Abstract
Brusatol, a triterpene lactone compound mainly from Brucea javanica, sensitizes a broad spectrum of cancer cells. It is known as a specific inhibitor of nuclear factor-erythroid 2-related factor 2 (Nrf2) pathway. In this review, we provide a comprehensive overview on the antitumor effect and molecular mechanisms of brusatol in vitro and in vivo. This review also covers pharmacokinetics studies, modification of dosages forms of brusatol. Increasing evidences have validated the value of brusatol as a chemotherapeutic agent in cancers, which may contribute to drug development and clinical application.
Keywords: antitumor, Brucea javanica (L.) Merr., brusatol, triterpene lactone
1. Introduction
Brucea javanica (L.) Merr. (Simaroubaceae), is mainly distributed from South-East Asia to northern Australia. In China, it is mainly found in Fujian, Taiwan, Guangdong, Guangxi, Hainan and Yunnan. Generally, its fruits and leaves can be used as traditional Chinese medicine with cold in property, bitter in prescription, belonging to liver and large intestine channels (Fig. 1). And as traditional Chinese medicine, it is called “Yadanzi” in Chinese, which has heat-clearing and detoxicating and preventing malaria effects. According to the theory of traditional Chinese medicine, tumor initiation is related to pathogenic toxin in vivo. This process can be explained as prolonged retention of phlegm and obstruction of damp leads to their accumulation and stasis in the body, and then it transforms into fire toxin and internal heat accumulation, eventually it forms a lump by degrees (Zhou & Jiang, 2015). Therefore, the heat-clearing and detoxicating effect of “Yadanzi” will contribute to the inhibition of lump or tumor formation. In modern pharmacology, plenty of compounds have been isolated from B. javanica, such as alkaloids, triterpenoids and flavonoids. Among them, triterpene lactones are a research hotspot, known as “quassinoids” (Du et al., 2017, Lahrita et al., 2019). They have extensive bioactivities such as anti-cancer, -malarial, -microbial, and -inflammatory effects. (Li et al., 2019).
Fig. 1.

Plants (fruits and leaves) of B. javanica.
The structure of brusatol is shown in Fig. 2. It includes an α,β-unsaturated cyclo-hexa-none ring (A), two cyclo-hexane rings (B and C), a six-membered lactone ring (D) and tetra-hydro-furan ring (E) which is known as a quassinoid compound. And it is principally obtained from the seeds and fruits of B. javanica. (Li et al., 2019). It was reported that the approximate quantitation of brusatol is 0.3% in fruits of B. javanica, suggesting that it has a high content in botanical which ensures the stability of the source and benefits to further development (Zhou et al., 2011). Since brusatol was first isolated and identified in 1968, intensive researches on it have been subsequently done (Keng et al., 2002). And it has been shown to have a good anti-tumor activity. The patents on brusatol showed it could be used as the chemotherapeutic drug synergist, one of the compositions for the treatment of breast cancer and active multiple myeloma and the drug for resisting tumor angiogenesis (John et al., 2003, Ren et al., 2011a, Yang et al., 2020, Zhu et al., 2017). Moreover, investigating its pharmacophore found that an enone carbonyl oxygen, an enolic oxygen at C-2, C-11 β-hydroxyl group, a C-8 to C-13 or C-8 to C-11 epoxymethano bridge contributes to the activity of the compound (Fukamiya et al., 2005, Hitotsuyanagi et al., 2006, Luyengi et al., 1996).
Fig. 2.
Structure of brusatol.
Compared with other potential anti-cancer compounds, brusatol shows its uniqueness and superiority. As a quassinoid compound, brusatol specifically inhibits Nrf2 signaling pathway, which sensitizes a broad spectrum of cancer cells (Cai, Liu, Han, & Yang, 2019). Meanwhile, it can also enhance radio-sensitivity and reduce chemoresistance. The combination of brusatol and first-line chemotherapeutic agents has preferable effects than using them alone.
With the development of natural products, more and more attention has been paid to the compounds from the plants. In recent years, brusatol has been a hot topic which has good biological activities, and many scholars have been trying to modify its structure and dosage form to improve its effect. We consider that comprehensive information on brusatol may be of concern to readers. Therefore, we provide this review that summarizes the antitumor activities and potential mechanisms of brusatol and discusses some contents related to pharmacokinetic.
2. Antitumor mechanisms of brusatol
2.1. Colorectal cancer
Colorectal cancer is the third most frequently diagnosed cancer in males worldwide while the second in females. The incidence and mortality have been steadily declining for the past years. However, tumors often exhibit resistance in the process of treatment due to intratumor heterogeneity and clonal evolution (Osumi et al., 2019, Torre et al., 2015).
In colorectal cancer, there is substantial evidence to support that brusatol inhibits colorectal cell lines effectively by inhibition of cell viability and proliferation. When the concentration of brusatol is more than 15 nmol/L in treating HCT116 cells, it can reduce cell viability significantly (Lu et al., 2016). In CT26 cells, it also has an excellent effect and its IC50 value is 373 nmol/L (Oh, Kim, Kim, Lee, & Park, 2017). Some studies also showed that PKO, SW480 and COLO205 cell lines are suppressed by brusatol (Evans et al., 2018). In addition, brusatol decreases xenograft and orthotopic tumors growth with 2 mg/kg injection dose in a mouse model in vivo (Evans et al., 2018, Oh et al., 2017). It is noteworthy that Cisplatin and Irinotecan have been used to cure colorectal cancer in a long time, and the combining effect of brusatol and Irinotecan or Cisplatin has been verified to improve the therapeutic effect (Chen et al., 2018, Evans et al., 2018).
The relevant mechanism is described as follows (Fig. 3). HIF-1 (hypoxia-inducible factor-1) is a crucial signaling pathway which is linked to angiogenesis, metastasis, tumor growth, chemoresistance and radio-resistance. Brusatol restrains the expression of c-myc and then decreases mitochondrial ROS and ROS-mediated transition of ferrous iron to its ferric state under hypoxia. And then it promotes HIF-1α degradation by activating PHD to induce colon cancer cells death (Oh et al., 2017). Besides, the results explicated that brusatol suppresses glucose uptake via inhibiting the transactivation function of HIF-1 and downregulating the expression of the well-known HIF-1 target genes, such as VEGF, GLUT1, HK2 and LDHA under hypoxia. Its inhibition of HIF-1 signaling pathway is also correlate with HIF-1α degradation and mitochondrial ROS levels (Lu et al., 2016).
Fig. 3.
