A., Senila L., Levei E. Validation and measurement uncertainty evaluation of the ICP-OES method for the multi-elemental determination of essential and nonessential elements from medicinal plants and their aqueous extracts. Journal of Analytical Science and Technology. 2014;5:37. [Google Scholar]
- Shahrokhi S.R, Ghanimatdan M., Akbaribazm M., Isaac K. Survey of indigenous medicinal plants and traditional foods in the Lorestan province, west Iran. Bulletin of Environment, Pharmacology and Life Sciences. 2014;(3):45–49. [Google Scholar]
- Shokri S., Maadi B. Effects of arbuscular mycorrhizal fungus on the mineral nutrition and yield of Trifolium alexandrinum plants under salinity stress. Agronomy Journal. 2009;8:79–83. [Google Scholar]
- Singh M., Malhotra P.K. Selenium availability in berseem (Trifolium alexandrinum) as affected by selenium and phosphorus application. Plant and Soil. 1976;44:261–266. [Google Scholar]
- Slinkard K., Singleton V.L. Total phenol analysis: Automation and comparison with manual methods. American Journal of Enology and Viticulture. 1977;28:49–55. [Google Scholar]
- Starlin T., Ragavendran P.A., Raj C.A., Perumal P.C., Gopalakrishnan V.K. Element and functional group analysis of Ichnocarpus frutescens R. Br. (Apocynaceae) International Journal of Pharmacy and Pharmaceutical Sciences. 2012;4:343–345. [Google Scholar]
- Tava A., Pecio Ł., Lo Scalzo R., Stochmal A., Pecetti L. Phenolic content and antioxidant activity in Trifolium germplasm from different environments. Molecules. 2019;24:298. doi: 10.3390/molecules24020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi S., Singh G., Sharma A., Aggarwal G. Phytochemicals as candidate therapeutics: An overview. International Journal of Pharmaceutical Sciences Review and Research. 2010;3:53–55. [Google Scholar]
- US Food and Drug Administration. (2006). Supporting document for recommended maximum level for lead in candy likely to be consumed frequently by small children. US Food and Drug Administration: Silver Spring, MD, USA
- Whittaker A., Vazzana C., Vecchio V., Benedettelli S. Evaluation of direct and indirect effects of flavonoids, mineral elements and dry weight on antiradical scavenging activity in leaf material of field-grown Trifolium pratense cultivars using path analysis. Field Crops Research. 2009;113:1. [Google Scholar]
- Yoshihara T., Yoshikawa H., Kunimatsu S., Sakamura S., Sakuma T. New amino acid derivatives conjugated with caffeic acid and DOPA from red clover (Trifolium pratense) Agricultural and Biological Chemistry. 1977;41:1679–1684. [Google Scholar]