Abstract
Objective
To investigate the immunomodulatory activity of polysaccharides from the roots of Brassica rapa.
Methods
The crude polysaccharide from roots of B. rapa (BRP) was extracted and purified to further investigate the active fraction of BRT for inducing macrophage phagocytosis.
Results
Effects on RAW264.7 cells demonstrated that BRP behaved better phagocytic capacity and had potent immunomodulatory activity, including increasing production of nitric oxide (NO), tumor necrosis factor α (TNFα) and upregulating mRNA levels of inducible NO synthase (iNOS) and TNFα. Furthermore, modulation of macrophage by BRP was indicated to be mediated via the activation of Akt and nuclear factor-kappa B (NF-κB).
Conclusion
The beneficial effects of BRP could be used as an immunotherapeutic adjuvant in treatment of inflammatory diseases.
Keywords: Akt/NF-κB, bioactivity, Brassica rapa L., immunomodulatory activity, macrophage, polysaccharide
1. Introduction
Macrophages play an essential role in the regulation of acquired immunity (Beurel & Jope, 2009), which could initiate the innate immune response by secreting pro-inflammatory factors, cytokines and chemokines. Stimulation of the innate immune system with immunomodulators can increase host resistance to unforeseen pathogenic threats (Schepetkin et al., 2008), and a number of innate system immunomodulators have been identified, including cytokines and natural substances isolated from microorganisms and botanical sources (Nworu et al., 2015, OuYang et al., 2013, Zhang et al., 2012). At present, traditional Chinese medicine (TCM) has been well accepted as a complementary and alternative therapy to improve the clinical symptoms (Jiang et al., 2016, Tian et al., 2012). A variety of polysaccharides isolated from TCM have also attracted a great deal of attention because of their broad spectrum of therapeutic properties and relatively low toxicity (Tzianabos, 2000), exhibiting various pharmacological activities, such as anti-tumor, anti-inflammatory and anti-atherosclerotic activities (Takata et al., 2005, Thakur, Connellan, Deseo, Morris, & Dixit, 2011, Wong et al., 2011).
Brassica rapa L., the family of Cruciferae, has been widely used as a Uyghur plant with homology to medicine and food, which has long been credited with health benefits especially by long-lived people. B. rapa has been traditionally used to cure cough and asthma in Xinjiang Uyghur Autonomous Region, and the root extract and capsules made of B. rapa have been clinically proven to have anti-tumor and immunity-enhancing activity (Sun et al., 2010, Xiao and Zhang, 2010, Zhang et al., 2013). Studies have shown that phytochemicals of B. rapa, including vitamins (Domínguez-Perles, Mena, García-Viguera, & Moreno, 2014), glucosinolates (Jaehoon et al., 2015), flavonoids (Hirokazu et al., 2011), and polysaccharides (Wang et al., 2016, Xie et al., 2010), are associated with biological functions such as antioxidative, immunomodulatory effects and inhibition of malignant transformation and mutations (Kestwal, Lin, Bagal-Kestwal, & Chiang, 2011). Wang et al have purified three fractions of BRP-1–1, BRP-2–1 and BRP-2–2 with average molecular weight of 1510, 1110 and 838 kDa, while the crude BRP (B. rapa polysaccharides) exhibited relatively higher antioxidant activity in vitro than purified fractions (Wang et al., 2016). One of the major mechanisms of plant-derived polysaccharides to play a role of immunomodulator has been reported to contribute to its ability to affect complement activation and macrophage function (Schepetkin, Faulkner, Nelson-Overton, Wiley, & Quinn, 2005).
In the present study, the polysaccharide from B. rapa was prepared, and the effects of both BRP and B. rapa tablet (BRT, the tablet from whole roots of B. rapa) on immunomodulatory activities were investigated, including phagocytic activities, inflammatory effect and its proper signaling pathway.
2. Materials and methods
2.1. Chemicals and reagents
Neutral Red Cell Proliferation and Total Nitric Oxide Assay Kit were purchased from Beyotime biotechnology (Shanghai, China). Quantitative Analysis Mouse ELISA Kit was ordered from Boster Biological Technology Co., Ltd (Wuhan, China). Immobilon™ Western Chemiluminescent HRP Substrate Kit (WBKL S0100) was purchased from Merck Millipore (CA, USA). Phospho-Akt (Ser473) (D9E) XP® Rabbit mAb (4060), Akt (pan) (C67E7) Rabbit mAb (4691), Phospho-IκBα (Ser32) (14D4) Rabbit mAb (2859), IκBα (44D4) Rabbit mAb (4812), Phospho-NF-kB p65 (Ser536) Rabbit mAb (3033), NF-κB p65 (D14E12) XP® Rabbit mAb (8242), TNFα (D2D4) XP® Rabbit mAb (11948), GAPDH (D4C6R) Mouse mAb (97166) Anti-rabbit IgG (HRP-linked Antibody, 7074) and Anti-mouse IgG (HRP-linked Antibody, 7076) were purchased from Cell Signaling Technology (MA, USA).
