Abstract
Botanical pentacyclic triterpenes possessed a broad range of pharmacological activities such as anti-oxidant, anti-tumor, anti-microbial and anti-inflammatory activities. It is believed that the mechanisms involved in these bioactivities are due to the modulation of immune system. Recently, the pharmacological validation on immunomodulatory of pentacyclic triterpenes derived from higher plants is very limited and several existence review papers related for this group of compound have not been focused for this activity. In this review, we have highlighted several studies on immunomodulatory potential of botanical pentacyclic triterpenes isolated from wide array of different species of medicinal plants and herbs based on various preclinical in vitro and animal models. This review also attempts to discuss on bioactivities of compouns related with their structure-activity relationship. Hence, the evaluation of pentacyclic triterpenes offers a great opportunity to discover adjuvants and novel therapeutic agents that presented beneficial immunomodulatory properties.
Keywords: botanical, immunomodulatory, pentacyclic triterpenes, structure activity relationship
1. Introduction
Recently, the high attention of plant-based natural products is paid by researchers due to numerous adverse side effects of modern therapeutic medicines. Also, the synthetic drugs are more expensive to obtain in comparison with herbal products. Large number of chemical entities with various pharmacological activities originated from plants are beneficial to promote human health. In 2013, 1453 new chemical entities patented from natural products approved by US Food and Drug Administration (Katz and Baltz 2016). They have been widely used as health supplements, nutritive products, medications since prehistoric times. Natural product-based drugs promote a significant role of the pharmaceutical industry. Lately, safe drugs with low side effects and high selective ligands that act on single disease target become a mission of drug development programs (David et al., 2015).
The immune system maintains homeostasis within the body in a normal condition of host. Various exogenous and endogenous agents contribute to the efficiency and function of the immune system that lead to the immunosuppression or immunostimulation (Jantan et al., 2015). The alteration of host's immune system contributes to the progression of abnormal conditions such as cancer. Thus, the modulation of host's immune response to increase the competence of this system in eliminating the aetiological agents that caused diseases might decrease this problem (Razali et al., 2016). Immunomodulation could be defined as the modification process of immune reactions that function to regulate immune responsiveness to treat illnesses (Rasheed et al., 2016). The application of immunomodulators for either as a treatment or prevention of various ailments that related with malfunctioning of immune reactions became the major attention (Sharma et al., 2015). The main purposes of immunomodulators are either to suppress immune responses as a treatment for autoimmune diseases or to enhance them that applied in immunodeficiency and infectious diseases (Ilyas et al., 2016). Additionally, the usage of immunostimulant agents also essentially act as an adjuvant to chemotherapy for various illnesses (Pujol et al., 2015).
Lately, the practice of immunomodulation drugs from medicinal plants and their derivatives have extended a great interest to treat numerous immunologic diseases. It is due to the adverse side effects of the immunomodulation drugs used in clinical setting. For example, azathioprine which is an immunosuppressant agent is associated with side effects such as medullar suppression, myalgia, pancreatitis, hepatitis and dizziness (Antonia et al., 2014). Immunostimulants derived from natural products are beneficial to treat or prevent the immunodeficiency conditions such as in cancer, bacterial and viral infections (Sepideh and Azam 2016). Previous studies showed that the botanical-derived phytochemicals such as triterpenoids, flavanoids, diterpenoids, glycosides, polysaccharides, lactones and alkaloids promoted alternative immunomodulating properties. Others like curcumin, capsaicin, quacertin, resveratrol, andrographolide, epigallocathecol-3-gallate, colchicine, and genistein also exhibited significant activities in different pathways of immunity in vitro (Jantan et al., 2015).
Pentacyclic triterpenes are secondary plant metabolites usually found in stem bark, leaves and fruit peel. This type of compounds contain 30-carbon skeleton comprising four six-membered rings and one five-membered ring (lupanes and hopanes) or five six membered rings (ursanes and lanostanes). Triterpenes include a huge number of diverse types of compounds which may be classified into families based on chemical structure. The major important groups of triterpenes and their glycosides are characterized by tetracyclic and pentacyclic derivatives. In brief, the pentacyclic derivatives are comprised of three main groups; lupane, ursane and oleaname (Niege et al., 2017). Currently, pentacyclic triterpenes gained a great attention because of their effects in promoting health such as immunomodulatory (Oladimeji et al., 2017), anti-tumor (Cho et al., 2015), anti-microbial (Wojnicz et al., 2017), anti-oxidant and anti-inflammation (Romero-Estrada et al., 2016). Based on the previous studies, almost all the bioactivities of this type of compound are believed originated from the alteration of immune systems. However, there are still limited review papers that compiled the immunomodulatory studies of this group of phytochemical. Under this background, the present work was carried out to review the immunomodulatory activities of botanical pentacyclic triterpenes isolated from wide array of different species of medicinal plants and herbs.
2. Immunomodulatory activities of selected plant-derived pentacyclic triterpenes
Immunomodulator is a component which is able to regulate the immune system involving both innate and adaptive immune responses. Currently, various bioactive compounds derived from medicinal plants have become a substance for scientific exploration to modulate immune system (Brindha et al., 2016). Inflammation is defined as a normal physiology process of our body's immune responses against infections. It comprises the body defend itself against foreign intruders such as bacteria and viruses, repair damaged tissue and effort to heal itself after an injury. However, excessive inflammation gives amplification to functional impairment, tissue damage, pain and discomfort (Bartold and Dyke 2017). Moreover, plant-derived pentacyclic triterpenes also have an ability to modulate immune responses in various inflammatory model studies that also included in this review.
Currently, the rise of researcher attention to obtain the immunomodulator agents from alternative sources to prevent or treat numerous of diseases because of the less-effective or adverse side effects presented by available synthetic drugs. For instance, recently, the antibiotic treatment has produced less or no significant effect on infections caused by targeted organisms because of the increase of multidrug resistant and tremendously drug resistant organisms (Lebeaux et al., 2014). The bioactive compounds isolated from natural products also provide positive feedback due to the potential of this agent to increase the normal body's immune defence system as a preparation to enhance host protection or as a microbial clearance agent against infection instead of using modern synthetic agents as a treatment (Adelina et al., 2013).
