Abstract
In recent years, the development of Chinese herbal medicine (CHM) has been challenged by shortages of CHM resources and drug safety concerns related to end products. There have been significant efforts by Chinese scholars to tackle these challenges, which are revealed by analyzing the research trend of CHM resources via surveying Chinese Traditional and Herbal Drugs (Zhong Cao Yao), a representative journal in CHM. Our study focused on 781 articles in CHM resources from 2013 to 2018 and included four subject areas: germplasm resources, quality analysis and evaluation, cultivation, and bioengineering of CHM. Discussion and prospective for future investigations were also presented, including: construct the core germplasm of medicinal plants and expand germplasms; combine molecular research with field experiments and promote the deeper study of cultivation of CHM plants; improve the quality evaluation method of CHM and strengthen the identification of Chinese patented medicines; promote the sustainable development of CHM resources by utilizing bioengineering and synthetic biology. This study helps international scholars understand the status quo of CHM research and provides theoretical support for the healthy, modern, and international development of CHM, and it will facilitate the sustainable development of the traditional Chinese medicine industry.
Keywords: bioengineering, cultivation, CHM resources, germplasm resources, identification, quality analysis
1. Introduction
In 2015, You-you Tu was awarded the Nobel Prize due to the discovery of artemisinin, a therapeutic drug of malaria extracted from the medicinal plant Artemisia annua L.. This landmark event shows that Chinese herbal medicine (CHM) resources play a dominant role in the new drug discovery. Currently more than one third of clinic drugs are extracted and/or derived from CHM resources, such as irinotecan and composite Danshen Pills. CHM resources have had crucial value in the treatment and prevention of various diseases for thousands of years, and they have significantly contributed to improving human health. Furthermore, CHM resources are also an important source for health care products, food flavors and pigments, and cosmetics, etc. With the progress of internationalization of traditional Chinese medicine, Chinese herbs are widely used all over the world. Many common herbs have been recorded in the United States Pharmacopoeia, Japanese Pharmacopoeia, India Pharmacopoeia, and Korea Pharmacopoeia (Chen et al., 2014).
The market demand for CHM in recent years has significantly increased. According to statistics, the annual consumption of CHM in China exceeds 40 0000 tons, of which the main consumption is wild CHM (Zhu, Wang, Liang, He, & Yan, 2018). The wild resources of CHM, such as Glycyrrhiza uralensis Fisch., are becoming gradually exhausted due to overexploitation (Zhang & Gong, 2016). As a result, the sustainable development of CHM and its resources has become an urgent problem to be solved. Cultivars have been the main source of many medicinal materials and their quality significantly affects the efficacy and use of these CHM plants. In this study, the Chinese domestic production of CHM resources is comprehensively introduced through summarizing the articles from the journal of Chinese Traditional and Herbal Drugs (Zhong Cao Yao) of the last 6 years (2013–2018), and the guidance is provided for the sustainable development of CHM resources.
2. Germplasm resources of Chinese herbal medicine
2.1. Genetic diversity analysis by molecular markers
The genetic diversity analysis of species germplasm resources can provide a theoretical basis for cultivation and breeding and new variety improvement. According to basic molecular biology techniques, methods utilizing molecular markers can be divided into three categories: 1. DNA markers based on molecular hybridization, such as Restriction Fragment Length Polymorphism (RFLP); 2. DNA markers based on polymer chain reaction (PCR), such as Random Amplified Polymorphic DNA (RAPD), Random Amplified Microsatellite Polymorphism (RAMP), Amplified Fragment Length Polymorphism (AFLP), Sequence Related Amplified Polymorphism(SRAP), Inter Simple Sequence Repeat (ISSR), and Simple Sequence Repeat (SSR); 3. DNA sequence markers, i.e., sequence analysis of some gene fragments in the nuclear genome, chloroplast genome, and mitochondria genome. The commonly used genes are rbcL and matK of the chloroplast genome, and ITS of the nuclear genome.
The application of molecular markers in the study of genetic diversity of medicinal plants in Chinese Traditional and Herbal Drugs is outlined in Table 1. No articles use molecular markers based on molecular hybridization techniques. Most studies have utilized the molecular marker technology based on PCR, including SSR, ISSR, SCoT, SRAP, RAPD, AFLP, CDDP, RAMP, and DALP, etc. For example, the genetic diversity of Gastrodia elata germplasm resources was studied by the SRAP molecular marker (Chan et al., 2014). The ISSR molecular marker was used to analyze genetic polymorphisms and the relationship of Anisodus tanguticus from Gansu and Qinghai Provinces in China (Zhang, Ren, Zhang, & Guan, 2018). In addition, the nuclear genomes or chloroplast genomes of medicinal plants were cloned and sequenced in some articles. The sequences of nuclear gene ITS and chloroplast psbA-trnH and trnS-trnG in cultivars and wild populations of Rehmannia glutinosa were amplified and sequenced. The haplotype (gene) diversity and nucleotide polymorphism of three genes from wild plants and cultivars of R. glutinosa were analyzed and compared (Xia, Huang, Li, Zhou, & Gao, 2018).
Table 1.
Application of molecular markers in study of genetic diversity of medicinal plants in Chinese Traditional and Herbal Drugs.
| No. | Medicinal plant species | Collection sites | Sample sizes | Sample sources | Molecule marker technologies |
|---|---|---|---|---|---|
| 1 | Aconitum brachypodum | Yunnan | 105 | Wild | AFLP |
| 2 | Aconitum carmichaelii | Sichuan | 126 | Cultivar | AFLP |
| 3 | Akebia trifoliate | Shaanxi | 6 | Wild | AFLP |
| 4 | Amomum tsaoko | Yunnan | 214 | Wild, cultivar | SSR |
| 5 | Angelica sinensis | Gansu | 41 | Cultivar | ISSR |
| 6 | Angelica sinensis | Gansu, Yunnan, etc. | 1037 | Wild, cultivar | ISSR |
| 7 | Anisodus tanguticus | Gansu, Qinghai | 127 | Wild | ISSR |
| 8 | Anoectochilus roxburghii | Fujian, Guangdong, etc. | 24 | Wild, cultivar | ISSR |
| 9 | Anoectochilus roxburghii | Yunnan, Vietnam, etc. | 18 | Wild | DALP |
| 10 | Anoectochilus roxburghii | Fujian, Guangdong, etc. | 20 | Cultivar | RAPD |
| 11 | Asarum L. | Zhejiang, Guizhou | 40 | – | rDNA ITS |
| 12 | Astragalus membranaceus | Inner Mongolia | 80 | Wild, cultivar | ISSR |
| 13 | Calanthe | Zhejiang, Guizhou, etc. | 55 | – | rDNA ITS |
| 14 | Chuanmingshen violaceum | Sichuan | 63 | Wild, cultivar, semi-wild | SRAP |
| 15 | Cichorium intybus, C. glandulosum | Xinjiang, Shandong, etc. | 14 | – | rDNA ITS |
| 16 | Citri reticulatae Semen | Sichuan | 35 | Cultivar | ISSR |
| 17 | Citrus reticulata cv. chachiensis | Guangdong, Zhejiang, etc. | 13 | Cultivar | SCoT |
| 18 | Clerodendrum L. | Jiangxi | 9 | Wild | SSR |
| 19 | Codonopsis lanceolata | Fujian, Sichuan, etc. | 12 | – | SSR |
| 20 | Codonopsis pilosula | Gansu, Shanxi | 48 | Wild | SSR |
| 21 | Codonopsis pilosula | Shanxi | – | Cultivar | SSR |
| 22 | Coix lacryma-jobi L. | Yunnan, Vietnam, Laos, etc. | 25 | – | SRAP |
| 23 | Curcuma wenyujin | Zhejiang | 11 | – | SRAP |
| 24 | Curcumae Rhizoma | Sichuan, Guangxi, etc. | 9 | Wild | RAPD |
| 25 | Dendrobium Lindl. | Yunnan, Hainan, etc. | 24 | Cultivar | ISSR |
| 26 | Dendrobium officinale | Fujian | 43 | Wild, cultivar | CDDP |
| 27 | Dioscorea opposita | Jiangxi | 22 | Tissue culture seedling | RAPD |
| 28 | Dioscoreae Rhizoma | Henan, Fujian, etc. | 14 | Wild | rDNA-ITS |
| 29 | Dipsacus | Yunnan, Sichuan, etc. | 90 | – | ISSR, SCoT, SRAP |
| 30 | Dipsacus asper | Sichuan | 14 | Cultivar | SRAP |
| 31 | Ephedra intermedia | Gansu | 11 | Wild, cultivar, semi-wild | ISSR |
| 32 | Epimedium acuminatum | Guizhou | 50 | Wild | ISSR |
| 33 | Eupolyphaga sinensis | Shandong | 10 | – | RAMP |
| 34 | Ferula syreitschikowii | Xinjiang | 96 | Cultivar | ISSR |
| 35 | Forsythia suspensa | Shaanxi, Shanxi, etc. | 14 | – | RAPD |
| 36 | Fritillaria L. | Xinjiang | 10 | – | ISSR |
| 37 | Gardenia jasminoides | Guangxi, Hunan, etc. | 47 | Cultivar | SCoT |
| 38 | Gardenia jasminoides | Fujian, Guangdong, etc. | 573 | Wild | SSR |
| 39 | Gastrodia elata | Sichuan, Guizhou, etc. | 24 | Wild, cultivar | SRAP |
| 40 | Gentiana straminea | Tibet, Qinghai, etc. | 83 | – | ISSR |
| 41 | Gentiana straminea | Tibet | 17 | Cultivar | nrDNA ITS |
| 42 | Geranium wilfordii | Liaoning, Shandong, etc. | 20 | – | ISSR |
| 43 | Goodyera L. | Zhejiang, Anhui | 35 | – | rDNA ITS |
| 44 | Gynostemma pentaphyllun | Yunnan, Guizhou, etc. | 48 | Wild | ISSR |
| 45 | Gynostemma pentaphyllun | Guangdong, Chongqing, etc. | 426 | Wild | SSR |
| 46 | Hedysari Radix | Gansu | 15 | Wild | ISSR |
| 47 | Hirudo nipponica | Heilongjiang | 10 | – | ISSR |
| 48 | Houttuynia cordata | Yunnan, Guizhou | 16 | – | SSR |
| 49 | Illicium difengpi | Guangxi | – | Wild | ISSR |
| 50 | Istais indigotica | Anhui, Beijing, etc. | 23 | Cultivar | ISSR |
| 51 | Leonurus artemisia | Henan | 8 | Wild | SCoT |
| 52 | Marsdenia R. Br. | – | 21 | Wild | ITS2, rbcL |
| 53 | medicinal leech | Yunnan | 14 | – | SSR |
| 54 | Microcos paniculata | Guangdong, Guangxi, etc. | 14 | – | psbA-trnH, ITS2 |
| 55 | Notopterygium incisum | Qinghai, Sichuan, etc. | 245 | Wild | trnT-trnL |
| 56 | Paeonia suffruticosa | Hunan | 47 | Cultivar | ISSR |
| 57 | Panax japonicus | Shaanxi, Sichuan, etc. | 19 | Wild, cultivar | ISSR |
| 58 | Panax quinquefolium | Canada, Beijing, etc. | 18 | Cultivar | RAPD, ISSR |
| 59 | Panax vietnamensis var. fuscidiscus | Yunnan, Laos | 13 | Wild | SSR |
| 60 | Paris polyphylla var. yunnanensis | Yunnan | 115 | – | SSR |
| 61 | Pesudostellaria heterophylla | Anhui, Jiangsu, etc. | 12 | Cultivar | ISSR |
| 62 | Pholidota cantonensis | Zhejiang | 68 | Wild | SRAP |
| 63 | Pinellia ternate and their relative species | Guizhou, etc. | 43 | – | psbK-psbI, atpF-atpH |
| 64 | Pleione bulbocodioides | Yunnan, Sichuan, etc. | 23 | Cultivar | ISSR |
| 65 | Polygala tenuifolia | Gansu, Hebei, etc. | 31 | Wild | cpDNA trnL |
| 66 | Polygonum multiflorum | Guangdong, Zhejiang, etc. | 116 | Wild | psbA-trnH |
| 67 | Potentilla L. | Hunan, Sichuan, etc. | 6 | – | ISSR |
| 68 | Psammosilene tunicoides | Yunnan, Guizhou | 184 | Wild | SSR |
| 69 | Pseudostellaria heterophylla | Fujian, Shandong | 9 | – | rDNA ITS |
| 70 | Rehmannia glutinosa | Henan | 62 | Wild, cultivar | ITS, psbA-trnH, trnS-trnG |
| 71 | Rhodiola L. | Tibet, Jilin | 17 | Wild | RAPD, ISSR |
| 72 | Rosa chinensis, and their relative species | Henan | 33 | Cultivar | ISSR |
| 73 | Rubia cordifolia | Henan | 7 | Wild | psbA-trnH |
| 74 | Rubus L. | Zhejiang | 15 | Wild | rDNA ITS |
| 75 | Sarcandra glabra | Fujian, Guangdong, etc. | 18 | Cultivar | ISSR |
| 76 | Sarcandra glabra | – | 18 | Cultivar | ITS |
| 77 | Siraitia grosvenorii | Guangxi | 28 | Cultivar | ISSR |
| 78 | Solanum nigrum | Heilongjiang, Hainan, etc. | 17 | Wild | ITS, trnH-psbA |
| 79 | Sophora alopecuroides L. | Ningxia, Gansu, etc. | 22 | Wild | ISSR |
| 80 | Stemona tuberosa | Guangxi, Guangdong, etc. | 34 | – | ISSR |
| 81 | Stephania kwangsiensis | Guangxi | 63 | Wild | SSR |
| 82 | Tamarix chinensis | Hebei | 32 | Cultivar | ISSR |
| 83 | Taxus chinensis var. mairei | Fujian | 23 | Cultivar | RAPD |
| 84 | Tetrastigma hemsleyanum | Jiangxi, Chongqing | 64 | Wild | ISSR |
| 85 | Tetrastigma hemsleyanum | Jiangxi, Hunan, etc. | 64 | Cultivar | SSR |
| 86 | Trichosanthis Fructus | Jiangxi, Hunan, etc. | 30 | Cultivar | SRAP, ITS |
2.2. Research gaps in germplasm resources of Chinese herbal medicine
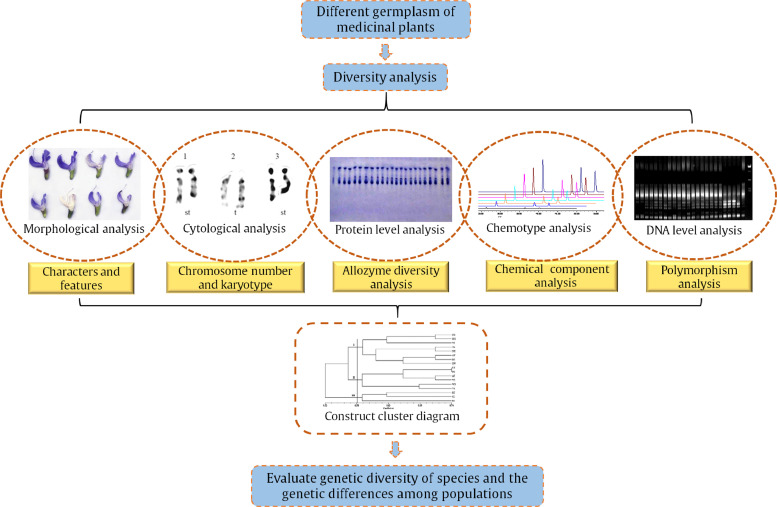
As can be seen from Table 1, these articles used various molecular markers in the genetic diversity analysis of species. Besides, the traditional research methods, including morphological markers, cytological markers, biochemical markers and chemotype markers, can also be used to reveal the variations of genetic material within a species at different levels. The research strategies used in the genetic diversity analysis of germplasm resources of CHM are shown in Fig. 1. However, few papers in the journal analyzed the genetic diversity of medicinal plants by using traditional methods. The multiple technologies should complement each other and be used to better evaluate the germplasm resources of medicinal plants. For instance, morphological analysis, DNA analysis, and HPLC quantitative analysis were combined to study the genetic diversity among Aconitum kiyomiense Kadota (Ranunculaceae), endemic to Takayama city, Gifu Prefecture, central Japan, and other members of subgenus Aconitum (Minami et al., 2019).
Fig. 1.
Research on genetic diversity of germplasm resources of CHM
Note: The image of morphological analysis comes from Palma-Rojas, Gonzalez, