Abstract
Kadsura belongs to the Schisandroideae subfamily of Magnoliaceae. Plants from genus Kadsura are widely distributed in the South and Southwest of China. The plants of the genus are widely used as folk medicine for a long time in history, with the functions of relieving pain, promoting ‘qi’ circulation, activating blood resolve stasis, and applications in the treatment of rheumatoid arthritis and gastroenteric disorders. Lignans are the primary characteristic constituents with various biological activities of plants from genus Kadsura. This paper summarized 81 lignans isolated from the plants of genus Kadsura over the past eight years (from 2014 to 2021), which belong to five types: dibenzocyclooctadienes, spirobenzofuranoid dibenzocyclooctadienes, aryltetralins, diarylbutanes and tetrahydrofurans. Each type of these lignans possess typical characteristics in proton magnetic resonance (1H NMR) and carbon-13 nuclear magnetic resonance (13C NMR) spectra, the NMR regularities of these types of lingans were summarized, which provided a useful reference for the structural analysis of lignans. The relationships between lignans and pharmacodynamics were also systematically analyzed, lignans were predicted to be the quality markers (Q-marker) of Kadsura genus.
Keywords: Kadsura, 1H NMR and 13C NMR spectrum characteristics, pharmacodynamics, Q-marker, lignans, structure classifications
1. Introduction
Kadsura contains about 29 plant species, and 10 species of this genus mainly distributed among the South and Southwest of China. Among these species of the genus Kadsura, lignans are the dominant constituents. Refers to Dong’s article (Dong, Shu, Liu, He, & Yan, 2014), the present paper aims to provide an up-to-date review of the structures of the lignans isolated from the genus Kadsura during 2014 to 2021. About 81 lignans have been isolated and identified, including 46 dibenzocyclooctadiens, 19 spirobenzofuranoid-dibenzocyclooctadienes, six aryltetralins, one tetrahydrofuran, three diarylbutanes and six new lignans. The lignan structures in the genus Kadsura have typical characteristics in their nuclear magnetic spectrum. Thus, this paper summarizes the structure types of lignans and the NMR regularities of each type, so as to provide reference for the future identification of lignan chemical structure.
2. Chemical constituents
A large number of compounds have been isolated from Kadsura genus, including lignans, triterpenoids, flavonoids, sesquiterpenoids, etc, with lignans and triterpenoids as the mainly chemical constituents. All these isolates enriched the diversity of constituents in Schisandraceae plants (Liu et al., 2014).
2.1. Lignans
Lignans, as significant characteristic class of secondary metabolites, were found in genus Kadsura. The lignans isolated from the Kadsura genus can be divided into five categories, including dibenzocyclooctadienes, spirobenzofuranoid dibenzocyclooctadienes, aryltetralins, diarylbutanes and tetrahydrofurans.
2.1.1. Dibenzocyclooctadiene
Dibenzocyclooctadiene lignans constitute more than half of the total lignans, they contain a basic skeleton biphenyl ring octene lignans which are divided into two series of biphenyl S and R configurations (I and II). Due to the existence of other chiral centers in molecules, there are many stereoisomers in this type of compound. There are three conformations of octet ring, including twisted boat chair type (TBC, III), twisted boat type (TB, IV) and 6,9 oxbridge biphenyl cyclooctadiene (V), the twisted boat chair type is the main conformation among the three ones. The main structural types and conformations of diphenylcyclooctene lignans in Kadsura are shown in Fig. 1.
Fig. 1.
Structural skeletons and conformations of dibenzocyclooctadiene lignans (I: S configuration, Ⅱ: R configuration, III: twisted boat chair, IV: twisted boat, Ⅴ: 6, 9 oxbridge biphenyl cyclooctadiene) from plants of Kadsura.
Except for the presence of two aromatic protons at C-4 and C-11 positions on the biphenyl ring, all other positions (C-1–3 and C-12–14) were substituted by oxygen-containing substitutions, including methoxyl, methylenedioxy, hydroxyl and ester groups (Chen, Qin, & Xie, 2000). Most hydroxyl groups are shown on C-1, C-12, C-14, and some are attached to the C-3. Biphenyl rings usually have one phenolic hydroxyl group, and a few have two phenolic hydroxyl groups or one phenolic hydroxyl group and one ester group. The most common substituents for the C-1, C-6 and C-9 are acetyl, angeloyl, tigloyl, propanoyl, benzoyl, isovaleryl and isobutyryl.
The recent published structures of biphenylcyclooctene lignans from Kadsura plants are shown in Fig. 2 and Table 1.
Fig. 2.
Structures of dibenzocyclooctadiene lignans in plants of Kadsura.
Table 1.
Dibenzocyclooctadiene lignans in plants of Kadsura.
| No. | Compound | Substituent groups | Structures of specific substituents | Sources | References |
|---|---|---|---|---|---|