There is also combination therapy particularly with compounds originated from natural products offers improved responses as compared to application of a single therapy of synthetic drug. It might increase the bioactivities and multi-targeted mode of actions (Bulusu et al., 2016). This statement supported by research conducted by Arunachalam et al., (2019) elucidated that the combination of corticosterone (0.001 mg/L) with escin, a natural combination triterpenoid glycosides derived from the seeds of Aesculus hippocastanum L. (0.1 mg/L) was significantly reduced the synthesis of immune-inflammatory markers such as tumor necrosis factor alpha (TNF-α) (24.43%), interleukin 1-beta (Il-1β) (46.9%) and nitrite oxide (NO) (31.8%) in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages. Hence, a combination of treatment appeared to be a better solution to treat inflammatory diseases as compared with an individual treatment with synthetic agent (Xin et al., 2011). The other study evaluated the prospects of QS-21 fraction, triterpene glycosides from Quillaja saponaria for a new alternative adjuvants in vaccines also promoted virtuous alternative to the vaccine development program. It is because the uses of aluminium salts as an adjuvant only elicit Th2-type adaptive immune response. However, there was an increment in immunogenicity level of polysaccharide, protein and glycoprotein in several animal models after treated with QS-21 fraction. Later, it also initiated the stimulation of both the humoral and cell mediated immunities as a response to specific antigens compared to the application of using aluminium salts as adjuvant (Ragupathi et al., 2011). Other research conducted by Adelina et al. (2013) reported that the combination of oleonolic acid and ursalic acid from Chamaedorea tepejilote and Lantana hispida aerial parts presented anti-microbial activities against streptomycin-resistant Mycobacterium tuberculosis H37Rv strain with mean inhibitory concentration (MIC) of 12.5 µg/mL for in vitro evaluation. Next, in vivo study conducted to the infected animal and further treated with drug-sensitive H37Rv strain and combined of both compounds for one and two months showed a significant reduced number of live bacilli in the lungs. These results might be related with the rise of gene expression of immunoregulatory markers such as interferon-gamma (IFN-γ):, TNF-α and inducible nitrite oxide synthase (iNOS) which also detected in this study.
Instead of that, several pentacyclic triterpenes derived from natural products have also applied into the clinical applications and targets for variety type of diseases. There was a past research presented that liposomal ursolic acid in healthy adult volunteers and in patients with advanced solid tumors possessed low toxicities after received a single-dose of ursalic acid liposomes via intravenous infusion. Therefore, the findings suggest that ursalic acid has a great potential to be established into a potent immunomodulation related anti-cancer and anti-inflammatory drug (Wang et al., 2012). There was also finding related with QS-21, purified fraction comprised of triterpene glycosides isolated from Q. saponaria has been evaluated in clinical models. Such an example, combination treatment of QS-21 at 100 to 200 µg per vaccination among cancer patients presented antibody responses against peptide and carbohydrate antigens in the linked of KLH-conjugate vaccine. Additionally, the concentration of applied QS-21 also showed light controllable toxicity likes flu-like symptoms and local erythema (Ragupathi et al., 2011). The other study was using hawthorn tablet consisted mainly of ursalic acid, oleonolic acid and hyperoside presented reduced level of serum total cholesterol and triglyceride in hyperlipidemia patients who were treated thrice daily for three months (Sheng and Sun 2011).
There were numerous studies conducted to assess the immunomodulatory activities of pentacyclic triterpenes originated from herbs Centella asiatica (Linn.) Urban contains abundant of pentacyclic triterpenes compounds which contribute to its pharmacological properties including immunomodulatory activities. Mahmood et al. (2016) reported that asiatic acid at concentration of 40 and 80 µmol/L decreased the NO production of LPS-stimulated RAW 264.7 macrophage cells in a dose dependent level. This parameter was analyzed using Griess assay. However, the opposite phenomenon exhibited after treatment with asiatic acid at a higher dose (160 µmol/L). There was another in vitro findings validated that LPS-induced prostaglandin E2 (PGE2), NO, interleukin-6 (IL-6), and interleukin-8 (IL-8) production in RAW 264.7 macrophage cells were dose-dependently diminished by asiatic acid after treated with the compound at concentration of 25, 50 and 100 µmol/L, respectively. Asiatic acid also reduced p65 NF-κB phosphorylation in LPS-stimulated human gingival fibroblasts (HGFs) from explants of human normal gingival tissue. Meanwhile, the in vivo study using Male Sprague-Dawley rats (7 weeks old) induced-periodontitis as a model presented that asiatic acid significantly suppress LPS-induced IL-8 and IL-6 protein expression levels in rats gingival tissue. In this study, enzyme-linked immunosorbent assay (ELISA) and western blot have been used as approached methodologies to evaluate the pro-inflammatory cytokines and protein expression levels, respectively (Hao et al., 2017). Besides, asiatic acid from the similar plant at a dosage of 20 µmol/L also significantly inhibited the mRNA expression levels of IL-6, IL-8, IL-1β and TNF-α in a human corneal epithelial cells (HCECs) after stimulated with LPS for in vitro study (Chen et al., 2017).
Yun et al. (2008) discovered that asiatic acid (30, 60 and 120 µmol/L) isolated from the leaves of C. asiatica produced inhibitory activities in the production of pro-inflammatory cytokines of TNFα, IL-6 and IL-1β in LPS-stimulated RAW 264.7 macrophage cells in a dose-dependent manner. Later, this compound also reduced protein and mRNA expression of inducible cyclooxygenase-2 (COX-2) and iNOS, nuclear translocation of NF-κβ and phosphorylation of both inhibitor of nuclear factor kappa-B (NF-κB) kinase subunit beta and alpha (IKK-α/β) and inhibitor of nuclear factor of kappa light polypeptide gene enhancer in B-cells subunit alpha (IκB-α). The anti-inflammatory properties of asiatic acid was believed originated from down-regulation of NF-κB activation due to inhibition of IKK and MAP kinase phosphorylation in RAW 264.7 cells that promoted the suppression of IL-6, IL-1β, iNOS, COX-2, and TNF-α expressions.
Madecassoside which also one of main pentacyclic triterpenoid compounds from C. asiatica that promotes immunomodulatory activities in various inflammatory models for both in vitro and in vivo. Li et al. (2009) revealed that treatment of madecassoside at dosage of 10 and 30 mg/kg body weight in collagen-induced athritis in DBA/1J mice reduced the IL-6, TNF-α levels and lymphocyte cells proliferation and increased IL-10 levels significantly in a dose relationship effect. Cao et al. (2010) also conducted both in vitro and in vivo studies using LPS-induced cardiomyocytes cells and LPS-induced male Sprague-Dawley rats, respectively using this compound. They reported that 100 and 300 µmol/L of madecassoside were able to dose-dependently reduced mRNA level of TNF-α using reverse transcriptase-polymerase chain reaction (RT-PCR) analysis. The in vivo study which did a measurement of TNF-α level in plasma of rats after treatment with 20 and 40 mg/kg body weight of this compound for 5 d also exhibited similar outcome.
Jimenez-Arellanes et al. (2013) investigated anti-microbial properties of ursolic and oleanolic acid derived from aerial parts of Chamaedora tepejilote Liebm. Ex Mart. and Lantana hispida Kunth, separately that involved immunoregulatory pathway. The in vivo findings presented that BALB/c mice (6-8 weeks) induced pulmunory tuberculosis treated with tripled dosage of mean inhibitory concentration (MIC) of ursalic acid and oleanolic acid which determined previously in vitro exhibited higher gene expression of IFN-γ): , TNF-α and iNOS when compared to untreated group. Interestingly, the combination treatment of ursalic acid and oleanolic acid exhibited greater expression in all similar parameters than the value of untreated group.
In other studies, the immunomodulatory effects of combination of several pentacyclic triterpene compounds derived from Rosa canina L. using LPS-activated Mono Mac 6 cells as an in vitro mode has been conducted. The results exhibited that the mixture of oleanolic, betulinic and ursolic acid (30:49:21) inhibit the LPS-induced IL-6 release higher than activity of individual compound with IC50 value of (21 ± 6) µmol/L (Saaby et al., 2011). Marquez-Martin et al. (2006) found that several pentacyclic triterpenoid compounds isolated from “orujo” olive oil such as olenolic acid, erythrodial and uvaol at concentration of 10, 25, 50, 100 µmol/L reduced dose-dependently secretion of IL-1 β and IL-6 of peripheral blood mononuclear cells (PBMCs) from donor compared with control. Erythrodial at all doses possessed the most effective inhibitory effect significantly (P < 0.05) on IL-1β and IL-6 production of PBMCs compared with other compounds. Meanwhile, madecassoside and oleanolic acid at 100 µmol/L presented significant inhibition of IL-6 released by these cells.
There was exploration regarding immunomodulatory effects of pentacyclic triterpenoid, 3β,6β-dihydroxyolean-12-en-27-oic acid derived from rhizomes of Astilbe chinensis (Maxim) Franch. et Savat. to regulate anti-tumor activities in S180-bearing mice. The outcome displayed that the compound at dosages of 60 and 80 mg/kg body weight significantly increase natural killer cells and cytotoxic T lymphocytes activity after 11 d of treatment in a dose-dependent manner, respectively. Meanwhile, at concentrations of 40 and 60 mg/kg body weight, 3β,6β-dihydroxyolean-12-en-27-oic acid also significantly enhanced production of IL-2 and anti-SRBC antibody production in treated mice compared with negative control group in similar pattern (Sun et al., 2009).
Zhang et al. (2014) reported that the activity of madecassic acid from C. asiatica increased CD4+ and CD8+ T-lymphocytes subpopulation and the secretion of pro-inflammatory cytokines IFN-γ): and IL-4 significantly compared with the untreated group of tumor bearing mice using flow cytometry analysis. They proposed that the enhanced of rat's immune defence system against tumorigenesis were modulated by both T-helper 1 (Th1) and T-helper 2 (Th2) mediated immune responses that influenced by this bioactive compound. Furthermore, the other studies exhibited significant elevated activities of phagocytosis in macrophage cells of Kunming mice after treated with betulinic acid (0.5 and 1 mg/kg body weight) in a dose-dependent manner. Meanwhile, the splenic ratios of CD4+/CD8+ and production of TNF-α in treated mice also significantly enhanced in a dose-dependent manner (Yi et al., 2010).
According to Dash et al. (2015), betulinic acid derived from barks of Ziziphus jujube Mill. tree at a high concentration (50 µg/mL) presented an increased level of TNF-α and IL-12 and reduced level of IL-10 and TGF-β of both lymphocyte and macrophage cells. In addition, the compound also enhanced level of CD4+ lymphocyte population. These in vitro studies were using ELISA and flow cytometric analysis, respectively. Besides, the study conducted in vivo using mice exhibited elevated level of IgG immunoglobulin after treated with this bioactive compound (50 µg) when with control that received only phosphate buffer saline.