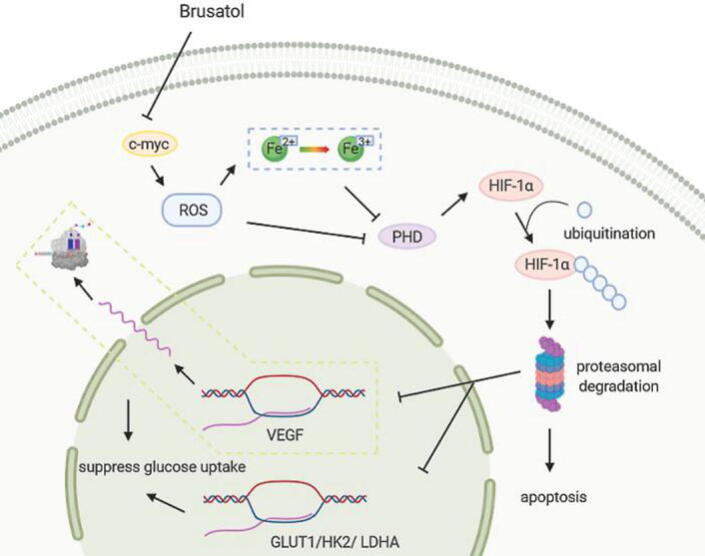
Major mechanisms of brusatol fights colorectal cancer. Brusatol inhibits the expression of c-myc and then decreases mitochondrial ROS and ROS-mediated transition of ferrous iron to its ferric state. Subsequently, HIF-1α degradation is promoted by activating PHD and linked to downregulate the expression of the HIF-1 target genes. Ultimately, it causes glucose uptake suppression and cell death.
2.2. Leukemia
Leukemia is one of the common cancers, statistically, the incidence and mortality of leukemia are 14.6% and 7.4% in more developed areas while 7.6% and 6.3% in less developed areas (Torre et al., 2015).
Related studies displayed that brusatol has strong antiproliferative and cytotoxic effects with low IC50 values in leukemia cell lines. For instance, NB4, BV173, SUPB13 cell lines show that their IC50 values are 0.03, 0.01, 0.04 µmol/L. Furthermore, HL-60, K562, Kasumi-1 and Reh cell lines are less sensitive to brusatol-induced cytotoxicity, however, their proliferation is inhibited primarily by the G1-phase arrest. And it is worth noting that brusatol induces their terminal differentiation effectively. Especially, HL-60 cell line possesses chemo-preventive potential supported by the inhibition of 7,12-dimethylbenz[a]anthracene-induced preneoplastic lesion formation in mouse mammary organ culture. In addition, NB4, U937, BV173, SUPB13, RS4;11, Daudi and DHL-6 cell lines which are extremely sensitive to brusatol, are responsive to brusatol with little induction of differentiation. And it was observed the apoptotic peaks with NB4 and BV173, an arrest in G1 phase with SUPB13, and an arrest in S phase with U937 and RS4;11 cell lines (Cuendet et al., 2004, Liu et al., 2011, Luyengi et al., 1996, Mata-Greenwood et al., 2002, Mata-Greenwood et al., 2001). Moreover, brusatol also shows potent antileukemic activity against P-388 lymphocytic leukemia in mice in vivo with the dose of 0.125 mg/kg (Lee, Hayashi, Okano, Nozaki, & Ju-Ichi, 1984). Additionally, brusatol restrains nucleic acid metabolism though inhibiting DNA polymerase, RNA polymerase, thymidylate synthetase, dihydrofolate reductase, phosphoribosyl pyrophosphate aminotransferase, and cathepsin protease activities and elevates cyclic AMP levels in P-388 cells (Hall et al., 1979). It also has an inhibitory effect on cell respiration via significantly inhibiting P-388 cell hexokinase, phosphofructokinase, malic dehydrogenase, and succinic dehydrogenase and increasing the concentration of reduced mitochondrial electron-transport cofactors (Elgebaly et al., 1979). Besides, inhibiting DNA and protein synthesis are one of the bioactivities of brusatol. Some studies validated that brusatol decreases the activity of peptidyl transferase, and it blocks the elongation step of protein synthesis in P-388 and L-1210 cell lines. This effect also exhibited in some cancerous tissues from the mice. The free 80 s ribosome is the binding site of brusatol in P-388 cells (Hall et al., 1983, Hall et al., 1982, Liou et al., 1982, Willingham et al., 1984).
In leukemic cell lines (such as HL-60), brusatol induces cell differentiation though activation of NF-κB. As shown in this research, brusatol activates NF-κB pathway via p50/p65 heterodimer and gathers phosphorylation of IκBa, which leads to NF-κB translocation from the cytoplasm to the nucleus eventually and induces cell differentiation. The cell differentiation in diverse cell lines above is involved in subsequent expression of various markers of differentiation, such as CD11b, CD13, CD14, and the differentiation effect is irreversible treated by brusatol after 48 h (Cuendet et al., 2004). Furthermore, the expression of c-myc is regulated by brusatol at the post-transcriptional level, which plays a central role in terminal differentiation (Mata-Greenwood et al., 2002).
Cytarabine (Ara-C), Daunorubicin (Dnr) and Arsenic trioxide (ATO) are regarded as the drugs to treat acute myeloid leukemia, but they have produced chemoresistance gradually (Sun et al., 2016). THP1 and U397 cell lines are resistant to Ara-C, Dnr and ATO with high IC50 values. Likewise, it is known that Nrf2 is a key target of chemoresistance (Tao et al., 2014). Hence, brusatol, as an inhibitor of Nrf2, is combined with chemotherapeutic agents to improve the sensitivity of acute myeloid leukemia cells. And at high concentration, brusatol has minimal effects in bringing about early apoptosis in acute myeloid leukemia cells (Karathedath et al., 2017) (Fig. 4).
Fig. 4.

Major mechanisms of brusatol that fights leukemia. NF-κB is activated by brusatol and then translocated to the nucleus to induce cell differentiation. Also, c-myc is regulated at the post-transcriptional level to induce S/G1 arrest and cell differentiation.
2.3. Liver cancer
Liver cancer is more common in men than in women with high incidence and mortality. It is estimated that China alone accounting for about 50% of the new liver cancer cases and deaths worldwide. Hepatocellular carcinoma (HCC) is the most common form of liver cancer whose incidence is increasing, and closely associated with advanced liver disease (Malek et al., 2014, Torre et al., 2015).
