Abstract
Objective
Mulberry (Morus spp.) fruits and leaves have been proven to possess nutraceutical properties. Due to its fast and easy growing characteristics, mulberry fruits (MF) and leaves (ML) potentially emerge as a great source of functional foods. This study aims to enhance bioactivities (antioxidant, anti-inflammation, and hypoglycemic activity) of MF and ML via submerged fermentation using bacteria (Lactobacillus plantarum TAR 4), yeast (Baker’s yeast and red yeast) and fungi (Tempeh and Tapai starter).
Methods
In this study, 25% (mass to volume ratio) of MF and ML were fermented (48 h) with 1% (mass to volume ratio) of different microbial cultures, respectively. Effects of different fermentations on MF and ML were determined based on the changes of total phenolics (TPC), flavonoids (TFC), anthocyanins, total sugar, DPPH activity, ferric reducing antioxidant power (FRAP), albumin denaturation inhibition activity (ADI), anti-lipoxygenase activity and α-amylase inhibition activity (AI).
Results
Generally, ML had higher AI than MF. However, MF exhibited higher DPPH, FRAP and anti-lipoxygenase activity than ML. After all forms of fermentation, DPPH and AI activity of MF and ML were increased significantly (P < 0.05). However, the effects of fermentation on TPC, FRAP, ADI and anti-lipoxygenase activity of MF were in contrast with ML. TPC, FRAP and anti-lipoxygenase activity of ML were enhanced, but reduced in MF after fermentation. Although the effects exerted by different microorganisms in MF and ML fermentation were different, the bioactivities of MF and ML were generally improved after fermentation. Fermentation by Tempeh starter enhanced TPC (by 2-fold), FRAP (by 2.3-fold), AI (at 10% increment) and anti-lipoxygenase activity (by 5-fold) of ML, whereas Tapai fermentation effectively enhanced the DPPH (at 17% increment) and ADI (by 2-fold) activity of MF.
Conclusion
Findings of this study provide an insight into the future process design of MF and ML processing into novel functional foods.
Keywords: anti-inflammation activity, antioxidant activity, fermentation, hypoglycemic activity, mulberry fruits, mulberry leaves
1. Introduction
Mulberry (Morus spp.), a plant of the Moraceae family has attracted interest from the community recently due to its high economy, nutraceutical, and medicinal values. Mulberry tree is a plant that grows easily. Due to its good tolerance and adaptability to harsh environment, it is widely cultivated throughout the world, ranging from tropical to temperate regions in different soil conditions (Krishna et al., 2020, Wani et al., 2017). A mulberry tree grown from seed takes approximately 10 years to bear fruits and the fruits spend around 2–4 weeks to ripe. The ripe fruits vary in white, red, purple, and black color depending on the species. The high moisture (70.0%−87.4%) and slight acidic pH (3.20–5.33) of the fruits contributed to the sweet–sour juiciness mouthfeel (Imran et al., 2010, Liang et al., 2012).
Numerous studies have proven that mulberry fruit is a good source of phenolics and flavonoids, which contributed to the antioxidant, anti-diabetic, anti-obesity, anti-tumor, anti-neuroinflammatory and anti-aging potential (Ahmed et al., 2016, Huang et al., 2013, Turan et al., 2017, Wang et al., 2013, Xu et al., 2020). The major phytochemicals in mulberry fruits are quercetin, kaempferol, chlorogenic acid, cinnamic acid, p-hydroxybenzoic acid, protocatechuic acid and vanillic acid (Sánchez-Salcedo, Mena, García-Viguera, Martínez, & Hernández, 2015a, Xu et al., 2020). In the study by Yu, Lim, Lee, Choi, and Kim (2021), ethanolic extract of mulberry fruits have proven to exhibit protective effects against oxidative stress and inflammatory responses in RAW 264.7 macrophages. Besides phenolics and flavonoids, other compounds such as loliolide, odisolane indole and its derivatives, which are the common pharmaceutical agents, were also detected in the extract. In addition, Xu et al. (2020) had also successfully identified 50 compounds, including the three new compounds in the methanolic extract of mulberry fruits. These compounds had been proven to significantly inhibit nitric oxide (NO) production induced by lipopolysaccharides (LPS), reduce expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), as well as block LPS-induced nuclear translocation of NF-ĸB. Complex interactions between the chemical constituents are postulated as the factors that contribute to the nutraceutical properties of mulberry fruits. Furthermore, Xu et al. (2020) also recommended that a reasonable consumption of mulberry fruits could prevent and reduce the prevalence of neuroinflammatory and oxidative related disorders. However, the chemical composition and nutritional value of mulberry fruits differ among cultivars. Each cultivar has its own nutrition strength (Liang et al., 2012). Thus, mulberry fruits are claimed to have great potential to be developed into novel nutritious raw ingredient for diverse health food applications.
For over 5000 years, mulberry leaves are widely served as the food source for silkworm in the silk industry and ruminant feed due to its high protein and fiber content. Mulberry leaves were found to contain approximately 27%−37% (dry weight) of protein and 11%−17% (dry weight) of crude fiber, which are significantly higher than most green leafy vegetables (Yu et al., 2018). Besides that, mulberry leaves are also widely used in traditional Chinese medicine as folk remedy to treat fever, cold, high blood pressure, diabetes mellitus, liver, and kidney damage, as well as other chronic diseases (Huang et al., 2013, Thaipitakwong et al., 2018, Yu et al., 2018). Mulberry leaves are proven rich in various phytochemicals. The most abundant phenolic acid in mulberry leaf is chlorogenic acid, while isoquercitrin is the major flavonol glycoside (Sánchez-Salcedo et al., 2015, Yu et al., 2018). 1-Deoxynojirimycin (DNJ) is the main polyhydroxyalkaloid in mulberry leaves that is well-known for its hypoglycemic property. DNJ has a structure similar to glucose which effectively inhibits intestinal ɑ-glucosidase activity, therefore control postprandial blood glucose level in human (Gao et al., 2016). DNJ content varies among mulberry leaf varieties. The China varieties were reported to contain 0.08 to 1.12 mg/g dry weight of DNJ whereas the Thailand varieties ranged from 0.3 to 1.7 mg/g dry weight (Vichasilp et al., 2012, Yu et al., 2018). In addition, DNJ also exhibits lipid-lowering activity, inhibits growth of colon cancerous cells and promotes growth of beneficial gut microbes (Hu et al., 2019, Kojima et al., 2010, Shuang et al., 2017). Furthermore, γ-aminobutyric acid detected in the mulberry leaves has also been proven to present anti-hypertensive and anti-fatigue properties (Chen et al., 2016, Yang et al., 2012). With the excellent nutraceutical benefits of mulberry fruits and leaves, both are foreseen to be the potential health-promoting ingredients for functional foods development.
