SUMMARY
Background.
Recovery trajectories of clinically unresponsive patients with acute brain injury are largely uncertain. Brain activation in response to spoken motor commands can be detected by electroencephalography (EEG), also known as cognitive motor dissociation (CMD). Here we explore the role of CMD in predicting time to recovery in acutely brain injured patients.
Methods.
We prospectively studied an observational cohort of clinically unresponsive, acutely brain injured patients (original cohort N=100, secondary cohort N=93). Machine learning was applied to EEG recordings to diagnose CMD by detecting brain activation to verbal commands. Functional outcomes were assessed with the Glasgow Outcome Scale-Extended at hospital discharge, 3, 6, and 12 months after injury. Survival statistics and shift analyses were applied to identify an association between CMD and time to and magnitude of recovery. The prediction accuracy of our model built on the original cohort was determined using the secondary cohort. Patients that underwent withdrawal of life sustaining therapies were censored and death was treated as a competing risk.
Findings.
At 12 months, 28 of 193 (15%) unresponsive patients had a GOS-E of 4 or above. CMD was seen in 27 (14%) patients and was an independent predictor of shorter time to good recovery (HR5.6, 95%-CI 2.5–12.5), together with underlying traumatic brain injury (HR4.4, 95%-CI 1.4–14.0), admission Glasgow Coma Scale score greater or equal to 8 (HR2.2, 95%-CI 1.0–4.7), and younger age (HR1.0, 95%-CI 1.0–1.1). Amongst patients discharged home or to a rehabilitation setting, those diagnosed with CMD consistently had higher of functional recovery that those without CMD seen as early as 3 months after the injury (OR 4.5; 95%-CI 2.0–33.6).
Interpretation.
Recovery trajectories of clinically unresponsive patients diagnosed with CMD early after brain injury are distinctly different from those without CMD. Diagnosis of CMD may offer more precise counselling for families of unresponsive patients and potentially could help identify patients that benefit from rehabilitation.
Funding.
National Institute of Health NS106014, NS112760.
Keywords: disorders of consciousness, coma, long term recovery, outcomes
INTRODUCTION
Predicting outcomes of acutely brain injured patients with disorders of consciousness (DoC) is imprecise.1 This presents challenges for guiding families in goals of care decisions and results in inadequate support to promote the recovery process of these patients, including inefficient allocation of rehabilitation resources. Access to and criteria that qualify patients for rehabilitation vary widely between health care systems,2,3 many requiring the ability to participate in rehabilitation services for a minimum amount of time. Increasingly it is recognized that recovery may be seen months and even years after the injury with earlier recovery typically seen in patients with traumatic brain injury (TBI).4 Late recovery and functional independence are possible for these patients including those that are unresponsive from brain injuries other than TBI3,5,6 and the potential of rehabilitation interventions to improve outcomes of brain injured patients cannot be overstated.6,7 The imprecision of predicting recovery of consciousness and long-term outcomes has been identified as a major gap in knowledge.8,9
Patients that demonstrate even inconsistent behavioural evidence of consciousness such as intermittent interactions with the examiner have a higher chance of later functional recovery.4 Unfortunately, bedside behavioural assessments alone are insufficient to accurately predict functional outcome trajectories.10–12 Detection of brain activation to motor commands using bedside EEG has been associated with 12 months functional outcomes and may increase the accuracy of predictions.13 This state, called cognitive-motor dissociation (CMD), indicates detection of volitional brain activity by task-based functional MRI or EEG in patients who appear unresponsive on bedside behavioural assessments, without the ability to meaningfully communicate with the examiner.14 Here we study the recovery trajectory over the first year for acutely brain injured, unconscious patients with and without CMD admitted to a single intensive care unit.
METHODS
Patient Population.
We prospectively screened all patients with acute brain injury admitted to the Neurological Intensive Care Unit at Columbia University Irving Medical Center/ New York Presbyterian Hospital between July 2014 and May 2019. We published initial results on the original patient cohort (N=100)13 that was collected between July 2014 and September 2017. The secondary cohort (N=93) was collected between October 2017 and September 2021. As part of our standard practice and in accordance with guidelines regarding the EEG monitoring of ICU patients,15 all unresponsive patients were either monitored by continuous EEG or were anticipated to be connected to EEG monitoring within 12 hours after screening, unless death was imminently expected.
Inclusion.
We enrolled all patients who (1) were in a coma, vegetative state, or minimally conscious state–minus (defined as unresponsiveness with preserved visual fixation or pursuit, or localization to noxious stimuli), (2) were unable to follow spoken commands, (3) had sustained an acute brain injury of any kind, (4) were connected to or were expected to be connected to continuous EEG monitoring, and (5) spoke English or Spanish as their first language.
Exclusion.
We excluded all patients who (1) were less than 18 years of age, (2) had a pre-existing disorder of consciousness or confounding neurological condition (i.e., baseline aphasia or advanced dementia) prior to the onset of their presenting acute brain injury, (3) were deaf prior to the acute brain injury, (4) clinically recovered the ability to follow commands prior to enrolment, (5) did not want to participate or whose family did not want them to participate, (6) had uncontrollable seizures, or (7) had logistical reasons that hindered their enrolment.
Study Procedures and Data Collection.
Daily neurological examinations were performed, including an assessment of the patients’ ability or inability to follow simple spoken commands. The Coma Recovery Scale–Revised16 (CRS-R, a six-dimension, 23-point scale of hierarchically arranged assessments) was used to categorize the patients’ clinical state of consciousness at the time of EEG recording, and to determine the presence of minimally conscious state-minus. Sedation was interrupted or decreased for neurological and CMD assessments if it was deemed safe by the attending physician during rounds. Patients assessed receiving deep sedation or neuromuscular blockade were not included (please refer to the supplement for details). The treating physicians, patients, and their families were unaware of the results of the EEG recordings, and these results were also not made available to treating clinicians to guide decisions regarding the WLST. Data on demographics, admission diagnosis, outcomes, and complications that occurred during the hospital stay were prospectively collected. For purposes of analysis admission diagnoses were classified into structural brain injury with trauma, structural brain injury without trauma (i.e., intracerebral haemorrhage, subarachnoid haemorrhage, acute ischemic stroke), or non-structural brain injury (i.e., cardiac arrest, meningitis; see supplemental material for further details).17 Certain in-hospital variables (i.e., length of stay [LOS], diagnosis) were collected retrospectively for all patients through an electronic medical record review.
In addition, we used the same protocol to record EEGs in 10 and 5 healthy volunteers using motor commands presented in English and Spanish, respectively. Of patients alive at hospital discharge, we recorded the discharge disposition categorized for analysis into home-rehabilitation (e.g., home with or without services, and acute, subacute or outpatient rehabilitation) and higher-level support with limited rehabilitation services (e.g., skilled nursing facility, long term acute care hospital, and hospice).
Outcome Assessments.
Functional outcome was assessed with the Glasgow Outcome Scale–Extended (GOS-E; levels range from 1 to 8, with higher levels indicating better outcome).18,19 Data was obtained in a structured telephone interview at discharge, 3, 6, and 12 months post-injury by an interviewer trained in the collection of outcomes assessments. If the patient was unable to communicate, the patients’ functional status was obtained through a close relative or caretaker. Neither the interviewee nor the interviewer who performed the outcome assessments were aware of the results of the EEG assessment used to determine the patient’s clinical state of consciousness.
