Abstract
Traditional herbal medicine (THM) is an important part of the traditional Chinese medicine culture. Due to its high medicinal potential, it should not only serve for the Chinese people's medical use, but also contribute to the world medicine, THM for the international market must be standardized and large-scale, and produced according to the “Good Agriculture Practice” (GAP). The quality of THM directly affects the patient's treatment status and safety of use. Therefore, the quality assurance of THM runs through the entire process of research and development, production and clinical practice. The standardized production and cultivation of THM is the starting point of the THM industry chain and plays a decisive role in the economic development of the THM industry. This article summarizes the development history, limitations and future development of GAP, and clarifies the opportunities for THM in the rapid development of the international and domestic Chinese medicine industry. In addition, analyzing the deficiencies that were existing in the former GAP implementation process and by suggesting science-based quality measures, it is hoped to stipulate improved GAP guidelines in the future and to lay the foundation for a modern THM international trade.
Keywords: Belt and Road Initiatives, Good Agricultural and Collection Practices, Good Agricultural Practice, quality assurance, traditional herbal medicine
1. Introduction
In recent years, as botanicals have become more and more widely used worldwide, many wild medicinal materials have been unable to meet the demand, so the proportion of cultivated medicinal materials has gradually increased (Zhang et al., 2012). However, the quality, safety and efficacy of medicinal materials are very important, and the cultivation of medicinal plants makes the implementation of “Good Agricultural Practice” (GAP) imperative (Yang et al., 2016).
At present, in order to ensure and control the quality of herbal medicines, the World Health Organization (WHO) and the European Medicines Agency (EMA) have formulated the “Guidelines for Good Agricultural and Collection Practices for Medicinal Plants” (GACP). For example, the World Health Organization issued the “Guidelines for Good Agricultural and Harvesting Practices for Medicinal Plants” (referred to as WHO-GACP) on February 10, 2004. In May 2002, the Herbal Products Committee of the European Medicines Agency issued the “Guidelines for Good Agricultural and Collection Practices of Herbal Origin Materials (GACP)” (EU-GACP) in London, which was implemented on February 20, 2006 (Zhang et al., 2021). It mainly aims at the characteristics of medicinal plants, and puts forward standardized guidelines for its cultivation, harvesting, preliminary processing, packaging, transportation, equipment and personnel, so as to achieve the purpose of high quality, safety and controllability of cultivated and collected plants as starting materials for drugs. However, these regulations do not state the efficacy of the herbs or the level of their ingredients.
Since 2002, China has issued and implemented “Administrative Measures for the Certification of Good Agricultural Practice for Chinese Crude Drugs”. The standardization requirements for the cultivation, harvest, initial processing, packaging, transportation and other aspects of THM equipment and personnel are mainly put forward and standardized according to their implementation. In the 14 years of GAP implementation, the standardization of THM production has made remarkable achievements. GAP production concept has been deeply rooted. GAP certification was suitable to ensure the quality and safety of Chinese herbal medicines (Zhang et al., 2011). In order to strengthen the production quality management of THM, the State Administration of Market Supervision and Administration issued the “Good Agricultural Practice for Chinese Crude Drugs (Draft for Solicitation of Comments)” in 2018 to solicit opinions from the whole society to further improve the GAP system for the production of THM (Deng, 2018).
GAP has been implemented for more than 10 years and has a far-reaching impact. The concept of GAP has been deeply rooted in the hearts of the people. The theory and methods of cultivation of THM have been continuously improved, and the large-scale planting area of THM has been continuously expanded. However, the current planting area of the GAP base only accounts for a small part of the total planting area of THM (Du et al., 2020). The modernization of China's GAP needs to solve and standardize the current germplasm confusion, breeding experience, and the abuse of pesticides and fertilizers, so as to promote THM production and quality control to be in line with international standards, and to enter the international market more and better. The establishment of standards and norms for the cultivation of medicinal materials is the basic work of inheriting and promoting the motherland medicine (Li, 2012).
This article will expatiate the history, opportunity and limitation of GAP development in China, and the scientific quality measures of GAP development in China in the future. The establishment of breeding standards and norms of THM is the basic work of inheriting and developing THM, further improving the management level of THM, and laying the foundation for international trade of THM.
2. Development history
In November 1998, the Chinese National Medical Products Administration proposed to implement the GAP in China, and organized experts to set up the GAP drafting expert group. The “Good Agricultural Practice for Chinese Crude Drugs” was approved by the Chinese Council of the National Drug Administration on April 17, 2002 and is now released and implemented on June 1, 2002 (Liang & Zhao, 2014). After repeated discussion and modification, “Administrative Measures for the Certification of Good Agricultural Practice for Chinese Crude Drugs” and “Chinese Crude Drugs GAP certification inspection evaluation standard (Trial)” were issued to ensure that the certification base and the GAP between enterprises. In November 2003, the first GAP certification application was formally accepted. During the period of national implementation of GAP certification (2004–2016), the China Food and Drug Administration (CFDA) published 196 GAP bases in all 66 batches of GAP inspection announcements that passed the review and certification, 25 re-certification bases were removed, and 167 GAP bases were included in the remaining 56 batches of announcements (Du et al., 2020). This process ended in 2016, when the certification of GAP bases was canceled by the General Office of the State Council of the People's Republic of China. On October 27, 2017, in order to further promote the implementation of THM production quality management norms and to ensure the safety and stability of THM quality, CFDA organized the drafting of “Good Agricultural Practice for Chinese Crude Drugs (revised version)” and publicly solicited opinions. On July 23, 2018, the State Administration for Market Regulation (SAMR) once again publicly solicited opinions from Chinese and international stakeholders on the “Management Standards for Quality Management of Chinese Medicinal Materials (Draft for Comment)” (Li & Mou, 2019), hoping to formulate more effective and reasonable management standards. As of July 2020, a new GAP is still being formed. The Development history of GAP is shown in Fig. 1.
