Abstract
Phosphorylation of SpoIIAA catalyzed by SpoIIAB helps to regulate the first sporulation-specific ς factor, ςF, of Bacillus subtilis. The steady-state rate of phosphorylation is known to be exceptionally slow and to be limited by the return of the protein kinase, SpoIIAB, to a catalytically active state. Previous work from this laboratory has suggested that, after catalyzing the phosphorylation, SpoIIAB is in a form (SpoIIAB*) that does not readily release ADP. We now show that the rate of release of ADP from the SpoIIAB*-ADP complex was much diminished by the presence of unreacted SpoIIAA, suggesting that SpoIIAA can form a long-lived ternary complex with SpoIIAB*-ADP in which the SpoIIAB* form is stabilized. In kinetic studies of the phosphorylation of SpoIIAA, the ternary complex SpoIIAA-SpoIIAB*-ADP could be distinguished from the short-lived complex SpoIIAA-SpoIIAB-ADP, which can be readily produced in the absence of an enzymatic reaction.
Soon after the start of sporulation in Bacillus subtilis, the cell divides asymmetrically to give two compartments, the prespore and the mother cell, and thereafter a pattern of differential gene expression is set up in the sporangium. The establishment of this differential gene expression depends on regulation of the sporulation-specific transcription factor ςF (references 8, 12, and 20 and references therein). Although ςF is present in the predivisional cell and in the mother cell, it is inhibited there; in the prespore, however, its activity is released. Regulation of ςF depends on the three proteins SpoIIAB, SpoIIAA, and SpoIIE. SpoIIAB is bifunctional: it can bind to ςF to inhibit its activity, or it can act as a specific protein kinase to phosphorylate SpoIIAA (1, 4, 5, 18). SpoIIE is a specific protein phosphatase which hydrolyzes SpoIIAA-P to SpoIIAA (2, 6, 9). In the predivisional cell, most of the SpoIIAA is present in the form of SpoIIAA-P (17), which does not interact with SpoIIAB (16); as a result, SpoIIAB is free to inhibit ςF. But at or a little before the time of asymmetric division, SpoIIE is activated and SpoIIAA-P is hydrolyzed to give SpoIIAA. The interaction of this free SpoIIAA with SpoIIAB prevents the latter from inhibiting ςF in the prespore (4, 7, 10, 11, 17).
This system has a number of distinctive features that help to explain its biological function. First, in the prespore the SpoIIAB kinase and SpoIIE phosphatase are active simultaneously, and SpoIIAA is continually cycled between the nonphosphorylated and the phosphorylated forms (17). Second, the activity of SpoIIE is much higher than that of SpoIIAB (15), and hence in the prespore all (or almost all) of the SpoIIAA is in the nonphosphorylated form (9, 11, 13). Third, the intracellular concentrations of SpoIIAB and ςF are equal, so that there is only just enough SpoIIAB to inhibit ςF; thus, the sequestration of even a fraction of the SpoIIAB in an alternative reaction will liberate some ςF activity (17). Finally, the phosphorylation catalyzed by SpoIIAB is extremely slow, with the result that at any given time much of the SpoIIAB is in fact sequestered in this reaction (17, 19).
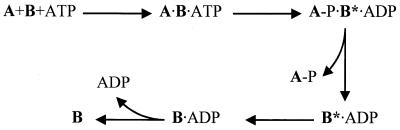
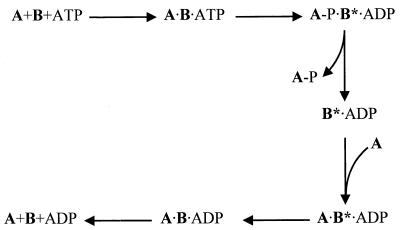
The kinetics of the phosphorylation of SpoIIAA by SpoIIAB in vitro follow a biphasic time course, with a moderate rate giving way to a very slow steady-state rate (about 0.6 × 10−3 mol of SpoIIAA phosphorylated per mol of SpoIIAB per s) after 1 mol of SpoIIAA has been phosphorylated per mol of SpoIIAB (17). This feature suggests that the rate-determining step in the enzymatic reaction is that in which the enzyme returns to a catalytically active form after each round of phosphorylation (17). In a detailed study of the reaction mechanism, Najafi et al. (19) suggested the minimal scheme shown in Fig. 1. A particular feature of this scheme was the proposal that, after liberating the product SpoIIAA-P, SpoIIAB is in a special conformation, SpoIIAB*, which releases ADP exceptionally slowly. It was the slow relaxation of this form to SpoIIAB that was presumed to allow the release of ADP and to determine the overall rate of the enzymatic reaction.