Motor Command Protocol and EEG Acquisition and Processing.
Motor commands consisting of “keep opening and closing your right hand” and “stop opening and closing your right hand” were presented to patients via single-use headphones throughout the EEG recording (3 blocks with 8 consecutive trials each for the left and right hand, respectively). Digital bedside EEG was recorded using a standard 21-electrode montage.15 Power in predefined frequency ranges was calculated for each EEG recording and was used to train a machine-learning algorithm (Support Vector Machine [SVM] with a linear kernel) to discern the EEG responses that followed the commands.20–22 EEG analysis was subsequently used to identify patients with cognitive-motor dissociation (CMD) detected on at least one recording. SVM performance for each EEG was estimated as the area under the receiver-operating-characteristic curve (AUC), significance of the AUC determined by a one-tailed permutation test (after random shuffling with 500 training and evaluation repetitions, significance set at greater than 0.5), and accounting for multiple recordings in a patient (Benjamini–Hochberg false-discovery-rate method). Further details of the motor command protocol, cognitive motor dissociation classification method, and EEG acquisition and processing have been reported previously.13
Study Oversight.
This study was approved by our local institutional review board. We obtained written informed consent from the patients’ surrogates. Patients who recovered consciousness at the time of follow up were given the opportunity to withdraw from the study. There was no industry support for or involvement in the study, and all authors vouch for the completeness and accuracy of the data as well as fidelity to the study protocol. Results are reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.23
Statistical Analysis.
The primary outcome investigated in the study was functional recovery defined as a GOS-E of greater than or equal to 4 (indicates the ability to be left alone up to 8 hours without assistance) assessed at hospital discharge, and at 3, 6, and 12 months following the injury. Secondary outcomes included shifts of GOS-E across all levels. Patients who underwent WLST or were lost to follow-up were censored,24 and death was treated as a competing risk.
To identify independent predictors of time to functional recovery (defined as a GOS-E greater or equal to 4) a sub-distribution hazard model was performed, accounting for death as a competing risk and stratifying by the original and secondary cohort.25 Additionally, restricted mean survival times (RMST) were used to compare the differences of the expected time to recovery over the complete follow-up time period of 12 months between CMD and non-CMD patients.26 Other predictors of recovery were adjusted for as covariates.
To determine models with the highest accuracy to predict time-to-recovery, first several regression models combining predictors identified above were built using the original cohort (N=100). Second, prediction accuracy for recovery of these models at hospital discharge, 3, 6 and 12 months after the injury was determined on the secondary cohort (N=93).27
To determine the significance of a shift across all levels of functional outcomes, a modified shift test was applied to the distribution of GOS-E scales comparing CMD to non-CMD patients that were alive at hospital discharge, accounting for possibly tied observations.28 To better interpret the shift effect, a common odds ratio across all cut points of GOS-E was estimated by a proportional-odds logistic regression model, which indicates the relative effect on the GOS-E increase for CMD patients.29
To determine an association between discharge disposition (higher-level care vs. a home-rehabilitation) and recovery amongst hospital survivors, the significance of CMD was tested by multivariate Mann-Whitney estimators,30 correcting separately for other predictors of recovery amongst patients discharged to a higher-level care or a home-rehabilitation setting. Additionally, a cumulative ordinal regression analysis was performed to predict GOS-E measurements across all follow-up time points, analysing discharge disposition and CMD.31
Categorical variables are presented as counts (percentages), and continuous variables presented as means (standard deviations) or medians (interquartile ranges [IQR]), as appropriate. Associations between variables and outcomes were assessed with a Wilcox rank-sum test for quantitative variables or chi-squared test for qualitative variables. All statistical tests were two-tailed. Variables with a P-value less than 0.10 in univariate analysis were considered for multivariate backward stepwise models. We also tested for two-way interactions and the interaction of each variable with time in our final models. Statistical analyses were performed with R(version 4.0.3) statistical software.
Role of the funding source.
The funder had no role in study design, data collection, data analysis, interpretation of the study results, writing of the report, or the decision to submit the manuscript for publication.
RESULTS
Patient characteristics.
193 of 598 patients with acute brain injury that were screened met criteria to be included in this study (Figure 1). Included patients were similar to patients who were not included with regards to age, sex, and admission Glasgow Coma Scale score (Supplemental Table 1). Included patients were more frequently admitted for ICH (33% vs 16%), cardiac arrest (21% vs 18%), TBI (13% vs 11%), and SAH (15% vs 5%) when compared to excluded patients, while excluded patients had more commonly other diagnoses (10% vs 40%). The mean age for included patients was 63+/−17 years and 105 (54%) patients were men (Table 1). The first language of included patients was English in 144 (75%) and Spanish in 49 (25%).
Figure 1.
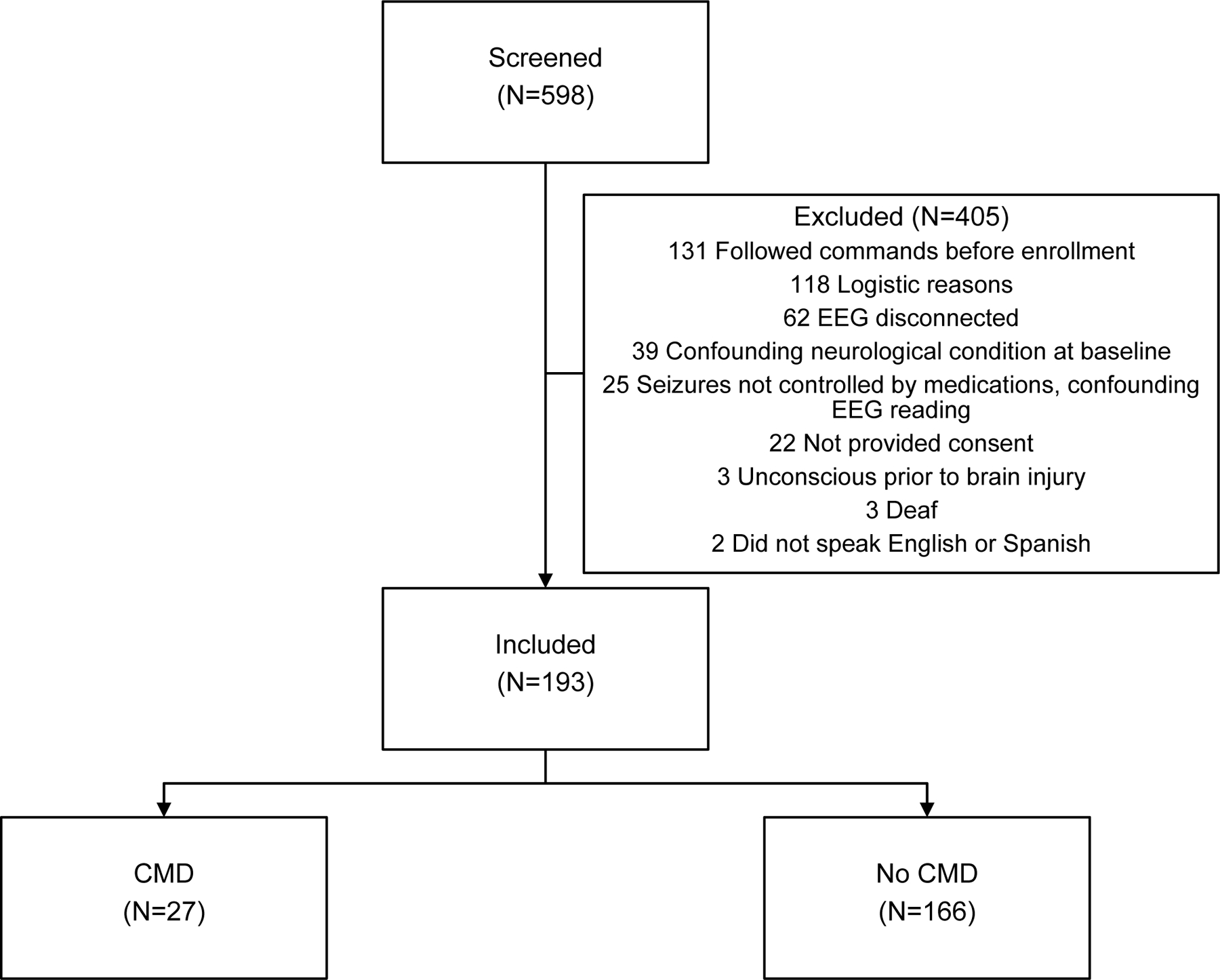
Distribution of study population.
