Abstract
Objective
The research indicated that the nature of Chinese medicine is mainly related to body's substance and energy metabolism. The purpose of the study is to elucidate the substance basis for warm nature of Poria cocos (called Fuling (FL) in Chinese).
Methods
In terms of the effects of its separated fractions on the substance and energy metabolism in rat models of cold-deficiency with Aconiti Lateralis Radix Praeparata (called Fuzi (FZ) in Chinese), with hot nature, as reference drug. Biochemical indexes in the material metabolism, energy metabolism, endocrine system, nervous system and nucleotide system were determined, then analyzed by additive, cluster and principal component analysis (PCA).
Results
The medicinal natures of oligosaccharides and amino acids fractions were attributable to plain and crude polysaccharides, volatile oils and triterpenoids fractions were attributable to mild warm.
Conclusion
The nature of FL was regarded as mild warm based on the old records of Chinese medicine and fractions of crude polysaccharides, volatile oils and triterpenoids might be the main substance basis for the warm nature of FL. It is the first time that substance basis of FL was elucidated from view point of medicinal nature.
Keywords: endocrine system, energy metabolism, material metabolism, medicinal properties, nervous system, nucleotide system, poria cocos components
1. Introduction
Traditional Chinese medicine (TCM) played a key role in preventing and curing diseases in China history. While the property theory of Chinese medicine is always pivotal to elucidate its therapeutic characteristics, encompassing four qi, five flavors and channel tropism etc. Among them, four qi, the nature of Chinese medicine, is the essential core in the property theory of Chinese medicine. Four natures of Chinese medicine referred to warm, hot, cool and cold, which are often applied to describe part of the efficacy of herbs in TCM. In general, herbs with the cool or cold nature are deemed to clear away heat, eliminate toxic substances, nourish yin, and remedy hot syndromes of patients, such as Anemarrhenae Rhizoma (called Zhimu (ZM) in Chinese), the rhizome of Anemarrhena asphodeloides Bge., which is generally used to cure deficiency-heat syndrome, has functions like clearing heat-fire, nourishing yin for moistening dryness, moistening dryness to quench thirst (Weng, Chen, Su, & Xu, 2018). On the contrary, herbs with the warm or hot nature were usually used to dispel cold, warm up the interior, support yang, and thus treat cold syndromes of patients. For example, Aconiti Lateralis Radix Praeparata (called Fuzi (FZ) in Chinese), the root of Aconitum carmichaelii Debx., which could be used in the treatment of cold-deficiency syndrome (Han et al., 2012). There is also a special kind nature of Chinese medicine defined as “plain”, i.e. the nature of Chinese medicine is no obvious hot or cold nature, however more and more research indicated that it finally could be ascribed to cool or warm nature.
Over 15 years successive supporting by National Basic Research 973 Program of China, it could be revealed that the nature of Chinese medicine is associated obviously to the metabolism of substance and energy of organism, i.e. hot (or warm) nature drugs could boost the body's basal metabolic rate or excite endocrine system and central nervous system, and cold (or cool)-nature drugs possessed the opposite action with hot-nature drug (Sui et al., 2010; Cui et al., 2013; Li et al., 2014), providing a new way to explore the nature of Chinese herb.
Poria (called Fuling (FL) in Chinese) is the dried sclerotia of fungus Poria cocos (Schw.) Wolf, whose nature is recorded as “plain” in Chinese Pharmacopoeia (Zhao et al., 2015), and has been regarded as mild warm nature based on textual and research of FL. Actually, experiment research indicated that FL could alleviate cold-deficiency syndrome and raise the decreased levels of glucokinase (GCK), phosphoglycerate kinase (PGK), cytochrome C reductase (CCR) and triiodothyronine (T3) in cold-deficiency model, but had no significant effect on deficiency-heat syndrome, indicated that the nature of FL inclined to warm nature (Han et al., 2018). However, presently, the substance basis of medicinal nature of FL remains unclear. The main efficacy of FL is clearing damp and promoting diuresis and their substance basis has been verified in our previous experimental studies by pharmacological comparison of separated fractions of FL decoction (Li et al., 2015). This paper deals with the elucidation of substance basis of warm nature of FL. Previously, most researches are mainly concerned on the nature of a whole herbal medicine and rarely on the separated fractions of herbal medicine, however, substance basis of the nature of herbal medicine could not only elucidate the efficacious components of herbal medicine, but also lay a TCM theory foundation for the new drug development in way of effective component compatibility. Taking FL for example, it is the first time that the substance basis of FL was elucidated from view point of medicinal nature.
2. Materials and methods
2.1. Materials
2.1.1. Instruments, plant materials, and reagents
Microplate reader was purchased from Kate Biomedical Electronic Technology Co., Ltd. (Shenzhen, China). TDZ4-WS low-speed centrifuge was purchased from Xiangyi Centrifuge Instrument Co., Ltd. (Changsha, China). DZKW-D-2 Electrothermal Constant Temperature Water Bath was purchased from Beijing Yongguang Medical Instrument Co., Ltd. (Beijing, China); SHZ-82 Constant Temperature Oscillator was purchased from Changzhou Guohua Company (Changzhou, China); UV-2100 spectrophotometer was purchased from Unico Co., Ltd. (Shanghai, China). Heparin anticoagulant tube (Batch No. 20150116) was purchased from Kehua Inspection of Medical Products Co., Ltd. (Shanghai, China); The 5 mL injectors were purchased from Kangli Medical Devices Co., Ltd. (Zhenjiang, China). Normal saline was obtained from Kelun Pharmaceutical Ltd. (Heilongjiang, China).
Gypsum Fibrosum, ZM, Gentianae Radix et Rhizoma and Phellodendri Chinensis Cortex were purchased from Anhui Songshan Hall herbal Pieces Co., Ltd. (Anhui, China. Batch No. 130506), and FZ was purchased from Henan Renhe Herbal Pieces Co., Ltd. (Henan, China. Batch No.140101). FL was purchased from Yunnan Xianghui Biotechnology Co., Ltd (Yunnan, China. Batch No. 201211). All the samples were identified by Professor Bing Wang, College of Pharmacy, Liaoning University of Traditional Chinese Medicine. FL separated fractions were provided by our laboratory.
