Abstract
Objective
Critical pharmaceutical process identification (CPPI) is an important step in the implementation of quality by design concept to traditional Chinese medicines (TCMs). Risk assessment methods are usually used in CPPI. However, risk evaluation is usually subjective. The purpose of this work is to present a more objective CPPI method.
Methods
A CPPI method considering chemical composition, biological activity, and batch-to-batch consistency was presented in this work. The manufacturing process of notoginseng total saponins (NTS) was investigated as an example. The changes of chemical composition, biological activity, and chemical composition consistency after main processes were measured and compared. A significant change of them indicated a critical process.
Results
After extraction process and chromatography process, saponin purity and chemical composition similarity remarkably increased, and saponin content variations decreased. Thrombin inhibitory activity was remarkably decreased after chromatography process. Because of the large influences on NTS quality, extraction process and chromatography process were identified to be critical processes of NTS.
Conclusion
Based on a comprehensive and objective examination of the role of each process, critical pharmaceutical processes can be identified. A similar method can also be applied to other TCM processes.
Keywords: batch-to-batch consistency, bioactivity, chemical composition similarity, critical process identification, Panax notoginseng (Burk.) F. H. Chen, quality by design
1. Introduction
Quality by design (QbD) concept has been worldwide accepted for the development of new drugs and analytical methods (Wang et al., 2019; Yu, 2008). Risk management and knowledge management were two cornerstones of QbD concept (Yu et al., 2014). Recently, different manufacturing processes of traditional Chinese medicines (TCMs) were optimized according to the QbD concept, such as ethanol precipitation process (Zhang, Yan, Gong, Yu & Qu, 2013), extraction process (Gong, Zhang, Pan & Qu, 2014), chromatography process (Chen, Gong, Chen, Zhang & Qu, 2016), and so on. The key steps of the implementation of QbD concept to TCMs manufacturing processes were summarized (Gong, Chen & Qu, 2017), including critical process determination, critical process parameter identification, model building, design space establishment, control strategy development, and continuous improvement.
To control the quality of TCMs, a quality control system covering the whole processes should be established, including planting, harvesting, material processing, manufacturing, storage, transportation, and so on (Liu et al., 2016). Except for extraction process, there are many other processes in the manufacturing of TCMs, such as ethanol precipitation, resin chromatography, activated carbon adsorption, liquid-liquid extraction, crystallization, concentration, drying, granulation, tableting, and so on. Critical pharmaceutical process identification (CPPI) of TCMs is an important step to strengthen process control. The transportation and transformation of substances in critical processes will be studied in depth. Control strategies, such as feedforward/feedback control of parameters (Yang, Tan, Wang & Luo, 2014) and process status monitoring (Xiong, Gong & Qu, 2012), can then be designed and implemented for these critical processes targetedly. Expensive process analytical technology (PAT) tools, such as near-infrared spectroscopy (Huang & Qu, 2011) or direct real-time mass spectrometry (Yan, Chen, Xu & Qu, 2014) can also be targetedly applied for critical processes.
Risk assessment is the most frequently used method to identify critical processes of TCMs products. According to the effects of each process on multiple drug quality indices, critical processes of Danhong Injection (Gong, Li, Guo & Qu, 2014) and Xueshuantong Powder (Gong, Chen, Chen & Qu, 2014) were determined. However, accurately assessing the effects of processes mainly based on experiences. Later, Chen (2016) used a failure mode and effect analysis (FMEA) method to find out critical processes of notoginseng total saponins. However, the evaluation of risk levels and occurrence frequency was also subjective. Therefore, a more objective method for critical pharmaceutical process identification is still required.
The quality evaluation of TCMs was performed based on their appearance, physical properties, chemical properties, biological activities, and microbial properties. The major part of current TCMs quality control system is the analysis of chemical properties, such as chemical marker content and fingerprints (Xiao et al., 2010; Yao, Shi, Shao, & Fan, 2011). Biological activity analysis can show integral features of TCMs without determining chemical marker contents (Luo et al., 2011). It is also in line with the TCM feature that drug effects are determined by the whole of chemical substances. Biological activity analysis is generally considered to be a beneficial supplement of current TCM quality control system. Therefore, the changes in chemical composition and biological activities should be both concerned in CPPI (Ren et al., 2011). On the other hand, due to the large quality variations of herbal raw materials, keeping batch-to-batch consistency of TCM quality is usually difficult. If a process can significantly lower the variations introduced by decoction pieces, it should also be a critical process.
