Abstract
Objective
In Chinese herbal medicine (CHM) history, Lonicerae Japonicae Flos and Lonicerae Flos were used clinically as one drug, but now they are admitted as two herbal medicines in Chinese Pharmacopoeia (2010 edition). This study used network pharmacology to investigate whether the two can be used interchangeably for the treatment of inflammatory diseases in TCM clinical practice.
Methods
Lonicerae Japonicae Flos and Lonicerae Flos were compared in the inflammation mechanism including core targets, Gene Ontology (GO), pathway and principle chemical components by the method of network pharmacology.
Results
Lonicerae Japonicae Flos and Lonicerae Flos shared in six targets accounting for 66.7% of the entire core targets and more than half of the GO terms and pathways are similar. Organic acids are dominent compounds responsible for anti-inflammatory effects. Three of the compounds that bind to core targets including luteolin, quercetin and kaempferol, are shared in both herbs.
Conclusion
Due to high similarity between Lonicerae Japonicae Flos and Lonicerae Flos, we believe that they can be used interchangeably for the inflammation in clinical treatment.
Keywords: inflammation, Lonicerae Japonicae Flos, Lonicerae Flos, network pharmacology
1. Introduction
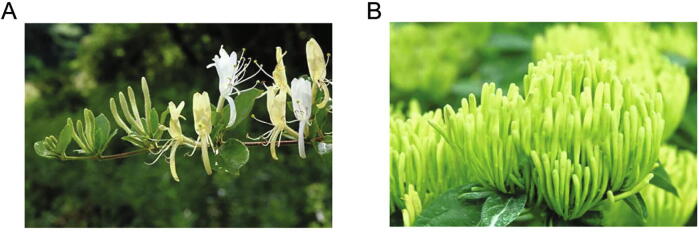
It is popular that a certain Chinese herbal medicine (CHM) generally has multiple botanical sources. However, it is not clear that why different botanical sources can serve as the same CHM. For example, Lonicerae Japonicae Flos (Jinyinhua in Chinese name) traditionally has several botanical sources including Lonicera japonica Thunb., L. macranthoides Hand.-Mazz, L. hypoglauca Miq., L. confuse DC. and L. fulvotomentosa Hsu et S.C. Cheng. Except L. japonica Thunb., the remaining plants were regarded as only a botanical source of Lonicerae Flos (Shanyinhua in Chinese name), a new item in Chinese Pharmacopoeia, 2005 edition. Some people believed that Lonicerae Japonicae Flos and Lonicerae Flos can no longer be used interchangeably, which opposite to traditional opinion. Fig. 1 showed the images of L. japonica and L. macranthoides plants.
Fig. 1.
Images of L. japonica (A) and L. macranthoides (B) plants. L. japonica has a pale yellowish surface, with long glandular hairs, the calyx tube being spherical; The surface of L. macranthoides is grayish green or brownish yellow, the calyx tube being ellipsoidal and glabrous.
They believed that Lonicerae Japonicae Flos and Lonicerae Flos are different in botanical origins and different in chemical composition (Liu et al., 2017, Xiao et al., 2019). Lonicerae Japonicae Flos has more abundant iridoid and flavonoid compounds and fewer chlorogenic acid than Lonicerae Flos (Li, Feng, & Zhou, 2018); Moreover, they are different in pharmacological effects. e.g. Lonicerae Japonicae Flos has a wider range of antimicrobial activity than Lonicerae Flos, and weaker antibioactivites of E. coli, typhoid bacillus, amoeba, type B streptococcus than Lonicerae Flos. The researches reported that Lonicerae Flos has significant anti-dysentery bacillus while Lonicerae Japonicae Flos hasn’t (Pan, Lei, Zhou, He, & Wu, 2004). However, those evidences do not seem particularly sufficient and strong for the following reasons. The ancient classification of traditional Chinese medicine is based on clinical efficacy which places more emphasis on the bias of the drug, e.g. Shennong’s Classic of Materia Medica. Chlorogenic acid is the dominent and efficient compound in Lonicerae Japonicae Flos and Lonicerae Flos and regarded as a marker by Chinese Pharmacopoeia rather than iridoid and flavonoid. There is no significant different in the content of chlorogenic acid between Lonicerae Japonicae Flos and Lonicerae Flos (Song et al., 2014). As the major pharmacological activity, anti-inflammation was similar between the two drugs. Zeng et al. reported that both Lonicerae Japonicae Flos extract and Lonicerae Flos extract showed similar and high anti-inflammatory effect in xylene-induced ear swelling experiment and lipopolysaccharide (LPS)-induced RAW264.7 cell inflammatory model. Hence, we tend to use network pharmacology to provide more evident for this conflict.
The network pharmacology approach is based on systems biology and multidirectional pharmacology which is resemblance with the idea of wholeness as emphasized in TCM theory (Hopkins, 2008). Shao Li, Chang-xiao Liu et al. successfully applied network pharmacology to the study of traditional Chinese medicine and prescriptions (Liu et al., 2015, Li and Zhang, 2013). As we all know, the cause of disorder is complex. Traditional pharmacology tends to focus on a single target for therapeutic breakthroughs which is different from the view of network pharmacology. By establishing component-target-pathway-disease networks, it provides a more comprehensive understanding of the disease and recommendations for treatment. Network pharmacology has been used to elaborate the mechanism of one drug or the mechanism of an herbal compound, but in our study, network pharmacology was used to compare two drugs for the first time.
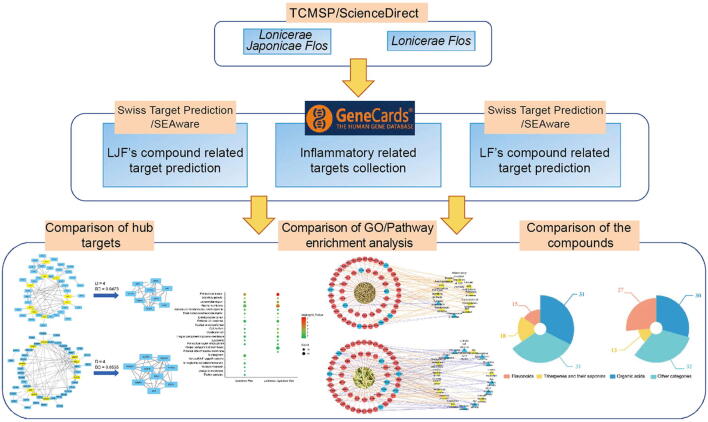
In this study, we took anti-inflammation, which is the modern pharmacological action corresponding to the traditional application of heat-clearing and detoxifying, as an example and adopted the network pharmacology method to compare the differences and similarities in the pharmacological effects of Lonicerae Japonicae Flos and Lonicerae Flos. Fig. 2 showed the flow chart of this study.