Liver glycogen assay kits (Batch No. 20160621), glucokinas (GCK) assay kits (Batch No. 20160621), phosphoglycerate kinase (PGK) assay kits (Batch No. 20160621), acetyl-coenzyme A (A-CoA) assay kits (Batch No. 20160621), citrate synthase (CS) assay kits (Batch No. 20160621), adipose triglyceride lipase (ATGL) assay kits (Batch No.20160621), cytochrome C reductase (CCR) assay kits (Batch No. 20160621), cytochrome C oxydase (COX) assay kits (Batch No. 20160621), triiodothyronine (T3) assay kits (Batch No. 20160621). thyroxine (T4) assay kits (Batch No. 20160621), 17-hydroxycorticosteroid (17-OHCS) assay kits (Batch No. 20160621), cyclic adenosine monophosphate (cAMP) assay kits (Batch No. 20160621), and cyclic guanosine monophosphate (cGMP) assay kits (Batch No. 20160621) were purchased from Beijing Cheng Lin Institute (Beijing, China). Pyruvate (PA) assay kits (Batch No. 20160621), acetylcholinesterase (AChE) assay kits (Batch No. 20160621) and Na+-K+-ATPase (Na+-K+-ATP) assay kits (Batch No. 20160505) were purchased from Nanjing Jian Cheng Bioengineering Institute (Nanjing, China).
2.1.2. Animals
Male Sprague-Dawley rats (weighing 170−210 g) were purchased from the Laborat-ory Animal Center of Changsheng Bio-Technique Co., Ltd. (Benxi, Liaoning, China; qualification NO. SCXK 2015-0001) and housed in air-conditioned room (temperature 22°C; relative humidity 55%), and fed ad libitum with standard feed and water in the course of the study. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). The study protocol was approved by the ethics regulations of Liaoning University of Traditional Chinese Medicine, China (131/2010; NO.2100009216026, date: 20161010).
2.2. Methods
2.2.1. Sample preparation
Decoction of drugs used to induce cold-deficiency rats: In the experiment, the rats with cold-deficiency syndrome were made by Chen et al., (2001). The Gypsum Fibrosum, ZM, Gentianae Radix et Rhizoma and Phellodendri Chinensis Cortex were weighed at the ratio 2: 1.2: 1:1.5, soaked in 8-fold water for 1 h, heated to boiling, and then kept micro-boiling for 20 min. After being filtered, 6-fold volume of distilled water was added and rapidly heated to boiling, and then kept micro boiling for 15 h, hot filter. The two filtrates were combined, then concentrated to 4 g/mL and stored at 4°C for reserve.
Decoction of reference drugs with hot nature: Quantitative FZ was weighed and soaked in 8-times of water for 1 h, then heated to boiling quickly, then kept in a slight boiling state until it tasted numb-free, hot filter. A total of 6-times water was added to the remaining drugs, quickly heated to boiling and then kept slightly boiling for 1 h, hot filter. All the filtrates were combined and then concentrated to 0.828 g/mL. The samples were kept at 4°C before use.
Preparation of FL separated fractions: The method for preparation of separated fractions of FL was performed according to reference (Li et al., 2015). Briefly, FL was pulverized and immersed in water for 1 h, boiled twice to give the volatile oils fraction and the filtrates were combined and evaporated in vacuum to 1.28 g/mL. Then 95% ethanol was added to the water decoction to regulate the alcohol concentration to 75%, standstill overnight to provide the precipitate and supernatant. The precipitate was washed three times with 95% ethanol and acetone respectively to furnish the crude polysaccharides fraction (CPF, the yield of CPF is 0.6631%). Then, the supernatant was evaporated on water bath at 50°Cto eliminate the alcohol and the resulting water layer was extracted eight times with 60-90 petroleum ether until there is no color in layer of petroleum ether to give petroleum ether fraction (PEF, the yield of PEF is 0.0241%). And then, the water layer was partitioned with ethyl acetate to yield ethyl acetate fraction (EAF, the yield of EAF is 0.0928%). The resulting water layer was subjected to a column of macroporous adsorption resin D101, washing with distilled water, 80% ethanol and 95% ethanol successively. The water eluate was concentrated at 50 and lyophilized to give water eluated fraction (WEF, the yield of WEF is 1.6868%) and 80% and 95% ethanol elutes were collected and evaporated in vacuo to give the alcohol eluted fraction (AEF, the yield of AEF is 0.2787%). In addition, based on the HPLC fingerprints of PEF, EAF, AEF and WEF, their overlapping degrees were evaluated to be less than 10%. Over 20 compounds were isolated and identified from the above fractions and thereof, it can be concluded that the constituent of PEF mainly contains aromatic esters, EAF mainly contains triterpenoids, WEF mainly contains oligosaccharides, AEF mainly contains amino acids and indoles compounds, CPF mainly contains crude polysaccharides fraction. The doses of FL separated fractions were calculated through equivalent dose ratio of human to rat according to body surface area. The one-day dose of FL for human is 15 g in accordance with the dose in 2015 edition of Chinese Pharmacopoeia, while the equivalent ten-fold dose (high dose) of FL for rat was 13.8 g/kg and equivalent five-fold dose (low dose) of FL was 6.9 g/kg. We decided the dose of FL separated fractions: volatile oils of low dose is 1.6629 × 10−4 g/mL, volatile oils of high dose is 3.3258 × 10−4 g/mL, triterpenoids of low dose is 6.4032 × 10−4 g/mL, triterpenoids of high dose is 1.2806 × 10−3 g/mL, crude polysaccharides of low dose is 4.5754 × 10−3 g/mL, crude polysaccharides of high dose is 9.1508 × 10−3 g/mL, oligosaccharides of low dose is 1.1639 × 10−2 g/mL, oligosaccharides of high dose is 2.3278 × 10−2 g/mL, amino acids of low dose is 1.9230 × 10−3 g/mL and amino acids of high dose is 3.8460 × 10−3 g/mL. The samples were kept at 4°C before use.
2.2.2. Dose calculation for rats
The doses of different samples were calculated through equivalent dose ratio of human to rat according to body surface area. The 1-day dose of FZ for humans is 15 g according to the 2015 edition of Chinese Pharmacopoeia. Therefore, the equivalent six-fold dose of FZ for rats was calculated as 8.28 g/kg. The 1-day dose of FL for human is 15 g in accordance with the dose in 2015 edition of Chinese Pharmacopoeia, while the equivalent ten-fold dose of FL for rat was 13.8 g/kg and equivalent five-fold dose of FL was 6.9 g/kg.
2.2.3. Animals and drug administration
Healthy male specific pathogen free grade SD rats, were housed for 7 d in an animal room prior to the experiments. Rats were randomly divided into 13 groups (n = 10 per group): the blank group, the model (MO) group, the FZ group, the crude polysaccharides of low dose group, the crude polysaccharides group, the volatile oils of low dose group, the volatile oils group, the triterpenoids of low dose group, the triterpenoids group, the oligosaccharides of low dose group, the oligosaccharides group, the amino acids of low dose group, and the amino acids group. The MO drugs were given by oral gavage i.g. every morning for 14 d, respectively, while blank group was administered i.g. with the equivalent volume of distilled water. The administration volume was 16 mL/kg of body weight. Since the 15th day, each administration group was administered i.g. with the dose as above every afternoon for seven consecutive days, while the MO and blank groups were given the equivalent volume of distilled water, the administration volume was 10 mL/kg of body weight.