3. Structure-activity relationship for immunomodulatory effects of plant-derived pentacyclic triterpenes
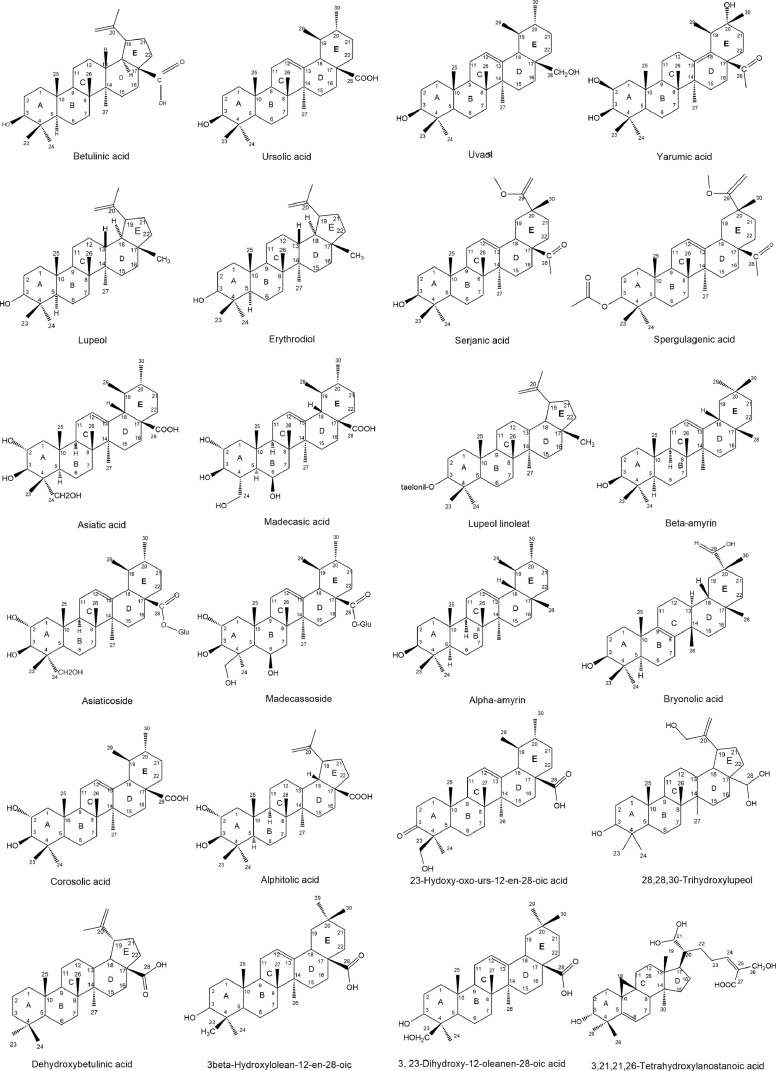
Pentacyclic triterpenes, aglycones of saponin are widely distributed in plants kingdom. These compounds have well known to have several pharmacological properties including anti-inflammation and immunomodulator. The chemical structures of pentacyclic triterpene compounds act as an immunomodulator are emphasized in Fig. 1. Ayatollahi et al. (2011) reported that three common pentacyclic triterpenes named betulinic acid, oleanolic acid and ursolic acid, have been successfully isolated from Euphorbia microsciadia Boiss, a perennial plant that cultivates in some parts of Iran. These compounds were then evaluated their effects on T-cell proliferation activity. Briefly, T-lymphocytes were isolated from fresh venous blood from a normal healthy volunteer and the analysis was done using liquid scintillation counter. The results showed that oleanolic acid even at the low concentration (0.5 µg/mL) stimulates T-cell proliferation. Conversely, betulinic acid and ursolic acid enabled to inhibit T-cell proliferation with IC50 values greater than 50 µg/mL and 3.01 µg/mL, respectively. Therefore, by comparing these results, oleanolic acid and ursolic acid which have the same chemical structure but only differ in ring E presented two different activities. Another study also proofs this statement by presented that oleanolic acid stimulated NO and TNF-α production of mouse macrophage cells (Choi et al., 2001). In contrast, ursolic acid showed suppression effects. It has been reported that ursolic acid has strong activity in inhibiting T-cell proliferation (Khajuria et al., 2007). These results indicated that different position of methyl group at ring E could be responsible for their activities. Substitution of one methyl group on C-19 and loss of one methyl group on C-20 might be important for inhibition effects of pentacyclic triterpenes. Another study also showed the evidence that dissimilar position of methyl group (ring E) pentacyclic triterpenes might be involved in their contrast activities. Present of methyl group at C19 increased inhibitory effect of α-amyrin on MPO activity (Holanda Pinto et al., 2008).
Fig. 1.
Chemical structures of pentacyclic triterpenes which have a potential as an immunomodulator.
On the other hand, inhibitory activities of betulinic acid and ursolic acid against T-cell proliferation, showed that ursolic acid which has six members E ring is more potent than betulinic acid (five members analogue in lupine type). This finding illustrated that ring E of pentacyclic triterpene is probably significant for its inhibitory effects. Moreover, four pentacyclic triterpenes that include oleanolic acid, maslinic acid, erythrodiol, and uvaol from olive pamoce oil have been evaluated for their immunomodulatory activity on cytokine production of human mononuclear cells. The results exhibited that among of these compounds, erythrodiol at 100 µmol/L is the most potent compound in decreasing IL-1β production. This compound at the same concentration also demonstrated strongest activity in reducing IL-6 production. In case of their activity on TNF-α production, uvaol and oleanolic acid at the highest concentration (100 µmol/L) significantly inhibited TNF-α production, while erythrodiol at the similar concentration did not affect these pro-inflammatory cytokines (Marquez-Martin et al., 2006). According to their chemical structures such as the present of carboxyl group and methanol at C-17, these compounds can be classified as acid and alcohol pentacyclic triterpenes. Although the relationship between their chemical structure and their activities are still unclear, however, it is worthy noted that the present of methanol at C-17 as well as substitution one methyl group at C-20 (ring E) might be important for inhibition effect of erythrodiol on pro-inflammatory cytokines productions. In addition, it can be observed that maslinic acid which has one additional hydroxyl group at C-2 (ring A) differ from others. Although this substitution is important for its antioxidant activity and inactivation of NF-κB, however its inhibition effects toward cytokine production was not found (Motilla et al., 2003; Fukumitsu et al., 2016). It can be assumed that the presence of hydroxyl group did not affect their anti-inflammatory activities through cytokines production and it might decrease its immunomodulatory activities.
Dendritic cells are classified in the group of leucocytes play a vital part in the activation and modulation of the immune responses. These cells are able to act as antigen-presenting cells and have a special ability to activate naive T cells to further stimulate adaptive immune response towards multiple pathways (Banchereau et al., 2000). Assessing immunomodulatory effect of pentacyclic triterpenes on dendritic cells has been performed by Pelaez et al. (2013). They have successfully isolated one novel compound named, yarumic acid, along with known compound such as serjanic acid, spergulagenic A, 20-hydroxy-ursolic acid and goreihic acid from Cecropia telenitida Cuatrec. The compounds were then preceeded to various assays including pro-inflammatory production test. The findings demonstrated that spergulagenic acid is the most potent compound which inhibits cytokines production (more than 50%) followed by serjanic acid and yarumic acid. Meanwhile, 20-hydroxy-ursolic acid and goreihic acid were the least active pentacyclic triterpenes. According to their chemical structures, their properties might be due related with the presence of carbonyl group at C-3 on ring A that is important for inhibition effects of spergulagenic on cytokines production. Moreover, in case of yarumic acid, substitution of hydroxyl group at C-2 and C-20, as well methyl group at C-19 might lessen its inhibition effect.