FIG. 1.
Scheme of the mechanism of phosphorylation of SpoIIAA (A) by SpoIIAB (B), proposed by Najafi et al. (19).
Duncan et al. (7) and Garsin et al. (10) have given an alternative account of the phosphorylation of SpoIIAA that does not include a proposal for an unusual conformation of SpoIIAB. These workers suggest that SpoIIAA-P is produced by a reaction of SpoIIAA with a SpoIIAB-ςF-ATP complex. This reaction liberates SpoIIAB-ADP, which interacts with another molecule of SpoIIAA to form a long-lived SpoIIAA-SpoIIAB-ADP complex. The dissociation of this complex is then necessary to allow phosphorylation of SpoIIAA to proceed.
In the present communication, we produce further evidence for the existence of SpoIIAB*, give a fuller account of the return of SpoIIAB*-ADP (via a ternary complex with SpoIIAA) to the catalytically active form, and assess the significance of the unusual properties of the system for the regulation of ςF in the cell.
SpoIIAB*-ADP forms a complex with SpoIIAA.
The experiments that led to the discovery of SpoIIAB* involved the phosphorylation of excess SpoIIAA in the presence of limiting concentrations of SpoIIAB. It seemed possible that, in such conditions, SpoIIAB*-ADP liberated after phosphorylating SpoIIAA could bind to another molecule of SpoIIAA to give a ternary complex and that it is the slow dissociation of this complex that determines the steady-state rate of the phosphorylation reaction. (This suggestion would be parallel to that made by Duncan et al. [7] and Garsin et al. [10], who, however, do not include SpoIIAB* in their scheme.) We therefore designed experiments to see whether the presence of excess SpoIIAA reduces the rate of release of ADP from its complex with SpoIIAB*. SpoIIAA proteins were purified as described previously (16). SpoIIAA and [α-32P]ATP (1 μM each) were incubated with 1 μM SpoIIAB in buffer A (19) at 30°C for 20 min. When samples of this mixture were separated by nondenaturing polyacrylamide gel electrophoresis, about 60% of the SpoIIAA was found to have been converted to SpoIIAA-P. By centrifuging a sample through a Centricon 10 filter and counting the radioactivity in the filtrate, we found that some 10% of the total radioactivity remained bound to protein. Of the 90% of the radioactivity that was free in solution, thin-layer chromatography (3) showed that about 40% was ATP and about 60% was ADP. It appears that under these conditions the phosphorylation of SpoIIAA by ATP fails to proceed to completion. (We note that the Km of the phosphorylation reaction for ATP is 1.4 μM and the Ki for ADP is 1.0 μM [19].)