| 1 | Kadheterin C | R1 = R3 = R4 = CH3, R2 = H, R5 = β-OAng, R6 = CH3, R7 = OH, R8 = OAng, R9 = H, R10 = CH3 |  |
K. heteroclita | (Luo et al., 2017) |
| 2 | Kadheterin D | R1 = R3 = R4 = CH3, R2 = H, R5 = β-OBz, R6 = CH3, R7 = OH, R8 = OAng, R9 = H, R10 = CH3 |  |
K. heteroclita | (Luo et al., 2017) |
| 3 | Kadheterin E | R1 = R3 = R4 = CH3, R2 = H, R5 = β-OAng, R6 = CH3, R7 = OH, R8 = OBz, R9 = H, R10 = CH3 | K. heteroclita | (Luo et al., 2017) | |
| 4 | Kadheterin F | R1 = R3 = R4 = CH3, R2 = H, R5 = β-OBz, R6 = OH, R7 = CH3, R8 = OBz, R9 = H, R10 = CH3 | K. heteroclita | (Luo et al., 2017) | |
| 5 | Kadheterin G | R1 = R3 = R4 = CH3, R2 = H, R5 = β-OIsobut, R6 = OH, R7 = CH3, R8 = OAng, R9 = H, R10 = CH3 | K. heteroclita | (Luo et al., 2017) | |
| 6 | Kadheterin H | R1 = R3 = R4 = CH3, R2 = H, R5 = β-OAng, R6 = OH, R7 = CH3, R8 = Olsoval, R9 = H, R10 = CH3 | K. heteroclita | (Luo et al., 2017) | |
| 7 | kadsutherin E | R1 = R2 = H, R3 = R4 = CH3, R5 = H, R6 = CH3, R7 = H, R8 = OBz, R9 = H, R10 = CH3 | K. interior | (Liu et al., 2018, Liu et al., 2018) | |
| 8 | 14-O-Demethyl polysperlignan D | R1 = H, R2 = R3 = R4 = CH3, R5 = β-OAng, R6 = CH3, R7 = H, R8 = OTig, R9 = H, R10 = CH3 |  |
K. coccinea | (Fang et al., 2014) |
| 9 | Heilaohulignan A | R1 = R3 = R4 = CH3, R2 = OIsobut, R5 = H, R6 = H, R7 = CH3, R8 = OH, R9 = H, R10 = CH3 | K. coccinea | (Liu, & Yang et al., 2018) | |
| 10 | Heilaohulignan B | R1 = R3 = R4 = CH3, R2 = OIsval, R5 = H, R6 = CH3, R7 = H, R8 = O, R9 = CH3, R10 = OH | K. coccinea | (Liu, & Yang et al., 2018) | |
| 11 | Heilaohulignan C | R1 = R3 = R4 = CH3, R2 = β-H, R5 = H, R6 = CH3, R7 = H, R8 = OTig, R9 = CH3, R10 = H | K. coccinea | (Liu, & Yang et al., 2018) | |
| 12 | Schizanrin O | R1 = R2 = R3 = R4 = CH3, R5 = OBz, R6 = OH, R7 = CH3, R8 = OProp, R9 = H, R10 = CH3 |
|
K. induta | (Minh et al., 2014) |
| 13 | Heilaohusu C | R1 = R3 = R4 = CH3, R2 = H, R5 = H, R6 = CH3, R7 = H, R8 = O, R9 = OAng, R10 = CH3 | K. coccinea | (Yang et al., 2019) | |
| 14 | Longipedlignan A | R1 = R3 = R4 = CH3, R2 = H, R5 = H, R6 = OH, R7 = CH3, R8 = OBz, R9 = H, R10 = CH3 | K. longipedunculata | (Liu, & Yang et al., 2018) | |
| 15 | Longipedlignan B | R1 = R3 = R4 = CH3, R2 = H, R5 = H, R6 = CH3, R7 = OH, R8 = OBz, R9 = H, R10 = CH3 | K. longipedunculata | (Liu, & Yang et al., 2018) | |
| 16 | Longipedlignan C | R1 = R3 = R4 = CH3, R2 = H, R5 = H, R6 = OH, R7 = CH3, R8 = OCin, R9 = H, R10 = CH3 |  |
K. longipedunculata | (Liu, & Yang et al., 2018) |
| 17 | Longipedlignan D | R1 = R3 = R4 = CH3, R2 = H, R5 = H, R6 = CH3, R7 = OH, R8 = OCin, R9 = H, R10 = CH3 | K. longipedunculata | (Liu, & Yang et al., 2018) | |
| 18 | Longipedlignan E | R1 = R3 = R4 = CH3, R2 = H, R5 = H, R6 = OH, R7 = CH3, R8 = OAng, R9 = H, R10 = CH3 | K. longipedunculata | (Liu, & Yang et al., 2018) | |
| 19 | Longipedunculatin D | R3 = R5 = CH3, R4 = H, R6 = OGlc, R7 = H, R8 = CH3, R9 = H, R10 = OH, R11 = H, R12 = CH3 | K. longipedunculata | (Liu et al., 2019) | |
| 20 | Renchangianin E | R1 = H, R2 = R3 = R5 = R6 = CH3, R4 = H, R7 = OBz, R8 = OH, R9 = H, R10 = OBz, R11 = H, R12 = CH3 | K. renchangiana | (Liu, Luo, Hu, Deng, & Chen, 2014) | |
| 21 | Heilaohusus D | R1 = H, R2 = R3 = R4 = R5 = R6 = CH3, R7 = OBz,R8 = CH3, R9 = OH, R10 = OAc, R11 = CH3, R12 = H |

|
K. coccinea | (Yang et al., 2019) |
| 22 | Heilaohuguosu H | R1 = OCap, R2 = β-OAc, R3 = OH, R4 = α-CH3 |  |
K. coccinea | (Jia et al., 2021) |
| 23 | Heilaohuguosu I | R1 = OAng, R2 = β-OAc, R3 = OH, R4 = α-CH3 | K. coccinea | (Jia et al., 2021) | |
| 24 | Heilaohuguosu J | R1 = OH, R2 = β-OCH3, R3 = OAc, R4 = α-CH3 | K. coccinea | (Jia et al., 2021) | |
| 25 | Heilaohuguosu K | R1 = OCH3, R2 = β-OCH3, R3 = OH, R4 = α-CH3 | K. coccinea | (Jia et al., 2021) | |
| 26 | Heilaohuguosu L | R1 = OCH3, R2 = β-OAc, R3 = OH, R4 = α-CH3 | K. coccinea | (Jia et al., 2021) | |
| 27 | Kadheterin B | R1 = OBz, R2 = OAng | K. heteroclita | (Luo et al., 2017) | |
| 28 | Heilaohuguosu A | R1 = OCH3, R2 = H, R3 = β-OH, R4 = OAng | K. coccinea | (Jia et al., 2021) | |
| 29 | Heilaohuguosu B | R1 = OCH3, R2 = OAng, R3 = α-OH, R4 = OH | K. coccinea | (Jia et al., 2021) | |
| 30 | Heilaohuguosu C | R1 = OH, R2 = OAng, R3 = β-H, R4 = OAc | K. coccinea | (Jia et al., 2021) | |
| 31 | Heilaohuguosu D | R1 = OCH3, R2 = OAng, R3 = β-H, R4 = methacry | K. coccinea | (Jia et al., 2021) | |
| 32 | Heilaohuguosu E | R1 = OCH3, R2 = OAng, R3 = β-H, R4 = OH |  |
K. coccinea | (Jia et al., 2021) |
| 33 | Heilaohuguosu F | R1 = OCH3, R2 = H, R3 = β-H, R4 = OIsobut | K. coccinea | (Chen, Luo, Zou, Lang, & Chen, 2014) | |
| 34 | Heilaohuguosu G | R1 = OCH3, R2 = H, R3 = β-H, R4 = OBz | K. coccinea | (Jia et al., 2021) | |
| 35 | Herteroclitin S | R1 = R2 = R4 = CH3, R3 = H, R5 + R6 = CH2, R7 = O, R8 = H, R9 = CH3, R10 = H | K. heteroclita | (Chen et al., 2014) | |
| 36 | Longipedlignan K | R1 = H, R2 = R3 = R4 = R5 = CH3, R6 = H, R7 = H, R8 = OBz, R9 = CH3, R10 = H | K. longipedunculata | (Liu et al., 2019) | |
