Abstract
Objective
Mitochondrial dysfunction is evident in the early stage of Alzheimer’s disease (AD). Therefore development of drugs that protect mitochondrial function is a promising strategy for AD. The present work was to investigate the effects of 2, 3, 5, 4′-Tetrahydroxystilbene-2-O-β-d-glucosides (TSG) on a mitochondrial dysfunction cell model induced by sodium azide and elucidate the underlying mechanisms.
Methods
Mitochondrial membrane potential (MMP) was detected by a fluorescence method. Cellular adenosine triphosphate (ATP) level was measured using a firefly luciferase-based kit. Reactive oxygen species (ROS) was detected using dichlorofluorescin diacetate (DCFH-DA). The expression levels of Bcl-2 and Bax were measured by Western blotting assay. Flow cytometry was utilized to measure apoptosis.
Results
Pretreatment of TSG (25–200 μmol/L) for 24 h significantly elevated MMP and ATP content, reduced ROS level and Bax/Bcl-2 ratio, and inhibited apoptosis in SH-SY5Y cells exposed to sodium azide.
Conclusion
These results suggest that TSG protects SH-SY5Y cells against sodium azide-induced mitochondrial dysfunction and apoptosis. These findings are helpful to understand the protective effect of TSG on mitochondria, which are involved in the early stage of AD.
Keywords: Alzheimer’s disease, mitochondria, tetrahydroxystilbene glucoside, sodium azide
1. Introduction
Alzheimer's disease (AD) is recognized by the World Health Organization as a global public health priority (Lane et al., 2018). The incidence of AD doubles every five years after the age of 65 years. Moreover, the number of cases is projected to more than 115 million by 2050 (Sun et al., 2018). The primary cause of AD is presently unknown. Impaired mitochondrial function is frequently observed in cellular and animal models of AD as well as in tissues of AD patients. The most consistent defect in mitochondrial electron transport enzymes in AD is a deficiency in cytochrome c oxidase (COX), which was reported in both AD platelets and postmortem brain samples (Maurer et al., 2000, Fišar et al., 2016). COX activity was decreased significantly in hippocampus of the AD patients (Bosetti et al., 2002). The dysfunction of mitochondrial energy metabolism reduces ATP production, followed by the generation of reactive oxygen species (Bellaver et al., 2016). In contrast to β-amyloid plaques and tau tangles seen in the late stage of AD, mitochondrial dysfunction is an early event in the pathology of AD (Leuner et al., 2007). Drugs for treatment of AD should be administered in the prodromal stages of AD (Onyango, 2018). Given the importance of mitochondrial dysfunction in the pathogenesis of AD, new treatment strategies have been proposed to improve or ameliorate mitochondrial function (Van Giau et al., 2018). 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glucoside (TSG) is the main component extracted from Polygonum multiflorum, a traditional anti-aging Chinese herb. TSG is an antioxidant and can scavenge free radicals (Tao et al., 2011, Ryu et al., 2002). Our previous studies demonstrated that TSG improved learning and memory abilities in both APP transgenic mice and aged rats (Zhang et al., 2006, Wang et al., 2007). TSG has been developed as a new drug (Taisi Capsule) to treat AD. It is now under phase III clinical trials in China and showed good effect. We wonder whether TSG can improve mitochondrial function. In the present study, we investigated the effects of TSG in a mitochondrial dysfunction cell model induced by sodium azide, for the purpose of expanding the understanding of the potential therapeutic value of TSG for AD.
2. Materials and methods
2.1. Reagents
2,3,5,4′-Tetrahydroxystilbene glucoside (TSG), purity >98%, was purchased from National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Sodium azide (NaN3) was obtained from Ameresco (USA). Antibodies against Bcl-2, Bax, β-actin and horseradish peroxidase-conjugated goat anti-rabbit IgG were from Zhongshan Goldenbridge Biotechnology Co., Ltd. (Beijing, China). Annexin V/PI detection apoptotic kit, 2′,7′-dichlorofluorescein diacetate (DCFH-DA) and 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimi- dazolylcarbocyanine iodide (JC-1) were purchased from Beyotime Institute of Biotechnology (Jiangsu, China).
