Abstract
Kadsura coccinea belongs to medicinally important genus Kadsura from the Schisandraceae family. It has been used in traditional Chinese medicine (TCM) for the treatment of rheumatoid arthritis and gastroenteric disorders. The initial phytochemical work focused on the identification of some structurally novel and diverse natural products, which turned the attention of many researchers towards this plant. Thus far, 202 compounds have been reported in this plant. Lignans and terpenoids were found as the main chemical constituents of this plant. Some of the triterpenoids and sesquiterpenoids with novel structures are of particular interest for natural product researchers. The isolated compounds of this plant have shown different bioactivities including anti-tumor, anti-HIV, anti-inflammatory, nitric oxide (NO) production inhibitory and other pharmacological effects. This review systematically summarizes all the phytochemical and pharmacological work done so far on K. coccinea, and can be used as a reference for future research on this plant.
Keywords: Kadsura coccinea (Lem.) A.C. Smith, Heilaohu, lignans, pharmacology, rheumatoid arthritis, triterpenoids
1. Introduction
The national medicine system in China consists of many smaller sub-units. One important unit of this type is the Tujia ethnomedicine system (Chen and Zhang, 2007). The Tujia ethnomedicine system has its own unique theories and methods for the treatment and prevention of diseases, which is based on the accumulated knowledge and experience of the ethnic Tujia peoples living in southern provinces of China. This system has relied on herbal therapy as a healing tool for hundreds of years. Along the way, it has identified and classified the essential properties of many herbs and just how they act on the body-mind-spirit (Wang, 2013).
Kadsura coccinea (Lem.) A.C. Smith is an evergreen climbing shrub from the medicinally important plant family Schisandraceae. It is widely distributed in the south-western provinces of China. In China, this plant is named as “Heilaohu”, with the strange shape fruit, like a football, and famous as the world's first strange fruit. At the same time, its function effects are very powerful. The roots of K. coccinea have also been used for the treatment of gastroenteric disorders, duodenal ulcers and gynecological problems in traditional Chinese medicine (TCM) (Pharmacopoeia of the People’s Republic of China, 1977). Particularly, the Tujia peoples used “Heilaohu” to treat rheumatoid arthritis (RA) (Liu et al., 2018a, 2018b). The roots of K. coccinea have been developed into different TCM products, such as “Heilaohu liquid” and “heilaohu plaster” that are good for the treatment of RA.
K. coccinea is a rich source of lignans and triterpenoids (Shi et al., 2008, Remy et al., 2016). The presence of interesting and structurally diverse compounds and their pharmacological importance have prompted many natural products researchers in recent years to turn their attention towards this plant. So far 202 different compounds have been isolated from this plant. The chemical constituents of this plant have been reported with several different bioactivities, including anti-HIV, anti-tumor, cytotoxic, anti-inflammatory, anti-hepatitis, nitric oxide inhibitory, anti-platelet aggregation, and neuroprotective effects (Liu et al., 2014). Currently, the researchers mainly focus on the chemical composition of K. coccinea, and the mechanism of pharmacological action is seldom studied. Therefore, further and more comprehensive studies are needed to find out the relationship between traditional uses and modern pharmacological activities. This review summarizes all the phytochemical and pharmacological works done so far on different parts of K. coccinea. It provides information, which can be used as a reference for future phytochemical and pharmacological research on K. coccinea.
Cosbaea coccinea Lem, Kadsura chinensis Hance ex Benth, Schizandra hanceana Baill, and Kadsura cavaleriei Levl were some of the names previously used for this plant. A. C. Smith in 1947 assigned the name Kadsura coccinea, which is now widely accepted (Smith, 1947). It is an evergreen climbing plant that is hairless. The shape of leaves is oblong to ovate-lanceolate leathery (Fig. 1). Its flowering season is from April to July. The fruiting season is from July to November (Richar, 1998). The aggregated fruit is nearly spherical, which color is red or dark purple. As the favorite wild fruit, it tastes unique and has high nutritional value. It is rich in vitamin C, vitamin E, amino acids and various trace elements. This plant is widely distributed in Jiangxi, Hunan, Guangdong, Hainan, Sichuan, Guizhou and Yunnan Provinces, and Guangxi Zhuang Autonomous Region in China.
Fig. 1.
Photos of K. coccinea. (A: fruits, B: whole plant, C: flowers, D: dried stems).
2. Chemical constituents
2.1. Lignans
Lignans and its derivatives are among the main chemical components of K. coccinea. According to skeleton types, K. coccinea lignans can be divided into four categories: dibenzocyclooctadiene, spirobenzofuranoid dibenzocyclooctadienes, diarylbutanes and aryltetralins lignans.