Lupeol and lupeol linoleate, two common pentacyclic triterpenes, have also been studied for their anti-inflammatory activity. The result elicited that lupeol linoleate has strong activity (58% reduce paw swelling) which is comparable to indomethacin as a positive control (Geetha and Varalakshmi 2001). In a different study, Lucetti et al. (2010) reported that lupeol acetate has significant anti-inflammatory effect in formalin test animal model. The present of linoleate at C-3 (ring A) might be contributed for its anti-inflammation properties in vivo. Human neutrophil elastase (HNE) is serine protein in the granule of neutrophil. It plays important in phagocytosis activity by assisting neutrophil migration towards infection side. In certain condition, over expression of this enzyme may lead to chronic inflammation. The effect of pentacyclic triterpenes which includes ursolic acid, oleanolic acid, betulinic acid and lupeol on HNE was performed. The results revealed vary inhibition activities against HNE which ursolic acid showed strongest effect in inhibiting serine protein production. The presence of carboxyl group at C-28 might be contributed for its activity. This result was then confirmed using molecular docking study in which 28-COOH and double bond in their skeleton enabled to increase its inhibitory activity (Feng et al., 2013).
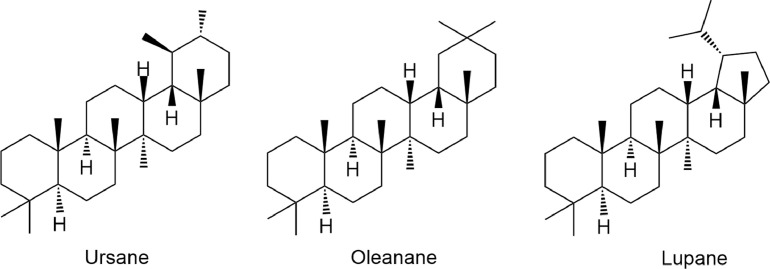
According to previous studies, asbetulin, betulinic acid, ursolic acid and lupeol have been known to possess anti-inflammatory activity. Using animal model, it has been shown that betulinic acid at a concentration of 30 µmol/L inhibited the expression of COX-2, while ursolic acid displayed an anti-inflammatory activity on NF-κB at somewhat higher concentration (50 µmol/L). In general, petacyclic triterpenes are classified into three classes, such as ursanes, oleananes, and lupanes (Catteau et al., 2018) (Fig. 2). The different activity of these compounds might be because of the different substitution on their basic skeleton. It has been known that betulinic acid has four six-rings and one five-rings, while ursolic acid consists of five six-rings (Laszczyk, 2009). Moreover, betulin, betulinic acid and oleanolic acid have also demonstrated anti-inflammatory effect with vary activities. This variation is not only due to their concentrations, but also the presence of different substitution groups on their main structure probably contribute for their action (Catteau et al., 2018).
Fig. 2.
Chemical structures of main classes of pentacyclic triterpenes.
Another study has also been performed to find relationship between chemical structure of some lupeol derivatives including lupeol, betulinic acid, ethyl betulinate, and lupenyl acetate and their immunomodulatory effects. Phagocytes chemiluminescene and lymphocyte proliferation assays were used in this study. The result showed that ethyl butinilate is the strongest compound in inhibiting phagocytosis (IC50 value of 3.85 µg/mL) compared with other tested compounds of pentacyclic triterpenes. This compound also revealed strongest effect to inhibit T-cell proliferation with IC50 value of 12.9 µg/mL. The presence of esteric group at C-28 of ethyl betulinate probably plays important role to increase inhibition effect of this pentacyclic triterpene (Shahlaei et al., 2013).
According to previous studies, asiatic acid isolated from C. asiatica strongly inhibited LPS-induced NO and PGE2 production in RAW 264.7 macrophage cells. This compound was more potent than its derivative namely asiaticoside. By comparing their chemical structures, the present of glucose at C-28 (ring E) of asiaticoside might reduce its anti-inflammatory effect (Yun et al., 2008). In vivo study has also performed to evaluate anti-inflammation of asiatic acid derivates. Asiatic acid along with corosolic acid and alphitolic acid have been tested their anti-inflammatory effect in animal model. All compounds revealed inhibition effect in TPA-induced inflammation. However, corosolic acid presented most potent activities when measured using arachidonic acid assay. Lack of one alcoholic group at C-24 (ring A) probably contributed for its activity (Aguirre et al., 2006).
Recently, five pentacyclic triterpenes, such as 3, 23-dihydroxy-12-oleanen-28-oic acid, 3β-hydroxylolean-12-en-28-oic, stigmasterol, 3-O-β-D-glucopyranosyl stigmasterol, and 23-hydroxy-3-oxo-urs12-en-28-oic acid, have been isolated from Cussonia arborea. These compounds were then determined for their immunomodulatory effect, including suppression of reactive oxygen species (ROS). Among of the compounds, 3, 23-dihydroxy-12-oleanen-28-oic acid, and 3β-hydroxylolean-12-en-28-oic showed strongest inhibition effect on ROS production with IC50 values of 24.4 and 37.5 µg/mL, respectively. While, 3β-hydroxylolean-12-en-28-oic exhibited highest effect on T-cell proliferation (IC50 value of 12.6 µg/mL). The structure activity relationship, presence of methyl group at C-23 (ring A) might be important for immunosuppression effect of pentacyclic triterpene (Oladimeji et al., 2017). Additionally, similar study was also conducted by Mawa and team. Three new triterpenoids that consist of 28,28,30-trihydroxylupeol, 3,21,21,26 tetrahydroxylanostanoic acid and dehydroxybetulinic acid, along with seven known compounds have been observed their immunomodulatory effects using chemiluminescence and chemotactic assay. The findings revealed that 3,21,21,26 tetrahydroxylanostanoic acid had strong inhibition activity on ROS production of PMNs, as well as chemotactic activity with IC50 values of 2.8 µg/mL and 0.9 µg/mL, respectively. According to general analysis of chemical structure and immunodulatory effect, the presence of an oxygenated group at C-3 as well as carboxyl group at C-28 of ring A might increase its immunosuppression activity on ROS production and chemotaxis of human neutrophil (Mawa et al., 2016).
