Abstract
Type 2 diabetes mellitus (T2DM) and nonalcoholic fatty liver disease (NAFLD) are the most problematic metabolic diseases in the world. NAFLD encompasses a spectrum of severity, ranging from simple steatosis to non-alcoholic steatohepatitis (NASH) and fibrosis, increasing the risk of cirrhosis and hepatocellular carcinoma. Importantly, NAFLD is closely linked to obesity and tightly interrelated with insulin resistance and T2DM. T2DM and NAFLD (T2DM-NAFLD) are called as the Xike Rixijing Disease and Tonglaga Indigestion Disease respectively, in Mongolian medicine. Xike Rixijing Disease maybe develop into Tonglaga Indigestion Disease. Forturnately many Mongolian medicines show efficient treatment of T2DM-NAFLD, such as Agriophyllum squarrosum, Haliyasu (dried powder of camel placenta), Digeda-4 (herbs of Lomatogonium carinthiacum, rhizomata of Coptis chinensis, ripe fruits of Gardenia jasminoides, herbs of Dianthus superbus), Guangmingyan Siwei Decoction Powder (Halite, ripe fruits of Terminalia chebula, rhizomata of Zingiber officinale, fruit clusters of Piper longum), Tonglaga-5 (ripe fruits of Punica granatum, barks of Cinnamomum cassia, ripe fruits of Amomum kravanh, fruit clusters of Piper longum, flowers of Carthamus tinctorius), Tegexidegeqi (rhizomata of Inula helenium, ripe fruits of Gardenia jasminoides, rhizomata of Platycodon grandiflorum, rhizomata of Coptis chinensis, heartwood of Caesalpinia sappan), Ligan Shiliu Bawei San (ripe fruits of Punica granatum, barks of Cinnamomum cassia, ripe fruits of Amomum kravanh, fruit clusters of Piper longum, flowers of Carthamus tinctorius, ripe fruits of Amomum tsao-ko, rhizomata of Zingiber officinale), etc. Principles of Mongolian medicine in treating diseases: by balancing “three essences or roots” and “seven elements”, strengthening liver and kidney function, transporting nutrients to enhance physical strength and disease resistance, and combined with drugs for comprehensive conditioning treatment. However, their molecular mechanisms remain unclear. In this review, we prospect that Mongolian medicines might be a promising treatment for T2DM-NAFLD by activating P2X7R/NLRP3/NF-κB inflammatory pathway via lipid-sensitive nuclear receptors (i.e., FXR and LXR).
Keywords: Mongolian medicine, nonalcoholic fatty liver disease, type 2 diabetes mellitus, Xike Rixijing Disease
1. Introduction
Diabetes is a metabolic disease characterized by increased glucose levels (Sun et al., 2020). Diabetes is known as Xike Rixijing Disease in modern Mongolian medicine. In Mongolian medicine, diabetes is believed to be induced by the imbalance of “three essences or roots” and “seven elements” in the human body, which leads to overaccumulation of Badagan and fat. Meanwhile, Badagan and fat may interact with Heyi and Xila (Ao, ALG, & Chen, 2019), causing heat and dryness in the liver and kidney, leading to liver and kidney function decline and making the needed essence unabe to produce and the dross decomposition abnormal. In other words, when Badagan and fat levels increase, they cannot become the essence but become urine transported to the bladder, leading to diabetes. Fat overaccumulation in the liver can easily cause Tonglaga (food essence) Indigestion Disease (Ba, 1988, Yuan, 1977), which is nonalcoholic fatty liver disease (NAFLD). Here, patients may develop costal distention or dull pain, anorexia, abdominal distention, fatigue, dull pain in the liver area, and a sense of compression or fullness in the right upper abdomen. Insulin resistance (IR) and inflammation can continue to aggravate NAFLD and promote its progression to nonalcoholic steatohepatitis (NASH), and advance liver fibrosis and cirrhosis (Kim et al., 2020, Manne, Handa, & Kowdley, 2018). NAFLD affects 25%–30% of the general population and may increase to 80%–90% of patients with type 2 diabetes mellitus (T2DM) (Polyzos & Mantzoros, 2016, Younossi et al., 2016). The risk factors for T2DM complicated with NAFLD (T2DM-NAFLD) mainly include obesity, IR, and fat metabolic disorder (Wang, 2014). They are mutually causal, not only increasing the difficulty of blood glucose control but also accelerating the progression to cirrhosis which may further increase the risk of other critical complications (Wang & Li, 2019). Studies have demonstrated that the degree of liver fibrosis is the most powerful predictor for all-cause death. Although most patients with NAFLD do not reach the stage of cirrhosis, patients with T2DM show a relatively faster progression as well as higher risks of NASH and liver-related death, one of the main causes of death in patients with diabetes (Ciardullo et al., 2020). The mechanisms of NAFLD incidence may be related to oxidative stress, inflammation, and metabolic disorder. However, the specific underlying mechanisms remain unclear (Neuschwander-Tetri, 2017, Otterdal et al., 2015). Several studies have highlighted the strong interaction between T2DM and NAFLD and describe a complex bidirectional relationship. Indeed, the coexistence of these two conditions pejoratively affect the course and prognoses of both diseases (Birkenfeld & Shulman, 2014, Caussy et al., 2021, McPherson et al., 2015, Wang et al., 2012, Watt et al., 2020).
IR is the common pathophysiological basis of T2DM and NAFLD. During the pathogenesis, fatty acid stimulation and inflammatory response activation can cause or aggravate IR. P2X7, a subtype of P2X receptors, plays an important role in inflammation (Xue et al., 2018). The NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome has been linked to metabolic disease, including high uric acid and IR (Yu, Chen, Zhao, Zhu, & Dong, 2021). The NLRP3 inflammasome together with the P2X7 receptors (P2X7R) release a large of cytokines such as interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-18 (IL-18), and those cytokines release activated inflammatory nuclear transcription factor (NF) κB (NF-κB) pathway and amplified the inflammatory process (Gora, Ciechanowska, & Ladyzynski, 2021). Farnesoid X receptor (FXR) is a master regulator of bile acid metabolism, lipid metabolism, and hepatic glucose metabolism. In patients with NAFLD, triglycerides accumulate in the liver leading to steatosis from increased de novo lipogenesis and fatty acid uptake in addition to reduced fatty acid oxidation and very-low-density lipoprotein (VLDL) export (Albillos et al., 2020). Liver X receptors (LXRs) are a group of ligand-activated transcription factors (Dixon et al., 2021). In NAFLD patients, the nuclear receptors FXR and LXR are master regulators of metabolism and liver physiology (Welch et al., 2022). Therefore, regulating fat metabolism and improving inflammatory response are the conventional approaches in the treatment of T2DM-NAFLD.
The mainstay of NAFLD management is currently to reduce modifiable metabolic risk. Achieving good glycaemic control and optimising weight loss are pivotal to restricting disease progression (Hazlehurst, Woods, Marjot, Cobbold, & Tomlinson, 2016). There is no cure for T2DM so far, but it can be treated and controlled. Synthetic drugs and/or insulin may be required to maintain the blood glucose level as near as possible to normal and to delay or possibly to prevent the development of diabetes-related health problems (Artasensi et al., 2020). Furthermore, Biguanide, glucagon-like peptide 1 receptor (GLP-1R) agonists, dipeptidyl peptidase 4 (DPP-4) inhibitors, and sodium-glucose cotransporter 2 (SGLT2) inhibitors have been successfully introduced for the treatment of T2DM. However, those drugs have the adverse effect, such as nausea, vomiting, diarrhea (Gilbert & Pratley, 2020, Ma et al., 2021).
