Abstract
Objective
The moisture content in the soil directly affects the yield and quality of Panax notoginseng, especially at the age of three years old. However, the suitable moisture for the growth of P. notoginseng is unknown. In this study, the effects of different soil moisture on the growth of P. notoginseng were studied.
Methods
Four different water treatments (0.45 field capacity (FC), 0.60 FC, 0.70 FC, and 0.85 FC) were set up in Shilin County, Yunnan Province, China. The water consumption and daily dynamic of water consumption were determined daily (from April 21 to October 18, 2012), and the daily dynamic of water consumption under different weather conditions (sunny and rainy) was determined. The transpiration coefficient and water use efficiency were calculated through dry matter accumulation and total water consumption. Accumulation of saponins of roots of P. notoginseng were analyzed by HPLC after treated, and the soil moisture content suitable for the growth of P. notoginseng was estimated by regression fitting of the active ingredient accumulation and the soil moisture content.
Results
The water consumption of 0.85 FC, 0.70 FC, 0.60 FC and 0.45 FC were 2.89, 3.68, 3.37 and 2.73 kg/plant per day, respectively. The water consumption of P. notoginseng from June to August was greater than other months. The daily dynamic of water consumption on sunny days and sunny days after rain showed a “double peak” feature, and it showed a “single peak” feature on rainy days. The water uses efficiency (WUE) of 0.85 FC, 0.70 FC, 0.60 FC and 0.45 FC were 2.51, 3.32, 4.59, 3.39 gDW/kg H2O, respectively. The increase of soil moisture content would reduce the WUE of P. notoginseng. With the increase of soil water content, the content of notoginsenoside R1 and ginsenoside Rg1 did not change significantly, while the content of ginsenoside Rb1 and Rd showed a decreasing trend.
Conclusion
Soil moisture content significantly affected the water consumption of P. notoginseng, and when it was 56.4% of the maximum water holding capacity in the field, the sum of the four saponins of 100 strains of P. notoginseng was the highest.
Keywords: Panax notoginseng (Burk.) F. H. Chen, saponins, transpiration coefficient, water consumption dynamics, water use efficiency
1. Introduction
The moisture content in the soil directly affects the yield and quality of Panax. notoginseng (Burk.) F. H. Chen, especially at the age of three years old, during which the content of effective ingredients in P. notoginseng and its efficacy were seriously affected. However, the suitable moisture for the growth of P. notoginseng is unknown at this key period.
Most of the water in plants is absorbed by the roots of plants, and the state of soil moisture directly affects the water status of plants (Jin et al., 2016, Mi et al., 2016, Saf and Ünlükara, 2013). Excessive soil moisture or severe drought will inhibit the growth and development of plants, such as Salvia miltiorrhiza Bge., Salvia plebeian R. Br, Aralia elata (Miq.) Seem and so on (Liu et al., 2011, Shi et al., 2010, Chen et al., 2008). But for the cultivation of P. notoginseng, previous research mainly focused on biological characteristics, pests and diseases, ingredient accumulation (Xia et al., 2017, Wan et al., 2006, Kim, 2012, Zhou et al., 2018, Zhao et al., 2018, Liu et al., 2020, Ding et al., 2020), and did not pay attention to the soil moisture content. P. notoginseng is a perennial plant, usually cultivated in nursery beds in the first year, and moved to the field for cultivation in the second year, and harvested after October of the third year. The third year is the critical period for the growth of P. notoginseng, so the three-year-old P. notoginseng was used as the research object to study the optimal soil moisture content. Its roots and rhizomes are commonly used in clinical traditional Chinese medicine, which could stanch blood, disperse gore and reduce the pain caused by blood disease (Ng, 2006, Konoshima et al., 1999, Jee et al., 2014). Saponins are the main active ingredients of P. notoginseng, ginsenoside R1 (7%−10%), ginsenoside Rb1 (30%−36%), Rg1 (20%−40%), Rd (5%−8.4%) and Re (3.9%−6%) are main saponins, accounting for 90% of the total saponins of P. notoginseng (Ning et al., 2017, Wang et al., 2016, Xia et al., 2014).
Previous studies on soil moisture usually designed three gradients (Liu & Gao, 2019). In this study, we designed four gradients (0.85 field capacity (FC), 0.70 FC, 0.60 FC, and 0.45 FC) because it is not certain whether higher soil moisture will be beneficial to the growth of P. notoginseng. The three-year-old P. notoginseng enters the peak growth period in April and is harvested in October. Therefore, the study of soil moisture content on P. notoginseng was chosen during this period. This study is aimed to discover the effects of different soil moisture contents on water consumption characteristics and saponins accumulation in P. notoginseng, and provide a theoretical basis for water management of P. notoginseng cultivation, and also benefit the research on the optimal ecological niche of P. notoginseng.