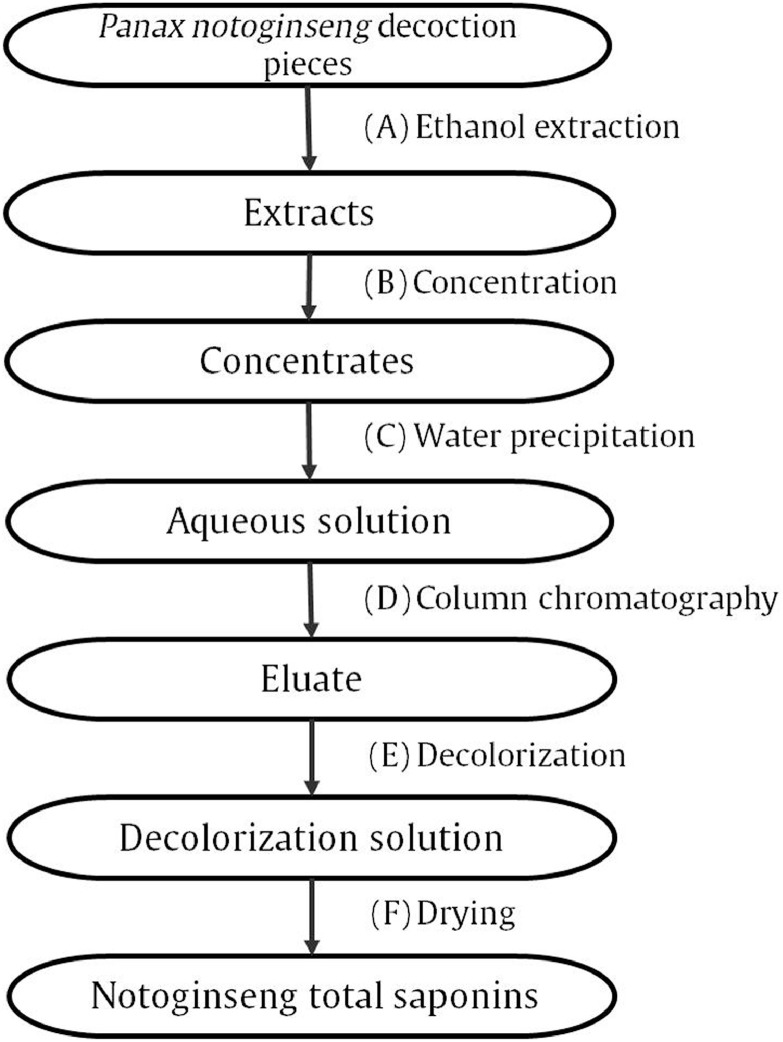
In this work, an objective and comprehensive CPPI method was presented. The manufacturing process of notoginseng total saponins (NTS) was used as an example. NTS is a herbal extract made from P. notoginseng, which is usually used for treating coronary artery disease, stroke and cerebrovascular diseases (He et al., 2017; Li et al., 2018; Si et al., 2014; Zhang, Wu & Zhang, 2015). Many P. notoginseng preparations used NTS as their material, such as Xuesaitong Capsule, Xuesaitong Lyophilized Powder, Xueshuantong Injection, and so on. In the 2015 edition of the Chinese Pharmacopoeia, notoginsenoside R1, ginsenoside Rg1, ginsenoside Rb1, ginsenoside Rd, and ginsenoside Re were chemical markers for quality control of NTS (Chinese Pharmacopoeia Commission, 2015; Wang et al., 2015). The results of pharmacology research also confirmed that these five saponins were the main active components of NTS (Chen, Dang & Zhu, 2010; Han, Sakah, & Liu, 2014; Shi et al., 2012; Wang et al., 2013). As seen in Fig. 1, the preparation of NTS contains six main processes, including ethanol extraction, concentration, water precipitation, column chromatography, decolorization, and drying. In this work, the changes in chemical composition and biological effects of materials, intermediates, and NTS were all determined. Critical processes of the manufacturing processes of NTS were identified. The results were discussed and compared with published works.
Fig. 1.
Flowchart of preparation of P. notoginseng total saponins.
2. Materials and methods
2.1. Materials and chemicals
Raw material of P. notoginseng was collected from Yunnan Province of China. No specific permissions were required for the described field studies. The locations are neither privately owned nor protected by the Chinese government. No endangered or protected species were sampled. Reference substances of notoginsenoside R1, ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, and ginsenoside Rd were purchased from Shanghai Winherb Medical Technology Co., Ltd (Shanghai, China). Analytical grade ethanol was purchased from Shanghai Lingfeng Chemical Reagents Co., Ltd (Shanghai, China). Analytical grade methanol was purchased from Sinophram Chemical Reagent Co., Ltd. HPLC-grade acetonitrile was purchased from Merck (Darmstadt, Germany). HPLC-grade formic acid was purchased from Tedia Company (Fairfield, USA). Deionized water was produced from Milli-Q academic water purification system (Milford, MA, USA). HPD-100 resin was purchased from Cangzhou Bon Adsorber Technology Co., Ltd. Thrombin was purchase from Beijing Solarbio Science & Technology Co., Ltd. Thrombin generation chromogenic substrate (T3068) was purchase from Sigma-Aldrich (Buchs, Switzerland). β-Ala-Gly-Arg-p-nitroaniline was purchased from Merck (Darmstadt, Germany).
2.2. Preparation of P. notoginseng saponins
The roots of P. notoginseng were pulverized to obtain decoction pieces, which was the material to prepare NTS. P. notoginseng decoction pieces (100 g) were extracted with 600 mL of 90% (volume percent) ethanol solution twice. The first extraction was heated to reflux at 85 °C. The reflux extraction time was 10 h. The second extraction was carried out under the same conditions except that the reflux time was 6 h. The two ethanol extracts were filtered and combined. The combined extracts were evaporated to obtain concentrates.
