Abstract
Objective
To establish HPLC fingerprints of different parts of chicory stems, leaves, roots, flowers and seeds, and compare the similarities and differences of chemical components in different parts, so as to provide a scientific basis for the comprehensive utilization of chicory.
Methods
To establish the HPLC fingerprint of chicory, the chromatographic column was chosen with Agilent ZORBAX Eclipse XDB-C18, the mobile phase was methanol (A) – 0.2% formic acid (B), the flow rate was 1 mL/min, the column temperature was 30 °C, and the detection wavelength was 254 nm. The Similarity Evaluation System of Chromatographic Fingerprint of Traditional Chinese Medicine (2012 Edition) was used to evaluate the similarity of different parts of decoction pieces, and the determination method of multi-component content was established based on fingerprint identification chromatographic peaks, and the determination results were analyzed.
Results
The HPLC fingerprinting method of chicory was established. Sixteen chromatographic peaks were identified and 10 of them were identified as: caftaric acid (1), esculin (2), chlorogenic acid (3), esculetin (4), caffeic acid (5), cichoric acid (8), hyperoside (11), rutin (12), isochlorogenic acid C (14) and luteolin (16). The similarity of different parts was 0.084–0.701. At the same time, the total content of detected chemical components was ranked as flower > leaf > stem > root > seed. Roots did not contain caftaric acid, rutin, and luteolin, flowers did not contain luteolin, and seeds did not contain caftaric acid, cichoric acid, and luteolin. The content of cichoric acid in leaves was the most, and esculin in flowers was the most.
Conclusion
The results of HPLC fingerprint and multi-component content determination revealed the similarity and difference of different parts of chicory from chemical composition, indicating that there were certain differences in different parts of chicory. The established HPLC fingerprinting method can provide a reference for quality control and evaluation of different parts of the chicory.
Keywords: caffeic acid, cichoric acid, chicory, chlorogenic acid, esculetin, fingerprint, HPLC, hyperoside, isochlorogenic acid C, luteolin, multi-component content determination, rutin
1. Introduction
Chicory is the dry aboveground parts or roots of Cichorium glandulosum Boiss. et Huet or Cichorium intybus L. Chicory contains phenolic acids, flavonoids, phenylpropanoids, terpenoids, coumarins, and polysaccharides, etc. It has the effect of protecting liver and promoting gallbladder, strengthening stomach and digesting food, inhibiting bacteria, diuretic and detumescence (Fan et al., 2016, Zhang et al., 2015, Youliduzi et al., 2010, Wang, 2008, Han et al., 2019, Zhu et al., 2017, Zhu et al., 2018). Chicory is a Uighur commonly used medicine. It is also a legal homology of medicine and food.
In recent years, people have paid more and more attention to the active ingredients and pharmacological effects of chicory. But, there are few researches on the chemical composition and pharmacodynamics studies of different parts of chicory. Chicory can be collected with different parts including stems, leaves, roots, flowers, and seeds, while the Chinese Pharmacopoeia recorded that only the above-ground parts and roots can be used as medicinal parts. According to reports, chicory seeds are rich in amino acids and oils, and the extract has a protective effect on acute liver injury (Man, 2010, Wu et al., 2005). Fresh petals contain a large number of anthocyanins (Gao et al., 2010), which have high nutritional value and health care efficacy. However, related research and application are very scarce (Board and of Chinese Medical Encyclopedia and Yishakejiang, 2005, Abudureyimu, 2005), and there is no in-depth research on the clinical application value so that the medicinal and edible value of chicory cannot be effectively developed.
The fingerprint technology of traditional Chinese medicines (TCMs) can reflect the types and quantities of the chemical components in TCMs, and it has become an internationally accepted evaluation model to control the quality of TCMs or natural medicines (Fan et al., 2016, Abudureyimu, 2005, Liu et al., 2016, Li et al., 2013). To maximize the utilization of resources, the differences in chemical composition between different parts were explored. This study set up a method for quality control of different parts, the different parts include stem, leaf, root, flower, and seed of chicory. It would provide data support and method reference for the full development and utilization of chicory as a high-quality medicinal resource and food material.
2. Materials and methods
2.1. Instruments
The HPLC fingerprints were established by Shimadzu SPD-20A High-Performance Liquid Chromatography (Shimadzu, Japan); Model GFL-620 electric heat blast drying box was from Tianjin Laiboterrui Instrument Equipment Co., Ltd (Tianjin, China); KQ-500DV ultrasonic cleaning instrument was from Kunshan Ultrasonic Instruments Co., Ltd (Shanghai, China).