2.2.4. Blood and tissue sampling
One hour after the last drug administration, the rats were intraperitoneally injected with 1% pentobarbital sodium (4 mL/kg body weight). After anesthesia, the abdominal cavity was exposed, then blood was collected from the abdominal aortic and cryogenically centrifuged at 2500 r/min at 4°C for 20 min to separate the plasma from the blood cells. The dissection of the liver was performed immediately after the blood was collected. The liver was removed and approximately 0.1 g of the liver was homogenized in nine times of saline and then centrifuged at 3500 r/min for 15 min. All the dissection parts of the liver were taken from the same spot of the liver of the rats and kept at –80°C before use.
2.2.5. Determination of indicators in vivo
All the indexes were measured strictly according to manufacturers’ instructions. The levels of glycogen, PA, GCK, PGK, A-CoA, CS were assayed to evaluate the effects on material metabolism. The level of ATGL was assayed to evaluate the effects on lipid metabolism. The levels of COX, CCR and Na+-K+-ATP were assayed to evaluate the effects on energy metabolism. The levels of T3, T4 and 17-OHCS were assayed to evaluate the effects on endocrine system. The levels of AChE were assayed to evaluate the effects on nervous system. The levels of cAMP and cGMP, and the ratio of cAMP/cGMP were assayed to evaluate the effects on cyclic nucleotide system.
2.2.6. Determination of rat rectal temperature, toe temperature and appearance score of each rat
Rectal temperature was measured with electronic rectal thermometer at 4:00 PM on the days 0, 14, and 21 (7th day of treatment). Simultaneously, toe temperature was measured with an infrared thermometer. The appearance score of each rat was measured on the days 7, 14, and 21 (7th day of treatment), according to the standard of TCM syndrome (Deng, 1990) and national standard for clinical diagnosis and treatment of TCM (State Bureau of Technical Supervision, GB/T16751.2-1997) .
2.2.7. Statistical methods
Measurement data were expressed as means ± S.D. Statistical analysis was performed using ANOVA with the LSD test using SPSS version 20.0. The values of P < 0.05 were considered statistically significant.
2.3. Analysis of nature attribution of FL separated fractions
2.3.1. Analysis of nature attribution of FL separated fractions by additive analysis
The data in vivo were analyzed by additive analysis to found the medicinal natures of FL separated fractions. All data of FL separated fractions groups were compared with that of the model group, when P < 0.05, the value was 1. When P < 0.01, the value was 2. There was no significant effect and the value was 0. Then sum, the larger the sum value was, the hotter the medicinal nature tends to be.
2.3.2. Analysis of nature attribution of FL separated fractions by clustering analysis method
The data in vivo were analyzed by clustering analysis to found the medicinal natures of FL separated fractions. All data of FL separated fractions groups were compared with that of the model group, when P < 0.05, the value was 1. When P < 0.01, the value was 2. There was no significant effect and the value was 0. Results were visualized in the form of dendrogram. Cluster analysis was performed with SPSS 19.0.
2.3.3. Analysis of nature attribution of FL separated fractions by PCA method
The data in vivo were analyzed by PCA to found the medicinal natures of FL separated fractions. PCA was performed to generate an overview for group clustering of all the index data. Results were visualized in the form of the score plots, where each point represented an individual sample. Multivariate analysis was performed with EZ info Software of Masslynx V4.1 version (Waters Corp., Milford, USA).
3. Results
3.1. Effects on substance metabolism
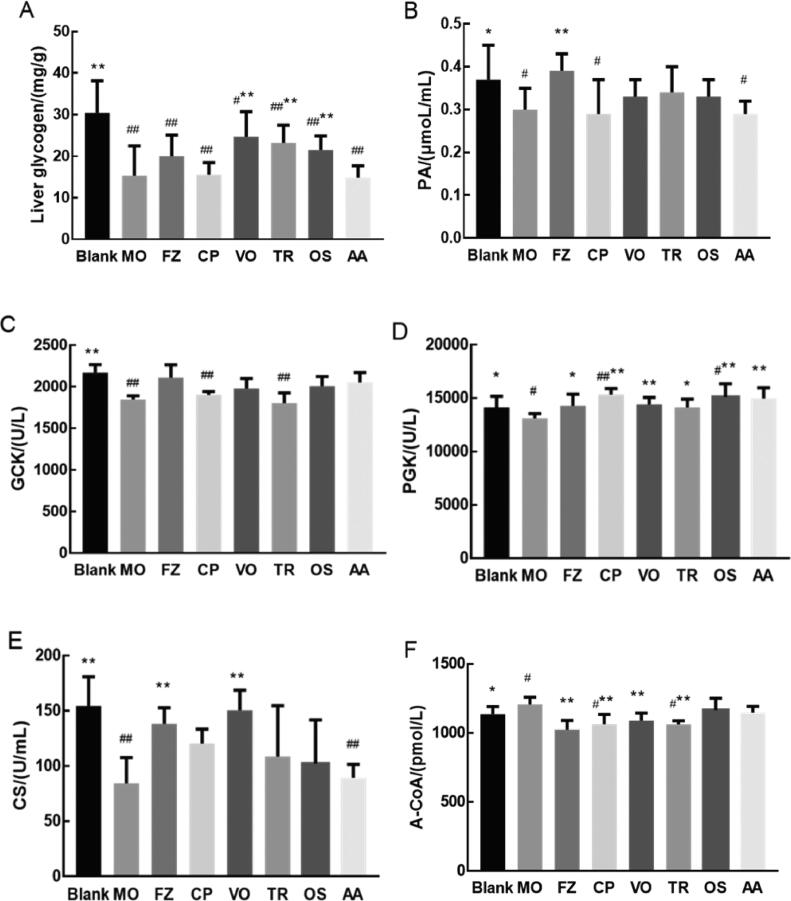
As shown in Fig. 1, compared with the blank group, the levels of liver glycogen, PA, GCK, PGK and CS in the MO group were significantly reduced (P < 0.05 or P < 0.01). The level of A-CoA in the MO group was significantly increased (P < 0.05). The levels of liver glycogen and GCK of the FZ group were increased compared with that of the MO group, but the difference was not significant (P > 0.05). The levels of CS, PA and PGK of the FZ group were significantly increased compared with that of the MO group (P < 0.05 or P < 0.01). As shown in Fig. 1A, compared with MO group, liver glycogen levels in volatile oils group, triterpenoids group and oligosaccharides group were significantly increased (P < 0.05 or P < 0.01). As shown in Fig. 1C, compared with MO group, the GCK levels in volatile oils group, amino acids group and oligosaccharides group were increased (P > 0.05). As shown in Fig. 1D, compared with MO group, the PGK levels in crude polysaccharides, volatile oils group, triterpenoids group, oligosaccharides group and amino acids group were significantly increased (P < 0.05 or P < 0.01). As shown in Fig. 1E, compared with MO group, the CS levels in crude polysaccharides, triterpenoids group and oligosaccharides group were increased (P > 0.05). the CS level in volatile oils group was significantly increased (P < 0.01). As shown in Fig. 1F, compared with MO group, the A-CoA levels in crude polysaccharides, volatile oils group and triterpenoids group were significantly decreased (P < 0.05 or P < 0.01).