In the water precipitation process, water was added to the concentrates, and the mixture was stirred at a constant speed for 10 min using a magnetic stirrer (85–1, Hangzhou Instrument Motor Co., Ltd.). Then the mixture was settled for 1 h. An aqueous solution was obtained by filtration.
A certain amount of HPD-100 microporous adsorption resin was loaded on the column. The column internal diameter was 6 cm, and the bed volume (BV) was 720 cm3. The aqueous solution was pumped into the column through the peristaltic pump (YZ-15, Changzhou VCL Fluid Technology Co., Ltd.) at a flow rate of 1.0 BV/h. After the sample was loaded, water was pumped at a flow rate of 1.0 BV/hr into the column. After being washed, the column was eluted with 60% (volume percent) ethanol solution at a flow rate of 1.0 BV/h. The eluate of the first 30 min was discarded. After that, eluate was collected for 120 min. After the eluate was collected, the resin was regenerated with 90% (volume percent) ethanol solution at a flow rate of 1.5 BV/h for 80 min. The column was then washed with water at 2 BV/h for 1.5 h before the next use.
The eluate was evaporated to remove ethanol. After that, activated carbon was added to remove pigments with an amount of 1 g of activated carbon per 100 g of medicinal material. The mixture was stirred for 10 min, and then filtered to obtain decolorization solution. The decolorization solution was placed in a polytetrafluoroethylene evaporation dish and dried in a vacuum oven (DZF-6050, Shanghai Jinghong Laboratory Instrument Co., Ltd.) at 75 °C. The dried powder was NTS. NTS powder was stored in a desiccator for use.
A total of six batches of P. notoginseng were used. The P. notoginseng decoction pieces and NTS were sampled and analyzed. Process intermediates of extracts, concentrates, aqueous solutions, eluates, and decolorization solutions were also sampled and analyzed for CPPI.
2.3. Analytical methods
2.3.1. Saponin content
To determine the content of saponin in P. notoginseng decoction pieces, the preparation of test samples was carried out as described in the Chinese Pharmacopoeia (2015th Edition) (Chinese Pharmacopoeia Commission, 2015) and then analyzed with an HPLC method.
The HPLC system was composed of a Waters Acquity UPLC apparatus with a UV detector (Waters, Milford, MA, USA) (Shen, Gong, Pan & Qu, 2017). Analyses were performed on a Waters CSH C18 column (50 mm × 2.1 mm i. d., 1.7 μm) with the column temperature controlled at 40 °C. The solvent flow rate was maintained at 0.35 mL/min, while the sample injection volume was set at 5 μL. 0.01% (volume percent) formic acid was both added in water phase and acetonitrile phase to form mobile phases A and B, respectively. The solvent gradients were as follows: 0 − 6 min 18%−20% B, 6 − 6.8 min 20%−30% B, 6.8 − 11 min 30%−35% B, 11−17 min 35%−90% B, 17−25 min 90% B. The detection wavelength was fixed at 203 nm. Samples of process intermediates or NTS were diluted with 18% (volume percent) acetonitrile solution, and then analyzed.
2.3.2. Dry matter content
The dry matter content in process intermediate samples was determined gravimetrically (Gong, Chen, Pan, & Qu, 2015). Samples were dried in an oven (DHG - 9146A, Shanghai Jinghong Laboratory Instrument Co., Ltd.) at 105 °C for 3 h, and then weighed precisely.
2.3.3. Biological activity analysis
An appropriate amount of dry powder obtained in each stage of the process was weighed accurately, and was diluted to about 20 mg/mL with water to obtain a test solution. Thrombin was accurately weighed and dissolved in water to form a solution of about 45 NIH (National Institute of Health) U/mL. Thrombin chromogenic substrate was also weighed accurately, and then diluted with water to obtain a 20 mg/mL solution. A total of 50 μL of the sample solution, 50 μL of thrombin chromogenic substrate solution, and 10 μL of thrombin solution were rapidly added to a well of a 96-well plate. Totally 50 μL of sample solution and 60 μL of water were rapidly added to another well as a blank. Then the plate was put in a mixer (Thermo Mixer FP, Eppendorf) at 37 °C for 2 h. The absorbance was measured at 405 nm using a microplate reader (M1000, Tecan, Switzerland). The background value was subtracted from the detected value as the detection result of the sample. The determination was repeated three times.
2.4. Data analysis
2.4.1. Saponin purity
For process intermediates, saponin purity was defined as the mass ratio of a saponin in dry matter, as seen in Eq. (1).
| (1) |
Where P and C refer to purity and concentration, respectively; subscript DM is dry matter; subscript i (i = =1, 2, …, 5) refers to notoginsenoside R1, ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, and ginsenoside Rd. For decoction pieces or NTS, saponin purity was the saponin mass content in decoction pieces or NTS. Total saponin purity was calculated as the sum of five saponin purity, as seen in Eq. (2).
| (2) |
Where subscript TS is total saponin.
2.4.2. Chemical composition similarity
To quantitatively characterize the chemical composition similarity between decoction pieces or process intermediate and the final product NTS, Pearson's correlation coefficient was calculated. The chemical composition feature of a decoction piece, process intermediate, or NTS was written as a vector V, where V= []. The term of represents the total content of the components in dry matter except total saponin. The similarity value between decoction piece and NTS was calculated as the Pearson's correlation coefficient of VDP and VNTS, where subscript DP refers to a decoction piece. The similarity value between a process intermediate and NTS was calculated as the Pearson's correlation coefficient of VPI and VNTS, where subscript PI refers to a process intermediate.
