Abstract
Objective
The quality evaluation of herbal products remains a big challenge. Traceable markers are the core concept of the authentication of herbal products. However, the discovery of traceable markers is labor-intensive and time-consuming. The aim of this study is to develop a convenient approach to rapidly screen the traceable markers for herbal product authentication.
Methods
Commercial Jing Liqueur and its 22 species of herbal ingredients were analyzed using HPLC-QTOF-MS and GC–MS to characterize nonvolatile and volatile chemicals. The acquired data were imported into MZmine 2 software for mass detection, chromatogram building, deconvolution and alignment. The aligned data were exported into a csv file and then traceable markers were selected using the built-in filter function in Excel. Finally, the traceable markers were identified by searching against online databases or publications, some of which were confirmed by reference standards.
Results
A total of 288 chemical features transferred from herbal materials to Jing Liqueur product were rapidly screened out. Among them, 52 markers detected by HPLC-QTOF-MS were annotated, while nine volatile markers detected by GC–MS were annotated. Moreover, 30 of these markers were confirmed by comparing with reference standards. A chemical fingerprint consisting of traceable markers was finally generated to ensure the authentication and quality consistency of Jing Liqueur.
Conclusion
A strategy for rapid discovery of traceable markers in herbal products using MZmine 2 software was developed.
Keywords: GC–MS, herbal products, HPLC-QTOF-MS, Jing Liqueur, MZmine 2, traceable markers
1. Introduction
Herbal products have been widely used as both therapeutic medicines and functional foods in healthcare worldwide (Aparicio-Soto et al., 2016, Guo et al., 2017). As the application of herbal products is increasingly integrated into the modern health care system, their quality has been one of the most important concerns in this field (Cañigueral et al., 2008, Liang et al., 2004). However, herbal products, commonly generated from multiple raw materials, have extremely complex chemical compositions and undergo sophistic manufacturing processes, which makes quality evaluation a challenging task.
Chemical markers are often the bioactive basis for both raw materials and their related products, become a core concept of herbal product authentication (Fan et al., 2006, Liu et al., 2018, Wang et al., 2017, Zhao et al., 2019). Traceable chemical markers are compounds that exist in any form of an herbal medicine such as the raw materials, pieces, extracts and finished products. In general, traceable chemical markers are selected by analyzing the variation of targeted chemicals throughout the whole production process, which is labor-intensive and time-consuming (Liu et al., 2020, Yu et al., 2013, Zhao et al., 2019). Herein, we proposed that potential traceable markers could be rapidly discovered by comparing the chemical profiles in the raw and finished forms of herbal products.
Liquid/gas chromatography coupled with mass spectrometry (LC/GC–MS) has become the most powerful tool to profile the chemical components in herbal products because of its high selectivity, sensitivity and throughput, as well as the ability to generate specific information including molecular mass and structural characteristics (Gomathi et al., 2015, Lai et al., 2015, Müller and Bracher, 2015, Weitzel, 2011). MZmine 2 is an open-source toolbox for the processing and visualization of mass spectrometry based on molecular profile data, which can automatically process a large number of mass spectra in numerous ways, including mass detection, chromatogram building, deconvolution, alignment and compound identification (Pluskal, Castillo, Villar-Briones, & Orešič, 2010). As mentioned above, traceable chemical markers exist in both raw materials and finished products. That is, after feature detection and alignment in MZmine 2, the potential traceable markers can be highlighted by comparing the feature distribution among the raw materials and their related products. Although there were numerous analogous free software suites like XCMS (Tautenhahn, Patti, Rinehart, & Siuzdak, 2012), OpenMS (Röst et al., 2016) or SMART (Liang et al., 2016), MZmine 2 is one of the most popular and user-friendly packages for its straightforward operations for nonexpert users and a flexible and modular platform for MS data processing (Olivon, Grelier, Roussi, Litaudon, & Touboul, 2017).
Jing Liqueur, composed of 22 species of herbal materials, is a very popular healthcare liqueur with anti-fatigue, anti-inflammation and immunity-enhancing activities (Feng et al., 2013, Shan et al., 2018). The trace levels, large number, and diverse structures and properties of the chemical components in Jing Liqueur increase the difficulty of its authentication. In this study, LC-MS and GC–MS were complementarily applied to characterize nonvolatile and volatile compounds in both the herbal materials and finished product of Jing Liqueur. The MZmine 2 tool was utilized to detect the mass features in all the samples and those features were aligned according to the mass-to-charge ratio (m/z) and retention time (rt). The chemical transitivity from raw materials to products was visualized to select potential traceable markers, which were then identified by comparing with reference standards or by searching against the available MS/MS databases and publications (Fig. 1). Consequently, a total of 288 features were transferred into Jing Liqueur from the 22 raw materials and 61 were annotated. In addition, considering the practicability of authentication, a chemical fingerprint consisting of traceable markers of Jing Liqueur was constructed.
Fig. 1.
Workflow of transitive chemical discovery.
2. Materials and methods
2.1. Regents and materials
Jing Liqueur samples (No. P1707122/05) were provided by Jing Brand Co., Ltd. (Hubei, China). The 22 species of herbal materials (Table 1) were collected from different areas of China and authenticated by Prof. Hui-jun Li according to Chinese Pharmacopoeia (2015). Voucher specimens were deposited in the State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, China.
Table 1.