2.2. Cell culture and treatment
Human neuroblastoma SH-SY5Y cells were gifts from Dr. Bengt Winblad (The Karolinska Institute, Sweden). SH-SY5Y cells were cultured in DMEM/F12 (Gibco, USA) supplemented with 10% fetal bovine serum (Institute of Hematology & Blood Diseases, Chinese Academy of Medical Sciences (Tianjin, China), 100 U/ml penicillin and 100 U/ml streptomycin at 37 °C in an atmosphere containing 5% CO2 and 95% air. Serial dilutions of TSG in DMEM/F12 were added to SH-SY5Y cells, at final concentrations from 25 to 200 μmol/L, and incubated with cells for 24 h. The media were removed and cells were washed twice with DMEM/F12. The cells were then cultured in the media containing sodium azide (NaN3) for 16 h.
2.3. Detection of cellular ATP level
Cellular ATP level was measured using a firefly luciferase-based ATP assay kit (Beyotime Institute of Biotechnology, China) according to the manufacturer's instruction. Briefly, after NaN3 treatment, SH-SY5Y cells were schizolysised and centrifuged at 12,000 g for 5 min. In 96-well black plates, 100 μL of each supernatant was mixed with 100 μL ATP detection working dilution. Luminance was measured by a microplate reader (Spectra Max M5, Molecular Devices Corporation, USA). Standard curve was also generated and the protein concentration of each treatment group was determined with a bicinchoninic acid assay kit (Applygen Technologies Inc., China).
2.4. Measurement of mitochondrial membrane potential
To monitor mitochondrial membrane depolarization in cells following sodium azide treatment, JC-1 (5, 5′6, 6′-tetrachloro-1, 1′3, 3′- tetraethylbenzimi- dazolylcarbocyanine iodide) was used. JC-1 is a cationic dye whose mitochondrial uptake is related directly to the magnitude of the mitochondrial membrane potential. The greater the mitochondrial uptake, the greater concentration of JC-1 aggregate forms that have a red fluorescent emission signal, as opposed to the JC-1 monomer that fluoresces green. As previously described (Sun et al., 2014), SH-SY5Y cells were incubated with JC-1 dye (final concentration 5 μg/mL) for 30 min at 37 °C in the dark. To quantify the mitochondrial membrane potential (MMP), a fluorescence plate reader was used (Spectra Max M5, Molecular Devices Corporation, USA). JC-1 fluorescence was measured from a single excitation wavelength (488 nm) with dual emission (a shift from green at 530 nm to red at 590 nm). Mitochondrial depolarization is indicated by a decrease in the red/green fluorescence intensity ratio.
2.5. Measurement of intracellular reactive oxygen species
Reactive oxygen species (ROS) was measured using a 2,7-dichlorofluorescin dictate (DCFH-DA) as previously described (Shi et al., 2016). Briefly, after treatment, cells were incubated in DMEM/F12 containing 10 μmol/L DCFH-DA for 20 min at 37 °C in the dark. Then the supernatant was removed and the loaded SH-SY5Y cells were washed with PBS for three times. The fluorescence was read at 485 nm excitation and 530 nm emissions with a fluorescence plat reader (Infinite, TECAN, Swiss). The increasing production of ROS was expressed as a percentage of control.
2.6. Western blot analysis
After treatment, protein of cultured cells was extracted and quantified by a bicinchoninic acid assay kit. The same amount of protein from each dish was subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. After transferred to a PVDF membrane (Hybond, USA), blots were probed with antibodies to Bcl-2 (1:500 dilution) and Bax (1:500) respectively. Membranes were then incubated with appropriate secondary antibodies (1:2000). The immune complex was detected using ECL Western blotting detection reagents (Millipore Co., USA). β-Actin was used to normalize against gel loading variability.
2.7. Flow cytometric detection of apoptotic cells
The SH-SY5Y cells were harvested at a concentration of 1 × 107 cell/mL and resuspended in PBS. After centrifuged at 1000 g for 5 min, 195 μL FITC-conjugated Annexin V binding buffer and 5 μL of Annexin V-FITC were added. Following gentle vortex, the mixture was incubated for 10 min at (20–25) °C in the dark. After centrifuged at 1000 g for 5 min, 190 μL FITC-conjugated Annexin V binding buffer and 10 μL propidium iodide were added. Following gentle vortex, samples were analysed using a dual-laser FACS VantageSE flow cytometer (Becton Dickinson, Mountain View, CA, USA). The percentages of apoptotic cells for each sample were exhibited.
2.8. Statistical analysis
Data are expressed as mean ± standard deviation (SD). Statistical differences between groups were analysed by one-way analysis of variance (ANOVA) followed by Tukey's tests. Difference was considered statistically significant at P < 0.05.
3. Results
3.1. TSG elevates ATP level in NaN3–treated SH-SY5Y cells
Cellular ATP content is a sensitive indicator of mitochondrial function, and was measured using a firefly luciferase-based ATP assay kit in the present experiment. As shown in Fig. 1, cellular ATP level in model group was decreased to 42.64% of control cells after NaN3 treatment (P < 0.01). Pretreatment with TSG (50–200 μmol/L) for 24 h increased cellular ATP level in a dose dependent manner (P < 0.01). F value between groups was 69.844.
Fig. 1.

TSG elevates ATP level in NaN3–treated SH-SY5Y cells. The cells were incubated with TSG for 24 h, and then exposed to 8 mmol/L NaN3 for 16 h. Cellular ATP content was detected using a firefly luciferase-based ATP assay kit. Data were expressed as mean ± S.D. ##P < 0.01 vs the control group; **P < 0.01 vs the model group.
3.2. TSG increases mitochondrial membrane potential in NaN3–treated SH-SY5Y cells
In the present study, NaN3 decreased MMP significantly (P < 0.05), whereas preincubation with TSG (50–100 μmol/L) for 24 h increased MMP significantly (P < 0.05) (Fig. 2). F value between groups was 4.003.
Fig. 2.

TSG increases mitochondrial membrane potential in NaN3–treated SH-SY5Y cells. SH-SY5Y cells were incubated with TSG for 24 h, and then exposed to 8 mmol/L NaN3 for 16 h. Mitochondrial membrane depolarization was observed by JC-1 staining. To quantify the effect of TSG on MMP, a fluorescence plate reader was used. JC-1 fluorescence was measured from a single excitation wavelength (488 nm) with dual emission (a shift from green at 530 nm to red at 590 nm). Mitochondrial depolarization is indicated by a decrease in the red/green fluorescence intensity ratio. Data were presented as mean ± S.D. #P < 0.05 vs control group; *P < 0.05 vs model group.
3.3. TSG decreases reactive oxygen species in NaN3–treated SH-SY5Y cells
Reactive oxygen species (ROS) was measured using a fluorescent probe DCFH-DA. The level of ROS increased in SH-SY5Y cells after 8 mmol/L NaN3 incubation (P < 0.01). Preincubation with TSG (25–200 μmol/L) for 24 h attenuated this increase in ROS induced by NaN3 (P < 0.01) (Fig. 3). F value between groups was 4.527.
Fig. 3.
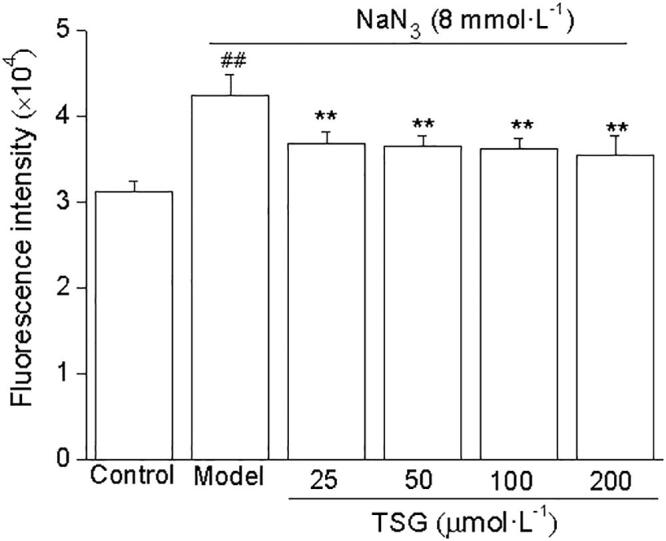
TSG decreases reactive oxygen species in NaN3–treated SH-SY5Y cells. The cells were incubated with TSG for 24 h, and then exposed to 8 mmol/L NaN3 for 16 h. The level of intracellular ROS was determined using a fluorescent probe DCFH-DA. Data were expressed as mean ± S.D. ##P < 0.01 vs control; **P < 0.01 vs the model group.
3.4. TSG reduces Bax/ Bcl-2 ratio in NaN3–treated SH-SY5Y cells
Western blot analysis showed that the expression of Bcl-2 was at a relative high level in the control cells and decreased after exposure to 8 mmol/L NaN3 for 16 h. On the other hand, the level of Bax was increased markedly after exposure to NaN3 for 16 h. Preincubation with TSG for 24 h increased the level of Bcl-2 and suppressed Bax expression. Therefore the Bax/Bcl-2 ratio was elevated in NaN3 model group (P < 0.01), whereas preincubation with TSG (25–100 μmol/L) decreased the Bax/Bcl-2 ratio (Fig. 4). F value between groups was 16.734.
Fig. 4.

TSG decreased Bax/ Bcl-2 ratio in NaN3–treated SH-SY5Y cells. SH-SY5Y cells were preincubated with TSG for 24 h before 8 mmol/L NaN3 exposure. The levels of Bax and Bcl-2 in cell lysates were determined by Western blot analysis. (A) Representative image of immunoblots for Bax and Bcl-2. (B) The Bax/Bcl-2 ratio was quantified by densitometric analysis. The Bax/Bcl-2 ratio in control cells was set as 100%. Data represent mean ± S.D. ##P < 0.01, vs control group; **P < 0.01, vs the model group.
3.5. TSG reduces the percentage of apoptotic cells in NaN3–treated SH-SY5Y cells
A method combining Annexin V-FITC/PI double-labeling with flowcytometric analysis was used to detect apoptosis. As shown in Fig. 5, the percentage of apoptotic cells increased from 1.05% to 16.18% after exposure to NaN3. TSG (25–200 μmol/L) preincubation decreased the percentage of apoptotic cells to 9.98%, 8.86%, 6.98% and 5.91% respectively. F value between groups was 28.082.
Fig. 5.
TSG inhibited apoptosis in NaN3-treated SH-SY5Y cells. Cells were preincubated with TSG for 24 h, and then exposed to NaN3 for 16 h. Apoptotic cells were detected by the flow cytometric analysis. Cells that stain positive for annexin V-FITC and negative for PI are undergoing apoptosis. Cells that stain negative for both annexin V-FITC and PI are alive and not undergoing measurable apoptosis. (A) Apoptotic cells (the lower right quadrant). (B) The percentage of apoptotic cells in total cells. Data represent mean ± S.D. ##P < 0.01 vs control group; *P < 0.05, **P < 0.01 vs model group.
4. Discussion
The pathogenesis of AD is complex. Impaired mitochondrial metabolism associated with respiratory chain dysfunction and the consequent oxidative stress is being considered to play a critical role in AD (Wang et al., 2014). Compounds those are able to induce and/or restore their bioenergetic capacity present an attractive AD therapy (Onyango, 2018).
In the present experiment, we treated cells with sodium azide (NaN3), an inhibitor of COX (i.e. mitochondrial electron transport chain complex IV), to mimic the selective decrease in activity of this enzyme observed in AD. Human neuroblastoma SH-SY5Y cells have been broadly used in studies of neuronal diseases and pharmacology (Wang et al., 2011). SH-SY5Y cells were treated with 8 mmol/L NaN3 to obtain decreased mitochondrial function but no significant decrease in cell viability.
The most important function of mitochondria in all types of cells is energy metabolism. Mitochondrial dysfunction is characterized by impaired biogenesis and inefficient bioenergetics and is accompanied by the generation and accumulation of reactive oxygen species (ROS) (Chen & Yan, 2010). Cellular ATP content is a sensitive indicator of mitochondrial function, and was measured using a firefly luciferase-based ATP assay kit in the present study. Pretreatment with TSG (50–200 μmol/L) for 24 h increased cellular ATP level in a dose dependent manner, which may contribute to maintain the normal function of mitochondria. Previously, we reported that TSG intragastrical administration increased the COX activity significantly (Zhang et al., 2018). Since COX pumps protons from the matrix into the intermembrane space to form the electrochemical proton gradient, which is used for the synthesis of ATP (Hüttemann et al., 2011), we suppose that TSG elevates cellular ATP content at least partly through increasing COX activity.