4. Conclusion
The significances of the outcome of these studies recommended pentacyclic triterpenes have a potential to further discover as a future immunomodulator agent for developing new natural products based on pharmaceuticals. However, there is still shortage of compilation of scientific data to offer validations on immunomodulatory activities of this group of compounds. Actually, sufficient research has not been implemented on normal state model in laboratory studies regarding all factors comprising pro-inflammatory enzymes, pro-inflammatory cytokines, proteins and genes expression. The evaluation of the effects of botanical pentacyclic triterpenes on the activity and gene expression of enzymes and cytokines involved in a normal model might be useful to explore the capability of this compound as a potential to enhance body's immune system. Therefore, this compound could be a good candidate to stimulate immunity of the host as prevention against attacking pathogens that cause various diseases. If there are enough preclinical and safety data, more clinical trials are fortified to be done to prove their activities.
Declaration of Competing Interest
The authors declared no conflict of interest.
Acknowledements
This study was funded by USM bridging grant (304.PPSK.6316150).
References
- Adelina J.A., Julieta L.H., Jorge C.G., Sonia L.G., Maria Eugenia C.M., Mariana M.F., Dulce M.E., Brenda M., Javier T., Rogella H.P. Ursolic and oleonolic acids as antimicrobial and immunomodulatory compounds for tuberculosis treatment. Complement. Alternat. Med. 2013;13:258. doi: 10.1186/1472-6882-13-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre M.C., Delporte C., Backhouse N., Erazo S., Letelier M.E., Cassels B.K., Silva X., Alegria S., Negrete R. Topical anti-inflammatory activity of 2α-hydroxy pentacyclic triterpene acids from the leaves of Ugni molinae. Bioorg. Med. Chem. 2006;14(16):5673–5677. doi: 10.1016/j.bmc.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Antonio B.M., Riikka M., Rafael G.F., Jukka H.M. Immunomodulatory drugs: Oral and systemic adverse effects. Med. Oral Patol. Oral Cirugia Bucal. 2014;1(19):24–31. doi: 10.4317/medoral.19087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunachalam K., Boopathi B., Rajendhran R., Ramanathan S., Sybiya V.P.I.A., Krishnaswamy B., Shunmugiah K.P., Arumugam V.R. In-vitro and in-vivo biofilm inhibitory efficacy of geraniol-cefotaxime combination against Staphylococcus spp. Food Chem. Toxicol. 2019;125:322–332. doi: 10.1016/j.fct.2019.01.008. [DOI] [PubMed] [Google Scholar]
- Ayatollahi A.M., Ghanadian M., Afsharypour S., Abdella O.M., Mirzaid M., Askarie G. Pentacyclic triterpenes in euphorbia microsciadia with their T-cell proliferation activity. Iranian J. Pharmaceut. Res. 2011;10(2):287–294. [PMC free article] [PubMed] [Google Scholar]
- Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y.J., Pulendran B., Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Bartold M.P., Dyke V.T.E. Host modulation: controlling the inflammation to control the infection. Periodontology. 2017;75(1):317–329. doi: 10.1111/prd.12169. [DOI] [PubMed] [Google Scholar]
- Brindha P., Venkatalakshmi P., Vadivel V. Role of phytochemicals as immunomodulatory agents: A review. Int. J. Green Pharmacy. 2016;10(1):1–2. [Google Scholar]
- Bulusu K.C., Guha R., Mason D.J., Lewis R.P.I., Muratov E., Kalantar Motamedi Y., Cokol M., Bender A. Modelling of compound combination effects and applications to efficacy and toxicity: State-of-the-art, challenges and perspectives. Drug Discovery Today. 2016;21:225–238. doi: 10.1016/j.drudis.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Cao W., Li X.Q., Zhang X.N., Hou Y., Zeng A.G., Xie Y.H., Wang S.W. Madecassoside suppresses LPS-induced TNF-α production in cardiomyocytes through inhibition of ERK, p38, and NF-κB activity. Int. Immunopharmacol. 2010;10(7):723–729. doi: 10.1016/j.intimp.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Catteau L., Zhu L., Van Bambeke F., Quetin-Leclercq J. Natural and hemi-synthetic pentacyclic triterpenes as antimicrobials and resistance modifying agents against Staphylococcus aureus: A review. Phytochem. Rev. 2018;17(5):1129–1163. [Google Scholar]
- Chen H., Hua X., Ze B., Wang B., Wei L. The anti-inflammatory effects of asiatic acid in lipopolysaccharide-stimulated human corneal epithelial cells. Int. J. Ophthalmol. 2017;10(2):179–185. doi: 10.18240/ijo.2017.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J., Tremmel L., Rho O., Camelio A.M., Siegel D., Slaga T.J., Digiovanni J. Evaluation of pentacyclic triterpenes found in Perilla frutescens for inhibition of skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Oncotarget. 2015;6(36):39292–392306. doi: 10.18632/oncotarget.5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C.Y., You H.J., Jeong H.G. Nitric oxide and tumor necrosis factor-α production by Oleanolic acid via nuclear factor-κB activation in macrophages. Biochem. Biophys. Res. Commun. 2001;288(1):49–55. doi: 10.1006/bbrc.2001.5727. [DOI] [PubMed] [Google Scholar]
- Dash S.K., Chattopadhyay S., Tripathy S., Dash S.S., Das B., Mandal D., Mahapatra S.K., Bag B.G., Roy S. Self-assembled betulinic acid augments immunomodulatory activity associates with IgG response. Biomedicine Pharmacotheraphy. 2015;75:205–217. doi: 10.1016/j.biopha.2015.07.033. [DOI] [PubMed] [Google Scholar]
