Abstract
There is growing evidence that pregnancy may have a significant impact on the maternal brain, causing changes in its structure. To investigate the patterns of these changes, we compared nulliparous women (n = 40) with a group of primiparous women (n = 40) and multiparous mothers (n = 37) within 1–4 days postpartum, using voxel-based and surface-based morphometry (SBM). Compared with the nulliparous women, the young mothers showed decreases in gray matter volume in the bilateral hippocampus/amygdala, the orbitofrontal/subgenual prefrontal area, the right superior temporal gyrus and insula, and the cerebellum. These pregnancy-related changes in brain structure did not predict the quality of mother–infant attachment at either 3 or 12 weeks postpartum nor were they more pronounced among the multiparous women. SBM analyses showed significant cortical thinning especially in the frontal and parietal cortices, with the parietal cortical thinning likely potentiated by multiple pregnancies. We conclude that, compared with the brain of nulliparous women, the maternal brain shows widespread morphological changes shortly after childbirth. Also, the experience of pregnancy alone may not be the underlying cause of the adaptations for mothering. As regards the exact biological function of the changes in brain morphology, longitudinal research will be needed to draw any definitive conclusions.
Keywords: gray matter volume, maternal brain, postpartum period, pregnancy
Introduction
Pregnancy is characterized by a remarkable increase in circulating steroid hormones, including estradiol, progesterone, and cortisol (Lindsay and Nieman 2005; Galea and Frokjaer 2019). Both in animals and humans, these continually increasing hormones are thought to change the pregnant brain by crossing the blood–brain barrier (Galea and Frokjaer 2019; Sacher et al. 2020). In humans, at least 2 independent studies have concluded that pregnancy is accompanied by significant decreases in brain size (Oatridge et al. 2002) and gray matter volume (GMV) (Hoekzema et al. 2017) with a number of other studies showing an increase in GMV after childbirth. An increase in GMV has been seen between 2 and 4 weeks and 3 and 4 months postpartum (Kim et al. 2010), and even as early as between 1 and 2 days and 4 and 6 weeks postpartum (Luders et al. 2020). Collectively, these studies suggest that a decrease of GMV/brain size in pregnancy is followed by a postpartum increase (restoration) of GMV/brain size. Multiple areas (such as the [para]-hippocampus, the fusiform gyrus (Hoekzema et al. 2017), the temporal lobe (Kim et al. 2010; Hoekzema et al. 2017), the amygdala (Kim et al. 2010), the precuneus/posterior cingulate (Hoekzema et al. 2017; Luders et al. 2020), the medial prefrontal/orbitofrontal areas (Kim et al. 2010; Hoekzema et al. 2017), the inferior frontal gyrus (Kim et al. 2010; Hoekzema et al. 2017; Luders et al. 2020), the pre- and postcentral gyri (Kim et al. 2010; Luders et al. 2020), the insula (Kim et al. 2010; Hoekzema et al. 2017), the hypothalamus (Kim et al. 2010), the striatum, and the thalamus (Kim et al. 2010; Luders et al. 2020) have been suggested to be involved in these peripartum/postpartum adaptation processes, compromising the so-called “maternal brain” (Kim et al. 2016). In sum, current research clearly suggests that pregnancy and the postpartum period change the maternal human brain. However, given that the postnatal adaptation processes involving structural change begin very early following childbirth (Oatridge et al. 2002; Luders et al. 2020) and last up to 6 months (Oatridge et al. 2002) or longer (Hoekzema et al. 2017), a better characterization of individual milestones of those changes is necessary to understand the trajectory of antenatal/postnatal brain plasticity. Thus, it would be important to first understand which changes are intrinsically associated with pregnancy, and then how the processes of neuroplasticity continue after childbirth. The previous studies, on the other hand, mostly focused on the postpartum brain, frequently including groups that were very heterogeneous in terms of the time points at which they were investigated. For instance, Hoekzema et al. (2017) performed the first magnetic resonance imaging (MRI) before pregnancy and the second between 1 and 4 months postpartum, disregarding the fact that the maternal brain 1 month postpartum may differ considerably from the one 4 months postpartum. Only one research group (Luders et al. 2018, 2020) assessed the very early postpartum period (1–2 days after delivery), demonstrating an early postpartum adaptation occurring between 1 and 2 days and 4 and 6 weeks after childbirth, but it did not address the question as to which changes may be intrinsically linked to pregnancy. In addition, the biological function of GMV reduction in pregnancy is not entirely clear. According to Hoekzema et al. (2017), the GMV reduction seen in humans at 1–4 months postpartum, compared with the prepregnancy level, may be related to the absence of hostility in new mothers in the first 6 months following childbirth. Such observations have been interpreted as proof of a link between pregnancy-related GMV reduction and the quality of mother–child attachment. Kim et al. (2010), on the other hand, suggested that the postpartum increase/restoration of GMV is likely associated with a mother’s positive perception of her child, which is in line with the findings in animal studies. In female mice, for instance, gestation and lactation periods have been seen to induce strong, albeit transient, gray matter concentration hypertrophy within key regions involved in emotion, motivation, reward, and mnestic functions (Barrière et al. 2021). Thus, it remains to be ascertained whether it is the GMV reduction in pregnancy, as suggested by Hoekzema et al. (2017), or the postpartum neuroplasticity, modulated by the interaction with the child, as suggested by Kim et al. (2010) or shown in animal models (Barrière et al. 2021), that is instrumental in the development of maternal attachment toward the child. In addition, none of the previous studies compared first-time pregnant women with their multiparous counterparts nor were there comparisons with control groups comprising yet-to-be-pregnant women. This question is of particular relevance given that, according to some studies, multiple pregnancies exert long-lasting, pregnancy-related effects on the brain. Notably, a higher number of previous pregnancies have been found to be associated with less apparent brain aging, especially in the striatal and limbic regions, including the nucleus accumbens and the amygdala, and also in the hippocampus (Lange et al. 2020), whereas grand multiparity (i.e., 5 or more childbirths) has been suggested to contribute to cognitive decline or increased dementia risk (Bae et al. 2020b). Thus, the findings with respect to the possible effects of parity/multiparty on brain aging are somewhat contradictory, calling for further research. Comparing primiparous and multiparous women shortly after delivery may yield conclusive results. In light of this, the overarching goal of this project was to explore pregnancy-related GMV changes in young mothers, taking into account such factors as multiparty, attachment toward the child, and postpartum mood. Assessing the very early postpartum period (e.g., the first few days following childbirth) was of particular relevance as it might shed valuable light on the entire spectrum of changes associated with pregnancy. Although this period is crucial for the development of mother–infant attachment (Fleming et al. 1993; Morsbach and Bunting 2008; Adeli and Aradmehr 2018), it can also give rise to negative feelings or mood swings (the so-called baby blues) (Hahn et al. 2021; Stickel et al. 2021), putting young mothers at a higher risk of postpartum depression (PPD) (DSM 5, APA 2013).
The data for the current investigation were drawn from the data pool of the risk of postpartum depression (RiPoD) study, in which postpartum women were subjected to a multimodal MRI scan within the first few days of childbirth and then observed for 12 weeks. Based on a clinical interview 12 weeks postpartum, participants were classified as mentally healthy or having either PPD or adjustment disorder (AD). Although the focus of our previous research was the investigation of early structural and functional brain alterations in PPD and AD (2021), the focus of the current study was the assessment of GMV changes associated with pregnancy itself in a subgroup of mentally healthy participants without any psychiatric history. After repeating the analysis of Schnakenberg et al. (2021a) and Schnakenberg et al. (2021b) in a bigger sample (see Supplementary Material), we compared 78 healthy mothers, with no psychiatric history, with healthy nulliparous women (n = 40), also with no history of psychiatric disorder. To determine whether the number of pregnancies can have any impact on the specific pregnancy-related changes in brain volume shortly after childbirth, we compared primiparous women with their multiparous counterparts (2–3 children). No other study had sought to address the GMV reduction seen shortly after delivery in the specific context of postpartum maternal behavior. Given that the data were collected as part of a longitudinal study, including observation of maternal attachment at several time points within the first 12 weeks postpartum, we also focused on the link between pregnancy-related GMV changes and the development of attachment based on multiple Maternal Postnatal Attachment Scale (MPAS) assessments. Choosing a different approach as compared with Hoekzema et al. (2017), we sought to determine if the MPAS scores at 3 or 12 weeks postpartum could be predicted based on the GMV at childbirth. Factors such as baby blues, scores of the Edinburg Postnatal Depression Scale (EPDS), and child’s sex were also taken into account.