2. Materials and methods
2.1. Plants and soil
Three-year-old P. notoginseng were used in this study. The experimental site was located in the town of Guishan in Shilin County, Yunnan, China (24°44′14′’ N, 103°38′54′’ E, altitude 2,128 m). The soil type was red clay soil. The field capacity (FC) was 36.8%. The contents of different substances in the soil were shown in Table 1.
Table 1.
Contents of different substances in soil.
| Substances in soil | Content |
|---|---|
| Organic matter | 3.29% |
| Total nitrogen | 0.125% |
| Total phosphorus | 0.111% |
| Total potassium | 0.697% |
| Available nitrogen | 9.376 mg/kg |
| Available phosphorus | 35.966 mg/kg |
| Available potassium | 166.58 mg/kg |
2.2. Experimental design
The experiment was carried out in the rainproof shelter (Fig. 1). A total of 200 3-years-old individuals with uniform morphology were randomly selected for the experiment, which were planted on March 12, 2016, in plastic pots with dimensions of 22 cm in height, 27 cm in mouth diameter, and 18 cm in bottom diameter. The total weight of stones and pot was 1.7 kg, and each pot was then filled with 6.3 kg of filtered soil sample. The pots were put under rain shelter after covering 150 g dried pine needle on potted soil. The pots without P. notoginseng were set as the control group. After transplanting, the soil moisture content was kept at about 70% of the maximum field water holding capacity. The water control experiment was performed on April 20, 2012, when the seedling leaves were sprouting. Four stress levels were designed-0.45 FC, 0.60 FC, 0.70 FC, and 0.85 FC. Each level had 25 pots with two plants in each. The water loss was supplemented at 17:00 daily. The water control ended on October 20, 2012, in total of 183 d. In each treatment, six pots with good growth and no disease were randomly selected, the whole plant was excavated, and the root morphology was observed. The root dry weight, root drying rate, root-shoot ratio and the accumulation of active components were analyzed.
Fig. 1.

Rainproof shelter of experiment in April 2012.
2.3. Measuring methods
Water consumption was measured every day at 17:00, the potted plants were weighed by an electronic platform scale, and the water loss was made up through the pipe in the bucket, the pot weight and water supplement were recorded, and the water consumption of P. notoginseng per plant was calculated and corrected according to the blank comparison of water loss and weight gain of P. notoginseng at each growth and development stage.
Daily water consumption change was measured with weighing every two hours from 7:00 to 19:00, recording the data and calculating the water consumption per plant during each period.
The transpiration coefficient was the ratio of total water consumption to dry matter accumulation during the test period; The water use efficiency was the ratio of the dry matter accumulation of P. notoginseng to the total water consumption during the test period. The dry matter accumulation of P. notoginseng during the test period was the difference between the biomass at the end of the experiment and the biomass at the beginning of the test. At the beginning of the experiment and at the end of the experiment, three pots of six plants were collected for each treatment, and the biomass was measured by oven drying.
2.4. Determination of notoginsenoside R1, ginsenosides Rg1, Rb1 and Rd
Sample preparation referred to the requirements of the Chinese Pharmacopoeia (2015 edition). HPLC analysis was performed on a Waters chromatography system (Milford, MA, USA), equipped with Waters 1525 Binary Pump, Waters 2487 Dual λ Absorbance Detector and Waters 2707 Autosamplerc. The column temperature was maintained at 30 °C. The UV absorption was measured at 203 nm. The chromatographic conditions were consistent with our previous tests for saponins (Xia et al., 2017). The flow rate was kept at 1 mL/min and the sample injection volume was 20 μL. The chromatographic column was Waters SYMMETRY C18 (4.6 mm × 250 mm, 5 μm). The standard products used were purchased from National Institutes for Food and Drug Control (notoginsenoside R1:110745-200617, ginsenoside Rg1:110703-201027, ginsenoside Rb1:110704-201122, ginsenoside Rd:111818-201001).
2.5. Data analysis
The test was performed in a completely randomized design. All experimental data were calculated using Microsoft Excel 2016. The data were analyzed by one-way ANOVA and Duncan’s multiple comparison tests using IBM SPSS Statistics 16.0 software. The fit of the regression model was done under the Regression menu. Statistical significance was considered as P < 0.05.