- David B., Wolfender J.L., Dias D.A. The pharmaceutical industry and natural products: Historical status and new trends. Phytochem. Rev. 2015;14(2):299–315. [Google Scholar]
- Feng L., Liu X., Zhu W., Guo F., Wu Y.C., Wang R., Chen K., Huang C., Li Y. Inhibition of human neutrophil elastase by pentacyclic triterpenes. Publ. Library Sci. 2013;8(12):1–11. doi: 10.1371/journal.pone.0082794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumitsu S., Villareal M.O., Fujitsuka T., Aida K., Isoda H. Anti-inflammatory and anti-arthritic effects of pentacyclic triterpenoids maslinic acid through NF-κB inactivation. Molecular Nutr. Food Res. 2016;60(2):399–409. doi: 10.1002/mnfr.201500465. [DOI] [PubMed] [Google Scholar]
- Geetha T., Varalakshmi P. Anti-inflammatory activity of lupeol and lupeol linoleate in rats. J. Ethnopharmacol. 2001;76:77–78. doi: 10.1016/s0378-8741(01)00175-1. [DOI] [PubMed] [Google Scholar]
- Hao C., Wu B., Hou Z., Xie Q., Liao T., Wang T., Ma D. Asiatic acid inhibits LPS induced inflammatory response in human gingival fibroblasts. Int. Immunopharmacol. 2017;50:313–318. doi: 10.1016/j.intimp.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Holanda Pinto S.A., Pinto L.M.S., Cunha G.M.A., Chaves M.H., Santos F.A., Rao V.S. Anti-inflammatory effect of α, β-Amyrin, a pentacyclic triterpene from Protium heptaphyllum in rat model of acute periodontitis. Inflammopharmacology. 2008;16(1):48–52. doi: 10.1007/s10787-007-1609-x. [DOI] [PubMed] [Google Scholar]
- Ilyas U., Katare D.P., Aeri V., Naseef P.P. A review on hepatoprotective and immunomodulatory herbal plants. Pharmacognosy Rev. 2016;10(19):66–70. doi: 10.4103/0973-7847.176544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantan I., Ahmad W., Bukhari S.N.A. Plant-derived immunomodulators: An insight on their preclinical evaluation and clinical trials. Frontier Plant Sci. 2015;6:655. doi: 10.3389/fpls.2015.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Arellanes A., Luna-Herrera J., Cornejo-Garrido J., Lopez-Garcia S., Castro-Mussot M.E., Meckes-Fischer M., Mata-Espinosa D., Marquina B., Torres J., Hernandez-Pando R. Ursolic and oleanolic acids as antimicrobial and immunomodulatory compounds for tuberculosis treatment. BMC Complement. Alternat. Med. 2013;13:258. doi: 10.1186/1472-6882-13-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L., Baltz R.H. Natural product discovery: past, present, and future. J. Ind. Microbiol. Biotechnol. 2016;43(2-3):155–176. doi: 10.1007/s10295-015-1723-5. [DOI] [PubMed] [Google Scholar]
- Khajuria A., Gupta A., Garai S., Wakhloo B.P. Immunomodulatory effects of two sapogenins 1 and 2 isolated from Luffa cylindrica in BALB/C mice. Bioorg. Med. Chem. Lett. 2007;17(6):1608–1612. doi: 10.1016/j.bmcl.2006.12.091. [DOI] [PubMed] [Google Scholar]
- Laszczyk M.N. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009;75(15):1549–1560. doi: 10.1055/s-0029-1186102. [DOI] [PubMed] [Google Scholar]
- Lebeaux D., Ghigo J.M., Beloin C. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Molecular Biol. Rev. 2014;78:510–543. doi: 10.1128/MMBR.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Gong X., Zhang L., Zhang Z., Luo F., Zhou Q., Chen J., Wan J. Madecassoside attenuates inflammatory response on collagen-induced arthritis in DBA/1 mice. Phytomedicine. 2009;16:538–546. doi: 10.1016/j.phymed.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Lucetti D.L., Lucetti E.C.P., Bandeira M.A., Veras H.N.H., Silva A.H., Leal L.K.A.M., Lopes A.A., Alves V.C.C., Silva G.S., Brito G.A., Viana G.B. Anti-inflammatory effects and possible mechanism of action of Lupeol acetate isolated from Himatanthus drasticus (Mart.) Plumel J. Inflammat. 2010;7:1–11. doi: 10.1186/1476-9255-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A., Tiwari A.K., Sahin K., Kucuk O., Ali S. Triterpenoid saponin-rich fraction of Centella asiatica decreases IL-1β and NF-κB and augments tissue regeneration and excision wound repair. Turk. J. Biol. 2016;40:399–409. [Google Scholar]
- Marquez-Martin A., La Puerta R.D., Fernandez-Arche A., Ruiz-Gutierrez V., Yaqoob P. Modulation of cytokine secretion by pentacyclic triterpenes from olive pomace oil in human mononuclear cells. Cytokine. 2006;36(5–6):211–217. doi: 10.1016/j.cyto.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Mawa S., Jantan I., Husain K. Isolation of terpenoids from the stem of Ficus aurantiaca Griff and their effects on reactive oxygen species production and chemotactic activity of neutrophils. Molecules. 2016;21(1):9. doi: 10.3390/molecules21010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montilla M.P., Agil A., Navarro M.C., Jimenez M.I., Garcia-Granados A., Parra A., Cabo M.M. Antioxidant activity of maslinic acid, a triterpene derivative obtained from Olea europaea. Planta Med. 2003;69(5):472–474. doi: 10.1055/s-2003-39698. [DOI] [PubMed] [Google Scholar]