Aiming to characterize the GMV changes in direct relation to childbirth, we had the following hypotheses: First, comparing the young mothers with nulliparous controls, we expected to see pregnancy-associated GMV reduction in regions including the hippocampus and the prefrontal and temporal cortices (Hoekzema et al. 2017). However, as the MRI scan was performed close to delivery, we expected a clearer definition of the regions affected by pregnancy-induced GMV reduction. In addition, we expected to see an effect of pregnancy on cortical thickness. Second, we hypothesized that the number of pregnancies might have an impact on the pregnancy-related changes in brain volume (Lange et al. 2020) and on cortical thickness. However, as previous findings had been contradictory with respect to the effect of single versus multiple pregnancies on the brain, it was not possible to ascertain exactly which regions (if any) are likely to be more affected by multiple pregnancies and to what degree. In addition, focusing on the link between the maternal brain shortly after pregnancy and the development of attachment toward the infant over the 12-week postpartum period, we expected to find out if the degree of pregnancy-induced GMV reduction predicts the quality of attachment during the first postpartum weeks. Given that there is increasing evidence from research in animals and humans that the postpartum period is associated with further reorganization of the brain in relationship to the attachment toward the child, we assumed that pregnancy-related GMV reduction may not be as crucial for mother–child attachment and, therefore, may not predict the quality of attachment postpartum.
Materials and Methods
Participants Included in the Analysis
Written informed consent was obtained from all participants. The study, approved by the Ethics Committee of the University Hospital RWTH Aachen, conformed to the ethical standards of the Helsinki declaration.
This study included a group of 78 healthy postpartum women (age range 21–42 years). The imaging data of these participants were selected from the brain imaging data pool (n = 169) of an ongoing longitudinal study pertaining to the early detection of PPD (RiPoD-study) based on the following criteria: euthymic women with no signs of postpartum psychiatric disorder based on the clinical interview at childbirth and at 12 weeks postpartum, and no psychiatric history. The postpartum women included in the current analysis were further split into 2 groups based on their parity: 41 primiparous women (22–39 years) and 37 multiparous women (21–42 years). In the multiparous group, 33 women had 2 children and 4 women had 3 children. To minimize the time window between childbirth and the MRI scan, only women whose structural MRI session had taken place between 1 and 4 days after childbirth were selected for the analyses.
RiPoD Study
All participants of the RiPoD study were recruited in the Department of Gynecology and Obstetrics at the University Hospital Aachen within 1–6 days of childbirth (for the analyses, only those recruited and examined in the MRI between 1 and 4 days postpartum were selected). Twelve weeks after childbirth, the participants were invited to a final semistandardized clinical interview for the final diagnosis by an experienced psychiatrist or psychologist. Women with current depression or any other manifest psychiatric condition at the moment of recruitment, based on the clinical interview, abuse of alcohol, drugs, psychotropic substances, antidepressant or antipsychotic medication during pregnancy, history of psychosis or manic episodes, were excluded from the RiPoD study. In addition, only mothers of healthy children (determined by the routine German Child Health tests [U2] conducted within the first 3–10 days of life) were included. Barring the pregnancy- and child-related exclusion criteria, the recruitment was equitable and inclusive, representing a cross-section of the local population. As part of the RiPoD study (for a comprehensive study overview please refer to Stickel et al. 2021), all participants had to complete the Edinburgh Postnatal Depression Scale (Cox et al. 1987), a 10-item self-report to help assess depressive symptomatology in the postpartum period, immediately after childbirth and every 3 weeks for 12 weeks postpartum. Using the MPAS (Condon and Corkindale 1998), a total score of maternal attachment including subscores for quality of attachment, absence of hostility, and pleasure in interaction was evaluated at 3, 6, 9, and 12 weeks postpartum. At 3 weeks postpartum, the experience of baby blues symptoms was assessed with both a self-report and a cut off score of >10 on the Maternity Blues Questionnaire (MBQ) (Kennerley and Gath 1989; Takács et al. 2016).
Nulliparous Control Subjects
The nulliparous control subjects were 40 healthy female adults (age range 19–35 years, M = 26.7 years, standard deviation [SD] = 4.53 years) recruited through advertisement in the study center. The control subjects had no sign of depression or any other manifest psychiatric disorder based on the clinical interview at the time of recruitment, no history of psychiatric disorders (identified by the short version of the German Structured Clinical Interview for DSM 4 Disorders [SCID-I]; Wittchen et al. 1997), had never been pregnant before, and had neither a history of (spontaneous) abortion nor an unfavorable pregnancy outcome (e.g., extrauterine pregnancy).
Behavioral Data Analysis
The analysis was conducted using SPSS 25 IBM Corporation (Armonk, NY) for Windows. The nulliparous and postpartum groups were compared with regard to age (using the Welch F-test due to violation of homogeneity of variance), education (Fisher’s exact test), and marriage status (Chi-square test).
The postpartum groups (primiparous and multiparous) were compared with regard to age, gestational age, child’s birth weight, birth mode, EPDS scores and MPAS total, as well as subscale scores. For each of the continuous measures, independent sample t-tests were conducted. In case of violation of the assumption of homogeneity of variance (Levene’s test), t-statistic not assuming equality of variance was computed. For categorical measures, Chi-square tests were conducted.
Separate analyses of variance (ANOVAs) for repeated measures were calculated within each parity group with measurement time points after birth and EPDS scores or MPAS total scores and subscale scores as within-subject variables. The Greenhouse–Geisser correction was used to adjust degrees of freedom when significant nonsphericity was detected via the Mauchly’s test.
Significant findings were pursued with Bonferroni-corrected post hoc tests for ANOVA, Games–Howell corrected post hoc test for the Welch F-test, and standardized residuals for the Chi-square test, determining which category of variables in which group had the largest difference between the expected and actual numbers relative to the sample size (critical value z = ±1.96).
The effect sizes of the significant results are reported using partial eta squared (ηp2) for F-tests (small: 0.20–0.05, medium: 0.06–0.13, large: 0.14 and greater), Cohen’s d for pairwise comparisons (small: 0.20–0.49, medium: 0.50–0.79, large: 0.80 and greater), and Cramer’s V for Chi-square test (small: 0.1–0.29, medium: 0.3–0.49, large: 0.5 and greater) (Cohen 1988).
A significance level of P value less than 0.05 was used.
MRI Data Acquisition
Neuroimaging data were acquired using a 3 Tesla Prisma MR Scanner (Siemens Medical Systems, Erlangen, Germany) located in the Medical Faculty of RWTH Aachen University. T1-weighted structural images were acquired by means of a 3-dimensional magnetization-prepared rapid acquisition gradient echo imaging sequence (4.12 min; 176 slices, TR = 2300 ms, TE = 1.99 ms, TI = 900 ms, FoV = 256 × 256 mm2, flip angle = 9°, voxel resolution = 1 × 1 × 1 mm3). All images were inspected for structural abnormalities, scanner artifacts, and motion artifacts. In case of the latter two, imaging acquisition was repeated.
Voxel-Based Morphometry (VBM)
Anatomical imaging data were preprocessed using the Computational Anatomy Toolbox (CAT12 Version r1728) and statistical parametric mapping (SPM)12 toolbox implemented in Matlab 2015b (MathWorks, Inc., Natick, MA). The default settings of CAT12 were applied for spatial registration, segmentation, and normalization (Gaser et al. in review). All images were affine registered to standard tissue probability maps by correcting individual head positions and orientations and translated into Montreal Neurologic Institute (MNI) space. The images were segmented into gray matter, white matter (WM), and cerebrospinal fluid. For normalization, the Diffeomorphic Anatomic Registration Through Exponentiated Linear algebra algorithm (DARTEL) was used (Klein et al. 2009) as DARTEL affords a more precise spatial normalization to the template than the conventional algorithm (Ashburner 2007). Images were visually inspected for potential segmentation and registration errors. Following the suggestions of the CAT12 toolbox manual (Gaser et al. in review), a homogeneity check of the unsmoothed data identified no outliers, thus the GMVs of all participants were included in subsequent analyses. Finally, the modulated GMV was smoothed with an 8-mm full-width at half-maximum (FWHM) Gaussian kernel.
Surface-Based Morphometry (SBM)
The CAT12 toolbox was used to extract information regarding cortical thickness. Volumes were segmented using surface and thickness estimation in the writing options. Local maxima were projected to the gray matter voxels by using neighbor relationship described by the WM distance, equaling cortical thickness. The estimation of cortical thickness was performed based on projection-based thickness including partial volume correction, sulcal blurring, and sulcal asymmetries without sulcus reconstruction (Dahnke et al. 2013). Topological correction was performed through an approach based on spherical harmonics. For interparticipant analysis, an algorithm for spherical mapping of the cortical surface was included (Yotter et al. 2011). An adapted volume-based diffeomorphic DARTEL algorithm was then applied to the surface for spherical registration. All scans were resampled and smoothed with a Gaussian kernel of 15-mm FWHM. The surface data were visually inspected for artifacts and all scans passed through the automatic surface data homogeneity check of the CAT12 toolbox.
Statistical Analyses for VBM and SBM
For statistical comparison of the 3 groups (nulliparous, primiparous, and multiparous), we applied the general linear model (GLM) approach implemented in SPM 12. To compare the 3 groups, a whole-brain one-way ANOVA was performed with smoothed gray matter segments of all participants for the VBM analysis and resampled and smoothed thickness files of all participants for the SBM analysis. To test whether the baby’s sex or the experience of baby blues (yes/no) had an effect on GMV or cortical thickness, a whole-brain full-factorial GLM with factor group (2 levels) and factor baby blues (2 levels) or gender (2 levels) was performed. The significant effects of all GLM analyses were further pursued with t-contrasts. The effect of hormonal contraception intake (yes/no) in nulliparous control subjects was performed with a 2-sample t-test. The correlation between EPDS scores at T0 and GMV and cortical thickness changes was performed with regression analyses (in all postpartum, primiparous, and multiparous women).