2.4.3. Product consistency
To ensure the quality consistency of TCMs, manufacturing processes are expected to reduce variations brought by raw materials among different batches. The quality variations were quantitatively characterized as the relative standard deviation (RSD) of saponin purities of a same kind of material among different batches, such as decoction pieces, process intermediate, and NTS. RSD values were calculated for each saponin of each kind of materials.
3. Results
3.1. P. notoginseng decoction pieces
The saponin content of P. notoginseng decoction pieces was shown in Table 1. For all the six decoction pieces, ginsenoside Rg1 content was the highest and ginsenoside Re content was the lowest. Ginsenoside Rb1 content was a little lower than ginsenoside Rg1 content. It can be seen from Table 1 that the total saponin content of PN2 was the highest and PN5 was the lowest. For all the six decoction pieces, total saponin content was lower than 100 mg/g decoction pieces.
Table 1.
Saponin content of P. notoginseng decoction pieces.
| No. | Saponin content (mg·g−1 decoction pieces) |
|||||
|---|---|---|---|---|---|---|
| Notoginsenoside R1 | Ginsenoside Rg1 | Ginsenoside Re | Ginsenoside Rb1 | Ginsenoside Rd | Total saponin | |
| PN1 | 6.72 | 27.7 | 4.75 | 27.5 | 7.80 | 74.5 |
| PN2 | 9.35 | 36.9 | 6.64 | 32.4 | 8.07 | 93.3 |
| PN3 | 6.86 | 27.3 | 4.33 | 23.9 | 7.56 | 70.0 |
| PN4 | 7.19 | 27.7 | 5.00 | 27.3 | 7.37 | 74.6 |
| PN5 | 3.31 | 17.3 | 3.20 | 14.3 | 3.77 | 41.8 |
| PN6 | 7.64 | 30.4 | 4.52 | 29.8 | 7.05 | 79.4 |
3.2. Changes of chemical composition
3.2.1. Saponin purities
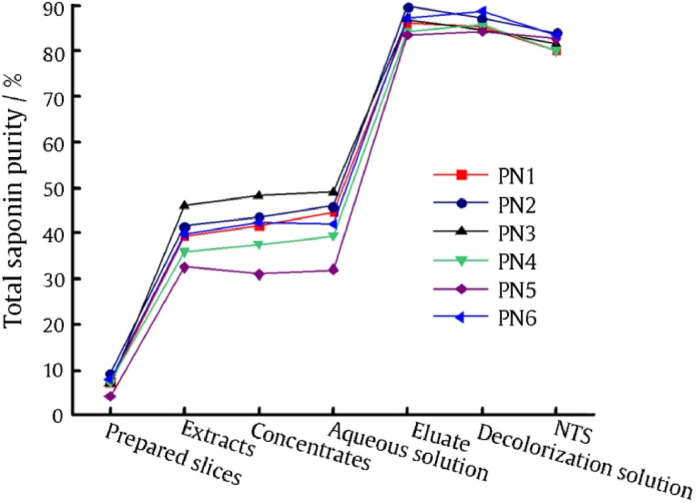
Fig. 2 showed the total saponin purities of decoction pieces, intermediates, and final products. Total saponin purity was increased at least 20% after the extraction process. After chromatography process, total saponin purity was increased about 40%. The increase of total saponin purities during extraction and column chromatography was far more than those during other processes. Purities of five saponins in different process intermediates can be seen in Table 2. Purity of each saponin was increased significantly after extraction process or chromatography process. It also means that impurities were removed remarkably in these two processes.
Fig. 2.
Changes of total saponin purities.
Table 2.
Chemical composition of processed intermediates and final products.