| 37 | Heilaohusu A | R1 + R2 = CH2, R3 = R5 = R6 = CH3, R4 = OAng, R7 = H, R8 = OH, R9 = CH3, R10 = H | K. coccinea | (Yang et al., 2019) | |
| 38 | Heilaohusu B | R1 + R2 = CH2, R3 = R5 = R6 = CH3, R4 = OIsoval, R7 = H, R8 = OIsoval, R9 = CH3, R10 = H | K. coccinea | (Yang et al., 2019) | |
| 39 | Heilaohuguosu M | R1 = H, R2 = α-OH, R3 = H | K. coccinea | ||
| 40 | Longipedunin E | K. longipedunculata | (Zhang et al., 2018) | ||
| 41 | Kadsulignan W | K. heteroclita | (Shehla et al., 2018) | ||
| 42 | Schisantherin R | R1 + R2 = CH2, R5 + R6 = CH2, R3 = R4 = R7 = R10 = CH3, R8 = R9 = R11 = H | K. coccinea | (Xu, Su, Wei, Zhang, & Li, 2018) | |
| 43 | Schisantherin S | R1 = R2 = R3 = R4 = R5 = R6 = R7 = R10=CH3, R8 = R9 = R11 = H | K. coccinea | (Xu et al., 2018) | |
| 44 | Kadheterin A | K. heteroclita | (Luo et al., 2017) | ||
| 45 | Longipedunin | K. longipedunculata | (Guo, Gao, Zhang, & Liu, 2016) | ||
| 46 | Heilaohuguosu N | R1 = R2 = CH3, R3 = R4 = H | K. coccinea | (Jia et al., 2021) |
2.1.2. Spirobenzofuranoid dibenzocyclooctadiene
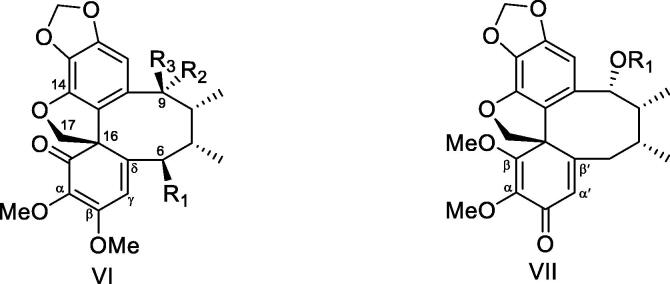
Spirobenzofuranoid dibenzocyclooctadiene lignan contains a new C-16 centric furan nucleus as compared with dibenzocyclooctadiene lignans, which forms by adding other oxygen-containing substituents to it. Meanwhile, it also has an enketonation on the aromatic ring of C-16. This kind of compounds were isolated from Kadsura plants. Most screw rings are formed from the oxygen-containing substituents of C-14 and C-16. There are two types of enketonations (Fig. 3) on the aromatic ring of these chemical compounds, the α, β, γ, δ-dienone (VI) and α, β, α', β'-dienone (VII) (Kuo et al., 2005a, Kuo et al., 2005b). The compositions of spirobenzofuranoid dibenzocyclooctadienes isolated from Kadsura plants in recent years are shown in Fig. 4 and Table 2.
Fig. 3.
Structural skeletons (VI: α, β, γ, δ-dienone, VII:α, β, α', β'-dienone) of spirobenzofuranoid dibenzocyclooctadiene lignans from plants of Kadsura.
Fig. 4.
Structures of spirobenzofuranoid dibenzocyclooctadiene lignans in plants of Kadsura.
Table 2.
Spirobenzofuranoid dibenzocyclooctadiene lignans isolated from plants of Kadsura.
| No. | Compound | Substituent groups | Structures of specific substituents | Source | References |
|---|---|---|---|---|---|
| 47 | Kadsutherin F | R1 = OAng, R2 = OH | K. interior | (Liu et al., 2018, Liu et al., 2018) | |
| 48 | Kadsutherin G | R1 = OBz,R2 = OH | 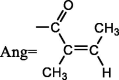 |
K. interior | (Liu et al., 2018, Liu et al., 2018) |
| 49 | Kadsutherin H | R1 = OAc,R2 = OH | 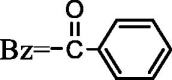 |
K. interior | (Liu et al., 2018, Liu et al., 2018) |
| 50 | Longipedlignan M | R1 = R2 = CH3, R3 = H, R4 = OH, R5 = CH3, R6 = CH3, R7 = H, R8 = OCin |  |
K. longipedunculata | (Liu et al., 2019) |
| 51 | Longipedlignan N | R1 = R2 = CH3, R3 = H, R4 = CH3, R5 = OH, R6 = CH3, R7 = H, R8 = OCin | 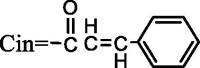 |
K. longipedunculata | (Liu et al., 2019) |
| 52 | Herteroclitin R | R1 = R2 = CH3, R3 = O, R4 = CH3, R5 = H, R6 = CH3, R7 = H, R8 = OAng | K. heteroclita | (Chen et al., 2014) | |
| 53 | Longipedlignan F | R1 = R2 = CH3, R3 = H, R4 = OH, R5 = CH3, R6 = CH3, R7 = H, R8 = OBz | K. longipedunculata | (Liu, & Pandey et al., 2018) | |
| 54 | Longipedlignan G | R1 = R2 = CH3, R3 = H, R4 = CH3, R5 = OH, R6 = CH3, R7 = H, R8 = OBz | K.longipedunculata | (Liu, & Pandey et al., 2018) | |
| 55 | longipedlignan H | R1 = R2 = CH3, R3 = OH, R4 = CH3, R5 = H, R6 = CH3, R7 = H, R8 = OAc | K. longipedunculata | (Liu, & Pandey et al., 2018) | |
| 56 | Longipedlignan I | R1 = R2 = CH3, R3 = OH, R4 = CH3, R5 = H, R6 = CH3, R7 = H, R10 = Obutanoyl | K. longipedunculata | (Liu, & Pandey et al., 2018) | |
| 57 | Longipedlignan J | R1 = R2 = CH3, R3 = OH, R4 = CH3, R5 = H, R6 = CH3, R7 = H, R8 = OAng | K. longipedunculata | (Liu, & Pandey et al., 2018) | |
| 58 | Longipedlignan L | K. longipedunculata | (Liu, & Pandey et al., 2018) | ||
| 59 | Longipedlignan O | R = Bz | K. longipedunculata | (Liu, & Pandey et al., 2018) | |
| 60 | longipedlignan P | R = Ac | K. longipedunculata | (Liu, & Pandey et al., 2018) | |
| 61 | Kadlongilignan E | (Shao, Qi, Sun, & Li, 2020) | |||
| 62 | Kadlongilignan F | (Shao et al., 2020) | |||
| 63 | Longipedunculatin A | R1 = H, R2 = Glc, R3 = Ang | (Liu, & Pandey et al., 2018) | ||
| 64 | Longipedunculatin B | R1 = Glc, R2 = H, R3 = Ang | (Liu, & Pandey et al., 2018) | ||
| 65 | Longipedunculatin C | R1 = H, R2 = Glc, R3 = 2-Methybutyryl | (Liu, & Pandey et al., 2018) |
2.1.3. Aryltetralin
Six lignans of aryltetrahydrothalene type (Table 3) were isolated and identified from Schisandra in rencent seven years, there are (7′S, 8′S, 8R)-(8β, 8′α)-dimethyl-4, 4′-dihydroxy-5, 3′-dimethoxy-5′- cyclolignan glucoside (66) (Yeon, Cheng, He, & Kong et al., 2014), heilaohusu E (67) (Yang, Liu, Daniyal, Yu, & Wang, 2019), and Heilaohuguosus O-R (68–71) (Jia et al., 2021). Their structures are shown in Fig. 5.
Tabe 3.