In all VBM analyses, age and total intracranial volume (TIV) were used as covariates. The SBM analyses were adjusted only for age, as TIV is not recommended as a covariate by the authors of the CAT12 toolbox (Gaser et al. in review).
For all analyses, the statistical threshold was set at P < 0.05 cluster-level family-wise error (FWE) correction for multiple comparisons, with a cluster-forming threshold at voxel-level P < 0.001, k = 920 voxels for VBM, and k = 116 voxels for SBM. Gray matter structures in the VBM analyses are labeled with reference to the Anatomy Toolbox for SPM (Eickhoff et al. 2005) and the Automated anatomical labeling atlas 3 (Rolls et al. 2020). In SBM analyses, surface structures are labeled with reference to the Desikan–Killiany atlas (Desikan et al. 2006).
All results are presented in the MNI space.
Additional analyses of all RiPoD study participants (n = 169; 124 nondepressed, 21 with AD, 24 with PPD) can be found in the Supplementary Material.
Multivariate Regression Analyses
The PRONTO toolbox 2.0 (http://www.mlnl.cs.ucl.ac.uk/pronto/) implemented in Matlab was used to predict the MPAS total and its attachment, hostility, and interaction subscales using whole-brain voxel-wise GMV, and controlling for age and TIV. A kernel ridge regression was computed using a leave-one-out approach with the default settings. Permutation-based nonparametric P values (1000 permutations) were computed for the correlation between observed and predicted scores.
Results
Behavioral Results
The average postpartum time frame of measurement was 2 days after childbirth (M = 2.58 days postpartum, SD = 0.79 days postpartum).
In the postpartum group, 63 (80.8%) were Germans, 2 (2.6%) were from other West European countries (Belgium, Netherlands), 1 (1.3%) was from Spain, 8 (10.3%) were from Eastern Europe (Poland, Lithuania, Russia, Slovakia), and 4 (5.1%) were from African, Arab, or Asian countries (Morocco, Iran, Liberia, Tajikistan).
In the nulliparous control group, 26 participants (70.3%) were from Germany, 3 (8.1%) from a West European country (France), 3 (8.1%) from the Mediterranean region (Spain, Greece, Turkey), 4 (10.8%) from Eastern Europe (Russia, Bulgaria), and 1 (2.7%) from Africa (no specific country was mentioned). Three nulliparous participants did not provide information regarding their country of origin. Table 1 summarizes the characteristics of the 3 groups.
Table 1.
Sample characteristics of postpartum women
| Nulliparous | Primiparous (n = 41) | Multiparous (n = 37) | Statistical test | |
| Mean (SD) | Mean (SD) | |||
| Age | 26.7 (4.53) | 31.24 (4.09) | 32.57 (4.66) | Welch F2,75.49 = 17.44, P < .001a |
| Gestational age in days | 276.68 (10.90) | 275.43 (10.63) | t 76 = 5.12, P = 0.610 | |
| Child’s birth weight | 3281.10 (454.91) | 3431.08 (508.45) | t 76 = −1.36, P = 0.173 | |
| MPAS 3 weeks pp | 86.07 (4.69) | 84.05 (5.82) | t 76 = 1.69, P = 0.094 | |
| Quality of attachment | 40.45 (2.68) | 39.11 (3.41) | t 76 = 1.93, P = 0.058 | |
| Absence of hostility | 22.85 (2.14) | 22.76 (1.89) | t 76 = 0.20, P = 0.841 | |
| Pleasure in interaction | 23.05 (1.72) | 22.19 (2.89) | t 57.76 = 1.57, P = 0.122 | |
| MPAS 6 weeks pp | 86.20 (4.33) | 83.73 (6.31) | t 62.84 = 1.99, P = 0.051 | |
| Quality of attachment | 40.35 (31.10) | 39.49 (3.45) | t 76 = 1.56, P = 0.251 | |
| Absence of hostility | 22.80 (1.92) | 22.05 (2.31) | t 76 = 1.54, P = 0.127 | |
| Pleasure in interaction | 23.05 (1.41) | 22.19 (2.85) | t 51.71 = 1.65, P = 0.104 | |
| MPAS 9 weeks pp | 85.93 (4.79) | 83.65 (6.29) | t 66.98 = 1.78, P = .079 | |
| Quality of attachment | 40.53 (3.43) | 39.22 (3.59) | t 76 = 1.64, P = 0.106 | |
| Absence of hostility | 22.55 (2.14) | 22.16 (2.48) | t 76 = 0.74, P = 0.463 | |
| Pleasure in interaction | 22.95 (1.68) | 22.30 (2.60) | t 60.75 = 1.29, P = 0.200 | |
| MPAS 12 weeks pp | 86.22 (4.66) | 85.05 (6.19) | t 76 = 0.95, P = 0.348 | |
| Quality of attachment | 40.55 (3.34) | 40.11 (3.51) | t 76 = 0.57, P = 0.573 | |
| Absence of hostility | 22.80 (2.42) | 22.27 (2.38) | t 76 = 0.97, P = 0.336 | |
| Pleasure in interaction | 23.25 (1.31) | 22.43 (2.89) | t 49.51 = 1.58, P = 0.121 | |
| EPDS at birth | 3.39 (2.48) | 4.32 (2.45) | t 76 = −.167, P = .099 | |
| EPDS 3 weeks pp | 4.34 (3.09) | 4.27 (2.28) | t 73.18 = 0.16, P = 0.908 | |
| EPDS 6 weeks pp | 3.51 (2.44) | 3.70 (2.59) | t 76 = −0.33, P = 0.739 | |
| EPDS 9 weeks pp | 2.71 (2.39) | 3.27 (2.68) | t 76 = −0.98, P = 0.330 | |
| EPDS 12 weeks pp | 2.24 (1.88) | 2.65 (2.29) | t 76 = −0.86, P = 0.394 | |
| Percent | Percent | |||
| Secondary education | Fisher’s exact = 9.41, P = 0.027b | |||
| Lowest (<9 years) | – | – | 11.1 | |
| Middle (10–12 years) | 12.5 | 15 | 2.8 | |
| Highest (>13 years) | 87.5 | 85 | 86.1 | |
| Birth mode | Fisher’s exact = 5.34, P = 0.125 | |||
| Spontaneous | 68.3 | 78.4 | ||
| Ventouse | 9.8 | – | ||
| C-section | 12.2 | 18.9 | ||
| Emergency C-section | 9.8 | 2.7 | ||
| Experience of baby blues (yes) | 48.8 | 54.1 | Chi2(1) = 0.22, P = 0.642 | |
| Intend to breastfeed at T0 (yes) | 92.7 | 83.8 | Fisher’s exact = 5.34, P = 0.295 | |
| Breastfeeding at T1 (yes) | 87.8 | 78.4 | Chi2(1) = 1.24, P = 0.265 | |
| Married (yes) | 7.7* | 61.0 | 75.7 | Chi2(2) = 30.10, P < 0.000c |
| Single (yes) | 65.4* | – | – | n. a. |
Note: MPAS (Condon and Corkindale 1998); EPDS (Cox et al. 1987); pp: postpartum; n.a.: no statistical analyses possible.
*About 14 nulliparous participants did not indicate their relationship status.
aGames–Howell corrected post hoc: significant differences between nulliparous and both postpartum groups.
bDue to low expected cell counts no post hoc test could be applied.
cSignificant standardized residual exceeding critical value in nulliparous controls.
The nulliparous control subjects were significantly younger than their postpartum counterparts (P < 0.001, d = 4.34–4.66) and significantly less likely to be married than expected (z = −3.2, Cramer’s V = 0.538, P < 0.001). The multiparous participants were more likely to have low educational status, although calculations were not possible here due to the low cell count.
The primiparous and multiparous participants did not differ significantly in age, gestational age, birth mode, the experience of baby blues (yes/no, based on self-report and MBQ cut off score > 10), intent to breastfeed at T0 and breastfeeding at T1 (Table 1). The groups did not differ in the self-reported values of mother–child attachment total scores and the respective subscales, although there was a trend for the primiparous mothers to have slightly higher MPAS scores than multiparous mothers 6 weeks postpartum (P = 0.051). In addition, the groups did not differ in the self-reported depression scores at any time point.
Repeated measures ANOVA was used within each group to analyze the postpartum course of the total mother–child attachment scores and the 3 subscales at all 4 time points. There was no significant effect of time in either group regarding the total scores (primiparous: F2.41,96.3 = 0.98, P = 0.935; multiparous: F3,108 = 2.00, P = 0.118). In addition, no significant interaction was detected between the subscales and the time points (primiparous: F6,234 = 0.26, P = 0.955; multiparous: F4.25,152.91 = 1.95, P = 0.101). In addition, the MPAS score correlated significantly throughout the postpartum course (T1—T2: r = 0.733, T2—T3: r = 0.818, T3—T4: r = 0.799, all P < 0.001).