Fig. 2.
Research flow chart of study. Compound and inflammation-related target information was first collected, then combined to obtain inflammatory targets for two herbs, and finally key targets, GO and pathway, and material basis analyses are performed.
2. Materials and methods
2.1. Establishment of chemical composition database
Information on the chemical composition of Lonicerae Japonicae Flos and Lonicerae Flos was retrieved from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform and the Encyclopaedia of Traditional Chinese Medicine (Ru et al., 2014, Xu et al., 2019). These databases are a repository of chemical information of traditional Chinese herbs. Moreover, more information on these herbs was collected from the literatures (Tang et al., 2018, Yang et al., 2016). Then the free chemical information online website Chemspider (Pence & Williams, 2010) and PubChem have been used to translate and check the file type database (Kim et al., 2016). Immediately afterwards, MOE 2019 software was been chosen to merge the data and established a database. Otherwise, descriptors of compounds, including weight, TPSA, logP, logS, number of Hydrogen bond, has also been calculated by MOE2019.
2.2. Target fishing
Most TCM compounds exert corresponding biological functions by acting on protein targets, which simultaneously induce a series of physiological change. Identifying the targets is meaningful to understand the mechanism of a compounds’ action. In this study, we employed the SEAware and Swiss Target Prediction webserver for the target fishing of the collected compounds (Gfeller, Michielin, & Zoete, 2013). The target fishing methods were based on ‘similarity hypothesis’ of similarity among small molecules. This hypothesis states that compounds with similar structures have similar physical and chemical properties and biological activities. The activity and targets of unknown molecules can be predicted by comparing the small molecules with activity data (Lengauer, Lemmen, Rarey, & Zimmermann, 2004). And the names of the targets were calibrated to the official gene name through the Uniprot database (“UniProt: the universal protein knowledgebase,” 2017).
2.3. Construction of disease target database
Several databases for information on inflammation-related targets were consulted. GeneCards database assembles data from 150 websites and provides comprehensive genomic, proteomics, genetic, clinical and functional information (Stelzer et al., 2016). The keywords ‘inflammation’ and ‘inflammatory’ in the retrieval were used, and the genes with high scores (≥10) were collected to build a database of targets related to inflammatory diseases. Finally, the targets from target fishing were integrated with the disease target database, and then the overlapping parts as the inflammation-related targets of Lonicerae Japonicae Flos and Lonicerae Flos were selected.
STRING (Franceschini et al., 2013) was used to obtain PPI network data of Lonicerae Japonicae Flos and Lonicerae Flos with high credibility score (≥0.7), and then these data were putted into Cytoscape (Doncheva, Morris, Gorodkin, & Jensen, 2019). Degree was used to indicate the number of connections between a node and other nodes and Betweenness Centrality to reflect the value of the bridge centrality of the node. These parameters are crucial indicators to screen, obtain and compare the core target relationship network of two Chinese medicines (Raman, Damaraju, & Joshi, 2014).
2.4. Enrichment analysis
The Database for Annotation, Visualisation and Integrated Discovery were used to analyze the Gene Ontology (GO) and Kyoto Encyclopaedia of Genes and Genomes (KEGG) of inflammation-related genes (Huang da, Sherman, & Lempicki, 2009). GO enrichment analysis included biological process (BP), molecular function (MF) and cellular component (CC) (Fazio et al., 2016). The anti-inflammatory mechanisms of Lonicerae Japonicae Flos and Lonicerae Flos were determined and compared.
2.5. Comparison of key compounds
Using degree as reference values, the key compounds that act on the anti-inflammatory targets of Lonicerae Japonicae Flos and Lonicerae Flos on the basis of their types and related features.
3. Results
3.1. Collection of compounds and targets
The databases of Lonicerae Japonicae Flos and Lonicerae Flos contained 243 and 200 compounds, respectively, 74 of which were common to both herbs (Supplementary Table 1). Based on the PCA analysis of the basic properties of the compounds (Fig. 3), it showed that all the chemical components in Lonicerae Japonicae Flos and Lonicerae Flos had a large diversity in chemical space, and most of the components meet the five principles of Lipinski medicinal properties. They overlap with most of the small molecule anti-inflammatory drugs derived from DrugBank and natural products with clear and obvious anti-inflammatory activity, indicating that the chemical components contained in Lonicerae Japonicae Flos and Lonicerae Flos have greater anti-inflammatory potential, and also provide future target screening references and basis. A total of 207 targets for Lonicerae Japonicae Flos and 198 targets for Lonicerae Flos were obtained as potential targets after screening and weight reduction, of which 176 were the same. A total of 1 006 genes related to inflammation were collected in GeneCards. We integrated the compound and inflammation targets to obtain the common targets with inflammation. Fifty-seven targets were identified from both Lonicerae Japonicae Flos and Lonicerae Flos, 49 of which were shared by them (Fig. 4).
Fig. 3.
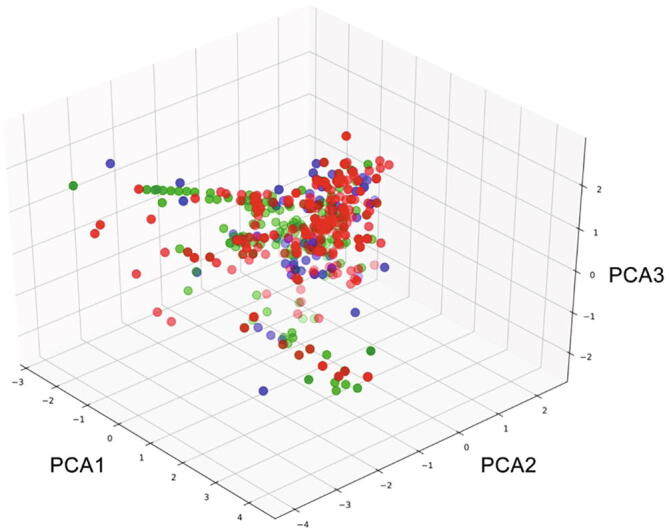
Chemical spatial distribute of Lonicerae Japonicae Flos and Lonicerae Flos by PCA. The red and green circles represent the compounds in Lonicerae Japonicae Flos and Lonicerae Flos, respectively, and the blue circle represents the drug molecule with anti-inflammatory activity.
