Abstract
Objective
To investigate the protective effects and possible mechanisms of Shenkang Injection (SKI) on the diabetic nephropathy in streptozotocin-induced mice.
Methods
STZ with the feeding of high fat diet (HFD) was used to induce diabetic mice. The balb/c mice and diabetic mice were then randomly divided into five groups: (1) control group, (2) model group, (3) alprostadil (Alp, 1.5 μg/kg) group, (4) SKI (30 ml/kg) group, (5) Alp (1.5 μg/kg) + SKI (15 ml/kg) group. After six weeks' treatment, blood, urine and kidney tissues were collected for biochemical assay, ELISA assay, and pathological analysis.
Results
Diabetic mice exhibited evident manifestations of diabetic nephropathy (DN), as indicated by increased 24-h urine volume, urinary albumin and kidney weight index (P < 0.01), which could be attenuated by SKI treatment (P < 0.01). SKI was further found to improve abnormal morphology in glomerulus with increased glomerular volume and to decrease urinary N-acetyl-b-D-glucpsaminidase (NAG), β2-microglobulin (β2-MG), and kidney injury molecules-1 (KIM-1) levels (P < 0.05, P < 0.01). Plasma levels of anti-oxidant enzymes significantly reduced in the diabetic mice, and those decreases could be reversed by SKI and Alp treatments. Additionally, SKI obviously suppressed the diabetes-induced increases of pro-inflammatory cytokines (IL-6, IL-1β and TNF-α) (P < 0.01). Meanwhile, SKI was found to effectively attenuate the diabetes-induced coagulation dysfunction, as evidenced by lengthening prothrombin and thrombin time, and decreasing plasma levels of fibrinogen (FIB), 6-K-PGF1α and thromboxane B2 (TXB2) (P < 0.05, P < 0.01). With SKI and Alp combined treatment, the anti-oxidant activities and improvements of coagulation dysfunction were enhanced.
Conclusion
SKI possesses a remarkable property to prevent diabetic nephropathy. The improvements of kidney function and hypercoagulability by SKI were enhanced with Alp combined treatment. The molecular mechanisms underlying the protection of SKI against DN may be related to enhancing the anti-oxidant and anti-inflammatory activities, and improving the coagulation dysfunction.
Keywords: ShenKang Injection, diabetic nephropathy, inflammation, oxidative stress
1. Introduction
Diabetic nephropathy (DN) is one of the devastating diabetic microvascular complications and the leading cause of end stage renal disease (ESRD) (Papadopoulou-Marketou et al., 2018). About 21.3% of the diabetes suffer from DN in China (Zhang et al., 2016). ESRD caused by DN has been the leading cause of death in nearly half of the diabetic patients (Ritz, 2013). Pathologically, the structural and functional changes of DN involve many aspects, including glomerulosclerosis, glomerular basement membrane thickening, tubulointerstitial fibrosis and albuminuria (Tervaert et al., 2010, Alomari et al., 2019). These pathological alterations can be induced by many factors including hyperglycemia, reactive oxygen species (ROS), and inflammation (Ding et al., 2018). Endogenous anti-oxidant enzymes play key roles in the protection of DN through clearing ROS. Thus, the effects of SKI on anti-oxidant enzymes including CAT, SOD and GSH-Px were detected in the present study. Previous studies have indicated that the herbs contained in SKI, such as Salvia miltiorrhiza Bge. (Danshen in Chinese), which possesses the anti-inflammatory potential. Chronic inflammation could promote the progression of DN. We speculated that SKI may reduce diabetes-induced inflammation. Thus, the inflammatory cytokines were also measured in the present study. Abnormality of glucose and lipids metabolism causes injury of vascular endothelial cells, which subsequently leads to platelet activation and aggregation, and further causes hypercoagulability (Bochenek & Schafer, 2019). Coagulation dysfunction can cause thrombogenesis, decreased renal blood flow and glomerulosclerosis, and eventually results in the progression of DN (Kaizu et al., 1993). Alprostadil (Alp) is widely used in the clinic based on its vasodilatory action to dilate blood vessels and its anti-platelet effects to suppress platelet aggregation. Clinical studies also indicate that Alp significantly improves renal function, reduces urinary protein, and alleviates the inflammatory response (Luo et al., 2014, Qin et al., 2017). Thus, Alp was selected as a positive drug to explore the protective roles of SKI in the treatment of DN.
Shenkang Injection (SKI), approved by State Food and Drug Administration of China (CFDA) in 1999, was used to treat chronic kidney diseases (CKD) (Permission No. YBZ08522004) (Fu et al., 2019). This treatment is composed of Rhei Radix et Rhizoma (Dahuang in Chinese, RRR), Salviae Miltiorrhizae Radix et Rhizoma (Danshen in Chinese, SMRR), Astragali Radix (Huangqi in Chinese, AR), and Carthami Flos (Honghua in Chinese, CF) (Wu et al., 2015). Clinical studies have indicated that 73.05% of patients with CDK treated with SKI exhibit improved renal function (Yong, Yang, & Chen, 2015). SKI protects against CDK through inhibiting oxidative stress-induced renal injury and preventing interstitial fibrosis (Wu et al., 2015). Although SKI has been reported to play beneficial roles in the treatment of CDK, little is known about its effects on DN. In the present study, we applied STZ-induced diabetic mouse model to investigate the protective effects of SKI on DN, and also study its possible molecular mechanisms.
2. Materials and methods
2.1. Animals
Male balb/c mice (20–22 g) were purchased from Beijing Huafukang Laboratory Animal Technology Co., Ltd. All animal experiments were approved by the Laboratory Animal Ethics Committee of the Institute of Medicinal Plant Development, Peking Union Medical College, and conformed to the Guide for the Care and Use of Laboratory Animals (Permit Number: SYXK 2017-0020).
2.2. Reagents
SKI (TYZ20181001) was supplied by Shijishenkang Co., Ltd. (Xi'an, China). Alprostadil injection (Alp) was purchased from Hainan Bikai Pharmaceutical Co., Ltd. (Hainan, China). STZ was purchased from Sigma-Aldrich (St. Louis, MO, USA). Primary antibody against collagen III (ab7778) was obtained from Abcam (Cambridge, UK). The albumin, N-acetyl-b-D-glucpsaminidase (NAG), HbA1c, catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GSH-PX) and malondialdehyde (MDA) kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Mouse interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), interleukin-4 (IL-4), interleukin-10 (IL-10), nitric oxide (NO), C-reactive protein (CRP), urinary β2-microglobulin (β2-MG), kidney injury molecules-1 (KIM-1), 6-K-PGF1α, and thromboxane B2 (TXB2) ELISA kits were purchased from Sinoukbio (Beijing, China).
2.3. Establishment of diabetic mouse model
Experimental diabetic mice were fed with high fat diet (20 kcal% protein, 20 kcal% carbohydrate and 60 kcal% fat) for 4 weeks and then they were induced by intraperitoneal (i.p.) injection of streptozotocin (STZ) (150 mg/kg, STZ was dissolved in 0.1 mmol/L citrate buffer, pH 4.5). Animals with glucose levels no less than 11.1 mmol/L were regarded as diabetes (DM) (Wang, Song, Ding, & Wei, 2019).
2.4. Grouping and treatment
The diabetic mice were randomly divided into five groups: (1) control group, (2) model group, (3) Alprostadil (Alp, 1.5 μg/kg, clinical equivalent dose) group, (4) SKI (30 ml/kg, clinical two-fold dose) group, (5) Alp (1.5 μg/kg) + SKI (15 ml/kg, clinical equivalent dose) group. Alp and SKI were injected intraperitoneally once a day for 6 weeks. Mice in the control and model groups were given the same dose of saline solution.
2.5. Measurement of body weight, fasting blood glucose and kidney weight index (KWI)
After 6 weeks’ treatment, the body weight in each group was determined, and fasting blood glucose was measured by an automatic glucometer (One Touch Ultra, Lifescan, USA). The kidney weight index (KWI) in each group was estimated by calculating the ratio of the kidney weight to the body weight.
2.6. Glucose tolerance test
Fasted overnight mice were subjected to a glucose tolerance test (GTT) through oral administration of 2 g/kg glucose. Blood glucose levels were measured by an automatic glucometer at indicated time points.
2.7. Measurement of 24-h urine volume and biochemical parameters in urine
The 24-h urine was collected and urine volume was recorded at the last administration of SKI. Urinary albumin and N-acetyl-b-D-glucpsaminidase (NAG) levels were detected using the kits from Nanjing Jiancheng bioengineering Institute (Nanjing, China). β2-Microglobulin (β2-MG), Kidney injury molecules-1 (KIM-1) levels were measured by ELISA kits according to the manufacturer's instructions (Sinoukbio, Beijing, China).
2.8. Measurement of biochemical parameters in blood
The animals were anesthetized with intraperitoneal injection of sodium pentobarbital (40 mg/kg). The blood sample was collected from the orbital plexus of the eyes and then genteelly mixed with 55 μL 3.8% citrate sodium anticoagulant in centrifuge tube. The plasma was separated by centrifugation at 3800 g for 15 min.
Activated partial thromboplastin time (APTT), prothrombin time (PT), thrombin time (TT), and fibrinogen (FIB) were assayed by a PUN-208A (BECKMAN COULTER, USA) automated blood coagulation analyzer.
The antioxidant enzymes (CAT, SOD and GXH-PX) and MDA levels were determined by the kits from Nanjing Jiancheng bioengineering Institute (Nanjing, China). Inflammatory cytokines (IL-4, IL-10, IL-1β, IL-6 and TNF-α), NO, CRP, 6-K-PGF1α and TXB2 levels were detected by ELISA kits according to the manufacturer's instructions (Sinoukbio, Beijing, China).
