Abstract
Face recognition is dependent on computations conducted in specialized brain regions and the communication among them, giving rise to the face-processing network. We examined whether modularity of this network may underlie the vast individual differences found in human face recognition abilities. Modular networks, characterized by strong within and weaker between-network connectivity, were previously suggested to promote efficacy and reduce interference among cognitive systems and also correlated with better cognitive abilities. The study was conducted in a large sample (n = 409) with diffusion-weighted imaging, resting-state fMRI, and a behavioral face recognition measure. We defined a network of face-selective regions and derived a novel measure of communication along with structural and functional connectivity among them. The modularity of this network was positively correlated with recognition abilities even when controlled for age. Furthermore, the results were specific to the face network when compared with the place network or to spatially permuted null networks. The relation to behavior was also preserved at the individual-edge level such that a larger correlation to behavior was found within hemispheres and particularly within the right hemisphere. This study provides the first evidence of modularity–behavior relationships in the domain of face processing and more generally in visual perception.
Keywords: connectome, face perception, face recognition, functional connectivity, structural connectivity
Introduction
Face perception is a complex cognitive function requiring the integration of multiple specialized brain regions exhibiting a higher response to faces, compared with other visual categories (Grill-Spector et al. 2017; Hesse and Tsao 2020). Recent studies have taken a network-based approach to examine how interactions between these regions give rise to face perception abilities. For example, structural connectivity (SC), assessed with diffusion-weighted imaging, revealed that the face network is organized in partially segregated ventral and dorsal streams (Pyles et al. 2013; Wang et al. 2020) corroborating existing neurocognitive models (Duchaine and Yovel 2015). Additionally, functional connectivity (FC) among these regions was found to dynamically increase while observing upright compared with inverted faces (Rosenthal et al. 2016) or compared with other visual categories (Rissman et al. 2004). Finally, functional and structural connectivity alterations are thought to underlie congenital prosopagnosia (Thomas et al. 2009; Rosenthal et al. 2017), an innate impairment in face recognition (Behrmann and Avidan 2005).
The connectome, a comprehensive map of the brain’s anatomical connections, can be thought of as the scaffold on which neuronal communication unfolds. Methods from network science have enabled characterizing the connectome and the way it gives rise to multiple subnetworks or communities that support specialized functional cognitive systems (Medaglia et al. 2015; Mišić and Sporns 2016). Communities are characterized by strong connectivity within the community nodes and weaker connectivity between communities and hence are characterized by a segregated or modular structure (Wig 2017). Computations within such modular communities can be performed efficiently with little interference from other systems (Bullinaria 2007) while still allowing integration between communities (Bullmore and Sporns 2012). The modular structure of the face processing network has been studied in previous work at the group level (Rosenthal et al. 2016; Maffei and Sessa 2021). However, it is not clear how such modular community structure underlies face perception abilities and how it may account for the vast intersubject variability documented in face perception in humans (Wilmer 2017).
The relationship between modularity and individual differences in cognitive abilities, such as executive function (Baum et al. 2016), memory (Chan et al. 2014), and motor learning (Bassett et al. 2011), was mostly described within large-scale multimodal association communities. These communities including, for example, the default mode or the frontoparietal networks are related to a broad range of cognitive functions and behaviors (Raichle 2015; Scolari et al. 2015). Hence, it is challenging to establish the specificity of the relation between community structure and cognitive ability. At the behavioral level, face recognition exhibits high within-subject reliability (Wilmer et al. 2012) and low correlation with the recognition of other visual categories (Shakeshaft and Plomin 2015). Moreover, this ability is associated with a specialized neural network (Grill-Spector et al. 2017). As such, face-processing offers a unique opportunity for studying how such a specialized network may be supported by a segregated structure of a specific community.
Studies relating individual-level face recognition performance and connectivity properties have been sparse and were mostly focused on characterizing the properties of major white-matter tracts traversing the ventral visual stream (Tavor et al. 2014; Unger et al. 2016; but see Gomez et al. 2015). These studies are limited, as these long-range tracts are not specific to the face processing network and subserve other systems along the ventral visual pathway (Herbet et al. 2018). Moreover, recent work revealed that the majority of connections among face-selective regions are actually dominated by short rather than long fibers (Wang et al. 2020). In contrast to the approach focused on specific tracts, a network approach entails quantifying the connectivity between specific face-selective regions (i.e., nodes) to construct a graph describing these nodes and all their dyadic relations. The global structure or the topology of this graph can then be examined and characterized. Finally, previous work was mainly focused on a single connectivity modality, mostly structural connectivity, while similar studies relating FC to face recognition abilities at the individual level are missing (see Ramot et al. 2019 for connections outside the face network).
The goal of the current study was to examine the relationship between individual differences in face recognition and the community structure of the face network (Fig. 1). In light of the studies reviewed above, the current work introduces three main innovations: 1) quantifying connectivity among specific face-selective regions; 2) focusing on the community structure of the face network and its structural and dynamic properties; and 3) employing multimodal connectivity measures that bridge structure and function. We utilized a large adult lifespan data set (N = 409; Cam-CAN Shafto et al. 2014; Taylor et al. 2015) with diffusion-weighted and functional magnetic resonance imaging (MRI) and behavioral recognition measure of unfamiliar faces. Using an automated meta-analysis, we defined a network of face-selective regions and derived multimodal measures of connectivity among them. Specifically, functional and structural connectivity were computed in addition to a novel measure of communication, previously shown to map structure to function and to better capture the relation to behavior (Levakov et al. 2021). This measure is based on the connectome embedding (CE) framework that finds compact vectorized representations of nodes that preserve their context in the brain structural graph. The nodes’ context is defined as their neighbors in random walks on the brain graph and as such represents a generative model of diffusive communication around nodes. Community structure was quantified using measures of segregation and modularity and was tested while taking age into account. We compared the results with those obtained with multiple alternative null networks and show that the correlation between modularity and face recognition performance is specific to the face network. Finally, we examined the association of individual edges with face recognition and demonstrated how they capture fundamental properties of the face system such as the well-documented right hemispheric dominance (Behrmann and Plaut 2020). From a broader perspective, the approach developed here, in which a specific relation between a cognitive function and a well-defined brain network can be quantified, could be adopted to examine other behavioral domains and their related neural systems.
Figure 1.
Rationale and workflow of the study. (a) Top: a graphical depiction of a brain graph. Edges within the brain graph can be defined in several ways: Structural connectivity (yellow nodes, solid black line) quantifies the physical connections among brain nodes. Node embeddings (light blue nodes) capture their context within the brain graph based on a random walk and hence can be interpreted as a generative model of diffusive communication around nodes. Here communication (light blue superimposed dashed line) is quantified as the cosine similarity among node embeddings. Functional connectivity (gray nodes, dashed gray line) captures a statistical dependence among the nodes’ time course. (b) Communication (middle) is a process generated on top of the structural backbone (left) that gives rise to functional connectivity (right), and hence bridges between the two. (c) Workflow: Based on existing literature, we defined a community within the brain graph associated with functional activation to a specific task (e.g., face perception). We then extracted measures of community structure for each of the three connectivity modalities described in (a) and tested their relation to behavior (e.g., face recognition task scores).