Fig. 1.
Development history of GAP in China.
3. Problems and improvements in GAP
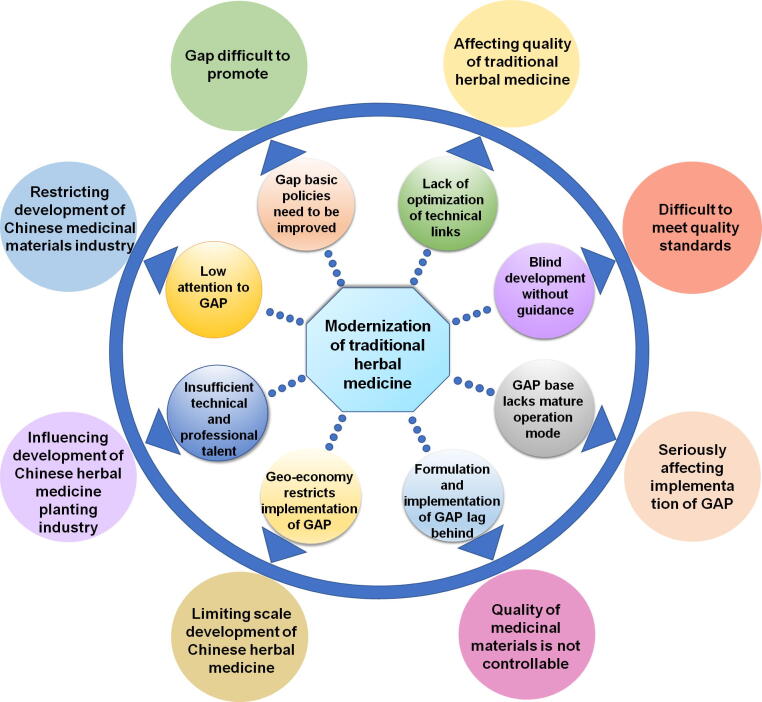
THM has a long history in China, and the research on THM application has also achieved certain results. However, the basic organization and management level of THM production is still relatively low. It is difficult to guarantee the quality of THM produced. The modernization and internationalization requirements of Chinese medicine are far apart, which affects the competitiveness of the international and domestic markets. It is undeniable that GAP has exposed many problems since 2002 (Fig. 2). In 2016, China GAP was suspended for certification in the trading system due to various reasons.
Fig. 2.
Problems in development of GAP.
3.1. Lack of correct understanding of GAP
GAP in THM is different from GAP in agriculture. This is because medicinal materials grown according to the regulations should not only have the same yield and safety requirements as agricultural crops but should also contain the adequate level of their effective curative ingredients; thus, the need to promote and strengthen the management of GAP standards is required to help people correctly understand GAP standards and recognize their importance (Wang et al., 2014).
The application of GAP should also be based on the internal needs of the developing enterprise, should control the quality of THM from the source, and improve the competitiveness of the enterprise. This requires companies to improve their understanding of GAP management norms (Wang et al., 2014). Currently, Chinese consumers and farmers do not show a high regard for GAP. Implementing GAP in THM raises the costs of cultivating THM materials, labor and other agricultural inputs, and the composition of production means. To obtain obvious price and sales advantages, the cost of THM produced according to GAP is significantly higher than that of conventional THM (Deng et al., 2013). Implementing GAP in non-THM and THM products certified by GAP in some regions are recognized by the international market. However, the domestic consumer market does not acknowledge the additional value of GAP based medicines. This leads to low income obtained by general THM growers, and seriously damages their enthusiasm to produce according to GAP standards of THM (Shi, 2010).
3.2. Insufficient technical and professional talent
GAP in THM involves many disciplines and departments, such as agronomy, THM, management science, resource science, and animal husbandry. In the implementation of GAP in THM, professional knowledge of medicine identification, medicinal botany, plant physiology, genetic breeding, medicinal plant cultivation, medicinal zoology, and entomology is required. The GAP base of THM generally lacks professional talent in the production of THM, especially those with compound talents who have medical knowledge as well as understand the cultivation technology involved in THM. It is difficult to recruit such compound talents, as long-term training is required. For many years, the problem of brain drain in THM cultivation industry has been serious. Especially the lack of talents engaged in the research and management of THM cultivation industry, resulted in a lack of technological innovation ability, so that key technical problems have not been completely advanced. In addition, in the process of planting THM, the lack of popularization of new technologies has led to the inability to effectively apply new technologies to the planting of THM. Large-scale planting of THM mainly adopts traditional field crop planting experience, and most THM are still in place. In the empirical cultivation stage, the planting and cultivation techniques of THM are not perfect, and the lack of scientific planning has affected the expansion of the scale of the industry, resulting in the inability to guarantee the quality of the medicinal materials produced (Han, 2021, Liu et al., 2021). It directly undermines the sustainable, high-level and scientific development of the THM cultivation industry (Deng et al., 2013, Wang et al., 2011, Gong et al., 2011).