2.2. Phagocytosis of peritoneal macrophages in vivo
BRT was made from the roots of B. rapa and was provided by Xinjiang Uygur Autonomous Region Keping County Shengquan Industry Co., Ltd. KM mice (weight 18–22 g) were provided by Experimental Animal Center of Xinjiang Medical University, allowed to acclimate to a new SPF surrounding [temperature: (22 ± 2) °C, humidity: 40%−60%, light/dark cycle: 12 h] for 1 week, with food and water supplied ad libitum. Then mice were randomly divided into four groups with ten animals in each group, including one control group and three treatment groups with different oral dosage of BRT (3, 6, 12 g/kg/d). For the control group, mice were given an equal volume of water. Phagocytosis by peritoneal macrophages was detected using the method described by Wang et al (Wang et al., 2009). One hour after the last oral administration, 0.4 mL 5 × 106 chicken red blood cells (CRBCs) was intraperitoneally injected into each mouse. One hour later the mice were euthanized with CO2. The experiments were conducted in full compliance with the local, national, ethical and regulatory principles with the approval of the Institutional Animal Care and Use Committee at Xinjiang Medical University.
Saline (2 mL) was injected into the abdominal cavity and 0.2 mL ascites was collected and prepared for smear microscopy. The smears were fixed with acetone-methanol (1:1, volume percent) solution, then stained with filtered Giemsa-phosphate (1:9) dye for 30 min. The number of macrophages swallowing CRBCs was counted under an optical microscope and the phagocytic rate (PR) and phagocytic index (PI) were calculated. PR (%) = the number of macrophages swallowing CRBCs/the number of total macrophages × 100%, PI = the number of swallowed CRBCs/the number of macrophages swallowing CRBCs (at least 100 cells were selected to test).
2.3. Extraction of BRP
B. rapa was collected in April 2015 from Keping county, Xinjiang Uyghur Autonomous Region (79°2′48"E, 40°30′30"N), and identified by associate professor Jiang He (Xinjiang Institute of Materia Medica). A voucher specimen (KP20150428-001) was deposited in Xinjiang Institute of Materia Medica (Xinjiang, China). The powder of roots of B. rapa was extracted with 25-time the volumes of distilled water (volume to mass ratio) at 100 °C twice (2 h each time). All extracts were combined, filtrated, precipitated with 80% ethanol and the mixture was kept overnight at 4 °C. The sediments were dried to constant weight under vacuum at 40 °C, and then resolved into distilled water to a rough concentration of 5 mg/mL for deproteinizing by Sevag method (Zha et al., 2014). After removal of the Sevag reagent, the extract was decolorized with 5% activated carbon (mass to volume ratio) at 60 °C for 30 min. The extracts were precipitated with 80% ethanol and dried to constant weight again. Finally, the polysaccharide from B. rapa was obtained, named as BRP, and its content was determined by phenol sulfuric acid method (Masuko et al., 2005).
2.4. Size exclusion chromatography analysis
BRP and BRT were dissolved in ultrapure water to a final concentration of 5 mg/mL. Size exclusion chromatography (SEC) analysis was performed at 35 °C on a Thermo Scientific Dionex UltiMate 3000, equipped with CAD detection Dionex Corona Veo RS. A TSK-Gel G3000SWXL column (7.8 mm × 300 mm, 5 μm, Tosoh Bioscience, Montgomeryville, PA, USA) was used at 25 °C. The mobile phase, comprised of 100 mmol/L ammonium formate-acetonitrile-ultrapure water (5:30:65), was delivered at a flow rate of 0.5 mL/min.
2.5. Cell culture and reagents
The murine macrophage-like cell line RAW264.7 was purchased from National Infrastructure of Cell Line Resource and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in a 5% CO2 humidified atmosphere.
2.6. Measurement of phagocytic capacity of macrophages in vitro
RAW264.7 cells were incubated with BRT and BRP (312.5, 625, 1250, 2500 and 5000 μg/mL) for 24 h, and then 100 μL of aseptic neutral red solution (0.075%) was added into each well and incubated for another 1 h. After washed with PBS, the cells were lysed by adding 150 μL cell lysis buffer (anhydrous ethanol/acetic acid, 1:1). The absorbance was measured at 550 nm using a Bio-Tek EXL800 microplate reader (Bio-Tek, Winooski, VT). The absorbance represented the phagocytic ability of the macrophages.