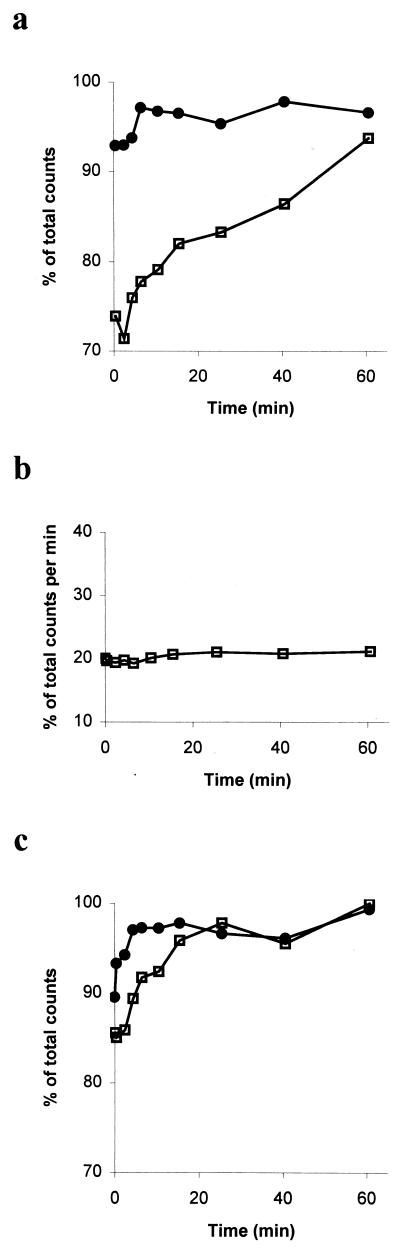
The mixture just described, which included protein-bound [α-32P]ADP, was then divided into two equal portions, and one of these was supplemented with additional SpoIIAA (10 μM). After 1 min, we added 10 μM ATP-γ-S [adenosine 5′-O-(3-thiotriphosphate)], a nonhydrolyzable analog of ATP, to both mixtures. At intervals, the mixtures were centrifuged through a Centricon 10 filter, and the radioactivity in samples of the filtrates was counted. The two curves in Fig. 2a show the results. In the portion to which excess SpoIIAA has not been added (upper curve), [α-32P]ADP (see below) was released rapidly after the addition of ATP-γ-S. By the time the first sample could be processed, only a small percentage of the radioactivity remained bound to protein, and this final quantity was released within 5 to 6 min (upper curve in Fig. 2a). But in the portion to which excess SpoIIAA had been added before the ATP-γ-S, the [α-32P]ADP was released only very slowly from the protein-bound form (lower curve in Fig. 2a); even after 60 min, some ADP was still bound to the protein complex.
FIG. 2.
(a) Effect of SpoIIAA on release of ADP from SpoIIAB*-ADP. [α-32P]ATP was used to phosphorylate SpoIIAA in the presence of SpoIIAB. After the addition of ATP-γ-S, samples were subjected to filtration through a Centricon filter. The radioactivity of the filtrate was measured and is expressed as a percentage of the radioactivity in the incubation mixture. The release of ADP was measured in the absence (●) and in the presence (□) of additional SpoIIAA. (b) Radioactivity released after incubation of SpoIIAA and SpoIIAB with [γ-32P]ATP. (c) Effect of SpoIIAAL90A on release of ADP from SpoIIAB*-ADP. The experiment shown in panel a was repeated, and the release of ADP was measured in the absence (●) and in the presence (□) of added SpoIIAAL90A.
We interpret these findings in the following way. In the first mixture, the addition of ATP-γ-S allows the release of [α-32P]ADP from SpoIIAB to be observed; this release is complete within some 6 min. In the second mixture (to which excess SpoIIAA is added), SpoIIAA forms a complex with SpoIIAB*-[α-32P]ADP. This ternary complex decays very slowly, and the rate of its decay can be followed by monitoring the release of [α-32P]ADP. By comparing the two curves, we see that the presence of SpoIIAA diminished the rate of release of ADP by a factor of more than 10-fold, suggesting that the SpoIIAA-SpoIIAB*-ADP complex is remarkably long-lived.