It is just the difference in electrical potential between the cytoplasm and the matrix that forms mitochondrial membrane potential (MMP). MMP is a highly sensitive indicator of the energetic state of mitochondria. Sodium azide can inhibit activity of cytochrome c oxidase, disturb proton circuit, and thus cause the loss of MMP. In the present study, we measured MMP by JC-1, a cationic dye which can produce red fluorescent J-aggregates in mitochondria with high potential and green fluorescence with low potential. We found that pretreatment of cells with TSG (50–100 μmol/L) for 24 h increased MMP, which may contribute to maintain the normal function of mitochondria. MMP regulates reactive oxygen species (ROS) production (Perry et al., 2011). Over production of ROS may cause oxidative damage to mitochondrial proteins, lipids, and DNA, thereby further disrupting mitochondrial function and energy production if the mitochondrial mechanisms of repair and defence fail (Atamna & Frey, 2007). The interaction between oxidative stress and mitochondrial dysfunction likely forms a vicious downward spiral that amplifies the alterations observed in AD (Mecocci et al., 2018). In the present study, pretreatment with TSG for 24 h increased MMP. This may partially contribute to maintain the normal function of mitochondria. TSG pretreatment also ameliorated abnormal ROS increase. It is believed that antioxidant therapy can be operated as a pharmacological approach to prevent or delay the oxidative stress events that lead to neurodegeneration (Oliveira et al., 2018). TSG was reported possesses strong antioxidant and free radical-scavenging activities (Zhang & Chen, 2018). Our result also demonstrated that TSG had antioxidant properties, which might be one of the mechanisms of TSG’s neuroprotective effect.
On the early stage of apoptosis, MMP is decreased, and then a series of biochemical changes in cells are induced, such as activation of Bcl-2 family. Bell reported that destabilization of MMP leads to the release of apoptotic factors thereby allowing apoptosis to occur (Bell et al., 2008). The best characterized protein family that plays an important role in apoptotic cell death regulation is Bcl-2 family proteins. Our present study focused on Bcl-2 and Bax, the two major members of Bcl-2 family. Bax, a pore-forming cytoplasmic protein localized on the outer mitochondrial membrane, leads to cell apoptotic death. The anti-apoptotic Bcl-2 is associated with the outer mitochondrial membrane where it stabilizes the membrane permeability, protecting mitochondrial integrity and inhibiting cell death (Yang et al., 1997). The alteration of the ratio of Bcl-2 to Bax is significant in determining whether apoptosis occurs (Yang & Korsmeyer, 1996). Our results showed that sodium azide decreased the expression of Bcl-2 and increased the expression of Bax, thus induced apoptosis in SH-SY5Y cells. Pretreatment with TSG reduced the expression of Bax and increased the expression of Bcl-2, and therefore inhibited apoptosis induced by sodium azide.
In the past, our research team observed effects of TSG in vivo and in vitro. Seven types of dementia animal models were used, including APP695V717I transgenic mouse model, Abeta brain injection model, basal forebrain cholinergic damage rat model induced by ibotanic acid, dementia rat model induced by mitochondrial complex IV inhibitor NaN3, naturally aged rat model, brain aging mouse model induced by D-galactose and hypercholesterolemia-induced dementia rat model. Results showed that TSG improved the learning and memory ability (Wang et al., 2007, Zhang et al., 2006), decreased MDA content in the cortex (Chu et al., 2003), increased gene expression of energy metabolizing enzymes, and decreased gene expression of inflammatory in the hippocampus of D-galactose model mice (Xie et al., 2005), inhibited the oxidative stress and apoptosis in the brain (Chu et al., 2003) and enhanced COX activity and expression of neurotrophic factors in the brain (Zhang et al., 2018). In conclusion, TSG is never only an antioxidant. TSG is able to act on multiple targets in the complicated pathogenesis of AD.