2.7. Biochemical assay
RAW264.7 cells were plated at a density of (5 × 105) cells/mL in 12-well culture plates and incubated in medium with and without BRT and BRP (312.5, 625, 1250, 2500 and 5000 μg/mL) for 24 h. 200 μL supernatants were saved for the measurement of nitric oxide (NO) according to the colorimetric method described previously (Schepetkin et al., 2005).
2.8. Enzyme-linked immunosorbent assay (ELISA)
RAW264.7 cells were seeded into six-well plates at (1 × 106) cells/mL. After 24 h irritants incubated the culture medium was collected and centrifugated, and the supernatant was stored at − 20 °C for the following test. The tumor necrosis factor α (TNF-α) level was detected using commercial enzyme-linked immunosorbent assay (ELISA) kit (Boster, Wuhan, China). All experimental procedures were performed according to the manufacture’s instructions and normalized by protein content (mg/mL).
2.9. Quantitative real-time PCR assay (qRT-PCR)
Total RNA from RAW264.7 cells was extracted using Mag-Bind Total RNA Kit, and then was used as substrate to synthesize the complementary DNA with First-Strand cDNA Synthesis SuperMix Kit. The relative levels of mRNA to glyceraldehyde-3-phosphate dehydrogenase (GAPDH, accession number: NC_000072.7) were analyzed using Faststart universal SYBR Green master Kit on Bio-Rad CFX96 qPCR system (CA, USA). The data were analyzed using the 2−ΔΔCt method (Subbiah et al., 2015). The primer sequences are as follows: inducible NO synthase (iNOS) forward 5′-TCAGCTACGCCTTCAACACC-3′ and reverse 5′-TTCCCAAATGTGCTTGTCACC-3′; TNF-α forward 5′-ACACCGTCAGCCGATTTGCTA-3′ and reverse 5′-CCAAAGTAGACCTGCCCGGAC-3′; GAPDH forward 5′-CATCTTCCAGGAGCGAGACCC-3′ and reverse 5′-AGACACCAGTAGACTCCACGACA-3′.
2.10. Western blot
After being treated with various irritants for 24 h, the cells were lysed by RIPA buffer (containing 4% protease and phosphatase inhibitor cocktail) for 10 min and centrifuged at 12 000 rpm for 10 min at 4 °C. The protein of each sample was detected using BCA protein assay kit. The samples were separated through a 12% SDS-PAGE gel, and then transferred to PVDF membranes. After being blocked with 5% skimmed milk or BSA solution for 2 h, the membranes were probed with diluted primary antibodies against phosphor-Akt, Akt, phosphor-IκBα, IκBα, NF-κB p65, phosphor-p65, TNFα and GAPDH in 5% BSA/TBST overnight at 4 °C. After being washed with TBST for 3 times, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h at room temperature. The blots were smeared with chemiluminescence (Millipore) and analyzed by ChemiDoc Imaging system (Bio-Rad, CA, USA).
2.11. Statistics
Data were shown as mean ± standard deviation of at least three independent experiments. One-way ANOVA and Student’s t-test were used to evaluate statistical significance with the SPSS statistics 23.0 software. Values of P < 0.05 were considered as statistically significant.
3. Results
3.1. BRT induces macrophage phagocytic activity in vivo
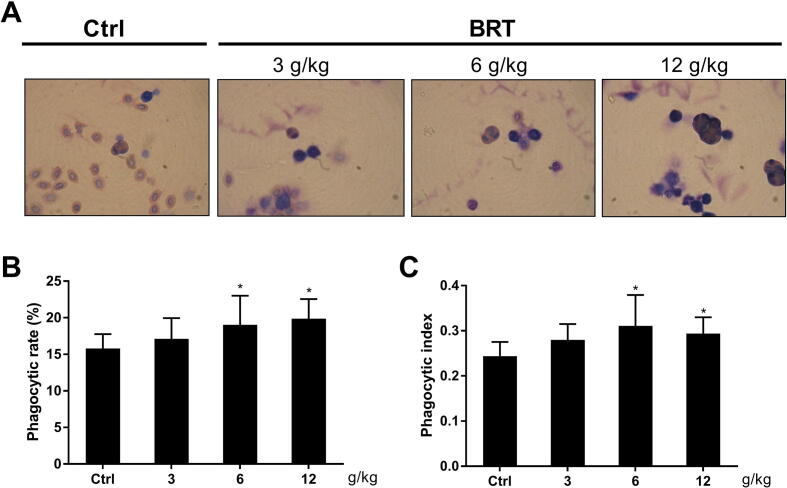
For phagocytosis of CRBCs, both PI and PR increased significantly in BRT 6 g/kg and 12 g/kg treatment groups when compared with control group (P < 0.05) (Fig. 1). Treatment of 3 g/kg BRT had no significant effect on rate and index of phagocytosis of CRBCs. The results indicated that BRT could enhance the ability of phagocytes to scavenge their none-self materials.
Fig. 1.
Macrophage phagocytic activity in vivo induced by BRT (mean ± SD, n = 6). Microscope images (A), PR (B), and PI (C) of CRBCs with BRT treatment (Ctrl stands for the group without BRT treatment; *P < 0.05 vs control).
3.2. Purification and characterization of BRP
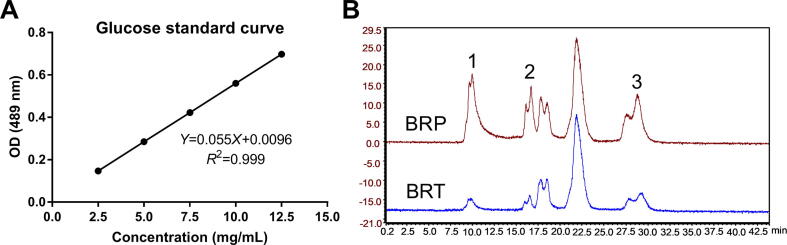
Polysaccharides from the roots of B. rapa were obtained following the method described in materials and methods, and determined by phenol sulfuric acid method. Based on the established standard curve (Fig. 2A), 7.03228 g BRP was calculated to be obtained from 20.0012 g roots of B. rapa, with a yield of 35.1619%. The content of main fractions 1, 2 and 3 in BRP was higher than that in BRT as shown in spectrum of size exclusion chromatography (Fig. 2B).
Fig. 2.
Detection of BRP extracted from B. rapa by SEC. Glucose standard curve (A) and main fractions 1, 2 and 3 showed on BRP and BRT grams (B).
3.3. Key active component of BRT inducing macrophage phagocytic activity
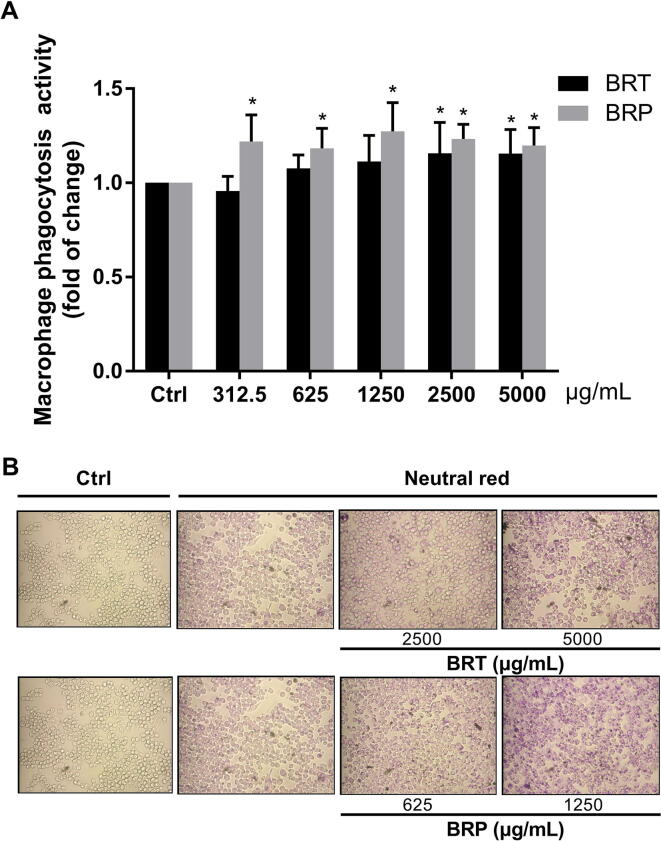
One of the most distinguished features of macrophage activation would be an increase in pinocytic activity. Pinocytic activity of polysaccharides-activated macrophages was examined by the uptake of neutral red (0.1%). BRP (1250 μg/mL) showed the similar activity to phagocytose neutral red with 5000 μg/mL BRT, and BRP behaved better in its phagocytic capacity than BRT at the same concentration (Fig. 3). The above results indicate that BRP significantly enhances the phagocytic ability of macrophages and could be the main active component of B. rapa promoting macrophage phagocytosis.
Fig. 3.
Phagocytic ability of macrophages with the treatment of BRP and BRT (mean ± SD, n = 3). (A) Absorbance compared with control and (B) microscope images of RAW264.7 cells after dyeing with neutral red (*P < 0.05 vs control).