Materials and Methods
Participants
The data were taken from the Cambridge Centre for Ageing and Neuroscience (Cam-CAN; Shafto et al. 2014; Taylor et al. 2015) dataset that includes functional, structural, and diffusion brain MRI along with demographic and face recognition scores. The Cam-CAN dataset includes 652 subjects (333 female, 322 male) aged 18–88 years roughly uniformly distributed from Cambridge, UK. All participants provided informed consent and the study was approved by the local ethics committee, Cambridgeshire 2 Research Ethics Committee (reference: 10/H0308/50). The data are freely available upon online access request (https://camcan-archive.mrc-cbu.cam.ac.uk/dataaccess/). Additional information on the recruitment, eligibility criteria, and demographics of the sample is available in the relevant publication (Shafto et al. 2014). Inclusion criteria were based on the availability of functional, structural, and diffusion-weighted imaging data; face recognition behavioral score; and successful completion of the preprocessing and quality control stages specified in the MRI Preprocessing and Face Recognition Scores. A total of 409 subjects complied with these criteria and were included in all following analyses.
MRI Acquisition
The data were acquired on a 3T Siemens TIM Trio System, equipped with a 32-channel head coil. T1 structural images were acquired using a gradient echo (MPRAGE) sequence with TR = 2250 ms, TE = 2.99 ms; TI = 900 ms; flip angle = 9°; FOV = 256 mm × 240 mm × 192 mm; and voxel size = 1 mm isotropic. Diffusion-weighted imaging (DWI) data were acquired using a twice-refocused spin-echo sequence with 60 diffusion gradient directions (b-values: 1000, 2000 s/mm2) and 3 reference b0 volumes, TR = 9100 ms, TE = 104 ms, voxel size = 2 mm isotropic, FOV = 192 mm × 192 mm × 66 mm, and number of averages = 1. The resting-state session was acquired using an EPI sequence with TR = 1970 ms, TE = 30 ms; flip angle = 78°; FOV = 192 × 192 × 142 mm3, voxel size = 3 × 3 × 4.44 mm3, slice thickness included 0.74 mm gap, and acquisition time of 9 min 20 s. In the resting-state session, subjects were instructed to keep their eyes closed and remain awake.
MRI Preprocessing
Here we report a general overview of the preprocessing pipeline extensively described in a previous publication (Levakov et al. 2021). T1w scans were preprocessed through the FreeSurfer’s (version 6.0) recon-all processing stream. FreeSurfer’s cortical segmentation and spherical warp were used to transfer parcellations to each subject’s volumetric anatomical space. The Connectome Mapping toolkit was used to render the Lausanne 462 node parcellation (448 cortical, 14 subcortical; Gerhard et al. 2011).
The DWI preprocessing pipeline included the following steps: denoising, motion and eddy current distortion correction, and alignment to the T1w using FreeSurfer’s white-matter segmentation (Bathelt et al. 2017; Ades-Aron et al. 2018). As fiber streamlines are less likely to extend into gray matter, the Lausanne parcellation ROI were expanded 3 mm into the white matter. The local orientation modeling and tractography were run via the Dipy package (Garyfallidis et al. 2014). Constrained spherical devolution was used to fit a local orientation model at each voxel with a spherical harmonic order of 6. Deterministic streamline tractography was conducted with a seeding density of 27. Streamlines shorter than 10 mm or ones that did not terminate in an ROI were discarded.
Functional images were preprocessed with the Configurable Pipeline for the Analysis of Connectomes (C-PAC version 1.6.2; Cameron et al. 2013). Briefly, the pipeline included the following steps: slice-timing correction, motion correction, skull stripping, and estimation of motion parameters and other nuisance signal time series. Preprocessed images were rendered in rsfMRI space.
Functional connectivity (FC) matrices were rendered after filtering the functional volumes for nuisance signals. This includes regression of the first five principal components of the signal from white matter and CSF (Behzadi et al. 2007), six motion parameters and linear and quadratic trends, and global signal regression, followed by temporal filtering between 0.1 and 0.01 Hz. Finally, a scrubbing threshold of 0.5 mm framewise displacement was applied (Power et al. 2014; removal of 1 TR before and 2 TR after excessive movement). Exclusion criterion for excessive movements was determined a priori to less than 50% (4 min and 20 s) of the resting-state session after the scrubbing procedure (453 subjects left out of 608). FC was defined as the Pearson correlation among pairs of ROIs’ time series followed by Fisher’s r-to-z transformation.
Structural connectivity (SC) matrices were constructed by counting the number of streamlines between regions normalized by the volume of these regions, rendering streamline density. Subjects with mean streamline density that was lower than 2.5 standard deviations (SDs) from the group mean were excluded (439 subjects left out of 453).
Face Recognition Scores
In the current study, face recognition ability was quantified as the accuracy score in the short form of the Benton facial recognition test (Levin et al. 1975), a standardized test for assessing recognition ability of unfamiliar faces. In each trial, a target face was presented followed by six test images. Subjects were required to identify one or more target faces among the six presented images despite changes in lighting and head orientation. Participants were required to find a single example of the target face in the first six trials and three face examples in the following seven trials. Scores were presented as proportions of correct responses out of 27 possible correct responses. Subjects with an accuracy score lower than 2 SDs from the mean were excluded (26 excluded out of 657).
Connectome Embedding (CE)
We used a variation of the word2vec algorithm (Mikolov et al. 2013) adapted for graphs (Node2Vec; Grover and Leskovec 2016) to create a vectorized representation of all brain nodes based on their high-level topological relations. The algorithm is given a set of target nodes v and their corresponding context nodes c. Its goal is to learn a set of parameters θ by maximizing the conditional probability P(v|c; θ). Simply put, the model’s goal is to predict the target node given its context. After fitting the model, a vectorized representation of each node is taken from the model’s parameters. The set of target and context nodes are taken from sequences of biased random walks on the brain structural graph. In each sequence, a sliding, fixed-size window (s = 3) is taken where the center node is the target v and the surrounding s × 2 nodes are the context c. Training samples were produced by initiating o random walk sequences of length l from each node (o = 800, l = 20). Two parameters (P = 0.1, q = 1.6) guided the random walk to remain in the vicinity of the initial starting point (local bias) or to explore distant nodes (global bias; see Grover and Leskovec 2016). All parameters used here were set as in previous publications (Levakov et al. 2021).
The model could be seen as a fully connected artificial neural network (ANN) with one hidden layer, that is, the embedding layer, with no activation function. Both the context {c1,…,c2 × s} and target v nodes are represented using a “one-hot encoding,” meaning that node i is encoded as a vector with zero in all its entries except the ith position that is equal to one. The number of neurons in the input and output layers is the number of nodes in the graph k and the number of neurons in the embedding layer is set to k', when typically k' < k (here k' = 30). The loss between the predicted and observed target node is the logarithmic loss. For each batch of training samples, the model’s parameters θ are updated, that is, the ANN weight matrices, by taking the derivative of the loss with respect to each parameter. After creating the CE for each subject, all embeddings are aligned to the same space (Levakov et al. 2021). The reference space for all embeddings was created by fitting the CE algorithm on a consensus SC matrix of all subjects (see Structure, Function, and Communication within the Face Processing Network). Finally, after CE alignment, CE matrices, that is, CE cosine angle similarity matrices, were constructed by taking the cosine of the angle between each pair of nodes. CE were created and aligned using the Cepy python package available online (https://github.com/gidlev/cepy). A detailed derivation of the model, random walk sampling and embedding alignment, is available in our previous work (Levakov et al. 2021).