Twenty-two species of raw materials involved in Jing Liqueur.
| No. | Raw materials | No. | Raw materials |
|---|---|---|---|
| 1 | Puerariae Lobatae Radix (PLR) | 12 | Epimedii Folium (EF) |
| 2 | Cuscutae Semen (CS) | 13 | Dioscoreae Rhizoma (DR) |
| 3 | Angelicae Sinensis Radix (ASR) | 14 | Curculiginis Rhizoma (CRh) |
| 4 | Caryophylli Flos (CF) | 15 | Codonopsis Radix (CR) |
| 5 | Cistanches Herba (CH) | 16 | Amomi Fructus (AF) |
| 6 | Salviae Miltiorrhizae Radix et Rhizoma (SMRR) | 17 | Astragali Radix (AR) |
| 7 | Achyranthis Bidentatae Radix (ABR) | 18 | Lycii Fructus (LF) |
| 8 | Alpiniae Oxyphyllae Fructus (AOF) | 19 | Allii Tuberosi Semen (ATS) |
| 9 | Morindae Officinalis Radix (MOR) | 20 | Euryales Semen (ES) |
| 10 | Imperatae Rhizoma (IR) | 21 | Rehmanniae Radix Praeparata (RRP) |
| 11 | Rosae Laevigatae Fructus (RLF) | 22 | Cinnamomi Cortex (CC) |
Reference standards of betaine (4), 5-hydroxymethylfurfural (9), 3′-hydroxy puerarin, 3,4-dihydroxybenzaldehyde, puerarin (17), magnoflorine, 3′-methoxypuerarin (19), calycosin-7-glucoside, β-ecdysone, lithospermic acid, salvianolic acid A (33), isoflavoues aglycone, sagittatoside B (39), 2″-O-rhamnosyl icariside II, epimedin C, icariside I, baohuoside II, and diosgenin (52) (purity ≥ 98%) were purchased from Shanghai Yuanye Bio-Technology Co., Ltd (Shanghai, China). The other reference standards were purchased from Shanghai Haoyuan Bio-Technology Co., Ltd (Shanghai, China). HPLC-grade acetonitrile (ACN) and methanol (MeOH) were supplied by Merck (Darmstadt, Germany). Formic acid of HPLC grade was purchased from ROE Co., Ltd. (Newark, USA). Ultra-pure water was purified by a Millipore water purification system (Millipore, Milford, MA, USA).
2.2. Sample preparation
Four different standard solutions, comprising a total of 52 reference standards at concentration of 20 μg/mL in MeOH, were prepared. The details are summarized in Table 2.
Table 2.
Preparation of reference solutions.
| Reference solutions | Compositions | Concentration /(µg·mL−1) |
|---|---|---|
| 1 | 3′-hydroxy puerarin, chlorogenic acid, puerarin, daidzin, calycosin-7-glucoside, β-ecdysone, isoflavoues aglycone, epimedin B, epimedin C, icariin | 20 |
| 2 | betaine, adenosine, 5-hydroxymethylfurfural, magnoflorine, 3,2′-dihydroxyflavone, Ononin, formononetin, coumarin, epimedin A1, epimedin A, icariside I, salidroside, diosgenin | 20 |
| 3 | sucrose, geniposide, geniposidic acid, 3,4-dihydroxybenzaldehyde, 3′-methoxypuerarin, purpureaside C, rutin, quercitrin, azelaic acid, rosmarinic acid, sagittatoside A, 2″-O-rhamnosyl icariside II, astragaloside A, astragaloside II, ginsenoside Ro | 20 |
| 4 | nystose, citric acid, protocatechuic acid, 4-dicaffeoylquinic acid, echinacoside, acteoside, isoacteoside, lithospermic acid, salvianolic acid A, sagittatoside B, astragaloside III, baohuoside II, chikusetsu saponin Ⅳa | 20 |
For HPLC-QTOF-MS analysis, the samples of Jing Liqueur and herbal materials were directly analyzed after centrifugation at 13 000 rpm/min for 10 min. For GC–MS analysis, it was necessary to remove all water in the sample solutions prior to injection. In detail, 4 g of Na2SO4 was added into 6 mL of the herbal material extracts or Jing Liqueur solution. After blending, the mixtures were settled at room temperature for 10 h and then centrifuged at 13 000 rpm/min for 10 min. The supernatant was collected for GC–MS analysis.
2.3. Chromatography and mass spectrometry conditions
2.3.1. HPLC-QTOF-MS method
Chromatographic separation was performed on an Agilent 1290 LC system (Agilent Technologies, USA). The samples were separated on an Agilent Zorbax Extend-C18 (octadecyl chemically bonded phase silica gel) analytical column (4.6 mm × 250 mm, 5 µm) at 25 °C. The mobile phase was composed of solvent A (water containing 0.1% formic acid) and solvent B (ACN containing 0.1% formic acid). The elution gradient was as follows: 0–2.5 min, 10% B; 2.5–20 min, 10%–20% B; 20–35 min, 20%–35% B; 35–40 min, 35%–60% B; 40–45 min, 60%–90% B; 45–50 min, 90%–95% B; 50–60 min, 95% B. The injection volume was set at 2 μL, and the flow rate was set at 0.8 mL/min.
The above HPLC system was coupled to an Agilent 6545 QTOF-MS spectrometer (Agilent Technologies, USA) equipped with an electrospray ionization (ESI) source. The capillary voltage was set at 4000 V. The fragmentor voltage was set at 135 V. The flow rate of drying-gas (N2) was 10 L/min with a temperature of 350 °C, and the nebulizer pressure was 35 psi. The sheath gas was 11.0 L/min with a temperature of 350 °C, the cone voltage was 65 V and OCT 1 RF Vpp was set at 750 V. Mass spectra were recorded over a mass-to-charge ratio (m/z) range of 50–1700 using both positive and negative ion detection modes. Multiple collision energies at 15, 25, 35, and 50 V were set to acquire MS/MS data.