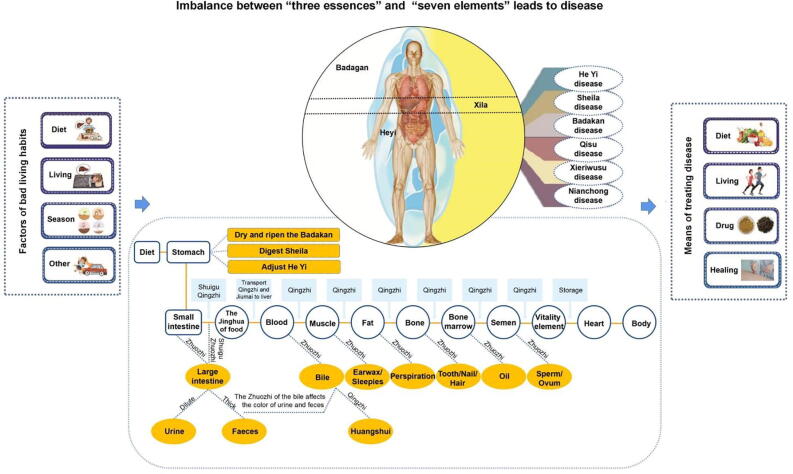
2. T2DM-NAFLD in Mongolian medicine
In Mongolian medicine, the human body is considered to consist of “three essences or roots” (i.e., Heyi, Sheila, and Badakan) and “seven elements” (fine food, blood, muscle, fat, bone, bone marrow, and semen). The “three essences or roots” refer to three energy and fundamental substances on which the human body relies for activities, whereas the “seven elements” refer to material basis on which “three essences” rely. All human life activities are regulated by the “three essences”, and all diseases are attributable to the imbalance of these “three essences” and “seven elements” induced by factors such as diet, daily life, and season change (Fig. 1). The whole digestive process represented by the biochemical process of the “seven elements”. Under the action of digestive function, Shuigu (i.e., food) received by the stomach is further decomposed into Jinghua (anima) and waste. Of these, Jinghua are transported to the liver, whereas carried the waste to the large intestines. Shuigu and Jinghua transported to the liver are biochemically transformed into blood in the liver by the action of heat energy and discoloration of Sheila and biochemically transformed into Jinghua such as muscles, fat, bone, bone marrow, and semen initially. Moreover, the bile generated in the liver (i.e., waste in blood) is delivered to the gallbladder simultaneously. Blood runs through the entire body under the action of the heart and the general Heyi and constantly breeds and supplements seven elements consumed in visceral functional activities. From this perspective, the importance of the liver in the biochemistry of seven elements is identical to that of modern medicine, which indicates that the liver is an important organ in the body responsible for detoxification, excretion, immunity, hematopoiesis, endocrine regulation, and the completion of the body’s material metabolism. Long-term overeating the foods with sweet, salty, cold, and heavy taste and sitting and lying on wetlands can cause imbalance of the “three essences” and “seven elements”. The overaccumulation of Badagan and fat, their interaction with Heyi and Sheila, and the abnormal decomposition of Jinghua and waste can lead to Xike Rixijing Disease. Moreover, overaccumulation of fat in the liver can easily cause Tonglaga Indigestion Disease. In other words, when glucose and fatty acids in the body cannot be utilized well, lipoprotein synthesis is impaired and most glucose and fatty acids are transformed into fat in the liver, which eventually induces fat accumulation in the liver. Overaccumulation of fat in the liver can easily result in T2DM-NAFLD. According to the theoretical characteristics of Mongolian medicine and relevant records in classical works, combined with clinical symptoms, fatty liver disease can be divided into Badagan Heyi, Badagan Sheila, Qisu Sheila, and Badagan Qisu. Moreover, the symptoms of the NAFLD, steatohepatitis, and steatohepatitis cirrhosis stages are similar to Badagan Heyi, Qisu Sheila, and Badagan Qisu, respectively (Wu & Ha, 2017). Drugs should be selected on the basis of the principle of regulating three essences and fat metabolism on the basis of strict control of blood glucose.
Fig. 1.
Fundamental theories of Mongolian medicine.
The principles of medication and treatment of T2DM-NAFLD in Mongolian medicine are roughly divided into three major steps: restoring physical strength, strengthening nutrition; strengthening kidney function; that is, patients should pay attention to the rules of life in life, refuse overwork, remember the combination of work and rest, refuse smoke and drink too much; in diet, they should pay attention to the combination of coarse and fine grains, meat and vegetables, main and non-staple food. They should eat black rice, black beans, black sesame seeds, black fungus often, eat less sugar, salt, white fat, and monosodium glutamate. Patients should prevent sudden climatic changes in life, prevent wind, cold, heat, damp, dry, fire climate damage caused by the invasion of the human body. It is also necessary to avoid diseases caused by strong mental stimulation of joy, anger, worry, thinking, sadness, fear, and shock, and combine physical therapy, spiritual (psychological) therapy, diet therapy, and drug therapy. Among various treatments the most important is drug treatment. Our research group found that Mongolian medicine Agriophyllum squarrosum (L.) Moq. has the effects of anti-oxidation, hypoglycemic, regulating blood lipids and improving insulin resistance, and can delay or inhibit the liver and kidney injury caused by diabetes. The pharmacological effects of A. squarrosum were summarized in Table 1. Furthermore, we found that Mongolian medicine extraction of Agriophyllum oligosaccharides could protect the liver in type 2 diabetes, in part by activating insulin in the INS-R/IRS2/PI3K/AKT/Glut4/PPAR-γ signal pathway, facilitating hepatocyte proliferation, and further reducing the blood glucose levels (Bao et al., 2020, Bao et al., 2021). In addition to this, there are many Mongolian medicines currently used for T2DM-NAFLD, such as traditional Mongolian medicine Haliyasu, Digeda-4, Guangmingyan Siwei Decoction Powder, Tonglaga-5, Tegexidegeqi, Ligan Shiliu Bawei San, etc. The pharmacological effects were summarized in Table 2. These medicines combined with a reasonable diet, regular exercise and psychotherapy have achieved good results in the treatment of diabetes, which shows that the effect of Mongolian medicine in the treatment of diabetes is objective and should be explored.
Table 1.