2.1.1. Dibenzocyclooctadiene lignans
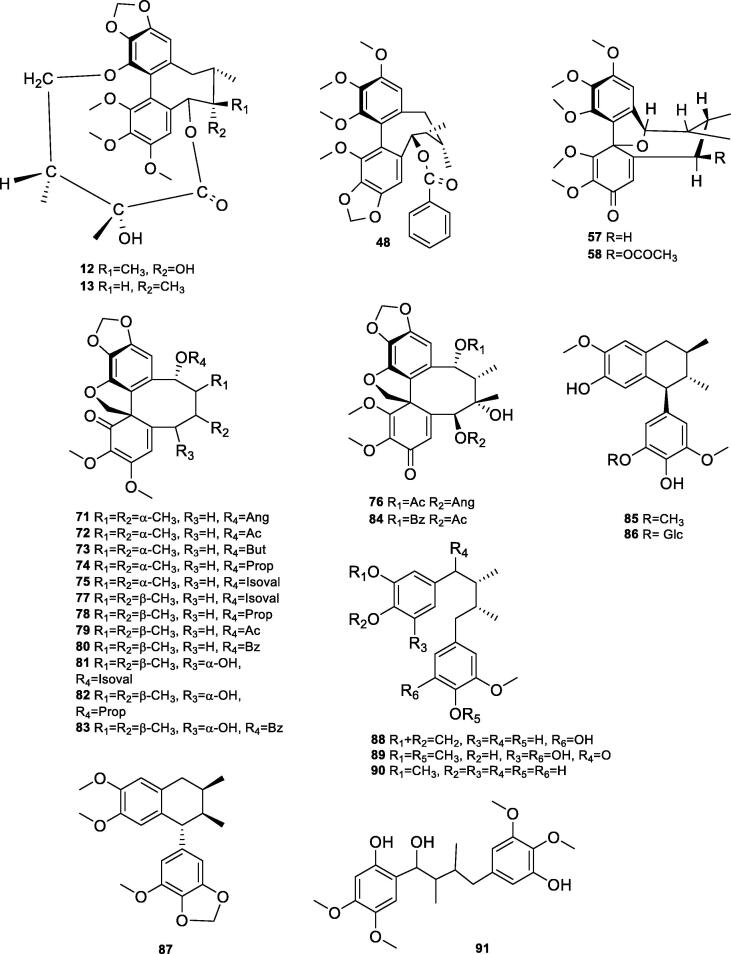
In this class, 58 different compounds have been reported so far. These compounds are different from each other on the basis of the attached substituent in dibenzocyclooctadiene lignan basic skeleton. Methoxy group is the most frequently found substituent, while other important substituents include, acetyl, angeloyl, tigloyl, propanoyl, benzoyl, cinnamoyl, and butyryl groups. In most compounds, hydroxyl and methoxy groups were found at C-1, C-2, C-3, and C-13, while substituents like acetyl, angeloyl, tigloyl, propanoyl, benzoyl, cinnamoyl, and butyryl were mostly present at C-6 or C-9. So far twenty-five different compounds (1–25) in this group have been reported having only hydroxyl and methoxy groups as substituents. These included gomisin M1 (1), 5,8-epoxy-6,7-dimethyl −2′,3′,2″,3″-dimethylenedioxy-4′,1″-dimethoxy-1,2,3,4-dibenzo-1,3-cyclooctadiene (2), kadsulignan N (3), kadsuphilin B (4), binankadsurin A (5), gomisin J (6), deoxyschisandrin (7), R-wuweizisu C (8), gomisin M2 (9), kadsuranin (10), isokadsuranin (11), gomisin D (12), gomisin E (13), neokadsuranin (14), kadsuralignan A (15), gomisin R (16), kadsulignan L (17), kadsulignan M (18), kadsulignan N (19), kadsuralignan F (20), schisantherin P (21), (6R,7R,8R)-2,10,11,12-tetramethoxy-6,7-dimethyl-5,6,7,8-tetrahydrodibenzo[a,c][8]annulene-3,8-diol (22), (6R,7R,8R)-1,2,10,11,12-pentamethoxy-6,7-dimethyl-5,6,7,8- tetrahydrodibenzo[a,c][8]annulene-3,8-diol (23), and (5R,6R,7R)-1,2,3,10,11,12- hexamethoxy-6,7-dimethyl-5,6,7,8-tetrahydrodibenzo[a,c][8]annulen-5-ol (24), (Fig. 2a, Fig. 2b) (Fang et al., 2014, Zhao et al., 2014, Ninh et al., 2009, Li et al., 1998, Li et al., 1985, Li et al., 2006, Liu and Li, 1995a, Liu and Li, 1995b, Liu et al., 2011). Fang et al. isolated 14-O-demethyl polysperlignan D (25), heteroclitin B (26), angeloyl-binankadsurin A (27), acetylschisantherin L (28), kadsurindutin A (29) from ethyl acetate extract of the roots of K. coccinea (Fang et al., 2014). These compounds contained angeloyl (Ang) groups at C-6 of dibenzocyclooctadiene lignan basic skeleton. Similarly, kadsutherin (30) has angeloyl moiety at C-14, while kadsuralignan I (31) possessed angeloyl group at C-1 (Li et al., 1998, Li et al., 2007). Liu et al. isolated further four lignans containing angeloyl group including schisantherin L (32), schisantherin M (33), schisantherin N (34), and acetylschisantherin L (35) (Liu and Li, 1994). Similarly, kadusurain A (36), kadusurain B (37), and kadusurain C (38) were identified containing tigloyl group in their structures (Zhao et al., 2014). Kadsurin (39) and diankadsurinone (40) were also isolated by Fang et al. (Fig. 2a) (Fang et al., 2014). Zhao et al. obtained kadsuphilin A (41) from the stems (Zhao et al., 2014). Hu et al. reported kadsuralignans G and L (42–43), angeloylbinankadsurin A (44), kadsuralignan J (45), and isovaleroylbinankadsurin A (46) from the stems (Hu et al., 2012). Acetylepigomisin R (47) (Ninh et al., 2009), benzoylisogomisin O (48) (Li et al., 1998), acetylbinankadsurin A (49) (Li et al., 1985), kadsuralignans J and K (50–51) (Li et al., 2007), kadsuralignan B (52) (Li et al., 2006), schizanrin F (53) (Li et al., 2006), schizanrin H (54) (Li et al., 2006), schisantherin O (55) (Liu and Li, 1994), schisantherin Q (56) (Liu and Li, 1995b), kadsulignan A (57) (Liu et al., 2011), and kadsulignan B (58) (Liu et al., 2011) were also isolated from the roots of K. coccinea. Liu et al. identified 3 new and 9 known analogues from the roots of EA and DCM layers of the Tujia ethnomedicine “Heilaohu”, heilaohulignans A-C (59–61), schizandrin (62), isobutyroylbinankadsurin A (63) longipedunin B (64), schisantherin F (65), schizanrin D (66), intermedin A (67), kadsurarin (68), kadsutherin A (69), kadsuphilol A (70) (Fig. 2a) (Liu et al., 2018). A most of compounds isolated from the roots or stems of K. coccinea except compounds schisantherins L-Q, kadsulignans A-B, kadsulignans L-N, acetylschisantherin L isolated from the seeds of K. coccinea by Liu et al.