| No. | Intermediates or products | Saponin purity /% |
||||
|---|---|---|---|---|---|---|
| Notoginseno-side R1 | Ginsenoside Rg1 | Ginsenoside Re | Ginsenoside Rb1 | Ginsenoside Rd | ||
| PN1 | Extracts | 3.78 | 15.43 | 2.39 | 14.27 | 3.55 |
| Concentrates | 3.95 | 16.35 | 2.54 | 14.99 | 3.70 | |
| Aqueous solution | 4.12 | 17.49 | 2.67 | 16.24 | 4.00 | |
| Eluate | 8.22 | 35.62 | 5.31 | 30.69 | 6.35 | |
| Decolorization solution | 8.23 | 35.51 | 5.31 | 30.29 | 6.10 | |
| Notoginseng total saponins | 8.88 | 33.89 | 5.03 | 28.10 | 4.14 | |
| PN2 | Extracts | 4.36 | 16.61 | 2.43 | 14.57 | 3.55 |
| Concentrates | 4.56 | 17.54 | 2.56 | 15.29 | 3.72 | |
| Aqueous solution | 4.77 | 18.52 | 2.63 | 16.19 | 3.95 | |
| Eluate | 10.02 | 39.81 | 5.53 | 29.64 | 4.76 | |
| Decolorization solution | 9.66 | 38.80 | 5.35 | 28.80 | 4.50 | |
| Notoginseng total saponins | 9.14 | 34.33 | 4.42 | 29.57 | 6.57 | |
| PN3 | Extracts | 4.89 | 18.28 | 2.28 | 16.14 | 4.45 |
| Concentrates | 5.09 | 19.22 | 2.44 | 16.83 | 4.62 | |
| Aqueous solution | 5.13 | 19.59 | 2.39 | 17.24 | 4.75 | |
| Eluate | 9.20 | 36.19 | 4.37 | 30.14 | 7.05 | |
| Decolorization solution | 8.89 | 35.46 | 4.16 | 29.43 | 6.75 | |
| Notoginseng total saponins | 8.60 | 32.87 | 4.67 | 29.96 | 5.37 | |
| PN4 | Extracts | 3.73 | 14.27 | 1.99 | 13.06 | 2.93 |
| Concentrates | 3.89 | 15.04 | 2.11 | 13.64 | 3.03 | |
| Aqueous solution | 4.00 | 15.79 | 2.13 | 14.41 | 3.22 | |
| Eluate | 8.56 | 34.97 | 4.74 | 30.30 | 5.80 | |
| Decolorization solution | 8.85 | 35.97 | 4.50 | 30.60 | 5.70 | |
| Notoginseng total saponins | 7.74 | 31.62 | 5.06 | 29.99 | 5.67 | |
| PN5 | Extracts | 2.16 | 15.16 | 2.17 | 11.06 | 2.03 |
| Concentrates | 2.00 | 14.55 | 2.23 | 10.43 | 1.84 | |
| Aqueous solution | 2.14 | 15.08 | 2.19 | 10.83 | 1.86 | |
| Eluate | 5.61 | 40.02 | 5.72 | 28.01 | 4.19 | |
| Decolorization solution | 5.92 | 41.03 | 5.80 | 27.55 | 3.92 | |
| Notoginseng total saponins | 7.05 | 34.44 | 5.00 | 31.26 | 4.91 | |
| PN6 | Extracts | 3.31 | 16.31 | 2.31 | 15.03 | 2.95 |
| Concentrates | 3.49 | 17.33 | 2.47 | 15.89 | 3.08 | |
| Aqueous solution | 3.41 | 17.16 | 2.42 | 15.83 | 3.09 | |
| Eluate | 7.42 | 36.67 | 5.13 | 32.69 | 5.49 | |
| Decolorization solution | 7.65 | 37.72 | 5.28 | 32.92 | 5.29 | |
| Notoginseng total saponins | 5.88 | 39.52 | 6.01 | 28.16 | 3.83 | |
3.2.2. Chemical composition similarity
Pearson correlation coefficients between decoction pieces or intermediates and NTS were shown in Fig. 3. The values of correlation coefficients were lower than 0.2 between decoction pieces and NTS, which means that their chemical composition were quite different. After the extraction process, the correlation coefficient was improved by at least 0.1. The column chromatography process increased the correlation coefficient values from less than 0.5 to over 0.9. Other processes had little effects on correlation coefficient values. As expected, correlation coefficient values kept increasing along with the manufacturing processes, which indicated that chemical composition of intermediates will be more and more similar with that of the final product.
Fig. 3.
Correlation coefficient change.
3.2.3. Summary of chemical composition change
As seen in Figs. 2 and 3, saponin purity and correlation coefficient values showed very similar trends. Both of them increased significantly in extraction process and chromatography process than those in other processes. Therefore, from the perspective of the chemical composition of P. notoginseng decoction pieces, process intermediates, and NTS, extraction process and column chromatography process were the critical processes for the preparation of NTS.
3.3. Changes of biological activity
P. notoginseng is a famous Chinese herbal medicine for promoting blood circulation and removing blood stasis (Guo et al., 2018; Li, Wang, Yang, & Wang, 2015). Therefore, thrombin inhibitory activity was chosen to represent its biological activity (Lau, Toh, Chua, Pang, & Woo, 2009; Li, Wang, & Xu, 2013; Wang et al., 2008). The thrombin inhibitory activity of P. notoginseng decoction pieces, the process intermediate and the final product were shown in Fig. 4. Thrombin inhibitory activity of prepared slices, extracts, concentrates, and aqueous solutions did not change much. However, thrombin inhibitory activity of eluate was much lower than those intermediates before chromatography process. It means that some components removed in the chromatography process can exhibit stronger enzyme inhibitory activity than that of saponins. After decolorization and drying, thrombin inhibitory activity did not change significantly. It can be concluded that the column chromatography process was a critical process.
Fig. 4.
Changes in anticoagulant activity of total saponins.