3.4. BRP stimulates secretion and transcription of NO and TNF-α in RAW 264.7
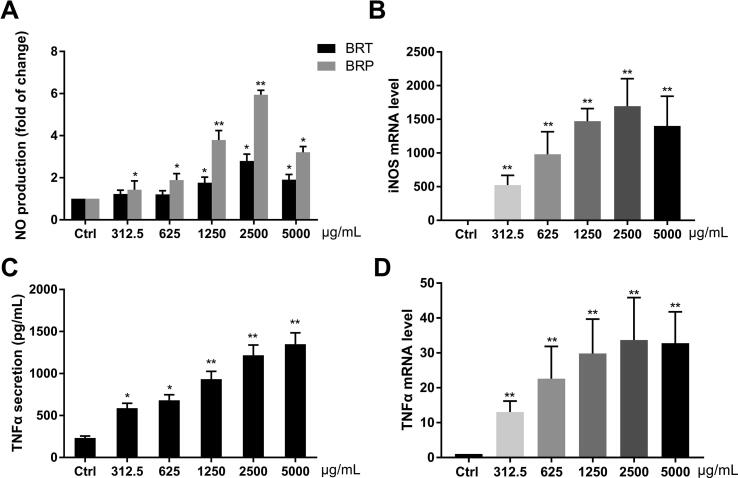
A minimum amount of NO production was determined in the culture supernatants of macrophages incubated with medium alone. Whereas, treatment with 1250 μg/mL BRP resulted in a 4-time increase in NO production (P < 0.01) and 2 times increase as treatment with BRT (Fig. 4A). iNOS mRNA level of RAW264.7 cells was enhanced by BRP significantly (P < 0.05) as compared with its control group, and presented a dose-dependent manner (Fig. 4B).
Fig. 4.
Effect of BRP on macrophage NO and TNFα in RAW264.7 cells (mean ± SD, n = 3). NO production of cells with BRP and BRT (A), iNOS mRNA levels of cells with BRP (B), TNFα production (C), and mRNA level (D) (*P < 0.05, **P < 0.01 vs control).
Untreated cells produced very little TNF-α. Incubation of these cells with BRP significantly enhanced TNF-α production (P < 0.05) and mRNA level (P < 0.01) in a concentration-dependent manner (Fig. 4C and D).
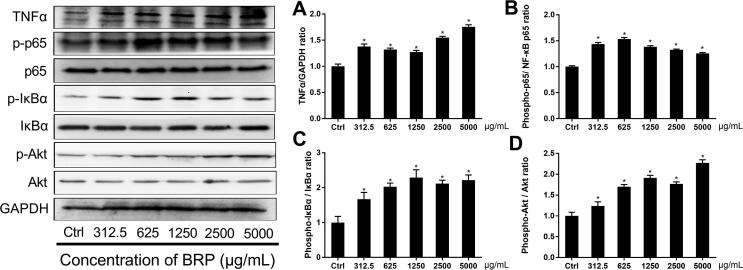
3.5. BRP activates Akt/NF-κB signaling pathway
Substance with immunomodulatory activity can regulate cytokine production. To further evaluate the signaling pathway involved in the immunomodulatory activity of BRP, the effect of BRP was assessed on macrophage TNF-α, phosphorylation of NF-κB, IκBα and Akt activation in RAW264.7 macrophage cells using Western blot.
BRP ranging from 312.5 μg/mL to 5000 μg/mL was observed to increase the protein expression of TNF-α and stimulate NF-κB directed alkaline phosphatase expression (P < 0.05) (Fig. 5A and B). Akt and IκBα phosphorylation were also increased by BRP (P < 0.05) (Fig. 5C and D). The results indicated that BRP was responsible for the observed effects and potently activation of the Akt/NF-κB pathway.
Fig. 5.
Effect of BRP on cell signaling pathway in RAW264.7 macrophage cells (mean ± SD, n = 3). The protein expression of TNFα (A), phosphorylation of NF-κB (B), IκBα (C) and Akt (D) (*P < 0.05, **P < 0.01 vs control).
4. Discussion
There are multiple receptors on the phagocytes surface could recognize and internalize particle and clear none-self substance such as apoptotic cells, senescent erythrocytes, and inflammatory products (Brock, Serezani, Carstens, & Peters-Golden, 2008). Macrophages, as the most important professional phagocytes, play an important role in phagocytosis of pathogens and apoptotic cells, production of cytokines, and proteolytic processing and presentation of foreign antigens (Hume, 2006). NO, synthesized by macrophage-induced nitric oxide synthase, has been identified as a critical mediator of a variety of biological functions, including pathogen elimination and destruction of tumor cells by activated macrophages (Niedbala, Cai, & Liew, 2006). TNFα, also considered as a major immune and inflammatory mediator, owns cytostatic and cytocidal effects in vivo and in vitro (Antwerp, Van Martin, Kafri, Green, & Verma, 1996). These reactive oxidants are involved in the regulation of apoptosis and immune homeostasis, which play critical roles in host defense and other physiological processes (Serhan & Savill, 2005).