Brusatol is capable of inhibiting the growth of hepatocellular carcinoma cells, and its IC50 values were found to be 0.69 µmol/L (Hep3B), 0.34 µmol/L (Huh-7), 12.49 µmol/L (LM3) and 18.04 nmol/L (Bel-7404) (Ye et al., 2018). Likewise, Huh6, Huh7.5, HepG2 and Hepa-1c1c7 cell lines are also suppressed by brusatol at a certain level. What is more, the nude mouse model was injected with brusatol (2 mg/kg), and the burden of Bel7404 xenograft tumor was relieved (Murakami et al., 2018, Olayanju et al., 2015). Some researchers suggested that brusatol triggers apoptosis via endogenous apoptotic pathway in liver cancer cells. Furthermore, cellular autophagy is linked to apoptosis though treating autophagy inhibitor chloroquine (CQ). Besides, brusatol makes a dent in the capacity of cell invasion and migration in hepatocellular carcinoma cells. EMT, as a key process in the metastasis of liver cancer, is also weakened by brusatol (Ye et al., 2018). What is worth mentioning is that combination of brusatol and Sorafenib can yield a better result in tumor treatment (Murakami et al., 2018).
Combating liver cancer with brusatol mainly involves the following signaling pathways. Phosphoinositide 3-kinase (PI3K)/AKT/mTOR signaling pathway is inhibited by brusatol, which leads to cell apoptosis and autophagy (Ye et al., 2018). In Hepa-1c1c7 hepatoma cells, related researches showed that brusatol exerts improving chemoresistance though inhibiting Nrf2 pathway, however, the inhibition is independent of its repressor Keap1, the proteasomal and autophagic protein degradation systems and signaling pathways that are known to modulate Nrf2 activity, such as AKT1/2, JNK1/2, which is beyond expectation. And it may involve a new form of regulation (Olayanju et al., 2015) (Fig. 5).
Fig. 5.

Major mechanisms of brusatol against liver cancer. Brusatol inhibits the PI3K/Akt/mTOR pathway to induce antophagy. CQ, as an autophagy inhibitor, not only inhibits cell autophagy, but also reverses the Brusatol-induced cell apoptosis. Brusatol also regulates EMT with cancer metastasis inhibition.
2.4. Lung cancer
Lung cancer is the leading cause of cancer death in males and females. In addition to known factors such as tobacco, air pollution is also taken into account which may cause lung cancer (Hamra et al., 2014, Torre et al., 2015).
In A549 cells, brusatol has a satisfactory result with IC50 value less than 0.06 µmol/L (Liu et al., 2011, Su et al., 2013). Also, brusatol decreases A549 cells viability with 20% at 20 nmol/L and 45% at 40 nmol/L, and induces apoptosis independent of caspase-3 due to the inhibition of Nrf2 activity (Zhou, Li, Ni, Ding, & Zhong, 2016). Additionally, several studies described the results of inhibiting tumors in vivo with the dose of 2 mg/kg. It was illustrated that at the time of tumors being not initiated, treatment with brusatol cannot prevent initiation of chemically induced lung cancer, whereas post-treatment with brusatol suppresses the progression of chemically and genetically induced lung cancer effectively (Tao, Rojo de la Vega, Chapman, Ooi, & Zhang, 2018).
With the use of clinical drugs, lung cancer has become less sensitive and even resistant to these drugs for the past decades. Multiple studies suggested that brusatol enhances the efficacy of chemotherapy and radio-sensitivity owing to the inhibition of Nrf2 (Sun et al., 2016). Co-treatment brusatol and cisplatin induces apoptosis, inhibits proliferation and suppresses tumor growth in A549 xenografts depending on blocking Nrf2 pathway. It was substantiated that brusatol promotes ubiquitination and degradation of Nrf2 but is not connected to Keap1 in A549 cells. (Ren et al., 2011). Likewise, A549 cells possess serious radioresistant due to the activation of Nrf2 after exposure to irradiation. And it was reported that radiation induces the compensatory increase of Nrf2. Brusatol promotes to downregulate the expression of Nrf2 and accumulate ROS, and then results in DNA damage to reinforce radio-sensitivity. Brusatol combined with irradiation inhibits proliferation of lung cancer cell lines and obtains a better effect of radiotherapy (Sun et al., 2016). Oncogenic KARS mutations activate Nrf2 signaling pathway to promote drug resistance. And the result showed that combinatorial therapy with brusatol and cisplatin overcomes KRAS tumor resistance to cisplatin (Tao et al., 2014). Likewise, some researchers established HCC827GRKU cells which are resistant to gefitinib, and cross-resistance to epidermal growth factor receptor tyrosine kinase inhibitors, Afatinib and Osimertinib. By proving experimentation, brusatol suppresses tumorigenicity, migration and invasion which is correlated with Nrf2 inhibition (Park et al., 2018).
It was also found that brusatol can be used as a good protein synthesis inhibitor. Brusatol induces cytotoxicity or overcomes chemical resistance by inhibiting protein translation. Its mechanism is that brusatol downregulating expression levels of majority of detected proteins, and the short half-life proteins, such as Nrf2, has the most obvious effect in A549 cells. Also, brusatol inhibits cap-dependent and cap-independent translation, but it is independent of Keap1/Nrf2 activity, which is similar to other translation inhibitors such as silvestrol in A549 cells. (Harder et al., 2017, Vartanian et al., 2016).
2.5. Pancreatic cancer
Pancreatic cancer is one of the fatal malignancies which is common in men and has an aggressive course. Unfortunately, it is difficult to detect owing to asymptomatic characteristic (Goral, 2015).
Brusatol has an antitumor activity with IC50 values of 0.36 µmol/L on PANC-1 and 0.10 µmol/L on SW1990 pancreatic cancer cell lines (Zhao, Lau, Leung, Che, & Lin, 2011). Brusatol is clarified to suppress growth and induce apoptosis in PANC-1 and PATU-8988 cell lines. And brusatol inhibits the cell proliferation in time- and concentration-dependent manner. Afterwards, the induction of apoptosis is attached to the endogenous apoptotic pathway. Thereafter, it suggests that brusatol activates JNK and p38 MAPK pathways while inhibits NF-κB and STAT3 pathways (Xiang et al., 2017). Pancreatic cancer, as it is known, is high resistant to chemotherapeutic agents. Compared to the first-line chemotherapeutic agents gemcitabine (GEM) and 5-fluorouracil (5-Fu), brusatol was validated to possess the similar effects. As a matter of fact, the effect of the combined treatment exceeds that of single-agent brusatol. More detailed mechanisms were substantiated that brusatol causes cell cycle arrest at G2/M phase and accentuates apoptosis, abrogates GEM- and 5-Fu-induced activation of NF-κB, suppresses EMT process and reverses brusatol-induced Nrf2 pathway activation with brusatol monotherapy or combination treatment. In addition, it was reported that the results in vivo are in keep with in vitro (Lu et al., 2017, Xiang et al., 2018) (Fig. 6).
