Abstract
Objective
Huidouba (HDB) is a Chinese folk medicine used to treat diabetes in Sichuan Province, China. Therefore, we investigated the anti-diabetic effects of HDB and its underlying mechanisms. We hypothesized that HDB treatment could enhance glucose tolerance and insulin sensitivity, and thus prevent a hyperglycemia state.
Methods
To test the hypothesis, streptozotocin (STZ)-induced diabetic mice and db/db mice, widely used models of hyperglycemia and insulin-resistant diabetes, were either treated with HDB, metformin, or acarbose. Blood glucose, oral glucose tolerance test, insulin tolerance test, pancreatic histopathology and serum biochemistry were detected to assess the hypoglycemic effect of HDB.
Results
HDB treatments were found to show the effect in reducing glucose levels. HDB also resulted in a significant reduction in body weight and food intake in the STZ-induced diabetic mouse model. Furthermore, it significantly improved glucose and insulin tolerance in the two diabetic mouse models. Importantly, insulin, glucagon, pancreatic polypeptide, and somatostatin immunohistochemistry revealed that HDB treatment improved the function and the location of the cells in the islets compared with the other two treatments. HDB treatment resulted in significant restoration of islet function. Our results illustrated the underlying mechanism of HDB in the progression of diabetes, and HDB can be an effective agent for the treatment of diabetes.
Conclusion
The results of this study suggested that HDB can reduce blood glucose levels in STZ-induced hyperglycemic mice and db/db mice.
Keywords: dyslipidemia, huidouba, hyperglycemia, insulin secretion, type 2 diabetes mellitus
1. Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic disease characterized by hyperglycemia caused by insulin resistance and insulin deficiency. Chronic hyperglycemia may cause microvascular complications, such as diabetic nephropathy, neuropathy, and retinopathy, macrovascular complications, including peripheral vascular disease, cerebrovascular disease and atherosclerosis, and miscellaneous complications (Papatheodorou, Papanas, Banach, Papazoglou, & Edmonds, 2016). Therefore, it is urgent to alleviate hyperglycemia, delay the progression of diabetes, and reduce the complications of diabetes.
The prevalence and ethnic pattern of diabetes and prediabetes in China in 2013 suggested that Tibetan and Muslim Chinese had significantly lower crude prevalence of diabetes than Han participants (14.7% for Han, 4.3% for Tibetan, and 10.6% for Muslim; P < 0.001 for Tibetan and Muslim compared with Han) (Wang et al., 2017). There are substantial differences in the genetic background, socioeconomic levels, climate and geographic features of the residential area, lifestyle, and dietary pattern among 56 ethnic groups, in China. The folk medicine Huidouba (HDB) which is considered to be Tibetan medicine is found only in Mount Emei in Sichuan Province with remarkable therapeutic effects against diabetes, in China (Wu, Tian, & Zhu, 2009). HDB is a strip-shaped, bag-shaped tube nest of Atypus, which is mainly formed by Atypus heterothecus (Wang et al., 2019). However, its underlying mechanism of blood glucose reduction is unclear, and researches on HDB are rare. In recent years, more and more researchers began to pay attention to the hypoglycemic activity of HDB. The α-glucosidase inhibitory activity and antioxidant activity in vitro of polysaccharides and several extractions from HDB were reported (Cai et al., 2019, Chen et al., 2018a, Li and Peng, 2012). The different extractions of HDB (ethanol extraction, water extraction and polysaccharide) improved the renal function of diabetic kidney mice by decreasing levels of serum glucose and advanced glycation end products (AGEs) and alleviating the glomerular basement membrane thickening (Zhou, Jia, Liu, & Feng, 2018). HDB treatment improved the impaired fasting glucose tolerance and ultrastructure damage of podocyte and glomerular basement membrane in diabetic nephropathy rats (Yang et al., 2020). In our study, we aimed to assess the antidiabetic efficacy of HDB in different animal models of diabetes. HDB treatments could lower blood glucose, increase insulin secretion and improve the morphology and function of islet cells.
2. Materials and methods
2.1. Preparation of HDB
The HDB was cut into pieces, added with 8, 6 and 6 times the volume of 70% ethanol, heated and boiled for extraction for 1 h respectively. After filtration, the solution was concentrated under reduced pressure until it had no alcohol flavor (Zhou et al., 2018).
2.2. Animals
4-Week-old male Kunming mice supplied by Beijing Vital River Laboratory Animal Technology Co., Ltd., and 4-week-old male db/db mice (C57BL/KsJ-db/db) and wild-type mice (C57BL/KsJ-db/+) purchased from Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China) were used. Procedures for the use of experimental animals conformed to the Guide for the Care and Use of Laboratory Animals and were approved by the Biological and Medical Ethics Committee, Minzu University of China (ECMUC2019010AO). The mice were maintained at 24 °C and (55 ± 5)% relative humidity on a 12-h light/12-h dark cycle. The animals had free access to standard rodent pellet food, except when fasted before experiments, and water ad libitum. The mice were acclimatized for a period of 3 d in the new environment before the initiation of the experiment.