Recently, a large amount of polysaccharides isolated from plants have been reported to process various biological activities, including immunomodulation, antitumor and anti-oxidation (Fei, Yuan, & Rashid, 2009). It has been reported that the union of polysaccharide and its receptor on the surface of macrophages could increase the secretion of NO and TNFα-related macrophage immune responses (Schepetkin & Quinn, 2006).
B. rapa root has been traditionally used as a Uyghur folk medicine as well as health-care food to cure cough and asthma by Uyghur nationality. In previous studies, the antioxidant activity of crude BRP was determined to be better than that of the purified fractions of BRP (Wang et al., 2016, Xie et al., 2010). In addition, the chalcone glycosides isolated from B. rapa exhibited the effect to suppress LPS-induced iNOS expression and NO production via inhibition of STAT1 (Hirokazu et al., 2014, Hirokazu et al., 2011), which left us a speculation that BRP could act an important role in activating immune system response. At first it was found that BRT (6 g/kg and 12 g/kg) had a significant effect on phagocytosis rate and index of CRBCs. Crude polysaccharides from the roots of B. rapa (BRP) were then precipitated with 80% ethanol and deproteinized by Sevag reagent, and immunomodulatory activity of BRP was investigated in this study. Compared with 1250 μg/mL of BRT, 312.5 μg/mL of BRP could already significantly enhance the phagocytic ability of macrophages, which indicated the benefit of BRT on immune and inflammatory diseases might mostly result from BRP dealing to the improvement of deficient phagocytosis of macrophage. BRP significantly enhanced TNFα production and mRNA level in a concentration-dependent manner within 312.5–5000 μg/mL, while 2500 μg/mL BRP exhibited the better effect on NO induction. iNOS is a key enzyme responsible for the production of NO and it plays an important role in the oxidative stress (Conforti & Menichini, 2011). As an immunomodulator, a dose between 2500 and 5000 μg/mL of BRP was thought of the optimal concentration. In addition, the efficacy of BRP can be explicated by interference with Akt/NF-κB signaling pathways in RAW264.7 macrophages by activating the phosphorylation of Akt, IκBα and NF-κB expression. Apparently, activation of NF-κB plays a key role in the transcriptional regulation of TNFα and iNOS genes. On the opposite, the generation of TNFα and NO due to cell activation can further activate the expression of NF-κB (Brasier, 2006).
5. Conclusion
By investigating the effects of BRT and BRP on RAW 264.7 macrophages, it was found that BRP behaved better phagocytic capacity, and had potent immune-stimulated activity via upregulating the iNOS and TNF-α pro-inflammatory cytokines, which was supported by the mRNA expression levels and secretion of cytokines. Furthermore, modulation of macrophage function by BRP was indicated to be mediated via activation of Akt/NF-κB signaling pathway. The present study showed beneficial effects of BRP which could be used as an immunotherapeutic adjuvant on inflammatory diseases, and provided a foundation for further studies of immunomodulatory mechanism of BRP.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by grants from the Natural Science Foundation of China (81760778), the Fundamental Research Funds for Public-interest Scientific Institution in Xinjiang Uygur Autonomous Region (KYGY2016169), and CAMS Innovation Fund for Medical Sciences (2017-I2M-1-012).
Contributor Information
Liping Bai, Email: lipingbai1973@163.com.
Xiqiang An, Email: axq0991@sina.com.