3. Results
3.1. Water consumption of different treatments
The average water consumption of 0.70 FC was 3.678 kg/plant during the 183 d of the experiment. The average water consumption of 0.45 FC was the smallest, which was 2.727 kg/plant, with a variation of 0.951 kg/plant. The results of variance analysis showed that there was a significant difference in water consumption between 0.45 FC and 0.85 FC treatments and 0.60 FC and 0.70 FC treatments (Table 2). The result showed that the water consumption of P. notoginseng had a significant relationship with the soil water environment. If the soil moisture content is too high or too low, the water consumption and water consumption intensity of P. notoginseng will be reduced, and the soil moisture content in the middle will increase the water consumption. Nowadays, the shortage of water resources has become a universal problem in the world. When soil moisture is maintained between 0.60 and 0.70 FC, it is not only beneficial to the growth of P. notoginseng, but also able to make rational use of soil moisture.
Table 2.
Water consumption of P. notoginseng of different treatments.
| Treatments | Water consumption / (kgH2O·plant−1) | Average water consumption per day / (kgH2O·plant−1·d−1) | Significance of differences |
|---|---|---|---|
| 0.45 FC | 2.7267 | 0.0149 | a |
| 0.60 FC | 3.3672 | 0.0184 | b |
| 0.70 FC | 3.6783 | 0.0201 | b |
| 0.85 FC | 2.8914 | 0.0158 | a |
Note: Different lowercase letters indicate significant difference (n = 183, P < 0.05).
3.2. Water consumption in different months
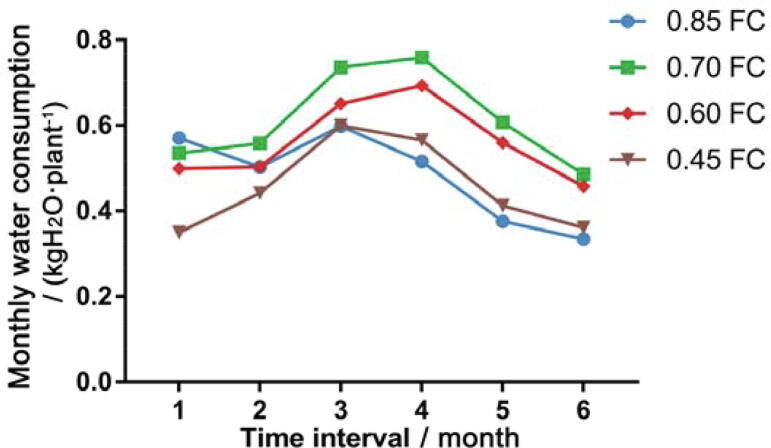
In this study, the period was divided into six uniform intervals on the 20th of each month, and the water consumption and water consumption intensity of each interval were counted. The results showed that from June 21 to July 21, the average water consumption of P. notoginseng was the largest, which was 0.645 kg/plant; the average water consumption from September 21 to October 20 was the lowest, which was 0.411 kg/plant, with a variation of 0.234 kg/plant. Analysis of variance showed that the average water consumption in the middle two intervals (June 21 to July 20 and July 21 to August 20) was significantly higher than the average water consumption of the other four intervals (Table 3). It showed that the water consumption of P. notoginseng had a significant relationship with its growth period. From June to August, P. notoginseng changed from vegetative growth to reproductive growth, and its water consumption was increased. Since August, the vegetative organs of P. notoginseng no longer grow, and the water content in the plant was relatively reduced, so the water consumption was reduced.
Table 3.
Water consumption of P. notoginseng of different times.