The collaborative and interdisciplinary work of relevant research institutes is crucial. Experts with professional GAP concepts and profound knowledge needed to provide technical guidance in site selection, cultivation and breeding, variety selection, and other aspects (Wang, Huang, & Wei, 2014). Therefore, the people with relevant professional skills are the necessary precondition for a more scientific implementation of GAP regulations at all levels.
3.3. Government supporting policies and market supervision
Although the standards for the quality management of GAP were published in 2002, this was only a trial version, and many areas still require improvement. For example, there are still many problems in government regulations. Some grass-roots government departments did not pay more attention to quality, but paid more attention to output. High output, high quality, and high-efficiency benefits are an important feature of constructing a GAP base. Some areas have constructed a GAP base using the incorrect path of “performance demonstration.” For example, some GAP bases built by the administrative orders of some government departments are vigorous on the surface, but they are not founded on economic management models (Wen, 2014). There is no value in constructing a base, which only focuses on appearance, performance, cost, and efficiency. In addition, because the relevant supporting policies of the different governments are inadequate and there are no corresponding policies to guarantee the GAP base, the enthusiasm of drug enterprises is poor and GAP is difficult to promote. There is also weak supervision from the relevant departments on THM markets and a lack of testing on various THM items, and there is a general lack of concern by farmers and the relevant departments about whether the current Chinese medicine entering the market meets the appropriate standards (Hu, 2009).
GAP differs from the GACP of other countries, in that it takes the form of a certified production base. In this way, some retail investors still do not produce according to GAP management norms to reduce the costs and avoid the risks. In contrast, large enterprises that produce according to GAP’s requirements have reduced their market competitiveness to a certain extent because they have raised their costs. Therefore, it is necessary to formulate supporting policies and measures as soon as possible to promote the construction of GAP bases of THM, such as economic and policy support for GAP base certified enterprises, and corresponding preferential policies for decoctions or Chinese patent medicines that use GAP base THM for production and processing. The enterprise's enthusiasm for GAP construction of THM ensures the quality of medicinal materials (Song and Wang, 2014, Deng, 2018).
3.4. Research and technology needs improvement
THM cultivation is a multidisciplinary, multi-level technology; However, from the protection of THM germplasm resources, its selection and breeding, seed seedling detection and standards, pest control, fertilization, irrigation, harvest, initial processing and other key cultivation techniques, the quality and yield of medicinal materials are affected. As the technology and know-how required is generally lacking, this resulted in a relatively backward situation at the production sites, which seriously restricts the scale and standards for the development of THM (Deng et al., 2013, Zhao and Yang, 2019). Due to the change in the traditional farming model of a small-scale economy and the higher requirements of GAP in the production process and product quality, GAP production has higher requirements on the level of science and technology. The traditional cultivation mode has phenomena such as irregular, chaotic, and scattered planting. The degree of organization, intensification, and mechanization is low. High-quality seedlings and good planting techniques have not been widely used. The proportion of standardized and large-scale production is low, and the level of technology is low. The production area's processing equipment, processing technology, medicinal material storage technology and facilities are not complete enough to meet the requirements of GAP. In addition, many GAP bases of THM will choose the production mode of large-scale cultivation, which leads to a decrease in biodiversity and the stress of disease and pest control (Liu, 2020). Currently, comprehensive control measures are not established and some bases are still very dependent on chemical controls, resulting in excessive pesticide residues in medicinal materials. There are also large technical barriers in the collection and production area processing of some medicinal materials (such as honeysuckle, safflower) and a large quantity of mature medicinal materials collected over a short period of time. This may be due to the materials not being collected on time, the best collection period is missed, or because they are not dried efficiently and effectively, and so the variety or quality declines. Due to a lack of optimization and GAP bases in the many specific technical links in production, the difficulties and challenges of production will undoubtedly increase, resulting in higher production costs (Cao & Huang, 2015).
3.5. Blind development without guidance
In 2002, China put forward the implementation of GAP for THM, which has been strongly supported by national policy. Many pharmaceutical enterprises, scientific research institutions, and THM growers have started the upsurge of building a base, a high-tech park, and herbal garden for THM. With the adjustment in the local agricultural industrial structure, local governments have considered the cultivation of medicinal materials as one of the adjustment objectives, and they guide farmers to develop large-scale cultivation of medicinal materials (Cao & Huang, 2015). Farmers then believe that growing medicinal materials can achieve higher returns, and they rush to plant medicinal materials. However, implementing the guiding principles of local government together with GAP is not enough, as the farmers lack the guidance on market information and standardized operation procedures; thus, the cultivation of medicinal materials violates GAP principles, resulting in a blind and chaotic situation. In addition, there is blind introduction and development of GAP, which fails to fully consider the authenticity and regional nature of THM, and farmers struggle to meet the quality requirements of the national regulations. Thus, the demands of the pharmaceutical enterprises for raw materials of THM are not met and the industrialization of THM is thereby hindered (Lin, 2003).
