Abstract
Objective
To compare the phytochemical and antimicrobial activity of Ethiopian Kale leaves infusions, investigate the antioxidant activity and profile the major phytochemicals existing in the better solvent system.
Methods
Ethiopian Kale leaves were collected from Addis Ababa, Ethiopia, and extracted using different solvents. The qualitative phytochemical analysis, antibacterial assays, and Fourier Transform Infrared (FTIR) analysis are executed for all extracts. Antioxidant assay and Gas Chromatography-Mass Spectrometry (GC–MS) analysis are carried out for the solvent system, which showed better activity in preliminary studies.
Results
The qualitative phytochemical analysis exposed the presence of different classes of phytoconstituents in most of the tested extracts. The broad spectrum of antibacterial activity (7–15 mm) was noted against the tested bacterial species. The functional groups of the extracts are reported by FTIR analysis. The antioxidant ability of ethanol extract was found to be (62.92 ± 0.34)% for 2,2-diphenyl-1-picrylhydrazyl (DPPH*) assay and (71.12 ± 0.41)% for 2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay. More than 17 major phytocompounds in ethanol extract were profiled by GC–MS analysis.
Conclusion
The ethanol extract of Ethiopian Kale leaves contain a good source of phytochemicals and it can be a significant source for various functional applications.
Keywords: antagonistic activity, antioxidant, functional groups, GC–MS analysis, leaf extract, therapeutics
1. Introduction
Plants are an excellent source of phytochemicals as natural antioxidants and antimicrobials, which may act as an impending drug in modern biomedicine. Recent years have witnessed enriched research work reported on potential integrative medicinal activities and applications of several plants and plant materials (Venkatesan et al., 2016). Still, the plants with traditional therapeutic usage are being screened. Especially, exploring the properties of plant food and in turn, contributes to the positive impacts of human health are still in search.
Ethiopian Kale (Brassica carinata A. Braun) thought to originate from highland plateaus of Ethiopia and adjacent parts of East Africa. It is an amphiploid species (BBCC, 2n = 34) derived from an ancestral interspecific hybridization of Brassica nigra (BB, 2n = 16) and Brassica oleracea (CC, 2n = 18) (Prakash, Wu, Bhat, 2011). Ethiopian Kale is cultivated as an oilseed crop in Ethiopia as a leaf vegetable, with a mild flavor providing micronutrients to the human and animal diet. Cultivation of Ethiopian Kale is hypothesized to have started in Ethiopia near 4000 BCE (Alemayehu & Becker, 2002), although precise information about its domestication is lacking, and cultivation may be more recent (Prakash et al., 2011). In Ethiopia it grows well in its native habitat, on the highland plateaus, in cool (14–18 °C), moist growing conditions (600–1000 mm average annual rainfall), a long growing season (180 d), and an elevation of 2200–2800 m above sea level. It also grows well in semi-arid climates on cultivated farmlands, and marginal lands (Alemayehu & Becker, 2002).
In general, Brassica plants are a rich source of phytochemicals, which are providing defense against diseases in humans. These non-nutritious chemicals are oxidation preventive, sweep out free radicals to provide safeguards against different cardiac, neurological, and many other physiological ailments and prevent the oxidative damage of important biomolecules (Uttara et al., 2009). A large number of Brassica plants have been studied for their bioactive phytochemicals and their potential in antioxidant, antimicrobial, antibacterial, antidiabetic, antiulcer potential, etc (Nawaz et al., 2018). But, in a similar context, no published information is available for the Ethiopian Kale. Profiling of bioactive metabolites from the plants delivers valuable information about their chemical diversities, medicinal potentials, and toxicity concerns that are relevant to various fields (Ezekwe & Chikezie, 2017). Therefore, the present work is aimed to examine the bioactive substances of Ethiopian Kale and their antibacterial and antioxidant efficiency.
