Abstract
Objective
Chinese yam (Shanyao in Chinese, SY) as one of the representatives for Chinese medicines can be used as both of medicine and food with rich nutritional and medicinal value. Most of Chinese herbal medicines need to be processed prior to be used in clinical practice. SY was divided into Maoshanyao (Hairy Shanyao, MSY) and Guangshanyao (Smooth Shanyao, GSY) based on different processing methods at the place of origin, and it also could be processed as stir-fried SY and bran stir-fried SY to meet the different clinical use. Moreover, during the processing of Chinese herbal medicines, more complicated Maillard reaction occurs compared to food processing. Therefore, the objective of this research is to quantify the firepower of SY processing, and combined this with the relevant parameters of Maillard reaction.
Methods
The MSY and GSY produced in Shanxi and Henan Provinces were chosen as the research objects. By using thermal analysis technology, we first established the correlation between pyrolysis and processing of SY and its mixtures. We also quantified the firepower of Shaoyao processing, and combined this with the relevant parameters of Maillard reaction (pH value, amino acid, and 5-HMF) and the changes in medicinal ingredients (allantoin).
Results
The SY was mainly fried with moderate-fire (190 °C−200 °C), and the starting temperatures of different SY–ingredient mixtures were (176.3 ± 5.33) °C for (honey) bran, and (205.9 ± 8.05) °C for rice. The upper limits of processing temperature were (289.9 ± 6.47) °C for (honey) bran and (298.9 ± 1.15) °C for rice. The cooking time was (10.80 ± 1.76) min for soil stir-fry, (10.31 ± 1.06) min for bran stir-fry, and (8.43 ± 0.68) min for rice stir-fry. Moreover, the pH values and the content of 5-HMF were increased (P < 0.001), while the content of glycine was decreased significantly (P < 0.001) after processing.
Conclusion
The results verified and quantified the firepower of traditional processing of SY, and also provided scientific reference for other studies related to SY processing.
Keywords: Chinese yam, processing methods, thermal analysis, Maillard reaction
1. Introduction
Dioscoreae Rhizoma (Shanyao in Chinese, SY) is the dried rhizome of Dioscorea opposita Thunb. (Dioscorea L., Dioscoreaceae). The applications of SY have been recorded for thousands of years in oriental medicine, which was represented by traditional Chinese medicine. SY was first recorded in Shennong Bencao Jing (Sun & Sun, 1986) as a highly rated medicinal material. It was mainly produced in Henan, Shanxi, Hunan, and Hubei Provinces etc., and SY produced in several counties of Jiaozuo City in Henan known as Huai SY is very famous. SY with sweet taste has the effects of replenishing spleen, nourishing stomach, promoting fluid, benefiting lung, tonifying kidney, and arresting seminal emission. Clinically, it could treat the disorders like spleen deficiency, eating less, lung deficiency, coughing, spermatorrhea, frequent micturition, yin deficiency, and consumptive thirst (Tang et al., 2015). SY has been listed as one of the essential medicines for mild supplement, and was also legally listed as one of the medicinal foods. According to different processing methods at the place of origin, SY was divided into Maoshanyao (Hairy Shaoyao, MSY) and Guangshanyao (Smooth Shanyao, GSY). After excavating from soil, the rough skin of SY was removed, and then sun-dried or air-dried to become MSY. MSY was then macerated, pressed, and polished to become GSY.
For the processing of SY, there are a lot of methods recorded in classic literatures, such as cleansing, cutting, frying, fire processing, roasting, baking, steaming, and processing with wine/medicine/vinegar/ginger/honey/milk, etc. (Wang, 1989). In the Chinese Pharmacopoeia (2015 version), bran stir-fried SY is listed under the SY item. Currently, raw SY and processed SY are mainly used in clinical applications. SY contains a variety of nutrients and pharmacologically active ingredients (Liao, Zhu, Liu, Liu & Wu, 2003) such as starch, protein, amino acids, multivitamins, trace elements, fatty acids, choline, imidazole heterocyclic compounds, and steroids. Moreover, the SY skin was rich in flavonoids (Liu & Huan, 2015). Thus, SY has the effects of anti-aging, anti-oxidation, antidiabetic, reducing blood lipid, enhancing immune system, and regulating gastrointestinal system, which have caught more and more attentions.
The Maillard reaction was first proposed by the French chemist Louis Maillard in 1912. It referred to the reaction between an amino group contained compound and a carbonyl group contained compound to form melanin by condensation and polymerization, which was also called non-enzymatic browning reaction (Guo, 2012). During the processing of Chinese medicines that were both medicinal and edible, more complicated Maillard reaction occurs compared to food processing (Shao, Zhou & Liu, 2012). As the Chinese medicinal pieces exhibit darker colors (blackening) and fragrant smells, which were characteristics of Maillard reaction, new ingredients with pharmacological activity were also produced. Thus, the Maillard reaction that occurs during the processing of Chinese medicine was a research focus in recent years (Gong et al., 2019).
Thermal analysis (Ma, Meng, Guo, Lan & Zhang, 2017; Zhou, Liu, Shen & Qu, 2001) was a technique for measuring the physical properties of a substance in relation to temperature or time. In recent years, this technique has been gradually applied in studying Chinese medicine, mainly including the thermal analysis of chemical constituents of Chinese medicines (Yu, Liang, Zeng & Luo, 2011), thermal decomposition (Zhang, Shi, Wei, Yin & liu, 2011), examination of thermal stability (Ma, Meng & Zhang, 2013), identifying Chinese medicines from different places (Song, Zhang, M. & Liu, 2003), and identifying closely related medicines (Wang, Gao, Chen, Yu & Xiao, 2003) etc. By using thermal analysis technique, our group has also explored the relations between Chinese medicines processing and pyrolysis (Meng et al., 2017, 2017b; Meng, Guo & Zhang, 2012, Meng, Guo, Cui, Ma, & Fan, 2014).
So far, in terms of the SY processing methods at the place of origin, the product was classified into MSY and GSY. However, how these methods affect the subsequent processing and chemical composition of SY was still unclear. In terms of SY processing, the quantification was mainly based on the combination of orthogonal design (Zhou, Yang, Hua, Cong & Cai, 2014), response surface method (Wang, Chen, Yuan, Lv & Liu, 2015), and traditional experience, but lack of the quantitative study on the pyrolysis characteristics of SY and auxiliary materials. Also, during the frying process, the color of SY turns to yellow or dark yellow, and there was a smell of fragrance, which were characteristics of Maillard reaction. However, the correlation between the processing technology and Maillard reaction was still unknown.