| Time interval | Water consumption / (kgH2O·plant−1) | Average water consumption per day / (kgH2O·plant−1·d−1) | Significance of difference |
|---|---|---|---|
| April 21th − May 20th | 0.459 | 0.0153 | a |
| May 21th − June 20th | 0.502 | 0.0162 | a |
| June 21th − July 20th | 0.645 | 0.0215 | b |
| July 21th − Aug 20th | 0.632 | 0.0204 | b |
| Aug 21th − Sept 20th | 0.490 | 0.0158 | a |
| Sept 21th − Oct 20th | 0.411 | 0.0137 | a |
3.3. Water consumption dynamics
The daily water consumption of P. notoginseng was very unstable and there was no obvious regular pattern (Fig. 2). The maximum daily water consumption occurred at 0.70 FC on July 26, which was 50.21 g/plant/d, and the lowest occurred at 0.45 FC on April 22, which was 0.45 g/plant/d, with a variation of 49.76 g/plant/d. The daily dynamics of water consumption and monthly water consumption of different treatments were analyzed. The relative change trend of 0.70 FC, 0.60 FC and 0.45 FC from the beginning of the study to the end of the study was consistent, and the water consumption of 0.70 FC was always higher than 0.60 FC and higher than 0.45 FC (Fig. 3). When the water supply was sufficient, the water consumption of P. notoginseng was relatively high. The relative change of 0.85 FC was relatively large. The water consumption of 0.85 FC was the highest at the beginning of the experiment, and the relative water consumption was decreased gradually over time. The water consumption of 0.85 FC became the lowest at the end of the study. At the beginning of the experiment, plants had small differences in each process, when the soil moisture content was large, the water consumption was large. Over time, the soil moisture content was too low, and the oxygen content in the soil inhibited the root respiration and affected the growth of P. notoginseng, making water consumption at a lower level.
Fig. 2.
Daily water consumption of P. notoginseng.
Fig. 3.
Monthly water consumption of P. notoginseng.
During the study, the water consumption of P. notoginseng was low in the early stage, high in the medium term, and low in the last. In the early stage of the experiment, due to the small leaf area and low ambient temperature of the P. notoginseng, the water consumption was low. After June 20, the leaf area of P. notoginseng reached its maximum level and began reproductive growth. At this time, the temperature of the environment was higher, resulting in a rapid increase in water consumption of P. notoginseng. From June 20 to August 20, the water consumption peak of P. notoginseng was formed, and the water consumption accounted for 40.69% of the water consumption during the whole study period. After August 20, the artificial disbudding stopped the reproductive growth of P. notoginseng, senescence of P. notoginseng began slowly, and the temperature became lower, which reduced the water consumption of P. notoginseng.
3.4. Daily dynamic of water consumption under different weather conditions
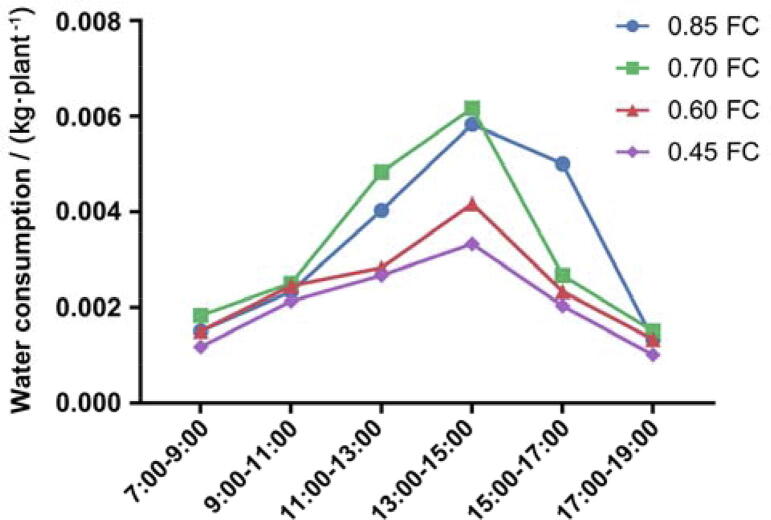
On the sunny day, the daily water consumption of P. notoginseng showed a distinct “double peak” feature (Fig. 4). The water consumption peaked at 13 to 15o'clock in all four treatments. The water consumption of four treatments in this section was 9.5 g/plant, 8.7 g/plant, 8.9 g/plant and 7.3 g/plant, which accounted for 23.6%, 24.5%, 25.9%, and 31.2% of the total water consumption, respectively. The less soil moisture content is, the higher the proportion of water consumption in the whole day is. The water consumption sub-high peak occurred from 9:00 to 11:00, and the water consumption of four treatments was 9.2 g/plant, 7.3 g/plant, 7.1 g/plant and 5.3 g/plant, which accounted for 22.8%, 20.7%, 20.8% and 22.7% of the total water consumption, respectively. From 11:00 to 13:00, the water consumption was low, which was the phenomenon of “noon break”. The water consumption reached the lowest level throughout the day, and the water consumption of the four treatments was about 1.0 g/plant.
Fig. 4.
Daily dynamic of water consumption of P. notoginseng under sunny day.