The repeated measures ANOVA showed a significant effect of time regarding the EPDS values in both groups (primiparous: F4,160 = 7.28, P < 0.001, ηp2 = 0.154; multiparous: F4,144 = 4.03, P = 0.004, ηp2 = 0.101). In the primiparous group, Bonferroni-corrected pairwise comparisons revealed significantly higher depressive values at 3 weeks compared with 9 (P = 0.003, d = 0.59) and 12 weeks postpartum (P < 0.001, d = 0.820), as well as higher values at 6 weeks compared with 12 weeks (P = 0.024, d = 0.583). In the multiparous group, depressive values at 12 weeks were significantly lower compared with after childbirth (P = 0.018, d = 0.705), 3 weeks (P = 0.01, d = 0.709), and 6 weeks postpartum (P = 0.041, d = 0.234). In addition, the EPDS score correlated significantly throughout the postpartum course (T0–T1: r = 0.241, P = 0.034, T1–T2: r = 0.399, T2–T3: r = 0.601, T3–T4: r = 0.514, all P < 0.001).
Whole-Brain VBM Results
GMV Alterations in Primiparous Participants
We tested whether pregnancy and childbirth lead to changes in the brain structure. In a whole-brain analysis, using t-contrast from random-effects GLM (ANOVA), we found a symmetrical pattern of significant GMV decrease in 3 clusters in the primiparous compared with the nulliparous women (Fig. 1A, Supplementary Table S1). The first cluster (20 607 voxel) included regions of the cerebellum extending to the inferior temporal gyrus, the fusiform gyrus, and the inferior occipital gyrus. The second cluster (12 159 voxel) included the basal ganglia, the subgenual and orbitofrontal cortices, the anterior and midcingulate cortices, the thalamus, the insula, the temporal cortex, the hippocampus, the parahippocampal gyrus, and the amygdala. Further decreases were observed in a cluster (3988 voxel) predominantly located in the superior parietal lobule, the paracentral and postcentral gyri, the midcingulate cortex, the precuneus, and the cuneus. No significant GMV increase was found in the primiparous compared with the nulliparous women.
Figure 1.
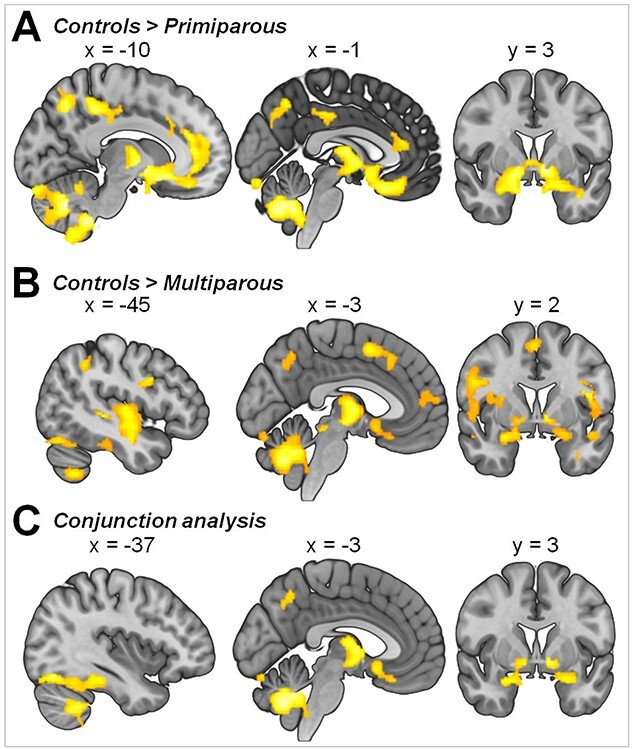
GMV differences in control subjects compared with (A) primiparous and (B) multiparous women. (C) Brain regions linked to pregnancy based on a conjunction analysis (controls > primiparous ∩ controls > multiparous). Illustration of clusters emerging from t-contrast from random-effects GLM, P < 0.05, cluster-level FWE correction, k = 920.
GMV Alterations in Multiparous Participants
Seeking to determine if GMV changes are potentiated by multiple pregnancies, we compared the nulliparous to the multiparous women. The whole-brain analysis of GMV differences between control subjects and multiparous women revealed a symmetrical pattern of significant decrease in multiparous women in 6 clusters (Fig. 1B, Supplementary Table S2). The first cluster (36 157 voxels) encompassed widespread GMV differences located in the cerebellum and the temporal lobe with extensions observed in the fusiform gyrus, the basal ganglia, the amygdala, the hippocampus, and the parahippocampal gyrus as well as the insula, the inferior frontal gyrus, and the orbital cortex. In both hemispheres of the multiparous women, significant decreases were seen in 1 cluster (7842 voxel) encompassing the temporal cortex, the rolandic operculum, the insula, the thalamus, the basal ganglia, the rectal gyrus, the parahippocampal gyrus, and the hippocampus, in 2 clusters (4838 and 1972 voxel) including the dorsal frontal, orbital, and cingulate cortices, and in 2 clusters (4300 and 1379 voxel) encompassing the parietal and occipital cortices. An additional cluster (3520 voxels) in the right hemisphere included the rolandic operculum, the insula, the inferior frontal gyrus (p. triangularis), and the putamen. No significant GMV increase was found in the multiparous compared with the nulliparous women.
GMV Alterations in Primiparous Compared With Multiparous Participants
Based on the GMV changes potentiated by multiple pregnancies, we further tested for long-lasting GMV changes by comparing primiparous and multiparous women. No significant group differences were identified between primiparous and multiparous participants in the t-contrast from random-effects GLM with a cluster-forming threshold at voxel-level P < 0.001. Likewise, when applying a more liberal cluster-forming threshold at voxel-level P < 0.005 as well as P < 0.01, no significant differences were found in the pairwise comparisons.
GMV Alterations Linked to Pregnancy
To isolate the GMV difference-based links to pregnancy in general, we performed a conjunction analysis across both contrasts (control > primiparous ∩ control > multiparous) and found the pregnancy-related GMV differences to be most pronounced in a cluster (16 736 voxel) encompassing the cerebellum, the bilateral fusiform gyrus, and the temporal cortex extending to the parahippocampal gyrus. Another cluster (4003 voxel) was found in both hemispheres in the thalamus, the temporal pole, the pallidum, and the rectal gyrus extending to the basal ganglia, the thalamus, the amygdala, the hippocampus, the parahippocampal gyrus, the insula, and the medial orbital gyrus. The third cluster (1507) included the left hemispheric superior parietal gyrus and the inferior gyrus (Fig. 1C, Supplementary Table S3).
Multivariate Regression Analyses
To test whether depressive symptoms at T0 and GMV changes were correlated in the postpartum group, 3 independent regression analyses were performed: 1) in all postpartum women, 2) in primiparous women only, and 3) in multiparous women only. We found no significant correlation between GMV and EPDS scores at T0 in any of the groups.
To determine whether maternal behavior toward the child is linked to structural changes due to pregnancy and childbirth, we examined the GMV in relation to maternal attachment. Kernel ridge regression was used to test whether the MPAS total score (Fig. 2) and its subscales (quality of attachment, absence of hostility, and pleasure in interaction; Table 2) at 3 and 12 weeks postpartum were predicted by whole-brain GMV at birth in the whole postpartum sample. The analysis showed that neither the overall quality of mother–infant attachment nor the subscales were predicted by GMV at any time point.
Figure 2.
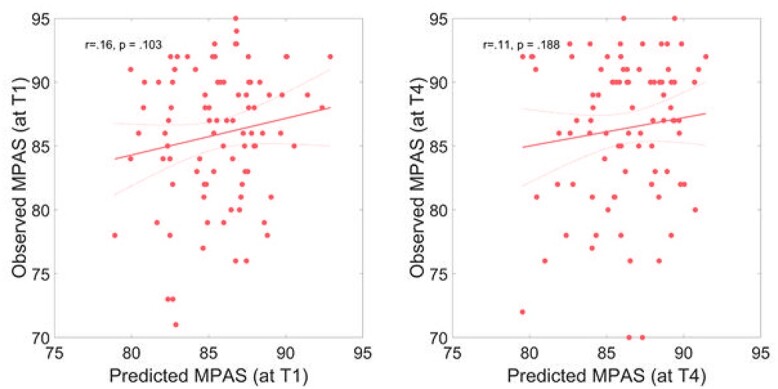
Multivariate prediction of MPAS scores based on the GMV at birth. Predicted versus actual scores are plotted at (A) 3 weeks postpartum and (B) 12 weeks postpartum. The solid red line indicates a linear fit and the dashed lines the 95% confidence interval.
Table 2.
Prediction of quality of attachment, absence of hostility, and pleasure in interaction at 3 and 12 weeks postpartum by GMV at birth
| Quality of attachment | Absence of hostility | Pleasure in interaction | |
| 3 weeks postpartum | r = .03, P = 0.369 | r = −.01, P = 0.712 | r = 0.18, P = 0.07 |
| 12 weeks postpartum | r = .02, P = 0.389 | r = −.07, P = 0.653 | r = 0.16, P = 0.116 |
Results of Whole-Brain SBM
Differences in Cortical Thickness Between Control Subjects and Primiparous Women
Finally, we assessed the structural changes in the maternal brain by means of surface-based analyses. In a whole-brain analysis, using t-contrast from random-effects GLM (ANOVA), we found reduced cortical thickness in the bilateral dorsolateral prefrontal cortex, the bilateral superior parietal gyrus, as well as the right inferior parietal sulcus and the supramarginal gyrus in the primiparous compared with the nulliparous women (Fig. 3A, Supplementary Table S4).