3.4. Product consistency
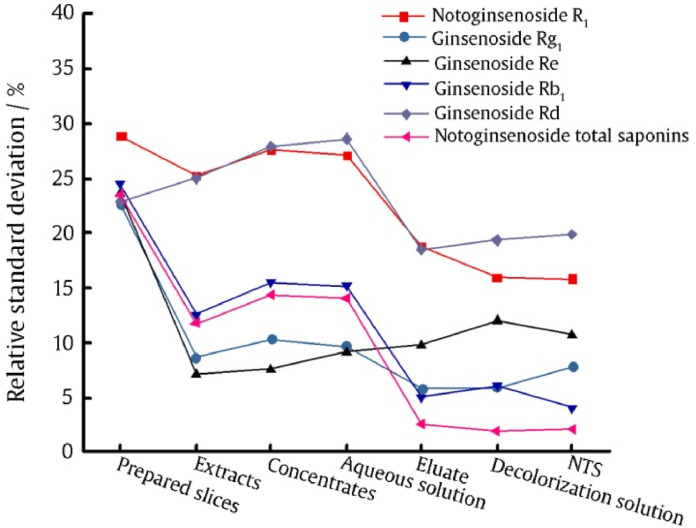
In this work, RSD values of saponin content among six batches were used to represent the quality variations brought by the decoction pieces. Fig. 5 showed the changes of RSD values of decoction pieces, process intermediates, and NTS. After ethanol extraction process, the RSD values of ginsenoside Rg1, ginsenoside Rb1, and ginsenoside Re content among six batches remarkably decreased for more than 10%. After chromatography process, the RSD values of notoginsenoside R1, ginsenoside Rb1, and ginsenoside Rd content decreased for more than 10%. For the RSD value of total saponin content among six batches, the extraction process reduced the RSD values from more than 20% to less than 15%. The column chromatography process reduced the RSD values from more than 10% to less than 3%. While other processes had little effect on total saponin content RSD. Obviously, ethanol extraction process and chromatography process contributed more to the quality consistency of NTS. Accordingly, these two processes should be critical processes.
Fig. 5.
Relative standard deviation of saponin content among different batches.
4. Discussion
The CPPI method presented in this work considers the changes of chemical composition, bioactivity, and consistency among different batches. It can also be used to identify the critical processes of other TCMs. However, the quality control of TCM cannot only focus on critical processes. Zhong, Chen, Zhang, Wang & Liu (2016) verified that the quality of Gegen Qinlian Decoction was mainly affected by the quality of decoction piece. For TCMs preparations, including pills, powders, ointments, granules, and decoctions, the manufacturing processes are usually simple. In these cases, drug quality variations are usually caused by the variations of decoction piece quality.
Decoction piece quality is influenced by many factors, such as herbal material quality, processing, store condition, and so on. The batch-to-batch consistency of decoction piece quality can be realized by mixing different batches of decoction pieces. Lau et al. showed the examples of material mixing for both single herb extraction and multiple herb extraction based on mechanism models (Lau et al., 2014; Lau, Ng, Lau & Wibowo, 2013). Other algorithms for mixing different batches of TCM materials can also be found (Qu, Ou & Cheng, 2006; Yan & Qu, 2013; Yang et al., 2007).
5. Conclusion
In this work, a comprehensive method used to determine critical processes for the manufacturing of traditional Chinese medicines was presented. In this method, the changes of chemical composition, bioactivity, and chemical composition consistency were all considered. The critical processes of the manufacturing of NTS were identified as an example. After extraction process and chromatography process, saponin purity and chemical composition similarity improved more than those of other processes. After chromatography, thrombin inhibitory activity changes more than that of other processes. The RSD value of saponin contents decreased more after extraction process and chromatography process. Overall, extraction process and chromatography process are critical processes for the manufacturing of NTS.
Declaration of Competing Interest
The authors have no conflict of interest to declare.
Acknowledgments
The authors would like to acknowledge the supports of the National Project for Standardization of Chinese Materia Medica (ZYBZH-C-YN-58) and Standardization Program of Ministry of Science and Technology of Yunnan Province, China (2017ZF001).
References
- Chen T. Zhejiang University; 2016. Studies on the macroporous resin chromatography process for the manufacturing of Panax notoginseng saponins using quality by design principles. pp. 14–22. Master's Thesis. [Google Scholar]
- Chen T., Gong X.C., Chen H.L., Zhang Y., Qu H.B. Chromatographic elution process design space development for the purification of saponins in panax notoginseng extract using a probability-based approach. Journal of Separation Science. 2016;39(2):306–315. doi: 10.1002/jssc.201500976. [DOI] [PubMed] [Google Scholar]
- Chen W., Dang Y.J., Zhu C.Y. Simultaneous determination of three major bioactive saponins of panax notoginseng using liquid chromatography-tandem mass spectrometry and a pharmacokinetic study. Chinese Medicine. 2010;5(1):12. doi: 10.1186/1749-8546-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Pharmacopoeia Commission . China Medical Science Press; Beijing: 2015. Pharmacopoeia of the people's republic of china, part i; pp. 393–394. [Google Scholar]