2.3. Induction of diabetes by STZ
Diabetes mellitus was induced using STZ (Sigma Aldrich, St. Louis, MO, USA) as described previously (Talchai, Xuan, Lin, Sussel, & Accili, 2012). After mice were fasted for at least 8 h in the morning, stable diabetes was induced by successive intraperitoneal injections of STZ (50 mg/kg body weight) dissolved in 0.1 mol/L sodium citrate buffer (pH 4.5) to the mice once a day, 5 d in a row. The mice in control group received only citrate buffer once a day, 5 d in a row. The mice then received a standard diet and water ad libitum for one week. One week after STZ injection, non-fasted plasma glucose and fasted plasma glucose levels were measured using a glucometer (Sinocare, China). Only mice with fed blood glucose levels over 16.7 mmol/L were considered as diabetic mice and used for the experiment. The mice used in the experiment were divided into one group of the normal control group (NCG) and six groups of diabetic mice (n = 10 in each group). There were diabetic group (DG), high dosage of HDB group (HDG, crude drug 48 g/kg), medium dosage of HDB group (MDG, crude drug 24 g/kg), low dosage of HDB group (LDG, crude drug 12 g/kg), metformin group (MG, 0.2 g/kg) and acarbose group (AG, 0.04 g/kg). Doses were administered orally for 35 d. The mice in the normal control group and diabetic group were given equal volumes of water. Food intake, water intake, fasting plasma glucose level, and the body weights of all animals were monitored throughout the experiment.
2.4. Experimental db/db mice and treatment
After adaptation for 3 d, blood samples were obtained from the tail vein every week to measure glucose levels. Thereafter, db/db mice were divided into five groups (n = 10/group) until the age of 8 weeks when fed blood glucose levels were over 16.7 mmol/L and fasting plasma glucose levels reached 11.1 mmol/L. The wild-type mice (n = 10), i.e., the normal control group (Control), were used for comparison with the diabetic groups. The db/db mice were grouped into the diabetic group (Model), HDB treatment group (HDB, crude drug 48 g/kg, the dosage of HDB extract was determined according to the preliminary experiment), metformin (Met, 0.2 g/kg). Body weight and fasting plasma glucose level were measured weekly during the administration period.
2.5. Oral amylum starch tolerance test (OATT), oral sucrose tolerance test (OSTT), oral glucose tolerance test (OGTT)
All mice were fasted in the morning for 8 h. Thereafter, mice were orally administered with amylum starch (3 g/kg), sucrose (2.5 g/kg) and glucose (2 g/kg). Blood samples were collected from the tail vein at 0, 30, 60, 90, and 120 min and glucose levels were measured using a blood glucose meter (Yusoff et al., 2015).
2.6. Insulin tolerance test (ITT)
For the insulin tolerance test, all mice were fasted in the morning for 6 h. Thereafter, insulin (0.15 U/kg) was injected intraperitoneally. Glucose measurements were performed at 0, 30, 60, 90, and 120 min after insulin injection.
The area under the curve (AUC) was obtained using Eq. (1):
AUC = (BG0min + BG30min) × 0.5 h × 0.5 + (BG30min + BG60min) × 0.5 h × 0.5 + (BG60min + BG90min) × 0.5 h × 0.5 + (BG90min + BG120min) × 0.5 h × 0.5.
2.7. Serum biochemical assay and ELISA
After treatment, blood was collected in a centrifuge tube, clotted at room temperature for 2 h and then centrifuged at 2000×g for 15 min at 4 °C to obtain serum. We detected high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol (TC) and triglycerides (TG) concentration using commercial kits (Nanjing Jiancheng). Insulin and proinsulin levels were measured using ELISA (Millipore and Mercodia) according to the manufacturer’s instructions. The molar insulin concentration subtracted the molar proinsulin concentration from molar total insulin and then the serum proinsulin-insulin ratio was calculated (Hasnain et al., 2014).
2.8. Histological examination and immunohistochemical analysis
Pancreas tissue samples were obtained from the db/db mice, fixed in 4% formaldehyde solution, and then embedded in paraffin. Paraffin sections (5-µm-thick) were used for immunohistochemical and morphometric analyses. For pancreatic polypeptide (PPY) and somatostatin (SST) positivity determination, the mean optical density (MOD) of islet PPY and SST were measured and calculated using Image-Pro Plus 6.0 software. Insulin and glucagon were studied by immunofluorescence staining, which was performed as previously described. The following primary antibodies were used: somatostatin (rabbit polyclonal, Abcam), pancreatic polypeptide (goat polyclonal, Abcam), insulin (guinea pig polyclonal, Abcam), and glucagon (goat polyclonal, Abcam).