In this study, we chose the MSY and GSY produced in Shanxi and Henan Provinces as the research objects. By using thermal analysis technology, we first established the correlation between pyrolysis and processing of SY and its mixtures. We also quantified the firepower of SY processing, and combined this with the relevant parameters of Maillard reaction (pH value, amino acid, and 5-HMF) (Hua, Kong, Yu, Chai & Cui, 2012; Morales & Romero, 1997; Sun, Kong, Han, Chen & Liu, 2013) and the changes in medicinal ingredients (allantoin) (Jiao & Wang, 2017; Shen & Wang, 2011; Zhang, Li, Yang, Zeng & Cai, 2010). These results verified and quantified the firepower of traditional processing of SY, and also provided scientific reference for other studies related to SY processing.
2. Materials and methods
2.1. Materials
The equipment used in this study included: STA-409 Multi-atmospheric Thermogravimetry-Differential Thermal Analyzer (NETZSCH, Germany), Ultra-3660 UV–Vis Spectrophotometer (Beijing Puyuan Jingdian Technology Co., Ltd.), Thermo Fisher Scientific U3000 High Performance Liquid Chromatograph, PHS-3C+ acidity meter (Chengdu Century Ark Technology Co., Ltd.), etc.
Other reagents used in the study included: 5-hydroxymethylfurfural (5-HMF) (mass fraction ≥ 98%, batch number: 14,099, Chengdu Pufeide Biotechnology Company); Glycine (mass fraction ≥ 98%, batch number: 1863, Shanghai Standard Technology Company); Ureidohydantoin (mass fraction ≥ 98%, batch number: 97-59-6, Shanghai Shidande Standard Technology Service Company). The SY samples and excipients used in this study were included in Table 1.
Table 1.
Information of samples and excipients of SY.
| Samples | Places of purchase | Manufacturers | Origins | Batch number | Specification |
|---|---|---|---|---|---|
| DO-1 | Hebei Anguo Medicinal Material Market | − | Shanxi | − | Guangshanyao |
| DO-2 | Beijing Tongrentang Pharmacy | Beijing Bencao Fangyuan Pharmaceutical Technology Company | Henan | 20,170,201 | Guangshanyao |
| DO-3 | Beijing Tongrentang Pharmacy | Anhui Shenghaitang Traditional Chinese Medicine Company | Henan | 2,017,071,521 | Maoshanyao |
| Rice and sticky rice | Hongxin Fresh Fruit and Vegetable Supermarket | − | Shanxi | 2017/10/28 | − |
| Honey | − | Shanghai Guanshengyuan Bee Products Company | − | 131300Y2 | − |
2.2. Preparations of SY water extract (WE) and alcohol extract (AW)
This process followed the water extract and alcohol extract extraction methods in four general extraction methods (2201) of Chinese Pharmacopoeia (2015 edition). (The four-part of Chinese Pharmacopoeia, 2015)
2.3. Extraction of total polysaccharide (TP) from SY
SY was pulverized, and degreased by refluxing with 95% ethanol. Distilled water was added at a ratio of 1:20, and the liquid was ultrasonically extracted twice, followed by centrifugation. The supernatant was combined and concentrated, precipitated with 95% ethanol at 4 °C overnight. Then, the supernatant was filtered and dried at 105 °C. (Yu, Zhang, Ma, Zhang & Meng, 2014)
2.4. Analysis of combustion and pyrolysis characteristics
A total of 30 (± 5) mg of each sample was put in the crucible. Then, the simulated air (N2:O2=4:1) was introduced at a constant temperature rate of 10 °C/min and a flow rate of 60 mL/min to rise the temperature from room temperature to 600 °C. The data were analyzed using Origin 8.0 software.
2.5. Preparations of wheat bran stir-fried SY and SY sample
These wheat bran stir-fried SY and SY sample were prepared to detect related indicators of Maillard reaction according to the processing methods obtained in the Chinese Pharmacopoeia (2015 edition) and the preparation was repeated. Statistical analysis differences were tested between these samples.
2.6. Determination of pH
A total of 2.5 g sample (DO-1, DO-2, and DO-3) was weighed and placed in a 25 mL flask. A total of 10 mL of distilled water was added, followed by sonication for 40 min. The supernatant was removed to a 50 mL centrifuge tube. After centrifuged at 3000 r/min, the supernatant was removed, and 5 mL of distilled water was added to the precipitate, followed by sonication for 40 min. After centrifugation, the supernatant was removed and 5 mL of distilled water was added to the precipitate, followed by sonication for 40 min. After centrifugation, the supernatant was removed and combined with the previous supernatants, placed in a 25 mL flask to volume 25 mL. After the pH was determined, the solution was stored at −20 °C.
2.7. Determination of glycine content
2.7.1. Preparation of glycine reference solution
The glycine reference substance was accurately weighed, dissolved by distilled water, and brought up to constant volume. Then a reference solution of 1.0 mg/mL was prepared for use.
2.7.2. Preparation of test solution
SY water extract (2 mL) was measured and added with 8 mL of 95% ethanol, mixed well and placed at 4 °C for 6 h. After centrifugation at 3000 r/min for 15 min, the supernatant was separated, and the precipitate was dissolved with 1 mL distilled water. After that, 4 mL of 95% ethanol was added to the liquid, mixed well, and placed at 4 °C for 6 h, followed by centrifugation. Then, the supernatant was separated and combined with the previous supernatant, dried at 45 °C, and dissolved in distilled water to the volume 5 mL.
2.7.3. Linear relationship investigation
The glycine reference substance was formulated into solutions with the concentrations of 0.003, 0.004, 0.005, 0.006, 0.007, 0.008, and 0.009 mg/mL, and then the absorbance was measured. Linear regression was performed on the absorbance and corresponding mass concentrations, and the equation was Y = 53.52857X + 0.1479 (R2 = 0.9995, n = 6). This indicates that glycine reference has a good linear relationship in the range of 0.003−0.009 mg/mL.
2.7.4. Methodological investigation
According to the requirements for methodology, the precision, repeatability, and sample recovery rate were examined (average recovery rate was 100.73%), and the RSD results were 0.21%, 1.16%, and 4.7%, respectively. In addition, the stability was examined during 0−12 h, and the RSD = 0.62%, indicating that the stability was good.