CMD, cognitive motor dissociation
Table 1.
Characteristics of original, secondary, and combined study cohort.
| Original cohort (N=100) |
Secondary cohort (N=93) |
Combined cohort (N=193) |
|
|---|---|---|---|
| Demographics | |||
| Age, years | 60 +/− 17 | 65 +/− 16 | 63 +/− 17 |
| Male | 56 (56%) | 49 (53%) | 105 (54%) |
| Aetiology of brain injury | |||
| Intracerebral haemorrhage | 25 (25%) | 38 (41%) | 63 (33%) |
| Cardiac arrest | 31 (31%) | 9 (10%) | 40 (21%) |
| Subarachnoid haemorrhage | 15 (15%) | 13 (14%) | 28 (15%) |
| Traumatic brain injury/SDH | 16 (16%) | 9 (10%) | 25 (13%) |
| Acute Ischemic Stroke | 3 (3%) | 14 (15%) | 17 (9%) |
| Other* | 10 (10%) | 10 (11%) | 20 (10%) |
| Behavioural Assessment | |||
| Admission Glasgow Coma Scale | 6 [3,8] | 6 [3,8] | 6 [3,8] |
| Coma Recovery Scale-revised score, median | 3 [0,5] | 2 [0,5] | 2 [0,5] |
| Coma Recovery Scale-revised score, worst | 1 [0,3] | 1 [0,3] | 1 [0,3] |
| Coma Recovery Scale-revised score, best | 3 [1,7] | 3 [1,9] | 3 [1,8] |
| Best DoC category | |||
| Coma | 44 (44%) | 36 (39%) | 80 (41%) |
| Vegetative state | 25 (25%) | 23 (25%) | 48 (25%) |
| Minimally conscious state, minus | 31 (31%) | 34 (37%) | 65 (33%) |
| EEG recordings | |||
| Number of EEG recordings per patient | 1 [1,2] | 2 [1,3] | 2 [1, 3] |
| Onset of acute brain injury to first recording >0 [days] | 65 (67%) | 67 (72%) | 132 (69%) |
| Primary language | |||
| English | 79 (79%) | 65 (70%) | 144 (75%) |
| Spanish/Other | 21 (21%) | 28 (30%) | 49 (25%) |
| CMD rate | 16 (16%) | 11 (12%) | 27 (14%) |
| GOS-E of 4 or above | |||
| Discharge | 2 (2%) | 2 (2%) | 4 (2%) |
| 3 months | 7 (7%) | 5 (5%) | 12 (6%) |
| 6 months | 12 (12%) | 9 (10%) | 21 (11%) |
| 12 months | 19 (19%) | 9 (10%) | 28 (15%) |
status epilepticus (N=6), toxic metabolic (N=3), encephalitis (N=4), sepsis (N=2), each one with thrombotic thrombocytopenic purpura, neurosarcoidosis, brain tumor, CAR-T cell toxicity, and uremia
Categorical data are shown as number (%). Continuous and ordinal variables are shown as mean +/−standard deviation and median [interquartile range] if normally or not normally distributed, respectively.
Amongst the 193 included patients, those enrolled as part of the secondary cohort (N=93) had more frequently intracerebral haemorrhage and less frequently cardiac arrest or traumatic brain injury causing unconsciousness than patients form the original cohort (N=100; Supplemental Table 2).
Cognitive motor dissociation.
Brain activation to motor commands was seen in all 14 healthy volunteers tested in English or in Spanish. A total of 27 of 193 patients (14%; 16 from the original and 11 from the secondary cohort) were diagnosed with CMD on at least one bedside EEG recording, first diagnosed on a median of 5 days post-ICU admission (interquartile range, 3 to 10). The median [IQR] time between onset of brain injury and the first CMD assessment (1 [0,3] vs 1 [0,4]) and the total number of auditory EEG recordings (2 [1,4] vs 1 [1,3]), did not differ between patients with and without CMD. When compared with non-CMD patients, CMD patients did not differ with respect to age, sex, race, primary language, premorbid modified Rankin scale, and underlying acute brain injury aetiology (Supplemental Table 3).
Hospital course and outcomes.
Mean duration of mechanical ventilation was 17 days [9–28] and 98 (51%) patients underwent tracheostomy prior to hospital discharge. Hospital complications included renal failure requiring dialysis in 23 (12%), cardiac arrest in 25 (13%), and sepsis in 75 (39%) patients. Overall hospital length of stay was 27 days [14, 41] and 56 patients (29%) underwent WLST. At hospital discharge, 71 (37%) patients were dead, 7 (4%) went to a hospice, 38 (19%) to long-term acute care hospital, 39 (20%) to a skilled nursing facility, 7 (4%) to subacute rehabilitation, 27 (14%) to outpatient rehab, and 4 (2%) to home with or without services.
Time to functional recovery.
By 3 months 12 (6%), at 6 months 21 (11%), and at 12 months 28 (15%) patients had recovered to a best GOS-E score of 4 or above. CMD and non-CMD patients continue to show increasing rates of functional recovery across all 3 follow-up time points (Figure 2, Supplemental Figure 1). Major improvements in CMD patients are seen already by 3 months after the injury, with 7 (26%) having recovered to a GOS-E of 4 or better amongst CMD patients compared to 5 (3%) without CMD (OR 11.3, 95%-CI 3.3–41.4, P=0.001). At 6 months, with 9 (33%) having recovered to a GOS-E of 4 or better amongst CMD patients compared to 12 (7%) without CMD (OR 6.4, 95%-CI 2.3–17.4, P<0.001). At 12 months, 11 (41%) CMD and 17 (10%) non-CMD patients had recovered (OR 6.0, 95%-CI 2.4–15.1, P<0.001). The majority of patients with CMD who recovered by one year (7 of 11, 64%) had recovered by 3 months, while the majority of patients without CMD who recovered (12 of 17, 71%) made their recovery after 3 months. The 12-month RMST to recovery was 8.0 months for CMD and 11.0 months for non-CMD patients. On average, CMD patients had a 3.0 months shorter (P=0.006, 95%CI 0.8–5.1) recovery compared to patients who did not have CMD.