To test this interpretation, we did two further kinds of experiment. First, we proved that it was ADP and not ATP that was released from the ternary complex by repeating the experiment reported in the lower curve of Fig. 2a, but substituting [γ-32P]ATP for [α-32P]ATP. The results (Fig. 2b) showed that no radioactivity was released in these circumstances. Second, we repeated the experiment again, but instead of adding excess wild-type SpoIIAA after the 20 min of initial incubation, we added SpoIIAAL90A, a mutant form of SpoIIAA that can be readily phosphorylated but does not make a stable complex with SpoIIAB in the presence of ADP (C.-S. Lee and M. D. Yudkin, unpublished results). The results (Fig. 2c) show that the release of ADP from its complex with SpoIIAB was scarcely affected by the presence of SpoIIAAL90A. The striking contrast between the effect of the wild-type protein and that of the mutant protein strongly suggests that the slow release of [α-32P]ADP shown in the lower curve of Fig. 2a reflects the gradual decay of a SpoIIAA-SpoIIAB*-ADP complex. The rate of loss of radioactivity from the complex that included wild-type SpoIIAA corresponded to about 0.4 × 10−3 mol of ADP released per mol of SpoIIAB per s, which is very similar to the steady-state rate of the overall phosphorylation reaction. It seems likely, then, that it is the dissociation of the SpoIIAA-SpoIIAB*-ADP complex that determines the rate of phosphorylation and accounts for its exceptional sluggishness. The mechanism of dissociation of the complex is considered in more detail below.
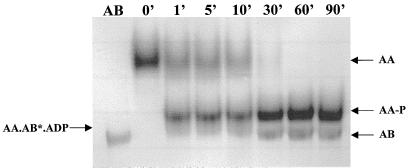
Given the results shown in Fig. 2, one might predict that in a mixture containing SpoIIAA, SpoIIAB, and ATP, the SpoIIAB would remain in a SpoIIAA-SpoIIAB*-ADP complex until all the SpoIIAA had been phosphorylated and thereafter would be set free. We tested this prediction by incubating 20 μM SpoIIAA and 1 μM SpoIIAB with 1 mM ATP at 30°C, taking samples at intervals, and subjecting them to nondenaturing gel electrophoresis, in which SpoIIAA-SpoIIAB-ADP complexes can be separated from SpoIIAB (4). The results (Fig. 3) show that SpoIIAB remained trapped in the complex until all the SpoIIAA had been converted to SpoIIAA-P, when it was released. It follows from this finding that in a situation in which there is an infinite supply of SpoIIAA (which is presumed to be the case in the prespore when SpoIIAB and SpoIIE are both active), SpoIIAB will never be released from the SpoIIAA-SpoIIAB*-ADP complex and will therefore remain unavailable to inhibit ςF.
FIG. 3.
Trapping of SpoIIAB in a complex with SpoIIAA and ADP and its release after phosphorylation is completed. Samples were taken at the times shown and subjected to nondenaturing gel electrophoresis. The positions of SpoIIAA, SpoIIAA-P, SpoIIAB, and SpoIIAA-SpoIIAB*-ADP are indicated by arrows.
Confirmation of the existence of SpoIIAB*.