2.7.5. Content determination
Sample content determination: 0.5 mL of 2% ninhydrin solution and 0.5 mL of pH 6.80 phosphate buffer were added to 0.5 mL of sample solution, mixed well, heated in water bath at 100 °C for 15 min, removed and cooled down at room temperature. Then, the solution was diluted with distilled water to 30 mL. After 15 min, the absorbance was measured at 567 nm. Each sample was measured three times and the results were averaged.
Blank sample content determination: 0.5 mL of absolute ethanol and 0.5 mL of pH 6.80 phosphate buffer were added to 0.5 mL of sample solution, mixed, heated and cooled down at room temperature. Then, the solution was diluted with distilled water to 5 mL. After 15 min, the absorbance was measured at 567 nm. Each standard solution was measured three times.
Actual light absorption of samples A value = A (sample) − A (sample blank)
Once the actual sample absorbance was obtained, the glycine concentration in the sample could be calculated from the linear regression equation.
2.8. Determination of 5-HMF content
2.8.1. Preparation of reference solution
The 5-HMF reference substance was accurately weighed, dissolved in 10% methanol and brought up to constant volume. The concentration of the reference solution was 3 µg/mL.
2.8.2. Preparation of test solution
Nine samples were numbered and accurately weighed for about 0.5 g. The samples were sonicated in 10% methanol for 30 min. After cooled down, the test solutions were brought up to 10 mL with 10% methanol.
2.8.3. Chromatographic conditions
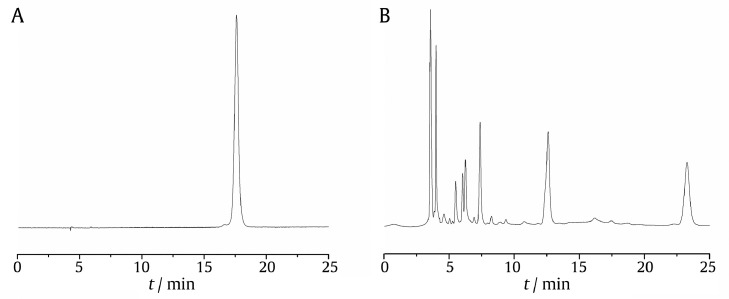
Column: Hypersil GOLD aQ-C18 column (4.6 mm × 250 mm, 5 µm); mobile phase: methanol-water (2:98); volume flow rate: 1.0 mL/min; detection wavelength: 284 nm; column temperature: 40 °C; injection volume: 10 µL. The chromatograms of the reference substance and test samples under this condition were shown in Fig. 1.
Fig. 1.
HPLC chromatogram of 5-HMF reference (A) and SY product (B).
2.8.4. Linear relationship investigation
The 5-HMF reference substance was prepared to solutions with the concentrations from 0.15 to 2.4 µg/mL. The peak areas of 5-HMF in each solution were then measured. Linear regression was performed on peak areas and concentrations, and the equation was Y = 23.214X-1.3932 (R2 = 0.9997, n = 6), indicating that 5-HMF had good linear relationship within 0.15−2.4 µg/mL.
2.8.5. Methodological investigation
According to the requirements for methodology, the precision, repeatability and sample recovery rate were examined (average recovery rate was 100.54%), and the RSD results were 2.6%, 2.0% and 1.06%, respectively. In addition, the stability was examined during 0 − 12 h, and RSD = 2.5%.
2.8.6. Content determination
The content of the test solution was measured under the above chromatographic conditions.
2.9. Determination of allantoin content
2.9.1. Preparation of reference solution
The allantoin reference substance was accurately weighed and dissolved with 20% ethanol solution to a final concentration of 0.2312 mg/mL.
2.9.2. Preparation of test solution
Sample (0.5 g) was measured and placed in a 25 mL volumetric flask. Ethanol (20%) was added till the marked line. The sample was mixed well and dissolved. After that, the flask was sonicated, 20 min each time for three times, with 30 min intervals. Then the solution was centrifuged at 3000 r/min for 10 min, and the supernatant was filtered. The filtrate was passed through a 0.45 µm microporous membrane and placed in a sample bottle, stored at −4 °C.
2.9.3. Chromatographic conditions
Column: Hypersil GOLD C18 column (4.6 mm × 250 mm, 5 µm); mobile phase: methanol-water (5:95); flow rate: 1.0 mL/min; detection wavelength: 210 nm; column temperature: 30 °C. The HPLC chromatograms of allantoin reference substance and the test samples were shown in Fig. 2.
Fig. 2.
HPLC chromatogram of allantoin reference (A) and SY sample (B).
2.9.4. Linear relationship investigation
The allantoin reference substance was formulated into solutions with content from 0.5 µg to 4.6 µg. The peak areas were measured, and linear regression was performed based on peak areas and concentrations. The equation was Y = 31.0007X + 1.9376. (R2 = 0.9995), indicating that allantoin had good linear relationship within the range 0.5−4.6 µg.
2.9.5. Methodological investigation
According to the requirements for methodology, the precision, repeatability and sample recovery rate were examined (average recovery rate was 100.93%), and the RSD results were 0.98%, 0.90% and 1.93%, respectively. In addition, the stability was examined during 0−12 h, and RSD = 2.16%, indicating good stability.
2.9.6. Content determination
The content of the test solution was measured under the above chromatographic conditions.
3. Results
3.1. Combustion and pyrolysis characteristics of auxiliary materials
As shown in Fig. 3 and Table 2, rice and sticky rice exhibited similar combustion and pyrolysis characteristics. During the main pyrolysis and combustion stage, the thermal loss rate curve DTG showed the maximum thermal weight loss rate peak of (25.42 ± 0.16)%/min at (306.5 ± 3.54) °C.
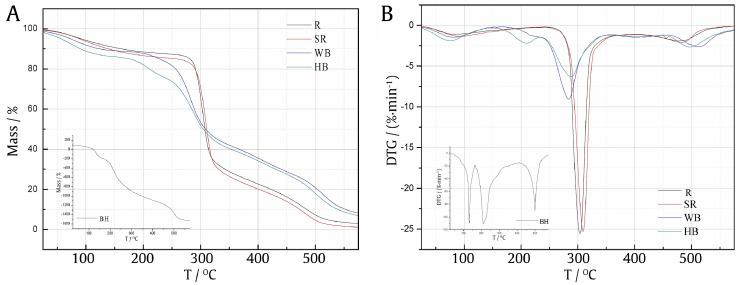
Fig. 3.
Pyrolysis and combustion TG curve (A) and DTG curve (B) for (sticky) rice and wheat bran (BH: honey, WB: wheat bran, HB: honey bran, SR: sticky rice, R: rice).
Table 2.