Figure 2.
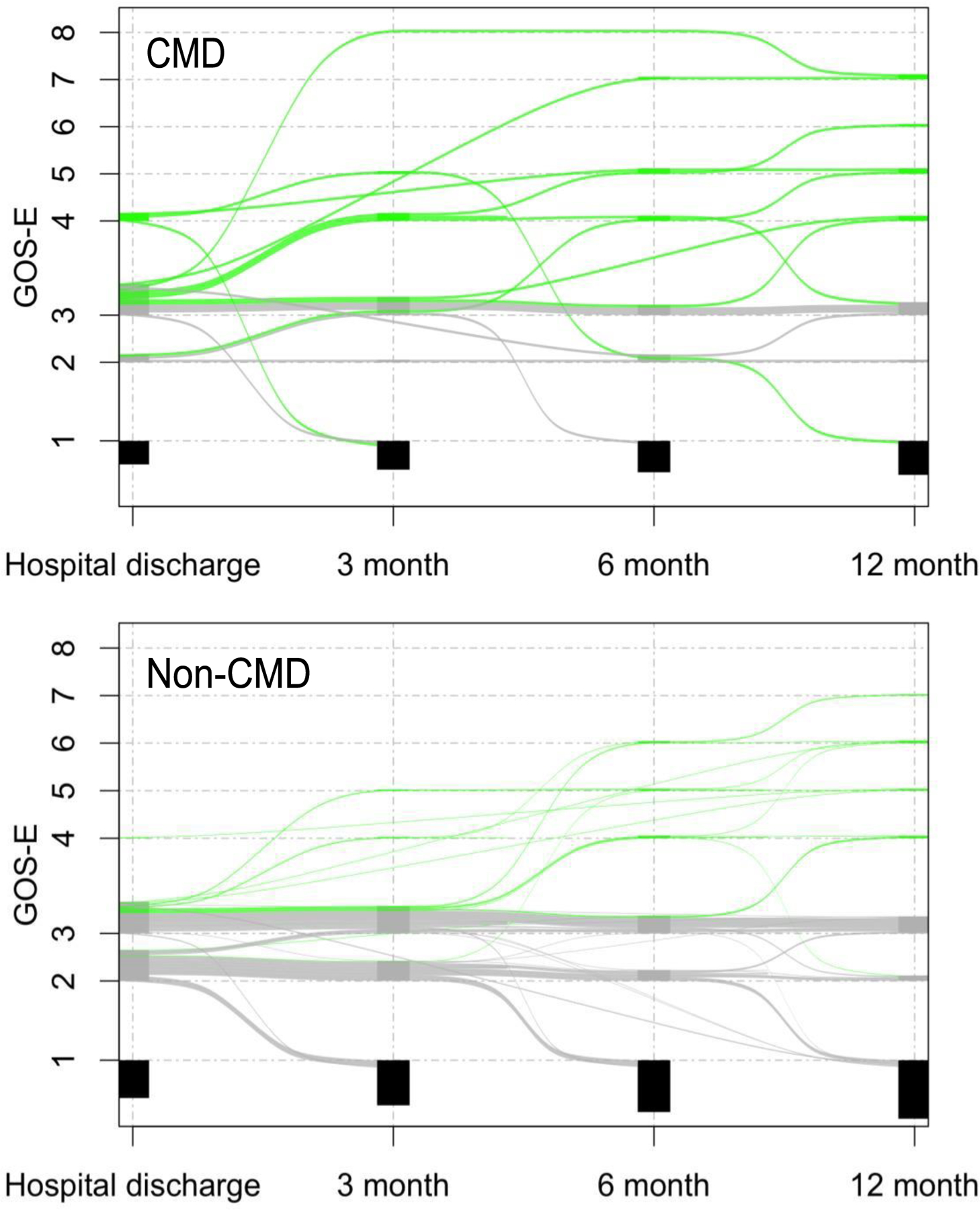
Recovery trajectory over the first year following acute brain injury
Alluvial plots representing individual GOS-E development across follow-up time points in patients with acute brain injury. Top plot CMD patients (N=27) and bottom plot non-CMD patients (N=166). Green lines indicate patients that recovered to a GOS-E of 4 or above at any point in time by one year after the injury, grey lines indicate patients were alive by 12 months but did not improve to a GOS-E of 4 or above, and black boxes indicate those patients that were dead by the time of these respective follow-up time-points. GOS-E, Glasgow Outcome Scale-Extended.
Predictors of recovery.
Presence of CMD, age, TBI/SDH injury aetiology, and admission GCS were associated in the univariate analysis with time to functional recovery and functional improvement in proportional hazards models (Supplemental Table 4). Independent predictors of earlier time to recovery were diagnosis of CMD (HR5.6, 95%-CI 2.5–12.5), traumatic brain injury as the underlying brain injury (HR4.4, 95%-CI 1.4–14.0), admission Glasgow Coma Score less than 8 (HR2.2, 95%-CI 1.0–4.7), and younger age (HR1.0, 95%-CI 1.0–1.1; Figure 3). Among patients alive at hospital discharge, on follow-up the main shift to good outcomes occurs at 3 months (OR 5.9, 95%-CI 1.8–20.7), 6 months (OR 2.6, 95%-CI 0.9–7.4), and 12 months (OR 1.1, 95%-CI 1.1–8.1; Supplemental Figure 2). At 3 and at 12 months after the injury, the distribution of shifts in GOS-E points integrated across all cut points of the scale was 1 point higher for CMD compared to non-CMD patients (Table 2). At hospital discharge there was no difference and at 6 months there was a trend for better outcomes in CMD patients.
Figure 3.
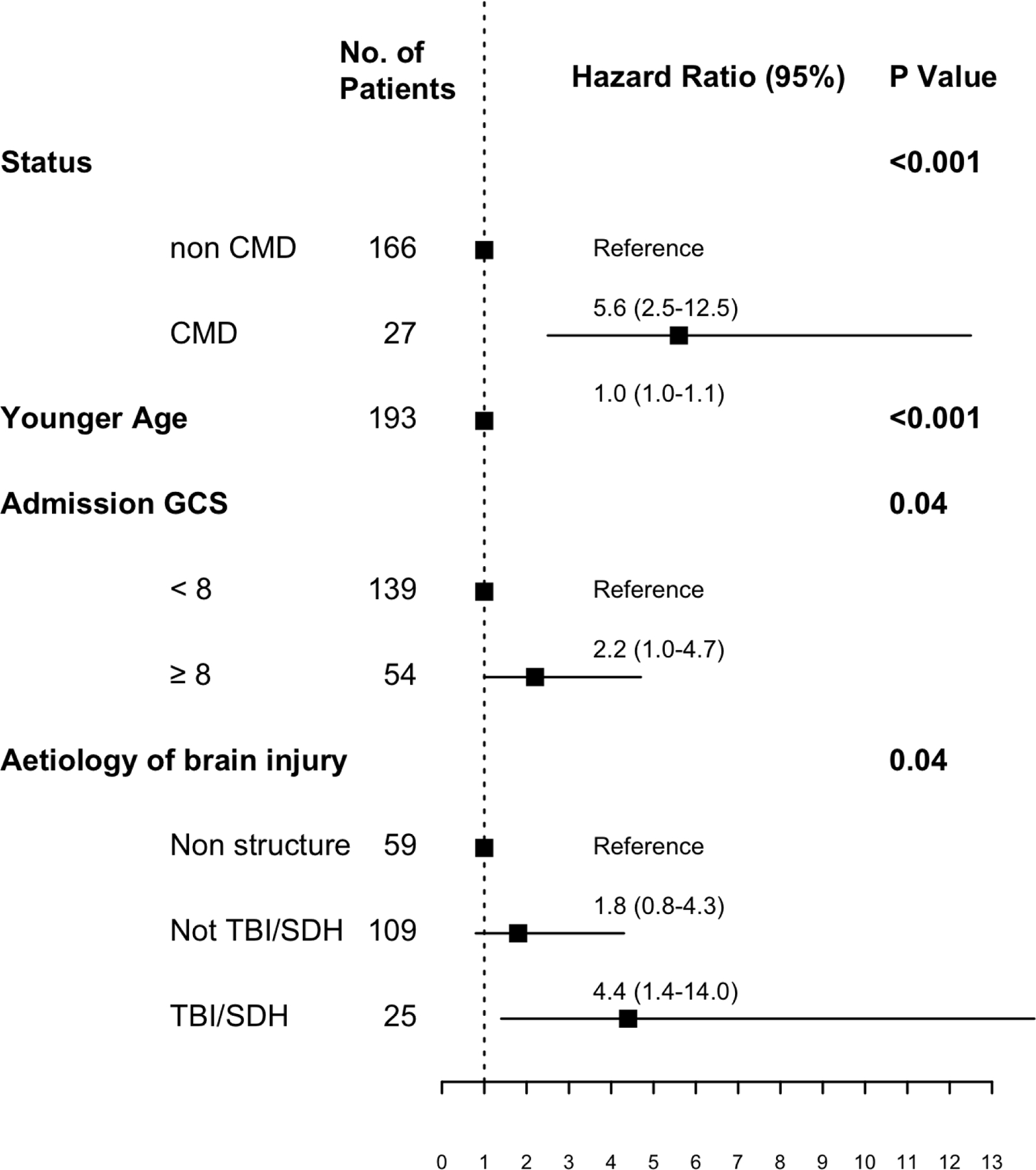
Predictors of time to functional recovery
Independent predictors of time to good functional recovery (defined as a GOS-E of 4 or above) based on a Cox proportional hazards regression model in the overall population. We included all variables associated with recovery from the univariate analysis (Supplemental Table 4) in the multivariable models.
Table 2.
Shift in GOS-E between CMD and non-CMD patients at different follow-up time points.
| OR [95%-CI] |
P Value | Distribution shift | Missing data |
||
|---|---|---|---|---|---|
| CMD (N=27) |
Non-CMD (N=166) |
||||
| Hospital discharge | 7.2 [2.2–29.3] | 0.001 | 0 | 0 (0%) | 0 (0%) |
| 3 months | 5.9 [1.8–20.7] | 0.005 | + 1.0 | 3 (11%) | 10 (6%) |
| 6 months | 2.6 [0.9–7.4] | 0.05 | + 1.0 | 2 (7%) | 24 (15%) |
| 12 months | 3.0 [1.1–8.1] | 0.02 | + 1.0 | 1 (4%) | 20 (12%) |
Accuracy of the prediction models was compared between CMD alone, demographic (e.g., age) and clinical factors (e.g., admission GCS and brain injury aetiology), and all measures combined using the AUC from risk regression models in the presence of competing risks (Supplemental Figure 3; Supplemental table 5). The AUC increased from 71.4 to 77.2 (P=0.02, increase of 5.8, 95%CI [0.9–10.6]) at 12 months by adding CMD to the final model including demographic and clinical factors.
Discharge disposition.
Amongst patients discharged alive (N=122), 38 (31%) were discharged to a home-rehabilitation and 84 (69%) to a higher-level care setting. Patients discharged to a home-rehabilitation setting had a higher chance of good 12 months outcomes when compared to those that were discharged to a higher-level care setting (OR 5.4 for GOS-E of 4 or above at 12 months, 95%-CI [2.2–13.6]; P<0.001). CMD status did not predict discharge disposition (OR 1.5, 95%-CI [0.5–4.2]; P=0.4). At the time of hospital discharge, there was a trend for a higher GOS-E scores amongst CMD patients compared to non-CMD patients that were discharged to a higher-level care setting (P=0.02, OR 2.0; 95%-CI 1.1–4.1). No difference in hospital discharge GOS-E was seen between CMD and non-CMD patients that were discharged home or to a rehabilitation setting (OR 1.2; 95%-CI 0.8–1.7; Figure 4).
Figure 4.