Definition of the Face and Place Networks
We used NeuroQuery (Dockès et al. 2020), an automated neuroimaging meta-analysis tool, to define the face and place regions from the Lausanne atlas (Hagmann et al. 2008). NeuroQuery is a predictive model that maps terms to the likelihood of observed brain location using semantic associations. We used the term “face perception” to generate z-score maps predicting where studies are likely to report activations given those terms. Within the MNI space, we computed the mean z-score value in each region-of-interest (ROI) and ranked the regions accordingly. We selected the regions that received the highest rank and their corresponding ROI in the opposite hemisphere. This procedure generated a symmetric face network that allowed us to directly compare edges across hemispheres. The number of ROIs in each hemisphere was set to 12 since a higher number resulted in regions residing outside of the ventral face pathway (Duchaine and Yovel 2015; see Supplementary Fig. 1 for the complete rank). The place network was similarly defined using the term “place perception” with an identical number of nodes as the face network. To avoid an overlap between the two networks, place network ROIs that were located within the face network were excluded from the place networks.
Modularity and Segregation Metrics
To assess how the face-processing network community structure relates to face recognition ability, we quantified modularity and segregation in each individual. Modularity can be defined as the degree or strength of edges within a network compared to the expected degree or strength if edges were placed at random (Newman 2006). We used the following formulation of community modularity from Betzel et al. (2014):
 |
Here, qc is the contribution of community c to modularity (Q), where aij+ and aij− are the positive and negative i, j entries of the adjacency matrix. m±and si±are the mean network and edge strength, respectively, again calculated separately for negative and positive edges. For graphs that only contain positive edges, as in SC, only the first term is used (Rubinov and Sporns 2011).
Segregation is a similar but distinct metric that is defined as the proportion of edges within compared with between networks (Chan et al. 2014). To account for the SC edge distribution, where edge strength varies through several orders of magnitude, segregation was formulated as the d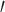 score from signal detection theory (Avidan et al. 2014):
score from signal detection theory (Avidan et al. 2014):
 |
where μbetween/within and σbetween/within represent the mean and the SD of the edges within the network or between networks, that is, between the community nodes and nodes outside the community. We further separated this term into two factors by taking the μwithin and μbetween, normalized by the denominator. This allowed us to examine the contribution of the within compared with between-network connectivity to the overall effect.
Structure, Function, and Communication within the Face Processing Network
CE-based connectivity was computed as the cosine similarity between each pair of node embedding. Group matrices were created by taking the mean edge value across all subjects for the CE cosine and FC matrices. The structural group consensus was created using distance-dependent thresholding that preserves length distribution and the density of individual subjects (Betzel et al. 2018; https://github.com/GidLev/consensus-thresholding). Correlation between structure and function was calculated using Spearman’s correlation for all direct edges, that is, edges in the SC consensus matrix that were larger than zero. Spearman’s rank correlation was employed due to the exponential distribution of the SC edges.
Community Structure of the Face Network and Behavioral Face Recognition Scores
Correlation of graph attributes to the Benton face recognition scores was reported using Spearman’s rank correlation as the Benton scores were not normally distributed. Correlation to behavior is reported on the raw Benton score and after correcting for age, age squared, and gender. We controlled for these confounds using linear regression by estimating the desired outcome, recognition scores, with each confounding variable as predictors, keeping only the residual. This approach was applied in all analyses, and we considered results significant only if they survived all corrections. Due to the use of Spearman’s correlation, in all scatterplot visualizations, we added a locally weighted scatterplot smoothing (LOWESS) line to indicate the relationships among variables. LOWESS is a method for adding a smooth local regression line to a scatterplot (Cleveland 1979).
Specificity of the Relation between Face Processing and Network Properties to the Face Network
We created a set of 10 000 null networks that preserve the spatial relations among nodes using a “spatial permutation” (Alexander-Bloch et al. 2018). Briefly, we first projected the atlas and the term activation retrieved from NeuroQuery to the FreeSurfer surface sphere with the mri_vol2surf command. Then, for each permutation, we applied a random rotation to the sphere of the right hemisphere and then the opposite rotation to the left hemisphere with respect to the sagittal plane to preserve hemispheric symmetry. Then, as in defining the empirical face network, the mean activations were computed within each new set of ROIs and their corresponding regions in the opposite hemisphere. The sphere rotation code is available in Python (https://git.io/rotate_sphere) and MATLAB (https://github.com/faskowit/brainRot-nullModel). The definition of the place network is described in Definition of the Face and Place Networks.
Edge-Level Connectivity–Behavior Correlation across Multiple Measures
The Spearman correlation between face recognition and the value of each edge across subjects was computed. Comparison of edge correlation with behavior among hemispheres and between versus within hemispheres was performed with an independent samples t-test. We used Nilearn for glass brain plotting (Abraham et al. 2014).
Results
Structure, Function, and Communication within the Face-Processing Network
Connectivity within the face-processing network was estimated using structural and functional connectivity (see Fig. 2 and Supplementary Fig. 2 for the whole brain). Brain communication modeled using embedding of the SC graph captures individual differences in behavior (Levakov et al. 2021) and better matches FC than SC alone (Rosenthal et al. 2018). Thus, we additionally quantified communication among brain nodes using the cosine similarities among their connectome embedding and tested their ability to capture FC. A significant correlation was found between the CE cosine and the FC edges (ρ(39) = 0.698, P < 0.001) and for SC and CE (ρ(39) = 0.399, P = 0.010) but not for SC and FC (ρ(39) = −0.040, P = 0.803; Fig 3c). Correlations were reported only for node pairs linked by a structural edge, that is, direct edges due to the sparsity of the SC graph. Accordingly, only 14.8% and 5.92% of edges were larger than zero for the face network and the whole brain, respectively.
Figure 2.
Quantifying connectivity within the face-processing network with structure, function, and communication. (a) The face network regions are depicted on an inflated surface (left) along with their names (right). All regions are part of the ventral face pathway and include subparcellation of the lateral occipital cortex (LOC), the fusiform gyrus, and the inferior temporal (IT) gyrus. (b) Communication among brain nodes is modeled as a diffusion process by sampling multiple random walks on the structural graph backbone. The random walk sequences are used to fit the CE model. (c) The CE cosine matrix representing the cosine similarity among the resulting node embeddings. (d) The face network functional (left) and structural (right) connectivity matrices. (e) Within the face network CE exhibits high correlation to FC (right; ρ(39) = 0.698, P < 0.001) while the correlation of SC to FC was nonsignificant (left; ρ(39) = −0.040, P = 0.803). The relation among edge values is illustrated using a LOWESS line (gray).
Figure 3.
Modularity within the right and left face network and face recognition abilities. Modularity was computed within the right (rh—upper row) and left (lh—lower row) hemispheres using the CE, SC, and FC measures (left, middle and right column respectively). In each plot, the x-axis depicts the Benton face recognition test scores and the y-axis the modularity. Spearman’s correlation between modularity and face recognition is shown at the bottom of each plot. The relation among variables is illustrated using a LOWESS line (gray). See Supplementary Fig. 3 for similar results with the segregation measure.