Fig. 2a.
Chemical structures of lignans isolated from K. coccinea.
Fig. 2b.
Chemical structures of lignans isolated from K. coccinea.
2.1.2. Spirobenzofuranoid dibenzocyclooctadiene lignans
Fang et al. isolated five spirobenzofuranoid dibenzocyclooctadiene lignans (71–75) (Fig. 2b) from ethyl acetate extract of the roots of K. coccinea, which included schiarisanrin B (71), heteroclitin D (72) kadsulignans H-I (73–74), and schiarisanrin A (75) (Fang et al., 2014). Similarly, Li et al. reported further seven compounds from this plant (76–82) (Li and Xue, 1990). Li et al also identified kadsulignans E (83) and F (84) from the roots of K. coccinea (Li et al., 2007).
2.1.3. Arylnaphthalene lignan
Li et al. isolated kadsuralignan C (85) from chloroform extract of the stems, which was the first report of arylnaphthalene lignan from this plant (Li et al., 2006). Yeon et al. obtained (7′S,8′S,8R) - (8β,8′α)-dimethyl-4,4′-dihydroxy-5,3′-dimethoxy-5′-cyclolignan glucoside (86) (Yeon et al., 2014). Similarly, Li et al. isolated kadsuralignan H (87) from the ethyl acetate fraction of the roots of K. coccinea (Li et al., 2007).
2.1.4. Diarylbutanes lignans
Kadsurindutin E (88), coccilignan A (89), and meso-dihydroguaiaretic acid (90) were isolated from K. coccinea. Fang et al identified these compounds from the ethyl acetate extract of the roots (Fang et al., 2014). Li et al. reported kadcoccilignan (91) (Gao et al., 2012).
2.2. Triterpenoids
Genus Kadsura is famous for the presence of structurally diverse triterpenoids. Many of these important triterpenoids are the first time reported from K. coccinea. These also included a number of highly oxygenated triterpenoids with different skeletons. In recent years, a series of nortriterpenoids and kadlongilactones with novel structures have also been isolated and identified from this plant (Song et al., 2010a). The reported triterpenoids mainly belong to intact lanostanes, seco-lanostanes, intact cycloartanes, and seco-cycloartanes types.
2.2.1. Intact lanostane triterpenoids
Song et al. reported 3-hydroxy-12-acetoxycoccinic acid (92) (Song et al., 2010a), 3,12-dihydroxycoccinic acid (93) (Liu and Li, 1994), and 3-hydroxy-neokadsuranic acid (94) (Song et al., 2010b) from ethyl acetate extract of the radix of K. coccinea. Ninh et al. obtained 20(R), 24(E)-3-oxo-9β-lanosta-7, 24-dien-26-oic acid (95) from the dichloromethane fraction (Ninh et al., 2009). Similarly, Wang et al. isolated coccinone A–D (96–99) (Fig. 3a), and coccinilactones A–B (100–101) from the petroleum ether layer of the roots of K. coccinea (Wang et al., 2009, Wang et al., 2008). In the result of further studies, coccinic acid (1 0 2), 12β-hydroxycoccinic acid (1 0 3), kadcoccinones D–F (104–106) were isolated from the stems of K. coccinea (Li and Xue, 1986, Liang et al., 2013, Hu et al., 2015).
Fig. 3a.
Intact lanostane (92–106) and Seco lanostane (107–124) triterpenoids isolated from K. coccinea.
2.2.2. Seco-lanostane triterpenoids
Ninh et al. obtained 3,4-seco-lanostane triterpenoid, seco-coccinic acid F (1 0 7) from methanol extract of the roots of K. coccinea (Ninh et al., 2009). The seco-coccinic acids A–K (108–118) were isolated and characterized by Wang et al. from petroleum ether extract of the roots (Wang et al., 2008, Wang et al., 2012). Li et al. isolated five seco-lanostane type triterpenoids including kadsuracoccinic acids A–C (119–121), kadsuric acid (1 2 2) and micranoic acid A (1 2 3) from chloroform extract of the dried stems (Li et al., 2008). Kadcoccilactones R (1 2 4) was identified by Gao et al. from the stems of K. coccinea (Gao et al., 2008a).