Fig. 1.
Effects of drug after administration on substance metabolism (A, liver glycogen; B, PA; C, GCK; D, PGK; E, CS; F, A-CoA) in cold-deficiency syndrome rats of each group.
Cumulative values are reported as means ± standard error (S.E.) for 10 rats in each group. *P < 0.05 and **P < 0.01 vs MO group, #P <0.05 and ##P < 0.01 vs CON group. Liver glycogen, PA, PGK, A-CoA using one-way analysis of variance (ANOVA) with the least significant difference (LSD) analysis. GCK, CS using ANOVA with the Dunnett's T3 analysis.
Notes: Crude polysaccharides group (CP), Volatile oils group (VO), Triterpenoids group (TR), Oligosaccharides group (OS), Amino acids group (AA).
3.2. Effects on lipid metabolism
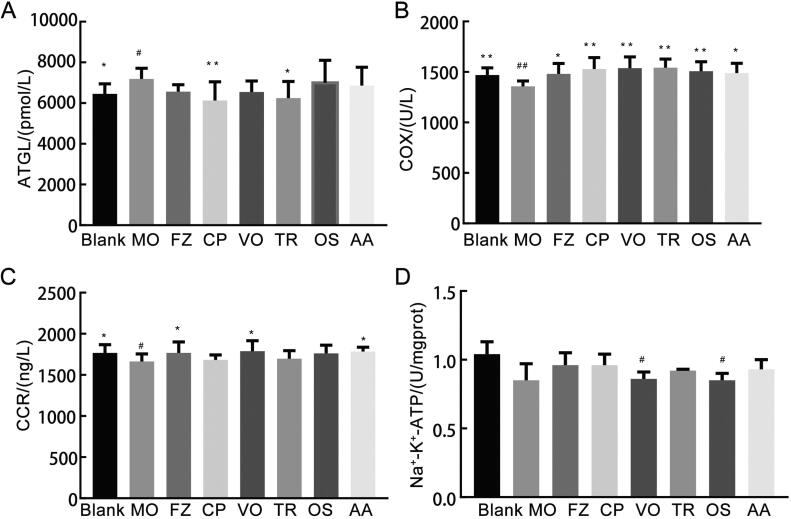
As shown in Fig. 2A, compared with the blank group, the level of ATGL in the MO group was significantly increased (P < 0.05). Compared with MO group, the levels of ATGL in crude polysaccharides group and triterpenoids group were significantly increased (P < 0.05 or P < 0.01). The levels of ATGL in volatile oils group, oligosaccharides group and amino acids group were increased compared with that of the MO group, but the difference were not significant (P > 0.05).
Fig. 2.
Effects of drug administration on lipid metabolism and energy metabolism in cold-deficiency syndrome rats of each group. A. Effect on ATGL. B. Effect on COX. C. Effect on CCR. D. Effect on Na+-K+-ATP. Cumulative values are reported as means ± S.E. for 10 rats in each group. *P < 0.05 and **P < 0.01 vs MO group, #P<0.05 and ##P <0.01 vs CON group, ATGL, COX, CCR using ANOVA with the LSD analysis. Na+-K+-ATP using ANOVA with the Dunnett's T3 analysis.
Notes: Crude polysaccharides group (CP), Volatile oils group (VO), Triterpenoids group (TR), Oligosaccharides group (OS), Amino acids group (AA).
3.3. Effects on energy metabolism
As shown in Fig. 2B, C, D, compared with the blank group, the activities of COX and CCR in the MO group were significantly reduced (P < 0.01 or P < 0.05). The Na+-K+-ATP activity of the MO group was reduced, with no significant difference (P > 0.05). Compared with MO group, the COX activities of FZ group, crude polysaccharides group, volatile oils group, triterpenoids group, oligosaccharides group and amino acids group were significantly increased (P < 0.05 or P < 0.01). The activities of CCR in FZ group, volatile oils group, amino acids group were increased significantly (P < 0.05 or P < 0.01), compared with that of MO group. That activity of oligosaccharides group was increased, but without significant difference (P > 0.05). Na+-K+-ATP activities of FZ group, crude polysaccharides group, triterpenoids group and amino acids group were increased, but without significant difference (P > 0.05).
3.4. Effects on nervous system
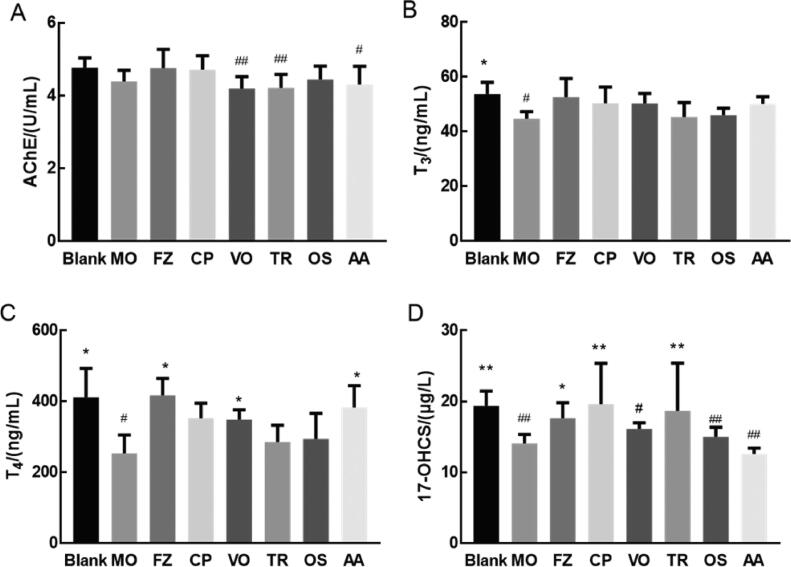
Fig. 3A shows that compared with the blank group, AChE activity in the MO group showed a decreasing trend, but there was no significant difference (P > 0.05). Compared with MO group, AChE activities in FZ group and crude polysaccharides group showed an increasing trend, but no significant difference (P > 0.05).