2.9. Pathological analysis and Immunohistochemistry staining
The kidney tissue samples fixed in 4% buffered paraformaldehyde, dehydrated, and then embedded in paraffin. Sections of about 5 μm thickness were sliced from each embedded sample and stained with hematoxylin and eosin (H&E).
Immunohistochemistry assay (IHC) was performed as previously (Zhang et al., 2015). Briefly, kidney sections were incubated with 3% hydrogen peroxide and 2.5% normal horse serum, followed by incubation with rabbit polyclonal anti-collagen III primary antibody overnight at 4 °C and with second antibody (goat anti-rabbit anti-body) at 37 °C for 30 min. Whole-slide digital images were collected at 400× magnification with an Aperio Scan Scope slide scanner (Aperio, Vista, CA, USA).
2.10. Statistical analysis
All data were expressed as Mean ± SD, and comparison among multiple groups was performed with One-way analysis of variance (ANOVA) followed by Student-Newman-Keuls test using statistical software (SPSS v.22, USA). P < 0.05 was considered statistically significant.
3. Results
3.1. Effects of SKI on body weight, fasting blood glucose (FBG), and kidney weight index (KWI)
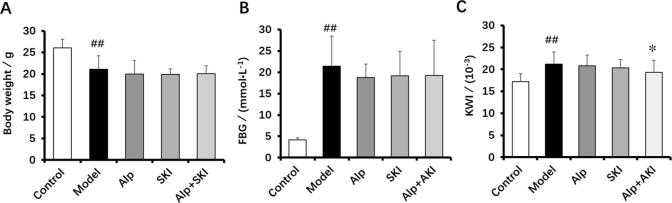
After 6 weeks’ treatment, we detected body weight and fasting blood glucose in each group. Compared with the control group, the diabetic mice in the model exhibited high levels of FBG, decreased body weight and increased KWI (P < 0.01, Fig. 1A and B). SKI and Alp treatments had no obvious impact on the FBG and body weight in comparison with the model group. After combined treatment with SKI and Alp, the KWI significantly decreased compared with the model group (P < 0.05, Fig. 1C).
Fig. 1.
Effects of SKI on body weight (A), fasting blood glucose (B) and kidney weight index (C) in diabetic mice (n = 10). ##P < 0.01 vs control group, *P < 0.05 vs model group.
3.2. Effects of SKI on glucose tolerance and plasma HbA1c levels
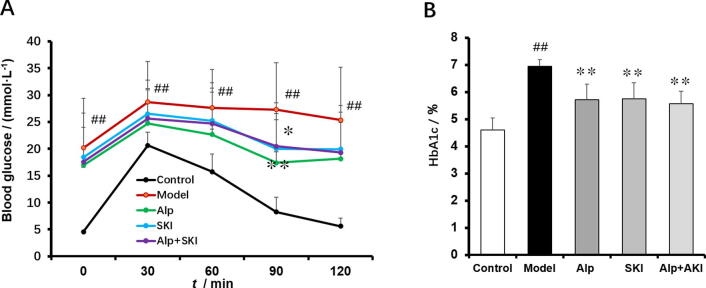
To assess the impact of SKI on elimination of blood glucose, oral glucose tolerance test (OGTT) was performed. Compared with the control group, the capability to reduce the blood glucose in the model markedly impaired, which could be improved by SKI, Alp and combined treatment (Fig. 2A). Meanwhile, SKI, Alp and combined treatment could reduce the high levels of HbA1c (Fig. 2B).
Fig. 2.
Effects of SKI on glucose tolerance (A) and HbA1c levels (B) in diabetic mice (n = 10). ##P < 0.01 vs control group, *P < 0.05, **P < 0.01 vs model group.
3.3. Effects of SKI on 24-h urine volume and urinary albumin levels
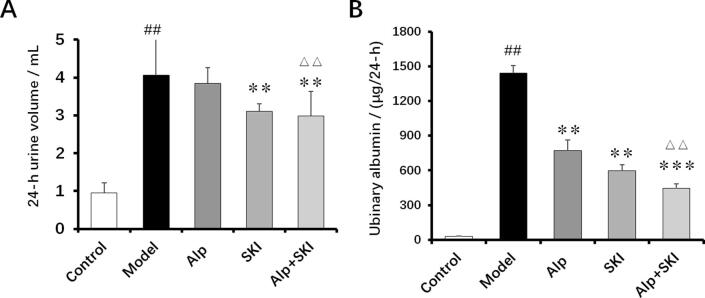
Compared with the control group, the mice in the model group showed increased 24-h urine volume and high levels of urinary albumin, those increases of which could be significantly attenuated by SKI treatment (Fig. 3). Noteworthily, the inhibitive effects of combined treatment on the 24-h urine volume and urinary albumin were more significant compared with the single Alp treatment (P < 0.01).
Fig. 3.
Effects of SKI on 24-h urine volume (A) and urinary albumin levels (B) in diabetic mice (n = 10). ##P < 0.01 vs control group, **P < 0.01, ***P < 0.001 vs model group, ΔΔP < 0.01 vs Alp group.