Aryltetralin lignanoids isolated from plants of Kadsura.
| No. | Compound | Substituent groups | Source | References |
|---|---|---|---|---|
| 66 | (7′S, 8′S, 8R)-(8β, 8′α)-dimethyl-4, 4′-dihydroxy-5, 3′-dimethoxy-5′- cyclolignan glucoside | K. coccinea | (Yeon et al., 2014) | |
| 67 | Heilaohusu E | R1 = R2 = R5 = R6 = OCH3, R3 = R4 = OH | K. coccinea | (Yang et al., 2019) |
| 68 | Heilaohuguosu O | R1 = R6 = OH, R2 = R3 = R4 = R5 = OCH3 | K. coccinea | (Jia et al., 2021) |
| 69 | Heilaohuguosu P | R1 = R2 = OCH3, R3 = R6 = OH, R4 = R5 = OCH3 | K. coccinea | (Jia et al., 2021) |
| 70 | Heilaohuguosu Q | R1 = R2 = OCH3, R3 = H, R4 = R5 = OCH3 R6 = OH, | K. coccinea | (Jia et al., 2021) |
| 71 | Heilaohuguosu R | R1 = R3 = R4 = R6 = OCH3, R2 = R5 = OH | K. coccinea | (Jia et al., 2021) |
Fig. 5.
Structures of aryltetralin, diarylbutane, tetrahydrofurans and new lignans in plants of Kadsura.
2.1.4. Diarylbutane
In recent years, three dibenzylbutane type lignans, such as kadsurindutin E (72) (Fang, Xie, Wang, Jin, Xu, Guo, & Ma, 2014), coccilignan A (73) (Fang et al., 2014), kadsuphilin J (74) (Shen et al., 2008) were isolated from Schisandrae. Structures are shown in Fig. 5.
2.1.5. Tetrahydrofurans
Only one tetrahydrofuran type lignan (Fig. 5), heilaohuguosu S (75) (Jia et al., 2021) was isolated from Schisandrae.
2.1.6. New lignans
Six new lignans have been found from the plants of the genus Kadsura. longipedlignan R (76) (Liu, Pandey, Wang, Adams, & Li, 2019), kadlongilignan A–D (77–80) (Qi, Liu, Chen, Hou, & Li, 2020), coumarinlignan (81) (Su et al., 2015). Their structures are shown in Fig. 5.
3. 1H NMR and 13C NMR spectrum characteristics of lignans from genus Kadsura
Lignans are dominant constituents of genus Kadsura, and a large number of 13C and 1H NMR chemical shift data of lignans have been reported, but these data are scattered in the literatures, we collected and analysis these NMR data, and it will be of value to provide easy access to elucidate the sturctures of lignans isolated from Kadsura. This paper reports the NMR data compilation of the main skeletons of these compounds.
3.1. 13C NMR characteristics of lignans
3.1.1. Biphenyclooctene lignans
3.1.1.1. Aromatic carbon (C-4 and C-11)
The chemical shift of carbon on biphenyl ring conforms to the empirical rule that substituent's chemical effects of the carbon atom on benzene ring (Chen, Qin, & Xie, 2001). Taking the structure of Fig. 6 as an example, the chemical shift of C4 and C11 is shown in Table 4.
Fig. 6.
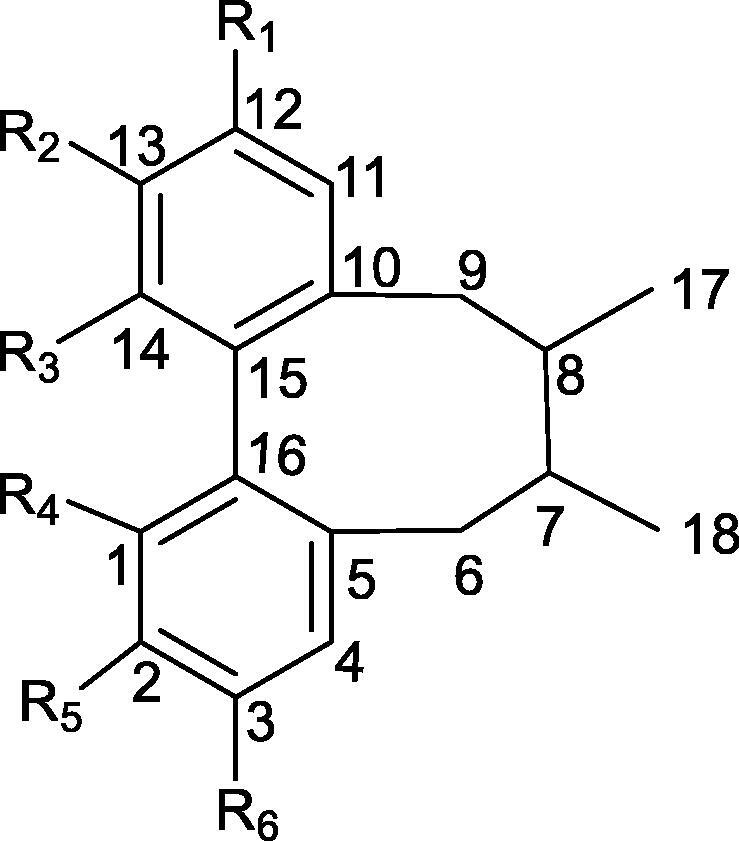
Structures of biphenyclooctene skeletonwise.
Table 4.
Chemical shift of C4 and C11 at different substituents on benzene ring.
| Substituent groups |
δC (C4 and C11) | ||
|---|---|---|---|
| R1,6 | R2,5 | R3,4 | |
| OMe | OMe | OMe | 106.9–111.9 |
| OH | OMe | OMe | 109.9–110.2 |
| OMe | OMe | OH | 105.9–107.7 |
| OCH2O | OMe | 101.1–103.1 | |
3.1.1.2. Aromatic quaternary carbon
The chemical shift of aromatic quaternary carbon is mainly affected by its own substituents, and the change of chemical shift is shown in Table 5.
Table 5.
Aromatic quaternary carbon chemical shift.
| Position of carbon | Substituents (R) | Chemical shift of carbon |
|---|---|---|
| C12, C3 | OMe | 153.3–150.2 |
| OCH2O | 147.9–149.6 | |
| C13, C2 | OMe | 133.6–142.1 |
| OCH2O | 132.8–136.9 | |
| C1, C14 | OR | 141.0–142.0 |
| OMe | 135.2–151.4 | |
| OH | 146.7–149.0 | |
| C15, C16 | 115.2–124.8 |
Note: R = Ang, Tig, Isoval, Bz, Ac…
3.1.1.3. Cyclooctene carbon (C-7 and C-8)
When there are methyl groups on the C-7 and C-8 bits, the chemical shift of C-7 and C-8 methyl carbon are around δC 32.2–43.9 ppm, if the hydrogen on C-7 are replaced by hydroxyl, the chemical shift of C-7 shows 71.8–80.9 ppm and C-8 is about 42.1–49.2 ppm.
3.1.1.4. C-6 and C-9
When C-6 and (or) C-9 are carbonyl groups, their chemical shifts are related to whether they are conjugated with the benzene ring. When there are different ester groups substituted C-6 and (or) C-9, the chemical shifts are between 80.2 and 86.6 (Table 6) (Chen et al., 2001).
Table 6.