Fig. 6.

Major mechanisms of brusatol against pancreatic cancer. Brusatol activates JNK/P38 MAPK pathway and inhibits NF-κB/STAT3 pathway to induce apoptosis. And JNK/P38 MAPK inhibitors restrain the inactivation of NF-κB/STAT3 pathway caused by brusatol.
2.6. Other cancers
2.6.1. Melanoma
Brusatol with low-dose Ultraviolet A (UVA) is more effective than treat with them alone in melanoma A357 cells. In vivo, brusatol combined with UVA also reduces melanoma-derived tumors burden. Its mechanisms have been certified that combination treatment boosts ROS generation and inhibits A375 cell growth and proliferation by regulating cell cycle related protein to result in G1 phase arrest. Moreover, co-treatment blocks AKT-Nrf2 signaling pathway and triggers cell apoptosis (Wang et al., 2018).
Multiple myeloma: Brusatol triggers the increase of oxidative stress and compromises multiple myeloma cell viability and proliferation. Also, brusatol combined with pyocyanin brings a better result in MM1S and U266 cell lines (Liu, Tuckett, Fennell, Garippa, & Zakrzewski, 2018).
2.6.2. Ovarian cancer
There are related studies in regard to the effect of brusatol on ovarian cancer. Nrf2 induces cisplatin resistance by suppressing the iron export related gene SLC40A1, as a consequence, brusatol along with Desferal (an iron chelating agent) ameliorates cisplatin resistance (Wu, Bao, Zhang, & Yi, 2017).
2.6.3. Endometrial cancer
Tamoxifen, as an agent to cure hormone receptor positive breast cancer, was found it unfortunate to cause side effect. It was reported to induce endometrial cancer. Brusatol selectively inhibits Nrf2 pathway and retards upregulation of SQSTM1 to prevent Tamoxifen-induced endometrial cancer. Additionally, combination of brusatol and metformin overcomes progestin resistance (a main obstacle in endometrial cancer therapy), though downregulating Nrf2/AKR1C1 in endometrial cancer (Fan et al., 2017, Feng et al., 2017).
2.6.4. Breast cancer
Brusatol demonstrates significant inhibitory activities against breast cancer MCF-7 cells and its IC50 value is 0.08 µmol/L. Besides, brusatol improves the sensitivity to Taxol as a result of suppressing Nrf2 pathway and enhancing ROS levels in MCF-7 and MDA-MB-231 breast cancer cell lines (Liu et al., 2011, Wu et al., 2015).
2.6.5. Nasopharyngeal carcinoma
Brusatol is also used to assess the cytotoxicity against KB cells derived from a human epidermoid carcinoma of the nasopharynx in vivo. It was found that brusatol has a positive effect on KB cells by measuring its IC50 value 0.20 µmol/L (Anderson, O'Neill, Phillipson, & Warhurst, 1991).
2.6.6. Glioma
It is common to observe mutations in genes encoding isocitrate dehydrogenases (IDHs) 1 and 2 in cancer. In gliomas, the vast majority of patients have IDH1 and IDH2 gene mutations. The evidence suggested that brusatol has inhibitory effect on IDH1-mutated U87, U251 and MGG152 cells, and IC50 value of IDH1-mutated U251 cells is about 20 nmol/L. Meanwhile, it was confirmed that brusatol can induce apoptosis and inhibit proliferation of IDH1-mutated cells and tumor xenografts (Liu et al., 2019, Tang et al., 2020).
Due to activation of the Nrf2 pathway, IDH1-mutated cells promote the glutathione (GSH) synthesis and scavenging of ROS to enhance cell proliferation. Brusatol blocks the Nrf2 pathway to suppress GSH synthesis, accumulate ROS and eventually results in cell damages. In addition, some researches substantiate that accumulating Amyloid-β (Aβ) in gliomas causes neurotoxicity, brusatol can ameliorate it by Nrf2/HO-1 pathway, which contributes to treat glioma (Liu et al., 2019, Liu et al., 2019, Tang et al., 2020).
2.6.7. Head and neck squamous cell carcinoma
When applied to head and neck squamous cell carcinoma cells, brusatol can decrease cell viability significantly with IC50 values 24, 38, 16, 14, 6, 22, 25 and 20 nmol/L on UMSCC47, UDSCC2, JMAR, TU167, LN686, YD-10B, HN-9 and FaDu cell lines, respectively. From the study, brusatol inhibits metastasis, angiogenesis, proliferation and apoptosis effectually. Related molecular mechanisms have been shown that brusatol blocks STAT3 by interfering with its phosphorylation and formation of dimers and its upstream kinase, furthermore, inhibits the ability of STAT3 to bind DNA (Lee et al., 2019) (Fig. 7, Table 1).
Fig. 7.
A brief summary of effects of erythromycin on major cancers.
Table 1.
In vitro effects of brusatol on cell growth of various cell lines.
| Tumor types | Cell lines | Cytotoxicity/(µmol·L−1) |
|---|---|---|
| Colorectal cancer | CT26 | 0.26* |
| HCT116 | 0.09* | |
| SW480 | 0.10* | |
| Leukemia | HL-60 | 0.10 (96 h) |
| Kasumi-1 | 0.14 (96 h) | |
| NB4 | 0.03 (96 h) | |
| U937 | 0.02 (96 h) | |
| BV173 | 0.01 (96 h) | |
| SUPB13 | 0.04 (96 h) | |
| RS4;11 | 0.05 (96 h) | |
| Reh | 0.19 (96 h) | |
| Daudi | 0.01 (96 h) | |
| DHL-6 | 0.01 (96 h) | |
| Liver cancer | Hep3B | 0.69 (48 h) |
| Huh7 | 0.34 (48 h) | |
| LM3 | 12.49 (48 h) | |
| Bel7404 | 0.02 (48 h) | |
| SMMC-7721 | < 0.07* | |
| Lung cancer | A-549 | < 0.07* |
| Pancreatic cancer | PANC-1 | 0.36 (72 h) |
| SW1990 | 0.10 (72 h) | |
| Breast cancer | MCF-7 | 0.08* |
| Nasopharyngeal carcinoma | KB | 0.20 (48 h) |
| Head and neck squamous cell carcinoma | UMSCC47 | 0.02 (24 h) |
| UDSCC2 | 0.04 (24 h) | |
| JMAR | 0.02 (24 h) | |
| TU167 | 0.01 (24 h) | |
| LN686 | 0.01 (24 h) | |
| YD-10B | 0.02 (24 h) | |
| HN-9 | 0.03 (24 h) | |
| FaDu | 0.02 (24 h) |
Effective time is not given.