The daily dynamic of water consumption in the rainy days of P. notoginseng showed an obvious “single peak” feature (Fig. 5). The trend of the four treatments was basically the same. From the morning, the water consumption was increased first and then decreased. At 13:00 to 15:00, the water consumption reached its peak. The water consumption was 5.8 g/plant, 6.1 g/plant, 4.1 g/plant, and 3.3 g/plant, accounting for 29.1%, 31.6%, 28.5% and 27.0% of the total daily water consumption, respectively. However, it didn’t show “noon break” from 11:00 to 13:00. Therefore, the water consumption of P. notoginseng can be further reduced by proper soil water stress in rainy days.
Fig. 5.
Daily dynamic of water consumption of P. notoginseng under rainy day.
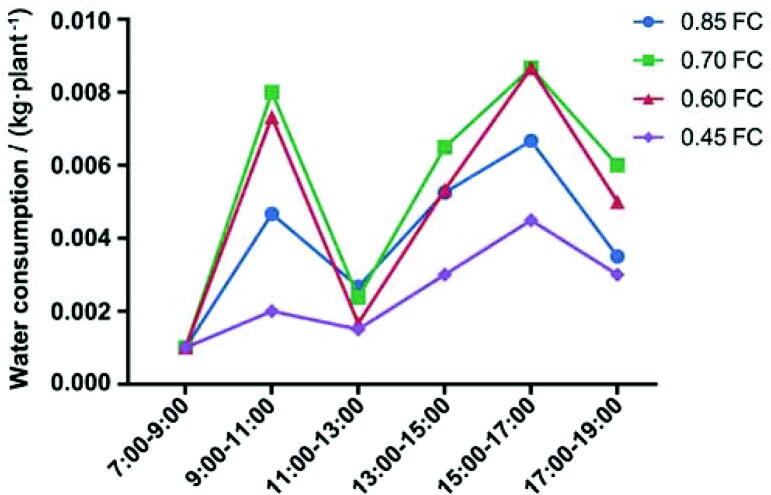
In the sunny days after the rain, the daily water consumption of P. notoginseng also showed obvious “double peak” characteristics (Fig. 6). The water consumption peaked at 15:00 to 17:00. The water consumption was 6.67 g/plant, 8.7 g/plant, 8.7 g/plant and 4.5 g/plant, respectively. The water consumption sub-high peak occurred from 9:00 to 11:00, and the water consumption of four treatments was 4.7 g/plant, 8.0 g/plant, 7.3 g/plant and 2.0 g/plant, respectively. In the two peaks, the water consumption of 0.70 FC and 0.60 FC was significantly larger. The water consumption also reached the lowest level throughout the day from 11:00 to 13:00.
Fig. 6.
Daily dynamic of water consumption of P. notoginseng in sunny days after the rain.
3.5. Transpiration coefficient and water use efficiency
The soil moisture content not only affected the water consumption of P. notoginseng, but also affected the dry matter accumulation, and further affected the transpiration coefficient and water use efficiency of P. notoginseng. The transpiration coefficients of the four treatments were 0.85 FC, 0.70 FC, 0.45 FC and 0.60 FC from high to low (Table 4). Water use efficiency is exactly the opposite of the change in transpiration coefficient. Excessive or too little water increased the transpiration coefficient of P. notoginseng and reduced the water use efficiency of P. notoginseng. Moderate water stress tends to reduce stomatal conductance, the plant can assimilate more molecules of CO2 for each unit of transpired water, being more efficient in the use of the available water. Thus, the WUE of 0.60 FC was the highest. Appropriate drought treatment not only improved the dry matter accumulation of P. notoginseng, but also improved the water use efficiency.
Table 4.
Transpiration coefficient and water use efficiency of P. notoginseng of different treatments.