Pyrolysis characteristics of various samples of SY and related excipients.
| Samples | Thermal decomposition periods | Mass/% | DTGmax /(%/min) |
|---|---|---|---|
| Honey | Water loss (room temperature−165.1 °C) | 290.81 | 18.71 |
| Pyrolysis & Combustion (165.1 °C − 410.7 °C) | 913.39 | 14.90 | |
| Carbonization & Combustion (410.7 °C − 600 °C) | 426.27 | 89.65 | |
| Wheat Bran | Water loss (room temperature−168 °C) | 11.22 | 0.19 |
| Pyrolysis & Combustion (168 °C − 354 °C) | 47.20 | 1.19 | |
| Carbonization & Combustion (354 °C − 600 °C | 33.44 | 2.65 | |
| Honey wheat bran | Water loss (room temperature−148.4 °C) | 13.66 | 0.32 |
| Pyrolysis & Combustion (148.4 °C − 355.0 °C) | 46.38 | 1.14 | |
| Carbonization & Combustion (355.0 °C − 600 °C) | 32.94 | 2.67 | |
| Rice | Water loss (room temperature−156.1 °C) | 9.69 | 0.63 |
| Pyrolysis & Combustion (156.1 °C − 411.1 °C) | 68.91 | 1.15 | |
| Carbonization & Combustion (411.1 °C − 600 °C) | 18.36 | 1.92 | |
| Sticky rice | Water loss (room temperature−150.8 °C) | 10.85 | 0.79 |
| Pyrolysis & Combustion(150.8 °C − 410.2 °C) | 70.14 | 1.11 | |
| Carbonization & Combustion (410.2 °C − 600 °C) | 17.85 | 2.10 | |
| WE-DO-1 | Water loss (room temperature−198.46 °C) | 7.07 | 0.75 |
| Pyrolysis & Combustion (198.46 °C − 420.58 °C) | 69.41 | 12.79 | |
| Carbonization & Combustion (420.58 °C − 600 °C) | 20.12 | 2.02 | |
| WE-DO-2 | Water loss (room temperature−217.78 °C) | 8.11 | 0.74 |
| Pyrolysis & Combustion (217.78 °C − 410.7 °C) | 70.3 | 14.62 | |
| Carbonization & Combustion (410.7 °C − 600 °C) | 18.39 | 1.60 | |
| WE-DO-3 | Water loss (room temperature−218.19 °C) | 12.41 | 1.61 |
| Pyrolysis & Combustion (1218.19 °C − 408.4 °C) | 54.23 | 10.27 | |
| Carbonization & Combustion (408.4 °C − 600 °C) | 25.07 | 4.61 | |
| AE-DO-1 | Water loss (room temperature−183.37 °C) | 20.75 | 4.99 |
| Pyrolysis & Combustion (183.37 °C − 478.15 °C) | 58.82 | 5.87 | |
| Carbonization & Combustion (478.15 °C − 600 °C) | 12.14 | 5.20 | |
| AE-DO-2 | Water loss (room temperature−160.23 °C) | 14.04 | 4.14 |
| Pyrolysis & Combustion (160.23 °C − 438.7 °C) | 53.81 | 2.93 | |
| Carbonization & Combustion (438.7 °C − 600 °C) | 17.54 | 2.84 | |
| AE-DO-3 | Water loss (room temperature−158.01 °C) | 13.27 | 4.42 |
| Pyrolysis & Combustion (158.01 °C − 455.52 °C) | 55.73 | 3.10 | |
| Carbonization & Combustion (455.52 °C − 600 °C) | 22.66 | 2.43 | |
| TP-DO-3 | Water loss (room temperature−234.35 °C) | 9.94 | 1.41 |
| Pyrolysis & Combustion (234.35 °C − 444.67 °C) | 50.81 | 7.56 | |
| Carbonization & Combustion (444.67 °C − 600 °C) | 7.7 | 1.85 | |
| TP-DO-2 | Water loss (room temperature−236.12 °C) | 9.54 | 1.56 |
| Pyrolysis & Combustion (236.12 °C − 436.77 °C) | 26.36 | 3.53 | |
| Carbonization & Combustion (436.77 °C − 600 °C) | 1.74 | 0.53 | |
| TP-DO-3 | Water loss (room temperature−231.42 °C) | 13.89 | 1.26 |
| Pyrolysis & Combustion (231.42 °C − 419.87 °C) | 60.72 | 8.85 | |
| Carbonization & Combustion (419.87 °C − 600 °C) | 3.1 | 0.78 | |
| DO-1 | Water loss (room temperature−170.8 °C) | 9.99 | 1.09 |
| Pyrolysis & Combustion (170.8 °C − 356.4 °C) | 61.57 | 25.65 | |
| Carbonization & Combustion (356.4 °C − 600 °C) | 27.04 | 3.33 | |
| DO-2 | Water loss (room temperature−151.2 °C) | 9.11 | 1.35 |
| Pyrolysis & Combustion (151.2 °C − 355.4 °C) | 62.42 | 23.77 | |
| Carbonization & Combustion (355.4 °C − 600 °C) | 25.40 | 4.53 | |
| DO-3 | Water loss (room temperature−187.3 °C) | 9.34 | 1.47 |
| Pyrolysis & Combustion (187.3 °C − 339.0 °C) | 52.47 | 19.10 | |
| Carbonization & Combustion (339.0 °C − 600 °C) | 33.16 | 8.70 | |
| R-DO-1 | Water loss (Room Temperature −210.3 °C) | 11.50 | 1.46 |
| Pyrolysis & Combustion (210.3 °C − 365.4 °C) | 59.03 | 21.31 | |
| Carbonization & Combustion (365.4 °C − 600 °C) | 26.91 | 2.86 | |
| R-DO-2 | Water loss (room temperature −211.3 °C) | 12.83 | 1.28 |
| Pyrolysis & Combustion (211.3 °C − 368.2 °C) | 62.09 | 27.24 | |
| Carbonization & Combustion (368.2 °C − 600 °C) | 23.68 | 5.26 | |
| R-DO-3 | Water loss (Room Temperature −196.1 °C) | 11.61 | 1.11 |
| Pyrolysis & Combustion (196.1 °C − 363.9 °C) | 59.58 | 16.47 | |
| Carbonization & Combustion (363.9 °C − 600 °C) | 27.14 | 12.58 | |
| SR-DO-1 | Water loss (room temperature−209.4 °C) | 12.66 | 1.48 |
| Pyrolysis & Combustion (209.4 °C − 367.3 °C) | 59.55 | 20.58 | |
| Carbonization & Combustion (367.3 °C − 600 °C) | 27.08 | 4.10 | |
| SR-DO-2 | Water loss (room temperature−210.2 °C) | 12.27 | 1.35 |
| Pyrolysis & Combustion (210.2 °C − 389.8 °C) | 64.66 | 20.78 | |
| Carbonization & Combustion (389.8 °C − 600 °C) | 21.49 | 1.51 | |
| SR-DO-3 | Water loss (room temperature−192.0 °C) | 11.28 | 1.24 |
| Pyrolysis & Combustion (192.0 °C − 362.7 °C) | 56.33 | 11.99 | |