Community Structure of the Face Network and Behavioral Face Recognition Scores
Communities that are densely interconnected and sparsely intraconnected are associated with increased cognitive ability (see Chan et al. 2014; Gu et al. 2015 for memory and general cognitive performance). Here we quantified the community structure of the face network using both modularity and segregation and examine its relation to the behavioral score of the Benton face recognition test. All results are reported for structural, functional, and CE cosine connectivity (SC, FC, CE), and for the right and left hemispheres (rh, lh). Consistent with previous reports, modularity (Betzel et al. 2014; Cao et al. 2014) and segregation (Chan et al. 2014; Wig 2017) decreased with age within our sample (all ρ’s(407) < −0.142, all P’s < 0.004; Supplementary Table 1); hence, we repeated all analyses after controlling for age. To account for the nonlinear effect of age (Hildebrandt et al. 2011), we controlled for age squared (Pfefferbaum and Sullivan 2015) in addition to gender and in-scanner motion (Supplementary Tables 2–4). The correlation between community structure measures across modalities (CE, SC, FC) is reported in Supplementary Tables 5 and 6.
Modularity
Modularity of the face network, quantifying the strength of connectivity within a network compared with chance, was computed for all subjects. We found a significant correlation of modularity and face recognition in both hemispheres for CE and SC (CE: ρrh(407) = 0.281, ρlh(407) = 0.193; SC: ρrh(407) = 0.224, ρlh(407) = 0.204; all P’s < 0.001; Fig. 3). In both hemispheres, the correlation of FC with behavior was not significant after correcting for age (see Table 1, Supplementary Table 2).
Table 1.
Correlation of modularity and segregation within the face network and face recognition performance
| Modality | Correction | Hemisphere | ρ-modularity | P-value—modularity | ρ—segregation | P-value—segregation |
|---|---|---|---|---|---|---|
| CE | No correction | rh | 0.281 | P < 0.001 | 0.248 | P < 0.001 |
| lh | 0.193 | P < 0.001 | 0.144 | 0.004 | ||
| Age2 | rh | 0.132 | 0.007 | 0.145 | 0.003 | |
| lh | 0.075 | 0.127 | 0.086 | 0.084 | ||
| SC | No correction | rh | 0.224 | P < 0.001 | 0.249 | P < 0.001 |
| lh | 0.204 | P < 0.001 | 0.247 | P < 0.001 | ||
| Age2 | rh | 0.116 | 0.019 | 0.137 | 0.006 | |
| lh | 0.104 | 0.036 | 0.152 | 0.002 | ||
| FC | No correction | rh | 0.112 | 0.023 | 0.069 | 0.167 |
| lh | 0.118 | 0.017 | 0.079 | 0.11 | ||
| Age2 | rh | -0.034 | 0.492 | -0.029 | 0.563 | |
| lh | -0.018 | 0.717 | -0.028 | 0.572 |
Notes: Both community structure measures were computed for all three modalities (column). Each row describes Spearman’s correlation (ρ) of modularity and segregation within the face network measured using each of the three connectivity modalities. Results are presented separately for the right and left hemispheres (rh/lh) and before and after correction for age squared.
Segregation
Segregation of the face network, capturing the strength of connections within this network compared to between the face network and the rest of the brain, was derived for all subjects. We found a significant correlation of segregation and face recognition within the right hemisphere for CE and in both hemispheres for SC (CE: ρrh(407) = 0.248; SC: ρrh(407) = 0.310, ρlh(407) = 0.304; all P’s < 0.001), while the remaining measures were not significant following age corrections (see Supplementary Fig. 3, Table 1 and Supplementary Table 3). We further examine whether the observed effect is driven by strong within connectivity of the face network or weak connectivity between the face network and the rest of the brain. We found a significant correlation of within connectivity and face recognition in the right hemisphere for CE and in both hemispheres for SC (all ρ(407) > 0.151; all P’s < 0.003). The remaining measures and all correlations to between connectivity were not significant following age corrections (see Supplementary Table 7). These findings suggest that a modular and segregated community structure of the face network is associated with better face recognition ability as measured by the Benton test. Moreover, it appears that this effect is mainly driven by strong within connectivity of the face network.
The Relation between Face Processing and Network Properties Is Specific to the Face Network
Comparison to the Place Network
We wanted to examine whether the relation between the reported network attributes and face recognition is unique to the face network. We repeated all analyses but this time within the place network, which served as a control network given that it also represents a high-level, category-specific network, which is spatially distinct from face regions (Levy et al. 2001). For both modularity and segregation, we did not find a significant correlation with face recognition performance in any of the modalities in both hemispheres following a correction to age (Table 2).
Table 2.
Correlation of modularity and segregation within the place network and face recognition
| Modality | Correction | Hemisphere | ρ-modularity | P-value—modularity | ρ—segregation | P-value—segregation |
|---|---|---|---|---|---|---|
| CE | No correction | rh | 0.114 | 0.021 | 0.091 | 0.065 |
| lh | 0.196 | P < 0.001 | 0.167 | P < 0.001 | ||
| Age2 | rh | 0.042 | 0.4 | 0.044 | 0.372 | |
| lh | 0.047 | 0.344 | 0.058 | 0.242 | ||
| SC | No correction | rh | -0.008 | 0.871 | -0.035 | 0.48 |
| lh | 0.134 | 0.007 | 0.138 | 0.005 | ||
| Age2 | rh | -0.021 | 0.669 | -0.033 | 0.502 | |
| lh | 0.032 | 0.515 | 0.051 | 0.3 | ||
| FC | No correction | rh | 0.085 | 0.086 | 0.121 | 0.014 |
| lh | 0.038 | 0.447 | 0.03 | 0.552 | ||
| Age2 | rh | -0.014 | 0.782 | 0.044 | 0.37 | |
| lh | -0.048 | 0.337 | -0.049 | 0.324 |
Notes: Each row describes Spearman’s correlation (ρ) of modularity and segregation within the place network measured using each of the three connectivity modalities. Results are presented separately for the right and left hemispheres (rh/lh) and before and after correction for age squared.
Comparison to Multiple Spatially Permuted Null Networks
In addition to the first comparison described above, which is theory driven, we tested the results obtained within the right face network against a null distribution of 10 000 random networks that preserved the nodes’ spatial arrangement (Fig. 4a). The correlation of modularity to face recognition was unique to the right face network for CE and SC (CE: prh = 0.009; SC: prh = 0.004). Similar results were found with segregation (CE: prh = 0.007; SC: prh = 0.003; Fig. 4b). This was not the case for FC (Supplementary Tables 2 and 3). All results were reproduced after controlling for age, age squared, and gender (Supplementary Tables 2 and 3). These findings indicate that the relation of face recognition to modular and segregated communities is not shared across the brain but is specific to the face network.
Figure 4.
Comparison of the results of the face network to networks derived from multiple spatially permuted null networks. (a) 10 000 null networks were created by extracting regions with the highest “face perception” term activation after random rotations of the parcellation map. (b) Correlation distribution of modularity (left) or segregation (right) and face recognition. Both measures were computed within the right hemispheres (see Supplementary Tables 2 and 3 for the left) with the CE (blue) or SC (orange) measures. The empirical value obtained with the original face network is marked by vertical lines for CE and SC. Note that these line overlap on right plot.
