Abstract
Durio zibethnus is mainly distributed in Southeast Asia. Traditional Chinese medicine believes that durian shells have the effects of clearing heat and purging fire, nourishing yin and moisturizing dryness. Therefore, it is often used as a pharmaceutic food in the Chinese folk to assist treating diseases. At present, the chemical constituents isolated from durian shell include phenolic acids, phenolic glycosides, flavonoids, coumarins, triterpenes, simple glycosides and other compounds. Modern pharmacological studies show that durian shell has many pharmacological activities, such as antioxidant, anti-inflammatory, regulation of glucose and lipid metabolism. The chemical composition and pharmacological effects of durian shells are summarized in order to provide references for the further research and application of durian shell.
Keywords: agricultural residues, chemical constituents, durian shell, Durio zibethnus Murr., pharmacological effects
1. Introduction
Durian, also named Shexiangmaoguo, is the fruit of Durio zibethnus Murr. (Bombacaceae). It is one of the most important tropical fruits in Southeast Asia and adjacent islands. It originated in the Malay Peninsula and now is mainly distributed in Thailand, Malaysia, Indonesia, Cambodia, Laos, Myanmar, Sri Lanka, the Philippines and Singapore (Wang, 2020). It is also planted in Guangdong, Guangxi, Hainan, and Taiwan in China. Durian is one of the most popular tropical fruits and an important economic fruit crop in Southeast Asian countries. China is the main country in durian consumption. In 2019, the import value of durian from Thailand was USD 1.6 billion (Han, 2020). Durian shells are often regarded as agricultural residues which refer to the non-main economic target product part of crops grown by farmers, and in traditional production activities (Deng, 2018), and this part is mainly not used for medicine or other valuable commodities. The medicinal research of agricultural residues can ensure the sustainable development of traditional Chinese medicine resources, which is conducive to protecting the environment and promoting the development of circular economy. Durian shells account for more than 50% of the quality of durians, but they are commonly used agricultural residues because of their economic added value. Through searching literatures, we found that durian has traditional medicinal value in Southeast Asia. The current research on durian mainly focuses on the processing and preservation of durian pulp (Yan et al., 2021), the identification of durian varieties (Zhou et al., 2021, Mao et al., 2020), and the study of durian shell energy (Shen et al., 2019) and materials (Zhao, Lyu, Lee, Cui, & Chen, 2019). The research and development of medicinal products of durian shells is relatively few, resulting in a waste of medicinal resources. Therefore, this paper mainly summarized the chemical components and pharmacological effects of durian shells to provide a basis for the products development of durian in the future.
2. Traditional application
Thailand, Malaysia and Indonesia are the main durian producing countries. There are 15 registered varieties in Malaysia and seven commercial varieties in Thailand (Husin, Rahman, Karunakaran, & Bhore, 2018). Similar varieties have different names in Malaysia and Thailand (Aziz & Jalil, 2019). In 2019, the durian planting area in Malaysia was 72,536 hm2, and the output reached 384,170 tons (He, 2020). In 2020, the durian output were 1.2 million tons and 110 000 tons in Indonesia and Thailand, respectively (He, 2021). Durian is not only used as fresh fruit and processed food, but also as a traditional folk medicine in Southeast Asia. Southeast Asian folk believe that the fruit is an aphrodisiac and abortion drug, which can improve menstruation (Lim, 2012) and treat infertility (Reshma, 2016). In Malaysia and China, the decoction of the leaves and roots is used for antipyretic, expectorant and cold (Ho, et al., 2015); And the peel ash can be used to treat infant fever (Siriphanich, 2011). In Malaysia, the leaf juice is applied locally to the head of fever patients. The traditional prescriptions include: Mix boiling the leaves of Hibiscus rosasinensis, Nephelium longan, Durio zibethinus, Nephelium mutabile and Artocarpus integrifolia to make poultice, or boiled decoctions of the roots of these species for fever patients. Or the local people use the leaves of Curculigo latifolia, Gleichenia linearis, Nephelium lappaceum and Durio zibethinus to boil water to wash the patients’ hair for a several days (Lim, 2012). They also use the leaves to boil water for medicated bath to treat jaundice. The decoction of the leaves and fruits can reduce swelling and skin diseases. In Java, durian shells are used externally to treat skin diseases (Sah, Pathak, Sankar, & Suresh, 2014); Epidermal swelling is treated with leaf and Acorus sp. (Mariod, Mirghani, & Hussein, 2017). The pulp can be used for anti-helminthic. The burnt peel ash is taken post-childbirth (Sah, Pathak, Sankar, & Suresh, 2014), and it can improve sexual function. In India some tribal sects believe that the decoction of seeds can enhance male sexual function and has an aphrodisiac effect (Ho & Bhat, 2015); Durian is used to the proven fertility enhancing activity in Nilgiris and India (Sah, Pathak, Sankar, & Suresh, 2014). The sap of the bark can be used to treat malaria. Rubbing the abdomen with the valves of durian can ameliorate constipation (Michael, 1997). Malaysia has a traditional fermented condiment “Tempoyak”, which is made from durian pulp as the main raw material (Leisner et al., 2001).
In traditional Chinese medicine (TCM), durian flesh is hot and sweet, and displays good effects of invigorating the spleen and tonifying qi, invigorating the kidney and strengthening yang, warming meridian and promoting blood circulation, dispersing cold and relieving pain, promoting diuresis for removing jaundice. Patients with hot constitution and yin deficiency constitution should take it with caution. People with diabetes, kidney disease, heart disease and high cholesterol are not allowed to eat durian (Qin & Zhang, 2017). Durian shells are warm in nature, pungent and sweet in taste; enter the lung, liver and kidney meridians (Jia, He, Liu, & Jia, 2020), have functions and indications of clearing heat and purging fire, clearing heat and purging fire, nourishing yin and moisturizing dryness, topical treatment for skin pruritus. Durian core has the effects of invigorating the kidney and invigorating the spleen (Cheng & Zou, 2014). In China, durian is often used as raw material to make medicated diet for adjuvant treatment of diseases. The roots of durian are used to treat fulminant dysentery, “cold air in the heart and abdomen”, the sap and leaves of the tree have the effects of detoxification and detumescence, and the leaves can cure jaundice. The dry powder of leaves and bark can be applied to the wound to stop bleeding. It is an effective first-aid hemostatic (Nan, 2014). Durian shells and light saline water boiled together to treat “Shang Huo”, and dry durian shells to boil in decoction to cure kidney deficiency (“Family Bookshelf” editorial board, 2007).
3. Chemical compositions
Durian shells contain a variety of chemical components, mainly including phenolic acids and phenolic glycosides, flavonoids, coumarins, triterpenes, simple glycosides, cellulose, pigments, etc. In addition, the volatile oil components are mainly composed of esters and acid compounds.
3.1. Phenolic acids and phenolic glycosides
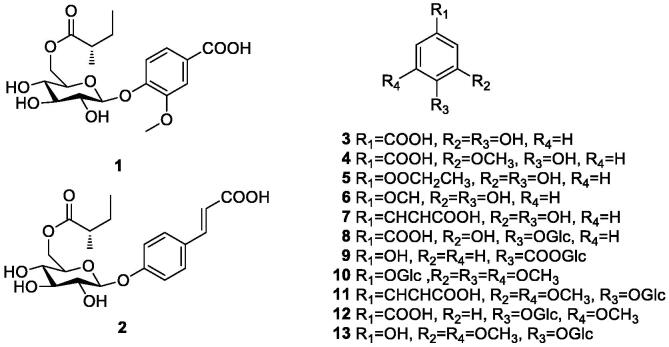
Feng et al. isolated nine phenolic acids and phenolic glycosides (1–6, 9–10, 13) from durian shells. Liu (2020) used UPLC-LTQ-Orbitrap-MS/MS to identify the main biologically active components of durian white sacs and shells (Feng, 2017, Feng et al., 2018, Feng et al., 2016). The extraction rates of polyphenols were 3.77 mg/g and 1.86 mg/g from durian white sac and the outer shell, respectively; And it was found that the durian white sac contained total polyphenols (135.52 ± 4.25) mg CAE/g·DW and the outer shell contained (101.06 ± 3.36) mg CAE/g·DW (Liu, Zhan, Xiong, Zhu, & Sun, 2020). At present, five phenolic acids (3–7) and eight phenolic glycosides (1–2, 8–13) were extracted from durian shells. The compounds are shown in Table 1 and Fig. 1.
Table 1.