2.3.2. GC–MS method
GC analyses were performed using an Agilent 7890B GC (Palo Alto, CA, USA) equipped with an Agilent-5977A MSD (Agilent Technologies, USA) and an Agilent 19091S-433UI HP-5MS (5% phenyl-methylpolysiloxan) Ultra Inert (30 m × 250 μm, 0.25 µm) column. Helium (purity ≥ 99.999%) was used as the carrier gas at a flow rate of 1 mL/min. A 1 μL aliquot of sample was injected in split mode at a split ratio of 10:1 and at an injection temperature of 250 °C. The oven temperature was programmed to increase from an initial temperature of 40 °C (held for 5 min) to 250 °C at 8 °C/min. All samples were analyzed with electron ionization (70 eV) in full scan mode.
2.4. Data processing in MZmine 2 software
Raw data files were converted into the open format mzML using MSConvert software, which was a part of the cross-platform ProteoWizard program (http://proteowizard.sourceforge.net/). An optimized MZmine 2.41.2 workflow was developed for feature list generation.
For HPLC-QTOF-MS data, centroid mass detection was performed with a noise level of 0 and then the ADAP chromatogram builder was implemented including a minimum group size of 5, a group intensity threshold of 103, and a m/z tolerance set to 0.01 Da or a relative tolerance of 2 × 10−5. Then, the Wavelets (ADAP) algorithm was applied for chromatogram deconvolution including a signal-to-noise ratio of 10, a minimum feature height of 5 × 103, a coefficient threshold of 110, a peak duration range of 0–10 min and an rt wavelet range of 0–0.1 min. The alignment was performed using RANSCAN algorithm with an m/z tolerance of 0.01 Da or a relative tolerance of 2 × 10−5, an rt tolerance of 0.5 min and an rt tolerance after correction of 0.1 min. RANSCAN iterations were estimated automatically and the threshold value was set at 4 s. In addition, isotopes were dismissed using an isotopic peak grouper with an m/z tolerance of 2, an rt tolerance of 0.1 and a maximum charge of 2; A duplicate peak filter was performed with an m/z tolerance of 0.01 and an rt tolerance of 0.1 min. The processed feature list was exported as a csv file containing rt, m/z and peak area data of different samples.
For GC–MS data, the workflow included the centroid mass detection algorithm, with a noise level of 0. ADAP chromatogram builder was performed with a minimum group size of 5, a group intensity threshold of 103, and an m/z tolerance of 0.1. The next step was chromatogram deconvolution by applying the Wavelets (ADAP) algorithm, including a signal-to-noise ratio of 10, a minimum feature height of 103, and a coefficient of 50. Finally, the detected features among different samples were aligned using the hierachical aligner algorithm with an m/z tolerance of 0.1 and an rt tolerance of 0.02. The aligned rt, m/z, and peak area data were exported as a csv file.
3. Results
3.1. Analysis of nonvolatile chemicals based on HPLC-QTOF-MS
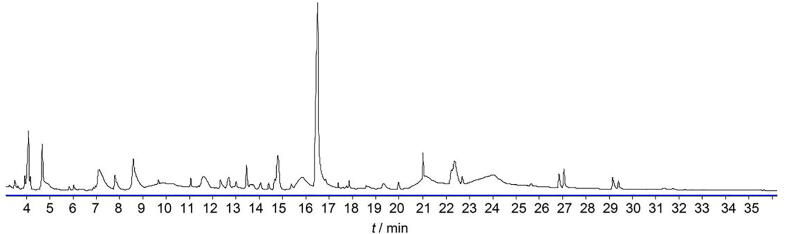
Due to the occurrence of complicated compounds in the 22 species of raw materials, HPLC-QTOF-MS analysis was performed in both positive and negative modes (Fig. 2 and Fig. S1). To reduce the false positive results from high-intensity noise signals, features with peak areas over 5000 were defined as real features in the samples. In positive scan mode, 3150 features were detected, only 120 features were discovered in Jing Liqueur. That is, over 90% of the chemical components in raw herbal materials were either lost or diluted to levels below the limits of detection after the complex manufacturing process. Similarly, 1816 features were observed in negative scan mode, but only 116 were observed in Jing Liqueur.
Fig. 2.
BPCs of Jing Liqueur analyzed by HPLC-QTOF-MS in positive scan mode (A) and negative scan mode (B).
3.2. Analysis of volatile chemicals based on GC–MS
Because herbal materials with pungent taste, such as CRh, CF, CC, AF, ASR and AOF are rich in volatile components, GC–MS analysis was performed to trace volatile chemicals from the six herbal materials in the final Jing Liqueur (Figs. 3 and S2). As a result, a total of 1542 features were detected: 179 in CRh, 263 in CF, 201 in CC, 319 IN AF, 325 in ASR, 246 in AOF and 481 in Jing Liqueur product.
Fig. 3.
BPCs of Jing Liqueur analyzed by GC–MS.
3.3. Discovery and identification of traceable markers in Jing Liqueur
The detected features were aligned using MZmine 2 software and a csv file containing retention time, m/z and peak area data of the different samples was generated. Traceable markers should exist both in some raw materials and in the Jing Liqueur product, which was used as a limitation to rapidly screen markers based on the filter function in Excel 2016. As a consequence, 205 traceable chemicals were discovered by HPLC-QTOF-MS analysis: 96 were detected in positive mode and 109 in negative mode; 83 volatile traceable chemicals were discovered by GC–MS analysis.
The 205 nonvolatile features detected by HPLC-QTOF-MS were identified by searching against online databases, including MassBank (Horai et al., 2010), ChemSpider (Pence & Williams, 2010) and ResPect (Sawada et al., 2012) with a relative m/z tolerance of 10−5 and similarity score of 0.7. Due to the limited natural product data in online databases, a custom database consisting of the 50 reference standards was constructed, including their rt, m/z and characteristic product ion data. The structures of other compounds that have been reported in the 22 species of herbal materials were also involved in the database. Then, 21 features detected in positive mode and 39 in negative mode were annotated. Since there were 8 overlapping features, 52 unique features were finally annotated, including 18 flavonoids, 7 organic acids, 3 triterpenoid saponins, 3 steroids, 2 phenylethanoid glycosides, 2 phenolic acids, 2 alkaloids, 2 glycosides, 2 amino acids, and 11 other types of compounds (Table 3).
Table 3.
Identification of nonvolatile chemical markers.
| No. | RT/min | Formula | [M+X]+/[M−X]− | Experimental m/z | Theoretical m/z | Relative error /(×10−6) | Product ions | Identification |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.537 | C6H14N4O2 | [M−H]− | 173.1041 | 173.1044 | 1.72 | 131.0820 | Arginine |
| 2 | 2.602 | C24H42O21 | [M−H]− | 665.2150 | 665.2146 | −0.63 | 485.1512, 383.1195, 179.0561, 89.0242 | Nystose # |
| 3 | 2.668 | C12H22O11 | [M−H] − | 341.1092 | 341.1091 | −0.77 | 113.0237, 89.0237, 71.0133, 59.0134 | Morindin |
| 4 | 2.799 | C5H11NO2 | [M+H]+ | 118.0857 | 118.0863 | 4.74 | 72.0815, 59.0738, 58.0663 | Betaine # |
| 5 | 2.875 | C10H13N5O4 | [M+H]+ | 268.1036 | 268.104 | 1.61 | 136.0617 | Adenosine # |
| 6 | 3.027 | C11H17NO8 | [M−H] − | 290.0881 | 290.0881 | 0.14 | 128.0344 | N-(1-deoxy-D-fructos-1-yl)pyroglutamic acid |
| 7 | 3.113 | C6H8O7 | [M−H] − | 191.0192 | 191.0197 | 2.74 | 111.0096, 87.0081, 85.0287 | Citric acid # |
| 8 | 3.877 | C4H6O4 | [M−H]− | 117.0184 | 117.0193 | 7.90 | 73.0288, 59.0120 | Succinic acid |
| 9 | 4.312 | C6H6O3 | [M+H]+ | 127.0385 | 127.0390 | 3.73 | 109.0280, 81.0338, 53.0396 | 5-hydroxymethylfurfural # |
| 10 | 4.499 | C16H22O10 | [M−H]− | 373.1138 | 373.1140 | 0.59 | 149.0595, 123.0444, 89.0235 | Geniposidic acid # |
| 11 | 5.021 | C14H21NO4 | [M+H]+ | 268.1530 | 268.1543 | 5.00 | 88.0758 | Codonopsine |
| 12 | 7.384 | C7H6O4 | [M−H]− | 153.0183 | 153.0193 | 6.70 | 109.0294 | Protocatechuic acid # |
| 13 | 10.060 | C16H18O9 | [M+H]+ | 355.1014 | 355.1024 | 2.71 | 163.0364, 145.0272, 135.0432, 117.0336, 89.0358 | Chlorogenic acid # |
| [M−H]− | 353.0872 | 353.0878 | 1.71 | 191.0553, 85.0294 | ||||
| 14 | 10.117 | C9H6O3 | [M+H]+ | 163.0373 | 163.0390 | 10.31 | 117.0345, 89.0391, 69.0331 | Tribenzaldehyde |
| 15 | 11.006 | C7H6O3 | [M−H]− | 137.0236 | 137.0244 | 5.92 | 108.0198 | Protocatechuic aldehyde # |
| 16 | 11.156 | C17H20O9 | [M−H]− | 367.1024 | 367.1035 | 2.87 | 193.0500, 134.0365 | 3-Feroyl-quinic acid |
| 17 | 12.280 | C21H20O9 | [M+H]+ | 417.1163 | 417.1180 | 4.11 | 297.0744, 267.0636, 239.0694 | Puerarin # |
| [M−H]− | 415.1032 | 415.1035 | 0.61 | 267.0654 | ||||
| 18 | 13.171 | C26H28O13 | [M+H]+ | 549.1584 | 549.1603 | 3.41 | 417.1172, 297.0747 | Puerarin-7-O-xyloside |
| [M−H]− | 547.1455 | 547.1457 | 0.39 | 295.0605, 267.0655 | ||||
| 19 | 13.384 | C22H22O10 | [M−H]− | 445.1133 | 445.1140 | 1.61 | 282.0526 | 3 '- Methoxypuerarin # |
| 20 | 14.332 | C16H18O8 | [M−H]− | 337.0923 | 337.0929 | 1.75 | 191.0553, 93.0341 | trans-5-p-Coumaroylquinic acid/4-O-p-Coumaroylqunic acid |
| 21 | 16.463 | C35H46O20 | [M−H]− | 785.2511 | 785.2510 | −0.17 | 623.2178, 161.0239 | Purpureaside C# |
| 22 | 16.475 | C7H6O2 | [M−H]− | 121.0287 | 121.0295 | 6.58 | 92.0273 | Benzoic acid |
| 23 | 19.796 | C18H34O11 | [M−H]− | 425.2050 | 425.2028 | −5.08 | 263.1492, 161.0441, 101.0239, 71.0141 | Sophorosin hexanol Glycoside |
| 24 | 20.665 | C27H30O16 | [M−H]− | 609.1478 | 609.1461 | −2.77 | 300.0249 | Rutin # |
| 25 | 20.971 | C27H44O7 | [M+H]+ | 481.3138 | 481.3160 | 4.54 | 445.2922, 371.2185, 165.1264 | β-Ecdysone # |
| [M+HCOOH-H]− | 525.3062 | 525.3069 | 1.47 | 479.2998, 319.1917, 159.1014 | ||||
| 26 | 22.004 | C27H44O7 | [M+H]+ | 481.3138 | 481.3160 | 4.54 | 445.2922, 371.2185, 165.1264 | 25R-Inokosterone |