3.4. Effects of SKI on urinary β2-MG, NAG and KIM-1 levels
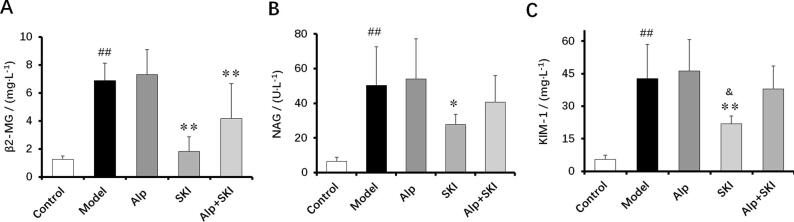
Clinically, β2-MG, NAG and KIM-1 are considered as key markers of renal dysfunction. Compared with the control group, urinary NAG, β2-MG and KIM-1 levels were drastically elevated in the model group (P < 0.01). The high levels of NAG, β2-MG and KIM-1 were obviously reduced in the diabetic mice with SKI treatment for 6 weeks (P < 0.05, P < 0.01), indicating that SKI can improve the renal function (Fig. 4).
Fig. 4.
Effects of SKI on urinary β2-MG (A), NAG (B) and KIM-1 (C) levels in diabetic mice (n = 10). ##P < 0.01 vs control group, *P < 0.05, **P < 0.01 vs model group, &P < 0.05 vs Alp group.
3.5. Effects of SKI on pathological alterations and collagen III protein levels in diabetic kidneys
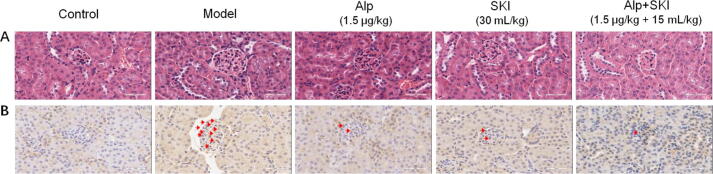
Compared with the control group, the kidney from the model group showed abnormal morphology in glomerulus with increased glomerular volume and mesangial cell proliferation. These pathological alterations were significantly attenuated with SKI and Alp treatment. The improvement of structural abnormalities of diabetic kidney by combined treatment was more obvious compared with the Alp group (Fig. 5A). Results from IHC showed that collagen III highly expressed in the diabetic kidney, and the protein levels of collagen III in the diabetic kidney were reduced by SKI and Alp treatments (Fig. 5B).
Fig. 5.
Effects of SKI on pathological changes (A) and collagen III protein levels (B) in the diabetic kidney tissues (×400).
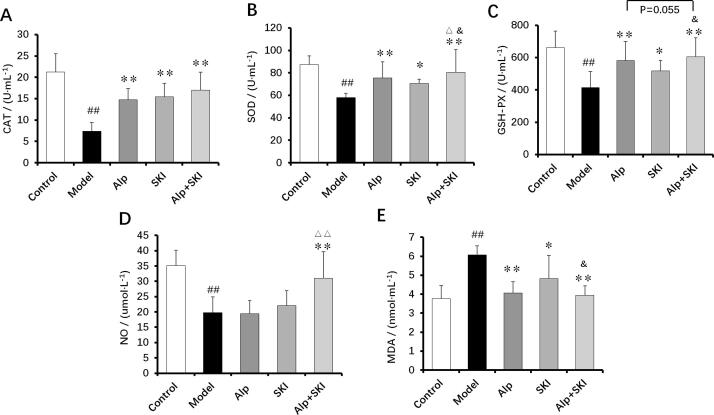
3.6. Effects of SKI on plasma levels of anti-oxidant enzymes, NO and MDA
Oxidative stress induced by hyperglycemia is closely associated with DN (Kashihara, Haruna, Kondeti, & Kanwar, 2010). Anti-oxidant enzymes including CAT, SOD and GSH-PX play key roles in the protection of DN. Compared with the control group, CAT, SOD, GSH-PX and NO levels were significantly decreased in the plasma from the model group, and oppositely, MDA levels were increased (P < 0.01). Both SKI and Alp treatments could elevate the anti-oxidant enzymes levels and reduce MDA levels (P < 0.05, P < 0.01). Compared with the Alp group, the anti-oxidant enzymes levels (SOD, GSH-PX) in the co-treatment group were found to be obviously elevated (P < 0.05, P = 0.055), and NO levels were also found to increase in the combined treatment group (P < 0.01). The MDA levels in the co-treatment group markedly reduced compared with the SKI group (P < 0.05, Fig. 6E). These data collectively indicated that the anti-oxidant effects can be enhanced with Alp and SKI combined treatment.
Fig. 6.
Effects of SKI on plasma levels of anti-oxidant enzymes (CAT (A), SOD (B) and GSH-PX (C)), NO (D) and MDA (E) in the diabetic mice (n = 10). ##P < 0.01 vs control group, *P < 0.05, **P < 0.01 vs model group, △△P < 0.01 vs Alp group, &P < 0.05 vs SKI group.
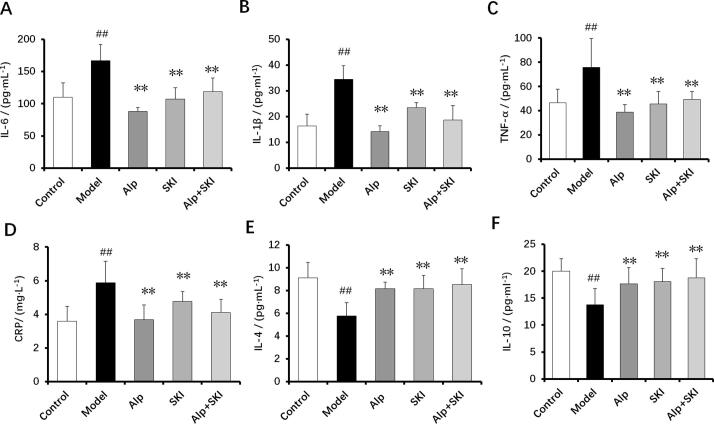
3.7. Effects of SKI on levels of inflammatory cytokines in diabetic mice
Chronic inflammation in the diabetes is related to the progression of DN (Wada & Makino, 2013). In line with these researches, pro-inflammatory cytokines including IL-6, IL-1β and TNF-α, and CRP levels were significantly increased in the model group, but anti-inflammatory cytokines including IL-4 and IL-10 levels were reduced (P < 0.01). Compared with the model group, SKI and Alp treatments reduced high levels of pro-inflammatory cytokines and CRP, and increased anti-inflammatory cytokines levels of IL-4, and IL-10 (P < 0.01, Fig. 7), indicating that SKI possesses remarkable anti-inflammatory activities.
Fig.7.
Effects of SKI on inflammatory cytokines in the diabetic mice (n = 10). A: IL-6, B: IL-1β, C: TNF-α, D: CRP, E: IL-4, F: IL-10, ##P < 0.01 vs control group, **P < 0.01 vs model group.
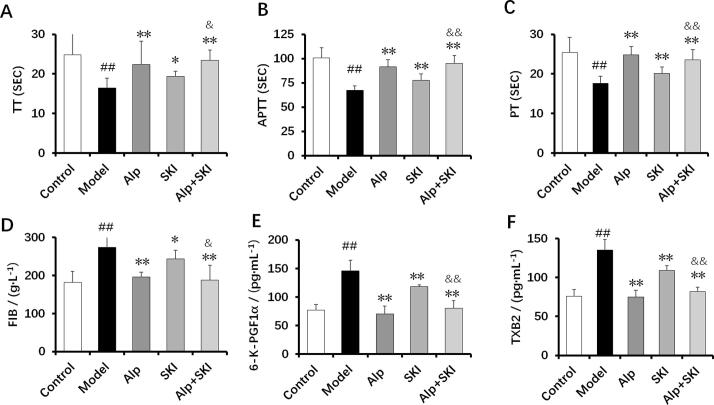
3.8. Effects of SKI on coagulation activity in diabetic mice
Clinically, numerous diabetic patients suffer from hypercoagulability (Pretorius et al., 2018). The diabetic mice in the model group were found in a hypercoagulable state, as indicated by reduced activated partial thromboplastin time (APTT), prothrombin time (PT) and thrombin time (TT) (P < 0.01). Meanwhile, plasma levels of fibrinogen (FIB), 6-K-PGF1α and thromboxane B2 (TXB2) were found increased in the model group (P < 0.01, Fig. 8A-D). With SKI and Alp treatments, the hypercoagulable state in the diabetic mice was obviously attenuated, as evidenced by the elevated APTT, PT and TT (P < 0.05, P < 0.01). Compared with the SKI group, the inhibitive effects of combined treatment on the 6-K-PGF1α and TXB2 were superior (P < 0.05, Fig. 8E and F).
Fig. 8.
Effects of SKI on coagulation activity in diabetic mice (n = 10). A: thrombin time (TT), B: activated partial thromboplastin time (APTT), C: prothrombin time (PT), D: plasma fibrinogen (FIB), E: 6-K-PGF1α, F: thromboxane B2 (TXB2). ##P < 0.01 vs control group, *P < 0.05, **P < 0.01 vs model group, &P < 0.05, &&P < 0.01 vs SKI group.