Figure 3.
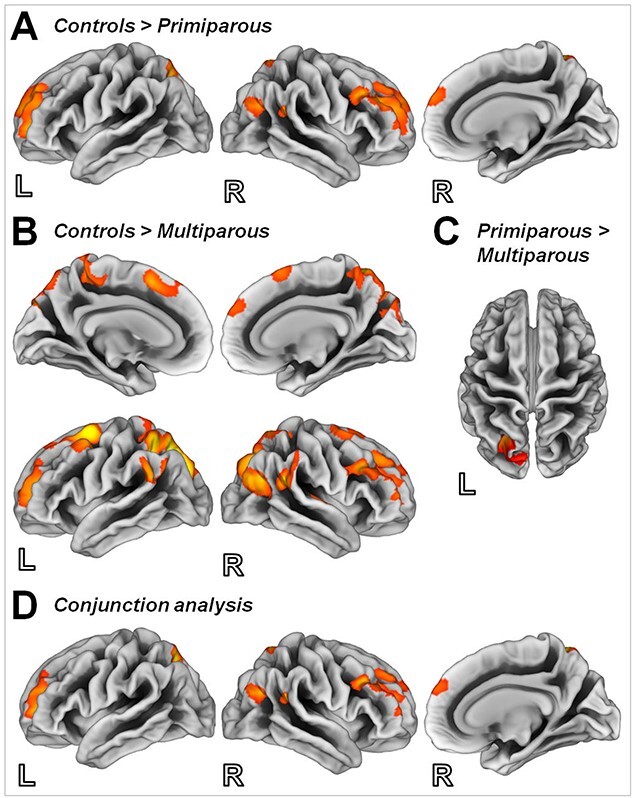
Surface maps depicting differences in cortical thickness in control subjects compared with (A) primiparous women and (B) multiparous women, and in (C) primiparous compared with multiparous women. (D) Brain regions linked to pregnancy based on a conjunction analysis (controls > primiparous ∩ controls > multiparous). Illustration of clusters emerging from t-contrast from random-effects GLM, P < 0.05, cluster-level FWE correction, k = 116 voxels.
Differences in Cortical Thickness Between Control Subjects and Multiparous Women
Multiple pregnancies were found to result in reduced cortical thickness in the bilateral dorsolateral and the dorsomedial prefrontal cortices. In addition, both the parietal and temporal cortices were reduced in the multiparous compared with the nulliparous women (Fig. 3B, Supplementary Table S4).
Differences in Cortical Thickness Between Primiparous and Multiparous Women
Investigating the possible long-lasting changes in brain structure, we found multiparous women to exhibit less cortical thickness compared with their primiparous counterparts in the superior parietal gyrus and the inferior parietal sulcus (Fig. 3C, Supplementary Table S4).
Differences in Cortical Thickness Linked to Pregnancy
To isolate pregnancy-related changes in cortical thickness in general, a conjunction analysis across both contrasts (control > primiparous ∩ control > multiparous) was performed, and the changes were found to be most pronounced in the bilateral dorsolateral prefrontal cortex, the bilateral superior parietal gyrus, as well as the right inferior parietal sulcus and the supramarginal gyrus (Fig. 3D, Supplementary Table S4).
Effects of Baby Blues in Mothers
To determine whether the experience of baby blues is associated with changes in brain structure, we first compared all postpartum women who experienced symptoms of baby blues (n = 40) with those who had not (n = 38) (and vice versa) and found no significant main effect of baby blues on GMV or cortical thickness. The comparison between primiparous women with baby blues (n = 20) and those without (n = 21) did not yield significant results in GMV either nor did the comparison between multiparous women with baby blues (n = 20) and those without (n = 17). With respect to cortical thickness, primiparous women with baby blues displayed reduced thickness in a cluster comprising the cuneus, the superior parietal gyrus, and the precuneus (149 voxel, T = 4.5). No differences in cortical thickness were observed in multiparous women.
Effects of Depressive Symptoms in Mothers at Childbirth
To investigate whether depressive symptoms at the time of childbirth (T0) have an effect on brain structure, we carried out regression analyses in the whole postpartum group and in the 2 parity groups. In addition, regression analyses were performed in the entire postpartum group recruited during the RiPoD study and in the diagnostic groups (AD, PPD) (see the Supplementary Material for characteristics of the whole group and those of AD and PPD). No significant association was found between EPDS and changes in GMV or cortical thickness in any of these comparisons.
Effects of Hormonal Contraception in Nulliparous Women
To investigate whether ovarian hormones can modify GMV, we compared nulliparous control subjects who used hormonal contraception (n = 17) with those who had not (n = 22) and found no significant differences in GMV or cortical thickness between these groups.
Effects of Child’s Sex
Forty-one of the postpartum women carried a girl (25 primiparous women) and 37 a boy (16 primiparous). Considering the previously observed effects of fetal sex on cognitive changes in pregnant women (Vanston and Watson 2005), we compared the brain structures of the mothers of boys with those of the mothers of girls and found no significant effect of sex on GMV or cortical thickness and no interaction between parity group and sex.
Discussion
During pregnancy and the early postpartum period, mothers undergo psychological and biological adaptations that trigger processes involving brain plasticity. To assess the structure underlying GMV changes in relation to pregnancy, we compared the brain structure of postpartum women within a very early postpartum period with that of nulliparous women. Shortly after childbirth (on average 2 days postpartum), young mothers displayed an extensive GMV decrease in the bilateral hippocampus/amygdala, the orbitofrontal cortex/the subgenual area (BA 25), the bilateral temporal lobe, the insula, the basal ganglia, and the cerebellum. In addition, the surface-based analyses revealed significant cortical thinning in the frontal and parietal cortices, with the parietal cortical thinning likely potentiated by multiple pregnancies.
The brain areas affected by pregnancies are not random, as they (the medial prefrontal cortex and the temporal lobe in particular) have been shown to be related to socio-cognitive processes and emotion perception (Mitchell and Phillips 2015) and be part (insula, amygdala, inferior frontal gyrus, superior temporal gyrus) of the neuroanatomy of the so-called maternal brain (Rocchetti et al. 2014) and the reward system (basal ganglia). Both in humans and other mammals, the sex steroid hormones and corticosteroids are suggested to be the main mediators of the observed morphometric changes (Galea and Frokjaer 2019; Sacher et al. 2020). In particular, the amygdala, the hippocampus, and the prefrontal cortex are densely covered with receptor cells for corticosteroid and ovarian hormones (McEwen and Milner 2007; Wharton et al. 2012; Toffoletto et al. 2014). As these brain areas are associated with a variety of major functions in humans, the effects of pregnancy on emotional and cognitive processes, and also on the development of maternal behavior, are likely to be significant, although they have yet to be sufficiently investigated.
Pregnancy, Multiparty, and Aging
The effects of pregnancy on cognitive demands are suggested by several studies (Pearson et al. 2009; Stickel et al. 2019). A meta-analysis by Davies et al. (2018) reports, for instance, that the overall cognitive function (memory, executive function and attention) is poorer in pregnant women compared with their nonpregnant counterparts, especially during the third trimester. On the level of brain morphology, our results along with those of animal studies suggest that pregnancy triggers (at least transitorily) a reduction of hippocampal volume (Galea et al. 2000; Barrière et al. 2021). However, it is unclear if the GMV reduction in the hippocampus or any other brain structure has any short- or long-term effect on the cognitive function of young mothers. It has been suggested that reduction in cerebral GMV, for example, in the hippocampus, can be long-lasting, seen even 2 years after childbirth (Hoekzema et al. 2017). Given that we did not find any GMV differences between primiparous and multiparous women, we cannot conclude that any morphological changes seen in the early postpartum period are more pronounced in multiparous mothers. The lack of GMV differences seen between the primiparous and multiparous women shortly after childbirth does not support the notion that pregnancy leads to a long-lasting reduction in cerebral GMV, for example, in the hippocampus. Some previous studies in older women have even suggested that parity is associated with fewer brain aging markers and, thus, have a protective influence on brain aging (Lange et al. 2020). However, this protective effect of pregnancy appears to be undermined by the number of pregnancies a woman undergoes, with grand multiparity being thought to be linked to dementia (Bae et al. 2020b), among other things, through an aggravation of amyloid-independent hippocampal or cortical atrophy (Jung et al. 2020). Other studies suggest that grand multiparity only increases the risk of vascular dementia, whereas nulliparity poses a higher risk of Alzheimer’s disease (Bae et al. 2020a) with a woman’s nationality (European vs. Asian) being a potent variable.