2.2.3. Kadlongilactone triterpenoids
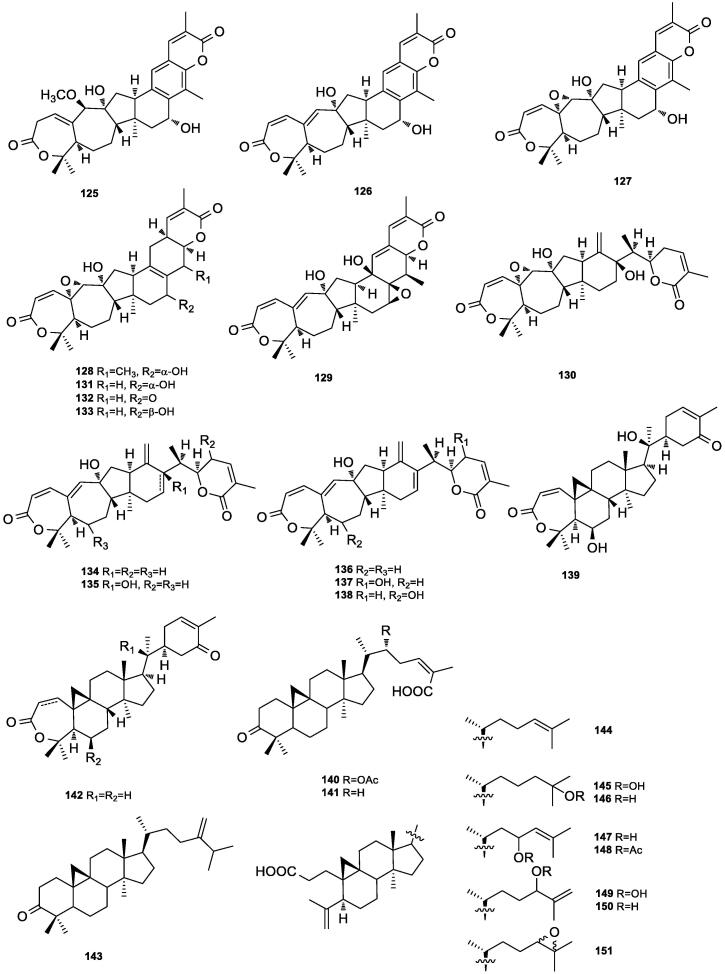
This type of triterpenoids has only been reported by Gao and his co-workers from this plant. They obtained fourteen kadlongilactone type triterpenoids from the ethyl acetate soluble extract of the stems. These compounds included kadcoccilactones K–P (125–130) (Fig. 3b), kadlongilactones A–B and D (131–133), and longipedlactones A–C, E and F (134–138) (Gao et al., 2008a).
Fig. 3b.
Kadlongilactone (125–138) and cycloartane (139–151) triterpenoids isolated from K. coccinea.
2.2.4. Intact cycloartane triterpenoids
Gao et al. isolated three intact cycloartane triterpenoids, kadcoccilactone Q (1 3 9), schisandronic acid (1 4 0) and heteroclic acid (1 4 1) from ethyl acetate extract of the stems of K. coccinea (Gao et al., 2008a). Tan et al. isolated kadsudilacton (1 4 2) from the ether extract of K. coccinea (Tan et al., 1991). Li et al. identified 24-methylenecycloartenone (1 4 3) from the ether extract of the stems of K. coccinea (Li and Xue, 1986).
2.2.5. Seco-cycloartane triterpenoids
Only eight compounds of this kind have so far been reported from this plant. Lai-King et al. isolated and characterized these compounds as coccinetane A–H (144–151) from the dichloromethane extract of the roots of K. coccinea (Sy and Brown, 1999).
2.2.6. Nortriterpenoids
The presences of highly oxygenated nortriterpenoids are characteristics of family Schisandraceae. Micrandiactone H (1 5 2) (Fig. 3c) was the first nortriterpenoid reported from K. coccinea. Yeon et al. isolated this compound from the ethyl acetate extract of the stems (Yeon et al., 2014). Gao et al. isolated further 10 nortirterpenoids, kadcoccilactones A − J (153–162) from the stems of K. coccinea (Gao et al., 2008b).
Fig. 3c.
Nortriterpenoids (152–163) and other (164–169) triterpenoids isolated from K. coccinea.
2.2.7. Other novel terpenoids
Hu et al. isolated three lanostane derived triterpenoids with novel structures from the plant (163–165) (Hu et al., 2015). Among these kadcoccinone C (1 6 3) featured a 6/6/9-fused carbocyclic core containing a rare oxabicyclo[4.3.1]decane carbon skeleton. The other two compounds, kadcoccinone A–B (164–165) had a 6/6/5/6-fused tetracyclic ring systems. Liang et al. isolated three triterpenoids (166–168) which featured an unprecedented carbon skeleton with a 6/6/5/5-fused tetracyclic ring system unit and a C9 side chain (Liang et al., 2013), along with kadcotriones A–C (169–171) containing a novel tricyclic ring system unit (Liang et al., 2012). Liang et al. isolated eleven novel triterpene acids with 6/6/5/6-fused tetracyclic ring systems, named as kadcoccinic acids A–J (172–181) (Fig. 3d), and seco-neokadsuranic acid A (1 8 2) (Liang et al., 2015). Hu et al. isolated a cage-like sesquiterpenoid possessing a tricyclo[4.4.0.03,10]decane scaffold, which was named kadcoccinin A (1 8 3), and also isolated the biosynthetically related kadcoccinin B (1 8 4) from the stems of K. coccinea (Hu et al., 2016a). Hu et al. also isolated kadcoccine acids A–N (185–198) from the ethyl acetate extract of the stems of K. coccinea (Hu et al., 2016b). Xu et al. reported that four new rearranged 6/6/5/6-fused lanostane-type triterpenoids, kadcoccitanes A−D (199–202), were isolated from the roots of Kadsura coccinea (Xu et al., 2019).