2.9. Statistical analysis
Statistical analysis was performed using IBM SPSS 20.0. Data are presented as mean ± SD values and were analyzed using the two-tailed Mann-Whitney U tests or one-way ANOVA as specified. The trapezoidal rule was applied to analyze the AUC. Fed blood glucose, fasting blood glucose and body weight were analyzed by using repeated measurements and multivariate ANOVA process of the general linear model. LSD pairwise comparison methods were used to compare the variance. Differences were considered statistically significant when P < 0.05.
3. Results
3.1. HDB modulation improves glycemic control in STZ-induced diabetic mice
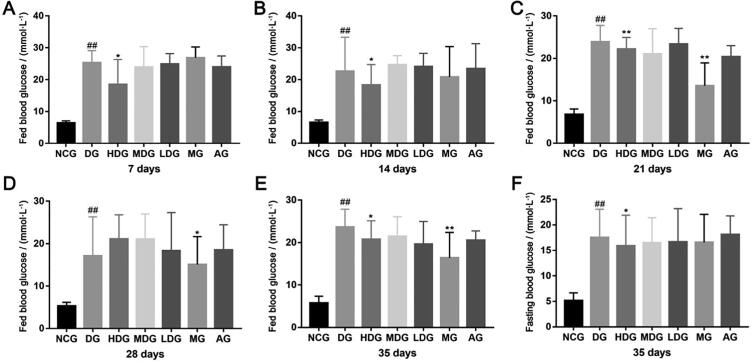
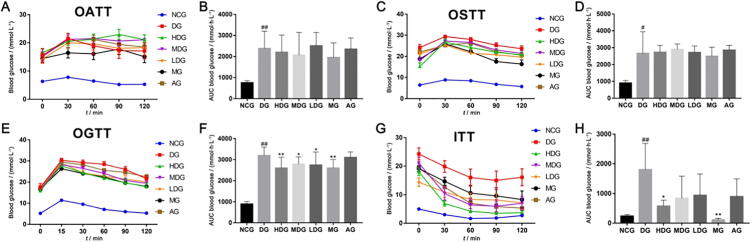
After four weeks of continuous administration, water and food intake in the HDG, MDG, LDG compared to the DG were significantly lowered (Tables 1 and 2, P < 0.01). HDB limited body weight gain in the STZ-induced diabetic mouse model. In comparison with DG, HDG, MDG, and LDG showed decreased body weights after 3 weeks of treatment (Table 3). Fed blood glucose and fasting blood glucose were reduced, especially, blood glucose fluctuation was improved with HDB (Fig. 1A–F). The HDG caused a marked reduction of fed blood glucose. However, there was no significant difference in fed blood glucose levels between the MG and DG during the first 14 days (Fig. 1A and B). The fasting glucose levels were significantly lower in the HDG than in the DG (Fig. 1F, P = 0.043). After 5 weeks of treatment, there were no significant effects on oral amylum starch tolerance (Fig. 2A and B) and sucrose tolerance (Fig. 2C and D). Obviously, glucose tolerance and the AUC values in the HDG, MDG, and LDG were better than the DG (Fig. 2E–F, P < 0.05). Insulin sensitivity was enhanced in comparison with that in DG showed a dose-dependent (Fig. 2G and H). The AUC values in the ITTs for the treatment groups were significantly lower than that for the STZ-induced diabetic group, the HDG being most significantly (Fig. 2H, P = 0.035).
Table 1.
Daily water intake per mouse during administration period (mean ± SD, n = 10).
| Groups | Daily water intake / (mL·mouse-1·d-1) |
|||
|---|---|---|---|---|
| 1st week | 2nd week | 3rd week | 4th week | |
| NCG | 9.0 ± 1.7 | 8.3 ± 0.5 | 8.7 ± 0.6 | 10.9 ± 1.6 |
| DG | 44.2 ± 2.7## | 52.3 ± 2.6## | 49.7 ± 3.8## | 55.5 ± 2.4## |
| HDG | 35.4 ± 8.4 | 24.5 ± 1.5** | 25.8 ± 1.4* | 33.3 ± 2.3** |
| MDG | 37.2 ± 5.0 | 31.5 ± 2.7** | 38.8 ± 0.5 | 40.0 ± 0.9** |
| LDG | 38.5 ± 1.4 | 36.2 ± 1.1** | 40.8 ± 4.0 | 45.8 ± 1.2** |
| MG | 37.1 ± 4.7 | 30.5 ± 1.6** | 33.0 ± 2.0* | 32.4 ± 1.9** |
| AG | 37.5 ± 3.0 | 40.4 ± 2.1** | 40.8 ± 0.8 | 37.9 ± 0.6** |
#P < 0.05, ##P < 0.01 vs normal control group (NCG), *P < 0.05, **P < 0.01 vs diabetic group (DG).