Even in the absence of GMV changes, multiparous women, compared with their primiparous counterparts, showed more pronounced cortical thinning particularly in the parietal cortices. Cortical thinning had been previously shown to be associated not only with pregnancy (Hoekzema et al. 2017), but also with adolescence (Carmona et al. 2019), suggesting similar hormonally primed biological processes in pregnancy and adolescence. Our data suggest that multiple pregnancies (entailing repeated exposures to elevated levels of hormones, mostly progesterone and estradiol) likely potentiate the effect of pregnancy on cortical thinning. Changes in cortical thickness, with age-related thinning in particular, are observed in normal development and aging (Fjell et al. 2015). Rapid thinning, however, is often apparent in middle-aged groups (Salat et al. 2004) with diseases such as Alzheimer’s (Dickerson et al. 2009). In sum, the findings involving the effects of parity/multiparty on brain aging are contradictory, with a host of factors, including the number of pregnancies, women’s life style, and genetic predisposition, being found to play their respective roles. Also, it may not be prudent to interpret the morphometric changes seen in our cohort of mothers as evidence of neurodegeneration. Further studies examining the similarities between pregnancy-related brain changes and those associated with neurodegenerative disorders would be needed to extend our findings and those of previous studies (Carmona et al. 2019). At the same time, it may be premature to conclude that pregnancy has neuroprotective effects. Factors such as the number of pregnancies a woman has, and their socioeconomic and biological conditions need to be considered for an assessment of the complex effects of pregnancy on the female brain.
Is mother’s Behavior Related to Structural Changes in the Brain During Pregnancy?
According to some previous studies, the typical behavior of a new mother is associated with structural changes in the maternal brain during and immediately after pregnancy (Kim et al. 2010; Hoekzema et al. 2017). However, it is unclear whether it is the pregnancy-related GMV reduction that is instrumental in the development of maternal attachment toward the child, as has been suggested by Hoekzema et al. (2017), or whether it is the postpartum neuroplasticity, as suggested by Kim et al. (2010), which facilitates mother–child attachment. As we did not find any association between the GMV reduction in young mothers shortly after giving birth and the quality of attachment both 3 and 12 weeks postpartum, we maintain that the experience of pregnancy itself may not be the sole underlying cause of adaptations for mothering and caregiving. A crucial difference between our study and the one by Hoekzema et al. (2017) is that, in their study, the examination of the mother’s brain took place considerably later (1–4 months postpartum) than in ours (2 days postpartum on average). Although Hoekzema et al. attributed the GMV alterations to pregnancy-related changes, we are of the opinion that what they actually examined was the postpartum brain. Given that fathers also develop attachment and caregiving skills (Paquette 2004; Cabrera 2020), other neurobiological mechanisms, and not only pregnancy-related brain structure alterations, are likely responsible for the transition to effective parenting. For instance, in a study by Kim et al. (2014), biological fathers showed GMV increases in the hypothalamus, the amygdala, the striatum, and the lateral prefrontal cortex between 2 and 4 weeks and 12 and 16 weeks after childbirth. Thus, the development of attachment is likely to be a time-dependent process rather than being purely driven by pregnancy-related changes in brain structure. The relevant question here is, to what degree the pregnancy-associated changes contribute, via the initiation of neuroplastic processes, to the development of maternal attachment.
Pregnancy-Associated Brain Alterations and the Development of PPD
As regards the effect of pregnancy on emotion processing and stress regulation, the subgenual anterior cingulate (ACC) (in addition to the amygdala and the hippocampus) is related to the regulation of emotions and stress (Bludau et al. 2016). In line with that, a recent study by our group found postpartum women with higher cumulative hair cortisol concentration in the last trimester of pregnancy to have lower activation in the subgenual ACC during emotional interference involving anxious emotions shortly after childbirth (Stickel et al. 2019). In addition, the subgenual anterior cingulate cortex, the hippocampus, and the amygdala are known to be related to affective disorders (Price and Drevets 2012). This raises the question as to whether these morphological changes seen shortly after childbirth in our sample are related to the development of conditions like PPD. We addressed this question in Schnakenberg et al. (2021a) as part of the RiPoD study and also in the current paper (see the Supplementary Material). Based on the data obtained shortly after childbirth, we did not find any structural brain differences between participants who would develop PPD or AD and healthy young mothers either in the analysis by Schnakenberg, Hahn, et al. (2021a) or in the current sample. In addition, analyzing data from both healthy postpartum mothers and those with PPD or AD (see Supplementary Material), we did not find any association between EPDS and structural changes related to pregnancy. As the RiPoD study was designed for early identification of PPD or AD cases, the imaging data obtained before PPD or AD had become clinically manifest (Hahn et al. 2021; Schnakenberg et al. 2021a). The lack of differences in GMV structure within that time frame may indicate that, if there are early structural alterations in PPD or AD, they are either too subtle to be detected based on a sample size such as ours or they develop later in the disease course. Taken together, our data suggest that no trait marker of PPD can be identified based on the structural changes that occur in relation to pregnancy. However, it is difficult to draw definite conclusions in this regard as research of trait markers or preexisting vulnerabilities with respect to both MDD and PPD is scarce.
Limitations
In sum, MRI examinations of women within a very narrow time frame after childbirth showed the early postpartum period to be associated with pronounced alterations in brain structure. These alterations, however, did not predict the development of attachment to the child in the first few weeks after delivery nor did they appear to be potentiated by multiple pregnancies. In addition, these alterations did not differ between women who developed PPD and those who developed AD within the first 12 weeks postpartum (see Supplementary Material). However, the ways in which the networks and the brain structures restore themselves following childbirth cannot be determined by our data given our study’s lack of longitudinal MRI assessment and the lack of prepregnancy MRI, which is a major limitation of the current work. Additional longitudinal neuroimaging data from several time points postpartum (in parallel with assessment of attachment) would be needed to determine if postpartum alterations in the brain are linked to the quality of maternal attachment to the child. The reports pertaining to the overall cognitive function in pregnant and postpartum women are not always consistent (Farrar et al. 2014; Logan et al. 2014), and a link between cognitive function and pregnancy-related reduction of hippocampal volume, or that of any other brain area, has yet to be confirmed. For definitive conclusions in this regard, it would be necessary to simultaneously examine brain structure and cognitive function during pregnancy and the postpartum period, which was not done in our study. These limitations notwithstanding the study involved a large sample of postpartum women (as part of the ongoing longitudinal study related to early recognition of PPD), controlling for psychiatric history or the development of PPD and postpartum AD within the first 12 weeks postpartum.
Another limitation worth mentioning is the much younger nulliparous control group compared with the postpartum women, which may cause the differences in GMV change to appear to be associated with age. In recent decades, maternal age has been found to be advancing (Statistisches Bundesamt 2021), leading to a right-skewed distribution and making it difficult to find either younger mothers or older nulliparous women. However, to avoid biasing the results, we controlled for age in all analyses and performed additional analyses with appropriate age-matched groups (see Supplementary Material), in which the same distribution of GMV changes was observed, suggesting that it is not age but the experience of pregnancy that leads to these changes.
Although the sample size of our study (118 in the whole analysis, 78 young mothers with no psychiatric history, 40 healthy nulliparous controls) can, conceivably, be seen as a further limitation, given that larger multimodal studies are likely to prove more conducive to investigations of this nature, ours is, to the best of our knowledge, the largest study to date to address this issue. The sample sizes of the studies by Oatridge et al. 2002, Kim et al. 2010, Luders et al. 2020, and Hoekzema et al. (2017) were considerably lower, typically including between 9 and 25 participants.
Conclusion and Perspectives
Based on our results and those of previous studies, we conclude that the exact biological/physiological function of the changes in brain morphology observed in humans shortly after childbirth remains unclear. Although, given that these changes have been seen to occur both in humans and other mammals, it can be safely assumed that they have a role to play. In gestating mice, motherhood has been seen to have a profound impact on brain organization (Barrière et al. 2021). Other animal studies have shown that increased interactions with pups over time during the early postpartum period lead to additional structural changes in the maternal brain (Fleming and Korsmit 1996; Featherstone et al. 2000; Lonstein et al. 2015). It is reasonable to assume, therefore, that similar structural changes occur in the brain of human mothers. In view of the fact that the acute pregnancy-related GMV differences were not found to predict the MPAS scores, it would be interesting to investigate the adaptive neuroplasticity of the mother’s brain with respect to the quality of maternal attachment to the child over a longer postpartum period. Thus, rather than the GMV reduction in pregnancy, postpartum adaptation is likely to play a crucial role in the development of mother–child attachment, facilitated in no small measure by the mother’s interaction with her child. In addition, evidence suggests that the cognitive functions may also be influenced by pregnancy (Barha and Galea 2017). However, it is unclear if the reduction of brain volume in pregnancy is necessarily related to the deterioration of cognitive or other functions. On the contrary, decreased GMV may result in more communication both between and within brain regions (Barha and Galea 2017). Another intriguing but yet to be fully resolved question is whether pregnancy (multiple pregnancies in particular, and how many pregnancies may be too many) can have lifelong consequences with respect to brain health and the aging process. For instance, parity-induced endocrine changes have been suggested to influence brain response to sex hormones later in life (Barha and Galea 2017), leaving a long-lasting footprint on the endocrine system. Large-scale epidemiological studies have reported associations between increasing parity and the risk of metabolic syndrome (Gunderson et al. 2009; Al-Farsi et al. 2010; Akter et al. 2013) and type 2 diabetes mellitus (Kritz-Silverstein et al. 1989; Nicholson et al. 2006), as well as dementia (Bae et al. 2020a, 2020b). In addition, the physiological adaptations associated with pregnancy can render pregnant women susceptible not only to psychiatric but also, and more frequently, to metabolic and cardiovascular diseases. Conditions such as pre-eclampsia, pregnancy-induced hypertension, and gestational diabetes are characterized by physiological responses indicative of the metabolic syndrome, potentially heralding future cardiovascular and metabolic diseases. However, the extent to which preexisting or pregnancy-induced medical conditions, multiparity or the age of women at the time of pregnancy, interact with pronounced GMV reductions in the brain during pregnancy, and their restoration after childbirth, remains unclear. Moreover, physiological changes such as the drastic decline in hormones, blood loss, changes in blood volume and blood pressure during labor and delivery may have an additional influence on the morphological alterations in the maternal brain. Another important aspect of this is whether pregnancy-induced alterations in brain morphology have long-lasting effects, extending beyond the reproductive event itself (Barha and Galea 2017). It is only through longitudinal studies that we can understand how the networks and brain structures that undergo pregnancy-related changes recover after childbirth, and which factors influence the recovery processes. Future studies tracking ovarian hormones from before pregnancy through pregnancy and the postpartum period, as well as longitudinal multimodal neuroimaging, cognitive, and lifestyle assessments, will likely help identify the factors that contribute to the observed neuroanatomical changes during pregnancy and the postpartum restoration of brain structure. Further research is needed also to investigate if the changes associated with pregnancy contribute to the development of psychiatric conditions, especially in women at higher risks for postpartum psychiatric disorders (e.g., women with a psychiatric history). Finally, the cumulative effect of multiple pregnancies on brain anatomy (if proven) may suggest that pregnancy has long-term consequences with respect to the maternal brain. And if pregnancy is indeed found to have negative long-term effects on aging, mental health, or any other aspect of women’s health, it would be imperative to explore how these effects can be counterbalanced by such factors as healthy life style, cognitive activities, and, importantly, the prevention or early treatment of pregnancy-related somatic and psychiatric disorders.