2.2. Materials
The standard compound of caftaric acid (purity ≥ 98%) was procured from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China); The standard compound of esculin (purity > 98.0%), chlorogenic acid (purity > 98.5%), esculetin (purity > 98.0%), caffeic acid (purity > 98.0%), cichoric acid (purity > 98.0%), hyperoside (purity > 99.0%), rutin (purity > 98.0%), isochlorogenic acid C (purity > 98.5%), luteolin (purity > 98.0%) were procured from Chengdu Push Biotechnology Co., Ltd. (Chengdu, China); Chromatographic methanol, formic acid was chromatographic pure, water was double distilled water, the methanol used in the extraction was of analytical grade. Chicory were collected from Fengqiu County, Henan Province. They were identified as stem, leaf, root, flower, and the seed of Chicory by Professor Yanze Liu, Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences. Sample information was shown in Table 1.
Table 1.
Information of samples.
| No. | Medicinal parts | Batches | Regions |
|---|---|---|---|
| S1 | leaf | 201907 | Fengqiu, Henan |
| S2 | leaf | 201910 | Fengqiu, Henan |
| S3 | leaf | 202002 | Fengqiu, Henan |
| S4 | leaf | 202003 | Fengqiu, Henan |
| S5 | leaf | 202004 | Fengqiu, Henan |
| S6 | leaf | 202005 | Fengqiu, Henan |
| S7 | leaf | 202007 | Fengqiu, Henan |
| S8 | leaf | 202008 | Fengqiu, Henan |
| S9 | leaf | 202010 | Fengqiu, Henan |
| S10 | leaf | 202011 | Fengqiu, Henan |
| S11 | stem | 201911 | Fengqiu, Henan |
| S12 | root | 201911 | Fengqiu, Henan |
| S13 | flower | 201911 | Fengqiu, Henan |
| S14 | seed | 201910 | Fengqiu, Henan |
2.3. Analytical conditions
Chromatographic columns: Agilent ZORBAX Eclipse XDB-C18 (Analytical 4.6 mm × 250 mm, 5 μm); mobile phase: methanol (A)-0.2% formic acid aqueous solution (B), the eluting conditions was programmed as follows: 10%→25% (A) in 0–20 min, 25%→39% (A) in 20–50 min, 39%→50% (A) in 50–80 min, 50%→10% (A) in 80–90 min; flow rate: 1 mL/min; column temperature: 30 °C; injection volume: 10 μL; detection wavelength: 254 nm.
2.4. Preparation of standard solutions
The 11 reference compounds were prepared accurately in HPLC grade methanol. The concentrations were: caftaric acid, 0.620 mg/mL; esculin, 0.658 mg/mL; chlorogenic acid, 0.528 mg/mL; esculetin, 0.510 mg/mL; caffeic acid, 0.660 mg/mL; cichoric acid, 0.586 mg/mL; hyperoside, 0.504 mg/mL; rutin, 0.704 mg/mL; isochlorogenic acid C, 0.708 mg/mL; luteolin, 0.492 mg/mL. Stock solutions were mixed and prepare the mixed standard stock solution. The mixed standard solution was serially diluted with methanol to yield a series of working standard solutions 1, 2, and 3.
2.5. Extraction and sample preparation
The plant materials were dried and grounded into powder, and 0.5 g of each powder was sonicated in 20 mL of methanol for 40 min. Then, the extract was filtered through a 0.22 μm PTFE syringe filter for HPLC analysis.
2.6. Method validation of quantitative analysis
2.6.1. Linear relationship examination
Totally10 μL of the mixed standard solutions 1, 2, 3 and 1, 2, 5, 10 μL of each stock solution under “2.4” were prepared for HPLC analysis. The injection volume and peak area were used to draw a standard curve, and using a standard curve to calculate the regression equation.
2.6.2. Precision test
To determine six times working standard solutions 1, the “Chinese Medicine Chromatographic Fingerprint Similarity Evaluation System” (2012 edition) formulated by the National Pharmacopoeia Commission was used to calculate the similarity. The results showed that the similarities were all greater than 0.99, and the RSD of the 10 analytes were in the range of 1.13%–2.91%.
2.6.3. Stability test
Stability was determined at room temperature, and the same sample solutions were analyzed within 24 h, the “Chinese Medicine Chromatographic Fingerprint Similarity Evaluation System” (2012 edition) formulated by the National Pharmacopoeia Commission was used to calculate the similarity. The results showed that the similarities were all greater than 0.99, and the RSD values of the 10 analytes were in the range of 0.51%–2.65%.
2.6.4. Repeatability test
Precisely weigh six copies of the same chicory sample powder, 0.5 g of each powder was sonicated in 20 mL of methanol for 40 min, the “Chinese Medicine Chromatographic Fingerprint Similarity Evaluation System” (2012 edition) formulated by the National Pharmacopoeia Commission was used to calculate the similarity. The results showed that the similarities were all greater than 0.99, and the RSD values of the 10 analytes were in the range of 1.18%–2.92%.
2.6.5. Sample recovery test
The accuracy of the established method was evaluated by the recovery test. The recoveries were carried out by spiking accurately known amounts of the 10 analytical standards into a sample, and then extracted and analyzed under this proposed method.
2.7. Sample determination
The test solution of chicory sample was prepared according to the method under “2.5”, the chromatographic conditions under ”2.3” were determined. The fingerprint of the sample was determined, and the peaks of the 10 components were integrated, and the content of the 10 components was calculatde according to the standard curve.
3. Results
3.1. Construction of fingerprint mapping
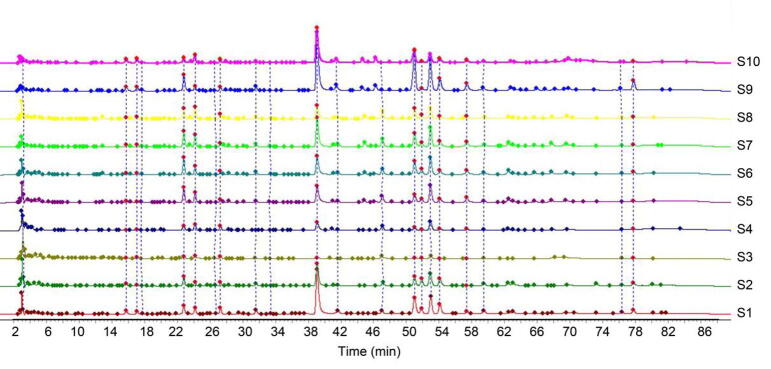
The fingerprint of 10 batches of chicory leaves was determined by HPLC. The “Chinese Medicine Chromatographic Fingerprint Similarity Evaluation System” (2012 edition) formulated by the National Pharmacopoeia Commission was used to establish the fingerprint of 10 samples. Taking S1 as the reference chromatogram, multi-point correction was used to establish the fingerprint chromatogram, and generate the superimposed chromatogram (Fig. 1).
Fig. 1.
HPLC fingerprints of 10 batches of chicory leaves.
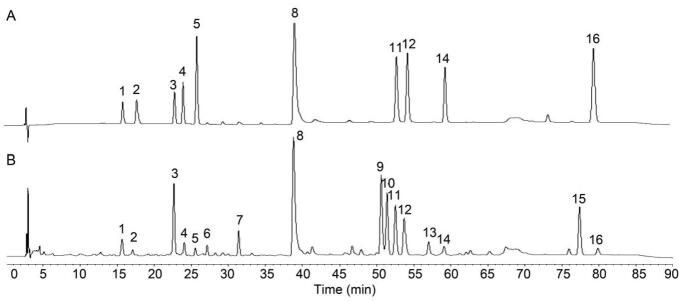
According to the test results of 10 batches of chicory leaf samples, the chromatographic peak of the main component with good separation (peak 8, cichoric acid) was selected as the characteristic peak, and a total of 16 common peaks were labeled, and 16 peaks were identified as the common peaks of chicory leaf fingerprint. The 10 common peak components were identified with reference substance (Fig. 2), which were identified as caftaric acid (peak 1), esculin (peak 2), chlorogenic acid (peak 3), esculetin (peak 4), caffeic acid (peak 5), cichoric acid (peak 8), hyperoside (peak 11), rutin (peak 12), isochlorogenic acid C (peak 14), luteolin (peak 16).
Fig. 2.
HPLC of mixed reference substances (A) and samples (B). 1. caftaric acid; 2. esculin; 3. chlorogenic acid; 4. esculetin; 5. caffeic acid; 8. cichoric acid; 11. hyperoside; 12. rutin; 14. isochlorogenic acid C; 16. luteolin.
3.2. Evaluation of similarities of different parts
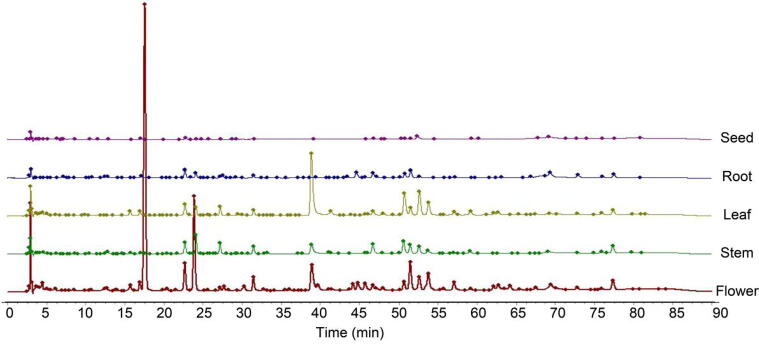
The median method and multi-point correction method were used to test the similarity of the fingerprints of chicory stems, leaves, roots, flowers, and seeds (Fig. 3, Table 2).
Fig. 3.
HPLC fingerprints of different parts of chicory.
Table 2.
Fingerprint similarity of different tissue.
| Parts | Stems | Leaves | Roots | Flowers | Seeds |
|---|---|---|---|---|---|
| Stems | 1.000 | 0.701 | 0.562 | 0.195 | 0.370 |
| Leaves | 0.701 | 1.000 | 0.229 | 0.191 | 0.131 |
| Roots | 0.562 | 0.229 | 1.000 | 0.147 | 0.226 |
| Flowers | 0.195 | 0.191 | 0.147 | 1.000 | 0.084 |
| Seeds | 0.370 | 0.131 | 0.226 | 0.084 | 1.000 |
From the results of similarity comparison, it can be seen that the similarity range of different parts of chicory was relatively wide, indicating that the fingerprints of different parts of chicory were quite different. The chemical components of stems and leaves were relatively similar, with a similarity of 0.701; The lowest similarity between flowers and seeds was 0.084; The similarity between roots and stems was 0.562; the similarity between flowers, seeds and stems, leaves, and roots was low, none exceeding 0.4. It showed that the leaves and stems of the chicory pieces had a similar chemical composition and similar chemical properties. It can be inferred that their medicinal effects were similar. The chemical composition of flowers and seeds was different, and their efficacy may be quite different.
3.3. Linear relationship
The results were shown in Table 3, indicating that the 10 tested components had a good linear relationship under this chromatographic condition.
Table 3.
Linear relationship for 10 components.
| Compounds | Regression equation | r | Linear range (μg) |
|---|---|---|---|
| Caftaric acid | Y = 780 224X-26 309 | 0.999 5 | 0.031–1.240 |
| Esculin | Y = 663 562X-64.866 | 0.999 9 | 0.003–6.580 |
| Chlorogenic acid | Y = 1 110 394.140 6X-1 870.192 3 | 0.999 9 | 0.003–2.640 |
| Esculetin | Y = 1 426 107.226 9X+658.466 0 | 0.999 9 | 0.003–2.550 |
| Caffeic acid | Y = 2 415 197.655 5X-16 538.257 2 | 0.999 4 | 0.003–1.320 |
| Cichoric acid | Y = 1 276 278.386 5X-75 466.526 9 | 0.999 9 | 0.059–5.860 |
| Hyperoside | Y = 2 943 470.551 5X-18 007.578 3 | 0.999 7 | 0.003–0.504 |
| Rutin | Y = 2 099 754.224 1X-2 287.037 6 | 0.999 0 | 0.004–1.408 |
| Isochlorogenic acid C | Y = 1 643 296.574 3X-13 125.421 7 | 0.999 3 | 0.004–1.416 |
| Luteolin | Y = 4 098 514.725 1X-21 956.072 3 | 0.999 3 | 0.002–0.984 |
3.4. Sample recovery test
The average recoveries of the 10 analytes were between 99.21% and 103.54%, with RSD values were in the range of 0.66%–2.78%, indicating that the developed method was accurate (Table 4).
Table 4.
Results of recovery of 10 components.
| Components | Sample weights (g) | Content in sample (mg) | Amount of addition (mg) | Measured amount (mg) | Recovery rate (%) | Average value (%) | RSD (%) |
|---|---|---|---|---|---|---|---|
| Caftaric acid | 0.250 3 | 0.221 0 | 0.217 0 | 0.444 1 | 102.82 | 102.14 | 2.08 |
| 0.250 5 | 0.221 2 | 0.217 0 | 0.445 8 | 103.55 | |||
| 0.250 1 | 0.220 8 | 0.217 0 | 0.443 5 | 102.64 | |||
| 0.250 6 | 0.221 2 | 0.217 0 | 0.448 0 | 104.51 | |||
| 0.250 5 | 0.221 2 | 0.217 0 | 0.439 5 | 100.63 | |||
| 0.250 3 | 0.221 0 | 0.217 0 | 0.435 1 | 98.69 | |||
| Esculin | 0.250 3 | 0.031 1 | 0.032 9 | 0.062 5 | 95.47 | 99.21 | 2.78 |
| 0.250 5 | 0.031 2 | 0.032 9 | 0.062 9 | 96.42 | |||
| 0.250 1 | 0.031 1 | 0.032 9 | 0.064 1 | 100.35 | |||
| 0.250 6 | 0.031 2 | 0.032 9 | 0.063 8 | 99.21 | |||
| 0.250 5 | 0.031 2 | 0.032 9 | 0.064 5 | 101.29 | |||
| 0.250 3 | 0.031 1 | 0.032 9 | 0.064 9 | 102.49 | |||
| Chlorogenic acid | 0.250 3 | 0.504 4 | 0.528 0 | 1.018 0 | 97.26 | 99.81 | 1.98 |
| 0.250 5 | 0.504 8 | 0.528 0 | 1.044 9 | 102.29 | |||
| 0.250 1 | 0.504 0 | 0.528 0 | 1.032 4 | 100.07 | |||
| 0.250 6 | 0.505 0 | 0.528 0 | 1.023 3 | 98.15 | |||
| 0.250 5 | 0.504 8 | 0.528 0 | 1.029 4 | 99.34 | |||
| 0.250 3 | 0.504 4 | 0.528 0 | 1.041 6 | 101.74 | |||
| Esculetin | 0.250 3 | 0.073 5 | 0.076 5 | 0.148 0 | 97.42 | 99.45 | 1.85 |
| 0.250 5 | 0.073 5 | 0.076 5 | 0.152 2 | 102.84 | |||
| 0.250 1 | 0.073 4 | 0.076 5 | 0.149 7 | 99.77 | |||
| 0.250 6 | 0.073 5 | 0.076 5 | 0.149 0 | 98.58 | |||
| 0.250 5 | 0.073 5 | 0.076 5 | 0.149 1 | 98.83 | |||
| 0.250 3 | 0.073 5 | 0.076 5 | 0.149 4 | 99.27 | |||
| Caffeic acid | 0.250 3 | 0.031 9 | 0.033 0 | 0.065 6 | 102.21 | 103.54 | 0.66 |
| 0.250 5 | 0.031 9 | 0.033 0 | 0.066 2 | 103.86 | |||
| 0.250 1 | 0.031 9 | 0.033 0 | 0.066 0 | 103.51 | |||
| 0.250 6 | 0.031 9 | 0.033 0 | 0.066 2 | 103.76 | |||
| 0.250 5 | 0.031 9 | 0.033 0 | 0.066 3 | 104.12 | |||
| 0.250 3 | 0.031 9 | 0.033 0 | 0.066 2 | 103.80 | |||
| Cichoric acid | 0.250 3 | 1.253 3 | 1.259 9 | 2.494 7 | 98.53 | 97.40 | 1.48 |
| 0.250 5 | 1.254 3 | 1.259 9 | 2.489 3 | 98.03 | |||
| 0.250 1 | 1.252 3 | 1.259 9 | 2.472 0 | 96.82 | |||
| 0.250 6 | 1.254 8 | 1.259 9 | 2.464 6 | 96.03 | |||
| 0.250 5 | 1.254 3 | 1.259 9 | 2.505 3 | 99.30 | |||
| 0.250 3 | 1.253 3 | 1.259 9 | 2.458 8 | 95.68 | |||
| Hyperoside | 0.250 3 | 0.143 4 | 0.151 2 | 0.292 9 | 98.89 | 99.94 | 1.35 |
| 0.250 5 | 0.143 5 | 0.151 2 | 0.291 6 | 97.98 | |||
| 0.250 1 | 0.143 3 | 0.151 2 | 0.294 0 | 99.66 | |||
| 0.250 6 | 0.143 6 | 0.151 2 | 0.295 5 | 100.46 | |||
| 0.250 5 | 0.143 5 | 0.151 2 | 0.296 5 | 101.20 | |||
| 0.250 3 | 0.143 4 | 0.151 2 | 0.296 8 | 101.44 | |||
| Rutin | 0.250 3 | 0.191 9 | 0.190 1 | 0.380 9 | 99.45 | 101.41 | 2.17 |
| 0.250 5 | 0.192 0 | 0.190 1 | 0.378 6 | 98.16 | |||
| 0.250 1 | 0.191 7 | 0.190 1 | 0.384 9 | 101.61 | |||
| 0.250 6 | 0.192 1 | 0.190 1 | 0.386 2 | 102.12 | |||
| 0.250 5 | 0.192 0 | 0.190 1 | 0.388 4 | 103.33 | |||
| 0.250 3 | 0.191 9 | 0.190 1 | 0.389 2 | 103.80 | |||
| Isochlorogenic acid C | 0.250 3 | 0.081 6 | 0.085 0 | 0.168 1 | 101.89 | 101.16 | 1.89 |
| 0.250 5 | 0.081 6 | 0.085 0 | 0.169 0 | 102.83 | |||
| 0.250 1 | 0.081 5 | 0.085 0 | 0.169 5 | 103.57 | |||
| 0.250 6 | 0.081 7 | 0.085 0 | 0.165 4 | 98.60 | |||
| 0.250 5 | 0.081 6 | 0.085 0 | 0.166 8 | 100.21 | |||
| 0.250 3 | 0.081 6 | 0.085 0 | 0.166 4 | 99.86 | |||
| Luteolin | 0.250 3 | 0.025 7 | 0.024 6 | 0.050 9 | 102.51 | 102.68 | 0.98 |
| 0.250 5 | 0.025 8 | 0.024 6 | 0.051 4 | 104.17 | |||
| 0.250 1 | 0.025 7 | 0.024 6 | 0.051 0 | 102.68 | |||
| 0.250 6 | 0.025 8 | 0.024 6 | 0.051 2 | 103.37 | |||
| 0.250 5 | 0.025 8 | 0.024 6 | 0.050 9 | 102.05 | |||
| 0.250 3 | 0.025 7 | 0.024 6 | 0.050 6 | 101.29 |
3.5. Simultaneous determination of 10 ingredients in different parts of chicory by HPLC
The results of 10 analytes in different parts of chicory were shown in Table 5. The chicory roots did not contain caftaric acid, rutin, and luteolin; Flowers did not contain luteolin; Seeds does not contain caftaric acid, cichoric acid and luteolin.
Table 5.
Determination results of 10 analytes in different parts of chicory.
| Samples | Quality scores (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Caftaric acid | Esculin | Chlorogenic acid | Esculetin | Caffeic acid | Cichoric acid | Hyperoside | Rutin | Isochlorogenic acid C | Luteolin | Total content | |
| Stem | 0.027 9 | 0.003 5 | 0.109 0 | 0.124 1 | 0.004 5 | 0.152 7 | 0.035 4 | 0.023 4 | 0.023 0 | 0.004 7 | 0.508 2 |
| Leaf | 0.075 7 | 0.009 3 | 0.112 9 | 0.059 8 | 0.005 6 | 0.785 7 | 0.109 0 | 0.089 2 | 0.040 7 | 0.003 6 | 1.291 5 |
| Root | − | 0.005 2 | 0.074 7 | 0.031 5 | 0.003 0 | 0.024 3 | 0.009 3 | − | 0.005 2 | − | 0.153 2 |
| Flower | 0.064 2 | 2.409 5 | 0.134 6 | 0.329 0 | 0.004 7 | 0.184 6 | 0.036 1 | 0.072 4 | 0.013 1 | − | 3.248 2 |
| Seed | − | 0.001 4 | 0.015 0 | 0.001 6 | 0.004 3 | − | 0.016 2 | 0.000 9 | 0.006 3 | − | 0.045 7 |
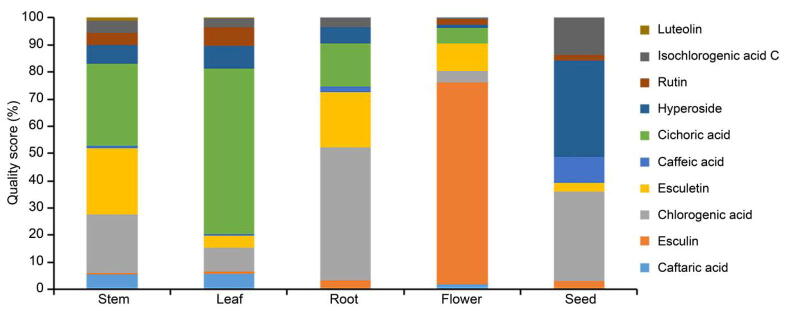
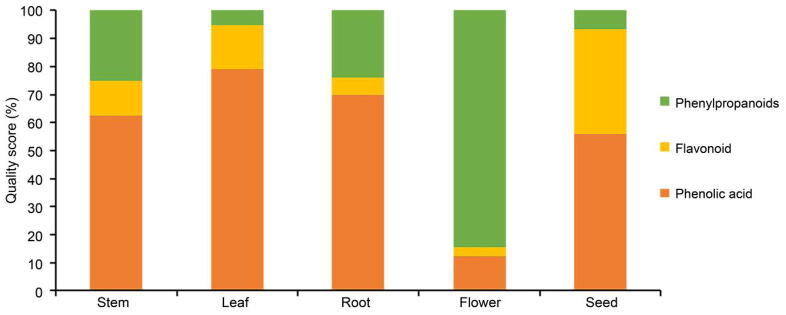
The content of 10 components was analyzed, and the content of components was ranked as flower > leaf > stem > root > seed. The content of esculin in flowers was the most, and the content of cichoric acid in leaves was the most (Table 5 and Fig. 4, Fig. 5). Caftaric acid, chlorogenic acid, caffeic acid, cichoric acid, isochlorogenic acid C belongs to phenolic acid, esculin and esculetin belong to phenylpropanoids, hyperoside, rutin, luteolin belong to the flavonoid. By analyzing the different kinds of components, the ranke of phenolic acid content was as leaf > flower > stem > root > seed, cichoric acid content was more; The flavonoids ranked as leaf > flower > stem > seed > root, the content of hyperoside was abundant;The phenylpropanoids ranked as flower > stem > leaf > root > seed, and the content of esculin was higher.