Fig. 4.
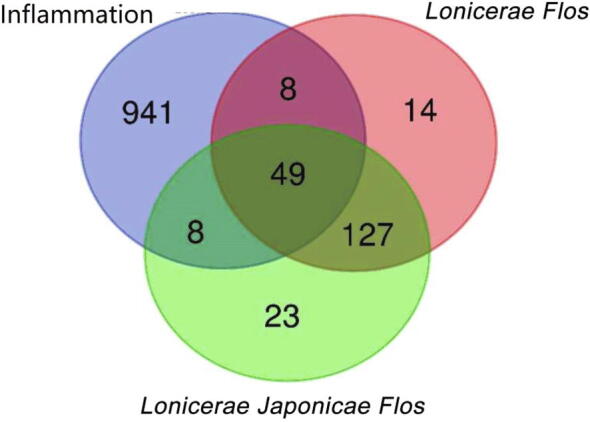
Venn diagram of the targets. The overlapping areas represent the same targets.
3.2. Analysis of key target network
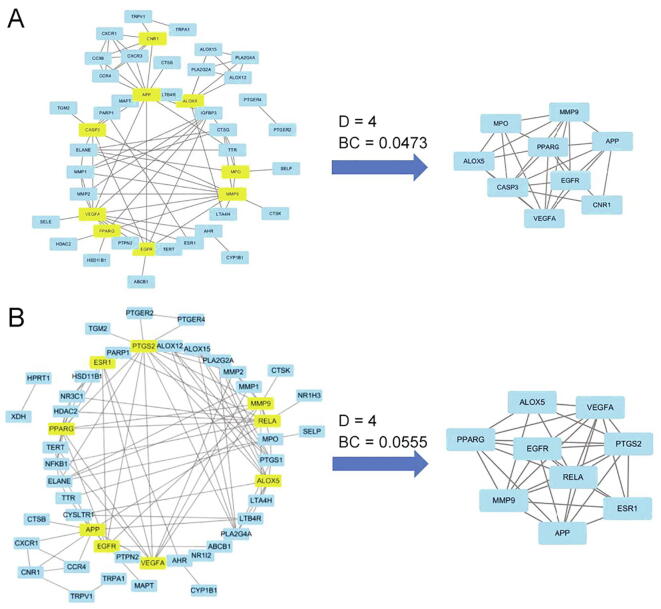
We introduced the inflammation targets of the two herbs to STRING. To illustrate the strength of the correlation between proteins, we acquired two PPI maps after setting the confidence level higher than 0.7. After filtering using the cut-off value, the PPI of Lonicerae Japonicae Flos consisted of 44 nodes with 90 edges, whereas that of Lonicerae Flos contained 48 nodes with 105 edges. The key targets were screened by analyzing the values of degree and betweeness centrality of each target in the respective networks and by using their medians as thresholds (Fig. 5). Results showed that Lonicerae Japonicae Flos and Lonicerae Flos had nine core targets. Six common targets, namely, APP, VEGFA, MMP9, EGFR, PPARG and ALOX5, were found. Information on the 12 target genes was listed in Table 1. These core targets that have been found are all related to inflammation (Fazio et al., 2016, Gamallat et al., 2016, Hur et al., 2007, Kremserova et al., 2016, Park et al., 2012, Ribas et al., 2010, Storr et al., 2010, Wang et al., 2004, Yoon et al., 2007)
Fig. 5.
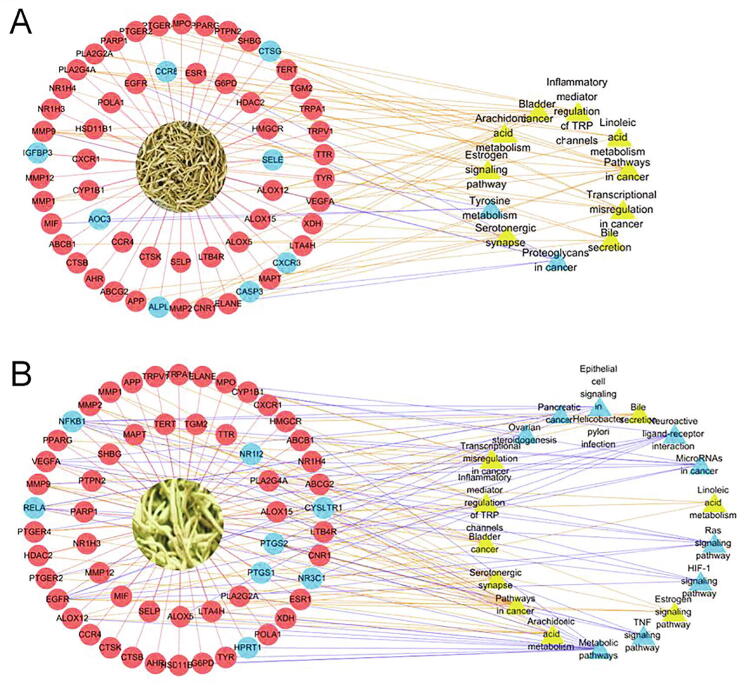
Network maps of targets and core targets of Lonicerae Japonicae Flos (A) and Lonicerae Flos (B). The yellow nodes are core targets, D is for Degree and BC is for betweeness centrality.
Table 1.