Author Contributions
N.C. and S.S. designed the experiment and carried out the experiment; S.T., C.E., and I.N. contributed data; J.D., N.C., and S.S. performed the analyses; N.C. and S.S. wrote the paper; all authors evaluated and approved the manuscript.
Supplementary Material
Contributor Information
Natalia Chechko, Department of Psychiatry, Psychotherapy and Psychosomatics, Medical Faculty, RWTH Aachen, Aachen 52074, Germany; Institute of Neuroscience and Medicine, JARA-Institute Brain Structure Function Relationship (INM 10), Research Center Jülich, Jülich 52428, Germany; Institute of Neuroscience and Medicine, Brain & Behavior (INM-7), Research Center Jülich, Jülich 52428, Germany.
Jürgen Dukart, Institute of Neuroscience and Medicine, Brain & Behavior (INM-7), Research Center Jülich, Jülich 52428, Germany; Institute of Systems Neuroscience, Medical Faculty, Heinrich Heine University Düsseldorf, Düsseldorf 40225, Germany.
Svetlana Tchaikovski, Department of Gynecology and Obstetrics, Medical Faculty, RWTH Aachen, Aachen 52074, Germany.
Christian Enzensberger, Department of Gynecology and Obstetrics, Medical Faculty, RWTH Aachen, Aachen 52074, Germany.
Irene Neuner, Department of Psychiatry, Psychotherapy and Psychosomatics, Medical Faculty, RWTH Aachen, Aachen 52074, Germany; Institute of Neuroscience and Medicine, JARA-Institute Brain Structure Function Relationship (INM 10), Research Center Jülich, Jülich 52428, Germany.
Susanne Stickel, Department of Psychiatry, Psychotherapy and Psychosomatics, Medical Faculty, RWTH Aachen, Aachen 52074, Germany; Institute of Neuroscience and Medicine, JARA-Institute Brain Structure Function Relationship (INM 10), Research Center Jülich, Jülich 52428, Germany.
Funding
The Rotation Program (2015–2017) of the Medical Faculty of the University Hospital RWTH Aachen, and the Deutsche Forschungsgemeinschaft (DFG, No. 410314797).
Notes
This work was supported by the Brain Imaging Facility of the Interdisciplinary Center for Clinical Research (IZKF) Aachen within the Faculty of Medicine at RWTH Aachen University. Conflict of Interest: None declared.
References
- Adeli M, Aradmehr M. 2018. A comparative study of maternal-neonate abdominal and kangaroo (skin-to-skin) skin contact immediately after birth on maternal attachment behaviors up to 2 months. J Educ Health Promot. 7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akter S, Jesmin S, Rahman MM, Islam MM, Khatun MT, Yamaguchi N, Akashi H, Mizutani T. 2013. Higher gravidity and parity are associated with increased prevalence of metabolic syndrome among rural Bangladeshi women. PLoS One. 8:e68319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Farsi YM, Brooks DR, Werler MM, Cabral HJ, Al-Shafei MA, Wallenburg HC. 2010. Effect of high parity on the occurrence of prediabetes: a cohort study. Acta Obstet Gynecol Scand. 89:1182–1186. [DOI] [PubMed] [Google Scholar]
- APA . 2013. Diagnostic and statistical manual of mental disorders. 5th ed ed. American Journal of Psychiatry. Washington (DC): American Psychiatric Association. [Google Scholar]
- Ashburner J. 2007. A fast diffeomorphic image registration algorithm. Neuroimage. 38:95–113. [DOI] [PubMed] [Google Scholar]
- Bae JB, Lipnicki DM, Han JW, Sachdev PS, Kim TH, Kwak KP, Kim BJ, Kim SG, Kim JL, Moon SW, et al. 2020a. Does parity matter in women’s risk of dementia? A COSMIC collaboration cohort study. BMC Med. 18:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae JB, Lipnicki DM, Han JW, Sachdev PS, Kim TH, Kwak KP, Kim BJ, Kim SG, Kim JL, Moon SW, et al. 2020b. Parity and the risk of incident dementia: a COSMIC study. Epidemiol Psychiatr Sci. 29:e176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Galea LAM. 2017. The maternal “baby brain” revisited. Nat Neurosci. 20:134–135. [DOI] [PubMed] [Google Scholar]
- Barrière DA, Ella A, Szeremeta F, Adriaensen H, Même W, Chaillou E, Migaud M, Même S, Lévy F, Keller M. 2021. Brain orchestration of pregnancy and maternal behavior in mice: a longitudinal morphometric study. Neuroimage. 230:117776. [DOI] [PubMed] [Google Scholar]
- Bludau S, Bzdok D, Gruber O, Kohn N, Riedl V, Sorg C, Palomero-Gallagher N, Müller VI, Hoffstaedter F, Amunts K, et al. 2016. Medial prefrontal aberrations in major depressive disorder revealed by cytoarchitectonically informed voxel-based morphometry. Am J Psychiatry. 173:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera NJ. 2020. Father involvement, father-child relationship, and attachment in the early years. Attach Hum Dev. 22:134–138. [DOI] [PubMed] [Google Scholar]
- Carmona S, Martínez-García M, Paternina-Die M, Barba-Müller E, Wierenga LM, Alemán-Gómez Y, Pretus C, Marcos-Vidal L, Beumala L, Cortizo R, et al. 2019. Pregnancy and adolescence entail similar neuroanatomical adaptations: a comparative analysis of cerebral morphometric changes. Hum Brain Mapp. 40:2143–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. 1988. Statistical power analysis for the behavioral sciences. 2nd ed. Mahwah (NJ): Lawrence Erlbaum Associates. [Google Scholar]
- Condon JT, Corkindale CJ. 1998. The assessment of parent-to-infant attachment: development of a self-report questionnaire instrument. J Reprod Infant Psychol. 16:57–76. [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. 1987. Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry. 150:782–786. [DOI] [PubMed] [Google Scholar]
- Dahnke R, Yotter RA, Gaser C. 2013. Cortical thickness and central surface estimation. Neuroimage. 65:336–348. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Lum JAG, Skouteris H, Byrne LK, Hayden MJ. 2018. Cognitive impairment during pregnancy: a meta-analysis. Med J Aust. 208:35–40. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 31:968–980. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, et al. 2009. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 19:497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. 2005. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- Farrar D, Tuffnell D, Neill J, Scally A, Marshall K. 2014. Assessment of cognitive function across pregnancy using CANTAB: a longitudinal study. Brain Cogn. 84:76–84. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Fleming AS, Ivy GO. 2000. Plasticity in the maternal circuit: effects of experience and partum condition on brain astrocyte number in female rats. Behav Neurosci. 114:158–172. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Grydeland H, Krogsrud SK, Amlien I, Rohani DA, Ferschmann L, Storsve AB, Tamnes CK, Sala-Llonch R, Due-Tønnessen P, et al. 2015. Development and aging of cortical thickness correspond to genetic organization patterns. Proc Natl Acad Sci U S A. 112:15462–15467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, Corter C, Franks P, Surbey M, Schneider B, Steiner M. 1993. Postpartum factors related to mother’s attraction to newborn infant odors. Dev Psychobiol. 26:115–132. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Korsmit M. 1996. Plasticity in the maternal circuit: effects of maternal experience on Fos-lir in hypothalamic, limbic, and cortical structures in the postpartum rat. Behav Neurosci. 110:567–582. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Frokjaer VG. 2019. Perinatal depression: embracing variability toward better treatment and outcomes. Neuron. 102:13–16. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Ormerod BK, Sampath S, Kostaras X, Wilkie DM, Phelps MT. 2000. Spatial working memory and hippocampal size across pregnancy in rats. Horm Behav. 37:86–95. [DOI] [PubMed] [Google Scholar]
- Gaser C, Dahnke R, Kurth K, Luders E, Alzheimer's Disease Neuroimaging Initiative. A Computational Anatomy Toolbox for the Analysis of Structural MRI Data. Neuroimage, in review. [DOI] [PMC free article] [PubMed]