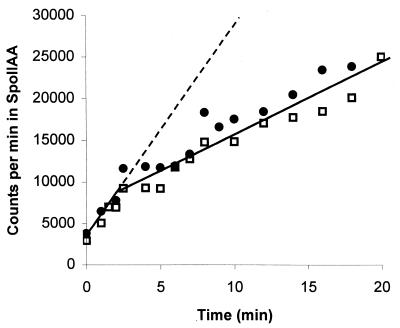
In the light of the above results, it might be suggested that one could interpret the biphasic kinetics of the phosphorylation of SpoIIAA in the following way. After 1 mol of SpoIIAA has been phosphorylated per mol of SpoIIAB, one equivalent of ADP is present in the mixture. The ADP and SpoIIAB could then interact with unreacted SpoIIAA to form SpoIIAA-SpoIIAB-ADP. This is a deadend complex, whose dissociation is essential for phosphorylation to proceed, and the subsequent diminished rate of the reaction (about 0.4 × 10−3 s−1—see above) could be explained if dissociation of the complex were very slow. Although this suggestion seems incompatible with the measured rate of dissociation of SpoIIAA-SpoIIAB-ADP, which is about 15 × 10−3 s−1 (17), we designed an experiment to see if we could distinguish between SpoIIAA-SpoIIAB*-ADP and SpoIIAA-SpoIIAB-ADP. If the two are identical and SpoIIAB* does not exist (that is, if the SpoIIAB that is released after phosphorylating SpoIIAA is no different from the normal SpoIIAB molecule), it should be possible to inhibit the phosphorylation reaction from the outset by adding 1 mol of ADP per mol of SpoIIAB. We therefore followed the phosphorylation of 50 μM SpoIIAA by 250 μM [γ-32P]ATP in the presence of 0.5 μM SpoIIAB, with and without 5 μM ADP (a 10-fold molar excess over SpoIIAB). Protein was precipitated with trichloroacetic acid, and its radioactivity was determined (17). As Fig. 4 shows, the addition of ADP had no effect on the initial rate of reaction. (A repetition of this experiment, but with SpoIIAB preincubated with ADP before the addition of SpoIIAA and [γ-32P]ATP, yielded similar results [not shown].) After about 3 min the rate of phosphorylation slowed to one-third of its original value, both in the reaction to which no ADP had been added and in that which had been supplemented with ADP (Fig. 4). At this point, measurement of the radioactivity that had been incorporated into SpoIIAA-P suggested that the ADP that had been generated by the phosphorylation reaction amounted to between 1.0 and 1.1 mol per mol of SpoIIAB.
FIG. 4.
Time course of phosphorylation of SpoIIAA. SpoIIAA (50 μM) was incubated with 250 μM [γ-32P]ATP and 0.5 μM SpoIIAB in the absence (□) or presence (●) of 5 μM ADP. The graph shows the radioactivity in trichloroacetic acid-insoluble protein (17). The dashed line is an extension of the gradient of incorporation over the first few minutes, to emphasize the difference between the pre-steady-state and the steady-state rates of incorporation.
In the light of the results presented in Fig. 2, we attribute the change in rate after 3 min to the formation of a long-lived SpoIIAA-SpoIIAB*-ADP complex, whose dissociation is now the rate-determining step in the phosphorylation of further SpoIIAA molecules. The formation of this complex evidently depends on the ability of SpoIIAB to bind to ADP even in the presence of a 500-fold excess of ATP, quite unlike the normal situation, in which SpoIIAB has about the same affinity for ADP and ATP (14). In other words, the SpoIIAB that has just phosphorylated SpoIIAA and thus generated ADP readily makes a complex with a molecule of (unphosphorylated) SpoIIAA, whereas the SpoIIAB present at the beginning of the reaction fails to make such a complex with SpoIIAA even when a 10-fold higher concentration of ADP is added to the mixture. We conclude that SpoIIAB that has taken part in the reaction in which SpoIIAA is phosphorylated is in an unusual conformation (the conformation that we have called SpoIIAB* [19]), in which it is tightly complexed with ADP and interacts readily with SpoIIAA.
A scheme that takes account of all these results is presented in Fig. 5. Given the characteristic biphasic time course of the phosphorylation reaction, it is clear that the rate-determining step in this scheme must be later than the step in which SpoIIAA-P is released—that is, it must be either the relaxation of SpoIIAB* to SpoIIAB or the dissociation of the SpoIIAA-SpoIIAB-ADP complex. However, the rate constant for the latter step is known to be some 20 times greater than the overall rate constant for the phosphorylation (17; see above). We therefore conclude, as before (19), that the rate of the reaction is limited by the conformational change from SpoIIAB* to SpoIIAB. The results presented in Fig. 2 suggest that the presence of SpoIIAA in the ternary complex stabilizes the SpoIIAB* conformation of SpoIIAB.
FIG. 5.
Scheme of the mechanism of phosphorylation of SpoIIAA (A) by SpoIIAB (B), taking into account the results described in this communication.
Significance of the results for the regulation of ςF in the cell.