Information of core targets.
| Gene ID | Gene names | Sources | Degree | Betweenness centrality |
|---|---|---|---|---|
| APP | Amyloid-beta precursor protein | Lonicerae Japonicae Flos | 13 | 0.445 |
| Lonicerae Flos | 12 | 0.347 | ||
| VEGFA | Vascular endothelial growth factor A | Lonicerae Japonicae Flos | 13 | 0.268 |
| Lonicerae Flos | 11 | 0.190 | ||
| MMP9 | Matrix metalloproteinase-9 | Lonicerae Japonicae Flos | 11 | 0.245 |
| Lonicerae Flos | 11 | 0.142 | ||
| ALOX5 | Arachidonate | Lonicerae | 6 | 0.185 |
| Japonicae Flos | ||||
| 5-lipoxygenase | Lonicerae Flos | 9 | 0.057 | |
| EGFR | Epidermal growth factor receptor | Lonicerae Japonicae Flos | 9 | 0.116 |
| Lonicerae Flos | 8 | 0.119 | ||
| PPARG | Peroxisome proliferator-activated receptor gamma | Lonicerae Japonicae Flos | 6 | 0.097 |
| Lonicerae Flos | 8 | 0.075 | ||
| CASP3 | Caspase-3 | Lonicerae Japonicae Flos | 8 | 0.123 |
| CNR1 | Cannabinoid receptor 1 | Lonicerae Japonicae Flos | 6 | 0.095 |
| MPO | Myeloperoxidase | Lonicerae Japonicae Flos | 5 | 0.051 |
| RELA | Transcription factor p65 | Lonicerae Flos | 8 | 0.100 |
| PTGS2 | Prostaglandin G/H synthase 2 | Lonicerae Flos | 16 | 0.294 |
| ESR1 | Estrogen receptor | Lonicerae Flos | 7 | 0.068 |
3.3. Enrichment analysis results
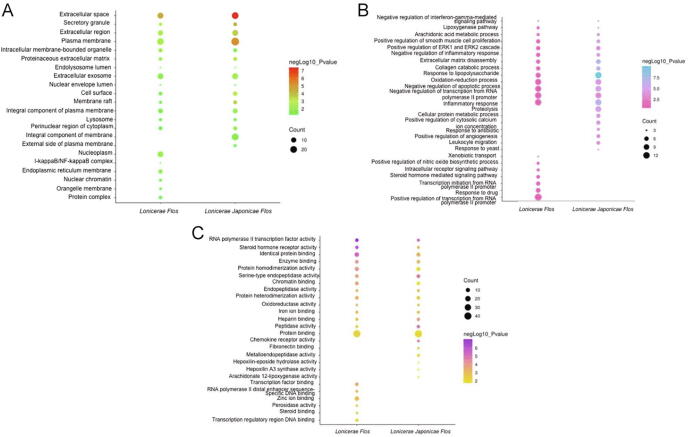
GO and KEGG data were obtained via David database enrichment analysis, with P-value ≤0.05 and Benjiamini ≤0.5 as the screening conditions. The P-value in the statistical analyses represented the probability of getting GO/pathway term error. Benjiamini value was used to globally correct the enrichment P-value of individual term members. Therefore, we were able to outcrop more accurate Lonicerae Japonicae Flos and Lonicerae Flos terms through the two condition pairs. A total of 135 GO terms were obtained from Lonicerae Japonicae Flos, 16 of which were for CC, 83 for BP and 36 for MF. By comparison, 163 GO terms were obtained for Lonicerae Flos, 20 of which were for CC, 70 for BP and 30 for MF. A total of 114 terms were common for both herbs, of which 14 were for CC, 70 for BP and 30 for MF. A comparison of the top 20 terms between Lonicerae Japonicae Flos and Lonicerae Flos in each GO term was shown in Fig. 6.
Fig. 6.
Compare of Lonicerae Japonicae Flos and Lonicerae Flos in GO terms. A is for cellular component; B is for biological process; C is for molecular function.
Lonicerae Japonicae Flos and Lonicerae Flos mainly act in extracellular space, including lysosomes and other cell components, for inflammation. The targets of Lonicerae Japonicae Flos were located on the external side of plasma membrane, including pivotal proteins with anti-inflammatory properties, such as ApoA-I (Mogilenko et al., 2012). The I-κB/NF-κB complex was found to be more relevant to Lonicerae Flos than to Lonicerae Japonicae Flos.
In MF, the two drugs specifically have RNA polymerase II transcription factor activity and combine various molecular functions, including enzyme, chromatin binding, transcription factor and other molecular functions. Furthermore, Lonicerae Japonicae Flos has special functions related to chemokine receptor activity, whereas Lonicerae Flos has peroxidase activity. We analyzed the involved biological processes and divided the co-participatory processes into five categories: apoptosis and proliferation (GO: 0022617, GO: 0043066), direct inflammation (GO: 0006954, GO: 0019369), immunity (GO: 0019372), oxidation (GO: 0055114) and signal transduction (GO: 0070374). These processes are directly or indirectly related to the development of inflammation. A total of 11 and 18 pathways of Lonicerae Japonicae Flos and Lonicerae Flos, respectively, were screened by KEGG pathway, of which nine terms were the same for both herbs. Two ‘target-path’ combination networks of Lonicerae Japonicae Flos and Lonicerae Flos were constructed (Fig. 7). The circle represents the corresponding anti-inflammatory target, whereas the triangle denotes the enrichment pathway. Nodes depicted in blue indicate the unique targets/pathways of Lonicerae Japonicae Flos and Lonicerae Flos. Lonicerae Flos showed more characteristic pathways than Lonicerae Japonicae Flos. The targets of Lonicerae Japonicae Flos and Lonicerae Flos in the same pathway were not exactly the same. The pathways common to both herbs included arachidonic acid metabolism, inflammatory mediator regulation of TRP channels and tumour-related pathways, which are closely related to inflammation. In addition, Lonicerae Flos has a unique TNF signalling pathway, and HIF-1 signalling pathway is related to inflammation.
Fig. 7.
‘Target-pathway’ network diagram combination of Lonicerae Japonicae Flos and Lonicerae Flos. Blue nodes indicated the unique targets/pathways of Lonicerae Japonicae Flos and Lonicerae Flos. Red and yellow nodes denoted the same targets/pathways.
3.4. Comparison of compounds
We analyzed the compounds of Lonicerae Japonicae Flos and Lonicerae Flos and their relevant anti-inflammatory targets. After that, eight and seven key compounds from Lonicerae Japonicae Flos and Lonicerae Flos has been obtained, respectively. These compounds could be divided into flavonoids or organic acid (Table 2). Three groups of Lonicerae Japonicae Flos and Lonicerae Flos compounds, namely, J185 and S144, J179 and S141 and J180 and S142, have the same structures (Fig. 8).