| 27 | 22.605 | C29H36O15 | [M−H]− | 623.1966 | 623.1981 | 2.47 | 461.1694, 161.0235 | Acteoside # |
| 28 | 24.053 | C27H29O13 | [M−H]− | 561.1612 | 561.1614 | 0.29 | 309.0755 | Formononetin-8-c-apiosy(1, 6)-O-glycoside |
| 29 | 24.516 | C29H36O16 | [M−H]− | 623.1978 | 623.1981 | 0.55 | 461.1675, 161.0254 | Isoacteoside # |
| 30 | 27.277 | C23H22O10 | [M+H]+ | 459.1265 | 459.1286 | 4.53 | 255.0632 | Acetyldaidzin |
| 31 | 27.778 | C9H16O4 | [M−H]− | 187.0968 | 187.0976 | 4.16 | 125.0972 | Azelaic acid # |
| 32 | 28.564 | C18H16O8 | [M−H]− | 359.0756 | 359.0772 | 4.56 | 161.0245 | Rosmarinic acid # |
| 33 | 29.130 | C26H22O10 | [M−H]− | 493.1129 | 493.1140 | 2.27 | 295.0628, 185.0234, 109.0289 | Salvianolic acid A# |
| 34 | 29.262 | C24H26O10 | [M+H]+ | 475.1589 | 475.1599 | 2.05 | 475.1756, 107.0514 | Puerarinoid D |
| 35 | 30.380 | C16H12O4 | [M+H]+ | 269.0805 | 269.0808 | 1.25 | 253.0487, 226.0616, 197.0591, 118.0399 | Formononetin # |
| 36 | 31.440 | C39H50O20 | [M+H]+ | 839.2930 | 839.2968 | 4.56 | 531.1808, 369.1310, 313.0640, 85.0276 | Epimedin A1# |
| 37 | 32.132 | C33H40O15 | [M−H]− | 675.2293 | 675.2294 | 0.21 | 366.1103, 351.0852, 323.0872 | Sagittatoside A # |
| 38 | 32.225 | C15H10O4 | [M+H]+ | 255.0641 | 255.0652 | 4.27 | 152.0608, 91.0532 | Daidzein # |
| [M−H]− | 253.0493 | 253.0506 | 5.24 | 223.0390, 195.0341, 132.0218, 91.0179 | ||||
| 39 | 32.454 | C32H38O14 | [M−H]− | 645.2178 | 645.2189 | 1.67 | 366.1099, 351.0863, 323.0904 | Sagittatoside B # |
| 40 | 32.566 | C38H48O19 | [M+H]+ | 809.2834 | 809.2863 | 3.53 | 677.2334, 531.1820, 369.1248 | Epimedin B # |
| [M+HCOOH-H]− | 853.2745 | 853.2772 | 3.32 | 645.2209 | ||||
| 41 | 32.855 | C33H40O14 | [M−H]− | 659.2339 | 659.2345 | 0.95 | 366.1098, 351.0864, 323.0885 | 2'-O-Rhamnosyl icariin Ⅱ# |
| 42 | 32.969 | C39H50O19 | [M+H]+ | 823.2992 | 823.3019 | 3.29 | 677.2395, 531.1808, 369.1315 | Epimedin C # |
| [M+HCOOH-H]− | 867.2909 | 867.2928 | 2.35 | 659.2337, 367.1164 | ||||
| 43 | 33.744 | C33H40O15 | [M+H]+ | 677.2418 | 677.2440 | 3.25 | 531.1831, 369.1308 | Icariin # |
| [M+HCOOH-H]− | 721.2325 | 721.2349 | 3.58 | 513.1756, 367.1182 | ||||
| 44 | 34.942 | C39H48O19 | [M+H]+ | 821.2838 | 821.2863 | 2.99 | 532.1850, 369.1328, 313.0671, 211.0577, 145.0472, 129.0536, 99.0450,85.0276 | Epimedin A1 derivative |
| [M+HCOOH-H]− | 865.2771 | 865.2772 | 0.10 | 751.9058, 659.2384, 513.1701, 367.1136 | ||||
| 45 | 36.439 | C11H12O4 | [M−H]− | 207.0654 | 207.0663 | 4.24 | 133.0288 | Ethyl 2,4-dihydroxycinnamate |
| 46 | 38.771 | C53H82O25 | [M−H]− | 1117.5060 | 1117.5072 | 1.11 | 1117.5088, 997.5003, 955.4873, 793.4506 | Achyranthosides D |
| 47 | 39.578 | C18H34O5 | [M−H]− | 329.2324 | 329.2333 | 2.87 | 229.1415, 211.1339, 171.1012 | 9, 12, 13-Trihydroxy-10-octadecenoic acid |
| 48 | 39.811 | C37H46O19 | [M−H]− | 793.4361 | — | — | 793.4337, 631.3732 | Epimedoside E |
| 49 | 42.175 | C42H66O14 | [M−H]− | 793.4361 | 793.4380 | 2.37 | 631.3886, 569.3873, 113.0246, 75.0289 | Chikusetsusaponin ⅣA# |
| 50 | 42.228 | C27H30O10 | [M+H]+ | 515.1893 | 515.1912 | 3.64 | 369.1317, 313.0692 | Icarisid Ⅱ |
| 51 | 42.396 | C48H76O19 | [M−H]− | 955.4527 | 955.4544 | 1.80 | 835.4529, 793.4492, 569.3802 | Ginsenoside Ro |
| 52 | 44.707 | C27H42O3 | [M+H]+ | 415.2101 | — | — | 119.0851 | Diosgenin # |
#Represents that this compound was confirmed by comparing with reference standard.