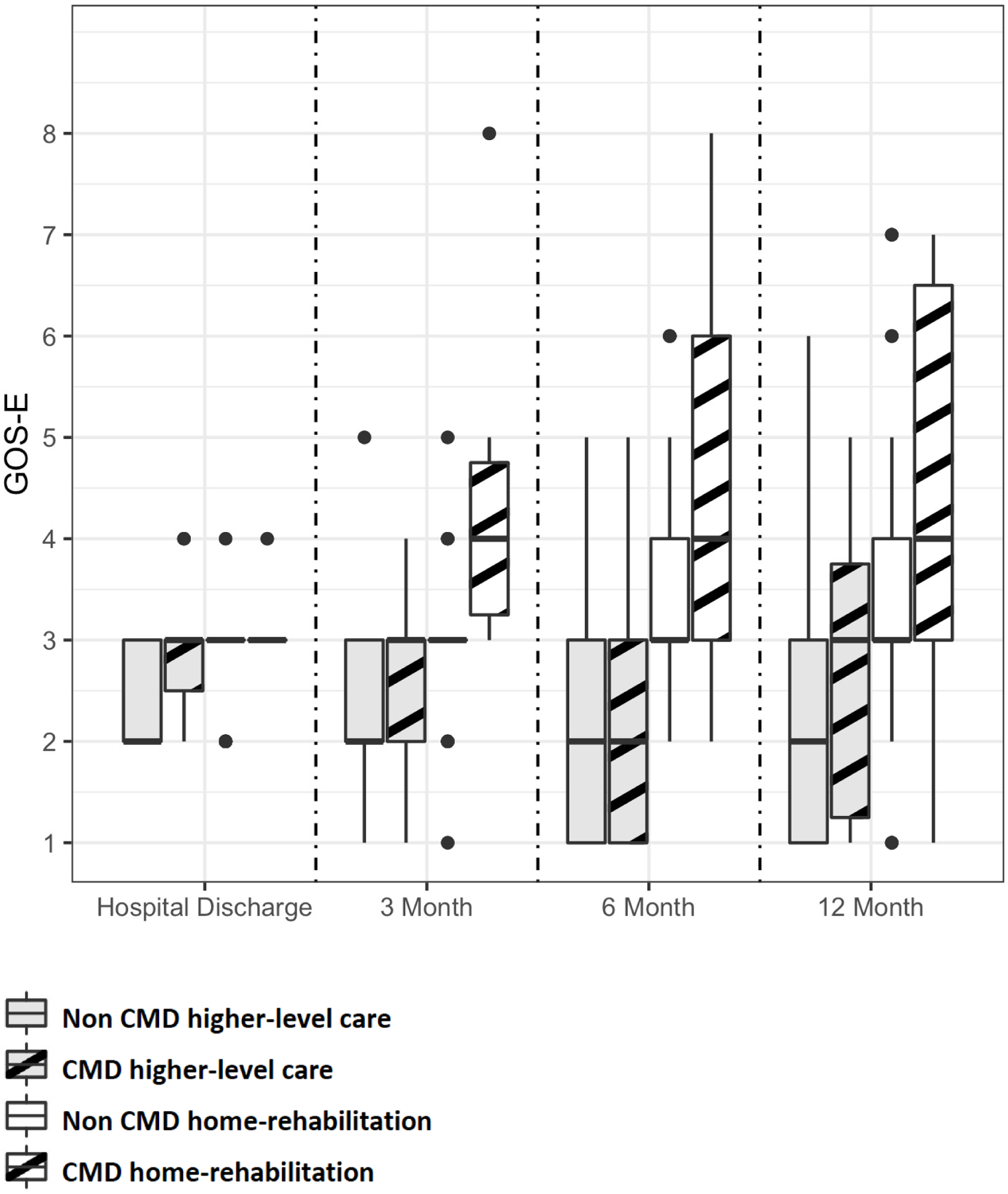
Functional outcomes stratified by CMD diagnosis and discharge disposition.
NS, not significant; * P<0.05; ** P<0.01; *** P<0.001
Patients with CMD that were discharged home or rehabilitation setting had better functional outcomes on the GOS-E score at 3 months (P<0.001, OR 4.5; 95%-CI 2.0–33.6), 6 months (P=0.07, OR 1.9; 95%-CI 1.0–4.4), and 12 months after the injury (P<0.001, OR 2.6; 95%-CI 1.4–6.2; Figure 4), accounting for other predictors such as age, admission GCS, and injury type. No significant difference was seen between CMD and non-CMD patients that were discharged to a higher-level care setting at any of the follow-up time points. In a multivariate longitudinal ordinal regression model amongst survivors, CMD (OR 7.2, 95%-CI [2.0–26.6] p=0.003), discharge to a home or rehabilitation setting (OR 5.6, 95%-CI [1.7–17.7] p<0.001), and the interaction between time and discharge disposition (OR 1.3, 95%-CI [1.2–1. 5] p<0.001) independently predicted of GOS-E improvement over time.
DISCUSSION
We found that 14% of patients without any behavioural signs of command following had evidence of command following detected by EEG within one week of injury in a consecutive series of patients with acute brain injury. Patients with EEG evidence of command following, also known as CMD, had higher rates of good functional recovery at all investigated time points, demonstrated a shorter time to recovery, and had a greater shift to recovery across all levels of functional outcome when compared to those without CMD. Shorter time to good recovery was predicted by CMD, underlying traumatic brain injury, and higher admission Glasgow Coma Score, with younger age having a minimal effect on time to recovery. Most patients with CMD that would recover by one year will have recovered by 3 months, while the majority of non-CMD patients that will recover do so after 3 months. CMD patients discharged home or to rehabilitation settings continued to improve at all follow-up timepoints, which was not seen for non-CMD patients or CMD patients not discharged to a home or rehabilitation setting. Detection of CMD may allow more accurate predictions of recovery trajectories and could help identify patients that benefit most from rehabilitation interventions.
Patient cohort.
We studied patients following acute brain injury during the acute phase of hospitalization, which is important to consider as most cohorts studied for recovery of consciousness in the past were recruited in the early or subacute rehabilitation setting.32 We enrolled close to one third (N=193) of the 598 unconscious patients screened for enrolment. Slightly more than a third (N=201) of the screened cohort did not qualify for this assessment as they started following commands clinically prior to enrolment, had a confounding neurological illness prior to the acute presentation, uncontrollable seizures, were deaf, or were unconscious prior to the injury. The remaining third (N=204) of screened patients were not included due to logistical reasons, EEG disconnection, or family not providing consent. Our results demonstrated that in healthy volunteers and patients brain activation to the paradigm was seen in both, English and Spanish, further supporting the generalizability of the approach. Comparing included to excluded patients, no differences in demographics or severity of neurological impairment on admission were seen but patients with brain haemorrhages (ICH or SAH), cardiac arrest, and TBI were slightly more commonly and patients with a mix of other diagnoses were less commonly enrolled than those that were excluded.
CMD was found to predict 12-month functional outcome in the original cohort of 100 acutely brain injured patients.13 There were slight differences between the original and secondary cohorts, most importantly in the underlying aetiology for unconsciousness (in the secondary cohort intracerebral haemorrhage was more, and cardiac arrest and traumatic brain injury less common than in the original cohort). Likely these differences reflect a more aggressive enrolment approach of patients with brain haemorrhages. The fact that despite minor differences between the two cohorts, CMD was predictive of better functional outcomes at all follow-up time points both in the original and secondary cohort (Supplemental Figure 6) supports the generalizability of our findings.
Recovery trajectory.
We confirm that brain activation in response to spoken commands can be detected early after brain injury,13,22 and also that the detection of CMD in these patients is associated with better recovery at 12 months.13 Delayed recovery may be seen for unconscious patients in the rehabilitation setting,6,33 but recovery trajectories of unresponsive patients with acute brain injury are uncertain8 and accurate predictions that families are seeking are lacking.9 We demonstrate here that recovery trajectories of patients with and without CMD are clearly different; importantly much earlier and that persistently greater degrees of recovery can be expected in CMD patients. CMD detection may represent part of the biological endotype as a reflection of underlying brain injury mechanisms allowing more precise predictions of recovery and identification of patients particularly amenable for therapeutic interventions such as intense rehabilitation efforts.9
We studied a cohort of unconscious patients with a mixture of underlying acute brain injuries, including structural with or without TBI and non-structural mechanisms. We demonstrated that CMD together with widely established predictors of recovery including a better neurological examination on hospital admission, traumatic brain injury causing the impairment of consciousness, and younger age, predicted time to recovery. Clinical trajectories of patients with varying underlying brain injuries may be quite different,4,6,33 as supported by our multivariate analysis that identified TBI/SDH as a predictor of earlier time to recovery and found that CMD was particularly predictive for patients with non-TBI structural brain injury. Most functional recovery trajectory data so far centers around patients with TBI.6,33 Studies show delayed recovery of consciousness in TBI patients, with 59% of unconscious patients having regained consciousness by 1 year, and 74% by 5 years.6 In patients with non-traumatic disorders of consciousness, 17% were found to have recovered consciousness by 6 months, and an additional estimated 7.5% after 6 months.4,34 Delayed functional recovery has been proven possible in patients with acute brain injury, however, current bedside behavioural assessments are unable to accurately predict the trajectory of functional recovery in these patients. We confirm here that younger patients and those with TBI causing unconsciousness not only have a greater chance for recovery but also a shorter time to recovery. In addition, the diagnosis of CMD particularly in patients with structural brain injury other than TBI may allow more accurate outcome predictions.