Edge-Level Connectivity–Behavior Correlation across Multiple Measures
The results described so far provide a general perspective on the properties of the face network, but they do not inform us of the possible contribution of individual nodes or edges to face recognition. Hence, we additionally employed a mass-univariate analysis for testing the correlation between edge values and the face recognition score. The results are depicted on a glass brain (Fig. 5) and in a matrix form (Supplementary Fig. 4). We found a higher signed correlation to behavior within compared with between hemispheres for CE (t = 18.2, P < 0.001) but not for FC (t = −1.2, P = 0.240). A similar comparison could not be conducted for SC as for 73 out of 144 between-hemisphere edges, no streamlines were reconstructed for any of the subjects. We found higher correlation values within the right compared to the left hemisphere for CE (t = 7.5, P < 0.001) and SC (t = 2.2, P = 0.032) but not for FC (t = −0.1, P = 0.942). The SC left versus right hemisphere comparison was not significant following correction to age, while all other results remained significant (see Supplementary Table 8).
Figure 5.

Glass brain plot of individual edge correlations with face recognition abilities. Edge correlation with behavior corrected for age squared was tested with CE (top panel), SC (middle panel), and FC (bottom panel). Edges with a P-value smaller than 0.05 without a correction for multiple comparisons are shown; this was done only for visualization purposes. Edges positively correlated with face recognition are depicted in red and negative correlations in blue. Node colors state whether they are a subdivision of the lateral occipital cortex (orange), the fusiform gyrus (red), or the inferior temporal cortex (green). Node size is proportional to their strength, that is, the sum of their absolute edge weights. Edges are presented on coronal, sagittal, and horizontal projections.
Discussion
The processing of a complex stimulus, such as a face, does not depend solely on the computation conducted by specific brain regions but also on communication among them. Perception of faces recruits these subparts of the face-processing network, forming increasingly complex representations along the ventral visual stream to support subsequent tasks such as identification (Riesenhuber and Poggio 1999). In this work, we focus on the community structure of the face network and its relation to face recognition ability. Community structure, assessed with modularity and segregation, was derived for multimodal measures of the face network. We examined how individual variability in community structure correlates to face recognition performance and the extent to which this correlation was specific to the face network.
Our main finding is that segregation and modularity of the face network, derived with SC and CE, positively correlated with recognition abilities. This was found in both hemispheres using SC and in the right hemisphere using CE. Furthermore, it appears that this effect is driven by strong within connectivity of the face network rather than weak connectivity between the face network and the rest of the brain. These results were still apparent after controlling for age and were specific to the face network when compared to the place network or to spatially permuted null networks. A segregated community structure was previously related to performance in several associative cognitive tasks (Chan et al. 2014; Nashiro et al. 2017). However, to the best of our knowledge, this is the first evidence of such relationships in perception. Nodes of the face network were defined as regions specifically activated during face perception, in contrast to previous work limited to existing canonical resting-state networks (Baum et al. 2016). This suggests that the community structure relation to behavior also exists in networks that are recruited by an engagement in a specific task and are less apparent during rest (Cohen and D’Esposito 2016; Hahn et al. 2020). This behavioral advantage of a segregated community structure was suggested to be mediated by the ability to preserve functional specialization (Gallos et al. 2012), reduce interference among cognitive systems (Hampson et al. 2010; Fornito et al. 2012), and balance efficiency and wiring cost (Bullmore and Sporns 2012).
The multimodal mapping of the face network provided a unique opportunity to examine how different aspects of interaction among face-selective regions capture recognition abilities. SC, quantified as streamline density, revealed a correlation between face recognition and both segregation and modularity. Prior work reported such relation to long-range white-matter tracts of the ventral visual system (Thomas et al. 2008; Hodgetts et al. 2015). However, our findings are novel as they reveal the structural topology of the face network. We applied the same analysis using a recently introduced measure of communication based on embedding similarity among brain nodes (CE; Levakov et al. 2021). We first demonstrate our ability to map structure to function within the face network as previously shown across the brain. Using this CE measure, within the right hemisphere we found a similar relation between community structure and recognition, corroborating the results obtained with SC. Finding this relation only in the right hemisphere is consistent with the well-documented right hemispheric dominance of face processing (Behrmann and Plaut 2020). Importantly, despite the fact the CE is calculated based on the sparse SC matrix, the communication matrix is fully dense. This, in turn, allows testing communication–behavior relations among all pairs of nodes. Finally, repeating the analysis using FC did not reveal similar results after taking age into account. This is in accordance with the sparse reports of FC relation with individual differences in face perception in typical subjects (but see Rosenthal et al. 2017 for congenital prosopagnosia). Accordingly, a recent study reported such a null finding for edges within the face network in a relatively large sample (N = 53; Ramot et al. 2019). This study, however, reported a correlation of functional connections between face-selective regions and non-selective face regions with perception performance. It is also possible that only FC obtained during a face perception task would reveal such a link to behavior (Greene et al. 2018). This possibility is consistent with reports of a correlation of face perception abilities to the extent of activation to faces (Elbich and Scherf 2017; but see McGugin and Gauthier 2016).
To gain a further, in-depth perspective of connectivity–behavior relation, we applied a mass univariate analysis, testing the correlation of each edge with face recognition. This has led to two findings, first, for both communication and SC, we found an age-dependent laterality effect such that edges within the right hemisphere had a larger correlation to behavior. After taking age into account, this effect was only maintained for CE. This suggests that the right hemisphere is not only more engaged in face processing (Maurer 2008) but also that the individual variability of its connectivity profile has a higher correlation to behavior. The second finding was a marked difference in the correlation to behavior among nodes connecting the two hemispheres to nodes within each hemisphere. Edges within hemispheres tended to have a higher and positive correlation to behavior compared to edges among hemispheres with the opposite effect. This was found only with CE as most of the interhemispheric connections cannot be reconstructed with diffusion imaging (Jones et al. 2013). A possible basis for this finding may be gleaned from lifespan cross-sectional studies showing that in addition to a decline in face perception abilities (Wilmer 2017), aging is also accompanied by decreased laterality of face processing (Lee et al. 2011; Daniel and Bentin 2012). It has been hypothesized that difficulty in face processing in aging might drive the recruitment of left face regions (Frässle et al. 2016; Lee et al. 2011; see Reuter-Lorenz and Cappell 2008 for the compensation hypothesis), which may result in enhanced cross-hemisphere connectivity. Hence, this compensatory effect might mediate the relation between lower perceptual abilities and the higher interhemispheric connectivity we observed.
It is important to consider several limitations of the current study. First, the nodes of the face network were defined using an automated meta-analysis, hence they represent a population average rather than subject-specific locations. Although the location of face-selective regions significantly overlaps among subjects, there is also substantial variability (Berman et al. 2010) and it is common to define them individually using a functional localizer (Saxe et al. 2006; but see Friston et al. 2006). Applying this approach to tractography might be challenging (Broser et al. 2012), yet it may enable to control for individual-level variation in the functional locations of face patches. A second limitation is the use of the Benton facial recognition test (Levin et al. 1975) originally designed for testing neurologically impaired individuals (Duchaine and Weidenfeld 2003 but see Murray et al. 2021). Assessing recognition abilities using a measure such as the Cambridge face memory test (Duchaine and Nakayama 2006) might be better suited for capturing existing variability in typical individuals. Finally, testing neural correlates of face perception across the lifespan provides valuable knowledge on the link between face perception, aging, and brain connectivity. However, conducting future studies in a more homogeneous age range might help to better account for the effect of age on the brain–behavior relation.