Chemical shift of C6 and C9.
| Substituent groups | δC (C6, C9) | |
|---|---|---|
| OR | 80.2–86.6 | |
| C O | Conjugated to aromatic | 200.4–203.7 |
| Not-conjugated to aromatic | 208.3–210.4 | |
Note: R = Ang, Tig, Isoval, Bz, Ac…
3.1.1.5. Methoxy carbon
The chemical shift of methoxy which has an unsubstituted ortho-carbon is bigger. For example, the chemical shift of methoxy group of C-3 and C-12 was 55.0 ppm, and the other methoxy group was around δC 60.0 ppm.
3.1.1.6. Methyl on cyclooctene (C17 and C18)
The chemical shift of methyl on the cyclooctene is affected by the relative configuration of methyl groups on the eight-member ring, when C-7, C-8 was only substituted by methyl, the chemical shift of α-methyl was between 8.2 and 15.7 ppm, β-methyl was 17.5–20.4 ppm. When the hydrogen on C-7 is replaced by hydroxyl group, the chemical shift of C-17 and C-18 is shown in Table 7.
Table 7.
Chemical shift of C17 and C18.
| Substituent groups | Corresponding substituents | Chemical shift |
|---|---|---|
| 7α-OH | 18-β-CH3 | 28.8–31.5 |
| 17-α-CH3 | 16.8–17.9 | |
| 7β-OH | 18-α-CH3 | 21.4–24.3 |
| 17-β-CH3 | 17.0–17.9 |
3.1.1.7. Typical substituents
Benzoyl group: δC 65.6 (C O), δC 129.9 (C-2', 6'), δC l28.1 (C-3 ', 5 '), δC 132.8 (C-1') (Chen, Zhang, Chen, Zhou, & Lee, 1996). acetyl group: δC 169.3 (C O), δC 21.2 (-Me); angeloy group: δC l5.8 (α-Me), 20.8 (β-Me), δC l27.6, δC138.7 (C C), δC l66.4 (C O) (Luo et al., 2017); isobutyryl group: δC 176.6(C O), δC 33.7(–CH); δC18.7 (α-Me), δC 19.2 (β-Me) (Yang et al., 2019), propanoyl group: δC 173.6(C O), δC 27.3(–CH2); δC 8.6 (-Me) (Hu et al., 2012).
3.1.2. 13C NMR spectral characteristics of spirobenzofuranoid dibenzocyclooctadienes lignans
Chemical shift of α, β, α’, β’-dienone carbonyl group is about 183.0 ppm, and that of α, β, γ, δ-dienone carbonyl group nears 196.0 ppm. The chemical shift of C-16 and C-17 in the spisobenzofuran ring are about 56.0–65.0 ppm and 78.0–85.0 ppm, respectively. When acyl groups were attached to C-6 and C-9, the chemical shifts of these two carbons are 78.0–85.0 ppm (Lin et al., 2013). Take the structure of Fig. 3 as example, the chemical shift is shown in Table 8.
Table 8.
13C NMR spectral characteristics of spirobenzofuranoid dibenzocyclooctadienes lignans.
| Structure types | Dienone carbonyl groups | Chemical shift |
||
|---|---|---|---|---|
| C17 | C16 | C6 and C9 | ||
| α, β, α’, β’ | 194.6–197.8 | 55.0–56.9 | 79.2–84.3 | 78.0–85.0 |
| α, β, γ, δ | 165.8–183.5 | 61.0–66.7 | 79.1–81.9 | 78.0–85.0 |
3.2. 1H NMR spectrum characteristics of lignans from genus Kadsura
3.2.1. Biphenyclooctene lignans
The chemical shift of hydrogen on biphenyclooctene lignans empirical rule is shown in Table 9.
Table 9.
1H NMR chemical shift of biphenyclooctene lignans.
| Positions | Chemical shift | |
|---|---|---|
| H4, H11 | 5.9–7.0 | |
| H7, H8 | 1.7–2.8 | |
| H6, H9 | Without substituents | 2.0–2.7 |
| Oxygen-containing substituents | 4.0–6.0 | |
| OCH3 | 3.2–3.9 | |
| OCH2O | 5.6–6.0 | |
| Cyclooctent moiety methyl proton | C7 is substituted for OH | H17 (1.1–1.4) |
| H18 (1.3–1.4) | ||
| H17, H18 | C7 only has methyl substitution | H17,18 (0.6–1.1) |
3.2.1.1. Aromatic proton
The chemical shift of the two aromatic protons H-4 and H-11 in biphenyl ring is 5.9–7.0 ppm (each 1H, s). The two aromatic protons H-4 and H-11 are equivalent in symmetric planar struc-ture, and the chemical shift of these two aromatic protons is related to ortho substituent. The ortho proton of methylenedioxy appears in the higher field than ordinary proton, and ortho proton of hydroxyl appears in the lower field (Chen et al., 2000). If an ester is substituted at C-6 or C-9, H-4 or H-11 should be deshielded, and their chemical shift increment is 0.1 ppm, when 6-OH is α-orientation, the chemical shift of H-4 is bigger than that of β-orientation (Wang & Chen, 1985).
3.2.1.2. Methoxy proton
Chemical shift of the methoxy group on the aromatic ring is 3.2–3.9 ppm, and that of methoxy groups on C-14 and C-1 are lower than the methoxy groups on other positions of same benzene ring, because of the shielding effect of the adjacent aromatic ring. When the methylenedioxy group and methoxy groups are on the same aromatic ring, the chemical shifts of methoxy groups in the same ring move to the lower field (Chen et al., 2000).
3.2.1.3. Methylene dioxy proton
Chemical shift of methylenedioxy group on the aromatic ring is about 5.6–6.0 ppm.
3.2.1.4. Cyclooctent moiety methyl proton
CH3-17 and CH3-18 are cis-form when there is no hydroxyl group substituted on C-7 and C-8, signals of CH3-17 and CH3-18 are two non-equivalent doublets (δH 0.7–1.0 ppm, J = 7.0 Hz). When a hydroxyl group substituted on C-7, CH3-18 is a singlet, δH 1.1–1.3 ppm.
3.2.1.5. H-6 and H-9
Chemical shift of H-6 and H-9 is about 2.0–2.7 ppm (each 2H, ABX), when there is no oxygen-containing substitution on C-6 and C-9. If there are hydroxyl or ester groups on C-6 or C-9, when H-6/ H-7 (or H-9/H-8) are trans-form, H-6 or H-9 are doublets (4.0–6.0 ppm, J = 8.0 Hz) and C-6 hydroxyl or ester group is β-oriented, C-9 hydroxyl or ester group is α-oriented. And when H-6/H-7 (or H-9/H-8) are cis-form, H-6 (or H-9) is singlet, and C-6 hydroxyl is α-oriented (Chen et al., 2000). Therefore, the configurations of C-6, C-9 and cyclooctene can be inferred from the multiplets and coupling constants.
3.2.1.6. H-7 and H-8
Chemical shifts of H-7 and H-8 are generally in the range of 1.7–2.2 ppm, which is related to the substituent, configuration, and conformation of the cyclooctent ring.
3.2.1.7. Typical acyl substituents
Benzoyl group: δH 7.20–7.50 (5H, m); Angeloy group: There are three group of signals which are δH 1.78 (3H, dq, J = 7.5, 1.5 Hz), 1.30 (3H, q, J = 1.5 Hz) and 5.80–6.00 (1H, m); Cis angelyl group: δH1.64 (3H, d, J = 7.0 Hz), 1.54 (3H, s), 6.78 (1H, m).
3.2.2. 1H NMR characteristics of spirobenzofuranoid dibenzocyclooctadienes lignans
Characteristic signal of spirobenzofuranoid dibenzocyclooctadienes lignans is the signal of 17-OCH3, its chemical shift is 4.0–5.0 ppm (2H, dd, J = 9.0 Hz), indicating the C-17 was connected to C-16.
3.2.2.1. h-4
Characteristic proton spectra of olefinic proton H-4 is δ 5.8–7.3 ppm, singlet or doublet (J = 1.5 Hz).
3.2.2.2. H-6 and H-9
The orientation of acyloxy group or hydroxyl group at C-6 or C-9 can be determined from the coupling constants J6, 7, and J8, 9. acyloxy group or hydroxyl groups on C-6 are generally β oriented, if there is no hydroxyl on C-7, J6, 7 is around 10.0 Hz, and if there is a hydroxyl on C-7, H-6 is a doublet (J = 1.5 Hz), for the long-range coupling between H-4 and H-6. Acyloxy group on C-9 is generally α-oriented, when the cyclooctent ring is a boat chair conformation, J8β, 9β is about 7.0 Hz or cyclooctent ring is a boat conformation J8β, 9β is about 0 Hz (Kuo, Kuo, & Chen, 1997).
4. Prediction lignans as Q-marker of genus Kadsura
In recent years, researchers are increasingly concerned about TCM quality control system. A few years ago, the new concept of a TCM quality marker was proposed by Liu et al. (2016). Q-marker of TCM is intrinsic chemicals that exist in herbs and in products made from herbs. In order to be indicators of quality control, these compounds should be associated with the functions and properties of the TCM in question, so that they can reflect its safety and efficacy.
Q-marker of Kadsura was predicted by plant phylogenetic relationship, pharmacodynamics, identifiable chemical compositions, injectable compositions.
4.1. Q-marker prediction analysis based on original plant phylogeny and characteristic chemical components
Kadsura Kaempf. ex Juss., belonging to the family Schisandroideae, has 29 species of plants, mainly growing in east and southeast of Asia. There are 10 species of this genus in China, most of them are distributed in the southeast and southwest of China, including Yunnan, Guizhou, Sichuan, Guangdong, Guangxi, Fujian and other provinces (Dong et al., 2014), Chinese Pharmacopeia (2015 Edition) lists Kadsura species such as K. interior, K. coccinea, K. longipedunculata, and K. heteroclita, etc. Among these species of the genus Kadsura, lignans are the dominant constituents. At present, more than 300 lignans have been isolated from Kadsura. Lignans are biosynthesised from shikimic acid (or cinnamic acid) pathway, shikimic acid (or cinnamic acid) is generated from phenylalanine through deamination and oxidation, then lignans are synthesized from cinnamic acid and benzoic acid. Based on the above analysis lignans are considered as Q-marker of Kadsura plants.
4.2. Q-marker prediction analysis based on chemical compositions and pharmacodynamics
Q-marker is the main index for evaluating and controlling the effectiveness of traditional Chinese medicine, it closely related to the effectiveness. The reported lignans from Kadsura plant possess a series of pharmacological activities, such as anti-cancer (Kuo et al., 2005a, Kuo et al., 2005b), anti-tumor (Xu, Peng, Chen, Wang, & Xiao, 2010), anti-HIV (Pu et al., 2008, Sun et al., 2011), anti-inflammatory (Lin, Shen, Shen, & Tsai, 2006), anti-platelet aggregation (Lu & Chen, 2009), nitric oxide inhibition (Awale et al., 2003, Mulyaningsil et al., 2010) and neuroprotective effects (Dong, Pu, Zhang, Du, & Sun, 2012).
The stem of Kadsura plant is mainly used for promoting blood circulation, relieving pain, removing wind and dehumidifying. Heilaohulignan C from Kadsura coccinea, showed good cytotoxicity in HepG-2 human liver cancer cells with IC50 values of 9.92 µM (Kuo et al., 2005). Interiorin A and interiorin B isolated from Kadsura heteroclita showed anti-HIV activity with EC50 1.6 and 1.4 μg/mL respectively (Pu et al., 2018). The in vitro anti-inflammatory assay of lignans longipedunculatin A, longipedlignan M, and longipedlignan J showed significant inhibitory effective with inhibition rates in 55.1%, 74.9%, and 89.8% respectively (Dong et al., 2014). Acetylepigomisin R, isovaleroylbinankadsurin A and binankadsurin A isolated from Kadsura coccinea have the effect of protecting rat liver injury caused by tert-butyl hydrogen peroxide, with ED50 135.7, 26.1 and 79.3 mol/L, respectively (Dong et al., 2014). The above studies indicate that lignans are important active substances of Kadsura plants and can be used as Q-marker.
4.3. Q-Marker prediction analysis of identifiable chemical composition
The identifiable of chemical components is basic conditions of Q-marker. The determination of chemical composition is mainly by chromatographic analysis. At present, the relevant literature on the chemical composition of Kadsura plants is summarized and found that lignans in Kadsura are qualitatively identified and determined by column chromatography, HPLC (Chen, Wang, & Song, 2018), UV spectrophotometry, infrared fingerprint method (Sun, Xu, Xu, Xin, & Huang, 2012), near infrared spectroscopy, ultra-high performance liquid chromatography (Deng, Wang, Yan, & Yin, 2017), liquid chromatography-ion trap mass spectrometric (LC-MS/MS) (Tian, Xu, Hu, Zhao, & Liu, 2012).
4.4. Q-Marker prediction analysis based on the injectable components
The complexity of the components of TCM is the basis on its various effects and pharmacological actions. Although the chemical composition is complex, it is only absorbed into the bloodstream and takes effect while reaching a certain blood concentration in the body (Shi et al., 2019). Studies have found that lignans are absorbed faster in the stomach than other organs. Lignans are mainly distributed in the liver, and exist in hepato-intestinal circulation, entero-intestinal circulation or gastro-intestinal circulation in the body (Wang et al., 2014). As Q-marker indicators, lignans provide significant reference for the quality control and surveillance research of genus Kadsura.
5. Conclusion and future perspectives
Lignans are the major effective components of genus Kadsura. Genus Kadsura plants are widely distributed in China, which possess unique resource superiority. Eighty-one lignans have been separated and identified from this genus in the past eight years, including dibenzocyclooctadienes, spirobenzofuranoid dibenzocyclooctadienes, aryltetralins, and neolignans. 1H NMR and 13C NMR spectral characteristics of lignans compounds are summarized in this paper. Based on Q-marker and the analysis of phylogenetic relationship and effective components of Kadsura, lignans were predicted to be one of the quality markers of Kadsura plants. Thus, the research and utilization of genus Kadsura based on lignans will have an extensive prospect.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 81703819, 81874369 and 82074122), and Natural Science Foundation of Hunan Province (No. 2020JJ4463 and 2020JJ4064).
Contributor Information
Wei Wang, Email: wangwei402@hotmail.com.
Chang-xiao Liu, Email: liuchangxiao@163.com.
References
- Awale S., Tezuka Y., Banskota A.H., Adnyana I.K., Kadota S. Nitric oxide inhibitory isopimarane-type diterpenes from Orthosiphon stamineus of Indonesia. Journal of Natural Products. 2003;66(2):255–258. doi: 10.1021/np020455x. [DOI] [PubMed] [Google Scholar]
- Chen M., Luo Y.P., Zou Y.L., Lang L.H., Chen D.F. Heteroclitins R-S: New dibenzocylooctadiene lignans from Kadsura heteroclita. Chinese Journal of Natural Medicines. 2014;12(9):689–692. doi: 10.1016/S1875-5364(14)60105-9. [DOI] [PubMed] [Google Scholar]
- Chen Y.G., Qin G.W., Xie Y.Y. 1H NMR spectroscopic characteristics of lignans from plants of Schisandraceae. Chinese Journal of Magnetic Resonance. 2000;5:427–432. [Google Scholar]
- Chen Y.G., Qin G.W., Xie Y.Y. 13C NMR spectroscopic characteristics of dibenzocyclooctadiene lignans from plants of Schisandraceae. Chemical Research. 2001;1:55–58. [Google Scholar]
- Chen M., Wang L., Song X.M. Determination of lignan content in Schisandra chinensis by HPLC. Journal of Northwest University. 2018;41(3):459–546. [Google Scholar]
- Chen D.F., Zhang S.X., Chen K.E., Zhou B.N., Lee K.H. Two new lignans, interiotherins A and B, as anti-HIV principles from Kadsura interior. Journal of Natural Products. 1996;59(11):1066–1068. doi: 10.1021/np9601667. [DOI] [PubMed] [Google Scholar]
- Deng L., Wang H.B., Yan S.C., Yin C.P. Simultaneous determination of seven lignans in Schisandra sphenanthera Rehd.et Wils. and Schisandra chinensis Bail. by UPLC. Chinese Journal of Hospital Pharmacy. 2017;37(12):1158–1162. [Google Scholar]
- Dong W.X., Shu Y.Z., Liu Y., He Q.Q., Yan Z.H. Research progress on chemical constituents in plants of Kadsura Kaempf. ex Juss. and their pharmacological activity. Chinese Traditional and Herbal Drugs. 2014;45(13):1938–1959. [Google Scholar]
- Dong K., Pu J.X., Zhang H.Y., Du X., Sun H.D. Dibenzocyclooctadiene lignans from Kadsura polysperma and their antineurodegenerative activities. Journal of Natural Products. 2012;75(2):249–256. doi: 10.1021/np200937h. [DOI] [PubMed] [Google Scholar]
- Fang L., Xie C., Wang H., Jin D.Q., Xu J., Guo Y., Ma Y. Lignans from the roots of Kadsura coccinea and their inhibitory activities on lps-induced no production. Phytochemistry Letters. 2014;9:158–162. [Google Scholar]
- Guo Y.J., Gao S.M., Zhang B.G., Liu H.T. Chemical constituents from stems of Kadsura longipedunculata. Journal of Chinese Medicinal Materials. 2016;39(6):1287–1289. [PubMed] [Google Scholar]
- Hu W., Li L., Wang Q., Ye Y., Fan J., Li H.X., Li H.R. Dibenzocyclooctadiene lignans from kadsura coccinea. Journal of Asian Natural Products Research. 2012;14(4):364–369. doi: 10.1080/10286020.2011.654334. [DOI] [PubMed] [Google Scholar]
- Jia Y.Z., Yang Y.P., Cheng S.W., Cao L., Xie Q.L., Wang M.Y., Wang W. Heilaohuguosus A-S from the fruits of Kadsura coccinea and their hepatoprotective activity. Phytochemistry. 2021;184:112678. doi: 10.1016/j.phytochem.2021.112678. [DOI] [PubMed] [Google Scholar]
- Kuo Y.H., Kuo L.M., Chen C.F. Four new C19 homolignans, schiarisanrins A, B, and D and cytotoxic schiarisanrin C, from Schizandra arisanensis. The Journal of Organic Chemistry. 1997;62(10):3242–3245. doi: 10.1021/jo9622542. [DOI] [PubMed] [Google Scholar]
- Kuo Y.H., Wu M.D., Huang R.L., Kuo L.M.Y., Ong C.W. Antihepatitis activity (anti-HBsAg and anti- HBeAg) of C19 homolignans and six novel C18 dibenzocyclooctadiene lignans from Kadsura japonica. Planta Medica. 2005;71(7):646–653. doi: 10.1055/s-2005-871271. [DOI] [PubMed] [Google Scholar]
- Kuo Y.H., Wu M.D., Hung C.C., Huang R.L., Yang Kuo L.M., Shen Y.C., Ong C.W. Syntheses of C(18) dibenzocyclooctadiene lignan derivatives as anti-HBsAg and anti-HBeAg agents. Bioorganic & Medicinal Chemistry. 2005;13(5):1555–1561. doi: 10.1016/j.bmc.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Lin Y.C., Cheng Y.B., Liaw C.C., Lo I.W., Kuo Y.H., Michael C.…Shen Y.C. New lignans from the leaves and stems of Kadsura philippinensis. Molecules. 2013;18(6):6573–6583. doi: 10.3390/molecules18066573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L.C., Shen C.C., Shen Y.C., Tsai T.H. Anti-inflammatory neolignans from piper kadsura. Journal of Natural Products. 2006;69(5):842–844. doi: 10.1021/np0505521. [DOI] [PubMed] [Google Scholar]
- Liu C.X., Chen S.L., Xiao X.H., Zhang T.J., Hou W.B., Liao M.L. A new concept on quality marker of Chinese materia medica: Quality control for Chinese medicinal products. Chinese Traditional and Herbal Drugs. 2016;47(9):1443–1457. [Google Scholar]
- Liu H.T., Liu J.S., Zhang J., Guo Y.J., Qi Y.D., Jia X.G., Zhang B.G. Chemical constituents in plants of genus Kadsura kaempf. ex Juss. Chinese Herbal Medicines. 2014;6(3):172–197. [Google Scholar]
- Liu S., Luo Y.P., Hu Y.J., Deng L.Q., Chen M. Renchangianin E: A new dibenzocyclooctadiene lignan from Kadsura renchangiana. Acta Pharmaceutica Sinica. 2014;49(10):1438–1441. [PubMed] [Google Scholar]
- Liu J., Pandey P., Wang X., Adams K., Li S. Hepatoprotective tetrahydrobenzocyclooctabenzofuranone lignans from Kadsura longipedunculata. Journal of Natural Products. 2019;82(10):2842–2851. doi: 10.1021/acs.jnatprod.9b00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.B., Pandey P., Wang X.J., Qi X.Z., Hua C., Zhang P.C.…Li S. Hepatoprotective dibenzocyclooctadiene and tetrahydrobenzocyclooctabenzofuranone lignans from Kadsura longipedunculata. Journal of Natural Products. 2018;81(4):846–857. doi: 10.1021/acs.jnatprod.7b00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yang Y., Tasneem S., Hussain N., Daniyal M., Yuan H.…Wang W. Lignans from Tujia Ethnomedicine Heilaohu: Chemical characterization and evaluation of their cytotoxicity and antioxidant activities. Molecules. 2018;23(9):2147. doi: 10.3390/molecules23092147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.S., Zhang J., Qi Y.D., Jia X.G., Zhang B.G., Liu H.T. Four new lignans from kadsura interior and their bioactivity. Molecules. 2018;23(6):1279–1288. doi: 10.3390/molecules23061279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Chen D.F. Analysis of Schisandra chinensis and Schisandra sphenanthera. Journal of Chromatography A. 2009;1216(11):1980–1990. doi: 10.1016/j.chroma.2008.09.070. [DOI] [PubMed] [Google Scholar]
- Luo Y.Q., Liu M., Wen J., Wang W.G., Hu K., Li X.N., Sun H.D. Dibenzocyclooctadiene lignans from kadsura heteroclita. Fitoterapia. 2017;119:150–157. doi: 10.1016/j.fitote.2017.04.013. [DOI] [PubMed] [Google Scholar]
- Minh P.T.H., Lam D.T., Tien N.Q., Tuan N.N., Kim S.H. New dibenzocyclooctadiene lignan from Kadsura induta and their cytotoxic activities. Bulletin of the Korean Chemical Society. 2014;35(6):1859–1862. [Google Scholar]
- Mulyaningsil S., Youns M., EI-Readi M.Z., Ashour M.L., Nibret E., Sporer F.…Wink M. Biological activity of the essential oil of Kadsura longipedunculata, (Schisandraceae) and its major components. Journal of Pharmacy and Pharmacology. 2010;62(8):1037–1044. doi: 10.1111/j.2042-7158.2010.01119.x. [DOI] [PubMed] [Google Scholar]
- Pu J.X., Yang L.M., Xiao W.L., Li R.T., Lei C., Gao X.M.…Huang H. Compounds from Kadsura heteroclita and related anti-HIV activity. Phytochemistry. 2008;69:1266–1272. doi: 10.1016/j.phytochem.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Qi X., Liu J., Chen J., Hou Q., Li S. New seco-dibenzocyclooctadiene lignans with nitric oxide production inhibitory activity from the roots of Kadsura longipedunculata. Chinese Chemical Letters. 2020;31(2):423–426. [Google Scholar]
- Shao S.Y., Qi X.Z., Sun H., Li S. Hepatoprotective lignans and triterpenoids from the roots of Kadsura longipedunculata. Fitoterapia. 2020;142:104487. doi: 10.1016/j.fitote.2020.104487. [DOI] [PubMed] [Google Scholar]
- Shehla N., Li B., Zhao J.P., Cao L., Jian Y.Q., Khan I.A., Wang W. New dibenzocyclooctadiene lignan from stems of Kadsura heteroclita. Chemistry of Natural Compounds. 2018;54(5):837–840. doi: 10.1080/14786419.2020.1758378. [DOI] [PubMed] [Google Scholar]
- Shen Y.C., Lin Y.C., Cheng Y.B., Chang C.J., Lan T.W., Liou S.S., Khalil A.T. New oxygenated lignans from Kadsura philippinensis. Helvetica Chimica Acta. 2008;91(3):483–494. [Google Scholar]
- Shi Y.P., Kong H.T., Li H.N., Li X.B., Zhang Y., Han L.W., Liu K.C. Research progress on chemical composition and pharmacological effects of Gardenia jasminoides and predictive analysis on quality marker (Q-marker) Chinese Traditional and Herbal Drugs. 2019;50(2):281–289. [Google Scholar]
- Su W., Zhao J., Yang M., Yan H.W., Pang T., Chen S.H., Wang W. A coumarin lignanoid from the stems of Kadsura heteroclita. Bioorganic & Medicinal Chemistry Letters. 2015;25(7):1506–1508. doi: 10.1016/j.bmcl.2015.02.022. [DOI] [PubMed] [Google Scholar]
- Sun R., Song H.C., Wang C.R., Shen K.Z., Xu Y.B., Gao Y.X., Dong J.Y. Compounds from Kadsura angustifolia with anti-HIV activity. Bioorganic & Medicinal Chemistry Letters. 2011;21(3):961–965. doi: 10.1016/j.bmcl.2010.12.055. [DOI] [PubMed] [Google Scholar]
- Sun H., Xu P.H., Xu H.X., Xin S.H., Huang Z.J. Identification on different habitats of Schisandra sphenanthera Rehd. et Wils. by FT-IR fingerprint. Chinese Journal of Hospital Pharmacy. 2012;32(15):1204–1205. [Google Scholar]
- Tian Z.H., Xu J., Hu R., Zhao Y.B., Liu W.E. Composition analysis of lignans from Schisandra chinensis (Turcz) Baill by LC-MS/MS. Natural Product Research and Development. 2012;24(B12):32–35. [Google Scholar]
- Wang H.J., Chen Y.Y. Studies of lignans from Schisandra rubriflora Rhed et Wils. Acta Pharmaceutica Sinica. 1985;20(11):832–841. [PubMed] [Google Scholar]
- Wang Q., Wang Y., Song X.M., Zhang W.D., Wang P.Y., Gu Y., Wang X.J. Simultaneous determination of six lignans compounds in rat plasma and phara cokinetics by UHPLC-MS/MS. Chinese Traditional Patent Medicine. 2014;36(2):266–271. [Google Scholar]
- Xu L.J., Peng Z.G., Chen H.S., Wang J., Xiao P.G. Bioactive triterpenoids from Kadsura heteroclita. Chemistry & Biodiversity. 2010;7(9):2289–2295. doi: 10.1002/cbdv.200900173. [DOI] [PubMed] [Google Scholar]
- Xu L., Su K.D., Wei X.C., Zhang J., Li H.R. Chemical constituents of Kadsura coccinea. Chemistry of Natural Compounds. 2018;54(2):242–244. [Google Scholar]
- Yang Y., Liu Y., Daniyal M., Yu H., Wang W. New lignans from roots of Kadsura coccinea. Fitoterapia. 2019;139:104368. doi: 10.1016/j.fitote.2019.104368. [DOI] [PubMed] [Google Scholar]
- Yeon J.H., Cheng L., He Q.Q., Kong Y.L. A lignin glycoside and a nortriterpenoid from Kadsura coccinea. Chinese Journal of Natural Medicines. 2014;12(10):782–785. doi: 10.1016/S1875-5364(14)60119-9. [DOI] [PubMed] [Google Scholar]
- Zhang J., Guo Y., Liu J., Jia X., Zhang B., Liu H. A new dibenzocyclooctadiene lignan from Kadsura longipedunculata. Chemistry of Natural Compounds. 2018;54(5):837–840. [Google Scholar]