Fig. 3d.
Other terpenoids (170–202) isolated from K. coccinea.
3. Biological activities
Compounds and extracts isolated from Kadsura coccinea had shown various biological activities including anti-tumor, anti-HIV, anti-hepatitis, anti-oxidant and neuroprotective effects. These bioactivities have been summarized herein.
3.1. Anti-tumor activities
Kadusurain A (36) exhibited significant antiproliferative effects with IC50 values ranging from 1.05 to 11.31 µg/mL against HCT116, A549, HL-60 and HepG2 human tumor cell lines (Zhao et al., 2014). Heilaohulignan C (61) demonstrated good cytotoxicity against the HepG-2 human cell line with an IC50 value of 9.92 µmol/L reported by Liu et al (Liu et al., 2018). Seco-coccinic acids F, G and K (107–108, 112) showed cell growth inhibitory effects against human leukemia HL-60 cells with GI50 values 16.6, 15.2 and 28.4 µmol/L, respectively (Wang et al., 2012). Kadlongilactone A–B (131–132) and longipedlactone A (1 3 6) were reported to exhibit potent cytotoxicities against K562, Bel-7402, and A549 cell lines with IC50 values 0.1, 0.1 and 1.0 µmol/L respectively (Gao et al., 2008b). Hu et al. reported that kadcoccinones A–F (104–106, 163–165) exhibited cytotoxic activities against six human cancer cell lines (HL-60, SMMC7721, A-549, MCF-7, SW-480 and Hela) (Hu et al., 2015). Structure-activity relationship of kadcoccinones A and B indicated that the cleavage and hemiketal reactions between C-12 and C-14 might decrease the cytotoxic potency. Furthermore, it was found that the cytotoxicity of kadcoccinone E was higher than the cytotoxicity of kadcoccinone D, suggesting that the 23S, 24R configuration of epoxide group can enhance cytotoxicity more than 23R, 24S configuration (Hu et al., 2015). It was reported that the treatment of individual cultured Xenopus laevis cells at the blastular stage by kadsuracoccinic acid A (1 1 9) arrested cleavage of these cells with an IC50 of 0.32 µg/mL (Li et al., 2008). Wang et al. reported the seco-coccinic acids A–C, E (113–115, 117) with antiproliferative effects against HL-60 cells with GI50 values of 6.8 to 42.1 µmol/L. The structure–activity relationship indicated that the side chain at C-17 will enhance its activities (Wang et al., 2008). Hu et al. evaluated kadcoccine acids A–N (185–198) for their cytotoxicity against six human cancer cell lines, including HL-60 (acute leukemia), SMMC-7721 (hepatic cancer), A-549 (lung cancer), MCF-7 (breast cancer), SW-480 (colon cancer), and HeLa (cervical cancer). Only kadcoccine acids B (1 8 6) and H (1 9 2) showed moderate inhibitory effects, with IC50 values ranging from 3.11 to 7.77 µmol/L (Hu et al., 2016b).
3.2. Anti-HIV activities
Liang et al. reported that kadcotriones A and C (166, 168) exhibited anti-HIV-1 activities with EC50 values of 47.91 and 32.66 µg/mL, respectively (Liang et al., 2013). In another study kadcoccitone B (1 7 0) and 12β-hydroxycoccinic acid (1 0 3) showed anti-HIV activities with EC50 values of 30.29 and 54.81 µg/mL (Liang et al., 2012). Liu et al. studied kadsulignan M (44) and other similar compounds for their anti-HIV activities. They reported that kadsulignan M (44) exhibited significant activity against HIV in vivo (IC50 1.19 × 10−4 mol/L, EC50 6.03 × 10−6 mol/L). Structure-activity relationship indicated that benzoyl group at C-6 and hydroxyl group at C-7 positions in kadsulignan M (44) enhances anti-HIV activity. It was also observed that 2, 3-methylenedioxy and 12,13-dimethoxy substitutions on the aromatic rings also contribute towards the enhancement of anti-HIV activity (Liu and Li, 1995a).
3.3. Nitric oxide (NO) production inhibitory effects
Hu et al. revealed that the ethyl acetate extract of the rhizomes of K. coccinea can inhibit nitric oxide (NO) production in lipopolysaccharide and recombinant mouse interferon-γ activated murine macrophage-like cell line, RAW264.7. Further fractionation of the ethyl acetate extract led to the identification of dibenzocyclooctadiene lignans kadsuralignan G and L (16–17) with moderate NO production inhibitory activities (Hu et al., 2012). Fang et al. evaluated several compounds isolated from this plant for their inhibitory activities against LPS-induced NO production in murine microglial BV-2 cells using the SMT method. The results showed that acetylschisantherin L (6) schiarisanrin B (71), heteroclitin D (72) kadsulignans H − I (73–74), and schiarisanrin A (75) can strongly inhibit LPS-induced NO production in murine microglial BV-2 cells (Fang et al., 2014). Kadsuralignans H and G (87, 16) isolated by Li et al. were also found to exhibit significant activities against NO production (Li et al., 2007).