4. Discussion
DN has been the leading cause of ESRD, contributing to severe morbidity and mortality in the diabetes (Hofni, El-Moselhy, Taye, & Khalifa, 2014). Strategy used to treat DN remains limited in the clinical setting. Thus, exploring novel methods is urgent and has important clinical value in the treatment of DN. Chinese medicine experts depict the characteristics of symptoms of DN, including qi deficiency, blood stasis, yin deficiency, turbid dampness, phlegm dampness, yang deficiency, blood deficiency and qi stagnation (Chen et al., 2011). Based on the clinical characteristics of DN, the treatment principles of DN include tonifying qi, nourishing yin, reinforcing kidney, removing stasis, clearing collaterals and resolving dampness (Wen, Yan, Zhang, & Li, 2017). SKI is composed of Rhei Radix et Rhizoma, Salviae Miltiorrhizae Radix et Rhizoma, Astragali Radix, and Carthami Flos, which has been reported to tonify qi, nourish yin and promote circulation. Meanwhile, this drug has been used to treat chronic kidney diseases for many years. Thus, we speculated that SKI may protect the diabetic mice against DN.
We firstly observed the impact of SKI on the body weight. Although the diabetic mice were treated with high fat diet for four weeks, they showed obvious body weight loss because they were injected high dose of STZ. It was found that SKI had no obvious impact on the body weight. Increasing evidence indicates that HbA1c levels elevate in the diabetic complications. HbA1c can be used to predict the risk of DN (Kilpatrick, Rigby, & Atkin, 2008). Patients with high HbA1c variability index may be more likely to develop DN (Zhong et al., 2019). In the present study, diabetic mice in the model showed high levels of FBG and HbA1c, accompanied with severe glucose intolerance. Treatment with SKI and Alp significantly attenuated glucose intolerance and reduced the HbA1c levels. High levels of HbA1c can also predict microalbuminuria, an early clinical characteristic in patients with DN (Waden et al., 2009). In line with these studies, 24-h urine volume and albuminuria were highly increased in the diabetic mice, which could be alleviated by SKI administration. Meanwhile, SKI treatment could reduce the increased levels of β2-MG, NAG and KIM-1 in the diabetic animals, indicating that SKI can protect against renal injury induced by diabetes. Combined treatment with SKI and Apl enhanced the inhibitive effects on 24-h urine volume and albuminuria, suggesting that the renal protective effects are more potent in the combined treatment group.
Oxidative stress induced by chronic hyperglycemia is closely associated with the progression of DN (Kashihara et al., 2010). Oxidative stress can cause mitochondria injury in the kidney (Chen & Fang, 2018), and it can also activate transforming growth factor β (TGF-β) pathway, which mediates much collagen accumulation in the glomerulus (Kashihara et al., 2010). Anti-oxidant enzymes regulated by Nrf2 pathway are found to prevent DN through inhibition of inflammasome (Du et al., 2019). Thus, enhancing the anti-oxidative activity may suppress the progression of DN. In this research, SKI treatment could improve the deficiency of anti-oxidant enzymes, and meanwhile, it inhibited diabetes-induced inflammation, as indicated by reduction of IL-6, IL-1β and CRP levels. The anti-oxidative activities were enhanced with SKI and Alp combined treatment, as evidenced by the increased levels of SOD and GSH-PX, and the decreased levels of MDA. These data collectively indicated that Alp may enhance the renal-protective effect of SKI through reinforcing the anti-oxidant activities.
Clinical trials have showed that some diabetic patients suffer from hypercoagulability (Yamada et al., 2000, Pretorius et al., 2018). Mechanistic study suggests that chronic hyperglycemia can lead to vascular endothelial cells injury, the abnormality of which subsequently causes platelet activation and aggregation (Bochenek & Schafer, 2019). Thus, the coagulation dysfunction in the diabetes facilitates the progression of DN. Increased levels of coagulation factor Xa in the diabetes can cause severe inflammation, which in turn exacerbates DN (Oe et al., 2016). Hypercoagulability induced by hyperglycemia causes the decreased blood flow and subsequently leads to renal ischemia and hypoxia, which forms the basis for the DN, including oxidative stress, cell apoptosis and mesangial cell proliferation (Tanaka et al., 2005, Wu et al., 2020). Alprostadil (Alp) is widely used in the clinic because of its vasodilatory effects to dilate blood vessels and its anti-platelet effects to suppress platelet aggregation. Alp has been reported to exert the renal-protective roles through improving local capillary circulation, and increasing blood flow and the glomerular filtration rate. Accordingly, Alp was used as a positive drug, and it was also observed whether the renal-protective effects of SKI were enhanced when Alp and SKI were combined treatment. Herein, the diabetic mice in the model were found in a hypercoagulability state, as indicated by decreased APTT, PT and TT, and increased levels of FIB, 6-K-PGF1α and TXB2. These abnormal changes can be attenuated with the SKI and Alp intervention. Noteworthily, the inhibition of FIB, 6-K-PGF1α and TXB2 in the co-treatment group was more obvious than that in the SKI group. Nitric oxide (NO) has been reported to possess numerous key functions including the regulation of renal haemodynamics and medullary blood flow (Mount & Power, 2006). In the present research, NO levels were obviously elevated in the combined treatment group, suggesting that the effects of SKI on vascular dilation may be improved when combined with Alp. These data collectively suggest that the improvement of hypercoagulability was enhanced when Alp and SKI were combined treatment, although SKI was used in a low dose (15 ml/kg) in the combined treatment group.