Pharmacological effects of A. squarrosum.
| Effects | Reported pharmacological effects |
References | ||
|---|---|---|---|---|
| Used parts | Extracts | Experimental results | ||
| Anti-oxidation | Shells of seeds | Flavonoids compounds | These extracts have the ability to scavenge DPPH radicals and intracellular ROS and prevented DNA scission. Mechanism: Up-regulated of Nrf2 mediates the p38 / pJNK / MAPK pathway and increases the expression of Bcl-2 to inhibit apoptosis. | Xu, Zheng, Zhang, Zhang, & Ma, 2018 |
| Protocatechuic acid | ||||
| Seeds | Chlorogenic acid | Chlorogenic acid had a stronger ability to reduce potassium ferricyanide than Vc, when they were at the same concentration. At the concentration of 187.5 mg/L, its antioxidant capacity to lard was stronger than that of BHA and Vc at the same concentration, but weaker than Trolox. | Wang et al., 2007 | |
| Extracts of different solvents | The antioxidant components of A. squarrosum were extracted at different temperatures (30 °C, 60 °C) and different solvents (80% ethanol, 60% ethanol, 60% acetone, methanol solution) to determine the ability of each extract to remove hydroxyl radicals. The results showed that the antioxidant effect of the extract extracted with 60% ethanol was the strongest at 30 °C. | Ding, Hu, Wang, Sun, & Jiao, 2008 | ||
| Above-ground plant parts | Aqueous extract | It can increase SOD vitality, reduce MDA activity in the serum of streptozotocin induced diabetic rats, and enhance the ability of the body to scavenge oxygen free radicals. | Ji RGL, 2016 | |
| Hypoglycemic | Above-ground plant parts | Aqueous extract | It has significant hypoglycemic effect on streptozotocin induced SD rats, improves the disorder of glucose and lipid metabolism, and promotes the repair of islet β cells. | Ji, 2016 |
| It has hypoglycemic effects and improved IR in KKAy mice. Mechanism: regulate insulin signal transduction pathway IRS2 / PI3K / AKT / GSK3β / GLUT4. | Saqier et al., 2019 | |||
| Extracts of different solvents | Ethanol extract, ethyl acetate extract, n-butanol extract and aqueous extract showed significant hypoglycemic effect in alloxan-induced diabetic mice, with aqueous extraction and alcohol extract were more obvious, which significantly improved glucose tolerance. | Ao & Bao, 2014 | ||
| Agiophyllum oligo saccharides (AOS) | AOS can play its hypoglycemic effect by reducing random blood glucose, improving oral glucose tolerance, increasing insulin sensitivity and improving pathological changes of islet tissue in Goto-Kakizaki (GK) rats, and the effect is similar to or even better than glibenuron. | Bao, Han, Wang, Bao, & Ao, 2016 | ||
| In vitro and in vivo experiments proved that AOS reduces blood glucose level, improves IR and protects pancreatic tissue by improving the function of islet β cells (MIN6 cells) function. Mechanism: AOS increases insulin signaling INS-R / IRS / GLUT4 signaling, thus regulating glucose metabolism disorder and improving IR. | Bao et al., 2021 | |||
| Regulating blood lipids | Above-ground plant parts | AOS | AOS decreased TG, TC and HDL-c levels in the serum of GK rats, indicating its effect in improving hyperlipidemia. | Bao, Han, Wang, Bao, & Ao, 2016 |
| Crude drug Alcohol extract Aqueous extract |
A. squarrosum has the effect of reducing TC, TG and LDL in the serum of hyperlipidemia diet induced Wistar rats. It was found by screening the active ingredients that its main antilipid component was AOS, which had little toxicity and could relieve the accumulation of fat in the liver tissues of hyperlipidemia rats. | Bao, 2017 | ||
| Improve the liver and kidney damage caused by diabetes mellitus | Above-ground plant parts | AOS | AOS significantly decreased the contents of ALT, AST, Cre and UA in serum of GK rats, and the level of NF-B in liver and kidney tissues was also significantly inhibited, which could significantly improve the pathological injury of liver and kidney tissues in diabetic rats. | Bao, Han, Chao, Che, & Ao, 2018 |
| AOS improved glucose and lipid metabolism disorder and liver function in db / db mice, and reduced the production of inflammatory factors to inhibit the development of T2DM-NAFLD. Mechanism: AOS up-regulated PPARγ, increased the phosphorylation of IRS2 and AKT proteins, and then mediated the signal transduction of insulin signaling pathway INS-R / PI3K / AKT / GLUT4. | Bao et al., 2020 | |||
Table 2.
Pharmacological effects of traditional Mongolian medicine for treatment of T2DM-NAFLD.
| Traditional Mongolian medicines | Reported pharmacological effects |
|||
|---|---|---|---|---|
| Medicinal materials | Animal or cells | Experimental results | References | |
| Haliyasu (Dried powder of camel placenta) | − | db / db mice | Haliyasu has the characteristics of overall regulation, which can significantly reduce food intake, inhibit appetite, improve abdominal obesity, reduce blood glucose, TC, TG and LDL-C levels, improve the proliferative capacity and functional status of T and β cells in spleen, increase the ratio of CD4+ / CD8+ in peripheral blood, and improve endocrine and immune dysfunction. | Xu, Xie, Wang, & Zhang, 2021 |
| Digeda-4 | Herbs of Lomatogonium carinthiacum (Wulf) Reichb. Rhizomata of Coptis chinensis Franch. Ripe fruits of Gardenia jasminoides Ellis Herbs of Dianthus superbus L. |
3T3-L1adipose cell | Digeda-4 promotes fatty acid oxidation in adipocytes. Mechanism: increasing PPARα, PPARβ, IκBα, AKT, p-AKT and down-regulating LXRα and iNOS protein expression. | Chen, 2011 |
| Hyperlipid-induced NAFLD rats | Digda-4 reduced the levels of TG, LDL-C, FFA, AST, FINS, and FIRI in the serum of NAFLD rats, while reducing TG, TC, and MDA in liver tissue.Its effects in the treatment of NAFLD are related to lowering blood lipid, improving liver function, antioxidant effect, and improving IR. Mechanism: increases the expression of PPARs, IκBα, AKT, p-AKT and reduces the expression of LXRα and iNOS to improve IR and oxidative stress. | Tian, Su, Zhao, Meng, & Bao, 2010, Chen, 2011 | ||
| Carbon tetrachloride induced rats | Digeda-4 increase the value of TC and HDL in rat serum, reduce the abnormally elevated NEFA value, LPL and HL vitality in liver tissue, increase the level of TC in liver tissue, reduce pathological changes such as liver tissue degeneration and necrosis, and regulate lipid metabolism. | Zhao, Zhang, Wang, Zheng, & Bao, 2012 | ||
| Guangmingyan Siwei Decoction Powder | Halite Ripe fruits of Terminalia chebula Retz. Rhizomata of Zingiber officinale Rosc. Fruit clusters of Piperlonguml. |
Hyperlipid-induced NAFLD mice | Guangmingyan Siwei Decoction Powder can decrease the levels of TG and TC in the serum of NAFLD mice, reduce liver wet weight and liver index, have obvious lipid-lowering effect and inhibit the lipidation deposition in liver tissue, and lipid-lowering effect is better than Tiopronin enteric-coated tablets. | Na & Bao, 2017 |
| 3T3-L1adipose cell | Guangmingyan Siwei Decoction Powder can activate PPARα / PGC-1α signaling pathway to promote energy and fat metabolism, prevent intracellular fat deposition, and then correct liver lipid metabolism disorder. | Na, 2017 | ||
| Tonglaga-5 | Ripe fruits of Punica granatum L. Barks of Cinnamomum cassia Presl Ripe fruits of Amomum kravanh Pierre ex Gagnep. Fruit clusters of Piper longum L. Flowers of Carthamus tinctorius L. |
3T3-L1adipose cell | Tonglaga-5 can up-regulate PPARα, PPARβ, LXRα, IκBα, AKT, p-AKT and down-regulate iNOS expression in 3T3-L1 adipocytes, promote insulin-mediated glucose uptake and improve IR. | Chen, Wang, Jiang, Bao, & Chen, 2011, Tian, 2011 |
| Hyperlipid-induced NAFLD rats | Tonglaga-5 decreased the levels of TG, TC, LDL-C, FFA, AST, FINS and FIRI in serum, while reducing the levels of TG, TC and MDA levels in liver tissue, and increasing SOD level in NAFLD rats.The expression of PPARα, PPARγ, IκBα, AKT, p-AKT and LXR and i NOS were increased in liver tissues to treat NAFLD. | Tian, Su, Zhao, Meng, & Bao, 2010, Chen, 2011 | ||
| Tegexidegeqi | Rhizomata of Inula helenium L. Ripe fruits of Gardenia jasminoides Ellis Rhizomata of Platycodon grandiflorum (Jacq.) A. DC. Rhizomata of Coptis chinensis Franch. Heartwood of Caesalpinia sappan L. |
Alloxan induced diabetic mice | Tegexidegeqi reduces the contents of FBG, TC, TG, LDL-C, IL-6, TNF-α in diabetic mice, increase HDL-C and insulin levels, increase the effects of SOD and GSH in liver and kidney, reduce pancreatic islet damage, reduce the oxidative stress state of liver and kidney tissues, increase the protein expression of GLUT-4 in skeletal muscle and adipose tissue, and improve IR. | Wu, 2020 |
| Ligan Shiliu Bawei San | Ripe fruits of Punica granatum L. Barks of Cinnamomum cassia Presl Ripe fruits of Amomum kravanh Pierre ex Gagnep. Fruit clusters of Piper longum L. Flowers of Carthamus tinctorius L. Ripe fruits of Amomum tsao-ko Crevost et Lemaire Rhizomata of Zingiber officinale Rosc. Halite |
3T3-L1adipose cell | Ligan Shiliu Bawei San promotes fglucose uptake in adipocytes. Mechanism: increasing PPARs, IκBα, AKT, p-AKT and down-regulating LXRα and iNOS protein expression. | Chen, 2011, Tian, 2011 |
| Hyperlipid-induced NAFLD rats | Ligan Shiliu Bawei San reduce the rat serum levels of TG, LDL-C, FFA and AST levels, as well as the contents of TC, TG, and MDA in the liver, elevated the SOD levels. Mechanism: the expression of PPARs, IκBα, AKT, p-AKT was increased and LXRα and iNOS were decreased in liver tissue of NAFLD rats, so as to improve IR and oxidative stress to treat NAFLD. | |||
3. Research progress on T2DM-NAFLD pathogenesis in modern medicine
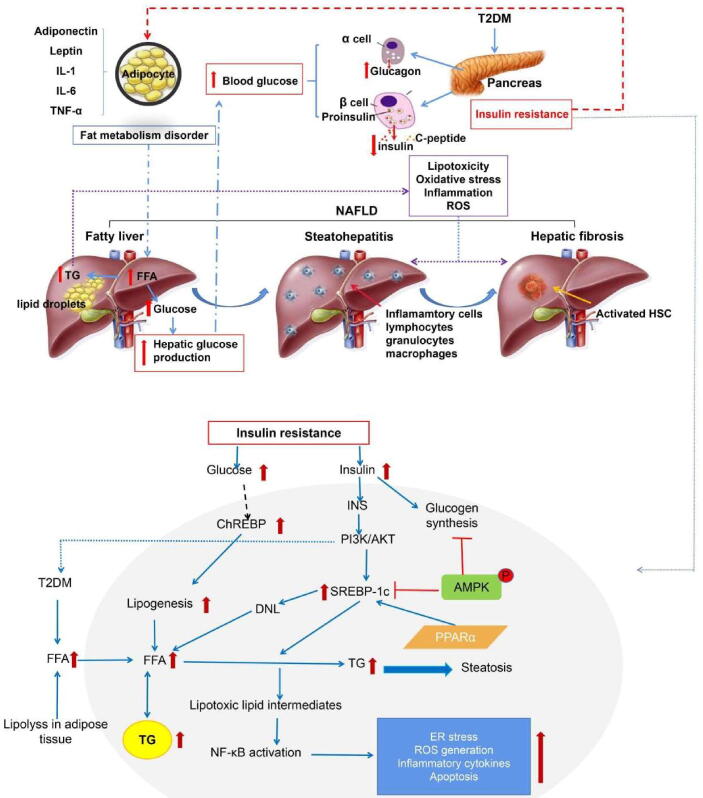
T2DM-NAFLD pathogenesis remains unclear, whereas metabolic disorder due to IR increasing triglyceride (TG) levels is a key reason (Jarvis et al., 2020, Mu et al., 2019). Scholars have also found that 27 targets are ubiquitous in T2DM-NAFLD–related pathways through target fishing and network construction and mainly involved in IR and inflammation (Qin et al., 2019). The liver is the target organ of insulin. It has an important role in lipid metabolism regulating (Titchenell, Lazar, & Birnbaum, 2017). Due to the weight gain caused by increased appetite and reduced physical activity, the insulin receptor on hypertrophic fat cell membranes in patients with T2DM is insensitive to insulin, which results in IR, leading to the weakening of insulin’s effect on inhibiting free fatty acid (FFA) release in the plasma and the increase in the levels of FFAs entering the liver. FFAs form considerable TG deposition in the liver, resulting in hepatocyte degeneration and fatty liver disease. The more serious IR, the more obvious is the lipid metabolic disorder, which indicates that IR may be important in potential liver diseases secondary to T2DM (Carolina et al., 2012, Li et al., 2021). In other words, IR, an independent risk factors for fatty liver disease, may be the link between T2DM and NAFLD. Pharmacological effect and mechanism are summarized in Fig. 2.
Fig. 2.
Effect and mechanism of IR in T2DM-NAFLD.
T2DM causes NAFLD (including NAFLD, NASH, liver fibrosis, and even cirrhosis); 10%–20% of patients with T2DM have NASH. NAFLD is only manifested as the fatty degeneration of liver cells, and liver cell damage does not generally occur at this stage. Liver function and transaminase can be normal or abnormal. In this stage, the symptoms of poor appetite, nausea, and bloating are similar to Badagan Heyi in Mongolian medicine of Tonglaga Indigestion Disease.
As NAFLD develops rapidly, the enriched FFA flows from the dietary fat and white fat tissue into the liver, further stimulating gluconeogenesis and increasing TG accumulation in the liver cells, gradually resulting in more pathological changes occurring and progressing to NASH (Thiyagarajan et al., 2020). According to the “two hits”, the first attack on the liver leads to IR. Fatty acids imported into the liver and TG synthesized in the liver are too high in diabetes, which results in TG accumulation in the liver and increases oxidative stress. Then, liver is hit twice, resulting in inflammation, necrosis, and fibrosis in the liver. IR leads to increased FFA intake by the liver, further leading to a compensatory increase in the rate of mitochondrial oxidation and thereby increasing reactive oxygen species (ROS) production. ROS can attack unsaturated fatty acids by itself, resulting in numerous peroxidation products. Therefore, some studies have indicated that hepatocytes change from NAFLD to NASH when they cannot resist the damage due to their defense ability in the process of oxidative stress. The clinical manifestations are fatigue, nausea, loss of appetite, fullness and pain in the liver area or right upper abdomen, abdominal distention, and other symptoms, including fever in some cases. The symptoms of liver area pain and fever at this stage are similar to Qisu Xila in Tonglaga Indigestion Disease in Mongolian medicine.
Studies have demonstrated that NAFLD due to T2DM is associated with adiponectin and peroxisome proliferator activated receptors α (PPARα) (Emmanuel, Alexander, Thierry, & Michael, 2021), NF-κB signaling pathway (Krzysztof et al., 2021), FXR (Alessandro & Andrea, 2021), and endoplasmic reticulum stress (Kumar et al., 2021). Some researchers are exploring new targets for T2DM and NAFLD treatment to regulate lipid metabolism and control inflammation (Hana et al., 2019).
One of the important characteristics of NAFLD is lipid metabolism disorder, and fat cells have been found to have various endocrine functions and secrete various cytokines, such as adiponectin and leptin. Leptin secretion is directly proportional to the body fat content, and it has a two-way regulatory effect on insulin. Insulin can stimulate leptin production, which can reduce insulin secretion by causing pancreatic β-cell hyperpolarization to form a fat–islet axis, IL-6 and tumor necrosis factor (TNF) α (Rohit, Brijesh, & Paul, 2018, Sheng et al., 2019). These fat cells can affect glucose and lipid metabolism through various ways. Therefore, these cytokines also play an important role in T2DM-NAFLD development. Diabetes hyperlipidemia and hyperglycemia can induce the transcription of proinflammatory cytokines and monocyte chemoattractant protein (MCP)-1 and then promote NAFLD pathogenesis. These cytokines also induce of adipokine fatty acid binding protein 4 (FABP4) transcription through NF-κB (Chang et al., 2021). Adipokine and proinflammatory cytokine transcription induces hepatocyte injury and IR. P2X7R is mainly involved in the inflammatory reaction released by adenosine triphosphate (ATP) from damaged cells, where it mediates a series of cellular signal transduction processes (Virgilio, Ben, Sarti, Giuliani, & Falzoni, 2017). For instance, it activates multiple pathways such as NLRP3 and NF-κB in the NLR inflammasome family, induces the release of IL-1β, IL-18, TNF-α, and other cytokines (Costa-Junior, Sarmento, & Coutinho-Silva, 2011), and play a key role in inflammatory reaction and immunoregulation. Studies have shown that ATP-P2X7R is an important signaling pathway for NLRP3 activation (Solini, Usuelli, & Fiorina, 2015). NLRP3 inflammasome belongs to NOD-like receptor protein, which can recognize relevant molecular patterns on pathogens. It is mainly composed of NLRP3 apoptosis-associated spot-like protein (ASC) and caspase-1, playing an important regulatory role in the occurrence and development of various inflammatory-related liver diseases (Franchi et al., 2009, Lee et al., 2012). NLRP3 inflammasome mainly has downstream effects by IL-1β, IL-18, and TNF-α secretion (Mangan et al., 2018). The proinflammatory factors TNF-α can activate NF-κB, thereby mediating cytokine release, inducing inflammatory reaction, and participating in NAFLD pathology (Zhang et al., 2021).
In NAFLD, P2X7 receptor activation caused by T2DM can activate NLRP3 and NF-κB inflammasome to cause hepatocyte injury. The P2X7/NLRP3/NF-κB signaling pathway plays an important role in maintaining immune homeostasis and downregulating inflammatory response and providing a new target for drug-based treatment of T2DM and NAFLD in the future (Fig. 2).
With the development and aggravation of NASH due to T2DM, the released TGF-β and the platelet derivative lead to hepatic stellate cell (HSC) activation, differentiation, and proliferation (Fujii et al., 2020). Studies (Campisano, Colla, Echarte, & Chisari, 2019) have confirmed that HSC is a key cell involved in fiber formation. After activation, it changes from vitamin A into fibroblasts (MFC) stored in static cells, which is the main driving factor of fibrosis in liver injury. HSC activation can produce extracellular matrix (ECM) protein and express α-smooth actin (α-SMA). ECM can also be used as a medium for promoting inflammation or fibrosis (Kisseleva, 2017, Trautwein, Friedman, Schuppan, & Pinzani, 2015). Patients at this stage are characterized by anorexia, fatigue, liver discomfort or pain, abdominal distension, hepatomegaly, gangrene, bleeding, loss of appetite, hepatomegaly, hemorrhage, and other symptoms, which are similar to Badagan Qisu in Mongolian medicine of Tonglaga Indigestion Disease.
Hepatic steatosis is closely related to the imbalance of TG (Koo, 2013) synthesis and lipolysis. FXR plays a certain regulatory role in different liver disease stages. Activated FXR can regulate lipid metabolism related proteins to reduce TG levels (Ding et al., 2014). FXR reduces TG levels via two means, including the downregulation of the expression of sterol regulatory element binding protein 1C (SREBP 1C) and its downstream target genes (Watanabe et al., 2004). FXR activation can also induce peroxisome proliferator activated receptor α (PPAR-α) expression, which in turn increases lipid oxidative consumption and reduces lipid accumulation (Torra et al., 2003). In addition, FXR protects hepatocytes from damage by increasing LKB1 levels (Lee et al., 2012). LKB1, an upstream kinase of AMPK, regulates AMPK phosphorylation. AMPK activation can inhibit SREBP1 expression and lead to decreased lipogenesis (Giri et al., 2006). Insulin and glucagon can affect AMPK activation. In the liver, AMPK mainly regulates liver fatty acid metabolism by regulating the transcription activity of SREBP-1c, liver specific transcription factor Ch REBP, and fatty acid receptor PPAR-α. Moreover, SIRT1 activates AMPK signaling pathway (Purushotham et al., 2009). In addition to FXR, LXR is a member of the nuclear receptor family. LXRs comprises two subtypes: LXRα and LXRβ, which are important to regulate cholesterol levels (Tardelli, Claudel, Bruschi, & Trauner, 2018). LXRα agonists can significantly inhibit increase in IL-1β levels caused by NLRP3 inflammasome activation by cholesterol crystals in the macrophage model, and this effect is related to NF-κB signaling pathway inhibition. In addition to sterol metabolism regulation, LXRs upregulate the expression of genes involved in the regulation of lipid metabolism, which include SREBP-1C (Repa et al., 2000). Evidence has shown that inflammation leads to liver fibrosis and that the extracellular signals of inflammatory cells can further regulate HSC activation (Seki & Schwabe, 2015). Nevertheless, the molecular mechanism of liver fibrosis warrants further discussion, and effective liver fibrosis treatment remains limited. Therefore, finding new targets and developing new drugs in antifibrosis therapy is of great significance.
4. Conclusion
Some studies have shown that Mongolian medicine–based treatment of T2DM-NAFLD is efficacious. Hence, developing novel Mongolian medicine-based treatment methods for T2DM-NAFLD is warranted. Advanced techniques should be used to investigate the effect of Mongolian medicine on the liver to reduce blood glucose levels and determine whether it may be useful for controlling the disease course involves lipid metabolism regulation and inflammation control. FXR and LXR are effective targets for lipid-related disease treatment as well as important regulators for liver fibrosis inflammation. P2X7R is also considered a key regulator of tissue fibrosis development associated with excessive collagen deposition, which can activate multiple pathway components including NLRP3 and NF-κB and play an essential role in inflammation and immunoregulation. Therefore, using P2X7R to activate NLRP3 and NF-κB inflammasome by regulating FXR or LXR can become a novel Mongolian medicine approach to treat T2DM-NAFLD.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant numbers 82004070, 82160814), the Science and Technology Project of Inner Mongolia (Grant numbers 2020GG0103), the Natural Science Foundation of Inner Mongolia (Grant numbers 2018MS08123), the Mongolian Medicine R & D National and Local Joint Engineering Research Center Open Fund Project (Grant numbers MDK2020001, MDK2021023), Science and Technology Research Project of Higher Education of Inner Mongolia Autonomous Region (Grant numbers NJZY21641) and Science and Technology Project of Tongliao (Grant numbers TLKJ2020001).
Contributor Information
Chengxi Wei, Email: cxwei@imun.edu.cn.