- Niege A.J.C.F., Laetitia P., Helene E., Lisa M.M., Cristina L.P., Veronique P., Yvan L., Christelle M.A. Pentacyclic triterpene bioavailability: An overview of in-vitro and in-vivo studies. Molecules. 2017;22(400):1–24. doi: 10.3390/molecules22030400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oladimeji A.O., Oladosu I.A., Jabeen A., Faheem A., Mesaik M.A., Ali M.S. Immunomodulatory activities of isolated compounds from the root-bark of Cussonia arborea. Pharmacy Biol. 2017;55(1):2240–2247. doi: 10.1080/13880209.2017.1400078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaez G.L.M., Jelver S., Fernando A., Ulrike H. Pentacyclic triterpenes from Cecropia telenitida with immunomodulatory activity on dendritic cells. Revista Brasileira Farmacog. 2013;23(7):754–761. [Google Scholar]
- Pujol J., Vansteenkiste J., Martino T., Atanackovic D., Reck M., Thomeer M., Douillard J., Fasola G., Potter V., Taylor P., Bosquee L., Scheubel R., Jarnjak S., Debois M., Alves P., Louahed J., Brichard V., Lehmann F. Safety and immunogenicity of MAGE-A3 cancer immunotherapeutic with or without adjuvant chemotherapy in patients with resected stage IB to III MAGE-A3-positive non-small-cell lung cancer. J. Thoracic Oncol. 2015;10(10):1458–1467. doi: 10.1097/JTO.0000000000000653. [DOI] [PubMed] [Google Scholar]
- Ragupathi G., Gardner J.R., Livingston P.O., Gin D.Y. Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer. Expert Rev. Vaccines. 2011;10:463–470. doi: 10.1586/erv.11.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed H.M.F., Rasheed F., Qureshi A.W., Jabeen Q. Immunostimulant activities of the aqueous methanolic extract of Leptadenia pyrotechnica, a plant from Cholistan desert. J. Ethnopharmacol. 2016;186:244–250. doi: 10.1016/j.jep.2016.03.039. [DOI] [PubMed] [Google Scholar]
- Razali F.N., Sinniah S.K., Hussin H., Zainal Abidin N., Shuib A.S. Tumor suppression effect of Solanum nigrum polysaccharide fraction on breast cancer via immunomodulation. Int. J. Biol. Macromol. 2016;92:185–193. doi: 10.1016/j.ijbiomac.2016.06.079. [DOI] [PubMed] [Google Scholar]
- Romero-Estrada A., Maldonado-Magana A., Gonzalez-Christen J., Bahena S.M., Garduno-Ramirez M.L., Rodriguez-Lopez V., Alvarez L. Anti-inflammatory and antioxidative effects of six pentacyclic triterpenes isolated from the Mexican copal resin of Bursera copallifera. BMC Compl. Alternative Med. 2016;16(1):1–10. doi: 10.1186/s12906-016-1397-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saaby L., Jager A.K., Moesby L., Hansen E.W., Christensen SB. Isolation of immunomodulatory triterpene acids from a standardized rose hip powder (Rosa canina L.) Phytotherapy Res. 2011;25(2):195–201. doi: 10.1002/ptr.3241. [DOI] [PubMed] [Google Scholar]
- Sapideh S., Azam B. Immunostimulants: Types and functions. J. Med. Microbiol. Infectious Diseases. 2016;4(3-4):45–51. [Google Scholar]
- Shahlaei M., Ghanadian S.M., Ayatollahi A.M., Mesaik M.A., Abdalla O.M., Afsharypour S., Rabbani M. Molecular modeling, structure activity relationship and immunomodulatory properties of some lupeol derivatives. Med. Chem. Res. 2013;22(4):1795–1803. [Google Scholar]
- Sharma K.R., Adhikari A., Jabeen A., Dastagir N., Kalauni S.K., Choudhary M., Pokharel Y.R. Immunomodulatory studies on triterpenoids from Scoparia dulcis Linn. Biochem. Pharmacol. 2015;4(4):182. [Google Scholar]
- Sheng H., Sun H. Synthesis, biology and clinical significance of pentacyclic triterpenes: A multi target approach to prevention and treatment of metabolic and vascular diseases. Natural Prod. Rep. 2011;28:543–593. doi: 10.1039/c0np00059k. [DOI] [PubMed] [Google Scholar]
- Sun H., Chen F., Yao M. Immunomodulatory Activity of 3b,6b-dihydroxyolean-12-en-27-oic acid in tumor-bearing mice. Chem. Biodivers. 2009;6:1243–1253. doi: 10.1002/cbdv.200800187. [DOI] [PubMed] [Google Scholar]
- Wang X.H., Zhou S.Y., Qian Z.Z., Zhang H.L., Qiu L.H., Song Z., Zhao J., Wang P., Hao X.S., Wang H.Q. Evaluation of toxicity and single-dose pharmacokinetics of intravenous ursolic acid liposomes in healthy adult volunteers and patients with advanced solid tumors. Expert Opin. Drug Metabol. Toxicol. 2012;9(2):117–125. doi: 10.1517/17425255.2013.738667. [DOI] [PubMed] [Google Scholar]
- Wojnicz D., Tichaczek-Goska D., Korzekwa K., Kicia M., Hendrich A. Anti-enterococcal activities of pentacyclic triterpenes. Adv. Clini. Exper. Med. 2017;26(3):483–490. doi: 10.17219/acem/62245. [DOI] [PubMed] [Google Scholar]
- Xin W., Zhang L., Sun F., Jiang N., Fan H., Wang T., Li Z., He J., Fu F. Escin exerts synergistic anti-inflammatory effects with low doses of glucocorticoids in-vivo and in-vitro. Phytomed. 2011;18:272–277. doi: 10.1016/j.phymed.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Yi J., Obminska-Mrukowicz B., Yuan L., Yuan H. Immunomodulatory effects of betulinic acid from the bark of white birch on mice. J. Veter. Sci. 2010;11(4):315–319. doi: 10.4142/jvs.2010.11.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun K.J., Kim J.Y., Kim J.B., Lee K.W., Jeong S.Y., Park H.J., Jung H.J., Cho Y.W., Yun K., Lee K.T. Inhibition of LPS-induced NO and PGE2 production by asiatic acid via NF-kappa B inactivation in RAW 264.7 macrophages: Possible involvement of the IKK and MAPK pathways. Int. Immunopharmacol. 2008;8(3):431–441. doi: 10.1016/j.intimp.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhang M., Tao Y., Wang G., Xia B. Madecassic acid inhibits the mouse colon cancer growth by inducing apoptosis and immunomodulation. J. Balkan Union Oncol. 2014;19(2):372–376. [PubMed] [Google Scholar]