- Gunderson EP, Jacobs DR, Chiang V, Lewis CE, Tsai A, Quesenberry CP, Sidney S. 2009. Childbearing is associated with higher incidence of the metabolic syndrome among women of reproductive age controlling for measurements before pregnancy: the CARDIA study. Am J Obstet Gynecol. 201:177.e1–177.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn L, Eickhoff SB, Habel U, Stickeler E, Schnakenberg P, Goecke TW, Stickel S, Franz M, Dukart J, Chechko N. 2021. Early identification of postpartum depression using demographic, clinical, and digital phenotyping. Transl Psychiatry. 11:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekzema E, Barba-Müller E, Pozzobon C, Picado M, Lucco F, García-García D, Soliva JC, Tobeña A, Desco M, Crone EA, et al. 2017. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 20:287–296. [DOI] [PubMed] [Google Scholar]
- Jung JH, Lee GW, Lee JH, Byun MS, Yi D, Jeon SY, Jung GJ, Joung H, Shin SA, Kim YK, et al. 2020. Multiparity, brain atrophy, and cognitive decline. Front Aging Neurosci. 12:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley H, Gath D. 1989. Maternity blues. Br J Psychiatry. 155:356–362. [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, Swain JE. 2010. The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behav Neurosci. 124:695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Rigo P, Mayes LC, Feldman R, Leckman JF, Swain JE. 2014. Neural plasticity in fathers of human infants. Soc Neurosci. 9:522–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Strathearn L, Swain JE. 2016. The maternal brain and its plasticity in humans. Horm Behav. 77:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang M-CC, Christensen GE, Collins DL, Gee J, Hellier P, et al. 2009. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 46:786–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritz-Silverstein D, Barrett-Connor E, Wingard DL. 1989. The effect of parity on the later development of non-insulin-dependent diabetes mellitus or impaired glucose tolerance. N Engl J Med. 321:1214–1219. [DOI] [PubMed] [Google Scholar]
- Lange AG, Barth C, Kaufmann T, Anatürk M, Suri S, Ebmeier KP, Westlye LT. 2020. The maternal brain: region-specific patterns of brain aging are traceable decades after childbirth. Hum Brain Mapp. 41:4718–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay JR, Nieman LK. 2005. The hypothalamic-pituitary-adrenal axis in pregnancy: challenges in disease detection and treatment. Endocr Rev. 26:775–799. [DOI] [PubMed] [Google Scholar]
- Logan DM, Hill KR, Jones R, Holt-Lunstad J, Larson MJ. 2014. How do memory and attention change with pregnancy and childbirth? A controlled longitudinal examination of neuropsychological functioning in pregnant and postpartum women. J Clin Exp Neuropsychol. 36:528–539. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Lévy F, Fleming AS. 2015. Common and divergent psychobiological mechanisms underlying maternal behaviors in non-human and human mammals. Horm Behav. 73:156–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Gingnell M, Poromaa IS, Engman J, Kurth F, Gaser C. 2018. Potential brain age reversal after pregnancy: younger brains at 4–6 weeks postpartum. Neuroscience. 386:309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Kurth F, Gingnell M, Engman J, Yong E-L, Poromaa IS, Gaser C. 2020. From baby brain to mommy brain: widespread gray matter gain after giving birth. Cortex. 126:334–342. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Milner TA. 2007. Hippocampal formation: shedding light on the influence of sex and stress on the brain. Brain Res Rev. 55:343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RLC, Phillips LH. 2015. The overlapping relationship between emotion perception and theory of mind. Neuropsychologia. 70:1–10. [DOI] [PubMed] [Google Scholar]
- Morsbach G, Bunting C. 2008. Maternal recognition of their neonates’ cries. Dev Med Child Neurol. 21:178–185. [Google Scholar]
- Nicholson WK, Asao K, Brancati F, Coresh J, Pankow JS, Powe NR. 2006. Parity and risk of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes Care. 29:2349–2354. [DOI] [PubMed] [Google Scholar]
- Oatridge A, Holdcroft A, Saeed N, Hajnal JV, Puri BK, Fusi L, Bydder GM. 2002. Change in brain size during and after pregnancy: study in healthy women and women with preeclampsia. Am J Neuroradiol. 23:19–26. [PMC free article] [PubMed] [Google Scholar]
- Paquette D. 2004. Theorizing the father-child relationship: mechanisms and developmental outcomes. Hum Dev. 47:193–219. [Google Scholar]
- Pearson RM, Lightman SL, Evans J. 2009. Emotional sensitivity for motherhood: late pregnancy is associated with enhanced accuracy to encode emotional faces. Horm Behav. 56:557–563. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. 2012. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 16:61–71. [DOI] [PubMed] [Google Scholar]
- Rocchetti M, Radua J, Paloyelis Y, Xenaki L, Frascarelli M, Caverzasi E, Politi P, Fusar-Poli P. 2014. Neurofunctional maps of the ‘maternal brain’ and the effects of oxytocin: a multimodal voxel-based meta-analysis. Psychiatry Clin Neurosci. 68:733–751. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Huang CC, Lin CP, Feng J, Joliot M. 2020. Automated anatomical labelling atlas 3. Neuroimage. 206:116189. [DOI] [PubMed] [Google Scholar]
- Sacher J, Chechko N, Dannlowski U, Walter M, Derntl B. 2020. The peripartum human brain: current understanding and future perspectives. Front Neuroendocrinol. 59:100859. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, Morris JC, Dale AM, Fischl B. 2004. Thinning of the cerebral cortex in aging. Cereb Cortex. 14:721–730. [DOI] [PubMed] [Google Scholar]
- Schnakenberg P, Hahn L, Stickel S, Stickeler E, Habel U, Eickhoff SB, Chechko N, Dukart J. 2021a. Examining early structural and functional brain alterations in postpartum depression through multimodal neuroimaging. Sci Rep. 11:13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnakenberg P, Jo H-G, Stickel S, Habel U, Eickhoff S, Brodkin ES, Goecke TW, Votinov M, Chechko N. 2021b. The early postpartum period – differences between women with and without a history of depression. J Psychiatr Res. 136:109–116. [DOI] [PubMed] [Google Scholar]
- Statistisches Bundesamt . 2021. Durchschnittliches Alter der Mütter und Väter bei der Geburt bis 2020 | Statista. https://de.statista.com/statistik/daten/studie/1180171/umfrage/durchschnittliches-alter-der-muetter-und-vaeter-bei-der-geburt-in-deutschland/. (2021-10-11, Date accessed). [Google Scholar]
- Stickel S, Eickhoff S, Goecke TW, Schneider F, Quinete NS, Lang J, Habel U, Chechko N. 2019. Cumulative cortisol exposure in the third trimester correlates with postpartum mothers’ neural response to emotional interference. Biol Psychol. 143:53–61. [DOI] [PubMed] [Google Scholar]
- Stickel S, Eickhoff SB, Habel U, Stickeler E, Goecke TW, Lang J, Chechko N. 2021. Endocrine stress response in pregnancy and 12 weeks postpartum – exploring risk factors for postpartum depression. Psychoneuroendocrinology. 125:105122. [DOI] [PubMed] [Google Scholar]
- Takács L, Smolík F, Mlikova Seilderova J, Cepicky P, Hoskovcova S. 2016. Postpartum blues – a Czech adaptation of the maternity blues questionnaire. Cesk Gynekol. 81:355–368. [PubMed] [Google Scholar]
- Toffoletto S, Lanzenberger R, Gingnell M, Sundström-Poromaa I, Comasco E. 2014. Emotional and cognitive functional imaging of estrogen and progesterone effects in the female human brain: a systematic review. Psychoneuroendocrinology. 50:28–52. [DOI] [PubMed] [Google Scholar]
- Vanston CM, Watson NV. 2005. Selective and persistent effect of foetal sex on cognition in pregnant women. Neuroreport. 16:779–782. [DOI] [PubMed] [Google Scholar]
- Wharton W, Gleason CE, Olson SR, Carlsson CM, Asthana S. 2012. Neurobiological underpinnings of the estrogen – mood relationship. Curr Psychiatr Rev. 8:247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen H-U, Zaudig M, Frydrich T. 1997. Strukuriertes klinisches interview für DSM-IV. Göttingen: Hogrefe. [Google Scholar]
- Yotter RA, Dahnke R, Thompson PM, Gaser C. 2011. Topological correction of brain surface meshes using spherical harmonics. Hum Brain Mapp. 32:1109–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