5. Conclusion
In the present study, our findings demonstrated that TSG protected SH-SY5Y cells from sodium azide-induced mitochondrial dysfunction and apoptosis through increasing mitochondrial membrane potential and ATP level, decreasing the production of ROS and Bax/Bcl-2 ratio. Since mitochondrial dysfunction is an early event in the pathology of AD, strategies to develop drugs that protect mitochondria from damage are worth pursuing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 81273498, 81341087); National Science and Technology Major Project of China (No. 2015ZX09101-016). We thank Ya-li Li and Li Zhang for technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.chmed.2020.11.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Atamna H., Frey W.H. Mechanisms of mitochondrial dysfunction and energy deficiency in Alzheimer’s disease. Mitochondrion. 2007;7(5):297–310. doi: 10.1016/j.mito.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Bell E.L., Klimova T., Chandel N.S. Targeting the mitochondria for cancer therapy: Regulation of hypoxia-inducible factor by mitochondria. Antioxidants & Redox Signaling. 2008;10(3):635–640. doi: 10.1089/ars.2007.1655. [DOI] [PubMed] [Google Scholar]
- Bosetti F., Brizzi F., Barogi S., Mancuso M., Siciliano G., Tendi E.A., et al. Cytochrome C oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer's disease. Neurobiology of Aging. 2002;23(3):371–376. doi: 10.1016/s0197-4580(01)00314-1. [DOI] [PubMed] [Google Scholar]
- Bellaver B., Bobermin L.D., Souza D.G., Rodrigues M.D.N., de Assis A.M., Wajner M., et al. Signaling mechanisms underlying the glioprotective effects of resveratrol against mitochondrial dysfunction. Biochimica et Biophysica Acta. 2016;1862(9):1827–1838. doi: 10.1016/j.bbadis.2016.06.018. [DOI] [PubMed] [Google Scholar]
- Chen J.X., Yan S.S. Role of mitochondrial amyloid-beta in Alzheimer's disease. Journal of Alzheimers Disease. 2010;20:S569–578. doi: 10.3233/JAD-2010-100357. [DOI] [PubMed] [Google Scholar]
- Chu J., Ye C.F., Li L. Effects of stilbene-glycoside on learning and memory function and free radicals metabolism in dementia model mice. Chinese Journal of Rehabilitation Theory and Practice. 2003;9(11):643–645. [Google Scholar]
- FiŠar Z., Hroudová J., Hansíková H., Spá|ilová J., Lelková P., Wenchich L., et al. Mitochondrial respiration in the platelets of patients with Alzheimer’s disease. Current Alzheimer Research. 2016;13(8):930–941. doi: 10.2174/1567205013666160314150856. [DOI] [PubMed] [Google Scholar]
- Hüttemann M., Helling S., Sanderson T.H., Sinkler C., Samavati L., Mahapatra G., et al. Regulation of mitochondrial respiration and apoptosis through cell signaling: Cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and inflammation. Biochimica et Biophysica Acta. 2012;1817(4):598–609. doi: 10.1016/j.bbabio.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane C.A., Hardy J., Schott J.M. Alzheimer's Disease. European Journal of Neurology. 2018;25(1):59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- Leuner K., Hauptmann S., Abdel-Kader R., Scherping I., Keil U., Strosznajder J.B., et al. Mitochondrial dysfunction: The first domino in brain aging and Alzheimer’s disease? Antioxidants & Redox Signaling. 2007;9(10):1659–1676. doi: 10.1089/ars.2007.1763. [DOI] [PubMed] [Google Scholar]
- Maurer I., Zierz S., Möller H.J. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiology of Aging. 2000;21(3):455–462. doi: 10.1016/s0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Mecocci P., Boccardi V., Cecchetti R., Bastiani P., Scamosci M., Ruggiero C., et al. Long journey into aging, brain aging, and Alzheimer's disease following the oxidative stress tracks. Journal of Alzheimers Disease. 2018;62(3):1319–1335. doi: 10.3233/JAD-170732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira C., Cagide F., Teixeira J., Amorim R., Sequeira L., Mesiti F., et al. Hydroxybenzoic acid derivatives as dual-target ligands: Mitochondriotropic antioxidants and cholinesterase inhibitors. Frontiers in Chemistry. 2018;6 doi: 10.3389/fchem.2018.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyango I.G. Modulation of mitochondrial bioenergetics as a therapeutic strategy in Alzheimer's disease. Neural Regeneration Research. 2018;13(1):19–25. doi: 10.4103/1673-5374.224362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S.W., Norman J.P., Barbieri J., Brown E.B., Gelbard H.A. Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. Biotechniques. 2011;50(2):98–115. doi: 10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu G., Ju J.H., Park Y.J., Ryu S.Y., Choi B.W., Lee B.H. The radical scavenging effects of stilbene glucosides from Polygonum multiflorum. Archives of Pharmacal Research. 2002;25(5):636–639. doi: 10.1007/BF02976935. [DOI] [PubMed] [Google Scholar]