In the 21st century, consumer demand for foods no longer just for their satiety. Consumers are seeking for convenient, good tasting, healthy, nutritious, and value-added foods. To fulfill the present trend and demand for foods, there is a need to develop more economical and green processing technologies to improve nutritional quality of food materials. Fermentation has been long applied in food processing to diversify food variety, prolong shelf life, as well as enhance nutritional and organoleptic properties of foods, in a cost-effective manner. In recent years, effect of fermentation on various fruits such as grape, blueberry, apple, mango, and red dragon fruit have also been widely investigated. Fermented fruits were proven to exhibit higher bioactivities such as antioxidant, anti-inflammation, hypoglycemic, etc. (Ayed et al., 2020, Choo et al., 2018). Nevertheless, limited study was conducted to determine the effects of fermentation on the bioactive constituents and bioactive properties of mulberry fruits and leaves. To date, research on fermentation of mulberry fruits and leaves was mostly limited to the fruit juice fermentation and solid-state fermentation of the leaves (Chaudhary et al., 2019, Wang et al., 2015, Zheng et al., 2014). Effect of fermentation on the recovery of bioactive constituents and bioactivities of mulberry fruits and leaves have yet to be explored. Therefore, this study was designed to evaluate the effect of submerged fermentation using bacteria (Lactobacillus plantarum TAR 4), yeast (Baker’s yeast and red yeast) and fungi (Tempeh and Tapai starter) on the recovery bioactive compounds, and hence the bioactivities of mulberry fruits (MF) and mulberry leaves (ML). Effect of fermentation using different microorganisms on the antioxidant, anti-inflammation and hypoglycemic activities of MF and ML were determined. This study provides an insight into the setup of fermentation technology for future functional foods development using mulberry fruits and leaves.
2. Materials and methods
2.1. Materials
Dried mulberry fruits (Morus sp.) (origin: Xinjiang, China) used in this experiment were purchased from a local store, whereas the fresh mulberry leaves were collected from the mulberry trees planted in the garden of Tunku Abdul Rahman University College, Kuala Lumpur, Malaysia. The fresh leaves harvested were washed, then dried in a vacuum oven under pressure 5 kPa at 40 °C for 12 h. The dried leaves and fruits were ground into coarse powder by using a knife mill. Both dried mulberry fruits and leaves were kept refrigerated at 4 °C until further usage.
Lactic acid bacteria (LAB) used in this experiment was an isolated strain Lactobacillus plantarum TAR4 (NCBI GenBank database under the accession numbers MH012173). The LAB was grown in MRS broth (Oxoid, England) and the live cells were harvested after 48 h through centrifugation at 6373 × g for 5 min. Commercial food grade Baker’s yeast (Saccharomyces cerevisiae), red yeast (Monascus purpureus), Tempeh (Rhizopus oligosporus) and Tapai starter were purchased from the local market. Tapai starter (a.k.a ragi) was a traditional starter inoculum that was widely available in local market for Tapai production. It was a mixed culture comprised of diverse range of bacteria, yeast, and fungal species, include Amylomyces rouxii, Endomyces fibuligera, Chlamydomocular oryzae, Candida pelliculosa, Saccharomyces cerevisiae, Hansenula anomala, Bacillus sp. and Acetobacter sp. (Law, Abu Bakar, Mat Hashim, & Abdul Hamid, 2011).
Chemicals used include Folin-Ciocalteu reagent, sodium carbonate, gallic acid, quercetin, sodium hydroxide, methanol, sulfuric acid, ferrous sulfate, sodium acetate, ferric chloride, ascorbic acid, 2,4,6-tripyridyl-s-triazine (TPTZ), 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical, albumin, etc. All chemicals used were analytical grade with the brand name of Sigma (U.S.), Merck (Germany) and Chemsoln (Malaysia).
2.2. Fermentation
All the glassware and utilities were sterilized in boiling water for 5 min before substrate preparation. About 25% (mass to volume) of mulberry leaves (ML) and fruits (MF) were prepared by completely soaking the ML and MF in distilled water. Next, about 1% (mass to volume) of microbial culture (LAB, Baker’s yeast, red yeast, Tempeh and Tapai starter) was inoculated separately into ML and MF, respectively. Fermentation was carried out in the screw-capped Scott bottle. LAB fermentation was conducted at 37 °C in an incubator, whereas fermentation by Baker’s yeast, red yeast, Tapai and Tempeh starter were carried out at ambient temperature (25 °C). Both ML and MF were fermented for 48 h. Experimental control was carried out by incubating the ML and MF without any inoculation under the same condition.
2.3. Extraction
About 100 mL of distilled water was added into the fermented ML and MF, followed by a 1 h extraction by using an ultrasonicator (40 kHz and 500 W) at 50 °C. The ML and MF juices were obtained via pressing using 2-layers cheese cloth.
2.4. Chemical content determination
Total phenolics (TPC) and flavonoids (TFC) content of ML and MF juices were determined according to methods described by Wang et al. (2015). Gallic acid and quercetin were used as the calibration standard for TPC and TFC, respectively. Total anthocyanins content was determined through pH differential method described by Elisia, Hu, Popovich, and Kitts (2007). Total sugar content was determined according to Dubois method (Masuko et al., 2005).
2.5. Bioactivity characterization
DPPH scavenging activity of ML and MF juices was determined according to method Wang et al. (2015) with slight modifications. Briefly, 100 µL of sample was mixed with 3.9 mL of 0.6 mmol/L DPPH in methanol, then stand in dark at room temperature for 30 min. Absorbance of the mixture was measured at wavelength 517 nm. Methanol was used as the control. DPPH free radical scavenging activity was calculated using formula:
| (Acontrol − Asample)/Acontrol × 100% |
where Acontrol and Asample indicate absorbance of control and sample, respectively.
Ferric reducing antioxidant power (FRAP) of ML and MF juices was determined according to method Fernandes et al. (2016). Anti-inflammatory activity was evaluated based on albumin denaturation inhibition (ADI) and anti-lipoxygenase activities according to procedures Rashid and Shafi (2018), whereas hypoglycemic activity was determined based on α-amylase inhibition (AI) activity (Abdul & Kasim, 2017).
2.6. Statistical analysis
All experiments were carried out in triplicates and the results were expressed as mean ± standard deviation. The statistical difference between data was determined via one-way ANOVA and LSD test. The results were considered significant when P < 0.05. Besides, correlation between the studied factors was determined via Pearson correlation analysis. All statistical analysis was conducted by using SPSS software version 20 (IBM, New York).
3. Results and discussion
3.1. Effect of fermentation using different microorganisms on chemical changes in ML and MF
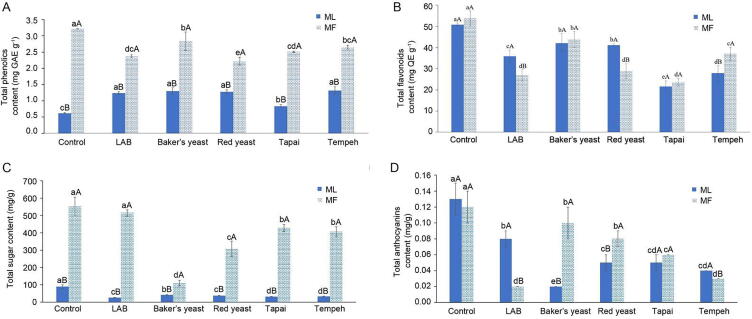
Fig. 1 showed the effect of fermentation using different microorganisms on the changes of total phenolics (TPC), flavonoids (TFC), sugar and anthocyanins content in ML and MF. Results indicated that MF juice contained higher phenolics and sugar than ML juice. However, their total flavonoids and anthocyanins content were not significant difference (P > 0.05). ML juice was found to contain (0.62 ± 0.02) mg GAE g−1 of phenolics, (50.85 ± 0.75) mg QE g−1 of flavonoids, (0.13 ± 0.02) mg/g of anthocyanins and (89.33 ± 11.22) mg/g of sugar, whereas MF juice contains (3.21 ± 0.01) mg GAE g−1 of phenolics, (53.85 ± 3.44) mg QE g−1 of flavonoids, (0.12 ± 0.02) mg/g of anthocyanins and (553.23 ± 52.08) mg/g of sugar. A higher total sugar content in MF juice as compared to ML juice was expected, because simple sugars in fruits were always higher than leaf. According to Wang et al. (2015), MF contained 75–118 g/L of reducing sugars, at which 34–66 g/L was glucose and 38–73 g/L was fructose. In the study by Espada-Bellido et al. (2017), about 1.3 mg/g of phenolics and 0.2 mg/g of anthocyanins was successfully extracted from MF via ultrasound-assisted technique. Gao et al. (2020) successfully extracted 8.352 mg/g of phenolics from ML via microwave-assisted deep eutectic solvent extraction technique. Chemical composition of an extract was greatly affected by the selected extraction method. Efficiency of an extraction method was influenced by the penetration of solvent, rate of mass transfer and solubility of the targeted compounds in the extraction solvent (Espada-Bellido et al., 2017). Therefore, lower TPC in ML juice compared with MF juice might be due to the limitation of the extraction method employed. The extraction efficiency might be higher on the fruits which had a softer texture than the waxy leaves.
Fig. 1.
Effect of fermentation using different types of microorganisms (LAB, Baker’s yeast, red yeast, Tapai and Tempeh) on the changes of total phenolics (TPC) (A), total flavonoids (TFC) (B), total anthocyanins (C), and total sugar of mulberry leaves (ML) and fruits (MF) (D) (mean ± SD, n = 3).
After fermentation, all chemical components (TPC, TFC, anthocyanins and sugar) of MF and ML juices were reduced significantly (P < 0.05), except TPC of ML juice. TPC of ML juice was increased significantly (P < 0.05) after fermentation. TPC of ML juice (0.62 ± 0.02 mg GAE g−1) was doubled (1.2–1.3 mg GAE g−1) after fermentation by red yeast, Baker’s yeast, LAB and Tempeh starter. The results were in accordance with study by Wang, Luo, Wu, Liu, and Wu (2018). According to the study, TPC of guava leaves increased after fermentation by Monascus anka and Saccharomyces cerevisiae. Besides, study by Degrain, Manhivi, Remize, Garcia, and Sivakumar (2020) also revealed that total phenolic acids of African nightshade leaves was increased by 1.3–1.4 times after lactic acid bacteria fermentation. Furthermore, Xiao et al. (2016) reported that Rhizopus oligosporus fermentation significantly enhanced total phenolics and isoflavone aglycone content of soybean. During fermentation, several carbohydrate-hydrolyzing enzymes, such as cellulase, estarase, xylanase and β-glucosidase are secreted by the microorganisms. These enzymes catalyze the hydrolysis of covalent bonds between the phenolics and lignocellulose in the plant cell wall matrix, hence release them into the extract. Therefore, both free and bound form of phenolics in the extract will be enhanced after fermentation (Thai, Camp, Smagghe, & Raes, 2014). According to Guo et al. (2020), cellulase and β-glycosidase activities were increased significantly during mulberry leaves fermentation. The hydrolytic action of these enzymes promotes cell wall break down, and subsequently dissociates the phenolics. However, the levels of enzymes secreted by the microorganisms during fermentation differ with the fermentation stage. At the early stage of fermentation, the microorganisms secreted enzymes such as amylase which dissociate nutrients from the substrate for its growth. Amylase activity was also proven highly correlated with the rate of liberation of soluble phenolics during fermentation (Bei, Chen, Lu, Wu, & Wu, 2018). However, high sugar level in MF juice may cease the amylase secretion by the microorganisms because the fermentation medium had supply adequate carbon source to support microorganism growth. Furthermore, fast growth rate of microorganisms with the high supply of carbon source might cause over-fermentation of MF, thus causing TPC and TFC reduction (Wang, Luo, Wu, & Wu, 2018). Nevertheless, further study needs to be conducted to determine the carbohydrate hydrolase activity and microbial growth rate in the extract to verify this hypothesis. Among the fermented MF, MF fermented with red yeast and LAB experienced the most drastic TPC and TFC reduction, which were approximately 30% and 50%, respectively. Although fermentation always associated with increased TPC and TFC yield, the recovery of TPC and TFC might also be reduced during fermentation if the soluble TPC and TFC bind with other soluble constituents in fermentation medium due to prolong fermentation, shifting of metabolic pathway of fermenting microorganisms to metabolize phenolics and degradation/hydrolysis of soluble TPC and TFC by the microbial enzymes (Adebo & Medina-Meza, 2020).
Sugar is the main carbon source for microorganisms during fermentation. Significant reduction of total sugar in MF and ML postulates microbial growth in the medium during fermentation. Among the samples, LAB fermentation in MF did not cause significant reduction of total sugar as compared with the unfermented MF. In contrary, LAB fermentation in ML caused substantial total sugar reduction. Among the fermented samples, LAB-fermented ML contained the lowest total sugar content [(25.53 ± 1.71) mg/g]. In study by Chaudhary et al. (2019), L. plantarum was proven to grow well in mulberry fruit juice. The unchanged total sugar content in MF after 48 h fermentation might be due to the action of amylase enzyme on carbohydrate breakdown in MF during fermentation (Plaza-Vinuesa, Hernandez-Hernandez, Moreno, Rivas, & Muñoz, 2019). Sugars released from the carbohydrate breakdown had countered sugar consumption by L. plantarum during fermentation. In a Pearson correlation analysis, total sugar content in fermented ML was found negatively correlated with TPC (r = −0.695 at P < 0.01), but positively correlated with TFC (r = 0.695 at P < 0.01) and anthocyanin (r = 0.731 at P < 0.01). This finding proposes that microbial growth in ML facilitates the release of phenolics, but concurrently promotes the degradation of flavonoids and anthocyanins during fermentation. Among the fermented samples, Baker’s yeast fermented MF contained the lowest total sugar content [(110.10 ± 16.09) mg/g]. Total sugar in MF was reduced by about 5-fold after Baker’s yeast fermentation. Results of Pearson correlation analysis revealed that there was no significant correlation between total sugar content in the fermented MF with its TPC, TFC and anthocyanins yield. This finding unveiled that microbial growth in MF may not be the only leading factors that caused the changes of phenolics, flavonoids and anthocyanins in MF during fermentation.