To conclude, the goal of the current work was to examine whether a specific link could be established between face recognition ability and the modular structure of the face network. The cognitive and neural basis of face processing is a topic of much research. This accumulated acknowledge provides a unique opportunity to study how a specific cognitive ability is supported by its dedicated neural architecture. We have shown that face recognition ability was positively correlated to the expression of modularity of the face network and not of other networks. These results are novel, both in the domain of perception and specifically in the study of face recognition within the general population. We demonstrated that CE, although based on SC, shows high correspondence with FC and captures behavior at both the network level and the individual edges level. These findings may advance our understanding of how neural networks form the basis for specific cognitive abilities and how they may account for individual differences in these abilities.
Notes
Data collection and sharing for this project were provided by the Cambridge Centre for Ageing and Neuroscience (CamCAN). Conflict of Interest: None declared.
Funding
The U.S.–Israel Binational Science Foundation (BSF) (2017242 to G.A. and O.S.); National Institutes of Health (NIH) (R01MH122957 to O.S.); CamCAN funding was provided by the UK Biotechnology and Biological Sciences Research Council (BB/H008217/1); the UK Medical Research Council and University of Cambridge, UK.
Data availability
The unprocessed data are openly available upon online access request (https://camcan-archive.mrc-cbu.cam.ac.uk/dataaccess/). A python package implementation of the CE framework (https://github.com/GidLev/cepy) is available online.
Supplementary Material
Contributor Information
Gidon Levakov, Department of Cognitive and Brain Sciences, Ben-Gurion University of the Negev, P.O.B. 653, Beer-Sheva 8410501, Israel; Zlotowski Center for Neuroscience, Ben-Gurion University of the Negev, P.O.B. 653, Beer-Sheva 8410501, Israel.
Olaf Sporns, Department of Psychological and Brain Sciences, Indiana University, 107 S Indiana Ave, Bloomington, IN 47405, USA; Program in Neuroscience, Indiana University, 107 S Indiana Ave, Bloomington, IN 47405, USA.
Galia Avidan, Department of Cognitive and Brain Sciences, Ben-Gurion University of the Negev, P.O.B. 653, Beer-Sheva 8410501, Israel; Zlotowski Center for Neuroscience, Ben-Gurion University of the Negev, P.O.B. 653, Beer-Sheva 8410501, Israel; Department of Psychology, Ben-Gurion University of the Negev, P.O.B. 653, Beer-Sheva 8410501, Israel.
References
- Abraham A, Pedregosa F, Eickenberg M, Gervais P, Mueller A, Kossaifi J, Gramfort A, Thirion B, Varoquaux G. 2014. Machine learning for neuroimaging with scikit-learn. Frontiers. Neuroinformatics. 8(Feb):14. 10.3389/fninf.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ades-Aron B, Veraart J, Kochunov P, McGuire S, Sherman P, Kellner E, Novikov DS, Fieremans E. 2018. Evaluation of the accuracy and precision of the diffusion parameter EStImation with Gibbs and NoisE removal pipeline. Neuroimage. 183:532–543. 10.1016/j.neuroimage.2018.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch AF, Shou H, Liu S, Satterthwaite TD, Glahn DC, Shinohara RT, Vandekar SN, Raznahan A. 2018. On testing for spatial correspondence between maps of human brain structure and function. Neuroimage. 178:540–551. 10.1016/j.neuroimage.2018.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidan G, Tanzer M, Hadj-Bouziane F, Liu N, Ungerleider LG, Behrmann M. 2014. Selective dissociation between core and extended regions of the face processing network in congenital prosopagnosia. Cereb Cortex. 24(6):1565–1578. 10.1093/cercor/bht007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Wymbs NF, Porter MA, Mucha PJ, Carlson JM, Grafton ST. 2011. Dynamic reconfiguration of human brain networks during learning. Proc Natl Acad Sci U S A. 108(18):7641–7646. 10.1073/pnas.1018985108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathelt J, Barnes J, Raymond FL, Baker K, Astle D. 2017. Global and local connectivity differences converge with gene expression in a neurodevelopmental disorder of known genetic origin. Cereb Cortex. 27(7):3806–3817. 10.1093/cercor/bhx027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum GL, Ciric R, Roalf DR, Betzel RF, Moore TM, Shinohara RT, Kahn AE, Quarmley M, Cook PA, Elliot MA, et al. 2017. Modular segregation of structural brain networks supports the development of executive function in youth. Curr Biol. 27(11):1561–1572. http://arxiv.org/abs/1608.03619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann M, Avidan G. 2005. Congenital prosopagnosia: face-blind from birth. Trends Cogn Sci. 9(4):180–187. 10.1016/j.tics.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Plaut DC. 2020. Hemispheric organization for visual object recognition: a theoretical account and empirical evidence. Perception. 49(4):373–404. 10.1177/0301006619899049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. 2007. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 37(1):90–101. 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman MG, Park J, Gonzalez R, Polk TA, Gehrke A, Knaffla S, Jonides J. 2010. Evaluating functional localizers: the case of the FFA. Neuroimage. 50(1):56–71. 10.1016/j.neuroimage.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel RF, Byrge L, He Y, Goñi J, Zuo XN, Sporns O. 2014. Changes in structural and functional connectivity among resting-state networks across the human lifespan. Neuroimage. 102(P2):345–357. 10.1016/j.neuroimage.2014.07.067. [DOI] [PubMed] [Google Scholar]
- Betzel RF, Griffa A, Hagmann P, Mišić B. 2018. Distance-dependent consensus thresholds for generating group-representative structural brain networks. Network Neurosci. 3(2):475–496. 10.1162/netn_a_00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broser PJ, Groeschel S, Hauser TK, Lidzba K, Wilke M. 2012. Functional MRI-guided probabilistic tractography of cortico-cortical and cortico-subcortical language networks in children. Neuroimage. 63(3):1561–1570. 10.1016/j.neuroimage.2012.07.060. [DOI] [PubMed] [Google Scholar]
- Bullinaria JA. 2007. Understanding the emergence of modularity in neural systems. Cognit Sci. 31(4):673–695. 10.1080/15326900701399939. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. 2012. The economy of brain network organization. Nat Rev Neurosci. 13(5):336–349. 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- Craddock C, Sikka S, Cheung B, Khanuja R, Ghosh SS, Yan C, Li Q, Lurie D, Vogelstein J, Burns R et al. 2013. Towards automated analysis of connectomes: the configurable pipeline for the analysis of connectomes (c-pac). Front Neuroinform. 42:10–3389. 10.3389/conf.fninf.2013.09.00042. [DOI] [Google Scholar]
- Cao M, Wang JH, Dai ZJ, Cao XY, Jiang LL, Fan FM, Song XW, Xia MR, Shu N, Dong Qet al. 2014. Topological organization of the human brain functional connectome across the lifespan. Dev Cogn Neurosci. 7:76–93. 10.1016/j.dcn.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. 2014. Decreased segregation of brain systems across the healthy adult lifespan. Proc Natl Acad Sci U S A. 111(46):E4997–E5006. 10.1073/pnas.1415122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland WS. 1979. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 74(368):829–836. 10.1080/01621459.1979.10481038. [DOI] [Google Scholar]