References
- Antwerp D.J., Van Martin S.J., Kafri T., Green D.R., Verma I.M. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274(5288):787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- Beurel E., Jope R. Glycogen synthase kinase-3 promotes the synergistic action of interferon-gamma on lipopolysaccharide-induced IL-6 production in RAW264.7 cells. Cellular Signalling. 2009;21(6):978–985. doi: 10.1016/j.cellsig.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier Allan R. The NF-κB regulatory network. Cardiovascular Toxicology. 2006;6(2):111–130. doi: 10.1385/ct:6:2:111. [DOI] [PubMed] [Google Scholar]
- Brock T., Serezani C., Carstens J., Peters-Golden M. Effects of prostaglandin E-2 on the subcellular localization of Epac-1 and Rap1 proteins during Fc gamma-receptor-mediated phagocytosis in alveolar macrophages. Experimental Cell Research. 2008;314(2):255–263. doi: 10.1016/j.yexcr.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti F., Menichini F. Phenolic compounds from plants as nitric oxide production inhibitors. Current Medicinal Chemistry. 2011;18:1137–1145. doi: 10.2174/092986711795029690. [DOI] [PubMed] [Google Scholar]
- Domínguez-Perles R., Mena P., García-Viguera C., Moreno D.A. Brassica foods as a dietary source of vitamin C: A review. Critical Reviews in Food Science Nutrition. 2014;54(8):1076–1091. doi: 10.1080/10408398.2011.626873. [DOI] [PubMed] [Google Scholar]
- Fei L., Yuan Q., Rashid F. Isolation, purification and immunobiological activity of a new water-soluble bee pollen polysaccharide from Crataegus pinnatifida Bge. Carbohydrate Polymers. 2009;78(1):80–88. [Google Scholar]
- Hirokazu H., Ryoko I., Masayuki N., Tetsuro K., Mamoru K., Tetsuo A. Newly synthesized 'hidabeni' chalcone derivatives potently suppress LPS-induced NO production via inhibition of STAT1, but not NF-κB, JNK, and p38, pathways in microglia. Biological and Pharmaceutical Bulletin. 2014;37(6):1042–1049. doi: 10.1248/bpb.b14-00116. [DOI] [PubMed] [Google Scholar]
- Hirokazu H., Yoko N., Masayuki N., Ryosuke M., Tetsuro K., Elias A.…Tetsuo A. Inhibitory effects of chalcone glycosides isolated from Brassica rapa L. 'hidabeni' and their synthetic derivatives on LPS-induced NO production in microglia. Bioorganic & Medicinal Chemistry. 2011;19(18):5559–5568. doi: 10.1016/j.bmc.2011.07.036. [DOI] [PubMed] [Google Scholar]
- Hume D.A. The mononuclear phagocyte system. Current Opinion in Immunology. 2006;18(1):49–53. doi: 10.1016/j.coi.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Jaehoon J., Heajin P., Hanbit H., Jihye K., Haesung K., Hyun Il O.…Ha Hyung K. Effects of glucosinolates from Turnip (Brassica rapa L.) root on bone formation by human osteoblast-like MG-63 cells and in normal young rats. Phytotherapy Research. 2015;29(6):902–909. doi: 10.1002/ptr.5331. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Liu L.S., Shen L.P., Han Z.F., Jian H., Liu J.X., Mao Z.J. Traditional Chinese medicine treatment as maintenance therapy in advanced non-small-cell lung cancer: A randomized controlled trial. Complementary Therapies in Medicine. 2016;24:55–62. doi: 10.1016/j.ctim.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Kestwal R.M., Lin J.C., Bagal-Kestwal D., Chiang B.H. Glucosinolates fortification of cruciferous sprouts by sulphur supplementation during cultivation to enhance anti-cancer activity. Food Chemistry. 2011;126(3):1164–1171. [Google Scholar]
- Masuko T., Minami A., Iwasaki N., Majima T., Nishimura S.I., Yuan C.L. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Analytical Biochemistry. 2005;339(1):69–72. doi: 10.1016/j.ab.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Niedbala W., Cai B., Liew F.Y. Role of nitric oxide in the regulation of T cell functions. Annals of the Rheumatic Diseases. 2006;65(suppl_3):iii37–iii40. doi: 10.1136/ard.2006.058446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nworu C.S., Ihim S.A., Okoye F.B.C., Esimone C.O., Adikwu M.U., Akah P.A. Immunomodulatory and immunorestorative activities of β-D-glucan-rich extract and polysaccharide fraction of mushroom, Pleurutus tuberregium. Pharmaceutical Biology. 2015;53(11):1555–1566. doi: 10.3109/13880209.2014.991838. [DOI] [PubMed] [Google Scholar]
- Ouyang F.J., Wang G.B., Guo W., Zhang Y.Y., Xiang W.H., Zhao M. AKT signalling and mitochondrial pathways are involved in mushroom polysaccharide-induced apoptosis and G1 or S phase arrest in human hepatoma cells. Food Chemistry. 2013;138(4):2130–2139. doi: 10.1016/j.foodchem.2012.10.047. [DOI] [PubMed] [Google Scholar]