- Gong X.C., Chen H.L., Chen T., Qu H.B. Unit operation optimization for the manufacturing of botanical injections using a design space approach: A case study of water precipitation. PloS One. 2014;9(8) doi: 10.1371/journal.pone.0104493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X.C., Chen H.L., Pan J.Y., Qu H.B. Optimization of Panax notoginseng extraction process using a design space approach. Separation and Purification Technology. 2015;141:197–206. [Google Scholar]
- Gong X.C., Chen T., Qu H.B. Research advances in secondary development of Chinese patent medicines based on quality by design concept. China Journal of Chinese Materia Medica. 2017;42(6):1031–1036. doi: 10.19540/j.cnki.cjcmm.20170223.020. [DOI] [PubMed] [Google Scholar]
- Gong X.C., Li Y., Guo Z.T., Qu H.B. Control the effects caused by noise parameter fluctuations to improve pharmaceutical process robustness: A case study of design space development for an ethanol precipitation process. Separation and Purification Technology. 2014;132:126–137. [Google Scholar]
- Gong X.C., Zhang Y., Pan J.Y., Qu H.B. Optimization of the ethanol recycling reflux extraction process for saponins using a design space approach. PloS one. 2014;9(12) doi: 10.1371/journal.pone.0114300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., Zhang Q., Zhou W.L., Zheng M.C., Sun R.Y., Du S.Y. Research progress of Notoginseng Radix and its preparation combined with chemical drugs in prevention and treatment of cardiovascular diseases. China Journal of Traditional Chinese Medicine and Pharmacy. 2018;33(12):5518–5522. [Google Scholar]
- Han L.F., Sakah K.J., Liu L.L. Saponins from the roots of Panax notoginseng. Chinese Herbal Medicines. 2014;6(2):159–163. [Google Scholar]
- He X., Liu Y.F., Wang W., Zhao H., Yan X.X., Yi Y.Q., et al. Effect of total saponins of Panax notoginseng on expression of GFAP in hippocampus and brain water content in rats subjected global cerebral ischemia injury. Chinese Traditional and Herbal Drugs. 2017;48(22):4695–4700. [Google Scholar]
- Huang H.X., Qu H.B. In-line monitoring of alcohol precipitation by near-infrared spectroscopy in conjunction with multivariate batch modeling. Analytica Chimica Acta. 2011;707(1):47–56. doi: 10.1016/j.aca.2011.09.031. [DOI] [PubMed] [Google Scholar]
- Lau A.J., Toh D.F., Chua T.K., Pang Y.K., Woo S.O. Antiplatelet and anticoagulant effects of Panax notoginseng: Comparison of raw and steamed Panax notoginseng with P. ginseng, and P. quinquefolium. Journal of Ethnopharmacology. 2009;125(3):380–386. doi: 10.1016/j.jep.2009.07.038. [DOI] [PubMed] [Google Scholar]
- Lau Y.T., Ng K.M., Chen N., Lau D.T.W., Ko K.M., Leung P.C. Quality assurance of Chinese herbal medicines: Procedure for multiple-herb extraction. AICHE Journal. 2014;60(12):4014–4026. [Google Scholar]
- Lau Y.T., Ng K.M., Lau D.T.W., Wibowo C. Quality assurance of Chinese herbal medicines: Procedure for single-herb extraction. AICHE Journal. 2013;59(11):4241–4254. [Google Scholar]
- Li C.T., Wang H.B., Xu B.J. A comparative study on anticoagulant activities of three Chinese herbal medicines from the genus Panax and anticoagulant activities of ginsenosides Rg1 and Rg2. Pharmaceutical Biology. 2013;51(8):1077–1080. doi: 10.3109/13880209.2013.775164. [DOI] [PubMed] [Google Scholar]
- Li J., Wang R.F., Yang L., Wang Z.T. Structure and biological action on cardiovascular systems of saponins from Panax notoginseng. China Journal of Chinese Materia Medica. 2015;40(17):3480–3487. [PubMed] [Google Scholar]
- Li Y.Q., Cao H.T., Liu M.Z., Zhang B.Y., Zhang X.L., Shi D.L. Different modulation of Panax notoginseng on the absorption profiling of triptolide and tripterine from Tripterygium wilfordii in rat intestine. Chinese Medicine. 2018;13(1):1. doi: 10.1186/s13020-017-0157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.X., Chen S.L., Xiao X.H., Zhang T.J., Hou W.B., Liao M.L. A new concept on quality marker of Chinese materia medica: Quality control for Chinese medicinal products. Chinese Traditional and Herbal Drugs. 2016;47(9):1443–1457. [Google Scholar]
- Luo Y., Jin C., Zhou J., Wen R.Q., Li X.F., Li R.S. Quality evaluation of artificial musk based on its inhibitory effect on cyclooxygenase-2. Acta Pharmaceutica Sinica. 2011;46(46):438–442. [PubMed] [Google Scholar]
- Qu H.B., Ou D.L., Cheng Y.Y. A new quality control method of traditional Chinese medicine extracts. Chinese Pharmaceutical Journal. 2006;41(1):57–59. [Google Scholar]
- Ren Y.S., Zhang P., Yan D., Wang J.B., Du X.X., Xiao X.H. A strategy for the detection of quality fluctuation of a Chinese herbal injection based on chemical fingerprinting combined with biological fingerprinting. Journal of Pharmaceutical and Biomedical Analysis. 2011;56(2):436–442. doi: 10.1016/j.jpba.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Shen J.J., Gong X.C., Pan J.Y., Qu H.B. Optimization of lime milk precipitation process of Lonicera japonica aqueous extract based on quality by design concept. China Journal of Chinese Materia Medica. 2017;42(6):1074–1082. doi: 10.19540/j.cnki.cjcmm.20170223.013. [DOI] [PubMed] [Google Scholar]