Fig. 4.
Composition of 10 components in different parts of chicory.
Fig. 5.
Classification and proportion of 10 components in different parts of chicory.
4. Discussion
In order to optimize the sample preparation, different extraction solvents (water, 25% MeOH, 50% MeOH, 70% MeOH, 100% MeOH), extraction methods (ultrasonication and reflux) and extraction time (20, 30, 40 min) were investigated. The results suggested that ultrasonication using 100% MeOH for 40 min worked better for the other compounds. Different elution ratios and different detection wavelengths of 254 nm, 278 nm, 330 nm were investigated, and finally, the elution ratio and detection wavelength described in the article were selected as the chromatographic conditions. In this study, the HPLC fingerprint of different parts of chicory pieces was established, the optimized HPLC method was applied to determine the content of 10 analytes in different parts of the pieces, which more comprehensively reflected the chemical information of the samples and provided references for the scientific evaluation of the intrinsic quality of chicory.
The chemical components in the decoction pieces of traditional Chinese medicine are the material basis for the effect of the medicine. The study of the chemical components of decoction pieces can provide research directions for the development and utilization of decoction pieces. The fingerprint similarity and content determination results show that the chemical components of different parts of the chicory are quite different. The Chinese Pharmacopoeia only the above-ground parts and roots as medicinal parts, however, there are very differences in the chemical composition of the stems, leaves, and roots of the aerial parts. The stems and leaves of chicory are rich in phenolic acids and flavonoids, cichoric acid content is the most. Studies have shown that cichoric acid has the effects of lowering uric acid, anti-oxidation and scavenging free radicals, antibacterial, anti-viral, anti-inflammatory, and lowering blood sugar (Wang et al., 2020, Wang et al., 2020, Xiao et al., 2018). The content of chlorogenic acid is the highest among all components contained in roots. Studies have shown that chlorogenic acid has various pharmacological effects such as lowering uric acid, antioxidant, antibacterial, antiviral, antitumor, hypolipidemic, and immune regulation (Wang et al., 2020). It shows that the chemical composition of stems, leaves and roots may cause differences in pharmacological effects, and they cannot be mixed in clinically. The results show that the content of total components in chicory leaves higher than stems. With chemical components as an indicator, the leaves have better quality, and the yield of chicory leaves is large, leaves can be collected in stages, so leaves can be used as the main medicinal part of chicory. Flowers contain many phenylpropanoids such as esculin, which has pharmacological effects such as lowering uric acid (Zhu et al., 2018), the seeds contain the least chemical ingredients. The flowers and seeds are not used for medicinal purposes, but the flowers contain a large amount of cyanidin and its derivatives, which have antioxidant capacity (Rapisarda et al., 2000). Studies have shown that the cyclohexane extract of seeds has a certain protective effect on experimental acute liver injury (Youliduzi, Nuermaimaiti, & Erbiguli, 2010), it can be used in the development of special drugs in the future. Chicory not only has medicinal value but also edible value. Chicory leaves can also be made into chicory tea or eaten as healthy food. Chicory root contains a large amount of inulin that does not affect blood sugar levels. It can be used to produce high-purity fructose, low-fat foods, and food quality improver, etc. (Zeng et al., 2010), the flowers can be used to make scented tea, and the seeds mainly contain oil, amino acids, trace elements, etc. (Man, 2010, Xu et al., 2013), which can be used for oil extraction and essential oil extraction, as a new resource for development and utilization, so that the dual-use value of medicine and food can be fully developed.
5. Conclusion
The research and establishment of chicory fingerprint and multi-component content determination method can be used to distinguish and determine the components of different parts of chicory, reveal the similarity and difference of different parts of chicory from the chemical composition, establish the quality evaluation of different parts of chicory. Further, the exploration of the differences in the efficacy of different parts of chicory makes full use of chicory resources and provide data support and method reference.
Editor Note
Yanze Liu is Editorial Board Members of Chinese Herbal Medicines. He was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal's standard procedures, with peer review handled independently of this Editorial Board Member and their research groups.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully acknowledge the financial support of the industry special project for the public welfare of the State Administration of Traditional Chinese Medicine of China (00104296).
Contributor Information
Zhenling Zhang, Email: zhangzl6758@163.com.
Yanze Liu, Email: yanze_liu@163.com.