3.4. Other bioactivities
Ninh et al. reported that acetylepigomisin R (22), isovaleroylbinankadsurin A (20) and binankadsurin A (21) isolated from the plant exhibited protective effects on primary rat hepatocyte injury induced by t-Butyl hydroperoxide, with ED50 values 135.7, 26.1 and 79.3 µmol/L, respectively (Ninh et al., 2009). Myeong-Jin Goh et al. reported kadsuralignan F had an inhibitory effect on melanin synthesis through tyrosinase degradation (Goh et al., 2013). Sun et al. evaluated the antioxidant activity in K. coccinea fruits using the DPPH method. The results showed that phenolic acid exhibited significant antioxidant activities associated with the fruits (Sun et al., 2009). Kadcoccinins A (1 8 3) and B (1 8 4) were initially evaluated for antifungal effects, but both of these compounds were found to exhibit weak antifungal effects (Hu et al., 2016a).
4. Conclusion
The plant K. coccinea contains a range of chemical constituents with varied pharmacological effects. So far, 202 different compounds have been reported from the plant and most of these compounds have yet to be tested to determine if they possess any bioactivities. The previous studies on the chemical constituents and extracts of K. coccinea have shown good potential. The various dibenzocyclooctadiene lignans and lanostane triterpenoids are typical isolates in K. coccinea. In addition, a number of curiously structured compounds have been found in this plant, including kadlongilactone-type triterpenoids and schinortriterpenoids. The kadlongilactone-type triterpenoids possess the rare naturally occurred carbon skeletons, only reported from the plants of the genus Kadsura up to now. The highly oxygenated schinortriterpenoids are characteristic constituents of the Schisandraceae species. The discovery of new skeleton compounds is a major discovery in this phytochemical investigation, which brings the plant to the forefront of interest in the field of phytochemistry. These studies resulted in the identification of several compounds with good anti-HIV, anti-tumor, and anti-inflammatory effects. These findings suggest that there is an increasing interest in the triterpenoids and lignans from this plant even though it is exceedingly difficult to discover new skeleton structures. In vitro screening of anticancer activity, a series of potent leading anticancer compounds have been discovered, some of the compounds discussed their structure–activity relationships. On this occasion, studies implementing the structural modification of this compound may be expected in the future. As we known, many triterpenoid compounds isolated from other herbal medicine have anti-tumor effects, such as oleanolic acid, betulinic acid, ginsenoside Rh2 and Rg3. However, K. coccinea is a rich source of lanostane triterpenoids, that enriched the source of anti-tumor compounds. Although the pharmacological potential of triterpenoids and lignans still remains to be discovered, their structural diversity and uniqueness make a continuing discovery of their usage eminently rewarding.
There are more than 5,000 kinds of Chinese herbal medicines used in China. At present, more than 3,700 kinds of active ingredients have been identified, and many of them have been studied in pharmacology. But for the whole drug the in-depth research task is still very arduous. At present, due to the unclear basis of the pharmacodynamic material basis of K. coccinea, it has not been widely used. However, more comprehensive studies are needed to find out the chemical constituents responsible for its pharmacological activity to support its traditional use. Only further studies can elucidate the relationship between traditional uses and modern pharmacological activities. This review provides scientific pieces of evidence for the full development of this plant.
The investigation on the material basis of TCM is the premise and foundation of realizing the modernization of TCM. Using modern techniques and methods to guide and clarify the active components, it is possible to effectively carry out secondary development of related TCMs and promote the development of Chinese herbal medicines.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the Ministry of Science and Technology (2018YFC1707900 and 2018FY100703), National Natural Science Foundation of China (81874369, 81803708 and 81673579), Hunan Provincial Natural Science Foundation (2018JJ2293), Key Research and Development Programs of Hunan Science and Technology Department (2018SK2113, 2018SK2119, 2018WK2081), College Graduate Research and Innovation Projects of Hunan Province of China (CX20190537).
References
- Chen L.Q., Zhang J.H. The basic characteristics, research status, and development ideas of the Tujia medicine. Journal of Hubei Institute for Nationalities. (Medical Edition) 2007;24:1–3. [Google Scholar]
- Fang L.Z., Xie C.F., Wang H., Jin D.Q., Xu J., Guo Y.Q., et al. Lignans from the roots of Kadsura coccinea and their inhibitory activities on LPS-induced NO production. Phytochemistry Letters. 2014;9:158–162. [Google Scholar]
- Gao X.M., Pu J.X., Zhao Y., Yang L.P., Sun H.D. Lignans from Kadsura angustifolia and Kadsura coccinea. Journal of Asian Natural Products Research. 2012;14:129–134. doi: 10.1080/10286020.2011.637922. [DOI] [PubMed] [Google Scholar]
- Gao X.M., Pu J.X., Huang S.X., Lu Y., Lou L.G., Li R.T., et al. Kadcoccilactones A-J, triterpenoids from Kadsura coccinea. Journal of Natural Products. 2008;71:1182–1188. doi: 10.1021/np800078x. [DOI] [PubMed] [Google Scholar]