Fig. 3.
Effects of drug administration on nervous system and endocrine system in cold-deficiency syndrome rats of each group. A. Effect on AChE. B. Effect on T3. C. Effect on T4. D. Effect on 17-OHCS. Cumulative values are reported as means ± S.E. for 10 rats in each group. *P < 0.05 and ⁎⁎P < 0.01 vs MO group, #P < 0.05 and ##P < 0.01 vs blank group. AChE, 17-OHCS using ANOVA with the LSD analysis. T3, T4 using ANOVA with the Dunnett's T3 analysis.
Notes: Crude polysaccharides group (CP), Volatile oils group (VO), Triterpenoids group (TR), Oligosaccharides group (OS), Amino acids group (AA).
3.5. Effects on endocrine system
As shown in Fig. 3B, C, D, compared with the blank group, the levels of T3, T4, and 17-hydroxycorticosteroid (17-OHCS) in the MO group were significantly reduced (P < 0.05 or P < 0.01). The levels of T3 in FZ group, crude polysaccharides group, volatile oils group and amino acids group were increased with no significant difference (P > 0.05), compared with that of MO group. Compared with MO group, the levels of T4 in FZ group, volatile oils group, amino acids group were significantly increased (P < 0.05). The T4 levels in other groups showed an increasing trend, with no significant difference (P > 0.05). The levels of 17-OHCS in FZ group, crude polysaccharides group and triterpenoids group were significantly increased (P < 0.05 or P < 0.01).
3.6. Effects on nucleotide system
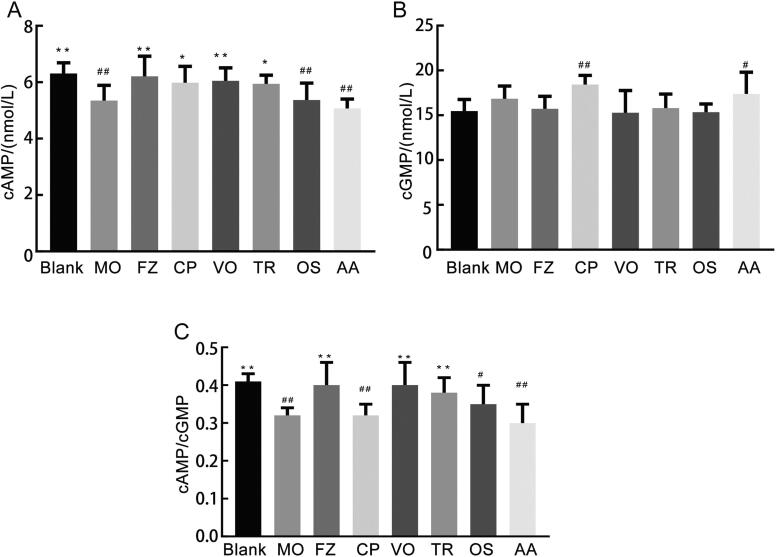
According to Fig. 4, compared with the blank group, the levels of cAMP and cAMP/cGMP ratio of the MO group were significantly reduced (P < 0.01). Compared with the MO group, the levels of cAMP in the FZ group, crude polysaccharides group, volatile oils group and triterpenoids group were significantly increased (P < 0.05 or P < 0.01). The cAMP/cGMP ratios in the FZ group, triterpenoids group and volatile oils group were significantly increased (P < 0.01).
Fig. 4.
Effects of drug administration on nucleotide system (A, cAMP; B, cGMP; C, cAMP/cGMP) in cold-deficiency syndrome rats of each group.
Notes: Cumulative values are reported as means ± S.E. for 10 rats in each group. *P < 0.05 and **P < 0.01 vs MO group, #P < 0.05 and ##P < 0.01 vs blank group, using ANOVA with the LSD analysis.
Notes: Crude polysaccharides group (CP), Volatile oils group (VO), Triterpenoids group (TR), Oligosaccharides group (OS), Amino acids group (AA).
3.7. Effects on rat rectal temperature, toe temperature and appearance score
According to supplementary material (Figs. S1, 2 and 3), on the 0 day of modeling, there was no significant difference in rectal and toe temperatures (P > 0.05). On the 14th day of modeling, the rectal and toe temperatures of the rats in the other groups were significantly lower than those in the blank group (P < 0.05 or P < 0.01). On the 7th day of treatment, compared with the model group, the rectal temperature of rats in the FZ group, crude polysaccharides group, volatile oils group and oligosaccharides group were increased significantly (P < 0.05 or P < 0.01). On the 7th day of treatment, compared with the model group, the toe temperature of rats in the FZ group was significantly increased (P < 0.05), while there was no significant difference between the other groups and the model group (P > 0.05).
On the 7th day of modeling, compared with the blank group, the apparent score of the model group was significantly increased (P < 0.01). On the 14th day of modeling, compared with the blank group, the apparent score of the model group was further increased, with an extremely significant difference (P < 0.01), suggesting the successful establishment of deficiency-cold syndrome model. On the 7th day of treatment, compared with the model group, the apparent scores of FZ group, crude polysaccharides group, volatile oils group, triterpenoids group, oligosaccharides group and amino acids group were significantly reduced (P < 0.01).
3.8. Multi-index comprehensive analysis after high dose of administration
3.8.1. Additive analysis for all indexes in vivo of different groups
The larger the sum value was, the hotter the medicinal nature tends to be. Combined with the additive analysis according to Table 1, Table 2, Table 3. We found the sums value of crude polysaccharides group, volatile oils group and triterpenoids group were higher than oligosaccharides group and amino acids group. But they all lower than FZ group and blank group. It can be considered that the natures of volatile oils, crude polysaccharides and triterpenoids were warm. The natures of oligosaccharides and amino acids group were plain.
Table 1.