Table 2.
Information of central compounds.
| Number | Compound names | Structure types | Structures | Degree |
|---|---|---|---|---|
| J185 | Luteolin | Flavonoids | 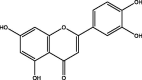 |
15 |
| J179 | Quercetin | Flavonoids | 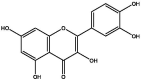 |
14 |
| J51 | 5-Hydroxy-4′,7-dimethoxy-flavone | Flavonoids | 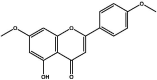 |
13 |
| J186 | Apigenin | Flavonoids | 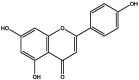 |
12 |
| J47 | Scoparol | Flavonoids | 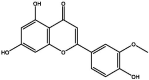 |
12 |
| J50 | 5-Hydroxy-3′,4′,7-trimethoxyflavone | Flavonoids | 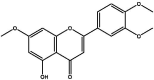 |
11 |
| J180 | Kaempferol | Flavonoids | 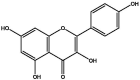 |
11 |
| J40 | 3-Methoxy-5,7,3′,4′-tetrahydroxy-flavone | Flavonoids | 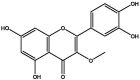 |
11 |
| S144 | Luteolin | Flavonoids | 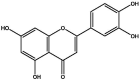 |
15 |
| S141 | Quercetin | Flavonoids | 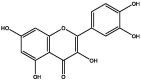 |
14 |
| S142 | Kaempferol | Flavonoids | 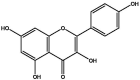 |
11 |
| S66 | 9,12-Octadecadienoic acid | Organic acid | 11 | |
| S61 | Ferulic acid | Organic acid | 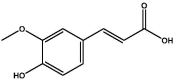 |
10 |
| S29 | Tricin | Flavonoids | 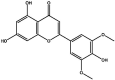 |
10 |
| S62 | 3-Phenylacrylic acid | Organic acid | 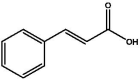 |
10 |
Fig. 8.
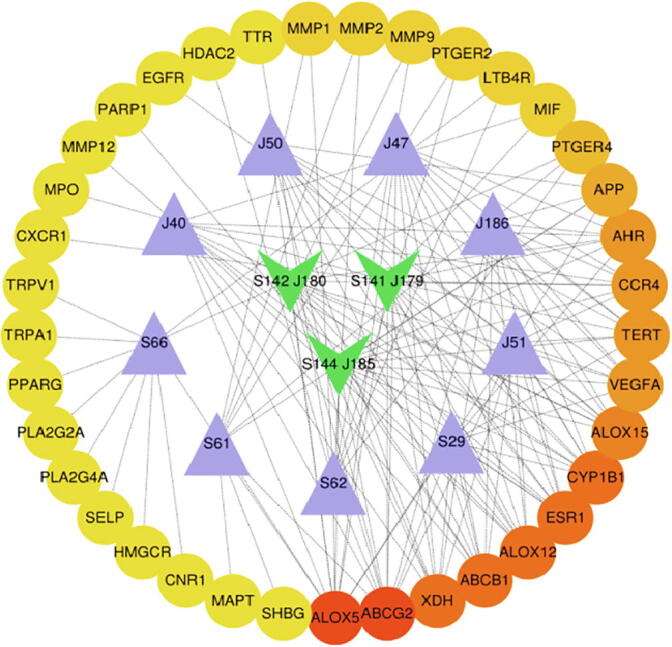
Diagram of target network of core compounds. Round nodes represent gene protein. Green nodes denote the same compounds from the two herbs. Purple nodes respectively indicate Lonicerae Japonicae Flos and Lonicerae Flos compounds.
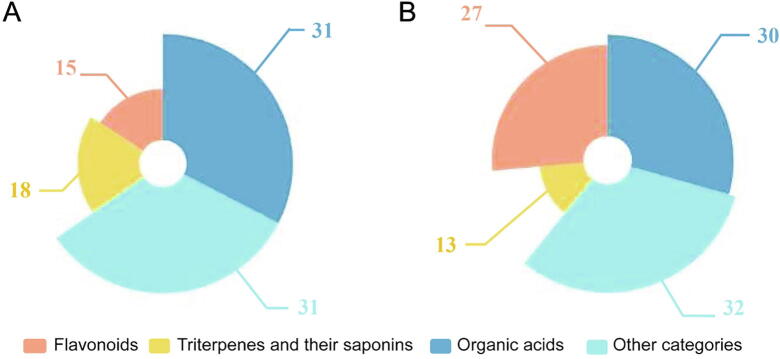
The compounds corresponding to anti-inflammatory targets were classified according to the main types of these herbs. As shown in Fig. 9, the class of each compound (organic acids, triterpenes and others) was compared, showing that the structures of these compounds only slightly differ. The quantity and types of flavonoids in Lonicerae Japonicae Flos were more plentiful than those in Lonicerae Flos.
Fig. 9.
Comparison of compounds that bind to core targets in Lonicerae Flos (A) and Lonicerae Japonicae Flos (B).
4. Discussion
4.1. Network pharmacology may be a new tool to reveal why different botanical sources can serve as the same drug
It is popular that a certain TCM generally has multiple botanical sources. However, it is not clear that why different botanical sources can serve as the same TCM. According to previous research, chemical and pharmacology assay were often used to the comparison of two species merely. Cui and corporates use spectrophotometry to compare similarities and differences in the chemical composition of the pulp and seeds of Schisandrae Sphenantherae Fructus (Cui et al., 2010). Zhao et al. compared the free radical scavenging activities of Citrus wilsonii Tanaka (CWT) and Citrus medica L. (CML) by biological experiments and high performance liquid chromatography (HPLC), and found that the content of naringin in CWT was the highest, and the radical scavenging activity of CWT was significantly higher than that of CML (Zhao et al., 2015). In order to comply with the holistic theory of TCM, the systemic pharmacology concept of network pharmacology can be better investigated. Through our study of Lonicerae Japonicae Flos and Lonicerae Flos, network pharmacology can indeed make a comprehensive comparison in terms of material basis, target of action, key pathways, and so on. Consequently, network pharmacology may be adopted to study similar issues.