- Cohen JR, D’Esposito M. 2016. The segregation and integration of distinct brain networks and their relationship to cognition. J Neurosci. 36(48):12083–12094. 10.1523/JNEUROSCI.2965-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel S, Bentin S. 2012. Age-related changes in processing faces from detection to identification: ERP evidence. Neurobiol Aging. 33(1):206.e1–206.e28. 10.1016/j.neurobiolaging.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockès J, Poldrack RA, Primet R, Gözükan H, Yarkoni T, Suchanek F, Thirion B, Varoquaux G. 2020. Neuroquery, comprehensive meta-analysis of human brain mapping. Elife. 9:e53385. 10.7554/eLife.53385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchaine BC, Weidenfeld A. 2003. An evaluation of two commonly used tests of unfamiliar face recognition. Neuropsychologia. 41(6):713–720. 10.1016/S0028-3932(02)00222-1. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Nakayama K. 2006. The Cambridge face memory test: results for neurologically intact individuals and an investigation of its validity using inverted face stimuli and prosopagnosic participants. Neuropsychologia. 44(4):576–585. 10.1016/J.NEUROPSYCHOLOGIA.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Duchaine B, Yovel G. 2015. A revised neural framework for face processing. Ann Rev Vision Sci. 1(1):393–416. 10.1146/annurev-vision-082114-035518. [DOI] [PubMed] [Google Scholar]
- Elbich DB, Scherf S. 2017. Beyond the FFA: brain-behavior correspondences in face recognition abilities. Neuroimage. 147:409–422. 10.1016/J.NEUROIMAGE.2016.12.042. [DOI] [PubMed] [Google Scholar]
- Fornito A, Harrison BJ, Zalesky A, Simons JS. 2012. Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proc Natl Acad Sci U S A. 109(31):12788–12793. 10.1073/pnas.1204185109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frässle S, Paulus FM, Krach S, Schweinberger SR, Stephan KE, Jansen A. 2016. Mechanisms of hemispheric lateralization: asymmetric interhemispheric recruitment in the face perception network. Neuroimage. 124:977–988. 10.1016/j.neuroimage.2015.09.055. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Rotshtein P, Geng JJ, Sterzer P, Henson RN. 2006. A critique of functional localisers. NeuroImage. 30(4):1077–1087. 10.1016/j.neuroimage.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Gallos LK, Makse HA, Sigman M. 2012. A small world of weak ties provides optimal global integration of self-similar modules in functional brain networks. Proc Natl Acad Sci U S A. 109(8):2825–2830. 10.1073/pnas.1106612109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garyfallidis E, Brett M, Amirbekian B, Rokem A, Walt S, Descoteaux M, Nimmo-Smith I. 2014. Dipy, a library for the analysis of diffusion MRI data. Front Neuroinform. 8(Feb):8. 10.3389/fninf.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard S, Daducci A, Lemkaddem A, Meuli R, Thiran J-P, Hagmann P. 2011. The connectome viewer toolkit: an open source framework to manage, analyze, and visualize connectomes. Front Neuroinform. 5:3. 10.3389/fninf.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez J, Pestilli F, Witthoft N, Golarai G, Liberman A, Poltoratski S, Yoon J, Grill-Spector K. 2015. Functionally defined white matter reveals segregated pathways in human ventral temporal cortex associated with category-specific processing. Neuron. 85(1):216–227. 10.1016/j.neuron.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene AS, Gao S, Scheinost D, Constable RT. 2018. Task-induced brain state manipulation improves prediction of individual traits. Nat Commun. 9(1):2807. 10.1038/s41467-018-04920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Weiner KS, Kay K, Gomez J. 2017. The functional neuroanatomy of human face perception. Ann Rev Vision Sci. 3(1):167–196. 10.1146/annurev-vision-102016-061214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover A, Leskovec J. 2016. node2vec: scalable feature learning for networks. In: KDD: Proceedings International Conference on Knowledge Discovery & Data Mining. Vol. 2016, p. 855–864. 10.1145/2939672.2939754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Satterthwaite TD, Medaglia JD, Yang M, Gur RE, Gur RC, Bassett DS. 2015. Emergence of system roles in normative neurodevelopment. Proc Natl Acad Sci U S A. 112(44):13681–13686. 10.1073/pnas.1502829112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Van Wedeen J, Sporns O. 2008. Mapping the structural Core of human cerebral cortex. PLoS Biol. 6(7):1479–1493. 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Breakspear M, Rischka L, Wadsak W, Godbersen GM, Pichler V, Michenthaler P, Vanicek T, Hacker M, Kasper Set al. 2020. Reconfiguration of functional brain networks and metabolic cost converge during task performance. Elife. 9:e52443. 10.7554/eLife.52443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen N, Roth JK, Gore JC, Constable RT. 2010. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magn Reson Imaging. 28(8):1051–1057. 10.1016/j.mri.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbet G, Zemmoura I, Duffau H. 2018. Functional anatomy of the inferior longitudinal fasciculus: from historical reports to current hypotheses. Front Neuroanat. 12:77. 10.3389/fnana.2018.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse JK, Tsao DY. 2020. The macaque face patch system: a turtle’s underbelly for the brain. Nat Rev Neurosci. 21(12):695–716. 10.1038/s41583-020-00393-w. [DOI] [PubMed] [Google Scholar]
- Hildebrandt A, Wilhelm O, Schmiedek F, Herzmann G, Sommer W. 2011. On the specificity of face cognition compared with general cognitive functioning across adult age. Psychol Aging. 26(3):701–715. 10.1037/a0023056. [DOI] [PubMed] [Google Scholar]
- Hodgetts CJ, Postans M, Shine JP, Jones DK, Lawrence AD, Graham KS. 2015. Dissociable roles of the inferior longitudinal fasciculus and fornix in face and place perception. Elife. 4(August 2015):e07902. 10.7554/eLife.07902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R. 2013. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. NeuroImage. 73:239–254. 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Lee Y, Grady CL, Habak C, Wilson HR, Moscovitch M. 2011. Face processing changes in normal aging revealed by fMRI adaptation. J Cogn Neurosci. 23(11):3433–3447. 10.1162/jocn_a_00026. [DOI] [PubMed] [Google Scholar]
- Levakov G, Faskowitz J, Avidan G, Sporns O. 2021. Mapping individual differences across brain network structure to function and behavior with connectome embedding. Neuroimage. 242:118469. 10.1016/J.NEUROIMAGE.2021.118469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HS, Hamsher KS, Benton AL. 1975. A short form of the test of facial recognition for clinical use. Journal of Psychology: Interdisciplinary and Applied. 91(2):223–228. 10.1080/00223980.1975.9923946. [DOI] [Google Scholar]
- Levy I, Hasson U, Avidan G, Hendler T, Malach R. 2001. Center-periphery organization of human object areas. Nat Neurosci. 4(5):533–539. 10.1038/87490. [DOI] [PubMed] [Google Scholar]
- Maffei A, Sessa P. 2021. Event-related network changes unfold the dynamics of cortical integration during face processing. Psychophysiology. 58(5):e13786. 10.1111/psyp.13786. [DOI] [PubMed] [Google Scholar]
- Maurer U. 2008. Category specificity in early perception: face and word N170 responses differ in both lateralization and habituation properties. Front Hum Neurosci. 2(Dec):18. 10.3389/neuro.09.018.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGugin RW, Gauthier I. 2016. The reliability of individual differences in face-selective responses in the fusiform gyrus and their relation to face recognition ability. Brain Imaging Behav. 10(3):707–718. 10.1007/s11682-015-9467-4. [DOI] [PubMed] [Google Scholar]
- Medaglia JD, Lynall ME, Bassett DS. 2015. Cognitive network neuroscience. J Cogn Neurosci. 27(8):1471–1491. 10.1162/jocn_a_00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolov T, Chen K, Corrado G, Dean J. 2013. Distributed representations of words and phrases and their compositionality. In: Advances in neural information processing systems. p. 3111–3119.