3. Pharmacokinetics
Considerable efforts have been devoted to the studies on antitumor activity of brusatol. Hence, a pharmacokinetic and distribution study is essential for purpose of in-depth researches and broad applications.
Groups of researchers investigated that the plasma concentration of brusatol decreases rapidly with the half-life time in 10 min. In organs, brusatol is distributed in liver, kidney, lung and spleen, and its concentration can peak in 15–30 min and decline tardily. Apart from the constant peak concentration in the liver, its concentration peaks in other tissues in a dose-dependent manner. Of concern is that brusatol in lung is 10 folds or higher than that in other organs, which informs that brusatol may target at lung. Unfortunately, brusatol cannot cross the blood–brain barrier (Guo et al., 2017, Zhang et al., 2016). Subsequently, excretion of brusatol in rats has been investigated, suggesting that average cumulative excretion rate in urine is 5.82% during 24 h, 0.71% in bile during 12 h and the rapid drop in plasma concentration. All the plasma/urine/bile/feces samples were collected in a tube to analysis metabolites. The results substantiated that brusatol has four metabolites, and according to their structures, hydroxylation, hydrolysis and glucuronidation is dominating metabolic pathways in vivo (Guo et al., 2018). In view of the short half-life and distribution of brusatol, multiple administration may be considered, but at the same time, attention should be paid to its potential accumulation in the lung and even toxic effects. We should also take into account the pharmacological activity of its metabolites. But there is no relevant literature on these problems until now, we infer that the metabolites of brusatol have similar biological activity to brusatol due to the good effect but short half-life of brusatol. These problems could be the subject of research in the future (Fig. 8).
Fig. 8.
Abridged general view of main pharmacokinetics studies. Brusatol was injected intravenously into mice at different doses (1.0 mg/kg; 1.5 mg/kg; 2.0 mg/kg), and the distribution of brusatol in liver, spleen, kidney and lung was analyzed. It was found that the highest concentration of brusatol is in lung and it may target at lung. Brusatol was injected into rats at 1.0 mg/kg to analyze the compound content in the plasma, bile, urine and feces, and the metabolites isolated from them.
4. Efficacy enhancement of brusatol
It was demonstrated in a number of studies that brusatol exhibits excellent activities on inhibition of proliferation and improvement of chemoresistance. Paradoxically, the low aqueous solubility and insufficient intracellular delivery of brusatol limits its potential application seriously. Hence, taking advantages of Pluronics as drug carriers and tumor microenvironment-responsive drug release profiles synthesizes redox-sensitive micelles composed of disulfdelinked Pluronic-linoleic acid (F68-SS-LA). Brusatol is loaded in micelles (Bru/SS-M) which has the advantages of high loading efficiency, narrow size distribution, and excellent storage stability. As a result, the fast-micellar destabilization and drug release can enhance cellular uptake and then improve intracellular concentration of brusatol in a high reduction environment. Pharmacological researches testify that Bru/SS-M enhances the effect of antitumor which is associated with the loss of mitochondrial membrane potential, intracellular ROS generation and upregulate of apoptosis rate in Bel-7402 and MCF-7 cell lines (Zhang et al., 2018). Also, for improving the poor aqueous solubility and rapid first-pass metabolism after oral administration of brusatol, a novel brusatol self-microemulsifying drug delivery system (BR-SMEDDS) is developed in order to acquire a better effect, which possesses smaller size, higher negative zeta potential and drug quantity, and excellent stability (Zhou et al., 2017, Zhou et al., 2018).
5. Conclusion
Brusatol, as monotherapy or in combination with conventional chemotherapy drugs, has beneficial effects in the treatment of different cancers. And it is a great promising natural compound for clinic treatment. It is worthy to make efforts on the study of enhancing the development of brusatol as a new drug. Effectiveness of brusatol prevents the different development stages of human cancers such as proliferation, migration. Likewise, it was also elucidated to play an antitumor role and ameliorate chemoresistance or radio-resistance by regulating varies signaling pathway such as AKT/mTOR/Nrf2 pathways. Pharmacokinetic studies have demonstrated that brusatol can be distributed in multiple tissues, which is the strong evidence of its potential in treating cancers in vivo. There are also studies on dosage form modification to improve its aqueous solubility and reduce the first-pass effect, ultimately, to improve its efficacy in vivo. In addition, what counts is that the problems of the plasma concentrations declining rapidly and the poor aqueous solubility about brusatol need to be solved. It should not be ignored that brusatol also requires more detailed pharmacokinetic studies to determine whether it can reach effective concentrations in the cancerous sites of human bodies. Dosage or structural modification of brusatol also needs to be studied further to achieve the breakthrough of the above barriers and better efficacy. It is equally important to explore the related mechanisms against cancers incessantly. Then, adequate clinical trials are expected before being applied to cure cancers.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research work was supported by National Natural Science Foundation of China (81872767).
Contributor Information
Guo-dong Yao, Email: guodong_yao@126.com.
Shao-jiang Song, Email: songsj99@163.com.
References
- Anderson M.M., O'Neill M.J., Phillipson J.D., Warhurst D.C. In vitro cytotoxicity of a series of quassinoids from Brucea javanica fruits against KB cells. Planta Medica. 1991;57(1):62–64. doi: 10.1055/s-2006-960020. [DOI] [PubMed] [Google Scholar]
- Cai S.J., Liu Y., Han S., Yang C. Brusatol, an NRF2 inhibitor for future cancer therapeutic. Cell & Bioscience. 2019;9:45. doi: 10.1186/s13578-019-0309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.M., Lai Z.Q., Liao H.J., Xie J.H., Xian Y.F., Su Z.R., et al. Synergistic antitumor effect of brusatol combined with cisplatin on colorectal cancer cells. International Journal of Molecular Medicine. 2018;41(3):1447–1454. doi: 10.3892/ijmm.2018.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuendet M., Gills J.J., Pezzuto J.M. Brusatol-induced HL-60 cell differentiation involves NF-kappaB activation. Cancer Letters. 2004;206(1):43–50. doi: 10.1016/j.canlet.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Du Y.Q., Cheng Z.Y., Zhao P., Huang X.X., Song S.J. Research review on the main chemical components of Brucea javanica (L.) Merr. Asian Journal of Traditional Medicines. 2017;12(6):240–249. [Google Scholar]
- Elgebaly S.A., Hall I.H., Lee K.H., Sumida Y., Imakura Y., Wu R.Y. Antitumor agents XXXV: Effects of brusatol, bruceoside A, and bruceantin on P-388 lymphocytic leukemia cell respiration. Journal of Pharmaceutical Sciences. 1979;68(7):887–890. doi: 10.1002/jps.2600680727. [DOI] [PubMed] [Google Scholar]
- Evans J.P., Winiarski B.K., Sutton P.A., Jones R.P., Ressel L., Kitteringham N.R., et al. The Nrf2 inhibitor brusatol is a potent antitumour agent in an orthotopic mouse model of colorectal cancer. Oncotarget. 2018;9(43):27104–27116. doi: 10.18632/oncotarget.25497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan R., Wang Y., Wang Y., Li W., Zheng W. Mechanism of progestin resistance in endometrial precancer/cancer through Nrf2-survivin pathway. American Journal of Translational Research. 2017;9(3):1483–1491. [PMC free article] [PubMed] [Google Scholar]
- Feng L., Li J., Yang L., Zhu L., Huang X., Jin H., et al. Tamoxifen activates Nrf2-dependent SQSTM1 transcription to promote endometrial hyperplasia. Theranostics. 2017;7(7):1890–1900. doi: 10.7150/thno.19135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukamiya N., Lee K.H., Muhammad I., Murakami C., Okano M., Pelletier J., et al. Structure-activity relationships of quassinoids for eukaryotic protein synthesis. Cancer Letters. 2005;220(1):37–48. doi: 10.1016/j.canlet.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Goral V. Pancreatic cancer: Pathogenesis and diagnosis. Asian Pacific Journal of Cancer Prevention. 2015;16(14):5619–5624. doi: 10.7314/apjcp.2015.16.14.5619. [DOI] [PubMed] [Google Scholar]
- Guo N., Xu X., Yuan G., Chen X., Wen Q., Guo R. Pharmacokinetic, metabolic profiling and elimination of brusatol in rats. Biomedical Chromatography. 2018;32(12) doi: 10.1002/bmc.4358. [DOI] [PubMed] [Google Scholar]
- Guo N., Zhang X., Bu F., Wang L., Cao Z., Wen Q., et al. Determination of brusatol in plasma and tissues by LC-MS method and its application to a pharmacokinetic and distribution study in mice. Journal of Chromatography B. 2017;1053:20–26. doi: 10.1016/j.jchromb.2017.04.012. [DOI] [PubMed] [Google Scholar]
- Hall I.H., Lee K.H., Elgebaly S.A., Imakura Y., Sumida Y., Wu R.Y. Antitumor agents XXXIV: Mechanism of action of bruceoside a and brusatol on nucleic acid metabolism of P-388 lymphocytic leukemia cells. Journal of Pharmaceutical Sciences. 1979;68(7):883–887. doi: 10.1002/jps.2600680726. [DOI] [PubMed] [Google Scholar]
- Hall I.H., Liou Y.F., Lee K.H., Chaney S.G., Willingham W.J. Antitumor agents LIX: Effects of quassinoids on protein synthesis of a number of murine tumors and normal cells. Journal of Pharmaceutical Sciences. 1983;72(6):626–630. doi: 10.1002/jps.2600720612. [DOI] [PubMed] [Google Scholar]
- Hall I.H., Liou Y.F., Okano M., Lee K.H. Antitumor agents XLVI: In vitro effects of esters of brusatol, bisbrusatol, and related compounds on nucleic acid and protein synthesis of P-388 lymphocytic leukemia cells. Journal of Pharmaceutical Sciences. 1982;71(3):345–348. doi: 10.1002/jps.2600710321. [DOI] [PubMed] [Google Scholar]
- Hamra G.B., Guha N., Cohen A., Laden F., Raaschou-Nielsen O., Samet J.M., et al. Outdoor particulate matter exposure and lung cancer: A systematic review and meta-analysis. Environmental Health Perspectives. 2014;122(9):906–911. doi: 10.1289/ehp/1408092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder B., Tian W., La Clair J.J., Tan A.C., Ooi A., Zhang D.D., et al. Brusatol overcomes chemoresistance through inhibition of protein translation. Molecular Carcinogenesis. 2017;56(5):1493–1500. doi: 10.1002/mc.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitotsuyanagi Y., Kim I.H., Hasuda T., Yamauchi Y., Takeya K. A structure–activity relationship study of brusatol, an antitumor quassinoid. Tetrahedron. 2006;62(17):4262–4271. [Google Scholar]
- John, M. P., James M.D., Muriel C. A., & Lawrence H. (2003). Cancer chemopreventative compounds and compositions and methods of treating cancers. Patent of the United States of America, US2003149096A1. 2003-08-07.