According to Fig. 1D, anthocyanins content in MF and ML juice (0.12–0.13 mg/g) had no significant difference (P > 0.05). However, LAB and Tempeh starter fermentation on MF and Baker’s yeast fermentation on ML had drastically reduced the anthocyanins content by about 80% to 0.02–0.03 mg/g. According to Zheng et al. (2014), release of hydrogen peroxide as the metabolite of LAB fermentation is the leading cause of anthocyanins degradation. Besides, pH change and action of microbial hydrolytic enzymes during fermentation are among the factors accelerate anthocyanins degradation (Kokkinomagoulos et al., 2020, Mushollaeni and Tantalu, 2020). In addition, anthocyanins will also react with the metabolites released during yeast fermentation, resulting in the formation of pyranoanthocyanins. The red–orange color pyranoanthocyanins are less sensitive to pH, hence less likely to be quantified through pH differential method (Ruta & Farcasanu, 2019). Moreover, anthocyanins in the fermentation medium may also be adsorbed on the yeast cell walls, thus reduces the anthocyanins content in the extract (Echeverrigaray et al., 2020, Hornedo-Ortega et al., 2017).
3.2. Effect of fermentation using different microorganisms on bioactivity changes of ML and MF
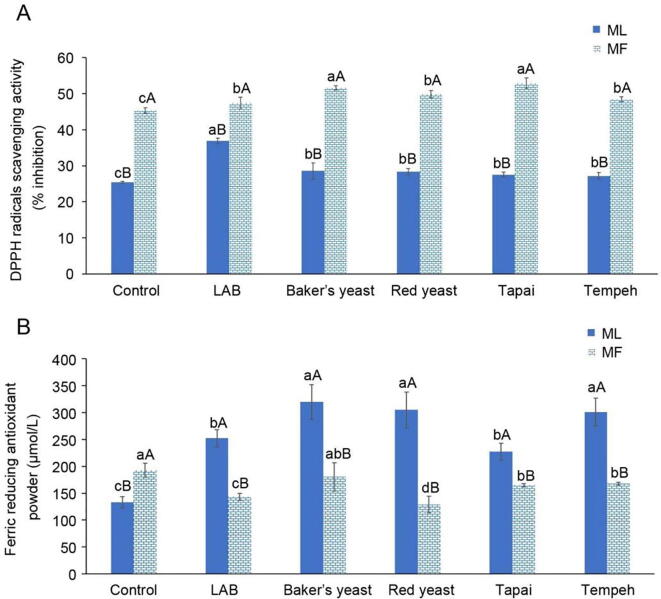
Fig. 2 indicated the effect of fermentation using different microorganisms on free radical scavenging activity (DPPH) and ferric reducing antioxidant power (FRAP) of ML and MF juices. The results showed that MF juice exhibited higher DPPH activity and FRAP than ML juice. DPPH activity of both ML and MF juices was increased significantly (P < 0.05) after fermentation by all types of microorganisms. DPPH activity of MF juice was increased from about 45% to 51%−53% after fermentation by Baker’s yeast and Tapai starter. For ML, LAB fermentation was the best to enhance DPPH activity. DPPH activity of ML juice was increased by about 45% from (25.43 ± 0.22)% to (36.90 ± 0.76)% after fermentation. However, the DPPH activity was not correlated with its TPC, TFC and anthocyanin content. This finding postulated that there might be other antioxidant compounds, other than phenolics, flavonoids and anthocyanins were present in the fermented ML and MF which contributed to DPPH activity. This hypothesis was supported by the previous report by Guo et al., 2020, Kwaw et al., 2018. In their study, DPPH activity of mulberry fruits and leaves was increased after fermentation, but the activity had a very weak correlation with its TPC, TFC and anthocyanin. According to Hur et al., 2014, Iqbal et al., 2012, compounds such as ascorbic acid, peptide, amino acids, and fiber were also contribute to DPPH free radical scavenging activity. Antioxidant compounds were not limited to phenolic compounds, flavonoids, or anthocyanins only. Furthermore, Adesulu-Dahunsi, Jeyaram, Sanni, & Banwo, 2018, Wang et al., 2017 also revealed that exopolysaccharides secreted by L. plantarum during fermentation also exhibited free radicals scavenging and ferrous ion chelating activities in a dose-dependent manner. However, an opposite trend was observed in FRAP of MF juice. Fermentation caused reduction of FRAP in MF juice. FRAP of ML juice (133.47 ± 10.54 µmol/ml) was increased by about 2.4-folds after Baker’s yeast, Tempeh starter and red yeast fermentation. However, the same fermentation caused 6.5% (Baker’s yeast), 13% (Tempeh starter) and 33% (red yeast) of FRAP reduction, respectively in MF juice. The increase of TPC released during fermentation due to the action of carbohydrate and cell wall degrading enzymes were believed to be the cause of the increment of FRAP in ML (Guo et al., 2020). This hypothesis was supported by the results of Pearson correlation analysis, in which the FRAP of ML was found correlated with its TPC (r = 0.900 at P < 0.01). Furthermore, Yu et al. (2018) also proved that chlorogenic acid isolated from the mulberry leaves exhibited a strong positive correlation with FRAP. Although study by Abd Razak et al., 2015, Kwaw et al., 2018 found that Rhizopus oligosporus and Monascus purpureus fermentation on rice bran and lactic acid bacteria fermentation on mulberry fruit juice enhanced FRAP of both products, results of this study were not in line with their studies. Results of Pearson correlation analysis in this study unveils that FRAP of MF juice was correlated with its TPC (r = 0.724 at P < 0.01) and TFC (r = 0.653 at P < 0.01), while fermentation was proven to cause a significant reduction in TPC, TFC and anthocyanins of MF. Thus, FRAP reduction after fermentation in MF was within expectation.
Fig. 2.
Effect of fermentation using different types of microorganisms (LAB, Baker’s yeast, red yeast, Tapai and Tempeh) on the changes of (A) DPPH scavenging activity and (B) ferric reducing antioxidant power (FRAP) of mulberry leaves (ML) and fruits (MF) (mean ± SD, n = 3).
Moreover, fermentation had successfully turn ML became a better source FRAP than MF. FRAP of all fermented ML juices was significantly (P < 0.05) higher than MF juices. Generally, FRAP was always positively correlated with TPC and TFC due to their strong reducing power, thereby forming stable compounds. However, the correlation between FRAP and TPC/TFC was not always compatible under all circumstances. Not all phenolics and flavonoids exhibit equally strong reducing power (Kwaw et al., 2018, Wang et al., 2015, Yu et al., 2018). In study by Yu et al. (2018), FRAP of ML was greatly affected by its rutin and isoquercitrin content, whereas quercetin-malonyl-glucoside and kaempferol-malonyl-glucoside were contributed to FRAP weakly. Sample varieties and species, types of microorganisms used, fermentation conditions and extraction methods were the factors that cause different post-fermentation changes in chemical profile and antioxidant activity (Hur et al., 2014).
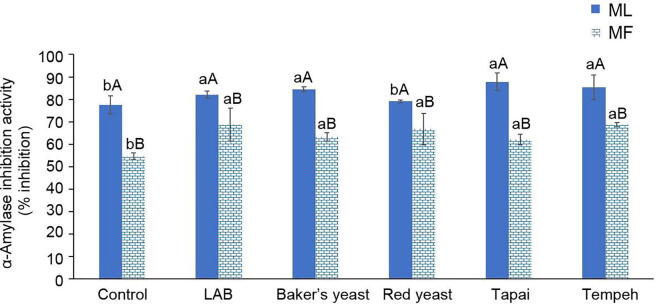
Fig. 3 demonstrated the effect of fermentation using different microorganisms on α-amylase inhibition activity of ML and MF juices. ML juice contained higher α-amylase inhibition activity than MF juice. α-amylase inhibition activity of both ML and MF was increased significantly (P < 0.05) after fermentation except red yeast-fermented ML. Red yeast fermentation did not cause significantly change (P > 0.05) in α-amylase inhibition activity of ML. Inhibiting carbohydrate-hydrolyzing enzymes such as α-amylase and α-glucosidase activity has been long adopted as one of the effective therapeutic approaches to monitor type 2 diabetes. Enzyme inhibition led to the delay in carbohydrate digestion and absorption, hence retard the substantial rise of postprandial blood glucose level (Adisakwattana, Ruengsamran, Kampa, & Sompong, 2012). Many studies have proven the remarkable effectiveness of fermentation in enhancing hypoglycemic property of foods. Shori (2020) proved that α-amylase inhibition activity of herbal fortified yogurt was five times higher than the plain yogurt, even after two weeks of storage. The fermented wheat sourdough bread had higher α-amylase inhibition activity than the common yeast bread (Diowksz, Malik, Jaśniewska, & Leszczyńska, 2020). Apart from this, Guo et al. (2020) found that hypoglycemic potential of mulberry fruits had been enhanced after Monascus anka fermentation. The α-glucosidase inhibition activity was increased by three-folds after fermentation. Results of Pearson correlation analysis reveals that α-amylase inhibition activity of MF was negatively correlated its TPC (r = −0.499 at P < 0.05), TFC (r = −0.551 at P < 0.05) and anthocyanins content (r = −0.639 at P < 0.01). This finding suggested that α-amylase inhibition activity of MF was not due to the phenolics, flavonoids and anthocyanins contained in it. High TPC, TFC and anthocyanins content in MF might slightly diminish its α-amylase inhibition activity. According to Jeong et al., 2014, Tian et al., 2016, anti-diabetic potential of mulberry leaves is contributed by its 1-deoxynojirimycin, fagomine, flavonoid and polysaccharide content. TPC and TFC were not always positively correlated with α-amylase inhibition activity because of the compound species and their interactive effects (Xiong et al., 2020). The configuration and polarity of phenolic compounds may also affect the ability of the compounds to bind to the active site of α-amylase enzyme (Li et al., 2018). Moreover, negative correlation between TPC and TFC in the plant extracts with their inhibitory effect toward α-amylase enzyme was also reported by Hyun et al., 2018, Loh and Hadira, 2011.
Fig. 3.
Effect of fermentation using different types of microorganisms (LAB, Baker’s yeast, red yeast, Tapai and Tempeh) on changes of α-amylase inhibition activity of mulberry leaves (ML) and fruits (MF) (mean ± SD, n = 3).
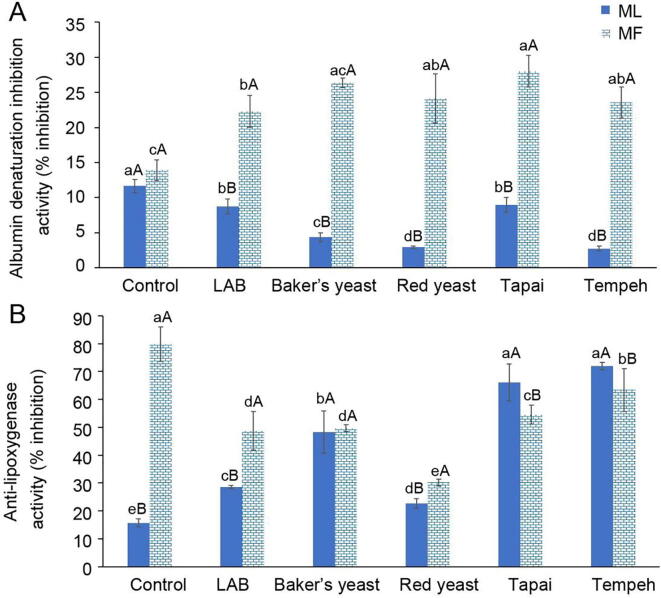
Fig. 4 elicited the effect of fermentation using different microorganisms on albumin denaturation inhibition and anti-lipoxygenase activity of ML and MF juices.
Fig. 4.
Effect of fermentation using different types of microorganisms (LAB, Baker’s yeast, red yeast, Tapai and Tempeh) on the changes of (A) albumin denaturation inhibition activity and (B) anti-lipoxygenase activity of mulberry leaves (ML) and fruits (MF) (mean ± SD, n = 3).
ML and MF demonstrated the same albumin denaturation inhibition activity before fermentation. The albumin denaturation inhibition activity of ML was decreased significantly (P < 0.05) after fermentation by all types of microorganisms. However, the activity was increased in MF. The albumin denaturation inhibition activity of MF juice was almost doubled after fermentation. Contrarily, MF juice exhibited higher anti-lipoxygenase activity than ML juice. Fermentation significantly increased (P < 0.05) the anti-lipoxygenase activity of ML juice. This finding proposes that both ML and MF exhibited anti-inflammation activity, but work via different pathways. Protein denaturation causes the production of autoantigens that trigger inflammation in condition such as rheumatic arthritis, cancer, and diabetes (Dharmadeva, Galgamuwa, Prasadinie, & Kumarasinghe, 2019). Lipoxygenase is one of the enzymes that plays important role in inflammation, particularly in the biochemical processes of leukotrienes. Leukotrienes are the main regulator of allergic response and inflammation (Sari, Elya, & Katrin, 2017). Either inhibiting protein denaturation or the activity of lipoxygenase enzyme could halt inflammation. Results of Pearson correlation analysis reveals that anti-lipoxygenase activity of MF was positively correlated with FRAP (r = 0.718 at P < 0.01), whereas albumin denaturation inhibition activity was correlated with DPPH activity (r = 0.878 at P < 0.01). These results disclosed that anti-inflammation activity of MF was closely related to its antioxidant activity. According to Arulselvan et al. (2016), antioxidant plays crucial role in inhibiting inflammation because over-production of reaction oxygen species will cause oxidative damage to body cells, eventually induce inflammation. Yu et al., 2021, Xu et al., 2020 suggested that indole, loliolide, odisolane, quercetin, kaempferol, eriodictyol and artonin are the compounds that contribute to anti-inflammation activity in mulberry fruits. However, there was no significant positive correlation found between anti-inflammation and antioxidant activity of ML. Besides phenolic compounds, other component such as polysaccharide of mulberry leaves has also been proven to exhibit anti-inflammation activity (He et al., 2018). However, Zhang et al. (2016) discovered that there was a synergistic interaction between flavonoid and mulberry leaf polysaccharide on the antioxidant activity. Mulberry leaf polysaccharide alone has weak antioxidative effect. Thus, it was strongly believed that anti-inflammation activity of ML was likely contributed by non-antioxidant compounds.
4. Conclusion
Both mulberry fruit and leaf juices demonstrated remarkable antioxidant, anti-inflammation, and anti-diabetic activities. Mulberry fruits exhibited higher DPPH free radical scavenging activity, ferric reducing antioxidant powder and anti-lipoxygenase activity than the leaves. However, mulberry leaves displayed higher α-amylase inhibition activity than the fruits. Although different microorganisms (lactic acid bacteria, red yeast, Baker’s yeast, Tapai starter and Tempeh starter) were found to exert different effects on the mulberry fruits and leaves, their antioxidant, hypoglycemic and anti-inflammation activities had been improved substantially after fermentation. In overall, fermentation by Tempeh starter was proven as the best technique to enhance total phenolics, ferric reducing antioxidant power, α-amylase inhibition activity and anti-lipoxygenase activity of mulberry leaves, whereas Tapai fermentation effectively enhanced the DPPH scavenging activity and albumin denaturation inhibition activity of mulberry fruits. Fermentation is recommended as an economic green approach to improve nutritional value of both mulberry fruits and leaves.
Future research to determine the chemicals profile in the Tempeh starter-fermented mulberry leaves and Tapai starter-fermented mulberry fruits is recommended. Besides, the strains in the Tempeh and Tapai starters should also be identified so that the biochemical reactions that happened during fermentation, which eventually contribute to the increase of antioxidant, anti-inflammation, and hypoglycemic activities of mulberry fruits and leaves can be elucidated.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors expressed gratitude to Mr. Booi Chin Hai, laboratory assistant in Department of Bioscience, Faculty of Applied Sciences, Tunku Abdul Rahman University College for his kind assistance in this project. This project is supported by teaching activity fund of the Faculty of Applied Science, Tunku Abdul Rahman University College.
References
- Abd Razak D.L., Abd Rashid N.Y., Jamaluddin A., Sharifudin S.A., Long K. Enhancement of phenolic acid content and antioxidant activity of rice bran fermented with Rhizopus oligosporus and Monascus purpureus. Biocatalysis and Agricultural Biotechnology. 2015;4(1):33–38. [Google Scholar]
- Abdul N., Kasim K.F. In-vitro antidiabetic activity of Clinacanthus nutans extract. International Journal of Pharmacognosy and Phytochemical Research. 2017;9(6):846–852. [Google Scholar]
- Adebo O.A., Medina-Meza I.G. Impact of fermentation on the phenolic compounds and antioxidant activity of whole cereal grains: A mini review. Molecules. 2020;25(4):927. doi: 10.3390/molecules25040927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adesulu-Dahunsi A.T., Jeyaram K., Sanni A.I., Banwo K. Production of exopolysaccharide by strains of Lactobacillus plantarum YO175 and OF101 isolated from traditional fermented cereal beverage. PeerJ. 2018;6:e5326. doi: 10.7717/peerj.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adisakwattana S., Ruengsamran T., Kampa P., Sompong W. In vitro inhibitory effects of plant-based foods and their combinations on intestinal α-glucosidase and pancreatic α-amylase. BMC Complementary and Alternative Medicine. 2012;12(1):110. doi: 10.1186/1472-6882-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A., Ali M., El-Kholie E., El-Garawani I., Sherif N. Anticancer activity of Morus nigra on human breast cancer cell line (MCF-7): The role of fresh and dry fruit extracts. Journal of Bioscience and Applied Research. 2016;2(6):352–361. [Google Scholar]
- Arulselvan P., Fard M.T., Tan W.S., Gothai S., Fakurazi S., Norhaizan M.E., Kumar S.S. Role of antioxidants and natural products in inflammation. Oxidative Medicine and Cellular Longevity. 2016;2016:1–15. doi: 10.1155/2016/5276130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayed L., M’hir S., Hamdi M. Microbiological, biochemical, and functional aspects of fermented vegetable and fruit beverages. Journal of Chemistry. 2020;2020:1–12. [Google Scholar]
- Bei Q., Chen G., Lu F., Wu S., Wu Z. Enzymatic action mechanism of phenolic mobilization in oats (Avena sativa L.) during solid-state fermentation with Monaskus anka. Food Chemistry. 2018;245:297–304. doi: 10.1016/j.foodchem.2017.10.086. [DOI] [PubMed] [Google Scholar]
- Chaudhary A., Sharma V., Saharan B.S. Probiotic potential of noni and mulberry juice fermented with lactic acid bacteria. Asian Journal of Dairy Food Research. 2019;38(2):114–120. [Google Scholar]
- Chen H., He X., Liu Y., Li J., He Q., Zhang C.…Wang J. Extraction, purification and anti-fatigue activity of γ-aminobutyric acid from mulberry (Morus alba L.) leaves. Scientific Reports. 2016;6(1) doi: 10.1038/srep18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo K.Y., Kho C., Ong Y.Y., Thoo Y.Y., Lim L.H., Tan C.P., Ho C.W. Fermentation of red dragon fruit (Hylocereus polyrhizus) for betalains concentration. International Food Research Journal. 2018;25(6):2539–2546. [Google Scholar]
- Degrain A., Manhivi V., Remize F., Garcia C., Sivakumar D. Effect of lactic acid fermentation on color, phenolic compounds and antioxidant activity in African Nightshade. Microorganisms. 2020;8(9):1324. doi: 10.3390/microorganisms8091324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmadeva S., Galgamuwa L.S., Prasadinie C., Kumarasinghe N. In-vitro anti-inflammatory activity of Ficus racemose L. bark using albumin denaturation method. AYU. 2019;39:239–242. doi: 10.4103/ayu.AYU_27_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diowksz A., Malik A., Jaśniewska A., Leszczyńska J. The inhibition of amylase and ACE enzyme and the reduction of immunoreactivity of sourdough bread. Foods. 2020;9(5):656. doi: 10.3390/foods9050656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverrigaray S., Scariot F.J., Menegotto M., Delamare A.P.L. Anthocyanin adsorption by Saccharomyces cerevisiae during wine fermentation is associated to the loss of yeast cell wall/membrane integrity. International Journal of Food Microbiology. 2020;314:108383. doi: 10.1016/j.ijfoodmicro.2019.108383. [DOI] [PubMed] [Google Scholar]
- Elisia I., Hu C., Popovich D.G., Kitts D.D. Antioxidant assessment of an anthocyanin-enriched blackberry extract. Food Chemistry. 2007;101(3):1052–1058. [Google Scholar]
- Espada-Bellido E., Ferreiro-González M., Carrera C., Palma M., Barroso C.G., Barbero G.F. Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chemistry. 2017;219:23–32. doi: 10.1016/j.foodchem.2016.09.122. [DOI] [PubMed] [Google Scholar]
- Fernandes R.P.P., Trindade M.A., Tonin F.G., Lima C.G., Pugine S.M.P., Munekata P.E.S.…de Melo M.P. Evaluation of antioxidant capacity of 13 plant extracts by three different methods: Cluster analyses applied for selection of the natural extracts with higher antioxidant capacity to replace synthetic antioxidant in lamb burgers. Journal of Food Science and Technology. 2016;53(1):451–460. doi: 10.1007/s13197-015-1994-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M.Z., Cui Q., Wang L.T., Meng Y., Yu L., Li Y.Y., Fu Y.J. A green and integrated strategy for enhanced phenolic compounds extraction from mulberry (Morus alba L.) leaves by deep eutectic solvent. Microchemical Journal. 2020;154 [Google Scholar]
- Gao K., Zheng C., Wang T., Zhao H., Wang J., Wang Z.…Wang W. 1-Deoxynojirimycin: Occurrence, extraction, chemistry, oral pharmacokinetics, biological activities and in silico target fishing. Molecules. 2016;21(11):1600. doi: 10.3390/molecules21111600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo N.A., Zhu Y.W., Jiang Y.W., Li H.K., Liu Z.M., Wang W.…Fu Y.J. Improvement of flavonoid aglycone and biological activity of mulberry leaves by solid-state fermentation. Industrial Crops and Products. 2020;148:112287. doi: 10.1016/j.indcrop.2020.112287. [DOI] [Google Scholar]
- He X., Fang J., Ruan Y., Wang X., Sun Y., Wu N.i.…Huang L. Structures, bioactivities and future prospective of polysaccharides from Morus alba (white mulberry): A review. Food Chemistry. 2018;245:899–910. doi: 10.1016/j.foodchem.2017.11.084. [DOI] [PubMed] [Google Scholar]
- Hornedo-Ortega R., Álvarez-Fernández M.A., Cerezo A.B., Garcia-Garcia I., Troncoso A.M., Garcia-Parrilla M.C. Influence of fermentation process on the anthocyanin composition of wine and vinegar elaborated from strawberry. Journal of Food Science. 2017;82(2):364–372. doi: 10.1111/1750-3841.13624. [DOI] [PubMed] [Google Scholar]
- Hu T.G., Wen P., Shen W.Z., Liu F., Li Q., Li E.N.…Zou Y.X. Effect of 1-deoxynojirimycin isolated from mulberry leaves on glucose metabolism and gut microbiota in a streptozotocin-induced diabetic mouse model. Journal of Natural Products. 2019;82(8):2189–2200. doi: 10.1021/acs.jnatprod.9b00205. [DOI] [PubMed] [Google Scholar]
- Huang H.P., Ou T.T., Wang C.J. Mulberry (Sang Shèn Zǐ) and its bioactive compounds, the chemoprevention effects and molecular mechanisms in vitro and in vivo. Journal of Traditional and Complementary Medicine. 2013;3(1):7–15. doi: 10.4103/2225-4110.106535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur S.J., Lee S.Y., Kim Y.C., Choi I., Kim G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chemistry. 2014;160:346–356. doi: 10.1016/j.foodchem.2014.03.112. [DOI] [PubMed] [Google Scholar]
- Hyun T.K., Ra J.H., Han S.H., Kim J.S. Antioxidant, antimicrobial, and antidiabetic activities of crowberry fruits. Indian Journal of Pharmaceutical Sciences. 2018;80(3):489–495. [Google Scholar]
- Imran M., Khan H., Shah M., Khan R., Khan F. Chemical composition and antioxidant activity of certain Morus species. Journal of Zhejiang University Science B. 2010;11(12):973–980. doi: 10.1631/jzus.B1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal S., Younas U., Sirajuddin, Chan K.W., Sarfraz R.A., Uddin M.K. Proximate composition and antioxidant potential of leaves from three varieties of mulberry (Morus sp.): A comparative study. International Journal of Molecular Sciences. 2012;13(6):6651–6664. doi: 10.3390/ijms13066651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J.H., Lee N.K., Cho S.H., Jeong D.Y., Jeong Y.S. Enhancement of 1-deoxynojirimycin content and α-glucosidase inhibitory activity in mulberry leaf using various fermenting microorganisms isolated from Korean traditional fermented food. Biotechnology and Bioprocess Engineering. 2014;19(6):1114–1118. [Google Scholar]
- Kojima Y., Kimura T., Nakagawa K., Asai A., Hasumi K., Oikawa S., Miyazawa T. Effects of mulberry leaf extract rich in 1-deoxynojirimycin on blood lipid profiles in humans. Journal of Clinical Biochemistry and Nutrition. 2010;47(2):155–161. doi: 10.3164/jcbn.10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkinomagoulos E., Nikolaou A., Kourkoutas Y., Kandylis P. Evaluation of yeast strains for pomegranate alcoholic beverage production: Effect on physicochemical characteristics, antioxidant activity, and aroma compounds. Microorganisms. 2020;8(10):1583. doi: 10.3390/microorganisms8101583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna H., Singh D., Singh R.S., Kumar L., Sharma B.D., Saroj P.L. Morphological and antioxidant characteristics of mulberry (Morus spp.) genotypes. Journal of the Saudi Society of Agricultural Sciences. 2020;19(2):136–145. [Google Scholar]
- Kwaw E., Ma Y., Tchabo W., Apaliya M.T., Wu M., Sackey A.S.…Tahir H.E. Effect of lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chemistry. 2018;250:148–154. doi: 10.1016/j.foodchem.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Law S.V., Abu Bakar F., Mat Hashim D., Abdul Hamid A. Popular fermented foods and beverages in Southeast Asia. International Food Research Journal. 2011;18:475–484. [Google Scholar]
- Li K.e., Yao F., Xue Q., Fan H., Yang L., Li X.…Liu Y. Inhibitory effects against α-glucosidase and α-amylase of the flavonoids-rich extract from Scutellaria baicalensis shoots and interpretation of structure–activity relationship of its eight flavonoids by a refined assign-score method. Chemistry Central Journal. 2018;12(1) doi: 10.1186/s13065-018-0445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L., Wu X., Zhu M., Zhao W., Li F., Zou Y., Yang L. Chemical composition, nutritional value, and antioxidant activities of eight mulberry cultivars from China. Pharmacognosy Magazine. 2012;8(31):215–224. doi: 10.4103/0973-1296.99287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh S.P., Hadira O. In vitro inhibitory potential of selected Malaysian plants against key enzymes involved in hyperglycemia and hypertension. Malaysian Journal of Nutrition. 2011;17(1):77–86. [PubMed] [Google Scholar]
- Masuko T., Minami A., Iwasaki N., Majima T., Nishimura S.I., Lee Y.C. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Analytical Biochemistry. 2005;339(1):69–72. doi: 10.1016/j.ab.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Mushollaeni, W. & Tantalu, L. (2020). Anthocyanin and nutritional contents of fermented lebui bean (Cajanus sp.) through SSF method and induced by Rhizopus sp. and Saccharomyces sp. IOP Conference Series: Earth and Environmental Science, 465, 012037.
- Plaza-Vinuesa L., Hernandez-Hernandez O., Moreno F.J., Rivas B., Muñoz R. Unravelling the diversity of glycoside hydrolase family 13 α-amylase from Lactobacillus plantarum WCFS1. Microbial Cell Factories. 2019;18:183. doi: 10.1186/s12934-019-1237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid M., Shafi S. Evaluation of phytochemical constituents and in vitro anti-inflammatory activity of Kashmiri pomegranate (Punica granatum Linn.) flower extract. IOSR Journal of Pharmacy and Biological Sciences. 2018;13(2):58–67. [Google Scholar]
- Ruta L.L., Farcasanu I.C. Anthocyanins and anthocyanin-derived products in yeast-fermented beverages. Antioxidants (Basel) 2019;8(6):182. doi: 10.3390/antiox8060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Salcedo, E.M., Mena, P., García-Viguera, C., Hernández, F. & Martínez, J.J. (2015b). (Poly)phenolic compounds and antioxidant activity of white (Morus alba) and black (Morus nigra) mulberry leaves: Their potential for new products rich in phytochemicals. Journal of Functional Foods, 18, part B, 1039-1046.