When applying prediction modelling based on population statistics to the bedside careful counselling of families is warranted. Importantly, failure to detect CMD is well established even in healthy volunteers14 as conscious patients may decide to not engage with the motor imagery or motor activation paradigm (false negatives, Supplemental Table 5). Patients with CMD may not recover (false positives) as functional recovery does not only rely on brain recovery and secondary complications during recovery may occur. These considerations need to be taken into account, when CMD is integrated into clinical practice.
Discharge disposition.
Criteria to qualify for rehabilitation services differ between health care systems but at a minimum our findings call into question the current practice of applying strict participation time cut-offs to identify the most promising candidates for rehabilitation services. We observed that CMD patients discharged home or to rehabilitation settings continued to improve at all follow-up timepoints, which was not seen for non-CMD patients or CMD patients not discharged to a home or rehabilitation setting. This observation needs to be interpreted with caution as our observational study design does not allow us to demonstrate that CMD patients that did not receive rehabilitation would have benefited from rehabilitation.
Limitations.
This study has several limitations. Firstly, it was conducted at a single centre with a relatively small sample, therefore its external validity is limited, and further larger observational studies are warranted to clearly define the reproducibility of this measure. It is worth noting that although the sample was relatively small, it is by far the largest to date and we were able to demonstrate reproducibility across two independent cohorts at our centre. Routine EEG is sufficient for the diagnosis when combined with time synchronized motor commands and the computational code to run the analysis is freely available for download.13 However, to serve as an early biomarker to predict later recovery, studies will now have to clearly define reproducibility across various sites and practice settings. Secondly, behavioural states are known to fluctuate and in chronic disorders of consciousness repeat assessments over several days have been recommended.35 For acutely brain injured patients in critical care this is often not practical due to the evolving illness with a non-stable neurological examination. Thirdly, longer term follow-up for years after the injury may establish a more complete recovery trajectory as increasingly small numbers of patients may demonstrate recovery up to ten years following brain injury.33 Fourthly, patients in the study may have experienced post-discharge hospitalizations or illnesses, which could have confounded measured outcomes. Outcomes in patients that were lost to follow-up are uncertain and secondary worsening is possible. However, the focus of this study is to investigate the potential to show recovery following brain injury. Fifthly, outcome measures were limited to crude outcome scales and future studies should include patient centred outcomes capturing cognition, mood, and quality of life. Sixthly, more complete phenotyping and endotyping including genetics, metabolomics, and standardized MR imaging protocols may support more accurate prediction schemes and allow further endotyping of acutely brain injured patients.9 Seventhly, we have included a heterogenous patient cohort but including patients with different underlying brain injuries allowed us to demonstrate that CMD is a behavioural state not restricted to a specific brain injury and predicts shorter time to recovery together with well-established predictors of more rapid recovery such as traumatic brain injury aetiology, younger age, and poor neurological function on hospital admission. This supports the generalizability of our findings. Eighthly, decisions about discharge disposition are complex and unaccounted factors including socio-economic elements specific to a particular healthcare context may be the deciding force. These may or may not be easy to change even with the most precise prediction algorithms. Unmeasured factors that associate with CMD may also drive discharge decisions. It remains unclear if re-triaging patients with CMD from higher-level care to a home-rehabilitation setting would result in better outcomes for these patients as we did not perform a randomization procedure. Lastly, some patients underwent WLST, which always needs to be considered in natural history studies of acute brain injury. To take this into account patients who underwent WLST or were lost to follow-up were censored and death was treated as a competing risk.
In conclusion, our study shows that patients with CMD recover earlier after acute brain injury and to a larger degree than those without CMD. Prediction of time to recovery may allow enrichment of interventional trials aimed at supporting recovery of consciousness. CMD may serve as a biomarker that quantifies the residual integrative function of the injured brain. This is not a static measure but may serve as an indicator of possible recovery if detected.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
We searched PubMed for articles published by April 2022 using the following search terms (“brain injury”) AND (“acute” OR “early”) AND (“coma” OR “disorder of consciousness” OR “unconscious” OR “vegetative state” OR “unresponsive wakefulness syndrome” AND (“cognitive motor dissociation” OR “covert consciousness” OR “brain activation”) AND (“recovery” OR “prognosis” OR “prognostication”). We did not restrict the search by language or publication date and identified 30 articles. Twelve of these were on chronic disorders of consciousness, 11 were reviews or editorials, 2 were unrelated and 1 focused on how the diagnosis of cognitive motor dissociation (CMD) would be perceived by health care proxies and caregivers. Four were prospective studies that diagnosed CMD using MRI or EEG in patients early after brain injury. These studies demonstrated that CMD could be detected early after brain injury in the ICU setting in approximately 15% of patients and three found an association with a behavioural outcome, command following or functional recovery. One study found that patients diagnosed with CMD during the ICU stay were more likely to be able to take care of themselves for at least 8 hours in a day one year after the injury. No studies prospectively investigated the role of “cognitive motor dissociation” OR “covert consciousness” on predicting the time to functional recovery.
Added value of this study
This study demonstrates that the diagnosis of CMD in the ICU independently predicts earlier time to recovery. Other predictors include younger age, better admission neurological status, and traumatic brain injury as the mechanism of injury. Amongst patients discharged home or to a rehabilitation setting, CMD diagnosed in the ICU was associated with better functional outcomes seen as early as 3 months after the injury. Even though CMD patients discharged to a higher-level care setting without access to rehabilitation service, such as skilled nursing facilities, had a marginally better functional status at hospital discharge than those without CMD, no outcome differences were seen at any of the follow-up time points.
Implications of all the available evidence
These findings provide families of unresponsive, acutely brain injured patients with more precise guidance regarding the recovery trajectory. CMD allows bedside quantification of the residual integrative function of the injured brain that may represent the foundation for a biologically meaningful classification of patients required for the successful design of clinical trials aimed at promoting recovery of consciousness. CMD diagnosis may allow identification of patients that have a high potential to benefit from rehabilitation interventions, possibly also amongst patients that currently do not meet criteria to be discharged to a rehabilitation setting.
Acknowledgements
The study was supported by funding from the NIH (NS106014, NS112760).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
DR is supported by grant funding from the NIH (HL151901). SA is supported by grant funding from the NIH (HL153311). SP is supported by grant funding from the NIH (NS113055). JC is a minority shareholder at iCE Neurosystems, and is supported by grant funding from the NIH (NS106014, NS112760), and the McDonnell Foundation.