| Treatments | Water consumption / (g·plant−1) | Dry matter accumulation / (g·plant−1) | Transpiration coefficient / (gH2O·gDW−1) | Water use efficiency / (gDW·kgH2O-1) |
|---|---|---|---|---|
| 0.85 FC | 2891.4 | 7.26 | 398.19 | 2.51 |
| 0.70 FC | 3678.3 | 12.20 | 301.43 | 3.32 |
| 0.60 FC | 3367.2 | 15.44 | 218.05 | 4.59 |
| 0.45 FC | 2726.7 | 9.25 | 294.90 | 3.39 |
3.6. Effect of soil moisture content on accumulation of active components in P. notoginseng
The effect of soil moisture content on the contents of four saponins in P. notoginseng was not closely consistent (Table 5). Among them, the content of notoginsenoside R1 and ginsenoside Rg1 did not change significantly with soil moisture content, and there was no significant difference between the treatments; The content of ginsenosides Rb1 and Rd were decreased gradually with the increase of soil moisture content. The content of ginsenoside Rb1 treated with 0.45 FC was significantly higher than that of the other three treatments. The content of ginsenoside Rb1 treated with 0.60 FC was significantly higher than 0.85 FC; The content of ginsenoside Rd of 0.45 FC was significantly higher than 0.85 FC. The sum of the four saponins content of soil moisture content of 0.45 FC, 0.60 FC, 0.70 FC and 0.85 FC was 81.74, 77.31, 71.71 and 67.87 g/kg, respectively. With the increase of soil moisture content, the saponin content showed a gradual decline, indicating that excessive soil moisture was not conducive to the accumulation of saponins in roots, while certain drought was beneficial to the accumulation of saponins in roots.
Table 5.
Influence of soil moisture content to saponins of roots.
| Treatments | R1 / (k·kg−1) | Rg1 / (k·kg−1) | Rb1 / (k·kg−1) | Rd / (k·kg−1) |
|---|---|---|---|---|
| 0.45 FC | 12.13 ± 2.43 a | 25.55 ± 2.35 a | 36.28 ± 2.83c | 7.78 ± 1.76b |
| 0.60 FC | 9.50 ± 3.42 a | 28.75 ± 2.59 a | 32.08 ± 3.19b | 6.98 ± 1.07 ab |
| 0.70 FC | 10.79 ± 1.31 a | 25.64 ± 4.60 a | 29.65 ± 1.41 ab | 5.63 ± 0.74 ab |
| 0.85 FC | 9.59 ± 2.27 a | 26.88 ± 3.81 a | 26.43 ± 5.05 a | 4.97 ± 2.20 a |
3.7. Calculation of suitable soil moisture content for P. notoginseng production
With the method of simple regression, the root dry weight of P. notoginseng, the sum of the contents of four saponins, the incidence of root rot and soil water content were respectively fitted by regression, and the following three relations were obtained:
Where Y was the sum of the contents of four saponins in P. notoginseng root per 100 g, and the unit was g. X was the ratio of the actual soil water content to the maximum field water holding capacity, and the unit was %.
According to the above formula, the function of the content of four saponins (g) and soil water content in 100 strains of P. notoginseng roots was constructed.
Substituting Y into the above function can obtain a function of the sum of the total dry weight (g) and the saponins content of 100 strains of P. notoginseng and the soil moisture content. The expression was as following:
The calculated F(X) had a maximum value when the ratio of soil moisture content to the maximum water holding capacity in the field is 56.4%, and the maximum value was 92.39 g. That is, when the soil moisture content was 56.4% of the maximum water holding capacity in the field, the sum of the four saponins of 100 strains of P. notoginseng was the highest, which was 92.39 g.
4. Discussion
4.1. Water consumption of different soil moisture content and weather
Water affects all aspects of plant metabolism. The response of plants to water is the result of complex interactions between plant organs through metabolism and signal events. The water consumption and water consumption intensity of P. notoginseng were significantly affected by soil moisture content. The rapid growth period of P. notoginseng vegetative organs was from April to June (Liao et al., 2016). The daily dynamic of water consumption had an obvious relationship with the weather. On the sunny day and sunny day after the rain, the daily water consumption of P. notoginseng showed a distinct “double peak” feature. However, the daily water consumption in the rainy days showed an obvious “single peak” feature. Water deficit affects many physiological processes in plants, generally increasing stomatal resistance and reducing transpiration (Santana et al., 2015). Too high or too low soil moisture content will significantly increase the transpiration coefficient of P. notoginseng and reduce its utilization rate of water. Maintaining proper soil moisture content is beneficial to the formation of P. notoginseng yield and can save water resources.
4.2. Influence of soil moisture on saponins
Secondary metabolites are usually the main medicinal components of Chinese medicine, and environmental factors have an important influence on their formation and accumulation. Studies have found that moderate drought stress was beneficial to the accumulation of active ingredients such as saponins in Gynostemma pentaphyllum (Thunb.) Makino, baicalin in Scutellaria baicalensis Georgi, and total saponins from Tribulus terrestris L. (Long et al., 2008, Shao et al., 2006, Yang et al., 2010). This study found that the sum of saponins in the roots of P. notoginseng tended to decrease with the increase of soil moisture content, which was consistent with the research results of Feng et al. (2006). Within the range of soil moisture content designed in this experiment, with the increase of soil moisture content, the contents of notoginsenoside R1 and ginsenoside Rg1 belonging to protopanaxtriol did not change significantly, while the contents of ginsenoside Rb1 and ginsenoside Rd belonging to protopanaxadiol were significantly decreased. It can be inferred that the ratio of the active ingredient content can be adjusted according to the difference in the response of each active ingredient to the soil moisture content.
The appropriate soil moisture content estimated in this study was 56.4% of the maximum field water holding capacity, which was smaller than the results of Cui et al. (2003) (about 65%), which may be caused by the experimental systematic error. But it also showed that more aspects, such as the influence of soil moisture content on the occurrence of diseases and insect pests, light and respiration characteristics, etc., should be explored to find the suitable range of soil moisture for the production of P. notoginseng and then ensure the yield and quality of P. notoginseng.
5. Conclusion
Soil moisture content significantly affected the water consumption of P. notoginseng, and when it was 56.4% of the maximum water holding capacity in the field, the sum of the four saponins of 100 strains of P. notoginseng was the highest.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgement
This work was supported by National Natural Science Foundation of China (81703641), Key project at central government level: The ability establishment of sustainable use for valuable Chinese medicine resources (2060302), China Postdoctoral Science Foundation (2020T130601), National key Research and development program (2017YFC1700704).
Contributor Information
Peng-guo Xia, Email: xpg_xpg@zstu.edu.cn.
Zong-suo Liang, Email: liangzs@ms.iswc.ac.cn.
References
- Chen J., Zhang G.C., Zhang S.Y., Wang M.J. Response processes of Aralia elata photosynthesis and transpiration to light and soil moisture. Chinese Journal of Applied Ecology. 2008;19:1185–1190. [PubMed] [Google Scholar]
- Cui X.M., Wang C.L., Feng G.Q. Yunnan Science and Technology Press; Kunming: 2003. Panax notoginseng GAP research and practice. [Google Scholar]
- Ding Y.S., Luo J., Zhao C.B.W., Li Q.Y., Shi J.L. Quality evaluation of different origins and commercial grades of Panax notginseng by HPLC and grey correlation analysis. Chinese Traditional and Herbal Drugs. 2020;51(4):1069–1075. [Google Scholar]
- Feng X.Q., Cui X.M., Chen Z.J., Zhang Y.L., Zhang W.S. Analysis of correlation between effective components of burk (Panax notoginseng) and meteorological factors. Journal of Agrometeorology. 2006;27:16–18. [Google Scholar]
- Jee H.S., Chang K.H., Park S.H., Kim K.T., Paik H.D. Morphological characterization, chemical components, and biofunctional activities of Panax ginseng, Panax quinquefolium, and Panax notoginseng roots: A comparative study. Food Reviews International. 2014;30:91–111. [Google Scholar]
- Jin X.X., Zuo Q., Ma W.W., Li S., Shi J.C., Tao Y.Y., et al. Water consumption and water-saving characteristics of a ground cover rice production system. Journal of Hydrology. 2016;540:220–231. [Google Scholar]
- Kim D.H. Chemical diversity of Panax ginseng, Panax quinquifolium, and Panax notoginseng. Journal of Ginseng Research. 2012;36:1–15. doi: 10.5142/jgr.2012.36.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konoshima T., Takasaki M., Tokuda H. Anti-carcinogenic activity of the roots of Panax notoginseng. II. Biological & Pharmaceutical Bulletin. 1999;22:1150–1152. doi: 10.1248/bpb.22.1150. [DOI] [PubMed] [Google Scholar]
- Liao P.R., Cui X.M., Yang Y., Qu Y., Wang C.X., Yang X.Y., et al. Effect of different water conditions on Panax notoginseng seeds after-ripening and germination physiology. China Journal of Chinese Materia Medica. 2016;41:2194–2200. doi: 10.4268/cjcmm20161204. [DOI] [PubMed] [Google Scholar]
- Liu D.H., Guo L.P., Huang L.Q., Jin H., Wu L.H., Zeng Y., et al. Effects of soil water content on seedlings growth and active ingredients of Salvia miltiorrhiza. China Journal of Chinese Materia Medica. 2011;36:321–325. [PubMed] [Google Scholar]
- Liu X.L., Gao M. Effect of different water content in soil on growth and four water-soluble active components of Salvia miltiorrhiza Bunge. Journal of Agricultural Science and Technology. 2019;281:1–6. [Google Scholar]