Table 2.
Daily food intake per mouse during administration period (mean ± SD, n = 10).
| Groups | Daily food intake/(g·mouse-1·d-1) |
|||
|---|---|---|---|---|
| 1st week | 2nd week | 3rd week | 4th week | |
| NCG | 6.8 ± 0.79 | 6.7 ± 0.4 | 6.8 ± 0.4 | 6.8 ± 1.2 |
| DG | 12.1 ± 1.4## | 13.3 ± 0.4## | 13.3 ± 0.2## | 13.6 ± 0.3## |
| HDG | 10.5 ± 1.7 | 8.1 ± 0.4** | 8.3 ± 0.2** | 9.9 ± 0.2** |
| MDG | 11.0 ± 1.6 | 9.0 ± 0.4** | 10.1 ± 0.5** | 10.6 ± 0.5** |
| LDG | 11.2 ± 1.0 | 11.3 ± 1.3** | 10.4 ± 0.3** | 12.1 ± 0.5** |
| MG | 12.1 ± 0.9 | 10.1 ± 0.6** | 10.4 ± 0.5* | 10.9 ± 1.7** |
| AG | 10.8 ± 1.1 | 11.1 ± 0.4** | 10.9 ± 0.1* | 11.2 ± 0.7** |
#P < 0.05, ##P < 0.01 vs normal control group (NCG), *P < 0.05, **P < 0.01 vs diabetic group (DG).
Table 3.
Body weight during administration period (mean ± SD, n = 10).
| Groups | Body weight/g |
|||||
|---|---|---|---|---|---|---|
| 0 day | 7 days | 14 days | 21 days | 28 days | 35 days | |
| NCG | 36.1 ± 0.7 | 39.7 ± 1.0 | 39.2 ± 0.9 | 40.9 ± 0.9 | 41.2 ± 0.9 | 43.5 ± 1.0 |
| DG | 32.2 ± 0.9## | 34.3 ± 3.0# | 31.7 ± 1.2# | 35.2 ± 3.1## | 36.3 ± 1.0## | 37.6 ± 1.4## |
| HDG | 31.8 ± 0.9 | 29.1 ± 2.5 | 29.5 ± 0.9 | 30.9 ± 2.9** | 31.3 ± 1.2* | 33.3 ± 1.1 |
| MDG | 31.5 ± 0.7 | 31.1 ± 2.5 | 29.3 ± 0.8 | 32.5 ± 2.6 | 33.0 ± 1.0 | 34.9 ± 1.0 |
| LDG | 31.5 ± 1.0 | 31.5 ± 0.9 | 30.5 ± 1.1 | 33.1 ± 0.8 | 33.9 ± 1.1 | 34.8 ± 1.2 |
| MG | 30.9 ± 0.6 | 30.5 ± 3.2 | 32.5 ± 0.9 | 33.0 ± 2.8 | 33.4 ± 1.0 | 34.9 ± 1.1 |
| AG | 31.8 ± 0.7 | 33.3 ± 2.6 | 31.3 ± 0.8 | 34.1 ± 2.7 | 35.2 ± 0.8 | 36.5 ± 1.0 |
#P < 0.05, ##P < 0.01 vs normal control group (NCG), *P < 0.05, **P < 0.01 vs diabetic group (DG).
Fig. 1.
HDB treatments lower fed blood glucose and fasting blood glucose in STZ-induced diabetic mice. (A) Fed blood glucose levels in the different treatment groups for 7 d; (B) Fed blood glucose levels in the different treatment groups for 14 d; (C) Fed blood glucose levels in the different treatment groups for 21 d; (D) Fed blood glucose levels in the different treatment groups for 28 d; (E) Fed blood glucose levels in the different treatment groups for 35 d; (F) Fasting blood glucose levels in the different treatment groups for 35 d. #P < 0.05, ##P < 0.01 vs normal control group (NCG), *P < 0.05, **P < 0.01 vs diabetic group (DG).
Fig. 2.
HDB treatments improve glucose tolerance and insulin tolerance in STZ-induced diabetic mice. (A) Oral amylum starch tolerance test (OATT); (B) The area under the curve of OATT; (C) Oral sucrose tolerance test (OSTT); (D) The area under the curve of OSTT; (E) Oral glucose tolerance test (OGTT); (F) The area under the curve of OGTT; (G) Insulin tolerance test (ITT); (H) The area under the curve of ITT. #P < 0.05, ##P < 0.01 vs normal control group (NCG), *P < 0.05, **P < 0.01 vs diabetic group (DG).
3.2. HDB treatment reduced fasting blood glucose and alleviated dyslipidemia to promote insulin secretion in db/db mice
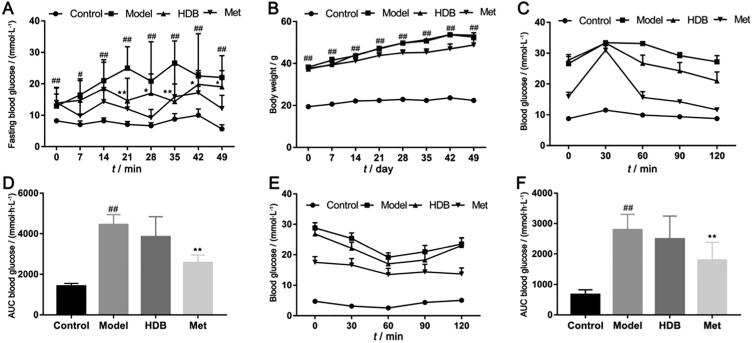
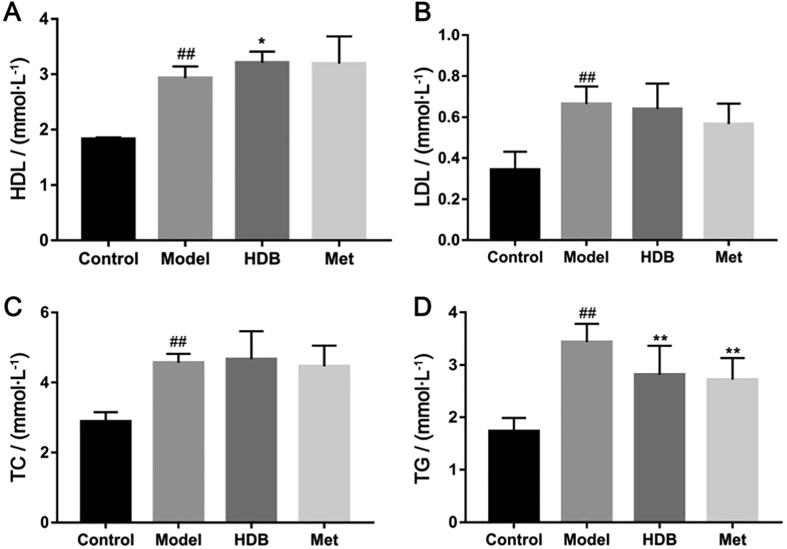
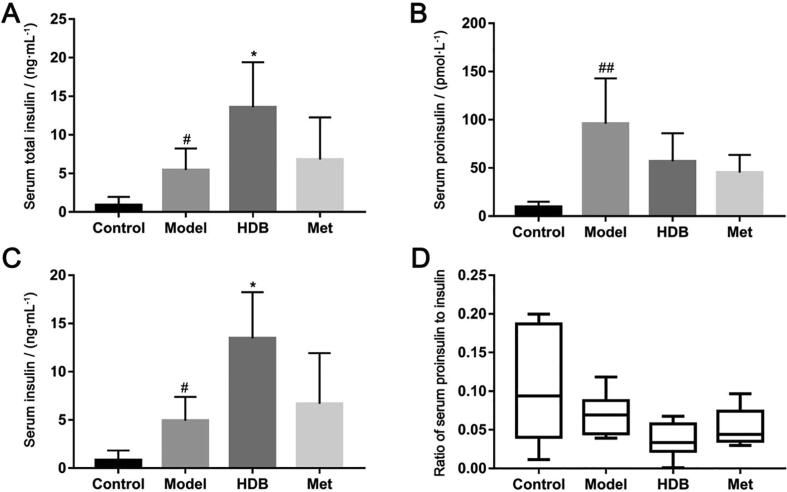
HDB treatment showed better effects on reducing the fasting blood glucose levels after administration for 21 d (Fig. 3A, P < 0.05). Importantly, blood glucose fluctuation in HDB was lower than the model group. However, there was no obvious influence on the body weight of db/db mice with HDB treatment (Fig. 3B). Oral glucose tolerance (Fig. 3C and D) and insulin tolerance of mice that received HDB treatment were improved from that in the model group (Fig. 3E and F). Serum HDL, LDL, TC and TG in the model group were higher compared with the control group (Fig. 4A–D). Serum HDL of db/db model mice was significantly higher than normal, while HDB further increased HDL (Fig. 4A, P = 0.047). Serum TG contents showed lower after HDB intervention (Fig. 4D, P = 0.008). There were no significant differences in serum LDL and TC between HDB and model (Fig. 4B and C). Fasting serum total insulin concentration was higher with HDB therapy than the model mice (Fig. 5A, P = 0.017). And Fasting serum proinsulin concentration was lower with HDB treatment than the model mice (Fig. 5B). Fasting serum insulin concentration was higher with HDB therapy than the model mice (Fig. 5C, P = 0.011). The fasting serum proinsulin-insulin ration was lower in HDB group (Fig. 5D).
Fig. 3.
HDB treatment in db/db mice reduces fasting blood glucose. (A) Fasting blood glucose levels in the different treatment groups; (B) Body weight in the various treatment groups; (C) Oral glucose tolerance test (OGTT) after 4 weeks of treatment; (D) The area under the curve of OGTT; (E) Insulin tolerance test (ITT) after 4 weeks of treatment; (F) The area under the curve of ITT. #P < 0.05, ##P < 0.01 vs control group, *P < 0.05, **P < 0.01 vs model group.
Fig. 4.
Effects of HDB on blood lipid levels in db/db mice. (A) Serum HDL content after treatment; (B) Serum LDL content after treatment; (C) Serum TC content after treatment; (D) Serum TG content after treatment. #P < 0.05, ##P < 0.01 vs control group, *P < 0.05, **P < 0.01 vs model group.
Fig. 5.
Effects of HDB on serum insulin and proinsulin contents in db/db mice. (A) Serum total insulin content after treatment; (B) Serum proinsulin content after treatment; (C) Serum insulin content after treatment; (D) Serum proinsulin and insulin ratio. #P < 0.05, ##P < 0.01 vs control group, *P < 0.05, **P < 0.01 vs model group.
3.3. HDB treatment in db/db mice improved morphology, function, and location of δ and pp cells in db/db mice
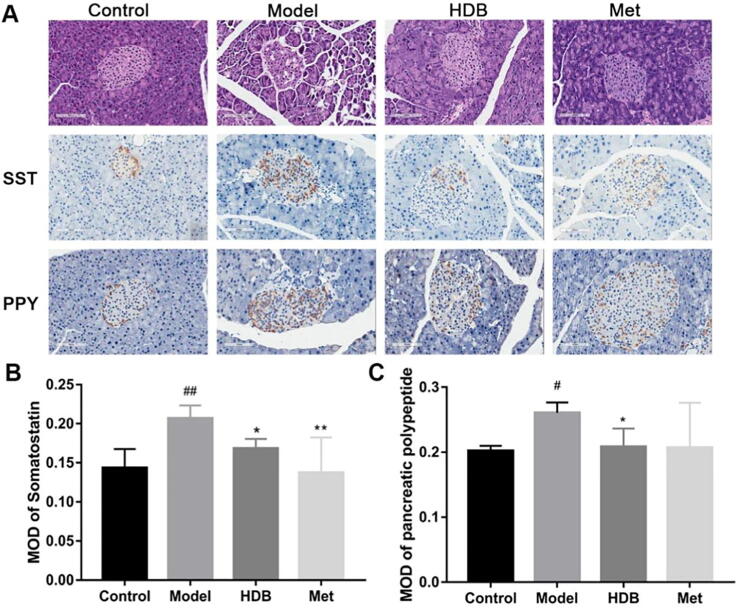
Histopathological examination of the pancreatic sections revealed a reduction in the size of pancreatic islets as well as the number of β-cells per islet count in the db/db model group. After treatment, the mice exhibited normal islet morphology in the HDB treatment group (Fig. 6A). HDB treatment decreased the PP- and somatostatin-positive areas in immunohistochemical staining of the pancreas. Moreover, the PP- and somatostatin-positive areas were located from the edge to the center in the model group. After HDB treatment, there were few PP- and somatostatin-positive cells in the center of islets. The MOD of somatostatin and PP in the HDB-treated groups was lower than that in the model group (Fig. 6B and C).
Fig. 6.
Immunohistochemical staining of decreased δ-cell and pp-cell numbers and positive area in db/db mice. (A) Representative images of the pancreas stained with hematoxylin and eosin (the first line of figure A), somatostatin immunohistochemistry (the second line of figure A), and pancreatic polypeptide immunohistochemistry (the third line of figure A) from different treatment groups. Magnification ×200, Bar = 100 µm; (B) Mean optical density values for somatostatin immunohistochemistry. (C) Mean optical density values for pancreatic polypeptide immunohistochemistry. #P < 0.05, ##P < 0.01 vs control group, *P < 0.05, **P < 0.01 vs model group.