Additive analysis of substance metabolism, lipid metabolism and nervous system of different groups
| Index | Substance metabolism |
Lipid metabolism | Nervous system | Sum | |||||
|---|---|---|---|---|---|---|---|---|---|
| liver glycogen | PA | GCK | PGK | CS | A-CoA | ATGL | AChE | ||
| Blank | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 0 | 10 |
| FZ | 0 | 2 | 0 | 1 | 2 | 2 | 0 | 0 | 7 |
| CP | 0 | 0 | 0 | 2 | 0 | 2 | 2 | 0 | 6 |
| VO | 2 | 0 | 0 | 2 | 2 | 2 | 0 | 0 | 8 |
| TR | 2 | 0 | 0 | 1 | 0 | 2 | 1 | 0 | 6 |
| OS | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 4 |
| AA | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 |
All data of FL separated fractions groups were compared with that of the model group, when P < 0.05, the value was 1. When P < 0.01, the value was 2. There was no significant effect and the value was 0. Notes: Crude polysacchaides group (CP), Volatile oils group (VO), Triterpenoids group (TR), Oligosaccharides group (OS), Amino acids group (AA).
Table 2.
Additive analysis of energy metabolism, endocrine system and nucleotide system of different groups
| Index | Energy metabolism |
Endocrine system |
Nucleotide system |
Sum | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| COX | CCR | Na+-K+-ATP | T3 | T4 | 17-OHCS | cAMP | cGMP | cAMP/cGMP | ||
| Blank | 1 | 1 | 0 | 1 | 1 | 2 | 2 | 0 | 2 | 10 |
| FZ | 1 | 1 | 0 | 0 | 1 | 1 | 2 | 0 | 2 | 8 |
| CP | 2 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 5 |
| VO | 2 | 1 | 0 | 0 | 1 | 0 | 2 | 0 | 2 | 8 |
| TR | 2 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 2 | 7 |
| OS | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| AA | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 |
All data of FL separated fractions groups were compared with that of the model group, when P < 0.05, the value was 1. When P < 0.01, the value was 2. There was no significant effect and the value was 0. Notes; Crude polysaccharides group (CP), Volatile oils group (VO), Triterpenoids group (TR), Oligosaccharides group (OS), Amino acids group (AA).
Table 3.
Additive analysis for all indices in vivo of different groups
| Index | Energy metabolism | Endocrine system | Nucleotide system | Substance metabolism | Lipid metabolism | Nervous system | Sum |
|---|---|---|---|---|---|---|---|
| Blank | 2 | 4 | 4 | 9 | 1 | 0 | 20 |
| FZ | 2 | 2 | 4 | 7 | 0 | 0 | 15 |
| CP | 2 | 2 | 1 | 4 | 2 | 0 | 11 |
| VO | 3 | 1 | 4 | 8 | 0 | 0 | 16 |
| TR | 2 | 2 | 3 | 5 | 1 | 0 | 13 |
| OS | 2 | 0 | 0 | 4 | 0 | 0 | 6 |
| AA | 2 | 1 | 0 | 2 | 0 | 0 | 5 |
All data of FL separated fractions groups were compared with that of the model group, when P < 0.05, the value was 1. When P < 0.01, the value was 2. There was no significant effect and the value was 0. Notes: Crude polysaccharides group (CP), Volatile oils group (VO), Triterpenoids group (TR), Oligosaccharides group (OS), Amino acids group (AA).
3.8.2. Cluster analysis for all indexes in vivo of different groups
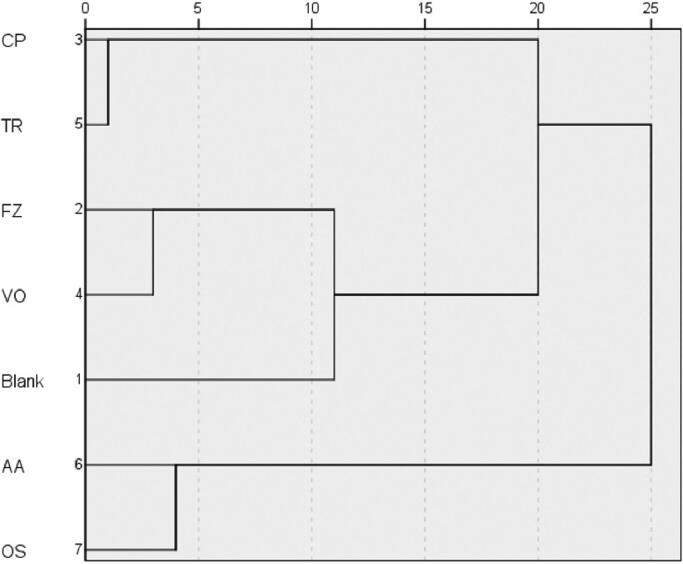
According to (Fig. 5), the natures of oligosaccharides group and amino acids group were similar, the natures of volatile oils group, the crude polysaccharides group, triterpenoids group, FZ group and blank group were similar. And the nature of volatile oils group was more closest to the FZ group. Combined with the analysis of significant differences, it can be considered that natures of volatile oils, crude polysaccharides and triterpenoids were warm. The natures of oligosaccharides and amino acids group were plain.
Fig. 5.
Cluster analysis graph.
Notes: Crude polysaccharides group (CP), Volatile oils group (VO), Triterpenoids group (TR), Oligosaccharides group (OS), Amino acids group (AA).
3.8.3. Principal component analysis (PCA) for all indexes in vivo of different groups
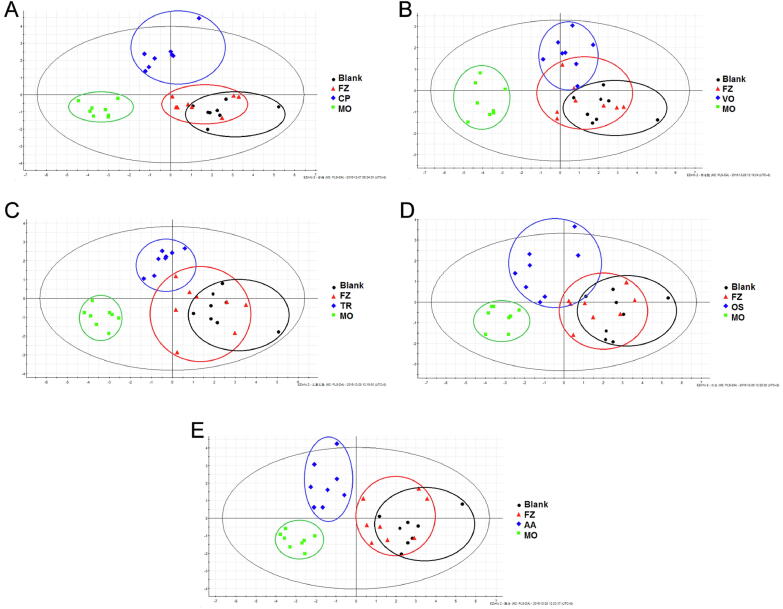
As shown in Fig. 6. The results showed that MO and blank groups were well separated. In addition, both groups could be separated from the blank group, while volatile oils group showed obvious overlap with MO group and obvious tendency to FZ group. It was concluded that the medicinal natures of the crude polysaccharides could be attributed to warm.