- Sánchez-Salcedo E.M., Mena P., García-Viguera C., Martínez J.J., Hernández F. Phytochemical evaluation of white (Morus alba L.) and black (Morus nigra L.) mulberry fruits, a starting point for the assessment of their beneficial properties. Journal of Functional Foods. 2015;12:399–408. [Google Scholar]
- Sari A.C., Elya B., Katrin Antioxidant activity and lipoxygenase enzyme inhibition assay with total flavonoids assay of Garcinia porrecta Laness. steam bark extracts. Pharmacognosy Journal. 2017;9(2):257–266. [Google Scholar]
- Shori A.B. Inclusion of phenolic compounds from different medicinal plants to increase α-amylase inhibition activity and antioxidants in yogurt. Journal of Taibah University for Science. 2020;14(1):1000–1008. [Google Scholar]
- Shuang E., Yamamoto K., Sakamoto Y., Mizowaki Y., Iwagaki Y., Kimura T.…Tsuduki T. Intake of mulberry 1-deoxynojirimycin prevents colorectal cancer in mice. Journal of Clinical Biochemistry and Nutrition. 2017;61(1):47–52. doi: 10.3164/jcbn.16-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai H.N., Camp J.V., Smagghe G., Raes K. Improved release and metabolism of flavonoids by steered fermentation processes: A review. International Journal of Molecular Sciences. 2014;15(11):19369–19388. doi: 10.3390/ijms151119369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaipitakwong T., Numhom S., Aramwit P. Mulberry leaves and their potential effects against cardiometabolic risks: A review of chemical compositions, biological properties and clinical efficacy. Pharmaceutical Biology. 2018;56(1):109–118. doi: 10.1080/13880209.2018.1424210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S., Tang M., Zhao B. Current anti-diabetes mechanisms and clinical trials using Morus alba L. Journal of Traditional Chinese Medical Sciences. 2016;3(1):3–8. [Google Scholar]
- Turan I., Demir S., Kilinc K., Burnaz N.A., Yaman S.O., Akbulut K.…Deger O. Antiproliferative and apoptotic effect of Morus nigra extract on human prostate cancer cells. Saudi Pharmaceutical Journal. 2017;25(2):241–248. doi: 10.1016/j.jsps.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichasilp C., Nakagawa K., Sookwong P., Higuchi O., Luemunkong S., Miyazawa T. Development of high 1-deoxynojirimycin (DNJ) content mulberry tea and use of response surface methodology to optimize tea-making conditions for highest DNJ extraction. LWT-Food Science and Technology. 2012;45(2):226–232. [Google Scholar]
- Wang, L., Sun, X., Li, F., Yu, D., Liu, X., Huang, W. & Zhan, J. (2015). Dynamic changes in phenolic compounds, colour and antioxidant activity of mulberry wine during alcoholic fermentation. Journal of Functional Foods, 18, Part A, 254–265.
- Wang L., Luo Y., Wu Y., Liu Y., Wu Z. Fermentation and complex enzyme hydrolysis for improving the total soluble phenolic contents, flavonoid aglycones contents and bio-activities of guava leaves tea. Food Chemistry. 2018;264:189–198. doi: 10.1016/j.foodchem.2018.05.035. [DOI] [PubMed] [Google Scholar]
- Wang L., Luo Y., Wu Y., Wu Z. Impact of fermentation degree on phenolic compositions and bioactivities during the fermentation of guava leaves with Monascus anka and Bacillus sp. Journal of Functional Foods. 2018;41:183–190. [Google Scholar]
- Wang X., Shao C., Liu L., Guo X., Xu Y., Lü X. Optimization, partial characterization and antioxidant activity of an exopolysaccharide from Lactobacillus plantarum KX041. International Journal of Biological Macromolecules. 2017;103:1173–1184. doi: 10.1016/j.ijbiomac.2017.05.118. [DOI] [PubMed] [Google Scholar]
- Wang Y., Xiang L., Wang C., Tang C., He X., Pintus G. Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (Morus alba L.) polyphenol enhanced extract. PLoS One. 2013;8(7):e71144. doi: 10.1371/journal.pone.0071144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani M.Y., Mir M.R., Baqual M.F., Ganie N.A., Bhat Z.A., Ganie Q.A. Roles of mulberry tree. The Pharma Innovation Journal. 2017;6(9):143–147. [Google Scholar]
- Xiao Y., Fan J., Chen Y., Rui X., Zhang Q., Dong M. Enhanced total phenolic and isoflavone aglycone content, antioxidant activity and DNA damage protection of soybeans processed by solid state fermentation with Rhizopus oligosporus RT-3. RSC Advances. 2016;6(35):29741–29756. [Google Scholar]
- Xiong Y., Ng K., Zhang P., Warner R.D., Shen S., Tang H.Y.…Fang Z. In vitro α-glucosidase and α-amylase inhibitory activities of free and bound phenolic extracts from the bran and kernel fractions of five sorghum grain genotypes. Foods. 2020;9(9):1301. doi: 10.3390/foods9091301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Huang Y., Xu J., He X., Wang Y. Anti-neuroinflammatory and antioxidant phenols from mulberry fruit (Morus alba L.) Journal of Functional Foods. 2020;68:103914. [Google Scholar]
- Yang N.C., Jhou K.Y., Tseng C.Y. Antihypertensive effect of mulberry leaf aqueous extract containing γ-aminobutyric acid in spontaneously hypertensive rats. Food Chemistry. 2012;132(4):1796–1801. [Google Scholar]
- Yu Y., Li H., Zhang B., Wang J., Shi X., Huang J.…Deng Z. Nutritional and functional components of mulberry leaves from different varieties: Evaluation of their potential as food materials. International Journal of Food Properties. 2018;21(1):1495–1507. [Google Scholar]
- Yu J.S., Lim S.H., Lee S.R., Choi C.I., Kim K.H. Antioxidant and anti-inflammatory effects of white mulberry (Morus alba L.) fruits on lipopolysaccharide-stimulated RAW 264.7 macrophages. Molecules. 2021;26(4):920. doi: 10.3390/molecules26040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.Y., Wan Y., Xu J.Y., Wu G.H., Li L., Yao X.H. Ultrasound extraction of polysaccharides from mulberry leaves and their effect on enhancing antioxidant activity. Carbohydrate Polymers. 2016;137:473–479. doi: 10.1016/j.carbpol.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Zheng X., Yu Y., Xiao G., Xu Y., Wu J., Tang D.…Zhang Y. Changes of anti-glucosidase content and some other characteristics in mulberry juice during fermentation with Leuconostoc mesenteroides. Acta Alimentaria (Budapest) 2014;43(4):668–675. [Google Scholar]