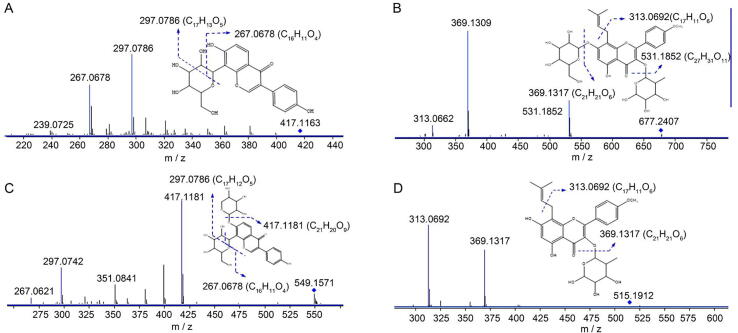
Flavonoids are a key group of compounds identified in Jing Liqueur. Of the 18 annotated flavonoids, 11 of them were present as flavonol glycosides and the others were present as isoflavone glycosides. Reference standards of flavonoids are easily obtained; Thus, 13 of the annotated flavonoids were identified by comparing with reference standards and the remaining were tentatively identified based on available databases or publications. For example, feature 17 (417.1164, [M + H]+, C21H20O9), a representative isoflavone glycoside, was identified as puerarin by its reference standard, and its typical fragmentation patterns were shown in Fig. 4A. Two diagnostic product ions at m/z 297.0786 and 267.0678 were observed, and deduced to be generated by cleavage at the glucopyranosyl moiety. Feature 18 (Fig. 4B) with a precursor ion at m/z 549.1584 ([M + H]+, C26H28O13) also generated product ions at m/z 297.0743 and 267.0657, which indicated its structural similarity with feature 17.
Fig. 4.
Characteristic fragmentation patterns of typical flavonoids.
The product ion at m/z 417.1146 ([M + H]+ - C5H8O4) demonstrated that feature 18 was a conjugated O-xyloside. Combined with the results obtained by database searching, feature 18 was finally annotated as puerarin-7-O-oxyloside.
Feature 43 (677.2418, [M + H]+, C33H41O15) was a representative flavonol glycoside and was identified as icariin by the corresponding reference standard. As shown in Fig. 4C, the characteristic product ions of m/z 531.1852 ([M + H]+ - C6H10O4), 369.1309 ([M + H]+ - C6H10O4 - C6H10O5) and 313.0662 ([M + H]+ - C6H10O4 - C6H10O5 - C4H10) were generated by the neutral loss of glycosides or the cleavage of the side chain of isoamylene. Feature 50 produced very similar product ions and its characteristic fragmentation patterns are shown in Fig. 4D. The product ions at m/z 369.1317 and 313.0692 demonstrated the similar chemical scaffold of feature 50 to that of feature 43. The mass difference of 162 (C6H10O5) indicated that feature 43 could be derived from feature 50 by the loss of glucose. Thus, feature 43 was finally annotated as icariside II, which has been reported to be an important component in Epimedii Folium (EF) (Choi et al., 2008).
In the same way, the other nonvolatile components were annotated. Their characteristic product ions are summarized in Table 2.
Among the 83 unique volatile features in Jing Liqueur detected by GC–MS, 23 could be detected in CR, 24 detected in CF, 27 detected in CC, 27 detected in AF, 22 detected in AOF and 57 detected in ASR. The compounds were identified by comparing their mass spectra with those in the instrument’s National Institute of Standards and Technology (NIST) library (Stein, 1995). A score value over 700 was considered necessary for a good match (Stein, 2011). To avoid false positive markers resulting from automatic data processing, the selected traceable features were confirmed by manual extraction of the raw data in MassHunter software. And then eight annotated volatile compounds were defined as traceable markers. The detailed information is shown in Table 4.
Table 4.
Identification of volatile chemical markers by searching against NIST database.
| No | RT/min | Molecular weight | Formula | Match score | Identification |
|---|---|---|---|---|---|
| 1 | 3.906 | 118 | C6H14O2 | 887 | 1,1-Diethoxyethane |
| 2 | 5.942 | 116 | C6H12O2 | 772 | Ethyl butanoate |
| 3 | 10.400 | 160 | C9H20O2 | 721 | 1,1-Diethoxy-3-methyl-Butane |
| 4 | 15.355 | 174 | C8H14O4 | 692 | Phenethyl alcohol |
| 5 | 17.370 | 196 | C12H20O2 | 755 | Bornyl acetate |
| 6 | 26.857 | 254 | C16H30O2 | 899 | Palmitoleic acid |
| 7 | 27.080 | 256 | C16H32O2 | 864 | Palmitic acid |
| 8 | 29.163 | 282 | C18H34O2 | 903 | Oleic acid |
| 9 | 29.409 | 284 | C18H36O2 | 845 | Stearic acid |
3.4. Generation of chemical marker fingerprint
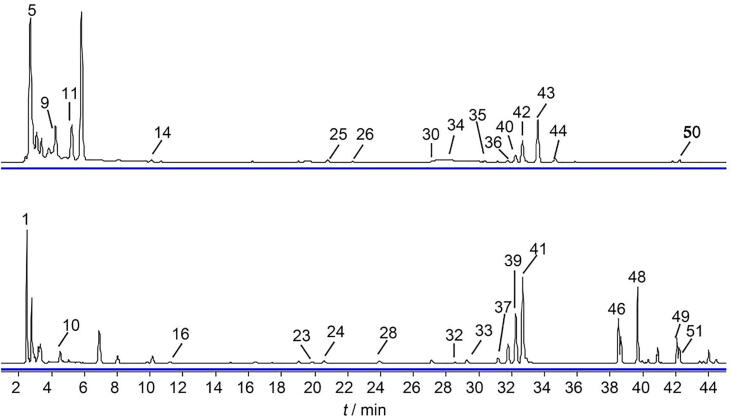
As shown in Fig. 5, among the 61 annotated markers, some were exclusively present in certain herbal species, and the others coexisted in all 22 species of raw materials. The chemical profile of Jing Liqueur was integrated by those of all 22 herbal species. The specificity of chemical markers was evaluated by their distribution ratio among the 22 raw herbal species. Theoretically, specific markers of an herbal material contributed the most to the relative intensity of the MS profile of Jing Liqueur. By limiting the distribution ratio to 50%, 29 chemical markers derived from all the herbal species except Allii Tuberosi Semen (ATS) were selected. With regard to ATS, the contribution intensity of the 52 features was evaluated by the ratio of the intensity of ATS features to the total intensity of features of the 22 herbal species. Feature 1 contributed the most to the corresponding component in Jing Liqueur, which was added to the marker group. The chemical profiles of the 30 markers were extracted from the raw LC-MS data of Jing Liqueur to generate the chemical marker fingerprint, which could be utilized to guide its authentication (Fig. 6).