- Liu Z.Z., Gu M.M., Zhang C., Ma M.L., Zhu R.Q., Ma X., et al. Analysis of microbial community characteristics of Panax notoginseng based on high-throughput sequencing technology. Chinese Traditional and Herbal Drugs. 2020;51(1):204–209. [Google Scholar]
- Long Y., Yang R., Zhong Z.C., Tan F. Effect of different water and mitrogen on biomass and gypenosides in Gynostemma pentaphyllum. Chinese Traditional and Herbal Drugs. 2008;39:1872–1876. [Google Scholar]
- Mi M.X., Shao M., Liu B.X. Effect of rock fragments content on water consumption, biomass and water-use efficiency of plants under different water conditions. Ecological Engineering. 2016;94:574–582. [Google Scholar]
- Ng T.B. Pharmacological activity of sanchi ginseng (Panax notoginseng) Journal of Pharmacy and Pharmacology. 2006;58:1007–1019. doi: 10.1211/jpp.58.8.0001. [DOI] [PubMed] [Google Scholar]
- Ning B.B., Chen Y., Zhang T. Research progress in the effects of Notoginseng Radix et Rhizoma and its main saponins in cardiovascular diseases. Journal of Traditional Chinese Medicine. 2017;24:126–129. [Google Scholar]
- Saf S., Ünlükara A. Determining the effects of water and salinity stress on plant growth, development, yield and water consumption in grasspea (Lathyrus sativus L.) Journal of Agricultural Faculty of Gaziosmanpasa University. 2013;30:1–12. [Google Scholar]
- Santana T.A.D., Oliveira P.S., Silva L.D., Laviola B.G., Almeida A.F., Gomes F.P. Water use efficiency and consumption in different Brazilian genotypes of Jatropha curcas L. subjected to soil water deficit. Biomass & Bioenergy. 2015;75:119–125. [Google Scholar]
- Shao X.W., Han M., Han Z.M., Kong W.W., Yang L.M. Effects of water supply on growth and photosynthesis in Scutellaria baicalensis. Acta Ecologica Sinica. 2006;26:3214–3220. [Google Scholar]
- Shi Y.Y., Zhang Q.H., Xiong Q.P., Zhang D.Y. Effect of soil moisture on the growth and homoplangaginin content of Salvia plebeia R. Br. Research and Practice on Chinese Medicines. 2010;24:16–19. [Google Scholar]
- Wan J.B., Yang F.Q., Li S.P., Wang Y.T., Cui X.M. Chemical characteristics for different parts of Panax notoginseng, using pressurized liquid extraction and HPLC-ELSD. Journal of Pharmaceutical and Biomedical Analysis. 2006;41:1596–1601. doi: 10.1016/j.jpba.2006.01.058. [DOI] [PubMed] [Google Scholar]
- Wang T., Guo R.X., Zhou G.H., Zhou X.D., Kou Z.Z., Sui F., et al. Traditional uses, botany, phytochemistry, pharmacology and toxicology of Panax notoginseng (Burk.) F.H. Chen: A review. Journal of Ethnopharmacology. 2016;188:234–258. doi: 10.1016/j.jep.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Xia P.G., Guo H.B., Ru M., Yang D.F., Liang Z.S., Yan X.J., et al. Accumulation of saponins in Panax notoginseng, during its growing seasons. Industrial Crops and Products. 2017;104:287–292. [Google Scholar]
- Xia P.G., Zhang S.C., Liang Z.S., Qi Z.H. Research history and overview of chemical constituents of Panax notoginseng. Chinese Traditional and Herbal Drugs. 2014;45:2564–2570. [Google Scholar]
- Yang L., Han Z.M., Yang L.M., Han M. Effects of water stress on photosynthesis, biomass, and medicinal material quality of Tribulus terrestri. Chinese Journal of Applied Ecology. 2010;21:2523–2528. [PubMed] [Google Scholar]
- Zhao Y.M., Cheng Y.X., Ma Y.N., Chen C.J., Xu F.R., Dong X. Role of phenolic acids from the rhizosphere soils of Panax notoginseng as a double-edge sword in the occurrence of root-rot disease. Molecules. 2018;23:819–830. doi: 10.3390/molecules23040819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.F., Yang Z.X., Dong L.Y., Zhou L.H., Ye Z.L. Rapid determination of moisture and ethanol extract content in Panax notoginseng by NIRS. Chinese Traditional and Herbal Drugs. 2018;41(11):1994–1999. [Google Scholar]