- Karathedath S., Rajamani B.M., Musheer Aalam S.M., Abraham A., Varatharajan S., Balasubramanian P., et al. Role of NF-E2 related factor 2 (Nrf2) on chemotherapy resistance in acute myeloid leukemia (AML) and the effect of pharmacological inhibition of Nrf2. PLoS One. 2017;12(5) doi: 10.1371/journal.pone.0177227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keng Y.S., James J.S., Geissman T.A. Constituents of Brucea sumatrana Roxb.Ⅰ. Brusatol. The Journal of Organic Chemistry. 2002;33(1):429–431. [Google Scholar]
- Lahrita L., Moriai K., Iwata R., Itoh K., Kato E. Quassinoids in Brucea javanica are potent stimulators of lipolysis in adipocytes. Fitoterapia. 2019;137 doi: 10.1016/j.fitote.2019.104250. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Rangappa S., Mohan C.D., Basappa S.G., Lin Z.X., Ahn K.S. Brusatol, a Nrf2 inhibitor targets STAT3 signaling cascade in head and neck squamous cell carcinoma. Biomolecules. 2019;9(10):550. doi: 10.3390/biom9100550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.H., Hayashi N., Okano M., Nozaki H., Ju-Ichi M. Antitumor agents, 65. Brusatol and cleomiscosin-A, antileukemic principles from Brucea javanica. Journal of Natural Products. 1984;47(3):550–551. doi: 10.1021/np50033a030. [DOI] [PubMed] [Google Scholar]
- Li Z., Ruan J.Y., Sun F., Yan J.J., Wang J.L., Wang T., et al. Relationship between structural characteristics and plant sources along with pharmacology research of quassinoids. Chemical & Pharmaceutical Bulletin. 2019;67(7):654–665. doi: 10.1248/cpb.c18-00958. [DOI] [PubMed] [Google Scholar]
- Liou Y.F., Hall I.H., Okano M., Lee K.H., Chaney S.G. Antitumor agents XLVIII: Structure-activity relationships of quassinoids as in vitro protein synthesis inhibitors of P-388 lymphocytic leukemia tumor cell metabolism. Journal of Pharmaceutical Sciences. 1982;71(4):430–435. doi: 10.1002/jps.2600710414. [DOI] [PubMed] [Google Scholar]
- Liu H.Y., Tuckett A.Z., Fennell M., Garippa R., Zakrzewski J.L. Repurposing of the CDK inhibitor PHA-767491 as a NRF2 inhibitor drug candidate for cancer therapy via redox modulation. Investigational New Drugs. 2018;36(4):590–600. doi: 10.1007/s10637-017-0557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.Q., Wang C.F., Li X.Y., Chen J.C., Li Y., Qiu M.H. One new pregnane glycoside from the seeds of cultivated Brucea javanica. Archives of Pharmacal Research. 2011;34(8):1297–1300. doi: 10.1007/s12272-011-0809-5. [DOI] [PubMed] [Google Scholar]
- Liu X., Xu H., Zhang Y., Wang P., Gao W. Brusatol inhibits amyloid-beta-induced neurotoxicity in U-251 cells via regulating the Nrf2/HO-1 pathway. Journal of Cellular Biochemistry. 2019;120(6):10556–10563. doi: 10.1002/jcb.28341. [DOI] [PubMed] [Google Scholar]
- Liu Y., Lu Y., Celiku O., Li A., Wu Q., Yang C., et al. Targeting IDH1-mutated malignancies with NRF2 blockade. Journal of the National Cancer Institute. 2019;111(10):1033–1041. doi: 10.1093/jnci/djy230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Wang B., Shi Q., Wang X., Wang D., Zhu L. Brusatol inhibits HIF-1 signaling pathway and suppresses glucose uptake under hypoxic conditions in HCT116 cells. Scientific Reports. 2016;6:39123. doi: 10.1038/srep39123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Lai Z.Q., Leung A.W.N., Leung P.S., Li Z.S., Lin Z.X. Exploring brusatol as a new anti-pancreatic cancer adjuvant: Biological evaluation and mechanistic studies. Oncotarget. 2017;8(49):84974–84985. doi: 10.18632/oncotarget.17761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyengi L., Suh N., Fong H.H.S., Pezzuto J.M., Kinghorn A.D. A lignan and four terpenoids from Brucea javanica that induce differentiation with cultured HL-60 promyelocytic leukemia cells. Phytochemistry. 1996;43(2):409–412. doi: 10.1016/0031-9422(96)00258-0. [DOI] [PubMed] [Google Scholar]
- Malek N.P., Schmidt S., Huber P., Manns M.P., Greten T.F. The diagnosis and treatment of hepatocellular carcinoma. Deutsches Arzteblatt International. 2014;111(7):101–106. doi: 10.3238/arztebl.2014.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata-Greenwood E., Cuendet M., Sher D., Gustin D., Stock W., Pezzuto J.M. Brusatol-mediated induction of leukemic cell differentiation and G (1) arrest is associated with down-regulation of c-myc. Leukemia. 2002;16(11):2275–2284. doi: 10.1038/sj.leu.2402696. [DOI] [PubMed] [Google Scholar]
- Mata-Greenwood E., Daeuble J.F., Grieco P.A., Dou J., McChesney J.D., Pezzuto J.M., et al. Novel esters of glaucarubolone as inducers of terminal differentiation of promyelocytic HL-60 cells and inhibitors of 7,12-dimethylbenz[a]anthracene-induced preneoplastic lesion formation in mouse mammary organ culture. Journal of Natural Products. 2001;64(12):1509–1513. doi: 10.1021/np010212p. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Sugiyama K., Ebinuma H., Nakamoto N., Ojiro K., Saito H., et al. Dual effects of the Nrf2 inhibitor for inhibition of hepatitis C virus and hepatic cancer cells. BMC Cancer. 2018;18(1):680. doi: 10.1186/s12885-018-4588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E.T., Kim C.W., Kim H.G., Lee J.S., Park H.J. Brusatol-mediated inhibition of c-Myc increases HIF-1alpha degradation and causes cell death in colorectal cancer under hypoxia. Theranostics. 2017;7(14):3415–3431. doi: 10.7150/thno.20861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olayanju A., Copple I.M., Bryan H.K., Edge G.T., Sison R.L., Park B.K., et al. Brusatol provokes a rapid and transient inhibition of Nrf2 signaling and sensitizes mammalian cells to chemical toxicity-implications for therapeutic targeting of Nrf2. Free Radical Biology & Medicine. 2015;78:202–212. doi: 10.1016/j.freeradbiomed.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osumi H., Shinozaki E., Yamaguchi K., Zembutsu H. Clinical utility of circulating tumor DNA for colorectal cancer. Cancer Science. 2019;110(4):1148–1155. doi: 10.1111/cas.13972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.H., Kim J.H., Ko E., Kim J.Y., Park M.J., Lee J.Y., et al. Resistance to gefitinib and cross-resistance to irreversible EGFR-TKIs mediated by disruption of the Keap1-Nrf2 pathway in human lung cancer cells. FASEB Journal. 2018 doi: 10.1096/fj.201800011R. fj201800011R. [DOI] [PubMed] [Google Scholar]
- Ren D., Villeneuve N.F., Jiang T., Wu T., Lau A., Zhang D.D., et al. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(4):1433–1438. doi: 10.1073/pnas.1014275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, D. M., Zhang, T. N., Wang, L. M., & Yang, S. (2011a). Application of brusatol as chemotherapeutic drug synergist. Patent of China, CN102106851A. 2011-06-29.