3.6. GAP base lacks a mature organization and operation mode
The so-called GAP operation mode is actually the economic interests of all parties involved in the development and establishment of the GAP base. The previous brief introduction to the organization and operation mode of GAP bases certified by the former CFDA shows that the mode of each GAP base is different, and this describes the operation mode of each enterprise according to its actual situation (Lin, 2003). Currently, China has not established a set of mature operation modes of GAP based organization to promote its development, and this is the most discussed issue on the GAP base. However, we conclude that although the operation modes of these GAP bases differ, they always focus on three aspects of “company,” “research,” and “farmer” (Lin, 2002). The base operation mode of “Company, Base, Scientific Research Unit, Farmer,” under the constraints of the laws and regulations, are followed to obtain maximum benefit outputs (Deng, 2009). Therefore, the problems that need to be solved urgently are how to grasp the relationship between these three aspects, how to combine them organically and harmoniously, how to consider the interests of all parties, and how to develop farmer enthusiasm. Without the involvement of all parties, the GAP base cannot be developed. If the organization’s operation mode is not stable and the economic relationship is not coordinated, it will seriously affect the implementation of GAP, and will ultimately affect people’s determination and confidence in building and implementing GAP (Hu, 2009).
3.7. Site selection for CAP base construction based on ecological environment selection
After the implementation of the industrialization project of THM, although a variety of standardized cultivation bases of THM have been established throughout the country, the formulation and implementation of GAP are not synchronized with the construction of standardized cultivation bases. GAP formulation and implementation appear some lag, this problem urgently needs to be solved (Zhang & Ding, 2003).
Although GAP provides for site selection conditions, it is generally based on health considerations. GAP requires the scale of the base to achieve industrialization, the need to achieve a certain scale of THM cultivation, to ensure the consistency of THM production base, but due to farmers and land dispersion, the unified management of the whole base is difficult (Deng et al., 2013). GAP is a THM-based large-scale agricultural production that exists in an open environment and is greatly affected by the natural environment and climate, and human control is limited. Drought, waterlogging and low temperature have great influence on the yield and quality of THM, and GAP of THM requires the quality of medicinal materials produced in the base is stable and consistent, which is difficult to achieve. Considering the increasing severity of climate change, many traditional areas suitable for THM might shrink or expand, and the subsequent changes to their intrinsic active ingredients are still unknown. Computer technology (such as GIS, GPS, and RS) has been developed in recent years to study the cultivation of THM. There is research on the suitable distribution area for Atractylodes lancea and the quality of each distribution area. According to the content of atractylodin in A. lancea, suitable cultivation areas can be determined based on the pharmacopeia standard (Zhu et al., 2017).
3.8. Geo-economy and smallholder economy are restricting implementation of GAP
Geo-economics and small-scale farming restrict the standardization and scale of THM. Geo-economic and regional differences also restrict the promotion of GAP. Due to differences in regional culture and national culture, it is difficult to establish a unified standard (Guo et al., 2014). Although the remote areas have concentrated land, good environment and easy scale cultivation, the infrastructure, transportation, information and concepts of the cultivation bases distributed in mountainous areas and underdeveloped areas also restrict the implementation of GAP management. China's small-scale peasant economy has a long history, mostly farmers scattered cultivation. Small-scale decentralized management of THM cultivation occupies a dominant position, which brings great difficulties to the standardized production of THM (Cao & Huang, 2015; Heinrich, 2015).
4. Future development of GAP
In the process of fighting the new coronavirus pneumonia epidemic in 2020, the diagnosis and treatment plan issued by the National Health Commission referred to giving full play to the role of THM (Song et al., 2020). It has been proved that Chinese medicine has played an unprecedented positive role in the early interventional treatment of diseases. It has become an indispensable part of China's successful fight against coronavirus pneumonia. At the same time, with the enhancement of people’s health awareness, the demand for Chinese medicine is also rising rapidly, and the Chinese medicine industry has entered an unprecedented period of rapid development (Zhang & Huang, 2020). The main manifestation is that the demand for health products and daily chemical products based on THM will be further expanded. The main manifestation is that the demand for health care products and daily chemical products using THM as raw materials will further expand. This has brought unlimited business opportunities for the cultivation, processing and production of THM. Therefore, effective quality monitoring of the whole process of production and processing of THM is carried out to ensure the quality and safety of THM and the reliability of clinical use of THM to achieve the purpose of promoting the protection and sustainable use of THM. It is also an important goal for the implementation of GAP for THM.
In recent years, the country has issued a number of policies to support the development of the THM industry. With the gradual establishment of industry standards, China’s traditional Chinese medicine industry has entered a critical period of transformation, upgrading and structural optimization after experiencing rapid expansion. GAP aims to achieve THM standardization, the top priority of modernization needs to be further implemented (Zhu, 2020).
4.1. Establishing correct GAP development concept
First of all, we must establish a correct GAP development concept, combine the best cultivation practices of THM with the protection of endangered THM resources, accelerate the legislative process of THM resource protection and sustainable use, and realize the sustainable use of THM. We should restrict the development and utilization of wild medicinal resources (Yang & Chen, 2019), and encourage the introduction, cultivation and large-scale production of THM, especially endangered Chinese herbal medicines, to promote the development of GAP (Kang et al., 2020). Secondly, relevant technical specifications need to be established to guarantee the collection, preservation and identification of medicinal germplasm resources. For example, research and formulate national and industry standards or regulations, such as THM planting technology guidelines, pesticide rational use technology guidelines, green pesticide standards, etc., to achieve GAP strategic goals (Kang et al., 2020). Finally, to avoid blindly introducing GAP and blindly pursuing large-scale production, we should respect the cultivation laws of medicinal materials, support and protect the scientific development of authentic medicinal materials in accordance with the law, and improve the quality of THM (Xiao, Zhang, & Lin, 2006).
4.2. Cultivating professional talent
Although China began to implement GAP in THM in 2002, relatively few professionals are familiar with GAP. THM cultivation is a highly technical and scientific occupation. In the past, Chinese medicinal plants were planted by pharmacists instead of technicians. However, cultivation medicinal plants are not equivalent to cultivation agricultural crops. THM is mainly used for disease treatment and prevention, and is therefore typically used by people with pathological conditions, its quality directly affects the health and safety of the patient. Thus, a lack of talent is not conducive to the sustainable and healthy development of the GAP industry (Zhao, 2017). In order to meet the needs of a large number of professional and technical personnel for GAP, the relevant departments and enterprises should speed up the professional GAP mainly the construction of talent team, actively cooperate with research institutions such as colleges and universities, and strengthen Chinese medicine technical personnel, management personnel and marketing personnel education and training, inevitably develop herbalist and students' ability to establish and manage the GAP base of Chinese medicine. Through holding THM cultivation technology training, distributing technical materials, exchanging experience and field practice, help to guide and improve THM cultivation technology level (Wang et al., 2005). To eliminate fake and inferior THM varieties from the source, and gradually develop the market of THM, the THM professional training base established by professionals will definitely promote the development of THM (Li, Liang, & Ouyang, 2019). Among them, the latest cultivation technology requires the promotion and teaching of professionals, especially training for farmers, hoping to change the status quo (Bai, 2020).
4.3. Improvements in relevant laws and supporting policies
Since the release of the GAP standard in 2002, the country has issued a number of regulations to ensure the implementation of the GAP standard. The country encourages the cultivation of THM and THM varieties that can be planted on a centralized scale, with controllable quality and meeting requirements. However, some government departments attach importance to the field of THM cultivation and ignore the benefits and quality, thus embarking on the wrong path of “performance demonstration”. We suggest that the Chinese government make good agricultural standards for the current scientific and technological work. It should perfect the corresponding institutions, provide financial support, equip the agricultural institutions with professional and technical personnel and equipment, formulate a plan of good agricultural standard development and annual implementation of good agricultural standards suitable to Chinese national conditions, and then implement it vigorously and practically. For the approval of GAP bases, supervision should be strengthened to ensure that Chinese herbal medicines that do not meet the GAP standard do not flow into the circulation field. There should be an obvious GAP logo on all THM products. In addition to GAP certification of the enterprise itself, the enterprise standards of the product shall be formulated according to the relevant technical requirements and the necessary technical audit should be carried out. In addition, technical standards that are widely used by developed countries and prestigious enterprises should include technical barriers. As GAP medicine has the characteristics of stable and excellent product quality, the formulation of standards may be appropriately higher than the Pharmacopoeia standards, depending on the situation, so that the Chinese medicine industry has strong market competitiveness and lasting vitality.
4.4. Optimizing good cooperation mechanisms
The industrialized business model is the basis for establishing the THM material industrialized business objective model. The promulgated standards for THM are the rules followed by the target model. Under the guidance of these norms, the business model of THM industrialization has been established and developed, forming a relatively complete industrial chain including THM seedling breeding, THM planting, THM pest control, THM purchase, THM storage, and THM sales (Cao, 2018). At present, GAP-based industrialized business models include: market + farmer model, enterprise + middleman + farmer model, leading enterprise + farmer model (Hu, Zhang, & Zhang, 2007). In addition, the leading enterprise + farmer model can be refined into a leading enterprise + farmer simple model, a leading company + base + farmer model, and a leading company + industry association + farmer model. Most of them adopt the model of leading enterprises + farmers, combining production, teaching and research to form a chain of planting, processing, and sales, and then organically combine them. Enterprises, farmers or both establish an effective base management organization to implement standardized management of the entire process of THM production, including GAP technical training, the development of standard operating procedures, production according to standards, and supervision of GAP standard operating procedures. Enterprises and farmers in line with the principle of voluntary and mutual benefit and win–win cooperation, take loose, close cooperation, to help farmers develop the GAP requirements of the medical plant scale production. Use brand advantages to enhance the competitiveness of the THM market (Chu, 2019), increase efforts to cultivate the market vitality of technology companies in the value chain of THM planting, encourage research institutes to expand the market, increase school-enterprise cooperation, and increase the number of THM breeding and seedling enterprises, promote the participation and integration of the value chain of THM by science and technology enterprises, and enhance the value creation ability of science and technology enterprises (Li, Zheng, & Li, 2007). At present, research on the value chain related to medicinal plant cultivation has been initiated. By studying the impact of different value chain combinations on the final value of medicinal materials, the production method of medicinal materials can be gradually changed. At the same time, both enterprises and farmers have higher profit income (Chu, 2019).