| Carbonization & Combustion (362.7 °C − 600 °C) | 29.33 | 9.04 | |
| WB-DO-3 | Water loss (room temperature−170.7 °C) | 11.40 | 0.12 |
| Pyrolysis & Combustion (170.7 °C − 354.4 °C) | 53.54 | 1.65 | |
| Carbonization & Combustion (354.4 °C − 600 °C) | 31.78 | 2.97 | |
| WB-DO-2 | Water loss (room temperature−177.0 °C) | 11.430 | 0.13 |
| Pyrolysis & Combustion(177.0 °C − 352.5 °C) | 53.09 | 1.69 | |
| Carbonization & Combustion (352.5 °C − 600 °C) | 30.01 | 4.07 | |
| WB-DO-3 | Water loss (room temperature−147.3 °C) | 10.23 | 0.38 |
| Pyrolysis & Combustion(147.3 °C − 352.4 °C) | 53.4 | 0.92 | |
| Carbonization & Combustion (352.4 °C − 600 °C) | 33.4 | 9.64 | |
| HB-DO-3 | Water loss (room temperature−166.4 °C) | 11.04 | 0.21 |
| Pyrolysis & Combustion (170.7 °C − 356.4 °C) | 56.46 | 21.19 | |
| Carbonization & Combustion (356.4 °C − 600 °C) | 31.03 | 1.67 | |
| HB-DO-2 | Water loss (room temperature−150.2 °C) | 10.75 | 1.50 |
| Pyrolysis & Combustion (150.2 °C − 356.4 °C) | 56.46 | 17.69 | |
| Carbonization & Combustion (356.4 °C − 600 °C) | 29.06 | 2.58 | |
| HB-DO-3 | Water loss (room temperature−150.3 °C) | 8.89 | 0.38 |
| Pyrolysis & Combustion (150.3 °C − 353.3 °C) | 53.36 | 16.58 | |
| Carbonization & Combustion (353.3 °C − 600 °C) | 32.74 | 7.78 |
The combustion and pyrolysis characteristic curves of wheat bran and honey bran were shown in Fig. 3. 168 °C−354 °C was the main pyrolysis and combustion stage. The thermal weight loss during this stage was 47.20%, and the corresponding thermal loss rate peak appeared at 283 °C, with a value of 9.06%/min. Honey wheat bran also showed similar pyrolysis characteristics with wheat bran. However, the former showed a thermal weight loss rate peak of 2.20%/min at 210.2 °C. Compared with the combustion and pyrolysis characteristics of honey (maximum thermal weight loss rate peak of 111.1%/min appeared at 211 °C), this thermal weight loss rate peak could be related to the addition of honey.
3.2. Combustion and pyrolysis characteristics of SY extract
The combustion and pyrolysis characteristic curve (TG-DTG) of SY water extract (WE) was shown in Fig. 4A and B and Table 2. The characteristics of each SY sample were similar: two thermal weight loss rate peaks, (12.79 ± 2.22)%/min and (4.77 ± 0.58)%/min, appeared at (294.1 ± 12.27) °C and (410.7 ± 7.87) °C, respectively.
Fig. 4.
Pyrolysis and combustion TG/DTG curves for water extract, alcohol extract and total polysaccharide of SY.
Note: A: TG curves of water extract; B: DTG curves of water extract (WE-DO-1: water extract of Shanxi GSY, WE-DO-2: water extract of Henan GSY, and WE-DO-3: water extract of Henan MSY); C: TG curves of alcohol extract; D: DTG curves of alcohol extract (AE-DO-1: alcohol extract of Shanxi GSY, AE-DO-2: alcohol extract of Henan GSY, and AE-DO-3: alcohol extract of Henan MSY); E: TG curves of polysaccharide extract; F: DTG curves of polysaccharide extract (TP-DO-1: polysaccharide extract of Shanxi GSY, TP-DO-2: polysaccharide extract of Henan GSY, TP-DO-3: polysaccharide extract of Henan MSY).
The combustion and pyrolysis characteristic curve (TG-DTG) of SY alcohol extract (AE) and total polysaccharide (TP) were shown in Fig. 4C−4F. The characteristics of alcohol extract and polysaccharide extract were variable. During the main combustion and pyrolysis stage, the alcohol extract showed the thermal weight loss rate peak of (4.59 ± 0.35)%/min around (153.24 ± 4.67) °C; Additionally, DO-1 and DO-3 showed thermal weight loss peak of (2.93 ± 0.30)%/min at (329.4 ± 20.22) °C, and DO-1 showed thermal weight loss peak of 3.46%/min at 203.2 °C. During the same stage, the SY polysaccharide extract showed the maximum thermal weight loss rate peak of (6.67 ± 2.80)%/min at (286.3 ± 13.47) °C. But for each polysaccharide extract sample, the thermal weight loss rate peak and the corresponding temperature varied a lot. For example, Shanxi GSY, Henan GSY, and Henan MSY showed thermal weight loss rate peaks of 7.59, 3.53 and 8.89%/min at 301.4, 281.8 and 275.6 °C, respectively.
3.3. Analysis of combustion and pyrolysis characteristics of SY
The combustion and pyrolysis characteristic curve TG-DTG for SY was shown in Fig. 5 and Table 2. The combustion and pyrolysis characteristics of the two types of GSY (DO-1 and DO-2) were similar, exhibiting maximum thermal weight loss rate peaks of (24.71 ± 1.33)%/min and (3.89 ± 0.81)%/min near (294.6 ± 1.20) °C and (397.94 ± 2.96) °C, respectively. However, for MSY (DO-3), the maximum thermal weight loss rate peak of 19.10%/min appeared at 280.6 °C, which were slightly lower than GSY in terms of intensity and temperature. Moreover, during the combustion stage, MSY showed the maximum thermal weight loss rate peak of 8.69%/min at 382.86 °C, which was higher than GSY in terms of intensity.
Fig. 5.
Pyrolysis and combustion TG curve (A) and DTG curve (B) for SY (DO-1: Shanxi GSY, DO-2: Henan GSY, and DO-3: Henan MSY).
3.4. Analysis of combustion and pyrolysis characteristics of SY with different auxiliary materials
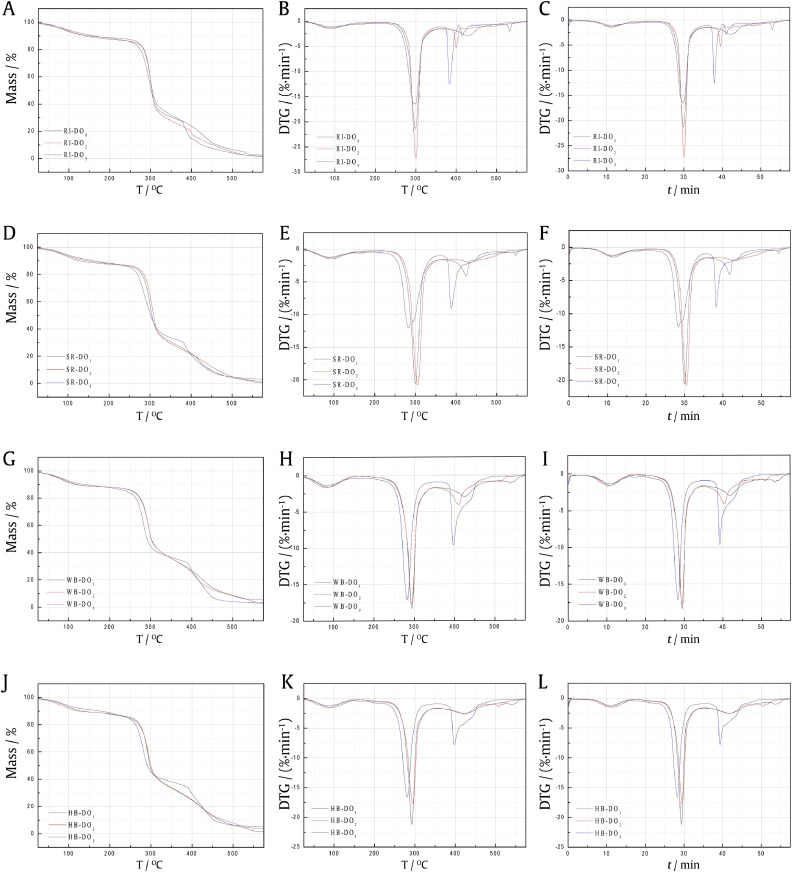
The combustion and pyrolysis characteristics TG-DTG-TIME curves of the (sticky) rice-SY mixture were shown in Fig. 6A–F and Table 2. The main pyrolysis and combustion phase started at (205.93 ± 8.05) °C, and the maximum thermal weight loss rate peak of (21.67 ± 5.39)%/min appeared at (298.9 ± 1.15) °C and (8.43 ± 0.68) min.
Fig. 6.
Pyrolysis and combustion TG/DTG/TIME curves for SY mixtures (R: rice, SR: sticky rice, WB: wheat bran, HB> honey bran).
A: TG curves of rice-SY mixtures; B: DTG curves of rice-SY mixtures; C: DTG-TIME curves of rice-SY mixtures; R-DO-1 to R-DO-3 were mixtures of rice and SY; D: TG curves of sticky rice-SY mixtures; E: DTG curves of sticky rice-SY mixtures; F: DTG-TIME curves of sticky rice-SY mixtures; SR-DO-1 to SR-DO-3 were mixtures of sticky rice and SY; G: TG curves of bran-SY mixtures; H: DTG curves of bran-SY mixtures; I: DTG-TIME curves of bran-SY mixtures; WB-DO-1 to WB-DO-3 were mixtures of wheat bran and SY; J: TG curves of honey wheat bran-SY mixtures; K: DTG curves of honey wheat bran-SY mixtures; L: DTG-TIME curves of honey wheat bran-SY mixture; HB-DO-1 to HB-DO-3 were mixtures of honey bran and SY.
The combustion and pyrolysis characteristic TG-DTG-TIME curves of the wheat bran-SY mixture and the honey wheat bran-SY mixture were shown in Fig. 6G–L and Table 2. The main combustion and pyrolysis stage of two mixtures started at (176.33 ± 5.33) °C, and the maximum thermal weight loss rate peaks of (14.38 ± 5.25)%/min and (18.49 ± 2.41)%/min appeared at (289.9 ± 6.47) °C and (10.31 ± 1.06) min, respectively.
The SY was mainly fried with moderate-fire (190°C−200 °C), and the starting temperatures of different SY–ingredient mixtures were (176.3 ± 5.33) °C for (honey) bran, and (205.9 ± 8.05) °C for rice. The upper limits of processing temperature were (289.9 ± 6.47) °C for (honey) bran and (298.9 ± 1.15) °C for rice. The cooking time was (10.80 ± 1.76) min for soil stir-fry, (10.31 ± 1.06) min for bran stir-fry, and (8.43 ± 0.68) min for rice stir-fry. Therefore, based on the results 2.1–2.3, the main chemical components were retained in this temperature and time range, satisfying the "preservation" requirement of Chinese medicine processing.
In addition, compared with the maximum thermal weight loss rate peak (22.84 ± 3.37)%/min of SY at (28.99 ± 8.14) °C, the order of the maximum thermal weight loss rate peak of each SY mixture and SY was: SY > (sticky) rice-SY mixture > honey wheat bran-SY mixture > wheat bran-SY mixture. Thus, the heat transfer abilities of solid ingredients were different during processing.
3.5. pH measurement
The pH of each sample was measured according to Method 2.6, and the results were shown in Fig. 7A. The pH values increased slightly after processing, but there was no significant difference between different SY samples.
Fig. 7.
Indexes (A: pH values; B: glycine content; C 5-HMF content; D allantoin content) of Maillard reaction and content of active ingredients of SY (mean ± SD; n = 4).
RDO-1 to RDO-3 represent three raw SY samples, and PDO-1 to PDO-3 represent three processed SY samples; *P < 0.05, ⁎⁎P < 0.01 and ⁎⁎⁎P < 0.001 vs. raw samples.
3.6. Glycine content determination
The glycine content was measured according to Method "2.7″, and the results were shown in Fig. 7B. Compared to the content of glycine in each raw SY, the glycine contents of processed products were significantly decreased (P < 0.001).
3.7. 5-HMF content determination
The content of 5-HMF was measured according to Method "2.8″, and the results were shown in Fig. 7C. The content of 5-HMF was very low or with trace amount in raw SY samples, but were significantly increased after processing (P < 0.001). The order of increased amount was DO-1>DO-2>DO-3.
3.8. Allantoin content determination
The content of allantoin in SY was measured according to Method "2.9″, and the results were shown in Fig. 7D. Except for sample DO-3 that showed significant increase of allantoin after processing (P < 0.001), there was no significant difference in allantoin content after processing for DO-1 and DO-2 samples.
4. Discussion
The processing of Chinese medicinal materials at production place and at preparation place were two closely related links in Chinese medicine industry. According to the different processing methods at the place of origin, SY could be divided into MSY and GSY. The main processing methods of SY during preparation include stir frying with or without solid auxiliary ingredients. SY has a unique effect and chemical composition (Feng et al., 2017; Yi et al., 2014). Based on different solid auxiliary ingredients, the processing could be further divided into (honey) bran stir-fry, (sticky) rice stir-fry, etc. According to the traditional theory, the effects of bran stir-fried and rice stir-fry SY were benefiting spleen and invigorating stomach. While transferring the heat during processing, the solid ingredients could also reduce the toxicity, alleviate the medicinal properties, enhance the curative effect, and improve the taste.
Our research team has used thermal analysis technology to study the processing crafts mechanism of traditional Chinese medicines. This technology can offer temperature quantification information by combustion and pyrolysis characteristics of herbs, which can complement the shortcomings of sensory evaluation for herbal medicines processing. According to the combustion and pyrolysis characteristics of SY and its mixtures, SY water extract, SY alcohol extract, and total polysaccharide, the starting temperatures of different SY processing methods were different. For example, (honey) bran stir-fry was at (176.3 ± 5.33) °C, and rice stir-fry was at (205.9 ± 8.05) °C. The upper limit of processing temperature was (289.9 ± 6.47) °C for (honey) bran stir-fry, and (298.9 ± 1.15) °C for rice stir-fry; The processing time was (10.31 ± 1.06) min for bran stir-fry, and (8.43 ± 0.68) min for rice stir-fry. These results validated, supplemented, and quantified the studies on firepower and heat of the traditional SY processing technology.
In addition, compared with the maximum thermal weight loss rate peak (22.84 ± 3.37)%/min of SY at (289.9 ± 8.14) °C, the order of the maximum thermal weight loss rates of SY mixtures and SY was: SY > (sticky) rice-SY mixture > honey wheat bran-SY mixture > wheat bran-SY mixture, indicating the differences in heat transfer ability between different solid ingredients during processing.
The Chinese Pharmacopoeia (2015 edition) includes two types of processed SY products: SY and bran stir-fried SY. Therefore, our study mainly focused on the quantification of bran-fried SY and its relation to Maillard reaction (Tang et al., 2013).
pH could affect Maillard reaction in many ways. Since the measured pH were all less than 7, the 1-amino-1-deoxy-2-ketosaccharide could react through the first route, which was the 1,2-enolization (enolization refers to a reaction in which a carbonyl compound that has an active hydrogen on enolization a-carbon atom was converted into an enol compound).
The nucleophilic addition of carbonyl-ammonia in this reaction was difficult, so the type and yield of nitrogen-containing substances were low, but the isomerization and dehydration of sugar were easier to occur, and further produce the furfural substances. This process could be viewed as the removal of three water molecules from the sugar to form a furfural derivative, in which the amino group-containing compound was first added and then removed, acting as a catalyst. Based on the results, the content of amino acids in SY was negatively correlated with the content of 5-HMF. When amino acids decreased, the 5-HMF increased.
In addition, the results showed that MSY and GSY had different components after Maillard reaction happened. The content of each parameter and the medicinal component in MSY was all higher than GSY, which was possibly due to the simpler processing method of MSY at production place that better retains the content of each component. Moreover, the SY from Henan and Shanxi also had different components after Maillard reaction, indicating that production place affects the SY quality.
In this study, we investigated different auxiliary materials and processing methods. By using thermal analysis technology, we quantified the firepower and heat, and analyzed the degree of Maillard reaction. These results solved some problems in the modernization of SY processing. However, currently, there was still very few objective studies on SY and its processing mechanisms. The role of Maillard reaction in Chinese medicine processing was still unclear. Therefore, it was necessary to use new technologies to bring SY to the field of modern medicine, and provide a scientific basis for its clinical treatment.
5. Conclusion
This study investigated the combustion and pyrolysis characteristics of SY and MSY, as well as their processing accessories. We quantified the SY processing methods and studied the combustion and pyrolysis characteristics of SY mixtures. The firepower and heat of bran-fried SY processing were determined, and the Maillard reaction happened during this process was discussed.
Declaration of Competing Interest
The authors declare no conflict of interests.
Acknowledgments
This work was supported by a grant from The Key Technology Research for TCM Modernization of Shanxi Province (Zhendong Special Project, 2014ZD0302) and The Key Research & Development plan of Shanxi Province (Social Development Project, 201603D3112002).
References
- Feng J.F., Tang Y.N., Ji H., Zhu L., Yi T., Xiao Z.G. Biotransformation of Dioscorea nipponica by Rat intestinal microflora and cardioprotective effects of diosgenin. Oxidative Medicine and Cellular Longevity. 2017;2017 doi: 10.1155/2017/4176518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong R.Z., Huo X.H., Zhang L., Liu C., Li S.S., Sun Y.S. Advances in research on the effects of Maillard reaction on the quality of traditional Chinese medicine. Chinese Herbal Medicine. 2019;50(01):243–251. [Google Scholar]
- Guo Y.X. Shandong University; 2012. Relationship between Maillard reaction and processing mechanism of Rehmannia glutinosa. 2012. [Google Scholar]
- Hua H.L., Kong C.P., Yu S., Chai C., Cui X.B. Determination of 5-hydroxymethyl furfural and furfural in different processed products of Dioscorea yam by HPLC. Nei Mongol Journal of Traditional Chinese Medicine. 2012;2(22):41. [Google Scholar]
- Jiao R.R., Wang C.Y. Determination of allantoin in Dioscorea yam and Dioscorea radix by HPLC. Journal of Anhui Health Vocational & Technical College. 2017;16(2):105–109. [Google Scholar]
- Liao C.H., Zhu B.F., Liu A.L., Liu Z., Wu C.G. Determination of main biochemical components of Dioscorea yam. Journal of Shaoguan University (Natural Science) 2003;24(6):67–69. [Google Scholar]
- Liu Q., Huan H.Y. Study on active ingredients extraction and antioxygenation of flavonoids in yam peels. Guangzhou Chemical Industry. 2015;43(24):98–100. [Google Scholar]
- Ma J., Meng X., Guo X., Lan Y., Zhang S. Thermal analysis during partial carbonizing process of rhubarb, moutan and burnet. PloS One. 2017;12(3) doi: 10.1371/journal.pone.0173946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.N., Meng X.L., Zhang S.S. Characteristic study on pyrolysis and combustion kinetics of Gallate. World Journal of Integrated Traditional and Western Medicine. 2013;8(05):468–471. [Google Scholar]
- Meng X.L., Guo X.H., Cui N.N., Ma J.J., Fan Z. Pyrolysis characteristics of Rhei Radix et Rhizoma, Moudan Radicis Cortex, and Sanguisorbae Radix and correlations with the carbonizing process of Chinese herbs. Chinese Journal of Natural Medicines. 2014;12(1):55–64. doi: 10.1016/S1875-5364(14)60010-8. [DOI] [PubMed] [Google Scholar]
- Meng X.L., Guo X.H., Zhang S.S. Study on processing mechanism of Glycyrrhiza uralensis based on TG-DTG. China Journal of Chinese Materia Medica. 2012;37(23):3558. [PubMed] [Google Scholar]
- Meng X.L., He M.J., Guo R., Duan R., Huo F.X., Lv C.Z., et al. Investigation of the effect of the degree of processing of Radix Rehmanniae Preparata (Shu Dihuang) on Shu Dihuangtan carbonization preparation technology. Molecules (Basel, Switzerland) 2017;22(7):1193. doi: 10.3390/molecules22071193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X.L., Ma J.N., Guo X.H. Modern research on processing of calamine: Processed by Huanglian Tang and Sanhuang Tang. Chinese Journal of Integrative Medicine. 2017;23(11):850–857. doi: 10.1007/s11655-017-2798-9. [DOI] [PubMed] [Google Scholar]
- Morales F.J., Romero C.S. Chromatographic determination of bound hydroxymethylfurfural as an index of milk protein glycosylation. Journal of Agricultural and Food Chemistry. 1997;45:1570–1573. [Google Scholar]
- Shao L.Y., Zhou J.W., Liu D.H. Advances in research on Maillard reaction mechanism and kinetic model in food. Chinese Journal of Food. 2012;12(12):103–112. [Google Scholar]
- Shen Y.Q., Wang M.J. Comparison of allantoin content between raw yam and bran. Chinese Medicine Modern Distance Education of China. 2011;9(15):75. [Google Scholar]
- Song A.X., Zhang J.W, Li M.J., Liu X.H. Characteristic study on pyrolysis and combustion kinetics of Gallate. Chinese Traditional and Herbal Drugs. 2003;34:169. [Google Scholar]
- Sun F.D., Kong B.H., Han Q., Chen Q., Liu Q. Influence of different initial pH and heating time on characteristic of the porcine bone protein hydrolysate Maillard products. Science and Technology of Food Industry. 2013;(22):106–115. [Google Scholar]
- Sun X.Y., Sun F.J. Vol. 1986. People’s Medical Publishing House; Beijing: 1986. Wei dynasty·PU WU; p. 26. (Shennong herbal classic [M]). [Google Scholar]
- Tang Y.N., He X.C., Ye M., Huang H., Chen H.L., Peng W.L., et al. Cardioprotective effect of total saponins from three medicinal species of Dioscorea against isoprenaline-induced myocardial ischemia. Journal of Ethnopharmacology. 2015;175:451–455. doi: 10.1016/j.jep.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Tang Y.N., Yi T., Chen H.M., Zhao Z.Z., Liang Z.T., Chen H.B. Quantitative comparison of multiple components in Dioscorea nipponica and D. Panthaica by ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Phytochemical Analysis. 2013;24:413–422. doi: 10.1002/pca.2428. [DOI] [PubMed] [Google Scholar]
- Wang J., Chen Y., Yuan Z.M., Lv J., Liu H. Optimization of processing technology of bile processed Rhizom Coptidis using response surface methodology. Chinese Archives of Traditional Chinese Medicine. 2015;33(6):1298–1300. [Google Scholar]
- Wang S.J., Gao W.Y., Chen H.X., Yu L., Xiao P.G. Application of TG, DTA in identification of Fritillaria chinensis. China Journal of Chinese Materia Medica. 2003;34(2):169. [Google Scholar]
- Wang X.T. Jiangxi Science and Technology Press; Nanchang: 1989. Ancient Chinese medicine processing: ancient part; pp. 12–13. [Google Scholar]
- Yi T., Fan L.L., Chen H.L., Zhu G.Y., Suen H.M., Tang Y.N., et al. Comparative analysis of diosgenin in Dioscorea species and related medicinal plants by UPLC-DAD-MS. BMC Biochemistry. 2014;15:19. doi: 10.1186/1471-2091-15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L.P., Liang X.L., Zeng S.C., Luo J.B. Ephedra scum components by thermal TG-DTG-DSC analysis and pyrolytic GC–MS analysis. Acta Scientiarum Naturalium Universitatis Sunyatseni. 2011;50:94. [Google Scholar]
- Yu L., Zhang J.T., Ma S.X., Zhang T., Meng D.X. Optimization of polysaccharide extraction from yam and its antibiotic activity. Chinese Traditional Patent Medicine. 2014;36(6):1194–1198. [Google Scholar]
- Zhang F.F., Li W.D., Yang G.M., Zeng Q.Q., Cai B.C. Determination of allantoin in Rhizoma Dioscoreae preparata by HPLC. Journal of Nanjing University of Traditional Chinese Medicine. 2010;26(2):146–149. [Google Scholar]
- Zhang J.H., Shi X.J., Wei Z., Yin l., liu Y.P. Component analysis and thermal stability of calamine before and after processing. Chinese Journal of Experimental Traditional Medical Formulae. 2011;17(24):16. [Google Scholar]
- Zhou C.P., Liu Y., Shen X.S., Qu S.S. Application of thermal analysis technology in drug research. Journal of Analytical Science. 2001;17:430–437. [Google Scholar]
- Zhou H.Y., Yang P.P., Hua Y.H., Cong X.D., Cai B.C. Study on preparation processing technique of Rhizoma Dioscoreae alatae by orthogonal experiment. Chinese Archives of Traditional Chinese Medicine. 2014;32(7):1597–1599. [Google Scholar]