- Mišić B, Sporns O. 2016. From regions to connections and networks: new bridges between brain and behavior. Curr Opin Neurobiol. 40:1–7. 10.1016/j.conb.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E, Bennetts R, Tree J, Bate S. 2021. An update of the Benton facial recognition test. Behav Res Methods. (Accepted). [DOI] [PubMed] [Google Scholar]
- Nashiro K, Sakaki M, Braskie MN, Mather M. 2017. Resting-state networks associated with cognitive processing show more age-related decline than those associated with emotional processing. Neurobiol Aging. 54:152–162. 10.1016/j.neurobiolaging.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MEJ. 2006. Modularity and community structure in networks. Proc Natl Acad Sci U S A. 103(23):8577–8582. 10.1073/pnas.0601602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. 2015. Cross-sectional versus longitudinal estimates of age-related changes in the adult brain: overlaps and discrepancies. Neurobiol Aging. 36(9):2563–2567. 10.1016/j.neurobiolaging.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. 2014. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 84:320–341. 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyles JA, Verstynen TD, Schneider W, Tarr MJ. 2013. Explicating the face perception network with white matter connectivity. PLoS One. 8(4):e61611. 10.1371/journal.pone.0061611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. 2015. The Brain’s default mode network. Annu Rev Neurosci. 38:433–447. 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Ramot M, Walsh C, Martin A. 2019. Multifaceted integration: memory for faces is subserved by widespread connections between visual, memory, auditory, and social networks. J Neurosci. 39(25):4976–4985. 10.1523/JNEUROSCI.0217-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. 2008. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 17(3):177–182. 10.1111/j.1467-8721.2008.00570.x. [DOI] [Google Scholar]
- Riesenhuber M, Poggio T. 1999. Hierarchical models of object recognition in cortex. Nat Neurosci. 2(11):1019–1025. 10.1038/14819. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. 2004. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 23(2):752–763. 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Rosenthal G, Sporns O, Avidan G. 2016. Stimulus dependent dynamic reorganization of the human face processing network. Cereb Cortex. 8:e59886. 10.1093/cercor/bhw279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal G, Tanzer M, Simony E, Hasson U, Behrmann M, Avidan G. 2017. Altered topology of neural circuits in congenital prosopagnosia. Elife. 6:e25069. 10.7554/eLife.25069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal G, Váša F, Griffa A, Hagmann P, Amico E, Goñi J, Avidan G, Sporns O. 2018. Mapping higher-order relations between brain structure and function with embedded vector representations of connectomes. Nat Commun. 9(1):2178. 10.1038/s41467-018-04614-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. 2011. Weight-conserving characterization of complex functional brain networks. Neuroimage. 56(4):2068–2079. 10.1016/j.neuroimage.2011.03.069. [DOI] [PubMed] [Google Scholar]
- Saxe R, Brett M, Kanwisher N. 2006. Divide and conquer: a defense of functional localizers. NeuroImage. 30(4):1088–1096. 10.1016/j.neuroimage.2005.12.062. [DOI] [PubMed] [Google Scholar]
- Scolari M, Seidl-Rathkopf KN, Kastner S. 2015. Functions of the human frontoparietal attention network: evidence from neuroimaging. Curr Opin Behav Sci. 1:32–39. 10.1016/j.cobeha.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafto MA, Tyler LK, Dixon M, Taylor JR, Rowe JB, Cusack R, Calder AJ, Marslen-Wilson WD, Duncan J, Dalgleish Tet al. 2014. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) study protocol: a cross-sectional, lifespan, multidisciplinary examination of healthy cognitive ageing. BMC Neurol. 14(1):204. 10.1186/s12883-014-0204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeshaft NG, Plomin R. 2015. Genetic specificity of face recognition. Proc Natl Acad Sci U S A. 112(41):12887–12892. 10.1073/pnas.1421881112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavor I, Yablonski M, Mezer A, Rom S, Assaf Y, Yovel G. 2014. Separate parts of occipito-temporal white matter fibers are associated with recognition of faces and places. Neuroimage. 86:123–130. 10.1016/j.neuroimage.2013.07.085. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Williams N, Cusack R, Auer T, Shafto MA, Dixon M, Tyler LK, Cam-CAN X, Henson RN. 2015. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) data repository: structural and functional MRI, MEG, and cognitive data from a cross-sectional adult lifespan sample. Neuroimage. 144:262–269. 10.1016/j.neuroimage.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Avidan G, Humphreys K, Jung KJ, Gao F, Behrmann M. 2009. Reduced structural connectivity in ventral visual cortex in congenital prosopagnosia. Nat Neurosci. 12(1):29–31. 10.1038/nn.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Moya L, Avidan G, Humphreys K, Kwan JJ, Peterson MA, Behrmann M. 2008. Reduction in white matter connectivity, revealed by diffusion tensor imaging, may account for age-related changes in face perception. J Cogn Neurosci. 20(2):268–284. 10.1162/jocn.2008.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger A, Alm KH, Collins JA, O’Leary JM, Olson IR. 2016. Variation in white matter connectivity predicts the ability to remember faces and discriminate their emotions. J Int Neuropsychol Soc. 22(2):180–190. 10.1017/S1355617715001009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Metoki A, Smith DV, Medaglia JD, Zang Y, Benear S, Popal H, Lin Y, Olson IR. 2020. Multimodal mapping of the face connectome. Nat Hum Behav. 4(4):397–411. 10.1038/s41562-019-0811-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig GS. Segregated systems of human brain networks. Trends Cognitive Sci .2017;21(12):981–996. 10.1016/j.tics.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Wilmer JB. 2017. Individual differences in face recognition: a decade of discovery. Curr Dir Psychol Sci. 26(3):225–230. 10.1177/0963721417710693. [DOI] [Google Scholar]
- Wilmer JB, Germine L, Chabris CF, Chatterjee G, Gerbasi M, Nakayama K. 2012. Capturing specific abilities as a window into human individuality: the example of face recognition. Cogn Neuropsychol. 29(5–6):360–392. 10.1080/02643294.2012.753433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The unprocessed data are openly available upon online access request (https://camcan-archive.mrc-cbu.cam.ac.uk/dataaccess/). A python package implementation of the CE framework (https://github.com/GidLev/cepy) is available online.