Jixing Nan, Email: jxnan@ybu.edu.cn.
Wuliji Ao, Email: wuliji@126.com.
References
- Albillos A., Gottardi A.D., Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. Journal of Hepatology. 2020;72:558–577. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- Alessandro M., Andrea D. Treatments for NAFLD: State of art. International Journal of Molecular Sciences. 2021;22:2350. doi: 10.3390/ijms22052350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q.P., Zhang Y., Wang Z., Zheng H.J., Bao N.R.S. Effects of Mongolian medicine Digda-4 on lipid metabolism disorder induced by carbon tetrachloride in rats. Lishizhen Medicine and Materia Medica Research. 2012;23:805–806. [Google Scholar]
- Ao, W., & Bao, S. Y. (2014). A Mongolian medicine for treating hyperglycemia and diabetes. China, ZL201210447839.0.
- Ao D.D., ALG, Chen W.Y. Research progress of Mongolian medicine Haliyasu in preventing and treating diabetes mellitus. Journal of Medicine and Pharmacy of Chinese Minorities. 2019;25:49–51. [Google Scholar]
- Artasensi A., Pedretti A., Vistoli G., Fumagalli L. Type 2 diabetes mellitus: A review of multi-target drugs. Molecules. 2020;25:1987. doi: 10.3390/molecules25081987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba Y.X. Inner Mongolia People’s Publishing House; Hohhot: 1988. Ganlusibu: Bai Jingjian (Mongolian version) p. 560. [Google Scholar]
- Bao M.M. Inner Mongolia University; 2017. Study on hypolipidemic activity of Agriopllyllum squarrosum. The Thesis of Master Degree. [Google Scholar]
- Bao S.Y., Han S.Y., Chao R.Y., Che L.G.R., Ao W. The protective effects of Agiophyllum oligo saccharides on rat liver and kidney. Chinese Pharmacological Bulletin. 2018;34:147–148. [Google Scholar]
- Bao S.Y., Han S.Y., Che L.G.R., Chao R.Y., Ao W. Effects of Agiophyllum oligo saccharides on insulin resistance of Goto-Kakizaki rats. Chinese Pharmacological Bulletin. 2016;32:403–409. [Google Scholar]
- Bao S.Y., Han S.Y., Wang H.G.J.L.T., Bao W.S., Ao W. Improvement effects of Agiophyllum oligo saccharides on general characterization and glucose and lipid metabolism of diabetic GK rats. Journal of Jilin University (Medicine Edition) 2016;42:1059–1065. [Google Scholar]
- Bao S.Y., Wang X.Z., Cho S.B., Wu Y.L., Wei C.X., Han S.Y., Nan J.X. Agriophyllum oligosaccharides ameliorate diabetic insulin resistance through INS-R/IRS/Glut4-mediated insulin pathway in db/db mice and MIN6 cells. Frontiers in Pharmacology. 2021;12 doi: 10.3389/fphar.2021.656220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S.Y., Wu Y.L., Wang X.Z., Han S.Y., Cho S.B., Ao W., Nan J.X. Agriophyllum oligosaccharides ameliorate hepatic injury in type 2 diabetic db/db mice targeting INS-R/IRS-2/PI3K/AKT/PPAR-γ/Glut4 signal pathway. Journal of Ethnopharmacology. 2020;257 doi: 10.1016/j.jep.2020.112863. [DOI] [PubMed] [Google Scholar]
- Birkenfeld A.L., Shulman G.I. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology. 2014;59:713–723. doi: 10.1002/hep.26672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisano S., Colla A.L., Echarte S.M., Chisari A.N. Interplay between early-life malnutrition, epigenetic modulation of the immune function and liver diseases. Nutrition Research Reviews. 2019;32:128–145. doi: 10.1017/S0954422418000239. [DOI] [PubMed] [Google Scholar]
- Carolina O.L., Romina L., Beverly O., Joan F., Zhi C., Valeria G.K., Kenneth C. Prevalence of prediabetes and diabetes and metabolic profile of patients with nonalcoholic fatty liver disease (NAFLD) Diabetes Care. 2012;35:873–878. doi: 10.2337/dc11-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caussy C., Aubin A., Loomba R. The relationship between Type 2 diabetes, NAFLD, and cardiovascular risk. Current Diabetes Reports. 2021;21:15. doi: 10.1007/s11892-021-01383-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G.R., Liu H.Y., Yang W.C., Wang C.M., Wu C.F., Lin J.W., Lin C.F. Clozapine worsens glucose intolerance, nonalcoholic fatty liver disease, kidney damage, and retinal injury and increases renal reactive oxygen species production and chromium loss in obese mice. International Journal of Molecular Sciences. 2021;22:6680. doi: 10.3390/ijms22136680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. (2011). Effect and mechanism of lipid and glucose metabolism on adipocytes and the liver of NAFLD rats by Mongolian drug “three modulation methods”. Third Military Medical University. The Thesis of Master Degree.
- Chen L., Wang H., Jiang Y.Z., Bao N.R.S., Chen B. Mongolian drug “Tonglaga-5” improves glucose uptaking in 3T3-L1 adipocytes. Journal of Third Military Medical University. 2011;33:686–689. [Google Scholar]
- Ciardullo S., Muraca E., Perra S., Bianconi E., Zerbini F., Oltolini A.…Perseghin G. Screening for non-alcoholic fatty liver disease in type 2 diabetes using noninvasive scores and association with diabetic complications. BMJ Open Diabetes Research & Care. 2020;8:e000904. doi: 10.1136/bmjdrc-2019-000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Junior H.M., Sarmento V.F., Coutinho-Silva R. C terminus of the P2X7receptor: Treasure hunting. Purinergic Signal. 2011;7:7–19. doi: 10.1007/s11302-011-9215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Pang S., Sun Y., Tian Y.L., Yu L., Dang N.N. Coordinated actions of FXR and LXR in metabolism: From pathogenesis to pharmacological targets for type 2 diabetes. International Journal of Endocrinology. 2014;2014 doi: 10.1155/2014/751859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L.Q., Hu L.J., Wang J.F., Sun A.M., Jiao Y. Studies on scavenging effects on hydroxylradical of the extraction from Agriopllyllum squarrosum. Food Research and Development. 2008;29:47–50. [Google Scholar]
- Emmanuel D.D., Alexander D.N., Thierry C., Michael T. The role of lipid sensing nuclear receptors (PPARs and LXR) and metabolic lipases in obesity, diabetes and NAFLD. Genes (Basel) 2021;12:645. doi: 10.3390/genes12050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L., Eigenbrod T., Munoz-Planillo R., Nuñez G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nature Immunology. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H., Kawada N., Japan Study Group of Nafld Jsg-Nafld The role of insulin resistance and diabetes in nonalcoholic fatty liver disease. International Journal of Molecular Sciences. 2020;21:3863. doi: 10.3390/ijms21113863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M.P., Pratley R.E. GLP-1 analogs and DPP-4 inhibitors in type 2 diabetes therapy: Review of head-to-head clinical trials. Frontiers in Endocrinology (Lausanne) 2020;11:178. doi: 10.3389/fendo.2020.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri S., Rattan R., Haq E., Khan M., Yasmin R., Won J.S., Singh I. AICAR inhibits adipocyte differentiation in 3T3L1 and restores metabolic alterations in diet-induced obesity mice model. Nutrition & Metabolism. 2006;3:31. doi: 10.1186/1743-7075-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gora I.M., Ciechanowska A., Ladyzynski P. NLRP3 inflammasome at the interface of inflammation, endothelial dysfunction, and type 2 diabetes. Cells. 2021;10:314. doi: 10.3390/cells10020314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hana X., Cuia Z.Y., Songa J., Piaoa H.Q., Liana L.H., Houa L.S., Wu Y.L. Acanthoic acid modulates lipogenesis in nonalcoholic fatty liver disease via FXR/LXRs-dependent manner. Chemico-Biological Interactions. 2019;311 doi: 10.1016/j.cbi.2019.108794. [DOI] [PubMed] [Google Scholar]