Phenolic acids and phenolic glycosides in durian shells.
| No. | Compounds | References | No. | Compounds | References |
|---|---|---|---|---|---|
| 1 | 3-methoxy-4-O-β-D-[6-(S)-2-methylbutanoylglucopyranosl] benzoic acid | (Feng, 2017, Feng et al., 2016) | 8 | protocatechuic acid-O-hexoside | (Liu, 2020) |
| 2 | 4-O-β-D-[6-(S)-2-methylbutanoyl]glucopyranosyl cinnamic acid | (Feng, 2017, Feng et al., 2016) | 9 | 1-O-(4-hydroxybenzoyl)-β-D-glucopyranose | (Feng, 2017, Feng et al., 2018) |
| 3 | 3,4-dihydroxybenzoic acid | (Feng, 2017, Feng et al., 2016) | 10 | 3,4,5-trimethoxyphenyl-1-O-β-D-glucopyranoside | (Feng, 2017, Feng et al., 2018) |
| 4 | 4-hydroxy-3-methoxybenzoic acid | (Feng, 2017, Feng et al., 2016) | 11 | sinapic acid hexoside | (Liu, 2020) |
| 5 | ethyl protocatechuate | (Feng, 2017, Feng et al., 2016) | 12 | vanillic acide-O-hexoside | (Liu, 2020) |
| 6 | 3,4-dihydroxybenzaldehyde | (Feng, 2017, Feng et al., 2016) | 13 | leonuriside A | (Feng, 2017, Feng et al., 2018) |
| 7 | caffeic acid | (Liu, 2020) |
Fig. 1.
Chemical structures of phenolic acids and phenolic glycosides in durian shells.
3.2. Flavonoids
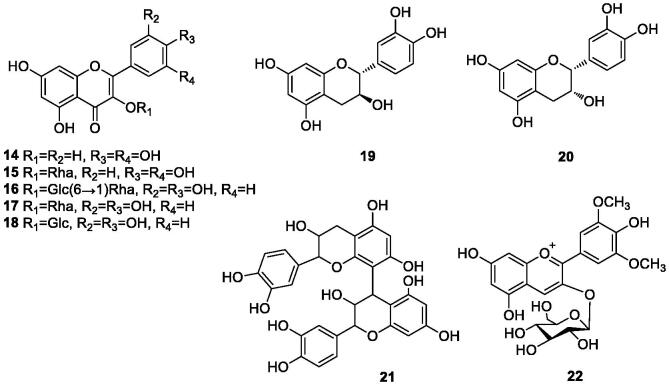
Masturi et al. (2020) extracted total flavonoids from three local durian shell in Indonesia, and the yields of total flavonoids were (0.405 ± 0.002) mg QE/g, (0.321 ± 0.003) mg QE/g and (0.324 ± 0.002) mg QE/g in Monti, Malik and Malon, respectively. Studies showed that the contents of total flavonoids were (56.79 ± 0.73) mg CAE/g·DW and (12.91 ± 0.03) mg CAE/g·DW from durian white sac and durian shells, respectively (Liu et al., 2020). A total of nine flavonoids were found (14–22). The flavonoids in durian shells are shown in Table 2 and Fig. 2.
Table 2.
Flavonoids in durian shells.
| No. | Compounds | References | No. | Compounds | References |
|---|---|---|---|---|---|
| 14 | quercetin | (Liu, 2020) | 19 | catechin | (Liu, 2020) |
| 15 | quercitrin | (Liu, 2020) | 20 | epicatechin | (Liu, 2020) |
| 16 | rutin | (Liu, 2020) | 21 | procyanidin B | (Liu, 2020) |
| 17 | quercetin-3-O-rhamnoside | (Liu, 2020) | 22 | malvidin-3-O-glucoside | (Liu, 2020) |
| 18 | quercetin-3-O-glucoside | (Liu, 2020) |
Fig. 2.
Chemical structures of flavonoids in durian shells.
3.3. Coumarin compounds
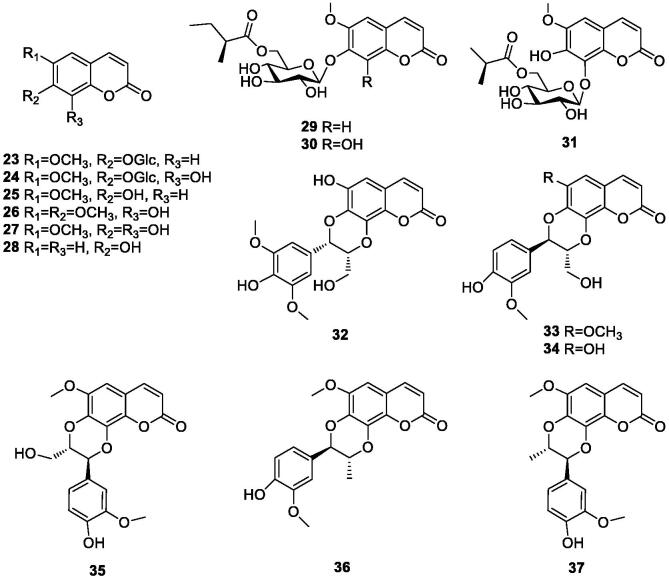
Coumarin has a wide range of pharmacological effects. At present, 15 coumarin compounds (23–37) have been isolated from durian shells. The specific compounds are shown in Table 3 and Fig. 3.
Table 3.
Coumarin compounds in durian shells.
| No. | Compounds | References | No. | Compounds | References |
|---|---|---|---|---|---|
| 23 | 7-O-β-D-glucosyl-6-methoxy coumarin | (Feng, 2017, Feng et al., 2018) | 31 | 7-hydroxyl-6-methoxy-8-O-β-D-[6-(S)-2-methylbutanoyl-glucopyranosyl]-coumarin | (Feng, 2017, Feng et al., 2018) |
| 24 | 8-hydroxy-7-O-β-D-glucosyl-6-methoxy coumarin | (Feng, 2017, Feng et al., 2018) | 32 | 5′-methoxy-7′-epi-jatrorin A | (Feng, et al., 2016) |
| 25 | scopoletin | (Feng, 2017, Feng et al., 2016) | 33 | cleomiscosin A | (Feng, 2017, Feng et al., 2016) |
| 26 | fraxetin | (Feng, 2017, Feng et al., 2016) | 34 | jatrocin A | (Feng, 2017, Feng et al., 2016) |
| 27 | fraxidin | (Feng, 2017, Feng et al., 2016) | 35 | cleomiscosin B | (Feng, 2017, Feng et al., 2016) |
| 28 | 7- hydroxycoumarin | (Feng, 2017, Feng et al., 2016) | 36 | propacin | (Feng, 2017, Feng et al., 2016) |
| 29 | 6-methoxy-7-O-β-D-[6-(S)-2-methylbutanoyl-glucopyranosyl]-coumarin | (Feng, 2017, Feng et al., 2016) | 37 | propacin isomer | (Feng, 2017, Feng et al., 2016) |
| 30 | 8-hydroxyl-6-methoxy-7-O-β-D-[6 -(S)-2-methylbutanoyl-glucopyranosyl]-coumarin | (Feng, 2017, Feng et al., 2018) |
Fig. 3.
Chemical structures of coumarin compounds in durian shells.
3.4. Triterpenoids
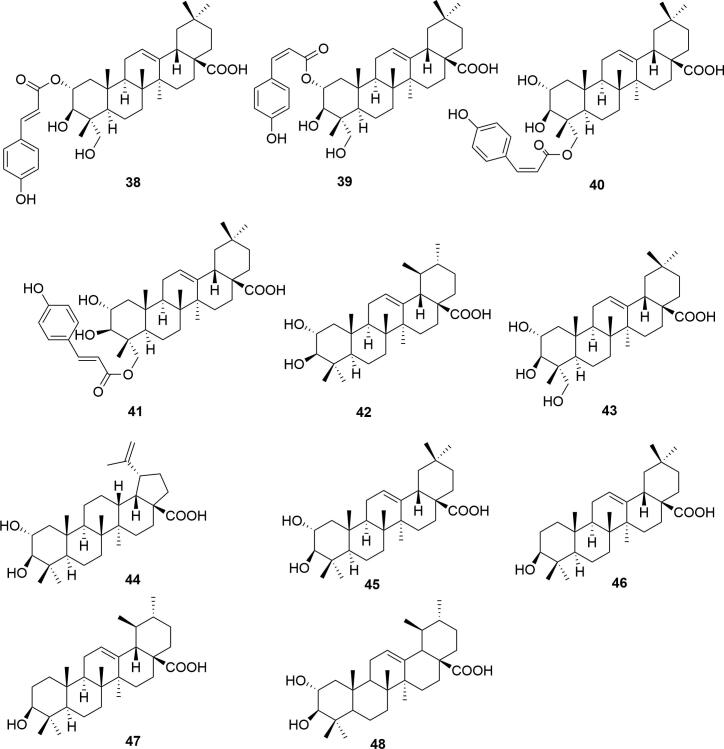
At present, 11 triterpenoids (38–48) have been isolated from durian shells. The specific compounds are shown in Table 4 and Fig. 4.
Table 4.