After asymmetric septation, SpoIIAB and SpoIIE are both active in the prespore, and SpoIIAA therefore cycles continually between the phosphorylated and the nonphosphorylated form (17). However, the activity of SpoIIE is much greater than that of SpoIIAB (15), and as a result SpoIIAB is constantly supplied with SpoIIAA—which is not only its substrate, but also its partner in the noncovalent complex. Every time a molecule of SpoIIAB catalyzes the phosphorylation of SpoIIAA, it is released as SpoIIAB*-ADP, and it can then interact with another molecule of SpoIIAA. The resulting noncovalent complex, SpoIIAA-SpoIIAB*-ADP, is enzymatically inactive, and the rate of its decay determines the rate at which SpoIIAB becomes available either to phosphorylate SpoIIAA or to inhibit ςF. Since the SpoIIAA-SpoIIAB*-ADP complex is long-lived by virtue of the slow relaxation of AB* to AB, and since the intracellular concentrations of SpoIIAB and ςF are equal (17), the effect of these interactions is to leave many ςF molecules uninhibited by SpoIIAB. Thus, activation of ςF depends on the sequestration of SpoIIAB in long-lived complexes that result from the phosphorylation reaction and which one might call postphosphorylation complexes.
Several years ago it was generally accepted that there were two ways in which SpoIIAA and SpoIIAB could interact—in the presence of ATP SpoIIAB catalyzed the phosphorylation of SpoIIAA, and in the presence of ADP the two proteins formed a noncovalent complex. These two interactions were regarded as alternatives, and the choice between them was believed to depend on the local concentration of ATP and/or ADP (1, 4, 7, 16). The results presented here show that the two interactions are not alternatives but rather (as hypothesized by Garsin et al. [10]) occur successively. Together, the two interactions constitute a subtle mechanism for releasing ςF from inhibition in the prespore. Once asymmetric septation has been completed, this activation of ςF enables differential gene expression to get under way in the two compartments of the sporangium.
Acknowledgments
We are grateful to D. A. Harris for much valuable advice and for his helpful comments on the manuscript.
This work was supported by grants from the Biotechnology and Biological Sciences Research Council.
REFERENCES
- 1.Alper S, Duncan L, Losick R. An adenosine nucleotide switch controlling the activity of a cell type-specific transcription factor in B. subtilis. Cell. 1994;77:195–205. doi: 10.1016/0092-8674(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 2.Arigoni F, Duncan L, Alper S, Losick R, Stragier P. SpoIIE governs the phosphorylation state of a protein regulating transcription factor ςF during sporulation in Bacillus subtilis. Proc Natl Acad Sci USA. 1996;93:3238–3242. doi: 10.1073/pnas.93.8.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeBoer P A J, Crossley R E, Hand A R, Rothfield L I. The MinD protein is a membrane ATPase required for the correct placement of the Escherichia coli division site. EMBO J. 1991;10:4371–4380. doi: 10.1002/j.1460-2075.1991.tb05015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diederich B, Wilkinson J F, Magnin T, Najafi S M A, Errington J, Yudkin M D. Role of interactions between SpoIIAA and SpoIIAB in regulating cell-specific transcription factor ςF of Bacillus subtilis. Genes Dev. 1994;8:2653–2663. doi: 10.1101/gad.8.21.2653. [DOI] [PubMed] [Google Scholar]
- 5.Duncan L, Losick R. SpoIIAB is an anti-ς factor that binds to and inhibits transcription by regulatory protein ςF from Bacillus subtilis. Proc Natl Acad Sci USA. 1993;90:2325–2329. doi: 10.1073/pnas.90.6.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan L, Alper S, Arigoni F, Losick R, Stragier P. Activation of cell-specific transcription by a serine phosphatase at the site of asymmetric division. Science. 1995;270:641–644. doi: 10.1126/science.270.5236.641. [DOI] [PubMed] [Google Scholar]