4.5. Power of science guarantees quality of medicinal materials
Chinese social productivity, agricultural cultivation mechanization, and production equipment have been developed significantly, laying a solid foundation for the development of THM cultivation with decentralization to scale. The rapid development of science and technology promotes the application of ecological fertilizers and high-efficiency, low-toxicity pesticides in the cultivation of THM, thereby guaranteeing the scientific cultivation of THM (Wang et al., 2020). We recommend that basic scientific research, such as variety selection, cultivation technology, disease and pest control, chemical weeding, quality evaluation, resource protection, and related machinery development should be applied to the cultivation of THM. Through the application of scientific research units for national funding, the selection of species, land, seed, and seedling standards, laws of water and fertilizer demands, disease and pest control, and optimal harvesting should be emphasized as influencing factors of the period and age (Wang, Huang, & Wei, 2011). Strengthen the integration of production, education and research, take the road of “prospering medicine with science and technology”, study the key techniques of THM cultivation, improve the cultivation level, improve the scientific and cultural quality of pharmaceutical farmers through professional training, and play the leading role of scientific and technological support to achieve high-yield based on high quality (Chen, 2007).
4.6. Improving organization and operation mode of GAP
The most direct reason for the decline in the quality of medicinal materials is scattered and irregular production and management. In order to change the disorder of THM production and circulation and the decline in quality, the state will regard the industrialization management of THM as the central task for a period of time in the future (Lyv, 1999, Ran, 2005). Based on the independent production of thousands of households and driven by leading enterprises and various intermediary organizations, we have taken various forms such as the integration of trade, industry, and agriculture; the integration of production, supply and marketing, through the pre-production, mid-production, and post-production links of THM; combining THM production with the construction of THM industrial enterprise raw material bases and commodity drug enterprise supply bases, so that the integrated operation mode of the value chain of the THM industry can be GAP regulations The implementation of this lay a good industrial foundation (Gidey, Asfaw, & Giday, 2016).
5. Discussion and conclusion
The international trade of THM shows a steady upward trend in general, and the overseas development of THM has ushered in new opportunities, thanks to the active implementation of the “Going Global” strategy and the promotion of the “One Belt and One Road” construction. At the same time, with the introduction of relevant plans and policies, the development of THM has ushered in a good opportunity. The release of a series of special THM development plans and policy documents such as the “Outline of the Strategic Plan for the Development of THM (2016–2030) and the National Authentic Chinese Medicine Production Base Construction Plan (2018–2025)” means that the state supports the development of the THM industry and provides a good policy guarantee system. The development and opportunity of THM industry is in the best period in history. THM is the material basis for the inheritance and development of THM, and the construction of THM quality assurance system should be strengthened during the development of THM. GAP is the means to ensure the quality of THM, can standardize the site selection of the base, cultivation technology and harvest and processing problems, the implementation of GAP, for changing the existing Chinese medicinal material production and development dilemma is one of the important measures to achieve the standardization of THM (Qi & Wang, 2018). GAP is an important project to ensure the quality of THM. It aims to standardize the whole process of THM planting and production, and fundamentally guarantee the quality of THM. Judging from the current development of the THM industry, the road to GAP exploration is still very long.
There are other countries or regions in the world also have GACP management label, such as the United States, the European Union, the World Health Organization and so on. Each GA(C)PS has its own advantages. THM GAP is to ensure the quality of THM means, is the basis of stable and reliable quality of THM. The standardized planting and production of THM needs to rely on the construction of GAP base (Wu, 2004), but the technological content and management level of THM production base construction still needs to be improved, which makes it difficult to guarantee the quality of THM, because we should focus on the accumulation of experience, considering the output under the premise of ensuring quality (Deng, 2018). In order to achieve standardization, modernization, and internationalization, THM must take the road of sustainable development. The implementation of GAP specifications and the innovative application of technology are important guarantees (Gu, Jiang, & Wang, 2006). Even if my country cancelled GAP in 2016, the cancellation of GAP certification does not mean the cancellation of GAP cultivation, and supervision after the cancellation of GAP certification still needs to be strengthened. Cancellation of certification is one of the measures to streamline administration and delegate power. Although there are still some problems at this stage, as an effective means and necessary measures to control the quality of medicinal materials, we should speed up the implementation of GAP, explore how to implement GAP better and faster, and contribute to the health of the country’s people and the prosperity of the economy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by Ministry of Agriculture and Rural Affairs of the People's Republic of China (No. CARS-21); National Administration of Traditional Chinese Medicine (No. Finance Society [2019], 39); National Natural Science Foundation of China (No. M1942003); National Natural Science Foundation of China (No. M2042003).
Contributor Information
Heidi Heuberger, Email: Heidi.Heuberger@lfl.bayern.de.
Huan-ting Li, Email: huanting_li@yeah.net.
Min-hui Li, Email: prof_liminhui@yeah.net.
References
- Bai Y.L. Research on application strategy of agricultural technology extension in rural cultivation industry. South China Agriculture. 2020;14(14):15–16. [Google Scholar]
- Cao M.C., Huang T.K. Discussion on the restrictive factors and countermeasures of promoting GAP in my country. China Pharmaceuticals. 2015;24(22):11–14. [Google Scholar]
- Cao P. Research on the development of Chinese herbal medicine planting industry from the perspective of value chain. Knowledge Economy. 2018;09:67–68. [Google Scholar]
- Chen L. Southwest Petroleum University; 2007. Research on the industrialization management model of Chinese traditional medicine. [Google Scholar]
- Chu, L., (2019). Research on cost management of RX traditional herbal medicine enterprises based on value chain. East China Institute of Technology.