Triterpenoids in durian shells.
| No. | Compounds | References | No. | Compounds | References |
|---|---|---|---|---|---|
| 38 | 2α-trans-p-coumaroyloxy-2α,3β,23α-trihydroxy-olean-12-en-28-oic acid | (Feng, 2017, Feng et al., 2018) | 44 | alphitolic acid | (Feng, 2017, Feng et al., 2018) |
| 39 | 2α-cis-p-coumaroyloxy-2α,3β,23α-trihydroxy-olean-12-en-28-oic acid | (Feng, 2017, Feng et al., 2018) | 45 | maslinic acid | (Feng, 2017, Feng et al., 2018) |
| 40 | 23α-trans-p-coumaroyloxy-2α,3β,23α-trihydroxy-olean-12-en-28-oic acid | (Feng, 2017, Feng et al., 2018) | 46 | oleanolic acid | (Feng, 2017, Feng et al., 2018) |
| 41 | 23α-cis-p-coumaroyloxy-2α,3β,23α-trihydroxy-olean-12-en-28-oic acid | (Feng, 2017, Feng et al., 2018) | 47 | ursolic acid | (Feng, 2017, Feng et al., 2018) |
| 42 | 2α,3β-di-hydroxyl-urs-12-en-28-oic acid | (Feng, 2017) | 48 | 2a-hydroxyursolic acid | (Feng, et al., 2018) |
| 43 | arjunolic acid | (Feng, 2017, Feng et al., 2018) |
Fig. 4.
Chemical structures of triterpenoids in durian shells.
3.5. Simple glycosides
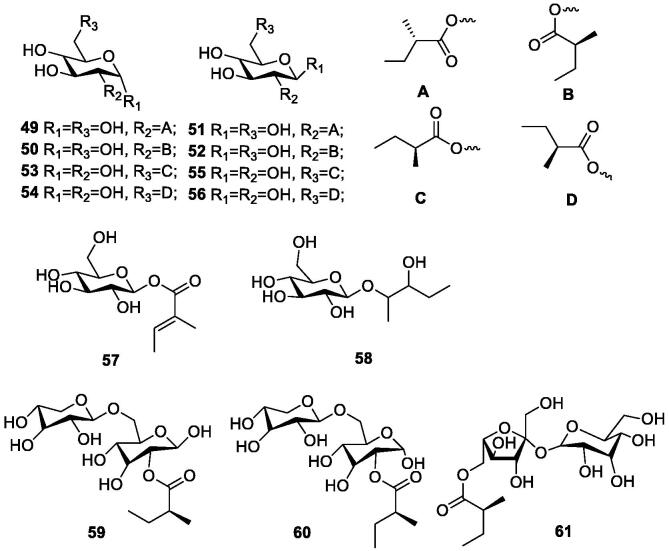
Song (2019) processed durian skin with water extraction and then alcohol precipitation method, deproteinization, depigmentation, and small molecular substances to obtain 42.28 g of durian skin crude polysaccharides, with an extraction rate of 1.41%. After the raw sugar was purified, it was found that the total sugar content of durian skin polysaccharide components DZM-A and DZM-B increased significantly, especially the total sugar content of DZM-A reached 93.65%, and its structure was a pectin polysaccharide. Hokputsa et al. (2004) used durian peels as the raw material to separate water-soluble crude polysaccharides (PG) with medicinal value by hot water extraction-ethanol precipitation method, and after separation and purification, the main component was pectin polysaccharide. And it was found that the crude polysaccharide extracted from durian shells has higher viscosity, which can be used as an alternative viscosity enhancer and has medicinal value, but it cannot be purified for too long, otherwise the intrinsic viscosity will be reduced. The simple glycosides (49–61) extracted from durian shells are shown in Table 5 and Fig. 5.
Table 5.
Simple glycosides in durian shells.
| No. | Compounds | References | No. | Compounds | References |
|---|---|---|---|---|---|
| 49 | 2-O-[(S)-2-methylbutanoyl]-α-glucose | (Feng, 2017) | 56 | 6-O-[(S)-2-methyl butanoyl]-β-D-glucose | (Feng, et al., 2018) |
| 50 | 2-O-[(S)-2-methyl butanoyl]-α-D-glucose | (Feng, et al., 2018) | 57 | tiglic acid 1-O-β-D-glucopyranoside | (Feng, 2017, Feng et al., 2018) |
| 51 | 2-O-[(S)-2-methylbutanoyl]-β-glucose | (Feng, 2017) | 58 | 2-glucosyl-3-pentanediol | (Feng, 2017) |
| 52 | 2-O-[(S)-2-methyl butanoyl]-β-D-glucose | (Feng, et al., 2018) | 59 | 2-O-[(S)-2-methylbutanoyl]-α-primeverose | (Feng, 2017, Feng et al., 2018) |
| 53 | 6-O- [(S)-2-methylbutanoyl]-α-glucose | (Feng, 2017) | 60 | 2-O-[(S)-2-methylbutanoyl]-β-primeverose | (Feng, 2017, Feng et al., 2018) |
| 54 | 6-O-[(S)-2-methyl butanoyl]-α-D-glucose | (Feng, et al., 2018) | 61 | 6-O-[(S)-2-methylbutanoyl]- sucrose | (Feng, 2017, Feng et al., 2018) |
| 55 | 6-O-[(S)-2-methylbutanoyl]-β-glucose | (Feng, 2017) |
Fig. 5.
Chemical structures of simple glycosides in durian shells.
3.6. Volatile components
Zhang et al. (2003) used steam distillation to extract the volatile chemical components of fresh durian shells and analyzed them by capillary gas chromatography-mass spectrometry. It was found that the isolated compounds were mainly alkanes, alcohols, aromatic and aromatic heterocyclic compounds and esters, which accounted for 95.97% of the total oil. Liu, Zhou, Xie, Zhu, and Huang (1999) used GC–MS to analyze durian shells and found that the inner and outer peels of durian contained saturated fatty acids. Among them, hexadecanoic acid has the highest content, 41.12% in the mesocarp and 25.41% in the exocarp; octadecanoic acid was 3.27% in the mesocarp, 3.20% in the exocarp; 0.50% arachic acid in the mesocarp, and 1.54% behenic acid in the exocarp. The content of unsaturated fatty acids in the mesocarp and exocarp was 51% and 56.37%. Among them, octadecanoic acid accounted for 37.07% in the mesocarp and 37.09% in the exocarp. 9,12-octadecadienoic acid (E, E) accounted for 8.03% in the mesocarp and 16.28% in the exocarp.
The chemical components of durian shells were extracted by simultaneous distillation extraction method, and analyzed by GC/MS: Fourteen main compounds were identified in the durian exocarp, and their content accounted for 97% of the total volatile oil content. The main compounds were ester compounds (59.71%) and acid compounds (26.56%). A total of 49 main compounds were identified in durian endocarp, accounted for 93.94% of the total volatile oil content, mainly ester compounds (53.73%) and acid compounds (26%) (Zhang, Li, Xin, Li, & Wu, 2012). The main chemical components (62–88) in the volatile oil are shown in Table 6.
Table 6.
Main components of volatile oil in durian shells.
| No. | Compounds | parts | References | No. | Compounds | parts | References |
|---|---|---|---|---|---|---|---|
| 62 | 13-octadecenoic acid methyl ester | outer/inner | (Zhang, et al., 2012) | 76 | methyl tetradecanoate | inner | (Zhang, et al., 2012) |
| 63 | methyl palmitate | outer/inner | (Zhang, et al., 2012) | 77 | 2-methyl butyric acid | inner | (Zhang, et al., 2012) |
| 64 | methyl stearate | inner | (Zhang, et al., 2012) | 78 | pentadecanoic acid | inner | (Zhang, et al., 2012) |
| 65 | methyl linoleate | inner | (Zhang, et al., 2012) | 79 | Z-11-hexadecenoic acid | inner | (Zhang, et al., 2012) |
| 66 | ethyl 2-hydroxy-3-methylbutyrate | outer | (Zhang, et al., 2012) | 80 | octadecenoic acid | outer | (Zhang, et al., 2012) |
| 67 | ethyl 2-methylbutyrate | outer | (Zhang, et al., 2012) | 81 | palmitic acid | outer/inner | (Zhang, et al., 2012) |
| 68 | ethyl palmitate | outer | (Zhang, et al., 2012) | 82 | palmitic aldehyde | outer | (Zhang, et al., 2012) |
| 69 | ethyl laurate | outer | (Zhang, et al., 2012) | 83 | phenylacetaldehyde | outer | (Zhang, et al., 2012) |
| 70 | ethyl linoleate | outer | (Zhang, et al., 2012) | 84 | 3-hydroxy-2-butanone | inner | (Zhang, et al., 2012) |
| 71 | ethyl oleate | outer/inner | (Zhang, et al., 2012) | 85 | butyl alcohol | inner | (Zhang, et al., 2012) |
| 72 | (2-methyl) dipropyl phthalate | inner | (Zhang, et al., 2012) | 86 | 2-methyl-1-butanol | inner | (Zhang, et al., 2012) |
| 73 | dibutyl phthalate | inner | (Zhang, et al., 2012) | 87 | (Z)-1-(1-ethoxyethoxy)-3-hexene | inner | (Zhang, et al., 2012) |
| 74 | butyl phthalate | inner | (Zhang, et al., 2012) | 88 | diethyl disulfide | outer | (Zhang, et al., 2012) |
| 75 | isobutyl butyrate | outer | (Zhang, et al., 2012) |
Note: The outer part refers to the outer shell of the durian shells, and the inner part refers to the white sac of the durian shells.
3.7. Cellulose
Li (2012) found that Lactobacillus bulgaricus and Streptococcus thermophilus were used as strains to ferment durian shells to produce dietary fiber. Its dietary fiber output is up to 29.50%.
Wanlapa et al., (2015) reported that in the dietary fiber of durian shells, the total dietary fiber (TDF) was (79.18 ± 3.46) g/100 g dry matter, insoluble dietary fiber (IDF) was (65.13 ± 2.21) g/100 g dry matter, and soluble dietary fiber (SDF) has (13.05 ± 1.21) g/100 g dry matter, insoluble pectin has (4.11 ± 0.08) g/100 g dry matter, hemicellulose has (18.51 ± 1.16) g/100 g dry matter, cellulose has (38.05 ± 1.35) g/100 g dry matter, Lignin has (2.36 ± 0.24) g/100 g dry matter, water retention was (11.41 ± 0.27), and oil retention was (3.20 ± 0.30). It was found that durian shells showed the greatest water and oil retention capacity, as well as high TDF and SDF content. In the oral acute toxicity test, all rats behaved normally, and the lethal dose was greater than 2000 mg/kg body weight, indicating that the dietary fiber sample was safe to eat and can be used as a low-calorie functional component to enrich dietary fiber.
3.8. Pigments
Zhang, Huang, Huang, and Chen (2012) extracted the pigment from the upper yellow skin of durian shells, and found that the pigment was bright yellow, bright in color and good stability. Temperature, redox agents, metal ions and various commonly used food additives have little effect on the stability of the pigment. Among them, citric acid has the effect of increasing the color. Among the metal ions, Fe3+ and Cu2+ have a greater impact on the pigment, and has a certain decolorization effect. Color effect, sunlight has a greater impact on pigments, so direct sunlight should be avoided during storage; When the pH is close to 12, the pigments will change color significantly. Researchers (Li, Li, Gu, Wang, & Liu, 2016) analyzed curcumin, demethoxycurcumin, and bisdemethoxycurcumin in durian shell extract by high performance liquid chromatography, and found that the content of curcumin was higher.
3.9. Pectin
Wang et al. (2012) used durian shell white sac as raw material, and the yield of pectin extracted by traditional acid method was 14.97%, and the yield of ultrasonic extraction method reached 19.68%. After comparison, it was found that the extraction effect of the ultrasonic-assisted extraction method was better than that of the traditional acid extraction method. With microwave-assisted method of acid hydrolysis to obtain slightly yellow pectin, and the extraction rate can reach 14.35% (Li, Zhou, Zhang, Chen, & Liu, 2012). Pectin was extracted by acid extraction and alcohol precipitation method. The pectin yield of durian skin was (2.59 ± 0.17)%, and the mass fraction of pectin acid was 84.12%. The pigment had no obvious interference with pectin, and the color was gray to white, with a weight average molecular weight of 622/kDa, which belongs to high methoxy pectin. It has high hardness (412.014 ± 10.27)/g and high chewiness (192.874 ± 5.394)/g. It was a good raw material for making candies (Gao et al., 2012).
Zhang et al., (2017) used acid extraction and alcohol precipitation to extract pectin from the white sac of durian shells. The adsorption experiment of pectin showed that when water: methanol was 1:1, pectin has the highest adsorption rate for benzoic acid (12%) and cinnamic acid (21%); When water: formaldehyde was 5:1, terephthalic acid (18%) and acetanilide (11%) has the highest adsorption rate.
3.10. Other compounds
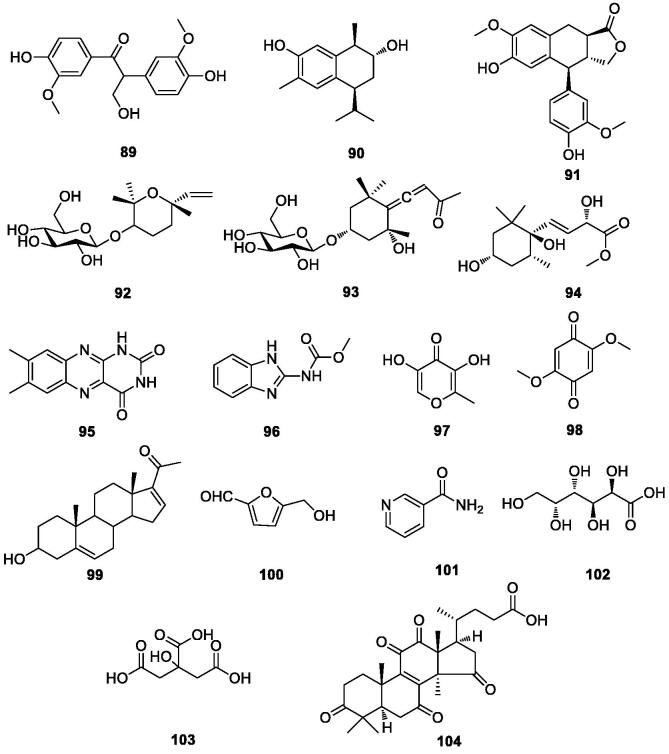
Approximate analysis of three local varieties of durian shells in Indonesia (Masturi, et al., 2020), showed that the overall moisture content of the durian shells was 7%, the fat content was 0.9%, the protein content was 4.9%, the ash content was 8.5%, and the carbohydrate content was 78%, and there was no significant difference in the analysis results of the three durian peel samples. The nutrient content of durian white sac was higher than the shells, and the content of crude ash, crude fiber and phosphorus was slightly lower than that of the shells. The whole durian skin contains 7.79% crude protein, 1.58% crude fat, 37.62% crude fiber, and 0.59% phosphorus; In mineral elements the highest content was magnesium and iron; The total amount of amino acids was 3.95%, which accounted for 50.71% of the crude protein, the essential amino acids accounted for 39.24% of the total amino acids, and the flavor amino acids (umami amino acids and sweet amino acids) accounted for 49.87% of the total amino acids (Zhang, et al., 2015). Other chemical components (89–104) found in durian shells are shown in Table 7 and Fig. 6.
Table 7.
Other compounds in durian shells.
| No. | Compounds | References | No. | Compounds | References |
|---|---|---|---|---|---|
| 89 | evofolin-B | (Feng, 2017, Feng et al., 2016) | 97 | 5-oxymaltol | (Feng, 2017) |
| 90 | 2-hydroxy-8α-hydroxycalamenene | (Feng, 2017, Feng et al., 2016) | 98 | 2,5-dimethoxy-1,4-benzoquinone | (Feng, 2017) |
| 91 | α-conidendrin | (Feng, 2017, Feng et al., 2016) | 99 | 16-dehydropregnenolone | (Feng, 2017) |
| 92 | (3S,6S)-cis-linalool-3,7-oxide-β-D-glucopyranoside | (Feng, 2017, Feng et al., 2018) | 100 | 5-hydroxymethylfurfural | (Feng, 2017) |
| 93 | icariside B1 | (Feng, 2017, Feng et al., 2018) | 101 | nicotinamide | (Feng, 2017) |
| 94 | (8E,9R)-methyl-(3S,5R,6S)-3,6-dihydroxy-1,1,5-trimethylcyclohex-yl-9-hydroxybut-8-enoate | (Feng, 2017) | 102 | galactonic acid | (Feng et al., 2016) |
| 95 | 7,8-dimethylbenzo[g]pteridine-2,4(1H,3H)-dione | (Feng, 2017) | 103 | citric acid | (Liu, 2020) |
| 96 | methyl(1H-benzo[d]imidazol-2-yl) carbamate | (Feng, 2017) | 104 | lucidenic acid D1 | (Feng, et al., 2016) |
Fig. 6.
Chemical structures of other compounds in durian shells.
4. Pharmacological effects
4.1. Antioxidant effect
Durian shells have antioxidant effect, which may be related to the flavonoids and phenolic compounds contained in durian shells. Hong, Du, and Hu (2014a) found that the flavonoids of durian shells (0.2–1.0 mg/mL) have strong scavenging ability to hydroxyl (OH) free radicals and ABTS free radicals. Feng (2017) found that the compounds in durian shells have strong ability to scavenge DPPH free radicals, and glycosides and triterpenoids (Feng et al., 2018) have strong inhibitory effects on nitric oxide (NO) produced by mouse RAW264.7 cells induced by LPS; Some phenolic compounds also have the ability to scavenge superoxide anion (O2–) free radicals, and their ability to inhibit NO was stronger than indomethacin (Feng et al., 2016). Evary et al., (2019) found that the total phenol content in the ethanol extract of durian shell white sac was significantly correlated with DPPH free radical scavenging ability and α-glucosidase inhibitory ability.
Liu (2020) found that durian shells white sac and durian shell extracts showed strong DPPH free radical and ABTS free radical scavenging ability and Fe3+ reduction ability; it can significantly inhibit the reactive oxygen species (ROS) in HepG2 cells induced by hydrogen peroxide (H2O2), malondialdehyde (MDA), super oxide dismutase (SOD), lactate dehydrogenase (LDH), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) can significantly reduce cell apoptosis caused by oxidative stress. Its mechanism of action is to inhibit cell apoptosis by regulating the expression of apoptosis-related genes in the mitochondrial pathway, which significantly increases the expression of the anti-apoptotic gene BCL-2, and inhibits the pro-apoptotic BAX, Caspase-3 and Caspase-9 genes and proteins expression. Durian shell has good antioxidant activity in HepG2 cells stimulated by H2O2, which can reduce cell damage and apoptosis caused by oxidative stress.
4.2. Anti-inflammatory effect
Wu (2010) found that the durian shell extract had a very significant inhibitory effect on the peritonitis in mice. It has a significant inhibitory effect on the swelling of the ears of mice caused by croton oil, the swelling of the feet of mice caused by carrageenan, and the chronic inflammation of the subcutaneous granuloma proliferation of mice caused by cotton balls. It has a certain inhibitory effect on allergic contact dermatitis in mice caused by 2,4-dinitrofluorobenzene (2,4-DNFB), and the dose–effect relationship was good. Xie et al., (2015) found that durian shell extracts (25 and 50 mg/L) can effectively inhibit the inflammatory factors tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β), NO and transcription factor nuclear factor-кB (NF-КB) content, increase the content of interleukin-10 (IL-10) and other anti-inflammatory factors, its effect may be related to the inhibition of NF-кB signaling pathway.
Huang (2020) found that the coumarin compound propacin in durian shells can significantly inhibit the release of NO and prostaglandin E2 (PGE2) induced by LPS in RAW264.7 cells. It can inhibit the release of NO, PGE2, ROS, IL-1β, IL-6 and TNF-α from macrophages induced by LPS, and inhibit the expression of nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). It was speculated that propacin inhibits the inflammatory response of macrophage RAW264.7 through mitogen-activated protein kinase (MAPK) and NF-κB signaling pathway. Propacin down-regulates the mRNA and protein expression of iNOS and PGE2, inhibits LPS-induced macrophages to produce NO, PGE2 and COX-2, reduces the overexpression of ROS induced by LPS, and maintains the integrity of mitochondria in active macrophages (Huang, Wang, Xu, Feng, & He, 2020). Its mechanism was to inhibit NF-κB p65 subunit enters the nucleus and phosphorylates MAPKs, especially JNK and ERK. Yang, Wang, Wang, He, and Xu (2021) found that propacin can inhibit LPS induced inflammation of RAW264.7 cells by enhancing autophagy.
Liu (2020) found that durian white flesh and shell extracts significantly inhibited the secretion of inflammatory mediators (NO) and the expression of inducible iNOS, COX-2 mRNA and protein in RAW264.7 cells induced by LPS; significantly reduce the inflammatory factors: TNF-α, IL-6, IL-1β secretion and mRNA expression; Significantly down-regulate the phosphorylation of inflammatory pathway MAPK, NF-κB related proteins (JNK, ERK, p38, IκBα, p65) And weaken the nuclear transfer effect of NF-κB p65. And it has good anti-inflammatory activity in RAW264.7 cells stimulated by LPS, and has an inhibitory effect on the phosphorylation of inflammatory signaling pathways (MAPK, NF-κB) related proteins (JNK, ERK, p38, IκBα, p65). Liu et al. (2020) found that durian shell extract was rich in polyphenolic compounds, which can reduce the expression of iNOS and COX-2, and reduce the production of NO and ROS in the LPS-induced RAW264.7 macrophage inflammation model. It also reduces the expression of inflammatory cytokines TNF-a, IL-6, and IL-1β at the gene and protein level, thus having strong anti-inflammatory activity. The underlying molecular mechanism may be through inhibiting IκBα and p65 protein phosphorylation, reducing the expression of NF-κB signaling pathway, and reducing the body's inflammatory damage.
4.3. Antibacterial effect
Pholdaeng and Pongsamart (2010) found that polysaccharide gel (PG) of durian-rinds can inhibit the growth of shrimp bacterial pathogen Vibrio harveyi 1526. The water extract of durian white sacs had a weak inhibitory effect on Staphylococcus aureus and Staphylococcus epidermidis, and a strong inhibitory effect on Pseudomonas aeruginosa (Wu, et al., 2010). The flavonoid extract of durian shell has obvious inhibitory effect on Staphylococcus aureus and Pseudomonas aeruginosa. But has no antibacterial effect on Escherichia coli and Bacillus megaterium at a concentration of 1.25–10 mg/mL (Hong et al., 2014a, Hong et al., 2014b, Lipipun et al., 2002). Lubis, Wirjosentono, Eddyanto, and Septevani (2020) found that the cellulose isolated from durian shells was grafted with 2-[acryloyloxy] ethyltrimethylammonium chloride (CIAETA) to obtain the product DRC-g-CIAETA for Staphylococcus aureus, Staphylococcus epidermidis and Candida albicans have good antibacterial and antifungal abilities. The ethanol extract of durian shell can inhibit Propionibacterium acnes (Fitrianingsih, Soyata, & Wigati, 2019) and Enterococcus faecalis (Rizky, Anastasia, & Merdekawati, 2020). Studies have found that durian shell has in vitro proliferation activity against A549 (human lung cancer cells), MCF-7 (human breast cancer cells), HepG2 (human hepatoma cells) and HT-29 (human colon cancer cells) (Li et al., 2013).
4.4. Regulation of glucose and lipid metabolism
Tippayakul, Pongsamart, and Suksomtip (2005) used semipermeable membrane dialysis technology to study the cellulose membrane and intestinal sacs of the rat jejunum in vitro. Using bile salts as surfactants, 0–2% W/V polysaccharide gel (PG) extracted from durian husks and lipids (cholesterol, oleic acid and stearic acid) are mixed in the dialysis membrane. HPLC analysis showed that as the concentration of PG increases, the amount of lipid retained in the membrane increases, and the amount of lipid released outside the membrane decreases. It showed that durian polysaccharide gel has the function of trapping lipids, and can be used as a dietary food for controlling blood lipids. In the experiment (Muhtadi, Primarianti & Sujono, 2015), it was found that the alcohol extract of durian shells can lower the blood sugar of diabetic model rats. The active ingredient with lowering blood sugar may be flavonoids. The mechanism of lowering blood sugar may be due to its astringent effect, which can precipitate intestinal mucosal proteins and form a protective film in the intestinal tract, thereby inhibiting glucose uptake enter; by speeding up the kidneys to filter and excrete glucose released in the circulation; Accelerate the release of glucose by accelerating metabolism or absorbing fat deposits. The alcohol extract of durian shells can significantly reduce the blood sugar level of alloxan-induced diabetic rats and the high-fat diet-induced hyperlipidemia cholesterol level (Muhtadi, Haryoto, Sujono, & Suhendi, 2016).
4.5. Anticoagulant effect
Song (2019) found that the two polysaccharides (DZM-A and DZM-D) isolated from durian shells could show potential anticoagulant effect in vitro through endogenous and exogenous coagulation pathway. DZM-A at doses of 150 mg/kg and 100 mg/kg can inhibit platelet aggregation and reduce whole blood viscosity and plasma viscosity by inhibiting endogenous and exogenous coagulation pathways.
4.6. Antitussive effect
Wu (2010) found that the durian shell extract has a better inhibitory effect on the incubation period and the number of coughs in mice caused by ammonia and SO2, and has a better dose-effect relationship. The results showed that durian shell extract had a good inhibitory effect on bronchial mucosal receptors caused by chemical irritants.
4.7. Analgesic effect
Wu (2010) found that durian shell extract can effectively prolong the incubation period of writhing response in mice caused by acetic acid, and can significantly prolong the pain threshold caused by the hot plate at 90 min. The results showed that durian shell can not only effectively inhibit the pain caused by chemical factors, but also improve the pain threshold of mice caused by hot plate to a certain extent. It has a certain effect on the pain caused by physical factors such as temperature, but its protective effect is weaker than that caused by acute chemical factors.
4.8. Anti-nitrosation
Nitrosamines (NDMA) is one of the chemical carcinogens at present. Some studies have proved that it can be synthesized in vitro. Therefore, blocking or removing nitrosamine – nitrite is one of the effective ways to prevent cancers. Chen, Chen, Liu, and Zhen (2005) found that the water extract of durian shells can block the synthesis of NDMA up to 82.15% and the nitrite removal rate can reach up to 82.62%. The effective active ingredients that block the synthesis of NDMA are mainly polar substances.
4.9. Liver protection
Xie et al. (2008) found that the alcohol extract of durian shells can significantly reduce the plasma ALT activity in restrained-loaded mice, effectively reduce the plasma MDA level and liver tissue NO content of restrained-loaded mice, and it also has an effect on the content of glutathione (GSH) in liver tissue with a certain degree of improvement. It indicated that the alcohol extract of durian shell has a certain protective effect on stress liver injury in mice induced by restraint load, and its mechanism may be related to scavenging free radicals and reducing the level of oxidative stress in restrained load mice.
4.10. Regulating immune function
Jiang (2020) found that polysaccharide of durian shells can regulate the immunity of immunosuppressive mice induced by cyclophosphamide, inhibit the content of SCFAs in mice, and improve metabolic pathways such as glucose metabolism, amino acid and lipid metabolism by reducing the relative abundance of Ruminococcus and Oscillospira, increasing the relative abundance of the beneficial bacteria Akkermansia, Bacteroides, Paraprevotella, improving the composition of the intestinal flora.
4.11. Laxative effect
Jiang et al. (2020) found that durian shell polysaccharides can significantly increase the intestinal peristalsis rate, motilin, gastrin, substance P levels and short-chain fatty acid concentrations in constipation model rats, reduce somatostatin levels and improve gastrointestinal motility. Compared with the model group, the Lachnospiraceae-NK4A136-group of durian shell polysaccharides was significantly higher, while Desulfovibrio was lower. It shows that durian shell polysaccharides have a certain therapeutic effect on functional constipation in rats and have a certain regulatory effect on intestinal flora.
4.12. Moisturizing effect
Futrakul et al. (2010) applied the polysaccharide gel prepared from durian shell to the facial skin of volunteers and found that the application had a significant effect on skin capacitance and firmness after 28 d and 56 d, and found that 56 d of treatment can significantly increase skin firmness without allergic reactions. It shows that polysaccharide gel of durian shells is a potential moisturizer for external use, and long-term application can improve skin firmness.
4.13. Toxic effects
Pongsamart et al. (2001) found that high-dose durian shell polysaccharides gel did not cause acute toxicity to mice and rats for 10 d. Durian shell polysaccharide gel, which was given to female and male mice for 0.25 and 0.5 g/kg/d for a long time, was not toxic and did not affect pregnancy and offspring (Pongsamart, et al., 2002). The above experiments showed that durian shell polysaccharide gel had no toxic effect (Pongsamart et al., 2001, Pongsamart et al., 2002).
4.14. Topical antipruritic effect
External use of durian shells has the effect of curing skin itching, and some researchers use it as the main raw material to make an ointment for external use. In clinical observations, it was found to be effective in treating senile pruritus, xerotic eczema and atopic dermatitis (Yang et al., 2004, Yang et al., 2008, Yang et al., 2016, Wang et al., 2014).
Yang et al. (2004) treated 85 patients with senile pruritus, and found that the total effective rate of the treatment group (Boil the durian skin Shell washed outside) was 84.4%, and the control group (oral Kemin combined with Vitamin E and topical Ailousong Ointment) was 57.5 % in 2004. In 2008, 70 patients with senile pruritus were treated in groups, and it was found that the total effective rate of the control group (Durian Skin Ointment) was 87.5%, and the control group (Urea Ointment) was 56.7%, indicating that Durian Skin Ointment was used to treat senile patients the curative effect of scrapie is better (Yang, et al., 2008). In 2016, 210 patients with atopic dermatitis were treated, and it was found that the SCORAD score and VAS of the patients in the treatment group (Compound Durian Skin Ointment) decreased significantly, compared with the control group (0.1% Mometasone Furoate Cream), it shows that the Compound Durian Skin Ointment has a definite curative effect on atopic dermatitis, can effectively improve the skin barrier function, has good long-term curative effect, and has no adverse reactions (Yang, et al., 2016).
In 2014, after treated 60 patients with xerotic eczema, the total effective rate of the treatment group (oral Yangxue Qufeng Granules combined with topical Durian Skin Ointment) was 93.33%, and the control group (oral Loratadine Dispersible Tablets combined with external Urea Ointment) was 70.00%, indicating that the effect of using Yangxue Qufeng granules and Durian Skin Ointment in the treatment of xerotic eczema is satisfactory (Wang, Yang, & Liu, 2014). Guo (2015) treated 90 patients with atopic dermatitis. After four weeks of treatment, it was found that the durian skin group (Compound Durian Skin Ointment) had a total effective rate of 46.6%, the total effective rate of the hormone group (Mometasone Furoate Cream) was 60%, and the total effective rate of the combined group (Compound Durian Skin Ointment combined with Mometasone Furoate Cream) was 80%, indicating that the Compound Durian Skin Ointment has a definite effect in the treatment of atopic dermatitis, and the dosage of hormone ointment can be reduced when combined. In 2016, when treated 210 patients with atopic dermatitis, at the fourth week, it was found that the water content angle, skin pH value and skin water loss TEWL of target skin lesions in popliteal fossa, cheek and elbow fossa in the treatment group (Compound Durian Skin Ointment) and the control group (0.1% Mometasone Furoate Cream) were significantly better than those before treatment. It shows that the compound durian skin ointment is safe and effective in the treatment of atopic dermatitis, and has a good effect on the repair of skin barrier function, which is significantly better than the control group (Huang, et al., 2016).
5. Conclusion
With the transformation of modern medical model and the increase of demand for prevention and health care, the demand for traditional Chinese medicine resources as the basis of traditional Chinese medicine industry has also increased. In order to promote the health of traditional Chinese medicine industry and realize the sustainable development of traditional Chinese medicine resources, we should not only protect the existing resources, but also explore new resources of traditional Chinese medicine. Such as agricultural residues, non-traditional medicinal parts of traditional Chinese medicine, etc. (Deng, 2018). It was reported that crop wastes such as mango leaves (Liu et al., 2017, Zhang et al., 2022), sugar cane leaf, rice bran, rice husk and corn stigma (Pan, et al., 2019) have medicinal value.
Durian is a tropical fruit, which is deeply loved by most consumers in various countries, and its planting resources are rich. Durian shells are not only agricultural residues, but also folk herbal medicines. They are used to treat fever, external use, skin diseases and so on. Modern research shows that durian shells mainly contain phenolic acids, phenolic glycosides, flavonoids, coumarins, triterpenoids, simple glycosides, volatile components, pectin and other compounds showing anti-oxidant, anti-inflammatory, antibacterial, regulation of glucose and lipid metabolism, anticoagulant, antipruritic and other biological activities, and has potential development value. As early as 1999, Liu et al. (1999) analyzed the fatty acid components in durian shell, but up to now, the research on their chemical components is not deep enough, and the pharmacological activity research mostly stays at the level of efficacy. Most biological activities have not been reported on the comprehensive and in-depth study of action mechanism. There are reports on the mechanism of action of durian shells on anti-oxidation, anti-inflammatory, regulation of glucose and lipid metabolism, protecting liver and regulating immune function, among which the mechanism of anti-oxidation and anti-inflammatory action of compound propacin (36) was relatively deep. With the development of “Belt and Road” and the China-ASEAN Expo (CAEXPO), the demand for durian is increasing year by year. Therefore, the systematic medicinal research of durian shells and the resource utilization of durian shells provide a research basis for developing new resources of traditional Chinese medicine and solving resource waste and environmental pollution, which is of great significance to the economic and social development of ASEAN.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgements
Authors thank for the support of the science foundation projects: STS Plan Project (No. KFJ-STS-QYZD-200); Guangxi Science and Technology Plan Project (Nos. GUIKE AA19254033 and GUIKE AD19110155); Innovation Project of Guangxi Graduate Education (No. YCSY2020096).
Contributor Information
Er-wei Hao, Email: ewhao@163.com.
Jia-gang Deng, Email: dengjg53@126.com.