- 7.Duncan L, Alper S, Losick R. SpoIIAA governs the release of the cell-type specific transcription factor ςF from its anti-sigma factor SpoIIAB. J Mol Biol. 1996;260:147–164. doi: 10.1006/jmbi.1996.0389. [DOI] [PubMed] [Google Scholar]
- 8.Errington J. Determination of cell fate in Bacillus subtilis. Trends Genet. 1996;12:31–34. doi: 10.1016/0168-9525(96)81386-2. [DOI] [PubMed] [Google Scholar]
- 9.Feucht A, Magnin T, Yudkin M D, Errington J. Bifunctional protein required for asymmetric cell division and cell-specific transcription in Bacillus subtilis. Genes Dev. 1996;10:794–803. doi: 10.1101/gad.10.7.794. [DOI] [PubMed] [Google Scholar]
- 10.Garsin D A, Duncan L, Paskowitz D M, Losick R. The kinase activity of the antisigma factor SpoIIAB is required for activation as well as inhibition of transcription factor ςF during sporulation in Bacillus subtilis. J Mol Biol. 1998;284:569–578. doi: 10.1006/jmbi.1998.2202. [DOI] [PubMed] [Google Scholar]
- 11.King N, Dreesen O, Stragier P, Pogliano K, Losick R. Septation, dephosphorylation, and the activation of ςF during sporulation in Bacillus subtilis. Genes Dev. 1999;13:1156–1167. doi: 10.1101/gad.13.9.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kroos L, Zhang B, Ichikawa H, Yu Y-T N. Control of ς factor activity during Bacillus subtilis sporulation. Mol Microbiol. 1999;31:1285–1294. doi: 10.1046/j.1365-2958.1999.01214.x. [DOI] [PubMed] [Google Scholar]
- 13.Lewis P J, Magnin T, Errington J. Compartmentalized distribution of the proteins controlling the prespore-specific transcription factor ςF of Bacillus subtilis. Genes Cells. 1996;1:881–894. doi: 10.1046/j.1365-2443.1996.750275.x. [DOI] [PubMed] [Google Scholar]
- 14.Lord M, Magnin T, Yudkin M D. Protein conformational change and nucleotide binding involved in regulation of ςF in Bacillus subtilis. J Bacteriol. 1996;178:6730–6735. doi: 10.1128/jb.178.23.6730-6735.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucet I, Borriss R, Yudkin M D. Purification, kinetic properties, and intracellular concentration of SpoIIE, an integral membrane protein that regulates sporulation in Bacillus subtilis. J Bacteriol. 1999;181:3242–3245. doi: 10.1128/jb.181.10.3242-3245.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magnin T, Lord M, Errington J, Yudkin M D. Establishing differential gene expression in sporulating Bacillus subtilis: phosphorylation of SpoIIAA (anti-anti-ςF) alters its conformation and prevents formation of a SpoIIAA/SpoIIAB/ADP complex. Mol Microbiol. 1996;19:901–907. doi: 10.1046/j.1365-2958.1996.434964.x. [DOI] [PubMed] [Google Scholar]
- 17.Magnin T, Lord M, Yudkin M D. Contribution of partner switching and SpoIIAA cycling to regulation of ςF activity in sporulating Bacillus subtilis. J Bacteriol. 1997;179:3922–3927. doi: 10.1128/jb.179.12.3922-3927.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Min K-T, Hilditch C M, Diederich B, Errington J, Yudkin M D. ςF, the first compartment-specific transcription factor of Bacillus subtilis, is regulated by an anti-ς factor that is also a protein kinase. Cell. 1993;74:735–742. doi: 10.1016/0092-8674(93)90520-z. [DOI] [PubMed] [Google Scholar]
- 19.Najafi S M A, Harris D A, Yudkin M D. Properties of the phosphorylation reaction catalyzed by SpoIIAB that help to regulate sporulation of Bacillus subtilis. J Bacteriol. 1997;179:5628–5631. doi: 10.1128/jb.179.17.5628-5631.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996;30:297–341. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]