However, the present study presents certain limitations. Dose-dependent effects of SKI have not been observed, and the impact of SKI on Nrf2 and NF-κB signaling has not been determined. Thus, our future work will address these questions.
In summary, SKI possesses a remarkable property to prevent diabetic nephropathy. The improvements of kidney function and hypercoagulability by SKI were enhanced with Alp combined treatment. The molecular mechanisms underlying the protection of SKI against DN may be related to enhancing the anti-oxidant and anti-inflammatory activities, and improving the coagulation dysfunction.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Key R&D Plan (No. 2018YFC1707408).
Contributor Information
Gui-bo Sun, Email: sunguibo@126.com.
Xiao-bo Sun, Email: sun-xiaobo@163.com.
References
- Alomari G., Al-Trad B., Hamdan S., Aljabali A., Al-Zoubi M., Bataineh N., et al. Gold nanoparticles attenuate albuminuria by inhibiting podocyte injury in a rat model of diabetic nephropathy. Drug Delivery and Translational Research. 2019;10(1):216–226. doi: 10.1007/s13346-019-00675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochenek M.L., Schafer K. Role of endothelial cells in acute and chronic thrombosis. Hamostaseologie. 2019;39(2):128–139. doi: 10.1055/s-0038-1675614. [DOI] [PubMed] [Google Scholar]
- Chen X., Fang M. Oxidative stress mediated mitochondrial damage plays roles in pathogenesis of diabetic nephropathy rat. European Review for Medical and Pharmacological Sciences. 2018;22(16):5248–5254. doi: 10.26355/eurrev_201808_15723. [DOI] [PubMed] [Google Scholar]
- Chen G., Wu X., Yang L., Yang X., Yan M., Li P. Research about the questionnaire of clinical experts and Delphi methods used on the characteristics of TCM symptom of diabetic kidney disease. China Journal of Traditional Chinese Medicine Pharmacy. 2011;26(10):2241–2244. [Google Scholar]
- Ding T., Wang S., Zhang X., Zai W., Fan J., Chen W., et al. Kidney protection effects of dihydroquercetin on diabetic nephropathy through suppressing ROS and NLRP3 inflammasome. Phytomedicine. 2018;41:45–53. doi: 10.1016/j.phymed.2018.01.026. [DOI] [PubMed] [Google Scholar]
- Du L., Wang J., Chen Y., Li X., Wang L., Li Y., et al. Novel biphenyl diester derivative AB-38b inhibits NLRP3 inflammasome through Nrf2 activation in diabetic nephropathy. Cell Biology and Toxicology. 2019;36:243–260. doi: 10.1007/s10565-019-09501-8. [DOI] [PubMed] [Google Scholar]
- Fu B., Yang J., Chen J., Lin L., Chen K., Zhang W., et al. Preventive effect of Shenkang injection against high glucose-induced senescence of renal tubular cells. Frontiers of Medicine. 2019;13(2):267–276. doi: 10.1007/s11684-017-0586-8. [DOI] [PubMed] [Google Scholar]
- Hofni A., El-Moselhy M.A., Taye A., Khalifa M.M. Combination therapy with spironolactone and candesartan protects against streptozotocin-induced diabetic nephropathy in rats. European Journal of Pharmacology. 2014;744:173–182. doi: 10.1016/j.ejphar.2014.10.021. [DOI] [PubMed] [Google Scholar]
- Kaizu K., Uriu K., Hashimoto O., Morita E., Eto S., Suzuki H. Role of intrarenal coagulation and anticoagulant therapy in the progression of diabetic nephropathy. Nihon Jinzo Gakkai Shi. 1993;35(1):35–42. [PubMed] [Google Scholar]
- Kashihara N., Haruna Y., Kondeti V.K., Kanwar Y.S. Oxidative stress in diabetic nephropathy. Current Medicinal Chemistry. 2010;17(34):4256–4269. doi: 10.2174/092986710793348581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick E.S., Rigby A.S., Atkin S.L. A1C variability and the risk of microvascular complications in type 1 diabetes: Data from the diabetes control and complications trial. Diabetes Care. 2008;31(11):2198–2202. doi: 10.2337/dc08-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C., Li T., Zhang C., Chen Q., Li Z., Liu J., et al. Therapeutic effect of alprostadil in diabetic nephropathy: Possible roles of angiopoietin-2 and IL-18. Cellular Physiology and Biochemistry. 2014;34(3):916–928. doi: 10.1159/000366309. [DOI] [PubMed] [Google Scholar]
- Mount P.F., Power D.A. Nitric oxide in the kidney: Functions and regulation of synthesis. Acta Physiologica (Oxf) 2006;187(4):433–446. doi: 10.1111/j.1748-1716.2006.01582.x. [DOI] [PubMed] [Google Scholar]
- Oe Y., Hayashi S., Fushima T., Sato E., Kisu K., Sato H., et al. Coagulation factor Xa and protease-activated receptor 2 as novel therapeutic targets for diabetic nephropathy. Arteriosclerosis Thrombosis and Vascular Biology. 2016;36(8):1525–1533. doi: 10.1161/ATVBAHA.116.307883. [DOI] [PubMed] [Google Scholar]
- Papadopoulou-Marketou N., Paschou S.A., Marketos N., Adamidi S., Adamidis S., Kanaka-Gantenbein C. Diabetic nephropathy in type 1 diabetes. Minerva Medica. 2018;109(3):218–228. doi: 10.23736/S0026-4806.17.05496-9. [DOI] [PubMed] [Google Scholar]
- Pretorius L., Thomson G.J.A., Adams R.C.M., Nell T.A., Laubscher W.A., Pretorius E. Platelet activity and hypercoagulation in type 2 diabetes. Cardiovascular Diabetology. 2018;17(1):141–151. doi: 10.1186/s12933-018-0783-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L., Qin W., Wang J., Lin L. Combined treatment of diabetic nephropathy with alprostadil and calcium dobesilate. Experimental and Therapeutic Medicine. 2017;14(5):5012–5016. doi: 10.3892/etm.2017.5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz E. Clinical manifestation and natural history of diabetic nephropathy. Medical Clinics of North America. 2013;97(1):19–29. doi: 10.1016/j.mcna.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Arai H., Liu N., Nogaki F., Nomura K., Kasuno K., et al. Role of coagulation factor Xa and protease-activated receptor 2 in human mesangial cell proliferation. Kidney International Supplements. 2005;67(6):2123–2133. doi: 10.1111/j.1523-1755.2005.00317.x. [DOI] [PubMed] [Google Scholar]
- Tervaert T.W., Mooyaart A.L., Amann K., Cohen A.H., Cook H.T., Drachenberg C.B., et al. Pathologic classification of diabetic nephropathy. Journal of the American Society of Nephrology. 2010;21(4):556–563. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- Wada J., Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clinical Science (Lond) 2013;124(3):139–152. doi: 10.1042/CS20120198. [DOI] [PubMed] [Google Scholar]
- Waden J., Forsblom C., Thorn L.M., Gordin D., Saraheimo M., Groop P.H., et al. A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes. 2009;58(11):2649–2655. doi: 10.2337/db09-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Song Z., Ding R., Wei C. Protective effects of aqueous extracts of Se-enriched Auricularia auricular on STZ – Induced diabetic nephropathy in mice. Journal of Shenyang Pharmacy University. 2019;36(5):410–414. [Google Scholar]
- Wen Y., Yan M., Zhang B., Li P. Chinese medicine for diabetic kidney disease in China. Nephrology (Carlton) 2017;22(4):50–55. doi: 10.1111/nep.13149. [DOI] [PubMed] [Google Scholar]
- Wu H., Wang Y., Zhang Y., Xu F., Chen J., Duan L., et al. Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting LPS-induced inflammation and oxidative stress. Redox Biology. 2020;32:101500–101513. doi: 10.1016/j.redox.2020.101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Guan Y., Yan J., Liu M., Yin Y., Duan J., et al. ShenKang injection suppresses kidney fibrosis and oxidative stress via transforming growth factor-beta/Smad3 signalling pathway in vivo and in vitro. Journal of Psychopharmacology. 2015;67(8):1054–1065. doi: 10.1111/jphp.12412. [DOI] [PubMed] [Google Scholar]
- Yamada T., Sato A., Nishimori T., Mitsuhashi T., Terao A., Sagai H., et al. Importance of hypercoagulability over hyperglycemia for vascular complication in type 2 diabetes. Diabetes Research Clinical Practice. 2000;49(1):23–31. doi: 10.1016/s0168-8227(00)00134-0. [DOI] [PubMed] [Google Scholar]
- Yong Z., Yang R., Chen J. Shenkang injection in the treatment of diabetic nephropathy meta-analysis. Journal of Liaoning University Traditional Chinese Medicine. 2015;132(4):165–167. [Google Scholar]
- Zhang J.M., Zhu Y.B., Li H.G., Luan S.M., Song C.Y., Deng X., et al. Protection of Sophocarpine on colonic varrier in DSS-induced acute colitis in mice by increasing expression of HNF4α. Chinese Herbal Medicines. 2015;7(3):261–266. [Google Scholar]
- Zhang L., Long J., Jiang W., Shi Y., He X., Zhou Z., et al. Trends in chronic kidney disease in China. New England Journal Medicine. 2016;375(9):905–906. doi: 10.1056/NEJMc1602469. [DOI] [PubMed] [Google Scholar]
- Zhong Y., Fan H., Zhang R., Wang H., Zhang W., Tian T., et al. Associations between hba1c variability index and risk of chronic complications of diabetes mellitus. Chinese General Practice. 2019;23(3):276–288. [Google Scholar]