References
- Abudureyimu K. Chinese herbal medicine Uyghur medicine roll. Shanghai Science & Technology Press; Shanghai: 2005. Xinjiang Institute of Traditional Uighur Medicine; Editorial Board Member of “Chinese Materia Medica” of National Administration of Traditional Chinese Medicine. [Google Scholar]
- Editorial Board of Chinese Medical Encyclopedia, Yishakejiang M. Shanghai Science & Technology Press; Shanghai: 2005. Encyclopedia of Chinese Medicine Uyghur Medicine. [Google Scholar]
- Fan H., Chen J., Liang C.Y., Ren B.R., Li W.L. Advance in studies on chemical constituents of Cichorii Herba and their pharmacological effects. Chinese Traditional and Herbal Drugs. 2016;47(4):680–688. [Google Scholar]
- Gao H.J., He H.J., Wang W.Q., Tang X.W., Song S.H. Preliminary study of an thocyanins present from the leaves of Cichoricum intybus var. foliosum Hegi. Science and Technology of Food Industry. 2010;31(1):141–143. [Google Scholar]
- Han C., Su L.J., Liu X., Dang T., Qin D.M., Zhang Y.S. Study on antibacterial and antioxidant effects of different polar solvent extracts from chicory stem. Lishizhen Medicine and Materia Medica Research. 2019;30(8):1825–1828. [Google Scholar]
- Li Q., Du S.M., Zhang Z.L., Lv C.M., Zhou Y.Q., Zhao Y., et al. Progress in fingerprint technology on Chinese materia medica and prospect of its future development. Chinese Traditional and Herbal Drugs. 2013;44(22):3095–3104. [Google Scholar]
- Liu D.F., Zhao L.N., Li Y.F., Jin C.D. Research progress and application in fingerprint technology on Chinese materia medica. Chinese Traditional and Herbal Drugs. 2016;47(22):4085–4094. [Google Scholar]
- Man, Q. L. (2010). Preliminary studies on the chemical composition of Cichorium glundulosum Boiss. et Hout. seeds in Xinjiang. Xinjiang University.
- Rapisarda P., Fanella F., Maccarone E. Reliability of analytical methods for determining anthocyanins in blood orange juices. Journal of Agricultural and Food Chemistry. 2000;48:2249–2252. doi: 10.1021/jf991157h. [DOI] [PubMed] [Google Scholar]
- Wang L.X. Hepatoprotective effect of chicory root extract. Drugs & Clinic. 2008;2:81. [Google Scholar]
- Wang Q.H., Du T.T., Zhang Z.H., Ji M., Hu H.Y., Chen X.G. Advances in research on the pharmacological effects and mechanism of action of chlorogenic acid. Acta Pharmaceutica Sinica. 2020;55(10):2273–2280. [Google Scholar]
- Wang Y.Z., Gao S., Li L.J., Zhang L., Sun Y., Bo F.M. Research advances on bioactivity and pharmacological effects of chicoric acid. Chinese Journal of New Drugs. 2020;29(15):1729–1733. [Google Scholar]
- Wu H.K., Fan Y.P., Ba H., Liao L.X., Haji A.A. Analysis of essential oil from seeds of Cichorium gladulosum Boiss et Hout by GS-MS. Chinese Journal of Spectroscopy Laborator. 2005;4:694–696. [Google Scholar]
- Xiao H.F., Yang S.Q., Wang X.G., Wang J., Song Y.D. Effect of chicoric acid on oxidative protein damage. Food Science. 2018;39(7):119–124. [Google Scholar]
- Xu Y., Li H.T., Yin X., Alifu A.B.D., Qing M.R. Determination of trace elements in Xinjiang Cichorium Intybus L. by ICP-AES. Chinese Journal of Spectroscopy Laborator. 2013;30(3):1305–1307. [Google Scholar]
- Youliduzi M.M.T., Nuermaimaiti A.M.T., Erbiguli Y.S.L.M. Study on the mechanism of chicory seed extract in protecting the liver and lowering enzymes. Journal of Medicine & Pharmacy of Chinese Minorities. 2010;16(3):39–40. [Google Scholar]
- Zeng X.Y., Luo D.L., Liu S.N., Liu J.X. Recent advance and future trend of inulin research and development. China Food Additives. 2010;4:222–227. [Google Scholar]
- Zhang Z.S., Lu Y.L., Gao Y.F., Zhang Y.X., Wang X.Y., Zhang L. Research progress of bitter substances in Cichorium intybus L. China Food Additives. 2015;5:174–178. [Google Scholar]
- Zhu C.S., Zhang B., Lin Z.J., Bai Y.F. Uric acid-lowering active ingredients and mechanism of Cichorium intybus. Chinese Traditional and Herbal Drugs. 2017;48(05):957–961. [Google Scholar]
- Zhu C.S., Zhang B., Lin Z.J., Bai Y.F. Pharmacodynamics authentication research on uric acid-lowering active ingredients of Cichorium intybus L. China Journal of Traditional Chinese Medicine and Pharmacy. 2018;33(11):4933–4936. [Google Scholar]