- Deng H.Q. Talking about the problems and countermeasures in the operation of the production base of Chinese medicinal materials. Modern Chinese Medicine. 2009;11(09):11–14. [Google Scholar]
- Deng Q.H., Huang Y., Xu Y.Y., Pan X.F. Problems and suggestions in the construction of GAP base of Chinese medicinal materials. Research and Practice on Chinese Medicines. 2013;27(06):3–6. [Google Scholar]
- Deng Y. The establishment of GAP system of medicinal materials needs efforts from all sides. Democracy and Legal Times. 2018;08–16(006) [Google Scholar]
- Du J.P., Ren W., Feng H.Q., Xia N.N. Analysis on the construction of Chinese traditional medicine production base based on GAP certification. Chinese Manipulation and Rehabilitation Medicine. 2020;11(22):14–16. [Google Scholar]
- Gidey M.H., Asfaw Z., Giday M. Value chain analysis of medicinal plants and the associated challenges. Journal of Medicinal Plants Studies. 2016;4(3):45–55. [Google Scholar]
- Gong J.W., Song J.Y., Yang Z.N. Current situation and demand analysis of talent team in traditional Chinese medicine planting industry. Shandong Journal of Traditional Chinese Medicine. 2011;30(10):736–738. [Google Scholar]
- Gu G.Q., Jiang C.Z., Wang X.F. Prospects of GAP came into effect in our country. Lishizhen Medicine and Materia Medica Research. 2006;17(5):877–878. [Google Scholar]
- Guo L.P., Zhang Y., Zhu S.D., Wang G.H., Wang X., Zhang X.B., et al. Good agricultural practice (GAP) of Chinese materia medica (CMM) for ten years: Achievements, problems and proposals. China Journal of Chinese Materia Medica. 2014;39(07):1143–1151. [PubMed] [Google Scholar]
- Han X.S. Discussion on the problems and measures of the industrialization development of Chinese medicinal materials under the background of agricultural economy. Shanxi Agricultural Economy. 2021;15:162–163. [Google Scholar]
- Heinrich M. Quality and safety of herbal medical products: Regulation and the need for quality assurance along the value chains. British Journal of Clinical Pharmacology. 2015;80(1):62–66. doi: 10.1111/bcp.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L., Zhang W.S., Zhang Z.J. Discussion on the development mode of Chinese herbal medicine cultivation enterprises under the supply chain management system. Modern Chinese Medicine. 2007;9(8):4–6. [Google Scholar]
- Hu X.J. Some Problems in GAP promotion and its countermeasures. Anhui Agricultural Science Bulletin. 2009;15(23):10–11. [Google Scholar]
- Kang C.Z., Lyu C.G., Huang L.Q., Wang S., Guo L.P. Pattern and matching technology of ecological cultivation for Chinese materia medica based on system level. China Journal of Chinese Materia Medica. 2020;45(09):1975–1981. doi: 10.19540/j.cnki.cjcmm.20200302.102. [DOI] [PubMed] [Google Scholar]
- Li H., Mou R. The development status and analysis of Chinese medicine related policies and regulations. Journal of Traditional Chinese Medicine Management. 2019;27(11):11–14. [Google Scholar]
- Li L.Y. Advances in standardized cultivation of Chinese medicinal materials and GAP certification. Chongqing Journal of Research on Chinese Drugs and Herbs. 2012;1:43–54. [Google Scholar]
- Li Q.X., Zheng S.F., Li R.Q. Study on the characteristics of herb medicine industry chain and counter measures for it development. Chinese Journal of Agricultural Resources and Regional Planning. 2007;28(02):47–51. [Google Scholar]
- Li Y.P., Liang Y.S., Ouyang P.Y. Exploration and practice of innovation and entrepreneurship talent training mode with traditional herbal medicine cultivation base as a pilot project. Vocational Technology. 2019;18(03):31–34. [Google Scholar]
- Liang D.J., Zhao X.L. Discusses the Good Agricultural Practice for Chinese crude drugs (GAP) implementation. Chinese Journal of Modern Drug Application. 2014;8(2):252. [Google Scholar]
- Lin H.M. Comparision of GAP implementation in China and abroad and base construction of Chinese medicinal materials. Modernization of Traditional Chinese Medicine and Materia Medica-World Science and Technology. 2002;4(6):62–65. [Google Scholar]
- Lin H.M. Chinese traditional medicine GAP and Some problems in base building. Gansu Agricultural Science and Technology. 2003;01:54–56. [Google Scholar]
- Liu J.H., Chen W.J., Wang J.L., Liu H.B. The present situation and development countermeasure of traditional Chinese medicine industry in Gansu Province. Gansu Agricultural Science and Technology. 2021;52(07):81–85. [Google Scholar]
- Liu T.T. Problems and suggestions in standardized cultivation of traditional herbal medicine. Contemporary Horticulture. 2020;43(12):197–198. [Google Scholar]
- Lyv Z.K. It is imperative to implement industrialized management of Chinese medicinal materials. Chinese Medicine Research and Information. 1999;01:20–21. [Google Scholar]
- Qi M., Wang X.W. Opportunities, challenges and solutions of internationalization of traditional Chinese medicine based on “Belt and Road” strategy. Medicine and Society. 2018;31(04):49–51. [Google Scholar]