Fig. 5.
Visualization of traceable markers detected by HPLC-QTOF-MS (A) and GC–MS (B) from raw materials to Jing Liqueur.
Fig. 6.
Chemical fingerprints for authentication of Jing Liqueur.
4. Discussion
The quality evaluation of herbal products has troubled scientists and researchers. Quality markers with transitivity and traceability, proposed by Liu et al. (2018), have been comprehensively explored to control the quality of herbal products. However, the discovery of quality markers was very tedious, which included the characterization of chemical ingredients, biosynthetic pathway analysis, bioactivity evaluation and conformation of quality markers (Li et al., 2019). The transitivity and traceability of chemical markers are the core concepts of herbal product authentication. Based on this view, we developed a novel strategy to rapidly discover traceable markers of herbal products.
MZmine 2 software is usually applied for automatic mass detection, chromatogram building and deconvolution before batch identification of complex mixtures (Korf et al., 2019, Pluskal et al., 2010). The alignment function in MZmine 2 software was designed to align the features among different sample runs. Traceable markers exist both in certain materials and in the final product. Therefore, by aligning the chemical features among herbal materials and the product using MZmine 2, traceable chemical markers were rapidly discovered.
In this work, MZmine 2 was first used to discover traceable markers of herbal products. This approach is very convenient. Once the raw data of the materials and products were imported, mass detection, chromatogram building, deconvolution and alignment could be completed in a few minutes. The aligned features were exported into a csv file. The traceable features could be rapidly screened using the filter function in Excel. In addition, the online and custom database search functions built in MZmine 2 software lead to an expedient identify cation of chemical markers.
There are still some points that need to be improved. First, the identification of traceable markers is hampered by the limitation of reference standards and available databases, which is a common issue in this field. With the increasing knowledge of natural products and the expanding databases, this issue will be resolved. Second, the chemical marker fingerprint of Jing Liqueur must be validated using additional commercial samples. Once the product fingerprint is confirmed, it can be embedded into data analysis software. Coupled with the fingerprint similarity evaluation system, real-time authentication of Jing Liqueur can be realized.
5. Conclusion
In this work, a novel strategy for the rapid discovery of traceable markers of herbal products was developed. Jing Liqueur, produced from 22 species of herbal materials, was taken as a case study. A total of 61 traceable markers were rapidly discovered and annotated. The chemical fingerprint of traceable markers was also generated, and could be used to authenticate Jing Liqueur products.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.chmed.2021.05.004.
Contributor Information
Yuan-cai Liu, Email: lyc@jingpai.com.
Hui-jun Li, Email: cpuli@126.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aparicio-Soto M., Sánchez-Hidalgo M., Rosillo M.Á., Castejón M.L., Alarcón-de-la-Lastra C. Extra virgin olive oil: A key functional food for prevention of immune-inflammatory diseases. Food and Function. 2016;7(11):4492–4505. doi: 10.1039/c6fo01094f. [DOI] [PubMed] [Google Scholar]
- Cañigueral S., Tschopp R., Ambrosetti L., Vignutelli A., Scaglione F., Petrini O. The development of herbal medicinal products: Quality, safety, and efficacy as key factors. Pharmaceutical Medicine. 2008;22(2):107–118. [Google Scholar]
- Choi H.J., Eun J.S., Kim D.K., Li R.H., Shin T.Y., Park H., et al. Icariside II from Epimedium koreanum inhibits hypoxia-inducible factor-1α in human osteosarcoma cells. European Journal of Pharmacology. 2008;579(1-3):58–65. doi: 10.1016/j.ejphar.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Fan X.H., Cheng Y.Y., Ye Z.L., Lin R.C., Qian Z.Z. Multiple chromatographic fingerprinting and its application to the quality control of herbal medicines. Analytica Chimica Acta. 2006;555(2):217–224. [Google Scholar]
- Feng S., Shan Y., Lu S., Liu Y., He G. The anti-inflammatory effect of moderate drinking. Liquor-Making Science and Technology. 2013;229:121–124. [Google Scholar]
- Gomathi D., Kalaiselvi M., Ravikumar G., Devaki K., Uma C. GC-MS analysis of bioactive compounds from the whole plant ethanolic extract of Evolvulus alsinoides (L.) L. Journal of Food Science and Technology. 2015;52(2):1212–1217. doi: 10.1007/s13197-013-1105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B.J., Bian Z.X., Qiu H.C., Wang Y.T., Wang Y. Biological and clinical implications of herbal medicine and natural products for the treatment of inflammatory bowel disease. Annals of the New York Academy of Sciences. 2017;1401(1):37–48. doi: 10.1111/nyas.13414. [DOI] [PubMed] [Google Scholar]
- Horai H., Arita M., Kanaya S., Nihei Y., Ikeda T., Suwa K., et al. MassBank: A public repository for sharing mass spectral data for life sciences. Journal of Mass Spectrometry. 2010;45(7):703–714. doi: 10.1002/jms.1777. [DOI] [PubMed] [Google Scholar]