Data sharing
Deidentified data and study protocols used in this publication will be made available to qualified researchers who provide a valid research question. Please direct inquiries to the corresponding author.
REFERENCES
- 1.O’Donnell JC, Browne KD, Kilbaugh TJ, Chen HI, Whyte J, Cullen DK. Challenges and demand for modeling disorders of consciousness following traumatic brain injury. Neurosci. Biobehav. Rev 2019;98:336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jourdan C, Bayen E, Bosserelle V, et al. Referral to rehabilitation after severe traumatic brain injury: Results from the PariS-TBI study. Neurorehabil Neural Repair 2013;27(1):35–44. [DOI] [PubMed] [Google Scholar]
- 3.Katz DI, Polyak M, Coughlan D, Nichols M, Roche A. Natural history of recovery from brain injury after prolonged disorders of consciousness: outcome of patients admitted to inpatient rehabilitation with 1–4 year follow-up. Prog Brain Res 2009;177:73–88. [DOI] [PubMed] [Google Scholar]
- 4.Giacino JT, Katz DI, Schiff ND, et al. Practice guideline update recommendations summary: Disorders of consciousness. Neurology 2018;91(10):450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estraneo A, Moretta P, Loreto V, Lanzillo B, Santoro L, Trojano L. Late recovery after traumatic, anoxic, or hemorrhagic long-lasting vegetative state. Neurology 2010;75(3):239–45. [DOI] [PubMed] [Google Scholar]
- 6.Nakase-Richardson R, Whyte J, Giacino JT, et al. Longitudinal outcome of patients with disordered consciousness in the NIDRR TBI Model Systems Programs. J Neurotrauma 2012;29(1):59–65. [DOI] [PubMed] [Google Scholar]
- 7.Kowalski RG, Hammond FM, Weintraub AH, et al. Recovery of Consciousness and Functional Outcome in Moderate and Severe Traumatic Brain Injury. JAMA Neurol 2021;78(5):548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammond FM, Katta-Charles S, Russell MB, et al. Research Needs for Prognostic Modeling and Trajectory Analysis in Patients with Disorders of Consciousness. Neurocrit Care 2021;35(Suppl 1):55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claassen J, Akbari Y; Alexander S, et al. Proceedings of the first Curing Coma Campaign NIH symposium: challenging the future of research for coma and disorders of consciousness. Neurocrit Care 2021;35(Suppl 1):4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schnakers C, Vanhaudenhuyse A, Giacino J, et al. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol 2009;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Childs NL, Mercer WN, Childs HW. Accuracy of diagnosis of persistent vegetative state. Neurology 1993;43(8):1465–7. [DOI] [PubMed] [Google Scholar]
- 12.Andrews K, Murphy L, Munday R, Littlewood C. Misdiagnosis of the vegetative state: Retrospective study in a rehabilitation unit. Br Med J 1996;313(7048):13–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Claassen J, Doyle K, Matory A, et al. Detection of Brain Activation in Unresponsive Patients with Acute Brain Injury. N Engl J Med 2019;380(26):2497–505. [DOI] [PubMed] [Google Scholar]
- 14.Edlow BL, Claassen J, Schiff ND, Greer DM. Recovery from disorders of consciousness: mechanisms, prognosis and emerging therapies. Nat Rev Neurol 2021;17(3):135–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Claassen J, Taccone FS, Horn P, et al. Recommendations on the use of EEG monitoring in critically ill patients: consensus statement from the neurointensive care section of the ESICM. Intensive Care Med 2013;39(8):1337–51. [DOI] [PubMed] [Google Scholar]
- 16.Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 2004;85(12):2020–9. [DOI] [PubMed] [Google Scholar]
- 17.Plum and Posner’s diagnosis and treatment of stupor and coma Posner JB, Saper CB, Schiff ND, J Claassen (Eds). Oxford University Press; 2019. [Google Scholar]
- 18.Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury: Observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry 1981;44(4):285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu J, Marmarou A, Lapane K, Turf E, Wilson L. A method for reducing misclassification in the extended Glasgow Outcome Score. J Neurotrauma 2010;27(5):843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedregosa F, Varoquaux G, Gramfort A, et al. Scikit-learn: machine learning in Python. J Mach Learn Res 2011;12:2825–30. [Google Scholar]
- 21.Cruse D, Chennu S, Chatelle C, et al. Bedside detection of awareness in the vegetative state: A cohort study. Lancet 2011;378(9809):2088–94. [DOI] [PubMed] [Google Scholar]
- 22.Edlow BL, Chatelle C, Spencer CA, et al. Early detection of consciousness in patients with acute severe traumatic brain injury. Brain 2017;140(9):2399–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int J Surg 2014;12(12):1495–9. [DOI] [PubMed] [Google Scholar]
- 24.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc 1999;94(446):496–509. [Google Scholar]
- 25.Zhou B, Latouche A, Rocha V, Fine J. Competing Risks Regression for Stratified Data. Biometrics 2011;67(2):661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong Y, Zhao O, Zhang B, Yao B. Adjusting for covariates in analysis based on restricted mean survival times. Pharm Stat 2022;21(1):38–54. [DOI] [PubMed] [Google Scholar]
- 27.Blanche P, Proust-Lima C, Loubère L, Berr C, Dartigues JF, Jacqmin-Gadda H. Quantifying and comparing dynamic predictive accuracy of joint models for longitudinal marker and time-to-event in presence of censoring and competing risks. Biometrics 2015;71(1):102–13. [DOI] [PubMed] [Google Scholar]
- 28.Howard G, Waller JL, Voeks JH, et al. A simple, assumption-free, and clinically interpretable approach for analysis of modified Rankin outcomes. Stroke 2012;43(3):664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lees KR, Zivin JA, Ashwood T, et al. NXY-059 for Acute Ischemic Stroke. N Engl J Med 2006;354(6):588–600. [DOI] [PubMed] [Google Scholar]
- 30.Kawaguchi A, Koch GG. sanon: An R Package for Stratified Analysis with Nonparametric Covariable Adjustment. J Stat Softw 2015;67(9):1–37. [Google Scholar]
- 31.Tutz G, Hennevogl W, Tutz G, Hennevogl W. Random effects in ordinal regression models. Comput Stat Data Anal 1996;22(5):537–57. [Google Scholar]
- 32.Kondziella D, Friberg CK, Frokjaer VG, Fabricius M, Møller K. Preserved consciousness in vegetative and minimal conscious states: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2016;87(5):485–92. [DOI] [PubMed] [Google Scholar]
- 33.Hammond FM, Giacino JT, Nakase Richardson R, et al. Disorders of Consciousness due to Traumatic Brain Injury: Functional Status Ten Years Post-Injury. J Neurotrauma 2019;36(7):1136–1146 [DOI] [PubMed] [Google Scholar]
- 34.Greer DM, Yang J, Scripko PD, et al. Clinical examination for outcome prediction in nontraumatic coma. Crit. Care Med 2012;40(4):1150–6. [DOI] [PubMed] [Google Scholar]
- 35.Wannez S, Heine L, Thonnard M, Gosseries O, Laureys S. The repetition of behavioral assessments in diagnosis of disorders of consciousness. Ann Neurol 2017;81(6):883–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