- Ran M.X. Exploration of Guizhou traditional Chinese medicine production and GAP base management model. Modernization of Traditional Chinese Medicine and Materia Medica-World Science and Technology. 2005;7(02):115–120. [Google Scholar]
- Shi W.P. Research on the effects and restrictive factors of GAP development in my country. Quality and Safety of Agro-Products. 2010;2010(4):52–54. [Google Scholar]
- Song X.L., Wang L.B. Current status and prospects of GAP base construction of Chinese medicinal materials. Forest By-Product and Speciality in China. 2014;04:107–109. [Google Scholar]
- Song Y.J., Lu Y.B., Wang X.X., Wang S.X., Wang Y. Literature analysis and prospect of traditional Chinese medicine in the treatment of COVID-19. Pharmacology and Clinics of Chinese Materia Medica. 2020;36(01):18–22. [Google Scholar]
- Wang F.C., Shen B.X., He C.Y., Zhen W.C., Nie S.L. Clinical efficacy of Lianhua Qingwen granules and its mechanism on COVID-19 based on network pharmacology. Pharmacology and Clinics of Chinese Materia Medica. 2020;36(02):93–101. [Google Scholar]
- Wang H.D., Huang J.Y., Wei Y.F. The status of the GAP base construction of Chinese pharmacy. Research and Practice on Chinese Medicines. 2011;25(04):6–8. [Google Scholar]
- Wang H.S., Lin Q.Q., Xu H.L., Wu S.S. Development status and suggestions on the GAP nase of Chinese medicinal materials in Fujian Province. Research and Practice on Chinese Medicines. 2014;28(03):5–7. [Google Scholar]
- Wang J.Q., Zhang A., Liu Y.L., Lu D.G., Zhang B., Cui J.H. Countermeasures and proposals for implementation of GAP normalized cultivation of Chinese medicinal materials. Modernization of Traditional Chinese Medicine and Materia Medica-World Science and Technology. 2005;7(4):74–77. [Google Scholar]
- Wen Q. Henan University; 2014. Comparison of GAP between China and foreign countries and my country's GAP development countermeasures. [Google Scholar]
- Wu J. Interpretation of the World Health Organization GACP Guidelines for medicinal plants. Chinese Journal of Information on Traditional Chinese Medicine. 2004;11(10):937–939. [Google Scholar]
- Xiao X.Y., Zhang N.P., Lin R.C. Promote the development of GAP supervising system of Chinese materia medica. Chinese Pharmaceutical Affairs. 2006;20(11):664–666. [Google Scholar]
- Yang F.J., Chen H.Y. Research status and strategy on endangered wild animal resources substitutes of traditional herbal medicine herbs. Chinese Medicine Modern Distance Education of China. 2019;17(16):47–49. [Google Scholar]
- Yang G., Guo L.P., Zhou X.T., Huang L.Q. Analysis of several key problems of good agricultural practice gap of Chinese medicinal materials. China Journal of Chinese Materia Medica. 2016;41(07):1173–1177. doi: 10.4268/cjcmm20160702. [DOI] [PubMed] [Google Scholar]
- Zhang B.G., Qi Y.D., Liu H.T., Jia X.Y. Discussion on the specification for quality control of cultivation and harvesting Chinese medicinal materials GACP. Modern Chinese Medicine. 2011;13(06):11–13. [Google Scholar]
- Zhang J., Wider B., Shang H., Li X., Ernst E. Quality of herbal medicines: Challenges and solutions. Complementary Therapies in Medicine. 2012;20(1-2):100–106. doi: 10.1016/j.ctim.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Zhang M., Wang C., Zhang R.u., Chen Y., Zhang C., Heidi H., et al. Comparison of the guidelines on good agricultural and collection practices in herbal medicine of the European Union, China, the WHO, and the United States of America. Pharmacological Research. 2021;167:105533. doi: 10.1016/j.phrs.2021.105533. [DOI] [PubMed] [Google Scholar]
- Zhang Q.X., Ding N. Implementation of GAP, production of “green” Chinese medicine to achieve modernization of Traditional Herbal Medicine. Chinese Traditional and Herbal Drugs. 2003;34(8):120–121. [Google Scholar]
- Zhang Z.L., Huang Y.L. Overview of the development of traditional Chinese medicine industry in Fujian and Taiwan. Strait Pharmaceutical Journal. 2020;32(07):43–46. [Google Scholar]
- Zhao J., Yang G.Y. Improving planting technology to expand the planting area of Mongolian medicinal materials in less developed areas with “more people and less land”. Journal of Medicine & Pharmacy of Chinese Minorities. 2019;25(10):53–54. [Google Scholar]
- Zhao Y.T. Strengthening the construction of GAP base for authentic medicinal materials is an effective way to improve the quality of Chinese medicinal materials. Journal of Traditional Chinese Medicine Management. 2017;25(20):15–17. [Google Scholar]
- Zhu L. Challenges, opportunities and promoting strategies for the international development of my country's traditional herbal medicine. Practice in Foreign Economic Relations and Trade. 2020;11:57–60. [Google Scholar]
- Zhu S.D., Peng H.S., Guo L.P., Xu T.R., Zhang Y., Chen M.L., et al. Regionalization of Chinese material medical quality based on maximum entropy model: A case study of Atractylodes lancea. Scientific Reports. 2017;7:42417. doi: 10.1038/srep42417. [DOI] [PMC free article] [PubMed] [Google Scholar]