- Korf A., Jeck V., Schmid R., Helmer P.O., Hayen H. Lipid species annotation at double bond position level with custom databases by extension of the MZmine 2 open-source software package. Analytical Chemistry. 2019;91(8):5098–5105. doi: 10.1021/acs.analchem.8b05493. [DOI] [PubMed] [Google Scholar]
- Lai K.M., Cheng Y.Y., Tsai T.H. Integrated LC-MS/MS analytical systems and physical inspection for the analysis of a botanical herbal preparation. Molecules. 2015;20(6):10641–10656. doi: 10.3390/molecules200610641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xie Y., He Y., Hou W., Liao M., Liu C. Quality markers of traditional Chinese medicine: Concept, progress, and perspective. Engineering. 2019;5(5):888–894. [Google Scholar]
- Liang Y.J., Lin Y.T., Chen C.W., Lin C.W., Chao K.M., Pan W.H., et al. SMART: Statistical metabolomics analysis-an R tool. Analytical Chemistry. 2016;88(12):6334–6341. doi: 10.1021/acs.analchem.6b00603. [DOI] [PubMed] [Google Scholar]
- Liang Y., Xie P., Chan K. Quality control of herbal medicines. Journal of Chromatography B. 2004;812(1-2):53–70. doi: 10.1016/j.jchromb.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Liu C., Guo D.A., Liu L. Quality transitivity and traceability system of herbal medicine products based on quality markers. Phytomedicine. 2018;44:247–257. doi: 10.1016/j.phymed.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Liu F.J., Jiang Y., Li P., Liu Y.D., Yao Z.P., Xin G.Z., et al. Untargeted metabolomics coupled with chemometric analysis reveals species-specific steroidal alkaloids for the authentication of medicinal Fritillariae Bulbus and relevant products. Journal of Chromatography A. 2020;1612:460630. doi: 10.1016/j.chroma.2019.460630. [DOI] [PubMed] [Google Scholar]
- Müller C., Bracher F. Determination by GC-IT/MS of phytosterols in herbal medicinal products for the treatment of lower urinary tract symptoms and food products marketed in Europe. Planta Medica. 2015;81(07):613–620. doi: 10.1055/s-0035-1545906. [DOI] [PubMed] [Google Scholar]
- Olivon F., Grelier G., Roussi F., Litaudon M., Touboul D. MZmine 2 data-preprocessing to enhance molecular networking reliability. Analytical Chemistry. 2017;1(89):7836–7840. doi: 10.1021/acs.analchem.7b01563. [DOI] [PubMed] [Google Scholar]
- Pence H.E., Williams A. Chemspider: An online chemical information resource. Journal of Chemical Education. 2010;87(11):1123–1124. [Google Scholar]
- Pluskal T., Castillo S., Villar-Briones A., Orešič M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics. 2010;11:395. doi: 10.1186/1471-2105-11-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röst H.L., Sachsenberg T., Aiche S., Bielow C., Weisser H., Aicheler F., et al. OpenMS: A flexible open-source software platform for mass spectrometry data analysis. Nature Methods. 2016;13(9):741–748. doi: 10.1038/nmeth.3959. [DOI] [PubMed] [Google Scholar]
- Sawada Y., Nakabayashi R., Yamada Y., Suzuki M., Sato M., Sakata A., et al. RIKEN tandem mass spectral database (ReSpect) for phytochemicals: A plant-specific MS/MS-based data resource and database. Phytochemistry. 2012;82:38–45. doi: 10.1016/j.phytochem.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Shan Y., Zhou H., Chen M., Chen K., Liu Y., Wang L. Study on anti-fatigue, regulating immunity and enhancing sexual function of Chinese Jing Liqueur. Chinese Traditional Patent Medicine. 2018;40:1600–1603. [Google Scholar]
- Stein S.E. Chemical substructure identification by mass spectral library searching. Jouranl of the American Society for Mass Spectrometry. 1995;6(8):644–655. doi: 10.1016/1044-0305(95)00291-K. [DOI] [PubMed] [Google Scholar]
- Stein S.E. 2.0g. https://chemdata.nist.gov/mass-spc/ ms-search/. 2011. National Institute and Standards and Technology (NIST) Mass Spectral Search Program, Version. [Google Scholar]
- Tautenhahn R., Patti G.J., Rinehart D., Siuzdak G. XCMS online: A web-based platform to process untargeted metabolomic data. Analytical Chemistry. 2012;84(11):5035–5039. doi: 10.1021/ac300698c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Wang B.o., Wang L., Xiong Z.Y., Gao W., Li P., et al. Discovery of discriminatory quality control markers for Chinese herbal medicines and related processed products by combination of chromatographic analysis and chemometrics methods: Radix Scutellariae as a case study. Journal of Pharmaceutical and Biomedical Analysis. 2017;138:70–79. doi: 10.1016/j.jpba.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Weitzel K.M. Bond-dissociation energies of cations-pushing the limits to quantum state resolution. Mass Spectrometry Review. 2011;30(2):221–235. doi: 10.1002/mas.20276. [DOI] [PubMed] [Google Scholar]
- Yu H.L., Wen C.J., Su H.Q. Establishment of traceable management information platform for quality safety of Panax Ginseng. Chinese Traditional and Herbal Drugs. 2013;44(24):3566–3574. [Google Scholar]
- Zhao C., Liu H., Miao P., Wang H., Yu H., Wang C., et al. A strategy for selecting “Q-Markers” of Chinese medical preparation via components transfer process analysis with application to the quality control of Shengmai Injection. Molecules. 2019;24(9):1811. doi: 10.3390/molecules24091811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.