References
- Aziz N.A.A., Jalil A.M.M. Bioactive compounds, nutritional value, and potential health benefits of indigenous durian (Durio Zibethinus Murr.): A review. Foods. 2019;8(3):1–18. doi: 10.3390/foods8030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.X., Chen X., Liu A.W., Zhen J.Y. Anti-function of forming NDMA caused by durian shell draw liquid. Food Science and Technology. 2005;2:89–91. [Google Scholar]
- Cheng J.S., Zou H.Y. Hakka Health Medicated Diet. China Medical Science Press; Beijing: 2014. p. 137. [Google Scholar]
- Deng J.G. Research on the Medicinal Uses of Agricultural Residues I Mango Leaf, Mango Kernel and its Extract Mangiferin. Beijing Science and Technology Publishing; Beijing: 2018. p. 1. [Google Scholar]
- Evary Y.M., Nugroho A.E., Pramono S. Comparative study on DPPH free radical scavenging and alpha-glucosidase inhibitory activities of ethanolic extracts from different parts of durian plant (Durio Zibethinus Murr.) Food Research. 2019;3(5):463–468. [Google Scholar]
- “Family Bookshelf” editorial board. Diet Materia Medica 2007 Beijing Publishing Beijing 227
- Feng J.Y. Study on the Bioactive Constituents of the Shell of Durio Zibethinus Murr. Guangdong Pharmaceutical University; Guangdong: 2017. pp. 7–88. [Google Scholar]
- Feng Jianying, Wang Yihai, Yi Xiaomin, Yang Weimin, He Xiangjiu. Phenolics from Durian exert pronounced no inhibitory and antioxidant activities. Journal of Agricultural and Food Chemistry. 2016;64(21):4273–4279. doi: 10.1021/acs.jafc.6b01580. [DOI] [PubMed] [Google Scholar]
- Feng Jianying, Yi Xiaomin, Huang Wenjie, Wang Yihai, He Xiangjiu. Novel triterpenoids and glycosides from Durian exert pronounced anti-inflammatory activities. Food Chemistry. 2018;241:215–221. doi: 10.1016/j.foodchem.2017.08.097. [DOI] [PubMed] [Google Scholar]
- Fitrianingsih F., Soyata A., Wigati S. The antibacterial activities of durian rinds extract (Durio Zibethinus) against Propionibacterium acne. IOP Conference Series: Earth and Environmental Science. 2019;391(1) [Google Scholar]
- Futrakul, B., Kanlayavattanakul, M. and Krisdaphong, P. 2010. Biophysic evaluation of polysaccharide gel from durian’s fruit hulls for skin moisturizer. International Journal of Cosmetic Science. 32. 211-215. [DOI] [PubMed]
- Gao J.H., Dai S.Q., Liu J.M., Li J.J., Li M., Cai Q., Ren J.Y. Comparison of physicochemical and gelation properties of pectins extracted from six pericarps. Transactions of the Chinese Society of Agricultural Engineering. 2012;28(16):288–292. [Google Scholar]
- Guo Y. The Clinical Efficacy of Compound Durian Shell Ointment in the Treatment of Atopic Dermatitis. Guangzhou University of Chinese Medicine; Guangzhou: 2015. pp. 9–16. [Google Scholar]
- Han X. Thai agricultural products come to China, not just durian. Agricultural Products Market. 2020;944(14):61. [Google Scholar]
- Husin N.A., Rahman S., Karunakaran R., Bhore S.J. A review on the nutritional, medicinal, molecular and genome attributes of durian (Durio Zibethinus L.), the king of fruits in Malaysia. Bioinformation. 2018;14(6):265–270. doi: 10.6026/97320630014265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B. Malaysia: Durian production is expected to exceed 440,000 tons in 2030. China Fruit News. 2020;37(10):47. [Google Scholar]
- He B. Thailand: Strive to become the world's largest durian producer. China Fruit News. 2021;38(5):42. [Google Scholar]
- Ho L.-H., Bhat R. Exploring the potential nutraceutical values of durian (Durio Zibethinus L.) – an exotic tropical fruit. Food Chemistry. 2015;168:80–89. doi: 10.1016/j.foodchem.2014.07.020. [DOI] [PubMed] [Google Scholar]
- Hokputsa Sanya, Gerddit Waraporn, Pongsamart Sunanta, Inngjerdingen Kari, Heinze Thomas, Koschella Andreas.…Paulsen Berit S. Water-soluble polysaccharides with pharmaceutical importance from durian rinds (Durio Zibethinus Murr.): Isolation, fractionation, characterisation and bioactivity. Carbohydrate Polymers. 2004;56(4):471–481. [Google Scholar]
- Hong J., Du H.X., Hu J.Y. Ultrasonic-assisted extraction of flavonoids from durian peel and their antioxidant and antimicrobial activities. Journal of Henan Agricultural University. 2014;48(5):653–657. [Google Scholar]
- Hong J., Hu J.Y., Zhang X., Ma X.M., Han S. Antioxidant and antibacterial activities of flavonoids extracted from durian peel. Guizhou Agricultural Sciences. 2014;42(6):41–43. [Google Scholar]
- Huang J., Yang Y.F., Chen B.Q., Zhao Y.F., Wang L.N., Wen H.C. Clinical study of compound durian skin ointment in repairing 120 cases of atopic dermatitis skin barrier function. Heilongjiang Journal of Traditional Chinese Medicine. 2016;45(2):24–25. [Google Scholar]
- Huang Y.Y. The Anti-Inflammatory Activities and Mechanism of Coumarin from Durian and Triterpenoid from Chinese Acorns. Guangdong Pharmaceutical University; Guangzhou: 2020. pp. 6–27. [Google Scholar]
- Huang Yuying, Wang Yihai, Xu Jingwen, Feng Jianying, He Xiangjiu. Propacin, a coumarinolignoid isolated from durian, inhibits the lipopolysaccharide-induced inflammatory response in macrophages through the MAPK and Nf-κB pathways. Food & Function. 2020;11(1):596–605. doi: 10.1039/c9fo02202c. [DOI] [PubMed] [Google Scholar]
- Jiang Huimin, Dong Jing, Jiang Shengjun, Liang Qiongxin, Zhang Yan, Liu Zhenhua.…Kang Wenyi. Effect of Durio Zibethinus rind polysaccharide on functional constipation and intestinal microbiota in rats. Food Research International. 2020;136:109316. doi: 10.1016/j.foodres.2020.109316. [DOI] [PubMed] [Google Scholar]
- Jia X.S., He X.H., Liu Q., Jia M.H. Selection of traditional Chinese medicine against new coronavirus based on traditional Chinese herb. Journal of Ningxia Medical University. 2020;42(9):956–961. [Google Scholar]
- Jiang H.M. Study of Immune Function Regulate of Polysaccharides Extracted from Durio Zibethinus Murr Rind. Henan University; Henan: 2020. p. 52. [Google Scholar]
- Leisner J.J, Vancanneyt M, Rusul G, Pot B, Lefebvre K, Fresi A, Tee L.K. Identification of lactic acid bacteria constituting the predominating microflora in an acid-fermented condiment (tempoyak) popular in Malaysia. International Journal of Food Microbiology. 2001;63(1-2):149–157. doi: 10.1016/s0168-1605(00)00476-1. [DOI] [PubMed] [Google Scholar]
- Li Fang, Li Sha, Li Hua-Bin, Deng Gui-Fang, Ling Wen-Hua, Wu Shan.…Chen Feng. Antiproliferative activity of peels, pulps and seeds of 61 fruits. Journal of Functional Foods. 2013;5(3):1298–1309. [Google Scholar]
- Li J.Z. Production of dietary fiber from durian shell. Modern Food Science and Technology. 2012;28(8), 994:995–997. [Google Scholar]
- Li S.Y., Zhou X.E., Zhang Q., Chen C.L., Liu P. Efficient extraction of pectin from durian skin. Farm Machinery. 2012;729(33):113–115. [Google Scholar]
- Li X.W., Li J., Gu Z.J., Wang K., Liu C. Determination of curcuminoids in durian by high performance liquid chromatography. Journal of Food Safety and Quality. 2016;7(12):4955–4959. [Google Scholar]
- Lim T.K. Fruits, Edible Medicinal and Non-Medicinal Plants. Springer; The Netherlands: 2012. p. 580. [Google Scholar]
- Lipipun V., Nantawanit N., Pongsamart S. Antimicrobial activity (in vitro) of polysaccharide gel from durian fruit-hulls. Songklanakarin Journal of Science and Technology. 2002;24(1):32–38. [Google Scholar]
- Liu W.Q. Identification of polyphenol extract from durian husks and its antioxidant and anti-inflammatory activities. Nanchang University: Nanchang. 2020;17:21–25. [Google Scholar]
- Liu W.Q., Zhan Y.L., Xiong H., Zhu X.M., Sun Y. Anti-inflammatory effect and molecular mechanism of durian hull polyphenols on LPS-induced RAW 264.7 macrophages. Food & Machinery. 2020;36(4), 15–20:50. [Google Scholar]
- Liu Q., Zhou J., Xie M.D., Zhu Y.F., Huang W.Y. The component analysis of fatty acid in durian. Chinese Journal of Analytical Chemistry. 1999;3:320–322. [Google Scholar]
- Liu Y., Wang S., Zhou X.L., Gong X.M., Deng J.G. Research progress on pharmacologic effects of Mangifera indica L. leaf. China Journal of Traditional Chinese Medicine and Pharmacy. 2017;32(02):662–665. [Google Scholar]
- Lubis R., Wirjosentono B., Eddyanto, Septevani A.A. Preparation, characterization and antimicrobial activity of grafted cellulose fiber from durian rind waste. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2020;604 [Google Scholar]
- Michael J.B. Durio, A Bibliographic Review. IPGRI office for South Asia; New Delhi: 1997. pp. 54–55. [Google Scholar]
- Mao H.T., Lin X.E., Ding Z.L., Ming J.H., Gao H.M., Sun X.H.…Zhou Z.X. Comparative analysis of main fruit characters of 9 durian varieties. Journal of Zhejiang. Agricultural Sciences. 2020;61(11):2360–2365. 2365. [Google Scholar]