Fig. 6.
PCA analysis results of CP group (A), VO group (B), TR group (C), OS group (D) and AA group (E).
Notes: KMO statistic of the dates > 0.5 and Bartlett spherical test of the date < 0.05, these met the requirements and were applicable to PCA analysis. Crude polysaccharides group (CP), Volatile oils group (VO), Triterpenoids group (TR), Oligosaccharides group (OS), Amino acids group (AA).
3.8.4. Biochemical indicators of rats in cold-deficiency syndrome after low dose administration
According to supplementary material (Figs. S4−10), the changes in biochemical indicators of rats suggested the successful establishment of cold-deficiency syndrome model. FZ and separated fractions of FL showed therapeutic effect on cold-deficiency syndrome.
3.8.5. Multi-index comprehensive analysis after low dose administration
According to supplementary material (Table s1−3), (Figs. S11−12), combined with the additive analysis, cluster analysis and PCA, we found when in low dose, the medicinal nature of the amino acids of low dose could be attributed to plain, while the medicinal natures of the crude polysaccharides of low dose, volatile oils of low dose, oligosaccharides of low dose and triterpenoids of low dose could be attributed to mild warm. But they have little effect on rats with cold-deficiency.
4. Discussion
Recent research indicated that the drugs nature was primarily associated with the metabolism of substances and the energy of organism (Xiao et al., 2017; Kuang and Cheng, 2009). For example, cold drugs can mostly inhibit the excitability of the central nervous system, weaken the functions of breathing, circulation, metabolism and muscle activity, reduce the body's calorific output, and weaken the body's response to pathogenic stimuli, whereas hot drugs can reverse this trend (Ma, 1984; Li et al., 1999; Liu, 1997). Studies have revealed that the nature of FL was inferred as trending to mild warm. This study was mainly focused on the effects of separated fractions of FL on substance and energy metabolism based on the models of cold-deficiency in rats, compared with FZ.
The biochemical indexes changed in substance metabolism, energy metabolism, endocrine system, nervous system, nucleotide system of rats in each group were measured. The experiment found the level of PA in rats with cold-deficiency syndrome was decreased, which was consistent with the reports (Liu et al., 2011). This may be due to the fact that rats with cold-deficiency syndrome mainly depend on glycolysis for energy supply. Liver glycogen is a storage form of sugar in animals. When the body needs glucose, it can be quickly used for supply and demand in order to maintain body temperature and ensure energy supply. The experiment found that the level of liver glycogen in model group rats was decreased. After treatment with FZ and separated fractions of FL, it was found that the levels of PA and liver glycogen in each group were affected to different degrees, thus promoting the recovery of substance metabolism in rats. The transformation of sugars, fats, and amino acids can be achieved with PA through A-CoA. A-CoA for citrate synthesis was generated by oxidation of PA, and the generated A-CoA can activate pyruvate dehydrogenase (Wang, 2016). The present study found that level of A-CoA in cold-deficiency model was increased, and FZ, crude polysaccharides, volatile oils and triterpenoids group could reverse the increasing tendency of A-CoA activity. The activity of GCK, PGK, and CS in the aerobic oxidation process of the cold-deficiency were decreased, and FZ could reverse the decreasing tendency of them.
CCR and COX exist on the complex III and IV of the oxidative respiratory chain. Some studies have found that the activity of complex I−V in the respiratory chain of rats with cold-deficiency was decreased, that is to say, the activity of respiratory chain complex in rats with cold-deficiency was inhibited (Yan, 2014). Na+-K+-ATP can maintain the different gradient concentrations of ions and adjust the transportation of amino acid and glucose (Einholm, 2016; Shi, 2012). The study found that the activities of CCR, COX and Na+-K+-ATP of rats with cold-deficiency syndrome were decreased, that is, the oxidative respiratory chain complexes III and IV were inhibited, which hindered the main synthesis of ATP. After treatment with FZ and separated fractions of FL, they can reverse the decreasing tendency of CCR, COX and Na+-K+-ATP activities in rats with cold-deficiency syndrome; Among which volatile oil, triterpenoids and crude polysaccharide composition group have strong influence.
Endocrine system is an important functional regulation system in the body, especially in metabolism, reproduction and maintaining the stability of the internal environment, including thyroid and adrenal glands (Tan, 2007). As hormones of thyroid synthesis and secretion, T3 and T4 can regulate substance metabolism, promote substance oxidation in body cells, increase oxygen consumption rate of rats, thus promote basic metabolism and raise body temperature. The experiment found that the levels of T4 and T3 were decreased in rats with cold-deficiency syndrome, that is, the herbs with cold nature were inhibited the function of thyroid hormones in rats, thus affecting the body's material and energy metabolism. The level of 17-OHCS in cold-deficiency syndrome group was decreased, showing that the modeling drug could inhibit the function of adrenal cortex. After treatment with FZ and separated fractions of FL, volatile oils and amino acids group could significantly increase the T4 level of rats with cold-deficiency syndrome, which indicated that the effect of FL separated fractions on endocrine system was weak.
AChE is a key enzyme in biological nerve conduction, which can degrade acetylcholine, terminate the excitability of neurotransmitters on the postsynaptic membrane, and ensure the normal transmission of nerve signals in the body. The strong activity of AChE indicates increased acetylcholine release and increased cholinergic nerve excitation (Xu and Yi, 1999). The AChE activity of rats in cold-deficiency model group was tended to decrease, and rats in model group were burnout and low reaction. After treatment, AChE activity of rats in FZ group and crude polysaccharides group were tended to increase, and the mental and activity status of rats in each group also recovered.
As the second messenger of cellular regulation, cyclic nucleotide cAMP and cGMP are intermediate links of hormones, neuromediators and drug effects, and played an important role in life activities. The levels of cAMP, cGMP and cAMP/cGMP are often used to identify yin deficiency and yang deficiency animal model. The experiment found that the level of cAMP in rats with cold-deficiency was reduced, the level of cGMP had no obvious change, the cAMP/cGMP ratio was decreased, the results were similar as the result of Zhang et al., (2015), the levels of cAMP and the cAMP/cGMP ratio were decreased in the rats with cold-deficiency in kidney in her experiment. It indicated that the cold-deficiency model was success. After treatment with FZ and FL separated fractions, the level of cAMP in FZ group, crude polysaccharides group, volatile oils group, triterpenoids group were increased, cAMP/cGMP ratio in FZ group, volatile oils group were increased. The results showed that volatile oils composition can restore the body's normal physiological effect.
5. Conclusion
Generally, the drugs with warm or hot nature could reverse the changes of substance metabolic system, energy metabolic system, endocrine system, nervous system and nucleotide system in cold-deficiency rats as FZ. Our experiment together with cluster analysis and PCA method revealed the natures of FL separated fractions. Oligosaccharides composition and amino acids composition could be classified as plain nature. The compositions of crude polysaccharides, volatile oils and triterpenoids of Poria cocos could be regarded as warm nature.
Declaration of Competing Interest
The authors declare no conflict of interests.
Acknowledgments
This research was funded by National Basic Research Program of China, grant number 2013CB531803.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.chmed.2020.04.002.
Appendix. Supplementary materials
References
- Chen X., Zhou Y., Fan Y., et al. Development of rat model of cold-deficiency syndrome. Chinese Journal of Experimental Animals. 2001;3:29–33. [Google Scholar]
- Cui G.Z., Li F., Song X.L., Wang X.Y. Effect of Pepper and Piper Longum on hepatic energy metabolism factors in rats. Modernization of Traditional Chinese Medicine and Materia Medica-World Science and Technology. 2013;15:1314–1316. [Google Scholar]
- Deng T. Guangdong science and technology press; Guangdong: 1990. Standard of TCM Syndrome. [Google Scholar]
- Einholm A.P., Nielsen H.N., Holm R., Toustrupjensen M.S., Vilsen B. Importance of a potential protein kinase A phosphorylation site of Na+-K+-ATPase and its interaction network for Na+ binding. Journal of Biological Chemistry. 2016;3:1–17. doi: 10.1074/jbc.M115.701201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B.B., Wang S.J., Zhang F.Y., et al. Influence of Aconiti Lateralis Radix Praeparata on asthenia cold syndrome rats with whole genome gene expression of liver by gene chip technique. China Journal of Chinese Materia Medica. 2012;37:500–504. [PubMed] [Google Scholar]
- Han X.Y., Wang Y.N., Dou D.Q. Regulatory effects of Poria on substance and energy metabolism in cold-deficiency syndrome compared with heat-deficiency syndrime in rats. Chinese Journal of Natural Medicimes. 2018;16:936–945. doi: 10.1016/S1875-5364(18)30135-3. [DOI] [PubMed] [Google Scholar]
- Kuang H.X., Cheng W. Studies on the capability of properties and flavors of Chinese medicine to be split and combined-A new assumption on the theory of properties and flavors of Chinese medicine and the research method. Modernization of Traditional Chinese Medicine and Materia Medica-World Science and Technology. 2009;11:768–771. [Google Scholar]
- Li Y., Yang Z., Xu J., Tang L.H. Effects of warm and heat Chinese herbs on liver mitochondria proteome of model rats with cold syndrome. China Journal of Chinese Materia Medica. 2014;45:373–378. [Google Scholar]
- Li B., Ding Y.X., Dou D.Q., Ran X.K., Xu Y.B., Li L.H., Kang T.G. Diuretic ingredients of Poria cocos. International Journal of Pharmacology. 2015;11:130–136. [Google Scholar]
- Li L., Liu G., Liang Y. Effects of cold and warm drugs on 5-hydroxytryptamine and norepinephrine neurons and fibers in rat brain, pituitary and adrenal glands. Chinese Journal of Traditional Chinese Medicine. 1999;24:40. [Google Scholar]
- Liu J. Research and Reflection on the nature and flavor of traditional Chinese medicine. Chengdu College Traditional Chinese Medicine. 1997;20:1–4. [Google Scholar]
- Liu Y.Y., Wang S.J., Han B.B. Effects of acetoacetate extract of Radix Aconite on hepatic contents of LA, LDH, PA, Gn, and ATPase activities in deficient cold model rats. Chinese Journal of Integrated Traditional and Western Medicine. 2011;31:1523–1526. [PubMed] [Google Scholar]
- Ma Z. A preliminary study on the cold, hot, warm and cool four qi of traditional Chinese medicine. Pharmaceutical Bulletin (Chinese Journal of Pharmacy) 1984;10:59. [Google Scholar]
- Shi W.J., Zhou H., Rong J.F. Effect of Bushen Decoction on Na+-K+-ATPase, Ca2+-Mg+-ATPase and succinate dehydrogenase in rats with heart failure. Chinese Journal of Rehabilitation Theory and Practice. 2012;18:544–547. [Google Scholar]
- Sui F., Yang N., Zhang C.B., Du X.I., LI L.F., Wang X.G., Guo S.Y., Huo H.R., Jiang T.L. Effects of ingredients from Chinese herbs with nature of cold or hot on expression of TRPV1 and TRPM8 J. China Journal of Chinese Materia Medica. 2010;35:1594–1597. doi: 10.4268/cjcmm20101220. [DOI] [PubMed] [Google Scholar]
- Tan X. Central University for Nationalities Press; Beijing: 2007. Basic and Clinical Medicine. [Google Scholar]
- Wang T., Sun H.G., Hua Y.L., Li P.L., Wei Y.M. Urine metabonomic study for blood-replenishing mechanism of Angelica sinensis in a blood-deficient mouse model. Chinese Journal of Natural Medicines. 2016;14:210–219. doi: 10.1016/S1875-5364(16)30018-8. [DOI] [PubMed] [Google Scholar]
- Weng L.L., Chen L., Su Y., Xu T.Y. Chemical constituents and pharmacological effects of Rhizoma Anemarrhenae. Jilin Traditional Chinese Medicine. 2018;38:90–92. [Google Scholar]
- Xiao H., Tan C., Yang G.L., Dou D.Q. The effect of red ginseng and ginseng leaves on the substance and energy metabolism in hypothyroidism rats. Journal of Ginseng Research. 2017;41:556–565. doi: 10.1016/j.jgr.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Yi J. The effect of cisapride on the release of some intestinal neurotransmitters. Journal of Clinical Digestive Disease. 1999;2:71–72. [Google Scholar]
- Yan P. Function of Mitochondrial respiratory chain module of cold-deficiency syndrome and deficiency-heat Witness group [D] Henan College of Traditional Chinese Medicine. 2014 [Google Scholar]
- Zhao Y.Y., Fan Z.X., et al. China Medical Science and Technology Publishing House; Beijing: 2015. Pharmacopoeia of the Peoples Republic of China. [Google Scholar]
- Zhang Z.Y., Chen B.J., Zhang Y.J., et al. Establishment and stability observation of rat models with kidney Yang deficiency and kidney Yin deficiency. Fujian Traditional Chinese Medicine. 2015;1:51–54. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.