4.2. Results of network pharmacology support traditional opinion that two herbs are same
Since ancient times, Lonicerae Japonicae Flos and Lonicerae Flos have been used as one medicine. The Chinese Pharmacopoeia admitted them as two drugs, but the descriptions are still the same that the two herbs are identical in nature and flavour, meridian tropism, directions and dosage. They can treat sores, rooted sores, wind–heat common cold and weakness. Moreover, Professor De-guang Wan who is famous Chinese medicine expert also proposed to merge Lonicerae Japonicae Flos and Lonicerae Flos (Wan & Yan, 2014). With our study, the two drugs are virtually indistinguishable with respect to the complex network of key target-pathways and other aspects of the anti-inflammatory side. There are some subtle differences between the two drugs in terms of key targets, material basis, etc., but these do not affect the main comparison. As results, we also exactly support the ancient tradition which Lonicerae Japonicae Flos and Lonicerae Flos can be used interchangeably in their traditional treatments.
4.2.1. Lonicerae Japonicae Flos and Lonicerae Flos are similar in core targets
We employed a common screening criterion to compare the protein–protein interaction network of Lonicerae Japonicae Flos and Lonicerae Flos. Among the nine core genes obtained, six were common in both herbs, accounting for 66.7%. Results showed that Lonicerae Japonicae Flos and Lonicerae Flos mainly all act on airway inflammation and tumour-induced inflammation. Both herbs were mainly involved in the NF-κB signalling pathway for anti-inflammatory effects. The extract of Lonicerae Flos blocks the activation of the NF-κB inflammatory signalling pathway by inhibiting IκBα phosphorylation with NF-κB p65 and IκBα degradation (Park et al., 2013). The extract of Lonicerae Japonicae Flos substantially reduces p50 and IKK expression levels on the NF-κB pathway (Lou et al., 2016). Therefore, Lonicerae Japonicae Flos and Lonicerae Flos act synergistically on the NF-κB signalling pathway but under different mechanisms. Lonicerae Japonicae Flos has a considerable effect on ovalbumin-induced asthma in a rat model (Hong et al., 2013). By contrast, research on Lonicerae Flos is few and there is no study has directly demonstrated that whether the Lonicerae Flos has a proportional effect on asthma or not. According to the results of the analysis of specific genes, Lonicerae Flos is more correlated with the NF-κB pathway and has an effect on neuro-inflammation, whereas Lonicerae Japonicae Flos is more biased towards systemic inflammation, such as enteritis, pneumonia and inflammation by microbial infection.
4.2.2. Lonicerae Japonicae Flos and Lonicerae Flos are similar in analysis of GO and pathway
GO and KEGG enrichment analyses of the gene targets revealed that both Lonicerae Japonicae Flos and Lonicerae Flos exert anti-inflammatory biological activity in a multitargeted multipathway manner. The targets of two herbs are mostly distributed in the extracellular gap and involve five types of biological processes and associated inflammatory pathways. Among the five major biological processes, two main aspects most directly related are involved in the immune process, namely, the inflammation caused by immune abnormalities and the immune response that accompanies the inflammatory response. The main pathological response of the former is autoimmune disease and tumour-associated inflammatory response. Lonicerae Japonicae Flos and Lonicerae Flos can exert anti-inflammatory effects by regulating the root cause of inflammation, that is, immunity. Both oxidation and signal transduction are also directly related to the occurrence and regulation of inflammation. These features are the similarities in anti-inflammatory mechanisms found in the GO and KEGG enrichment analyses of Lonicerae Japonicae Flos and Lonicerae Flos. These similarities can be visualised from the bubble and network diagrams. Their GO entries are mostly the same, although the mechanism of Lonicerae Japonicae Flos and Lonicerae Flos is different.
When it comes to the pathway of Lonicerae Japonicae Flos and Lonicerae Flos, here are some similarities. Fig. 7 is one of the representatives. But more often, the GO results showed that Lonicerae Japonicae Flos and Lonicerae Flos are not involved in exactly the same targets, such as inflammatory response (GO: 0006954) and Fig. 6. Hence, Lonicerae Japonicae Flos and Lonicerae Flos probably act in different ways on the same pathway and end up acting at different intensities because of the different correlations between the targets. In addition, given that Lonicerae Flos contains more anti-inflammatory-related entries, we analyzed the characteristic targets of the source of this phenomenon. Lonicerae Flos has more characteristic targets of action in the immune pathways than Lonicerae Japonicae Flos, and both are neurologically involved or related to fat metabolism (Capel et al., 2008). We inferred that the anti-inflammatory effects of Lonicerae Flos are more intense than those of Lonicerae Japonicae Flos, as well as targeted more to neurological and metabolic aspects of inflammation. However, no relevant studies are available to support this conjecture, and pharmacological experiments should validate these results.
4.2.3. Lonicerae Japonicae Flos and Lonicerae Flos are similar in analysis of compounds that bind to core targets
We further analyzed the material basis of the anti-inflammatory activity of Lonicerae Japonicae Flos and Lonicerae Flos. The types of central compounds of both Lonicerae Japonicae Flos and Lonicerae Flos are flavonoids and organic acids, which are certified exert anti-inflammatory effects by formerly studies (Chirumbolo, 2010, Devi et al., 2015, Park et al., 2015). The results revealed the similarity in substance bases of the two herbs and demonstrated the effectiveness of the screening method. The pharmacological activities of the other central compounds of Lonicerae Japonicae Flos and Lonicerae Flos are not reported in the literatures. Hence, future studies should investigate these aspects. Although the compounds are different, their biological activities are similar because their structures are similar. This condition leads to a large target repetitive rate. The other chemical types were not substantially different. The content of chlorogenic acid analogues in Lonicerae Flos is substantially more than that in Lonicerae Japonicae Flos (Zhang & Feng, 2012). We hypothesise that the material differences between Lonicerae Japonicae Flos and Lonicerae Flos are mainly due to considerably different flavonoids and organic acids.
4.3. This study is still insufficient in a methodological and holistic sense
This study is a new application of the network pharmacology method in the field of traditional medicine. Inevitably, there are still some flaws in this study. For instance, we only compared Lonicerae Japonicae Flos and Lonicerae Flos in terms of anti-inflammation, followed by other major efficacy analyses that should have been performed to make the study more complete. Also, the validity of the method can be further confirmed by applying it to the other polygenic plants such as Scutellaria baicalensis. Similarly, the method of network pharmacology currently has many shortcomings. The target prediction of chemical composition may not be completely correct, and the results obtained by enrichment analysis still have certain errors and limitations. These illustrated the great challenge for us to make the approach to online pharmacology more refined.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The study was supported by grants from the National Mega-Project for Innovative Drugs [2019ZX09735002] and the Science and Technology Innovation Project of the Chinese Academy of Medical Sciences [2016-I2M-3-015].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.chmed.2021.06.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Capel F., Viguerie N., Vega N., Dejean S., Arner P., Klimcakova E., et al. Contribution of energy restriction and macronutrient composition to changes in adipose tissue gene expression during dietary weight-loss programs in obese women. The Journal of Clinical Endocrinology & Metabolism. 2008;93(11):4315–4322. doi: 10.1210/jc.2008-0814. [DOI] [PubMed] [Google Scholar]
- Chirumbolo S. The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflammation & Allergy Drug Targets. 2010;9(4):263–285. doi: 10.2174/187152810793358741. [DOI] [PubMed] [Google Scholar]
- Cui J.C., Liang Q., Sun B.P., Feng G.L., Deng C., Song X.M. Comparative study of the chemical composition of seeds and pulp of Fructus Schisandrae Sphenantherae. Journal of Shaanxi College of Traditional Chinese Medicine. 2010:57–58. [Google Scholar]
- Devi K.P., Malar D.S., Nabavi S.F., Sureda A., Xiao J., Nabavi S.M., et al. Kaempferol and inflammation: From chemistry to medicine. Pharmacological Research. 2015;99:1–10. doi: 10.1016/j.phrs.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Doncheva N.T., Morris J.H., Gorodkin J., Jensen L.J. Cytoscape StringApp: Network analysis and visualization of proteomics data. Journal of Proteome Research. 2019;18(2):623–632. doi: 10.1021/acs.jproteome.8b00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio C., Piazzi G., Vitaglione P., Fogliano V., Munarini A., Prossomariti A., et al. Inflammation increases NOTCH1 activity via MMP9 and is counteracted by eicosapentaenoic acid-free fatty acid in colon cancer cells. Scientific Reports. 2016;6(1) doi: 10.1038/srep20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini A., Szklarczyk D., Frankild S., Kuhn M., Simonovic M., Roth A., et al. STRING v9.1: Protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Research. 2013;41(Database issue):D808–D815. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamallat Y., Meyiah A., Kuugbee E.D., Hago A.M., Chiwala G., Awadasseid A., et al. Lactobacillus rhamnosus induced epithelial cell apoptosis, ameliorates inflammation and prevents colon cancer development in an animal model. Biomedicine and Pharmacotherapy. 2016;83:536–541. doi: 10.1016/j.biopha.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Gfeller D., Michielin O., Zoete V. Shaping the interaction landscape of bioactive molecules. Bioinformatics. 2013;29(23):3073–3079. doi: 10.1093/bioinformatics/btt540. [DOI] [PubMed] [Google Scholar]
- Hong S.H., Kwon J.T., Shin J.Y., Kim J.E., Minai-Tehrani A., Yu K.N., et al. Therapeutic effect of Broussonetia papyrifera and Lonicera japonica in ovalbumin-induced murine asthma model. Natural Product Communications. 2013;8(11):1609–1614. [PubMed] [Google Scholar]
- Hopkins A.L. Network pharmacology: The next paradigm in drug discovery. Nature Chemical Biology. 2008;4(11):682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- Huang da W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocol. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Hur G.Y., Lee S.Y., Lee S.H., Kim S.J., Lee K.J., Jung J.Y., et al. Potential use of an anticancer drug gefinitib, an EGFR inhibitor, on allergic airway inflammation. Experimental & Molecular Medicine. 2007;39(3):367–375. doi: 10.1038/emm.2007.41. [DOI] [PubMed] [Google Scholar]
- Kim S., Thiessen P.A., Bolton E.E., Chen J., Fu G., Gindulyte A., et al. PubChem substance and compound databases. Nucleic Acids Research. 2016;44(D1):D1202–D1213. doi: 10.1093/nar/gkv951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremserova S., Perecko T., Soucek K., Klinke A., Baldus S., Eiserich J.P., et al. Lung neutrophilia in myeloperoxidase deficient mice during the course of acute pulmonary inflammation. Oxidative Medicine and Cellular Longevity. 2016;2016:1–13. doi: 10.1155/2016/5219056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengauer T., Lemmen C., Rarey M., Zimmermann M. Novel technologies for virtual screening. Drug Discovery Today. 2004;9(1):27–34. doi: 10.1016/S1359-6446(04)02939-3. [DOI] [PubMed] [Google Scholar]
- Li S., Zhang B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chinese Journal of Natural Medicines. 2013;11(2):110–120. doi: 10.1016/S1875-5364(13)60037-0. [DOI] [PubMed] [Google Scholar]
- Li W.P., Feng T.T., Zhou Y. Study on the efficacy and safety of Lonicerae Japonicae Flos and Lonicerae Flos. Journal of Mountain Agriculture and Biology. 2018;37(04):89–94. [Google Scholar]
- Liu C.X., Liu R., Fan H.R., Xiao X.F., Chen X.P., Xu H.Y., Lin Y.P., et al. Network pharmacology bridges traditional application and modern development of traditional Chinese medicine. Chinese Herbal Medicines. 2015 [Google Scholar]