- Mariod A.A., Mirghani M.E.S., Hussein I. Chapter 30 – Durio Zibethinus (Durian). Unconventional Oilseeds and Oil Sources. Academic Press; 2017. pp. 187–197. [Google Scholar]
- Masturi Alighiri D., Edie S.S., Drastisianti A., Khasanah U., Tanti K.A., Susilawati Maghfiroh R.Z.…Choirunnisa F. Identification of flavonoid compounds and total flavonoid content from biowaste of local durian shell (Durio Zibethinus) Journal of Physics: Conference Series. 2020;1567 [Google Scholar]
- Muhtadi, Primarianti Alfiani Urilia, Sujono Tanti Azizah. Antidiabetic activity of durian (Durio Zibethinus Murr.) and Rambutan (Nephelium Lappaceum L.) fruit peels in alloxan diabetic rats. Procedia Food Science. 2015;3:255–261. [Google Scholar]
- Muhtadi M., Haryoto H., Sujono T.A., Suhendi A. Antidiabetic and antihypercholesterolemia activities of rambutan (Nephelium Lappaceum L.) and durian (Durio Zibethinus Murr.) fruit peel extracts. Journal of Applied Pharmaceutical. Science. 2016;6(4):190–194. [Google Scholar]
- Nan C.J. Diet, Nutrition and Cooking. China Medical Science Press; Beijing: 2014. p. 902. [Google Scholar]
- Pan W.Y., Deng J.G., Hou X.T., Qin J.F., Hao E.W., Du Z.C.…Chen M.L. Chemical constituents of agricultural residues producing from 4 kinds of gramineous crops and their pharmacological effects. Chinese Journal of Experimental Traditional Medical Formulae. 2019;25(10):214–225. [Google Scholar]
- Pholdaeng Komsil, Pongsamart Sunanta. Studies on the immunomodulatory effect of polysaccharide gel extracted from Durio Zibethinus in Penaeus Monodon Shrimp against Vibrio Harveyi and WSSV. Fish & Shellfish Immunology. 2010;28(4):555–561. doi: 10.1016/j.fsi.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Pongsamart S., Sukrong S., Tawatsin A. The determination of toxic effects at a high oral dose of polysaccharide gel extracts from fruit-hulls of durian (Durio zibethinus L.) in mice and rats. Songklanakarin. Journal of Science and Technology. 2001;23(1):55–62. [Google Scholar]
- Pongsamart S., Tawatsin S., Sukrong S. Long-term consumption of polysaccharide gel from durian fruit-hulls in mice. Songklanakarin Journal of Science and Technology. 2002;24(4):649–661. [Google Scholar]
- Qin Z., Zhang S. Dietotherapy and Health Preservation of Traditional Chinese Medicine. China Pree of Traditional Chinese Medicine; Beijing: 2017. p. 58. [Google Scholar]
- Reshma M.A. Potential use of durian fruit (Durio Zibenthinus Linn) as an adjunct to treat infertility in polycystic ovarian syndrome. Journal of Integrative Medicine. 2016;14(1):22–28. doi: 10.1016/S2095-4964(16)60240-6. [DOI] [PubMed] [Google Scholar]
- Rizky A.M., Anastasia D., Merdekawati L.E. The potential antibacterial power of ethanol extract of durian bark (Durio zibethinus murr) against Enterococcus faecalis. Sriwijaya Journal of Dentistry. 2020;1(1):1–12. [Google Scholar]
- Sah B.P., Pathak T., Sankar S., Suresh B. Phytochemical investigations on the fruits of Durio zibenthinus Linn. for antimicrobial activity. International Journal of Pharma Sciences and Research. 2014;5(12):878–891. [Google Scholar]
- Shen J., Zhao C., Liu Y., Zhang R.H., Liu G.Q., Chen C. Biogas production from anaerobic Co-digestion of durian shell with chicken, dairy, and pig manures. Energy Conversion and Management. 2019;198:1–10. [Google Scholar]
- Siriphanich J. 5 Durian (Durio zibethinus Merr.). Postharvest Biology and Technology of Tropical and Subtropical Fruits. Woodhead Publishing:; Cambridge, GB: 2011. p. (pp. 82).. [Google Scholar]
- Song M.M. Structural Characterization and Anticoagulantactivity of Polysaccharide from Durio Zibethinus Murr pell. Henan University; Henan: 2019. pp. 10–50. [Google Scholar]
- Tippayakul T., Pongsamart S., Suksomtip M. Lipid entrapment property of polysaccharide gel (PG) extracted from fruit-hulls of durian (Durio Zibethinus Murr. Cv. Mon-Thong). Songklanakarin Journal of Science and Technology. 2005;27(2):292–300. [Google Scholar]
- Wang H.Y., Liao M.S., Zhang M., Wen B., Li P.S., Huang C.X., Wang X.B. Study on the extraction of pectin technology from durian shell. Science and Technology of Food Industry. 2012;33(12):246–250. [Google Scholar]
- Wang L.N., Yang Y.F., Liu R.Y. Effect of yangxue qufeng granules and durian skin ointment on xerotic eczema. Heilongjiang Journal of Traditional Chinese Medicine. 2014;43(4):22–23. [Google Scholar]
- Wang Q.J. The Effect of Drying Methods on the Flavor Compounds of Durian. Shenyang Agricultural University; Shenyang: 2020. p. 5. [Google Scholar]
- Wanlapa Sorada, Wachirasiri Kulaphat, Sithisam-ang Damrongchai, Suwannatup Thitichaya. Potential of selected tropical fruit peels as dietary fiber in functional foods. International Journal of Food Properties. 2015;18(6):1306–1316. [Google Scholar]
- Wu M.Z. Preparation of Durio zibethinus Murr shell Extract as well as its Anti-Inflammatory and Analgetic Activities. Southern Medical University; Guangzhou: 2010. pp. 25–46. [Google Scholar]
- Wu M.Z., Xie G., Li Y.X., Liao Y.F., Zhu R., Lin R.A.…Rao J.J. Cough-relieving, analgesic and antibiotic effects of durian shell extracts: A study in mice. Journal of Southern Medical University. 2010;30(4):793–797. [PubMed] [Google Scholar]
- Xie G., Bao L., He R.R., Lv Y.Q., Yao X.S., Li Y.B. Protective effect of alcohol-extract on liver injury in mice induced by restraint stress. Traditional Chinese Drug Research & Clinical Pharmacology. 2008;19(1):22–25. [Google Scholar]
- Xie G., Wu M.Z., Cheng J.L., Zhan R.T., Chen W.W. Study on the anti-inflammatory effect of durian skin extract. Journal of Guangzhou University of Traditional Chinese Medicine. 2015;32(1):130–135. [Google Scholar]
- Yan T.C., Wang Q.J., Duan X.J., Ben A.L., Chen S.J., Li S.B., Wang H.O. Three drying methods on free amino acids and soluble sugar of durian. Food and Fermentation Industries. 2021;47(14):137–144. [Google Scholar]
- Yang Y.F., Chen B.Q., Guo Y., Liu S.F. Clinical study of compound durian skin ointment repairing skin barrier in patients with atopic dermatitis. Journal of Hebei TCM and Pharmacology. 2016;31(3):16–19. [Google Scholar]
- Yang Y.F., Liang S.Q., Wen H.C., Guo D.J., Liu Y., Wang L.N. Clinical study on durian skin ointment in treating senile pruritus. Journal of Hebei TCM and Pharmacology. 2008;23(4):12–13. [Google Scholar]
- Yang Y.F., Wang L.X., Liu R.Y., Yang Y., Ding N.N., Ye Q.H. 85 cases of senile pruritus treated by external washing of durian skin. Chinese Journal of Information on Traditional Chinese Medicine. 2004;11(6):521–522. [Google Scholar]
- Yang X.T., Wang Y., Wang Y.H., He X.J., Xu J.W. Propacin, extracted from durian, inhibits the lipopolysaccharide-induced inflammatory response in macrophages by inducing autophagy. Pharmacy Today. 2021;31(8):565–569. [Google Scholar]
- Zhang B., Li S.Q., Xin G., Li T.C., Wu J. Analysis of volatile component in different part of durian fruit by GC/MS. Food Research and Development. 2012;33(1):130–134. [Google Scholar]
- Zhang J., Wang Y.D., Xue Q.W., Zhao T.R., Khan A., Wang Y.F.…Cheng G.G. The effect of ultra-high pretreatment on free, esterified and insoluble-bound phenolics from mango leaves and their antioxidant and cytoprotective activities. Food Chemistry. 2022;368 doi: 10.1016/j.foodchem.2021.130864. [DOI] [PubMed] [Google Scholar]
- Zhang J., Liu A.P., Yao J., Yang Y.L., Huang A.L., Sheng X.G. Analysis of volatile chemical components in durian peel. Food Science. 2003;6:128–131. [Google Scholar]
- Zhang L., Yang C.L., Liu H.X., Zhao G.H., Zheng Y.P. Study on the extraction of pectin from durian husk and grapefruit peel and its adsorption of organic compounds. Journal of Gansu Normal Colleges. 2017;22(12):28–33. [Google Scholar]
- Zhang Y.L., Zhu L.Q., Yang X.X., Wang G., Zhu F.H. Detection and evaluation of nutritional components in durian skin. Heilongjiang Animal Science and Veterinary Medicine. 2015;475(7):138–140. [Google Scholar]
- Zhang Y.Y., Huang M.L., Huang J.S., Chen Y.N. The research of the extraction and the stability of durian pericarp yellow pigment. China Condiment. 2012;37(5):113–118. [Google Scholar]
- Zhao Guili, Lyu Xiaomei, Lee Jaslyn, Cui Xi, Chen Wei-Ning. Biodegradable and transparent cellulose film prepared eco-friendly from durian rind for packaging application. Food Packaging and Shelf Life. 2019;21:100345. doi: 10.1016/j.fpsl.2019.100345. [DOI] [Google Scholar]
- Zhou X.W., Wu H., Chen H.Q., Yan Z., Jin B.H., Xie L.Q.…Chen H. Discrimination of different geographical origins of durian based on mineral element fingerprints characteristics. Food Science. 2021;42(14):255–262. [Google Scholar]