- Su Z., Hao J., Xu Z., Huang R., Zhang N., Qiu S. A new quassinoid from fruits of Brucea javanica. Natural Product Research. 2013;27(21):2016–2021. doi: 10.1080/14786419.2013.821119. [DOI] [PubMed] [Google Scholar]
- Sun X., Wang Q., Wang Y., Du L., Xu C., Liu Q. Brusatol enhances the radiosensitivity of A549 cells by promoting ROS production and enhancing DNA damage. International Journal of Molecular Sciences. 2016;17(7) doi: 10.3390/ijms17070997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Fu X., Liu Y., Yu D., Cai S.J., Yang C. Blockade of glutathione metabolism in IDH1-mutated glioma. Molecular Cancer Therapeutics. 2020;19(1):221–230. doi: 10.1158/1535-7163.MCT-19-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao S., Rojo M., Chapman E., Ooi A., Zhang D.D. The effects of NRF2 modulation on the initiation and progression of chemically and genetically induced lung cancer. Molecular Carcinogenesis. 2018;57(2):182–192. doi: 10.1002/mc.22745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao S., Wang S., Moghaddam S.J., Ooi A., Chapman E., Zhang D.D., et al. Oncogenic KRAS confers chemoresistance by upregulating NRF2. Cancer Research. 2014;74(24):7430–7441. doi: 10.1158/0008-5472.CAN-14-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- Vartanian S., Ma T.P., Lee J., Haverty P.M., Kirkpatrick D.S., Stokoe D., et al. Application of mass spectrometry profiling to establish brusatol as an inhibitor of global protein synthesis. Molecular & Cellular Proteomics. 2016;15(4):1220–1231. doi: 10.1074/mcp.M115.055509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Shi G., Bian C., Nisar M.F., Guo Y., Zhong J.L., et al. UVA irradiation enhances brusatol-mediated inhibition of melanoma growth by downregulation of the Nrf2-mediated antioxidant response. Oxidative Medicine and Cellular Longevity. 2018;2018:9742154. doi: 10.1155/2018/9742154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham W., Tobias Considine R., Chaney S.G., Lee K.-H., Hall I.H. Reversibility of protein synthesis inhibition by quassinoid antineoplastic agents in a rabbit reticulocyte system. Biochemical Pharmacology. 1984;33(2):330–333. doi: 10.1016/0006-2952(84)90494-5. [DOI] [PubMed] [Google Scholar]
- Wu J., Bao L., Zhang Z., Yi X. Nrf2 induces cisplatin resistance via suppressing the iron export related gene SLC40A1 in ovarian cancer cells. Oncotarget. 2017;8(55):93502–93515. doi: 10.18632/oncotarget.19548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Harder B.G., Wong P.K., Lang J.E., Zhang D.D. Oxidative stress, mammospheres and Nrf2-new implication for breast cancer therapy? Molecular Carcinogenesis. 2015;54(11):1494–1502. doi: 10.1002/mc.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y., Ye W., Huang C., Lou B., Zhang J., Zhou M., et al. Brusatol inhibits growth and induces apoptosis in pancreatic cancer cells via JNK/p38 MAPK/NF-kappab/Stat3/Bcl-2 signaling pathway. Biochemical and Biophysical Research Communications. 2017;487(4):820–826. doi: 10.1016/j.bbrc.2017.04.133. [DOI] [PubMed] [Google Scholar]
- Xiang Y., Ye W., Huang C., Yu D., Chen H., Zhou M., et al. Brusatol enhances the chemotherapy efficacy of gemcitabine in pancreatic cancer via the Nrf2 signalling pathway. Oxidative Medicine and Cellular Longevity. 2018;2018:2360427. doi: 10.1155/2018/2360427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y., Tian Z. Y., Huang J., Chen J. H., & Zhang Q. Y. (2020). The drugs or compositions of treating breast cancer. Patent of China, CN110935018A. 2020-03-31.
- Ye R., Dai N., He Q., Guo P., Xiang Y., Zhang Q., et al. Comprehensive anti-tumor effect of brusatol through inhibition of cell viability and promotion of apoptosis caused by autophagy via the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Biomedicine & Pharmacotherapy. 2018;105:962–973. doi: 10.1016/j.biopha.2018.06.065. [DOI] [PubMed] [Google Scholar]
- Zhang J., Fang X., Li Z., Chan H.F., Lin Z., Chen M., et al. Redox-sensitive micelles composed of disulfide-linked Pluronic-linoleic acid for enhanced anticancer efficiency of brusatol. International Journal of Nanomedicine. 2018;13:939–956. doi: 10.2147/IJN.S130696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Yuan Y., Cui J., Xiao T., Deng Z., Jiang D. Determination of a potential antitumor quassinoid in rat plasma by UPLC-MS/MS and its application in a pharmacokinetic study. Journal of Pharmaceutical and Biomedical Analysis. 2016;124:143–148. doi: 10.1016/j.jpba.2016.02.046. [DOI] [PubMed] [Google Scholar]
- Zhao M., Lau S.T., Leung P.S., Che C.T., Lin Z.X. Seven quassinoids from Fructus Bruceae with cytotoxic effects on pancreatic adenocarcinoma cell lines. Phytotherapy Research. 2011;25(12):1796–1800. doi: 10.1002/ptr.3477. [DOI] [PubMed] [Google Scholar]
- Zhou J., Tan L., Xie J., Lai Z., Huang Y., Xie Y., et al. Characterization of brusatol self-microemulsifying drug delivery system and its therapeutic effect against dextran sodium sulfate-induced ulcerative colitis in mice. Drug Delivery. 2017;24(1):1667–1679. doi: 10.1080/10717544.2017.1384521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Wang T., Dou Y., Huang Y., Qu C., Su Z., et al. Brusatol ameliorates 2, 4, 6-trinitrobenzenesulfonic acid-induced experimental colitis in rats: Involvement of NF-kappaB pathway and NLRP3 inflammasome. International Immunopharmacology. 2018;64:264–274. doi: 10.1016/j.intimp.2018.09.008. [DOI] [PubMed] [Google Scholar]
- Zhou X., Jiang D. Academic experience of Longjiang Medicine School Founder GAO Zhongshan's treatment of tumor. Liaoning Journal of Traditional Chinese Medicine. 2015;42(8):1411–1413. [Google Scholar]
- Zhou Y., Li Y., Ni H.M., Ding W.X., Zhong H. Nrf2 but not autophagy inhibition is associated with the survival of wild-type epidermal growth factor receptor non-small cell lung cancer cells. Toxicology and Applied Pharmacology. 2016;310:140–149. doi: 10.1016/j.taap.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.L., Shi R.B., Liu B., Zou J.M., Wang L.S., Xia J.M. Quantitative determination of contents of three components in Brucea javanica by HPLC. China Journal of Chinese Materia Medica. 2011;36(14):1979–1981. [PubMed] [Google Scholar]
- Zhu L., Lu Y. P., Wang D., Zhao Y., & Wang X. T. (2017). Use of brusatol in preparation of drugs for resisting tumor angiogenesis. Patent of China, CN106551926A. 2017-04-15.