- Liu Y.N., Shi D.H., Lei L.C. HPLC fingerprint identification of Lonicera Japonica Flos and Lonicera Flos. Chinese Traditional and Herbal Drugs. 2017;48(4):773–776. [Google Scholar]
- Lou, L., Zhou, J., Liu, Y., Wei, Y. I., Zhao, J., Deng, J., et al. (2016). Chlorogenic acid induces apoptosis to inhibit inflammatory proliferation of IL-6-induced fibroblast-like synoviocytes through modulating the activation of JAK/STAT and NF-κB signaling pathways. Experimental and Therapeutic Medicine, 11(5), 2054–2060. [DOI] [PMC free article] [PubMed]
- Mogilenko D.A., Orlov S.V., Trulioff A.S., Ivanov A.V., Nagumanov V.K., Kudriavtsev I.V., et al. Endogenous apolipoprotein A-I stabilizes ATP-binding cassette transporter A1 and modulates Toll-like receptor 4 signaling in human macrophages. FASEB Journal. 2012;26(5):2019–2030. doi: 10.1096/fj.11-193946. [DOI] [PubMed] [Google Scholar]
- Pan Q.P., Lei Z.J., Zhou R.B., He Y.S., Wu C.R. A comparative study of the antibacterial effects of Lonicera macranthodes Hand—Mazz and Lonicera japonica Thunb. Chinese Journal of Traditional Chinese Medicine. 2004;22(002):243–244. [Google Scholar]
- Park S.H., Baek S.I., Yun J., Lee S., Yoon D.Y., Jung J.K., et al. IRAK4 as a molecular target in the amelioration of innate immunity-related endotoxic shock and acute liver injury by chlorogenic acid. Journal of Immunology. 2015;194(3):1122–1130. doi: 10.4049/jimmunol.1402101. [DOI] [PubMed] [Google Scholar]
- Park S.J., Lee K.S., Kim S.R., Chae H.J., Yoo W.H., Kim D.I., et al. AMPK activation reduces vascular permeability and airway inflammation by regulating HIF/VEGFA pathway in a murine model of toluene diisocyanate-induced asthma. Inflammation Research. 2012;61(10):1069–1083. doi: 10.1007/s00011-012-0499-6. [DOI] [PubMed] [Google Scholar]
- Park S.H., Roh E., Kim H.S., Baek S.I., Choi N.S., Kim N., et al. Inhibition of IRAK-4 activity for rescuing endotoxin LPS-induced septic mortality in mice by Lonicerae Flos extract. Biochemical and Biophysical Research Communications. 2013;442(3-4):183–188. doi: 10.1016/j.bbrc.2013.11.045. [DOI] [PubMed] [Google Scholar]
- Pence H.E., Williams A. Chemspider: An online chemical information resource. Journal of Chemical Education. 2010;87(11):1123–1124. [Google Scholar]
- Raman K., Damaraju N., Joshi G.K. The organisational structure of protein networks: Revisiting the centrality-lethality hypothesis. Systems and Synthetic Biology. 2014;8(1):73–81. doi: 10.1007/s11693-013-9123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas V., Nguyen M.T., Henstridge D.C., Nguyen A.K., Beaven S.W., Watt M.J., et al. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERalpha-deficient mice. American Journal of Physiology Endocrinology and Metabolism. 2010;298(2):E304–E319. doi: 10.1152/ajpendo.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru J., Li P., Wang J., Zhou W., Li B., Huang C., et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. Journal of Cheminformatics. 2014;6(1) doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y.L., Ni F.Y., Zhao Y.W., Xie X., iao,, W. Research progress on chemical constituents from Lonicerae Flos. Chinese Traditional and Herbal Drugs. 2014;45(24):3656–3664. [Google Scholar]
- Stelzer G., Rosen N., Plaschkes I., Zimmerman S., Twik M., Fishilevich S., et al. The GeneCards Suite: From gene data mining to disease genome sequence analyses. Current Protocols Bioinformatics. 2016;54(1) doi: 10.1002/0471250953.2016.54.issue-110.1002/cpbi.5. [DOI] [PubMed] [Google Scholar]
- Storr M., Emmerdinger D., Diegelmann J., Pfennig S., Ochsenkühn T., Göke B., et al. The cannabinoid 1 receptor (CNR1) 1359 G/A polymorphism modulates susceptibility to ulcerative colitis and the phenotype in Crohn's disease. PLoS One. 2010;5(2):e9453. doi: 10.1371/journal.pone.0009453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.R., Zeng T., Zafar S., Yuan H.W., Li B., Peng C.Y., et al. Lonicerae Flos: A review of chemical constituents and biological activities. Digital Chinese Medicine. 2018;1(2):173–188. [Google Scholar]
- UniProt: the universal protein knowledgebase. (2017). Nucleic Acids Research, 45(D1), D158−169. [DOI] [PMC free article] [PubMed]
- Wan D., Yan Z. The suggestion on the naming of Lonicerae Japonicae Flos and Lonicerae Flos. Traditional Chinese Medicine and Clinic. 2014;005:1–2. [Google Scholar]
- Wang Y., Lerner S., Dan L., Dinney C.P., Grossman H.B., Wu X. Polymorphisms in the inflammatory genes IL-6, IL-8, TNF-α, NFKB1, and PPARG and bladder cancer risk. Cancer Research. 2004;64 [Google Scholar]
- Xiao Z.W., Xie M.Z., Gan L., Fang M.Y., Zhou X.Y., Zhou Y.M., et al. Determination of chlorogenic acid, total flavones, and anti-oxidant activity of Flos Lonicerae Japonicae and Flos Lonicerae. Chinese Traditional and Herbal Drugs. 2019;50(1):210–216. [Google Scholar]
- Xu, H. Y., Zhang, Y. Q., Liu, Z. M., Chen, T., Lv, C. Y., Tang, et al. (2019). ETCM: An encyclopaedia of traditional Chinese medicine. Nucleic Acids Research, 47(D1), D976−982. [DOI] [PMC free article] [PubMed]
- Yang Q.R., Zhao Y.Y., Hao J.B., Li.,, W., D. Research progress on chemical constituents and their differences between Lonicerae Japonicae Flos and Lonicerae Flos. China Journal of Chinese Materia Medica. 2016;41:1204–1211. doi: 10.4268/cjcmm20160708. [DOI] [PubMed] [Google Scholar]
- Yoon H.K., Cho H.Y., Kleeberger S.R. Protective role of matrix metalloproteinase-9 in ozone-induced airway inflammation. Environmental Health Perspectives. 2007;115(11):1557–1563. doi: 10.1289/ehp.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.H., Feng B.B. Rp-hplc determination on the content of chlorogenic acid in Flos Lonicerae Japonicae and Flos Lonicerae from different producing areas. Medicinal Plant. 2012;01:163–168. [Google Scholar]
- Zhao P., Duan L., Guo L., Dou L.L., Dong X., Zhou P., et al. Chemical and biological comparison of the fruit extracts of Citrus wilsonii Tanaka and Citrus medica L. Food Chemistry. 2015;173:54–60. doi: 10.1016/j.foodchem.2014.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.