- Hazlehurst J.M., Woods C., Marjot T., Cobbold J.F., Tomlinson J.W. Non-alcoholic fatty liver disease and diabetes. Metabolism. 2016;65:1096–1108. doi: 10.1016/j.metabol.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis H., Craig D., Barker R., Spiers G., Stow D., Anstee Q.M., Hanratty B. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of population-based observational studies. Plos Medicine. 2020;17:e1003100. doi: 10.1371/journal.pmed.1003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji R.G.L. Inner Mongolia University; 2016. Hypoglycemic activity of aqueous extract of Agriopllyllum squarrosum. [Google Scholar]
- Kim M.H., Seong J.B., Huh J.W., Bae Y.C., Lee H.S., Lee D.S. Peroxiredoxin 5 ameliorates obesity-induced non-alcoholic fatty liver disease through the regulation of oxidative stress and AMP-activated protein kinase signaling. Redox Biology. 2020;28 doi: 10.1016/j.redox.2019.101315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzysztof D., Katarzyna S., Andrzej C., Cezary O., Anna Z., Adam K., Mateusz M. Phloroglucinol strengthens the antioxidant barrier and reduces oxidative/nitrosative stress in nonalcoholic fatty liver disease (NAFLD) Oxidative Medicine and Cellular Longevity. 2021;2021:8872702. doi: 10.1155/2021/8872702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisseleva T. The origin of fibrogenic myofibroblasts in fibrotic liver. Hepatology. 2017;65:1039–1043. doi: 10.1002/hep.28948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo S.H. Nonalcoholic fatty liver disease: Mmolecular mechanisms for the hepatic steatosis. Clinical and Molecular Hepatology. 2013;19:210–215. doi: 10.3350/cmh.2013.19.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Xin X.F., Ma J.Y., Tan C., Osna N., Mahato R.I. Therapeutic targets, novel drugs, and delivery systems for diabetes associated NAFLD and liver fibrosis. Advanced Drug Delivery Reviews. 2021;176 doi: 10.1016/j.addr.2021.113888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.Y., Lee Y.S., Choi H.M., Ko Y.S., Lee H.Y., Jo S.K., Kim H.K. Distinct pathophysiologic mechanisms of septic acute kidney injury: Role of immune suppression and renal tubular cell apoptosis in murine model of septic acute kidney injury. Critical Care Medicine. 2012;40:2997–3006. doi: 10.1097/CCM.0b013e31825b912d. [DOI] [PubMed] [Google Scholar]
- Lee C.G., Kim Y.W., Kim E.H., Meng Z.P., Huang W.D., Hwang S.J., Kim S.G. Farnesoid X receptor protects hepatocytes from injury by repressing miR-199a-3p, which increases levels of LKB1. Gastroenterology. 2012;142:1206–1217. doi: 10.1053/j.gastro.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F.X., Ye J.Z., Sun Y.H., Lin Y.S., Wu T.F., Shao C.X., Zhong B.H. Distinct dose-dependent association of free fatty acids with diabetes development in nonalcoholic fatty liver disease patients. Diabetes & Metabolism Journal. 2021;45:417–429. doi: 10.4093/dmj.2020.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan M.S.J., Olhava E.J., Roush W.R., Seidel H.M., Glick G.D., Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nature Reviews Drug Discovery. 2018;17:588–606. doi: 10.1038/nrd.2018.97. [DOI] [PubMed] [Google Scholar]
- Ma X.X., Liu Z.H., Ilyas I., Little P.J., Kamato D., Sahebka A., Xu S.W. GLP-1 and the kidney: From physiology to pharmacology and outcomes in diabetes. International Journal of Biological Sciences. 2021;17:2050–2068. doi: 10.7150/ijbs.59965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manne V., Handa P., Kowdley K.V. Pathophysiology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Clinics in Liver Disease. 2018;22:23–37. doi: 10.1016/j.cld.2017.08.007. [DOI] [PubMed] [Google Scholar]
- McPherson S., Hardy T., Henderson E., Burt A.D., Day C.P., Anstee Q.M. Evidence of NAFLD progression from steatosis to fibrosingsteatohepatitis using paired biopsies: Implications for prognosis and clinical management. Journal of Hepatology. 2015;62:1148–1155. doi: 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- Mu W., Cheng X.F., Liu Y., Lv Q.Z., Liu G.L., Zhang J.G., Li X.Y. Potential nexus of non-alcoholic fatty liver disease and type 2 diabetes mellitus: Insulin resistance between hepatic and peripheral tissues. Frontiers in Pharmacology. 2019;9:1566. doi: 10.3389/fphar.2018.01566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na R.S. Effects of Guangmingyan Siweitang Powder on fat metabolism and PPARα/PGC-1α pathway in nonalcoholic fatty live. Beijing University of Chinese Medicine. The Thesis of Ph. Degree. 2017 [Google Scholar]
- Na R.S., Bao N.R.S. Effects of Guangmingyan Siweitang Powder on blood lipid and liver function in nonalcoholic fatty liver model mice. China Journal of Traditional Chinese Medicine and Pharmacy. 2017;32:3782–3784. [Google Scholar]
- Neuschwander-Tetri B.A. Non-alcoholic fatty liver disease. BMC Medicine. 2017;15:45. doi: 10.1186/s12916-017-0806-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterdal K., Haukeland J.W., Yndestad A., Dahl T.B., Holm S., Segers F.M., Aukrust P. Increased serum levels of LIGHT/TNFSF14 in nonalcoholic fatty liver disease: Possible role in hepatic inflammation. Clinical and Translational Gastroenterology. 2015;6:e95. doi: 10.1038/ctg.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyzos S.A., Mantzoros C.S. Nonalcoholic fatty future disease. Metabolism. 2016;65:1007–1016. doi: 10.1016/j.metabol.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Purushotham A., Schug T.T., Xu Q., Surapureddi S., Guo X.M., Li X.L. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metabolism. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H.Y., Chen H.J., Zou Y., Zhang X.Y., Wei C.Q., Chen W., Han B. Systematic investigation of the mechanism of Cichorium glandulosum on type 2 diabetes mellitus accompanied with non-alcoholic fatty liver rats. Food & Function. 2019;10:2450–2460. doi: 10.1039/c8fo02284d. [DOI] [PubMed] [Google Scholar]
- Repa J.J., Liang G., Ou J., Bashmakov Y., Lobaccaro J.M., Shimomura I., Mangelsdorf D.J. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRα and LXRβ. Genes & Development. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohit A.S., Brijesh K.S., Paul M.Y. Direct effects of thyroid hormones on hepatic lipid metabolism. Nature Reviews Endocrinology. 2018;14:259–269. doi: 10.1038/nrendo.2018.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saqier B., Han S.Y., S., Y.,, Ao, W. Effects of Agriophyllum squarrosum extracts on glucose metabolism in KKAy mice and the associated underlying mechanisms. Journal of Ethnopharmacology. 2019;241:112009. doi: 10.1016/j.jep.2019.112009. [DOI] [PubMed] [Google Scholar]