2. Materials and methods
2.1. Plant material collection and processing
Ethiopian Kale leaves (EKL) (Batch number- AA01) used in this study were collected from Addis Ababa, Ethiopia in January 2018. The plant was identified by the botanist at Addis Ababa Science and Technology University, Ethiopia, and a voucher specimen (EL0063/DBT/AASTU/2018) was deposited in the department herbarium collection unit. Ethiopian Kale leaves powder (EKLP) was prepared from the shade dried material by an electrical grinder.
2.2. Extraction and qualitative phytochemical analysis
EKLP was weighed (20 g) and soaked separately in 200 mL of different solvents like acetone, chloroform, ethyl acetate, petroleum ether, ethanol, and distilled water for 24 h at 30 °C under shaking conditions. The contents were kept in an orbitary shaker for 48 h and each extract was filtered through filter paper (Whatman No. 42, Maidstone, England) and the concentrated extracts are stored at 4 °C for further experiments. The phytochemical screening of EKL extracts is assayed by standard methods (Harborne, 1978).
2.3. In vitro antibacterial activity of EKL extracts
The antibacterial efficacy of the EKL extracts (30 µL) was tested against the selective Gram-Positive (Staphylococcus spp., Streptococcus spp. and Listeria spp.) and Gram-Negative (Escherichia coli, Salmonella spp., Neisseria spp. and Pseudomonas spp.) bacterial cultures (obtained from the bacterial collection unit of Department of Biotechnology, Addis Ababa Science and Technology University, Ethiopia) by disc diffusion method according to Rodriguez-Carpena et al. (2011) with minor changes. A sterile distilled water (30 µL) and standard antibiotic (Tetracycline-20 µg/mL) were loaded in a separate disc, serving as negative and positive control respectively. The experiment was performed in technical triplicates.
2.4. FTIR analysis
EKL extract powder (1 mg) was pressed into a pellet with 200 mg of potassium bromide and IR spectra were recorded using the FTIR instrument (Model Avatar, 370 Spectrometer, Thermo Nicolet Corporation, Madison, USA) to analyze the functional group presents on the extracts.
2.5. In vitro antioxidant assay
Antioxidant and Antiradicals activity of the EKL ethanol extract (EKLEE) was evaluated using 2,2–diphenyl-1-picrylhydrazyl (DPPH*) and 2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging methods as described by Brand-Williams et al. (1995) at different concentrations (20, 40, 60, 80, and 100 µL). Ascorbic acid was used as a standard reference compound and both assays were performed in technical triplicates.
2.6. GC–MS analysis
The main phytocompounds of EKLEE were identified by using the GC–MS detection system (GC Ultra DSQ II, Thermo Scientific, Germany). Helium gas was used as a carrier gas with a flow rate of 1 mL/min. The sample (1 µL) was injected with a standard rapid mode at the temperature of 240 °C. The column temperature of GC used in this study was programmed from 110 to 240 °C with an increasing rate of 5 °C/min. Elements were detected in electron ionization (EI) mode and the scan range was (m/z 40–600) with running time 32 min. Elucidation of phytocompounds was assayed by comparison of their retention times and mass with their regular authentic standard spectra using computer searches in NIST08.L and Wiley7n.L libraries (Sharma et al., 2018).
3. Results and discussion
3.1. Qualitative phytochemical screening
The results of the phytochemical analysis of EKL extracts exposed the presence of different classes of phytoconstituents namely alkaloids, flavonoids, glycosides, steroids, and carbohydrates in most of the tested extracts (Table 1). However, these phytoconstituents were copiously present in ethanol extracts than other extracts. Proteins and saponins were found to be absent in all the solvent extracts. Phenolics and terpenoids were present only in ethanolic extracts among the tested extracts. This is an agreement with previous reports on the phytochemicals in different plants of the Brassica genus such as B. oleracea var. capitata, B. oleracea var. italica, B. oleracea var. botrytis, B. juncea, B. rapa, and B. nigra, etc. (Nawaz et al., 2018).
Table 1.
Qualitative phytochemical screening of Ethiopian Kale leaves extracts.
| Tests | Acetone extract | Chloroform extract | Petroleum ether extract | Ethyl acetate extract | Ethanol extract | Aqueous extract |
|---|---|---|---|---|---|---|
| Alkaloids | + | + | − | + | ++ | − |
| Flavonoids | + | − | − | + | ++ | + |
| Terpenoids | − | − | − | − | ++ | + |
| Glycosides | + | − | + | + | ++ | + |
| Steroids | ++ | − | − | + | + | + |
| Carbohydrate | + | + | − | + | + | ++ |
| Protein | − | − | − | − | − | − |
| Phenolics | + | − | − | − | + | − |
| Saponins | − | − | − | − | − | − |
Copiously present (++), Moderately present (+), negative (−).
3.2. Antibacterial activity of EKL extracts
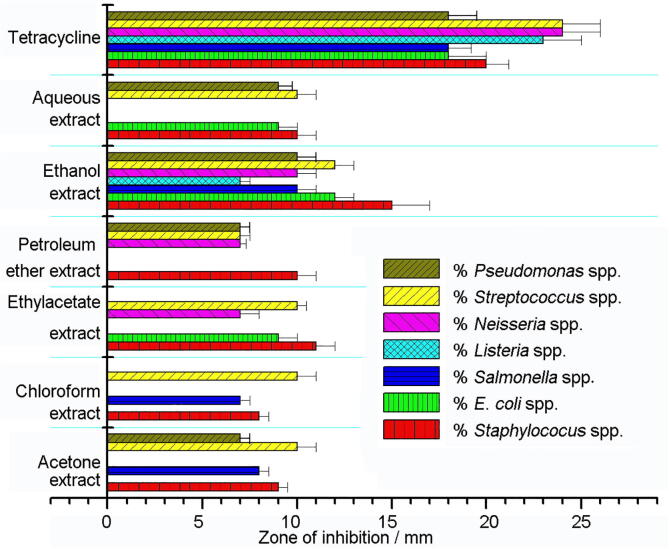
EKL extracts showed a broad spectrum of antibacterial activity (7–15 mm) against the tested bacterial species (Fig. 1). The results revealed that ethanol extract harbored more significant antibacterial activity than other extracts and maximum growth inhibition was observed against Staphylococcus spp. (15 mm) and followed by E.coli (12 mm). The moderate antibacterial potential was observed in other extracts and it’s not potential against all the tested bacteria. Especially, against Listeria spp. EKLEE alone shows the inhibition zone. Several researchers have reported the antimicrobial potential of various parts of different plants of the Brassica genus, which are widely used as a diet as well as a medicinal plant throughout the world (Khandayataray and Murthy, 2019, Nawaz et al., 2018).
Fig. 1.
Antibacterial activity of Ethiopian Kale leaves extracts in comparison with Tetracycline.
In this study, the antibacterial activity of the extracts was found higher against gram-positive bacteria, than in gram-negative bacteria, which may be due to the membrane structure (Paz et al., 2015, Rodríguez-Carpen et al., 2011). The observations suggested that antibacterial activity effectiveness is not due to one of its constituents, but maybe the combined action of many constituents such as alkaloids, flavonoids, terpenoids, etc., which are reported in our preliminary phytochemical analysis (Table 1).
3.3. FTIR analysis of functional groups
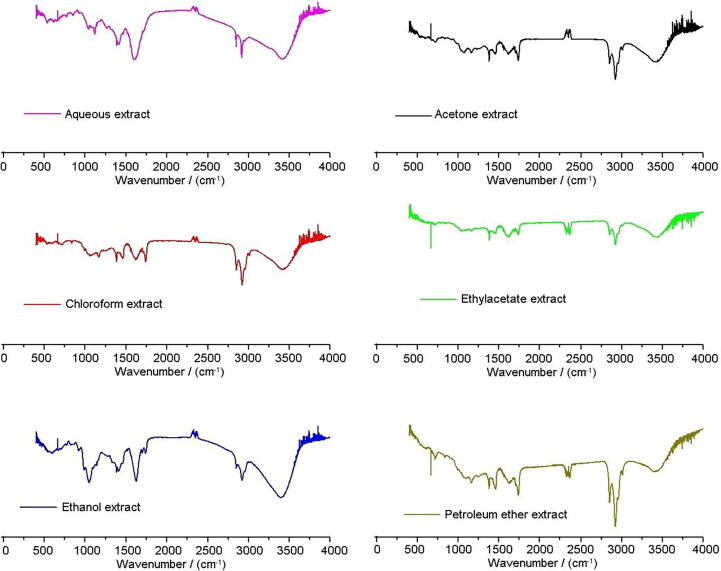
The FTIR spectra of EKL extracts showed multiple peaks, with relatively few different broad diagnostic peaks in the region above 2000 cm−1 and contrast, many sharp peaks with varying shapes and intensities in the region between 400 and 2000 cm−1 (Fig. 2).
Fig. 2.
Functional group profiling of different solvent extracts by FTIR analysis.
The vibrational band assignment for the prominent peaks, the chemical compounds identified and the absorbance (%) of the important vibrational frequencies were presented in Table 2. The absorbance (%) values obtained from the spectra representing the flavonoid and polyphenol contents were correlated to the antioxidant potential of the extracts. The high values of correlation efficient between FTIR absorbance (%) and antioxidant activity are reported by Karori et al., 2007, Senthilkumar et al., 2018. Since EKLEE showed high functional group absorbance (%) and better antibacterial activity than other extracts, it was selected for further testing of antioxidant activity and phytocompounds profiling.
Table 2.
FTIR absorbance of selected vibrational bands of Ethiopian Kale leaves extracts.
| Chemical compounds | Vibration bands/groups | Vibrational band / cm−1 |
Absorbance /% |
|||||
|---|---|---|---|---|---|---|---|---|
| Acetone extract | Chloroform extract | Ethyl acetate extract | Petroleum ether extract | Ethanol extract | Aqueous extract | |||
| Phenols, alcohols | Group O–H stretch, H–bonded | 3270–3320 | 37.47 | 41 | 34.47 | 43.23 | 50 | 36.64 |
| Flavonoids, Polyphenols, Catechins, Aromatics | C = O stretch (carbonyls) C = C stretch |
1629–1663 | 35.37 | 38.35 | 33.11 | 45.86 | 47.5 | 36.91 |
| Aromatics | C–C stretch (in ring) | 1448–1450 | 35.55 | 38.35 | 32.57 | 47.94 | 47.23 | 34.47 |
| Aromatics, Aliphatic amines | C–C stretch (in ring) C–N stretch |
1113–1148 | 31.8 | 37.27 | 31.84 | 31.76 | 42 | 30.47 |
3.4. Antioxidant potential of EKLEE
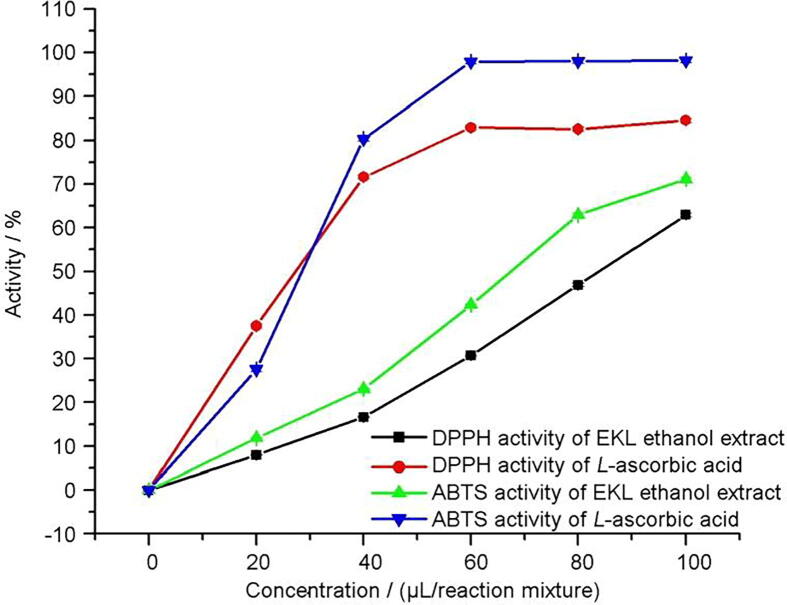
In DPPH* and ABTS assay, EKLEE displayed significant activity in a dose-dependent manner, which may serve as a significant indicator of the potential antioxidant property (Fig. 3). The percentage of radical scavenging activity of EKLEE on the DPPH radical varies from (7.95 ± 0.12)% (20 µg/mL) to (62.92 ± 0.34)% (100 µg/mL). In ABTS assay, it was found to increase from (11.78 ± 0.15)% (20 µg/mL) to (71.12 ± 0.41)% (100 µg/mL). The antioxidant and antiradical results indicated that both activities go parallel and were shown to be concentration-dependent and may be due to the pronounced content in the EKLEE. These findings were in accordance with previous studies reported by Bhandari and Kwak, 2015, Nawaz et al., 2018.
Fig. 3.
Antioxidant efficiency of ethanolic extracts of Ethiopian Kale leaves (EKL) in comparison with L-ascorbic acid.
3.5. Profiling of phytocompounds
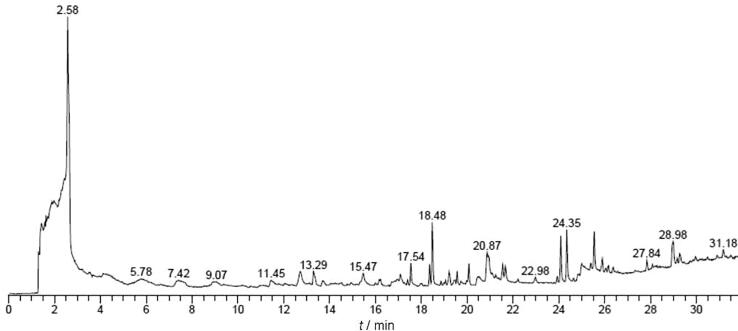
The GC–MS analysis of EKLEE (Fig. 4 and Table 3) showed the presence of nearly 17 major phytocompounds including estra-1,3,5(10)-trien-17á-ol (4.09%), 9,12,15-octadecatrienoic acid, ethyl ester (2.95%), 9,12,15-octadecatrienoic acid, 2,3-dihydroxypropyl ester, (Z,Z,Z) (2.59%) 2-pentadecanone, 6,10,14-trimethyl (2.41%), 2,3-dihydroxypropyl ester (2.19%), strychane, 1-acetyl-20à-hydroxyl-16-methylene (1.68%), 9,12,15-octadecatrienoic acid, methyl ester (1.62%), phytol (1.64%), hexadecanoic acid, ethyl ester (1.55%), and chimonanthine (1.30%) being the major compounds.
Fig. 4.
GC–MS chromatogram for phytocompounds profiling of ethanolic extracts of Ethiopian Kale leaves.
Table 3.
Major phytocompounds detected in GC–MS analysis of ethanolic extracts of Ethiopian Kale leaves.
| Serial number | tR/ min | Compounds | Area /% | SI |
|---|---|---|---|---|
| 1 | 5.80 | Strychane, 1-acetyl-20à-hydroxy-16-methylene- | 2.27 | 601 |
| 2 | 7.42 | Ascaridole epoxide | 1.37 | 651 |
| 3 | 8.99 | 2,7-Diphenyl-1,6-dioxopyridazino[4,5:2′,3′]pyrrolo[4′,5′-d]pyr idazine | 1.23 | 619 |
| 4 | 12.72 | 2,5-Dimethoxy-4-ethylamphetamine | 1.36 | 641 |
| 5 | 13.31 | 1-Dodecanol, 3,7,11-trimethyl- | 1.01 | 692 |
| 6 | 15.47 | Chimonanthine | 1.30 | 639 |
| 7 | 17.09 | Cyclopenta[d]anthracene-8,11-dione, 1,2,3,3a,4,5,6,6a,7,8,11,12-dodecahydro-3-(1-methylethyl)-12 –hydroxyl | 1.69 | 638 |
| 8 | 18.48 | 2-Pentadecanone, 6,10,14-trimethyl- | 2.41 | 842 |
| 9 | 20.50 | 9,12,15-Octadecatrienoic acid, 2,3-dihydroxypropyl ester, (Z,Z,Z)- | 1.19 | 744 |
| 10 | 20.91 | Estra-1,3,5(10)-trien-17á-ol | 4.09 | 723 |
| 11 | 21.59 | Hexadecanoic acid, ethyl ester | 1.55 | 758 |
| 12 | 24.08 | 9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)- | 1.62 | 833 |
| 13 | 24.35 | Phytol | 1.64 | 838 |
| 14 | 25.04 | 9,12,15-Octadecatrienoic acid, 2,3-dihydroxypropyl ester, (Z,Z,Z)- | 2.57 | 728 |
| 15 | 25.55 | 9,12,15-Octadecatrienoic acid, ethyl ester, (Z,Z,Z)- | 2.95 | 813 |
| 16 | 25.88 | 22S)-6à,11á,21-Trihydroxy-16à,17à-propylmethylenedioxypregna-1,4-diene-3,20-dione | 1.11 | 656 |
| 17 | 28.98 | 16-Nitrobicyclo[10.4.0]hexadecan-1-ol-13-one | 1.48 | 681 |
Most of the detected compounds in GC–MS analysis of this study are known as bioactives for strengthening the plant defense mechanism as a part of plant protective compounds such as phytoprotectants and phytoanticipins. Hexadecanoic acid is known for its potential antimicrobial, antioxidant, and antiandrogenic activities (Mahadkar et al., 2013). Similarly, 9,12-octadecadienoic acid is known for its anti-inflammatory, anticancer, and antiandrogenic activities (Sharma et al., 2018). A steroid, estra-1,3,5(10)-trien-17á-ol, present in the essential oil differs from estradiol, though similar in basic structure and however, it may be responsible for the observed estrogenic and/or anti-estrogenic activity (Ogunlesi et al., 2010). Various activities of phytol have been reported in the literature, including its activity against mycobacteria, and anticonvulsant, anxiolytic, antispasmodic, immune-modulating, and anticancer activities (Islam et al., 2018).
The major limitation of this study is that the antioxidant activity and chemical composition are explored only for the ethanol extract (based on its potential in antimicrobial activity and FTIR analysis) and not tested for the other extracts used in a study due to the limited access of chemicals and GC–MS analysis in due time of research. However, in-depth studies are recommended to denote the continuation of this Ethiopian heritage.
4. Conclusion
The findings of this investigation indicated that ethanol extract of Ethiopian Kale leaves contains a good source of phytochemicals that possess a significant antibacterial and antioxidant abilities and it will be a noteworthy source for various functional applications. This study recommends further research needed for isolation of phytocompounds and evaluation of their bioactivity through in vitro and in vivo models.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are thankful to Addis Ababa Science and Technology University, Ethiopia for providing partial financial support (Grant Reference No-AASTU-SSRF-CBCE-BT-2018) and lab facilities for this work.
References
- Alemayehu N., Becker H. Genotypic diversity and patterns of variation in a germplasm material of Ethiopian mustard (Brassica carinata A. Braun) Genetic Resources and Crop Evolution. 2002;49:573–582. [Google Scholar]
- Bhandari S.R., Kwak J.-H. Chemical composition and antioxidant activity in different tissues of brassica vegetables. Molecules. 2015;20(1):1228–1243. doi: 10.3390/molecules20011228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology. 1995;28(1):25–30. [Google Scholar]
- Ezekwe S.A., Chikezie P.C. GC-MS analysis of aqueous extract of unripe fruit of Carica papaya. Journal of Nutrition and Food Sciences. 2017;7(6):1–5. [Google Scholar]
- Harborne JB, T. G. (1978). Phytochemical Methods: A Guide to Modern Technique of Plant Analysis (3rd ed.). Chapman and Hall.
- Islam M.T., Ali E.S., Uddin S.J., Shaw S., Islam M.A., Ahmed M.I., et al. Phytol: A review of biomedical activities. Food and Chemical Toxicology. 2018;121:82–94. doi: 10.1016/j.fct.2018.08.032. [DOI] [PubMed] [Google Scholar]
- Karori S.M., Wachira F.N., Wanyoko J.K., N. R. M. Antioxidant capacity of different types of tea products. African Journal of Biotechnology. 2007;6(19):2287–2296. [Google Scholar]
- Khandayataray P., Murthy M.K. Qualitative and quantitative phytochemical screening, antioxidant and anti-inflammatory activities of acetone extract of Brassica juncea L. leaf. Asian Journal of Research. Biochemistry. 2019;5(1):1–15. [Google Scholar]
- Mahadkar, S., Valvi, S., J. V. (2013). Gas chromatoghraphy mass spectroscopic (GC-MS) analysis of some bioactive compounds from five medicinaly relevant wild edible plants. Asian Journal of Pharmaceutical and Clinical Research, 6(1), 136-139.
- Nawaz, H., Shad, M. A., & Muzaffar, S. (2018). Phytochemical composition and antioxidant potential of Brassica. In Brassica Germplasm - Characterization, Breeding and Utilization. Mohamed Ahmed El-Esawi (Ed.), IntechOpen https://doi.org/10.5772/intechopen.76120
- Ogunlesi M., Okiei W., Osibote E.A. Analysis of the essential oil from the leaves of Sesamum radiatum, a potential medication for male infertility factor, by GC-MS. African Journal of Biotechnology. 2010;9(7):1060–1067. [Google Scholar]
- Paz M., Gúllon P., Barroso M.F., Carvalho A.P., Domingues V.F., Gomes A.M., et al. Brazilian fruit pulps as functional foods and additives: Evaluation of bioactive compounds. Food Chemisty. 2015;172:462–468. doi: 10.1016/j.foodchem.2014.09.102. [DOI] [PubMed] [Google Scholar]
- Prakash, S., Wu, X. M., Bhat, S. R. (2011). History, evolution and domestication of Brassica crops. In Plant Breeding Reviews, J. Janick (Ed.), Wiley‐Blackwell.https://doi.org/10.1002/9781118100509.ch2
- Rodríguez-Carpen J.G., Morcuende D., Andrade M.J., Kylli P., E. M. Avocado (Persea americana Mill.) phenolics, in vitro antioxidant and antimicrobial activities, and inhibition of lipid and protein oxidation in porcine patties. Journal of Agriculture and Food Chemistry. 2011;59(10):5625–5635. doi: 10.1021/jf1048832. [DOI] [PubMed] [Google Scholar]
- Senthilkumar S.R., Thirumal S., Arulmozhi K.T., Mythili N. FT-IR analysis and correlation studies on the antioxidant activity, total phenolics and total flavonoids of Indian commercial teas (Camellia sinensis L.)-A novel approach. International Research. Journal of Biological Sciences. 2018;6(3):1–7. [Google Scholar]
- Sharma A., Rai P.K., Prasad S. GC-MS detection and determination of major volatile compounds in Brassica juncea L. leaves and seeds. Microchemical Journal. 2018;138(September):488–493. [Google Scholar]
- Uttara B., Singh A.V., Zamboni P., M. R. T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Current Neuropharmacology. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan A., Kathirvel A., Prakash S., Sujatha V. Antioxidant, antibacterial activities and identification of bioactive compounds from Terminalia chebula bark extracts. Free Radicals and Antioxidants. 2016;7(1):43–49. [Google Scholar]