3.4. HDB therapy resolved morphology of pancreas and islet α cells and β cells in db/db mice
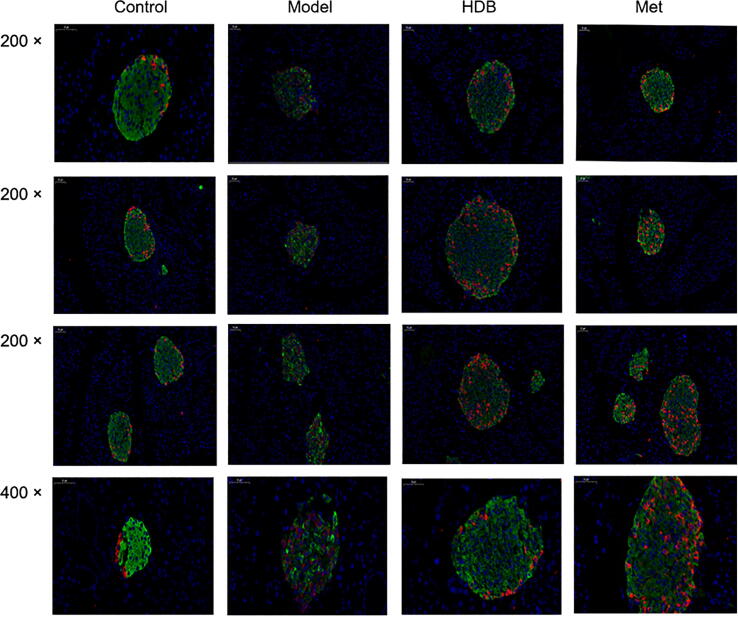
Immunofluorescence staining revealed disrupted architecture with a marked loss of insulin content and insulin-positive β-cells in the islets from the representative untreated diabetic pancreas in the model group (Fig. 7). However, HDB treatment improved islet morphology in db/db mice. The insulin content and insulin-positive β-cells were partly restored in the islets from the HDB-treated group, and the treatment also drove α-cells to the periphery of the islets. Such a β-cell centric architecture was typically observed in the islets of Langerhans in normal mice, and the insulin content of the pancreas in the treated groups was higher than that in the model. Glucagon staining in the treated groups was lower than that in the model group. After treatment, the glucagon-stained area moved to the edge of the islet with no glucagon immunoreactive cell staining, which was similar to the normal morphology of the wild-type mice.
Fig. 7.
HDB treatment improved the co-staining of pancreas glucagon (red) and insulin (green) immunofluorescence in db/db mice. Magnification × 200, Bar = 100 µm, Magnification ×400, Bar = 50 µm.
4. Discussion
In our study, the hyperglycemic diabetic mouse model was induced by low dosage STZ five times in a row. The diabetic mice whose blood glucose level meets the standard: fasting blood glucose is 11.1 mmol/L and random blood glucose is 16.7 mmol/L, were selected. However, the fasting blood glucose level fluctuates greatly. It is particularly critical to develop appropriate standards for diabetic mouse models to evaluate the pharmacodynamic activity.
The folk usage and dosage of HDB in Mount Emei is that the initial stage (under 5 years), patients were given 50 g per day with a course of treatment of 30 d; Patients in the intermediate stage (5–10 years) were given 60 g per day for a course of treatment of 40 d; In severe cases (over 10 years), 80 g per day for a course of 50 d. In our study, HDB treatment can significantly reduce blood glucose levels in different diabetic mice models. After HDB treatment for 7 d, the fed blood glucose levels of HDB were lower than MG in STZ-induced mice. During the 21 days of HDB administration, there was prominent difference in fed plasma glucose. After 28 days of intervention, fed blood glucose measurements showed no difference between HDB and the model group, which may be because STZ severely damaged the pancreatic islets. For the fasting blood glucose, HDB reduced the fasting blood glucose during 35 days of therapy. HDB significantly attenuated the rise in plasma glucose after glucose loaded, but there were no differences between the HDB treatment and the STZ-induced mouse model on oral amylum starch and sucrose. HDB extraction may not inhibit α-amylase activity. Here we evaluated the activities of HDB on glucose tolerance and insulin secretion in db/db mice. HDB showed better effects on blood glucose reduction, improved dyslipidemia and impaired glucose tolerance. After 21 days of HDB administration, the fasting glucose of db/db mice was significantly reduced. Type 2 diabetes are characterized by elevated blood glucose due to an insufficiency of the insulin. Insulin plays an important role in glucose homeostasis. HDB can effectively inhibit the increase of the proinsulin/insulin ratio in diabetic mice. And the release of incompletely processed proinsulin was lowered after HDB intervention. We also monitored the water and food intake in db/db mice (not shown), but there was no difference between the groups, possibly because db/db mice were leptin receptor deficient mutant mice whose appetite was not controllable.