- Seki E., Schwabe R.F. Hepatic inflammation and fibrosis: Functional links and key pathways. Hepatology. 2015;61:1066–1079. doi: 10.1002/hep.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng D.D., Zhao S.M., Gao L., Zheng H.F., Liu W.T., Hou J., Wei L.X. BabaoDan attenuates high-fat diet-induced non-alcoholic fatty liver disease via activation of AMPK signaling. Cell & Bioscience. 2019;9:77. doi: 10.1186/s13578-019-0339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solini A., Usuelli V., Fiorina P. The dark side of extracellular ATP in kidney diseases. Journal of the American Society of Nephrology. 2015;26:1007–1016. doi: 10.1681/ASN.2014070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y.N., Ma C., Sun H., Wang H., Peng W., Zhou Z.B., He X. Metabolism: A novel shared link between diabetes mellitus and alzheimer’s disease. Journal of Diabetes Research. 2020;2020:4981814. doi: 10.1155/2020/4981814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardelli T., Claudel F.V., Bruschi F.V., Trauner M. Nuclear receptor regulation of aqua-glyceroporins in metabolic organs. International Journal of Molecular Sciences. 2018;19:pii: E1777. doi: 10.3390/ijms19061777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiyagarajan G., Narendra K., Curtis P.O., Carol A.C., Terrence M.D., Edward N.H., Viswanathan S. Nanoformulated SOD1 ameliorates the combined NASH and alcohol-associated liver disease partly via regulating CYP2E1 expression in adipose tissue and liver. American Journal of Physiology Gastrointestinal and Liver Physiology. 2020;318:G428–G438. doi: 10.1152/ajpgi.00217.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H.P. The terapeutical effect and mechanism of traditional Mongolian medicine “San Gen Tiao Jie Fa” on non-alcoholic fatty liver disease in rats. Chengdu University of Traditional Chinese Medicine. The Thesis of Master Degree. 2011 [Google Scholar]
- Tian H.P., Su R.G.W., Zhao G.Q., Meng X.L., Bao N.R.S. Therapeutic effect of Mongolian medicine Digda-4 on non-alcoholic fatty liver model rats. Pharmacology and Clinics of Chinese Materia Medica. 2010;26:115–116. [Google Scholar]
- Titchenell P.M., Lazar M.A., Birnbaum M.J. Unraveling the regulation of hepatic metabolism by insulin. Trends in Endocrinology and Metabolism. 2017;28:497–505. doi: 10.1016/j.tem.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torra I.P., Claudel T., Duval C., Kosykh V., Fruchart J.C., Staels B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor α gene via activation of the farnesoid X receptor. Molecular Endocrinology. 2003;17:259–272. doi: 10.1210/me.2002-0120. [DOI] [PubMed] [Google Scholar]
- Trautwein C., Friedman S.L., Schuppan D., Pinzani M. Hepatic fibrosis: Concept to treatment. Journal of Hepatology. 2015;62:S15–S24. doi: 10.1016/j.jhep.2015.02.039. [DOI] [PubMed] [Google Scholar]
- Virgilio F.D., Ben D.D., Sarti A.C., Giuliani A.L., Falzoni S. The P2X7 receptor in infection and inflammation. Immunity. 2017;47:15–31. doi: 10.1016/j.immuni.2017.06.020. [DOI] [PubMed] [Google Scholar]
- Wang P., Kang D., Cao W., Wang Y., Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: A systematic review and meta-analysis. Diabetes Metabolism Research and Reviews. 2012;28:109–122. doi: 10.1002/dmrr.1291. [DOI] [PubMed] [Google Scholar]
- Wang S. Analysis of multiple factors and TCM syndrome differentiation in patients with type 2 diabetes mellitus combined with nonalcoholic fatty liver disease. Chinese Journal of Basic Medicine in Traditional Chinese Medicine. 2014;20:649–650. [Google Scholar]
- Wang Y., Li X.J. Clinical research progress of traditional Chinese Medicine compound in treating type 2 diabetes combine non-alcoholic fatty liver. Clinical Journal of Traditional Chinese Medicine. 2019;31:2020–2023. [Google Scholar]
- Wang Y., Zhao P., Zhao K., Song Y. Optimization of extraction process and antioxidant activity of chlorogenic acid from Agriophylla squarrosum. Food Fermentation Industries. 2007;33:131–134. [Google Scholar]
- Watanabe M., Houten S.M., Wang L., Moschetta A., Mangelsdorf D.J., Heyman R.A., Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. Journal of Clinical investigation. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt G.P., Cerda I.D.L., Pan J.J., Fallon M.B., Beretta L., Loomba R., Fisher-Hoch S.P. Elevated glycated hemoglobin is associated with liver fibrosis, as assessed by elastography, in a population-based study of Mexican Americans. Hepatology Communications. 2020;4:1793–1801. doi: 10.1002/hep4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R.D., Billon C., Losby M.K., Bedia-Diaz G., Fang Y.Y., Avdagic A., Griffett K. Emerging role of nuclear receptors for the treatment of NAFLD and NASH. Metabolites. 2022;12:238. doi: 10.3390/metabo12030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, R. L. G. (2020). Study on pharmacodynamic ealuation of Mongolian medicine Tegexi Degeqi (Danurea) Powder on type 2 diabetes model mice. Inner Mongolia Medical University. The Thesis of Master Degree.
- Xu Z.Y., Xie H.X., Wang W.S., Zhang X.H. Effect of Mongolian medicine Haliyasu on spontaneous diabetes in db/db mice. Journal of Inner Mongolia Medical University. 2021;43:171–175. [Google Scholar]
- Xue Y., Guo T., Zou L.F., Gong Y.X., Wu B., Yi Z.H., Li G.D. Evodiamine attenuates P2X7-mediated inflammatory injury of Human umbilical vein endothelial cells exposed to high free fatty acids. Oxidative Medicine and Cellular Longevity. 2018;2018:5082817. doi: 10.1155/2018/5082817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.D., Ha S.B.G. Discussion on the treatment of zifugui fatty liver by stages in Mongolian medicine. World Latest Medicine. 2017;17:34–35. [Google Scholar]
- Xu H.Y., Zheng H.C., Zhang H.W., Zhang J.Y., Ma C.M. Comparison of antioxidant constituents of Agriophyllum squarrosum seed with conventional crop seeds. Journal of Food Science. 2018;83:1823–1831. doi: 10.1111/1750-3841.14159. [DOI] [PubMed] [Google Scholar]
- Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- Yu Y.X., Chen D.R., Zhao Y.H., Zhu J.J., Dong X.H. Melatonin ameliorates hepatic steatosis by inhibiting NLRP3 inflammasome in db/db mice. International Journal of Immunopathology and Pharmacology. 2021;35 doi: 10.1177/20587384211036819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y.T. Inner Mongolia People’s Publishing House; Hohhot: 1977. Four medical classics (Mongolian version) p. 138. [Google Scholar]
- Zhang Y.Q., Wen J., Liu D.Q., Qiu Z., Zhu Q.Q., Li R.Y., Zhang Y.B. Demethylenetetrahydroberberine alleviates nonalcoholic fatty liver disease by inhibiting the NLRP3 inflammasome and oxidative stress in mice. Life Sciences. 2021;281:119778. doi: 10.1016/j.lfs.2021.119778. [DOI] [PubMed] [Google Scholar]