- Shi Y., Hu Y., Lv C., Tu G. Effects of reactive oxygen species on differentiation of bone marrow mesenchymal stem cells. Annals of Transplantation. 2016;21:695–700. doi: 10.12659/aot.900463. [DOI] [PubMed] [Google Scholar]
- Sun B.L., Li W.W., Zhu C., Jin W.S., Zeng F., Liu Y.H., et al. Clinical research on Alzheimer’s disease: Progress and perspectives. Neuroscience Bulletin. 2018;34(6):1111–1118. doi: 10.1007/s12264-018-0249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Chen R.C., Yang Z.H., Sun G.B., Wang M., Ma X.J., et al. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food and Chemical Toxicology. 2014;63:221–232. doi: 10.1016/j.fct.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Tao L., Li X., Zhang L., Tian J., Li X., Sun X., et al. Protective effect of tetrahydroxystilbene glucoside on 6-OHDA-induced apoptosis in PC12 cells through the ROS-NO pathway. PLoS One. 2011;6(10):e26055. doi: 10.1371/journal.pone.0026055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Giau V., An S.S.A., Hulme J.P. Mitochondrial therapeutic interventions in Alzheimer's disease. Journal of the Neurological Sciences. 2018;395:62–70. doi: 10.1016/j.jns.2018.09.033. [DOI] [PubMed] [Google Scholar]
- Wang P., Jiang S., Cui Y., Yue Z., Su C., Sun J., et al. The N-terminal 5-MER peptide analogue P165 of amyloid precursor protein exerts protective effects on SH-SY5Y cells and rat hippocampus neuronal synapses. Neuroscience. 2011;173:169–178. doi: 10.1016/j.neuroscience.2010.10.069. [DOI] [PubMed] [Google Scholar]
- Wang R., Tang Y., Feng B., Ye C., Fang L., Zhang L., et al. Changes in hippocampal synapses and learning-memory abilities in age-increasing rats and effects of tetrahydroxystilbene glucoside in aged rats. Neuroscience. 2007;149(4):739–746. doi: 10.1016/j.neuroscience.2007.07.065. [DOI] [PubMed] [Google Scholar]
- Wang X., Wang W., Li L., Perry G., Lee H.-gon., Zhu X. Oxidative stress and mitochondrial dysfunction in Alzheimer's disease. Biochimica et Biophysica Acta. 2014;1842(8):1240–1247. doi: 10.1016/j.bbadis.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W.J., Li L., Wei H.F., Chu J., Ye C.F., Zhang L., et al. Effect of 2, 3, 5, 4′-tetrahydroxystillbene-2-O-β-D-glucoside on hippocampal genes expression in mimetic-dementia mice induced by D-galactose. Chinese Journal of Pharmacology and Toxicology. 2005;19(1):24–28. [Google Scholar]
- Yang E., Korsmeyer S.J. Molecular thanatopsis: A discourse on the BCL2 family and cell death. Blood. 1996;88(2):386–401. [PubMed] [Google Scholar]
- Yang J., Liu X., Bhalla K., Kim C.N., Ibrado A.M., Cai J., et al. Prevention of apoptosis by Bcl-2: Release of cytochrome C from mitochondria blocked. Science. 1997;275(5303):1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Zhang L., Chen J. Biological effects of tetrahydroxystilbene glucoside: An active component of a rhizome extracted from Polygonum multiflorum. Oxidative Medicine and Cellular Longevity. 2018;2018:1–15. doi: 10.1155/2018/3641960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Xing Y., Ye C., Ai H., Wei H., Li L. Learning-memory deficit with aging in APP transgenic mice of Alzheimer's disease and intervention by using tetrahydroxystilbene glucoside. Behavioural Brain Research. 2006;173(2):246–254. doi: 10.1016/j.bbr.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Zhang R.Y., Zhang L., Zhang L., Wang Y.L., Li L. Anti-amyloidgenic and neurotrophic effects of tetrahydroxy stilbene glucoside on chronic mitochondrial dysfunction rat model induced by sodium azide. Journal of Natural Medicines. 2018;72(3):596–606. doi: 10.1007/s11418-018-1177-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