- Schepetkin I.A., Faulkner C.L., Nelson-Overton L.K., Wiley J.A., Quinn M.T. Macrophage immunomodulatory activity of polysaccharides isolated from Juniperus scopolorum. International Immunopharmacology. 2005;5(13-14):1783–1799. doi: 10.1016/j.intimp.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Schepetkin I.A., Quinn M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. International Immunopharmacology. 2006;6(3):317–333. doi: 10.1016/j.intimp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Schepetkin I.A., Xie G., Kirpotina L.N., Klein R.A., Jutila M.A., Quinn M.T. Macrophage immunomodulatory activity of polysaccharides isolated from Opuntia polyacantha. International Imunopharmacology. 2008;8(10):1455–1466. doi: 10.1016/j.intimp.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N., Savill J. Resolution of inflammation: The beginning programs the end. Nature Immunology. 2005;6(12):1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Subbiah R., Ganesan V., Shanmugha R.K., Tharmarajan R., Krishnan K., Sudhiranjan G. MiRNAs with apoptosis regulating potential are differentially expressed in chronic exercise-induced physiologically hypertrophied hearts. PLoS One. 2015;10(3):e0121401. doi: 10.1371/journal.pone.0121401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., An X.Q., Ma Y., Zhang T., Yao H., Jia H.Y., Zhang Y.H. Clinical observation of Brassica rapa L. capsule combined with FOLFIRI regimen in the treatment of colorectal cancer. China and Foreign Medical Treatment. 2010;7(6):20–22. [Google Scholar]
- Takata R., Yamamoto R.T., Konno T., Okubo T. Immunostimulatory effects of a polysaccharide-rich substance with antitumor activity isolated from black currant (Ribes nigrum L.) Bioscience Biotechnology and Biochemistry. 2005;69(11):2042–2050. doi: 10.1271/bbb.69.2042. [DOI] [PubMed] [Google Scholar]
- Thakur M., Connellan P., Deseo M.A., Morris C., Dixit V.K. Immunomodulatory Polysaccharide from Chlorophytum borivilianum roots. Evidence-Based Complementary Alternative Medicine. 2011;2011:1–7. doi: 10.1093/ecam/neq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q.E., Li H.D., Yan M., Cai H.L., Tan Q.Y., Zhang W.Y. Astragalus polysaccharides can regulate cytokine and P-glycoprotein expression in H22 tumor-bearing mice. World Journal of Gastroenterology. 2012;18(47):7079. doi: 10.3748/wjg.v18.i47.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzianabos A.O. Polysaccharide immunomodulators as therapeutic agents: Structural aspects and biologic function. Clinical Microbiology Reviews. 2000;13(4):523–533. doi: 10.1128/cmr.13.4.523-533.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Fu F., Zhang L., Han B., Zhu M., Zhang X. Effects of escin on acute inflammation and the immune system in mice. Pharmacological Reports. 2009;61(4):697–704. doi: 10.1016/s1734-1140(09)70122-7. [DOI] [PubMed] [Google Scholar]
- Wang W., Wang X., Ye H., Hu B., Zhou L., Jabbar S.…Shen W. Optimization of extraction, characterization and antioxidant activity of polysaccharides from Brassica rapa L. International Journal of Biological Macromolecules. 2016;82:979–988. doi: 10.1016/j.ijbiomac.2015.10.051. [DOI] [PubMed] [Google Scholar]
- Wong K.H., Lai C.K.M., Cheung P.C.K. Immunomodulatory activities of mushroom sclerotial polysaccharides. Food Hydrocolloids. 2011;25(2):150–158. [Google Scholar]
- Xiao C.X., Zhang H.L. Clinical observation of the effect of Brassica rapa L. extract on chemotherapy-related indicators in advanced colorectal cancer. Asia-Pacific Traditional Medicine. 2010;06(12):30–31. [Google Scholar]
- Xie Y., Jiang S., Su D., Pi N., Ma C., Gao P. Composition analysis and anti-hypoxia activity of polysaccharide from Brassica rapa L. International Journal of Biological Macromolecules. 2010;47(4):528–533. doi: 10.1016/j.ijbiomac.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Zha S., Zhao Q., Chen J., Wang L., Zhang G., Zhang H., Zhao B. Extraction, purification and antioxidant activities of the polysaccharides from maca (Lepidium meyenii) Carbohydrate Polymers. 2014;111:584–587. doi: 10.1016/j.carbpol.2014.05.017. [DOI] [PubMed] [Google Scholar]
- Zhang L.P., Ma J.W., Zhang H.L. Clinical observation of Brassica rapa L. capsule combined with FOLFIRI regimen in the treatment of colorectal cancer. In China & Foreign Medical Treatment. 2013;32:103–104. [Google Scholar]
- Zhang X.D., Xu D.Z., Li J.H., Wang T., Ge F.H., Yang L. Study on the immunocompetence of polysaccharide extracted from root of Salvia miltiorrhiza. Journal of Chinese Medicinal Materials. 2012;35(6):949. [PubMed] [Google Scholar]