- Shi S.M., Liu Y.Z., Zhao Y.Q., Tai W., Chen C.Q., Zhao Y.Q. Smashing tissue extraction and HPLC determination of active saponins from different parts of notoginseng. Chinese Herbal Medicines. 2012;4(4):340–344. [Google Scholar]
- Si Y.C., Li Q., Xie C., Niu X., Xia X.H., Yu C.Y. Chinese herbs and their active ingredients for activating xue (blood) promote the proliferation and differentiation of neural stem cells and mesenchymal stem cells. Chinese Medicine. 2014;9(1):13. doi: 10.1186/1749-8546-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Huang Z.G., Cao H., Wang Y.T., Hui P., Hoo C. Screening of anti-platelet aggregation agents from Panax notoginseng using human platelet extraction and HPLC–DAD–ESI‐MS/MS. Journal of Separation Science. 2008;31(6–7):1173–1180. doi: 10.1002/jssc.200700507. [DOI] [PubMed] [Google Scholar]
- Wang L.L., Li Z., Zhao X.P., Liu W., Liu Y.F., Yang J.H. A network study of Chinese medicine xuesaitong injection to elucidate a complex mode of action with multicompound, multitarget, and multipathway. Evidence-Based Complementray and Alternative Medicine. 2013;2013(2013):1–8. doi: 10.1155/2013/652373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.X., Zhang Y.J., Zhu X.H., Hu H.F., Wang Y.X., Wu Y., et al. Spray drying technology of shenpu penyan granule based on quality by design concept. Chinese Traditional and Herbal Drugs. 2019;50(6):1334–1340. [Google Scholar]
- Wang Z., Chen Y.Y., Pan H.J., Li W., Wang Y.H., Zeng C.H. Saponin accumulation in flower buds of Panax notoginseng. Chinese Herbal Medicines. 2015;7(2):179–184. [Google Scholar]
- Xiao X.H., Jin C., Yan D., Wang J.B., Yuan H.L., Zhao Y.L. Proposition and practice on “integrative quality” in quality control for Chinese materia medica. Chinese Traditional and Herbal Drugs. 2010;41(4):505–508. [Google Scholar]
- Xiong H.S., Gong X.C., Qu H.B. Monitoring batch-to-batch reproducibility of liquid-liquid extraction process using in-line near-infrared spectroscopy combined with multivariate analysis. Journal of Pharmaceutical and Biomedical Analysis. 2012;70:178–187. doi: 10.1016/j.jpba.2012.06.028. [DOI] [PubMed] [Google Scholar]
- Yan B.J., Chen T., Xu Z.L., Qu H.B. Rapid process development of chromatographic process using direct analysis in real time mass spectrometry as a process analytical technology tool. Journal of Pharmaceutical and Biomedical Analysis. 2014;94:106–110. doi: 10.1016/j.jpba.2014.01.033. [DOI] [PubMed] [Google Scholar]
- Yan B.J., Qu H.B. An approach to optimize the batch mixing process for improving the quality consistency of the products made from traditional Chinese medicines. Journal of Zhejiang Universityence B. 2013;14(11):1041–1048. doi: 10.1631/jzus.B1300059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.H., Yong W., Zhang H.Y., Liang Q.L., Wang Y.M., Luo G.A. Optimization method for blending traditional Chinese medicinal herbs to ensuring the content stability of multiple indicative constituents. Chemical Journal of Chinese Universities. 2007;28(10):1863–1868. [Google Scholar]
- Yang L., Tan J., Wang K., Luo G.S. Mass transfer characteristics of bubbly flow in microchannels. Chemical Engineering Science. 2014;109(16):306–314. [Google Scholar]
- Yao H., Shi P.Y., Shao Q., Fan X.H. Chemical fingerprinting and quantitative analysis of Panax notoginseng preparation using HPLC-UV and HPLC-MS. Chinese Medicine. 2011;6(1):9. doi: 10.1186/1749-8546-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L.X. Pharmaceutical quality by design: Product and process development, understanding, and control. Pharmaceutical Research. 2008;25(4):781–791. doi: 10.1007/s11095-007-9511-1. [DOI] [PubMed] [Google Scholar]
- Yu L.X., Amidon G., Khan M.A., Hoag S.W., Polli J., Raju G.K. Understanding pharmaceutical quality by design. The AAPS Journal. 2014;16(4):771–783. doi: 10.1208/s12248-014-9598-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Yan B.J., Gong X.C., Yu L.X., Qu H.B. Application of quality by design to the process development of botanical drug products: A case study. AAPS PharmSciTech. 2013;14(1):277–286. doi: 10.1208/s12249-012-9919-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.M., Wu J.R., Zhang B. Xuesaitong injection as one adjuvant treatment of acute cerebral infarction: A systematic review and meta-analysis. BMC Complementary and Alternative Medicine. 2015;15(1):36. doi: 10.1186/s12906-015-0560-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W., Chen S., Zhang J., Wang Y.S., Liu A. Which one is more important, raw materials or productive technology?–A case study for quality consistency control of gegen qinlian decoction. China Journal of Chinese Materia Medica. 2016;41(6):1027–1032. doi: 10.4268/cjcmm20160609. [DOI] [PubMed] [Google Scholar]