More and more studies have confirmed the hypoglycemic activity of HDB. However, its hypoglycemic active ingredient is rarely reported except HDB polysaccharides (Chen, Li, Zhou, Sun, et al., 2018). More studies will find out the hypoglycemic active ingredients of HDB. We needed to do more research to explore the mechanism of HDB in treating diabetes.
5. Conclusion
We showed a significant effect of HDB treatment on glucose homeostasis in STZ-induced diabetic mice and db/db mice. Our results showed an improvement in glucose tolerance, insulin tolerance and the protection of β-cells in diabetic mouse models.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81673957); State Key Laboratory of Natural and Biomimetic Drug (No. K20160210), Independent Research Projects for Ph.D. Students of Minzu University of China (No. 181084) and Undergraduate Research and Training Program (URTP) of Minzu University of China (No. GCCX2020110018).
Contributor Information
Bi-nan Lu, Email: binanlu@muc.edu.cn.
Zong-ran Pang, Email: pangzongran@muc.edu.cn.
References
- Cai, W. L., H. M. M., Sun, Y. M., Li, Z. M., and Peng, L. (2019). Study on the chemical constituents and inhibitory activity against α-glucosidase of ethyl acetate extract from Huidouba. Journal of Jiangxi Science & Technology Normal University, (06), 60−63.
- Chen J.C., Li L., Zhou X., Li B., Zhang X., Hui R. Structural characterization and alpha-glucosidase inhibitory activity of polysaccharides extracted from Chinese traditional medicine Huidouba. International Journal of Biological Macromolecules. 2018;117:815–819. doi: 10.1016/j.ijbiomac.2018.05.192. [DOI] [PubMed] [Google Scholar]
- Chen J.C., Li L., Zhou X., Sun P.Y., Li B., Zhang X. Preliminary characterization and antioxidant and hypoglycemic activities in vivo of polysaccharides from Huidouba. Food & Function. 2018;9(120):6337–6348. doi: 10.1039/c8fo01117f. [DOI] [PubMed] [Google Scholar]
- Hasnain S.Z., Borg D.J., Harcourt B.E., Tong H., Sheng Y.H., Ng C.P., Das I., Wang R., Chen A.C., Loudovaris T., et al. Glycemic control in diabetes is restored by therapeutic manipulation of cytokines that regulate beta cell stress. Nature Medicine. 2014;20(12):1417–1426. doi: 10.1038/nm.3705. [DOI] [PubMed] [Google Scholar]
- Li Z.M., Peng L. Inhibitory effect on α-glucosidase of Huidouba extracts in vitro. Lishizhen Medicine and Materia Medica Research. 2012;23(06):1379–1380. [Google Scholar]
- Papatheodorou K., Papanas N., Banach M., Papazoglou D., Edmonds M. Complications of diabetes 2016. Journal of Diabetes Research. 2016;2016:1–3. doi: 10.1155/2016/6989453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talchai C., Xuan S.H., Lin H.V., Sussel L., Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150(6):1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.W., Wu Q.R., Wang P., Zhang Y.Y., Xu L.C., Dou Y.L. Differentiation and analysis of Tibetan medicine Huidouba. Modern Chinese Medicine. 2019;21(9):1169–1172. [Google Scholar]
- Wang L., Gao P., Zhang M., Huang Z., Zhang D., Deng Q., Li Y., Zhao Z., Qin X., Jin D., et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317(24):2515–2523. doi: 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Tian S.Q., Zhu J.A. Preliminary test of chemical components for Tibetan medicine HDB. Journal of Southwest University for Nationalities (Natural Science Edition) 2009;35(5):1017–1019. [Google Scholar]
- Yang K.B., Pang Z.R., Yin C.J., Bai Y.H., Lu B.N., Yu N., Han G.Y. Ameliorative effects of Tibetan Huidouba on podocyte injury in diabetic nephropathy rats. Chinese Traditional Patent Medicine. 2020;42(9):2299–2305. [Google Scholar]
- Yusoff N.A., Ahmad M., Al-Hindi B., Widyawati T., Yam M.F., Mahmud R., et al. Aqueous extract of Nypa fruticans Wurmb. vinegar alleviates postprandial hyperglycemia in normoglycemic rats. Nutrients. 2015;7(8):7012–7026. doi: 10.3390/nu7085320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.L., Jia X.B., Liu J.P., Feng L. Protection of Huidouba, a Tibetan medicine on renal injury in STZ induced type Ⅱ diabetic mice. Chinese Traditional Patent Medicine. 2018;40(3):505–511. [Google Scholar]