- Gao X.M., Pu J.X., Xiao W.L., Huang S.X., Lou L.G., Sun H.D. Kadcoccilactones K-R, triterpenoids from Kadsura coccinea. Tetrahedron. 2008;64:11673–11679. doi: 10.1021/np800078x. [DOI] [PubMed] [Google Scholar]
- Goh M.J., Lee H.K., Cheng L., Kong D.Y., Yeon J.H., He Q.Q., et al. Depigmentation effect of kadsuralignan F on melan-A murine melanocytes and human skin equivalents. International Journal of Molecular Sciences. 2013;14:1655–1666. doi: 10.3390/ijms14011655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Li L., Wang Q., Ye Y., Fan J., Li H.X., et al. Dibenzocyclooctadiene lignans from Kadsura coccinea. Journal of Asian Natural Products Research. 2012;14:364–369. doi: 10.1080/10286020.2011.654334. [DOI] [PubMed] [Google Scholar]
- Hu Z.X., Hu K., Shi Y.M., Wang W.G., Du X., Li Y., et al. Rearranged 6/6/5/6-fused triterpenoid acids from the stems of Kadsura coccinea. Journal of Natural Products. 2016;79:2590–2598. doi: 10.1021/acs.jnatprod.6b00508. [DOI] [PubMed] [Google Scholar]
- Hu Z.X., Shi Y.M., Wang W.G., Li X.N., Du X., Liu M., et al. Kadcoccinones A-F, new biogenetically related lanostane-type triterpenoids with diverse skeletons from Kadsura coccinea. Organic Letters. 2015;17:4616–4619. doi: 10.1021/acs.orglett.5b02360. [DOI] [PubMed] [Google Scholar]
- Hu Z.X., Shi Y.M., Wang W.G., Tang J.W., Zhou M., Du X., et al. Structural characterization of kadcoccinin A: A sesquiterpenoid with a tricyclo[4.4.0.0(3,10)]decane scaffold from Kadsura coccinea. Organic Letters. 2016;18:2284–2287. doi: 10.1021/acs.orglett.6b00919. [DOI] [PubMed] [Google Scholar]
- Li H.R., Feng Y.L., Yang Z.G., Wang J., Daikonya A., Kitanaka S., et al. New lignans from Kadsura coccinea and their nitric oxide inhibitory activities. Chemical & Pharmaceutical Bulletin. 2006;54:1022–1025. doi: 10.1248/cpb.54.1022. [DOI] [PubMed] [Google Scholar]
- Li H.R., Wang L.Y., Miyata S., Kitanaka S. Kadsuracoccinic acids A-C, ring-A seco-lanostane triterpenes from Kadsura coccinea and their effects on embryonic cell division of Xenopus laevis. Journal of Natural Products. 2008;71:739–741. doi: 10.1021/np700739t. [DOI] [PubMed] [Google Scholar]
- Li H.R., Wang L.Y., Yang Z.Q., Kitanaka S. Kadsuralignans H-K from Kadsura coccinea and their nitric oxide production inhibitory effects. Journal of Natural Products. 2007;70:1999–2002. doi: 10.1021/np070269x. [DOI] [PubMed] [Google Scholar]
- Li L.N., Xue H. Triterpenoids from roots and stems of Kadsura coccinea. Planta Medica. 1986;52:492–493. doi: 10.1055/s-2007-969264. [DOI] [PubMed] [Google Scholar]
- Li L.N., Hung X., Tan R. Dibenzocyclooctadiene lignans from roots and stems of Kadsura coccinea. Planta Medica. 1985;51:297–300. doi: 10.1055/s-2007-969495. [DOI] [PubMed] [Google Scholar]
- Li L.N., Qi X.J., Ge D.L., Kung M. Neokadsuranin, a tetrahydrofuranoid dibenzocyclooctadiene lignan from stems of Kadsura coccinea. Planta Medica. 1998;54:45–46. doi: 10.1055/s-2006-962331. [DOI] [PubMed] [Google Scholar]
- Li L.N., Xue H. Dibenzocyclooctadiene lignans possessing a spirobenzofuranoid skeleton from Kadsura coccinea. Phytochemistry. 1990;29:2730–2732. [Google Scholar]
- Liang C.Q., Shi Y.M., Li X.Y., Luo R.H., Li Y., Zheng Y.T., et al. Kadcotriones A-C: Tricyclic triterpenoids from Kadsura coccinea. Journal of Natural Products. 2013;74:2350–2354. doi: 10.1021/np400546z. [DOI] [PubMed] [Google Scholar]
- Liang C.Q., Shi Y.M., Luo R.H., Li X.Y., Gao Z.H., Li X.L., et al. Kadcoccitones A and B, two new 6/6/5/5-fused tetracyclic triterpenoids from Kadsura coccinea. Organic Letters. 2012;14:6362–6365. doi: 10.1021/ol303168y. [DOI] [PubMed] [Google Scholar]
- Liang C.Q., Shi Y.M., Wang W.G., Hu Z.X., Li Y., Zheng Y.T., et al. Kadcoccinic acids A-J, triterpene acids from Kadsura coccinea. Journal of Natural Products. 2015;78:2067–2073. doi: 10.1021/acs.jnatprod.5b00392. [DOI] [PubMed] [Google Scholar]
- Liu J.S., Li L. Schisantherins L-O and acetylschaisantherin L from Kadsura coccinea. Phytochemistry. 1994;32:1293–1296. [Google Scholar]
- Liu J.S., Li L. Kadsulignans L-N, three dibenzocyclooctadiene ligans from Kadsura coccinea. Phytochemistry. 1995;38:241–245. [Google Scholar]
- Liu J.S., Li L. Schisantherins P and Q, two lignans from Kadsura coccinea. Phytochemistry. 1995;38:1009–1011. [Google Scholar]
- Liu J.S., Li L., Yu H.G. Kadsulignan A and B, two novel lignans from Kadsura coccinea. Canadian Journal of Chemistry. 2011;67:682–684. [Google Scholar]
- Liu J.S., Qi Y.D., Lai H.W., Zhang J., Jia X.G., Liu H.T., et al. Genus Kadsura, a good source with considerable characteristic chemical constituents and potential bioactivities. Phytomedicine. 2014;21:1092–1097. doi: 10.1016/j.phymed.2014.01.015. [DOI] [PubMed] [Google Scholar]
- Liu Y.B., Yang Y.P., Tasneem S., Hussain N., Daniyal M., Yuan H.W., et al. Lignans from Tujia ethnomedicine Heilaohu: Chemical characterization and evaluation of their cytotoxicity and antioxidant activities. Molecules. 2018;23:2147. doi: 10.3390/molecules23092147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.B., Yang Y.P., Yuan H.W., Li M.J., Qiu Y.X., Choudhary M.I., et al. A review of triterpenoids and their pharmacological activities from genus Kadsura. Digital Chinese Medicine. 2018;1:247–258. [Google Scholar]
- Ninh K.B., Bui V.T., Phan V.K., Chau V.M., Nguyen X.C., Nguyen X.N., et al. Dibenzocyclooctadiene lignans and lanostane derivatives from the roots of Kadsura coccinea and their protective effects on primary rat hepatocyte injury induced by t-butyl hydroperoxide. Planta Medica. 2009;75:1253–1257. doi: 10.1055/s-0029-1185537. [DOI] [PubMed] [Google Scholar]
- Pharmacopoeia of the People's Republic of China. 1977. 1, 594.
- Remy B.T., Souvik K.L., Michael S. Recent advances in research on lignans and neolignans. Natural Product Reports. 2016;33:1044–1092. doi: 10.1039/c6np00021e. [DOI] [PubMed] [Google Scholar]
- Richar M.K.S. Monograph of Kadsura (schisandraceae) Systematic Botany Monographs. 1998;54:1–106. [Google Scholar]
- Shi Y.M., Xiao W.L., Pu J.X., Sun H.D. Triterpenoids from the schisandraceae family: An update. Natural Product Reports. 2008;25:367–410. doi: 10.1039/c4np00117f. [DOI] [PubMed] [Google Scholar]
- Smith A.C. The families Illiciaceae and Schisandraceae. Sargentia. 1947;7:1–224. [Google Scholar]
- Song Y., Zhao Q.J., Jin Y.S., Feng C.W., Chen H.S. A new triterpenoid from Kadsura coccinea. Chinese Chemical Letters. 2010;11:1352–1354. [Google Scholar]
- Song Y., Zhao Q.J., Jin Y.S., Feng C.W., Chen H.S. Two new triterpenoid acids from Kadsura coccinea. Archives of Pharmacal Research. 2010;33:1933–1936. doi: 10.1007/s12272-010-1207-0. [DOI] [PubMed] [Google Scholar]
- Sun J., Yao J.Y., Huang S.X., Long X., Wang J.B., Garcia-Garcia E. Antioxidant activity of polyphenol and anthocyanin extracts from fruits of Kadsura coccinea (Lem.) A.C. Smith. Food Chemistry. 2009;117:276–281. [Google Scholar]
- Sy L.K., Brown G.D. Novel seco-cycloartanes from Kadsura coccinea and the assisted autoxidation of a tri-substituted alkene. Tetrahedron. 1999;55:119–132. [Google Scholar]
- Tan R., Xue H., Li L.N. Kadsulactone and kadsudilactone, two new triterpenoid lactones from kadsura species. Planta Medica. 1991;57:87–88. doi: 10.1055/s-2006-960031. [DOI] [PubMed] [Google Scholar]
- Wang N., Li Z.L., Li D.Y., Pei Y.H., Hua H.M. Five new triterpenoids from the roots of Kadsura coccinea. Helvetica Chimica Acta. 2009;92:1413–1418. [Google Scholar]
- Wang N., Li Z.L., Song D.D., Li W., Fu H.W., Koike K., et al. Lanostane-type triterpenoids from the roots of Kadsura coccinea. Journal of Natural Products. 2008;71:990–994. doi: 10.1021/np7007522. [DOI] [PubMed] [Google Scholar]
- Wang N., Li Z.L., Song D.D., Li W., Pei Y.H., Jing Y.K., et al. Five new 3,4-seco-lanostane-type triterpenoids with antiproliferative activity in human leukemia cells isolated from the roots of Kadsura coccinea. Planta Medica. 2012;78:1661–1666. doi: 10.1055/s-0032-1315260. [DOI] [PubMed] [Google Scholar]
- Wang W. The research and development of Tujia ethnomedicine. Planta Medica. 2013;79:22. [Google Scholar]
- Xu H.C., Hu K., Sun H.D., Puno P.T. Four 14(13→12)-abeolanostane triterpenoids with 6/6/5/6-fused ring system from the roots of Kadsura coccinea. Natural Products and Bioprospecting. 2019;9:165–173. doi: 10.1007/s13659-019-0203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeon J.H., Cheng L., He Q.Q., Kong L.Y. A lignin glycoside and a nortriterpenoid from Kadsura coccinea. Chinese Journal of Natural Medicines. 2014;10:782–785. doi: 10.1016/S1875-5364(14)60119-9. [DOI] [PubMed] [Google Scholar]
- Zhao Q.J., Song Y., Chen H.S. Cytotoxic dibenzocyclooctadiene lignans from Kadsura coccinea. Archives of Pharmacal Research. 2014;37:1375–1379. doi: 10.1007/s12272-013-0186-3. [DOI] [PubMed] [Google Scholar]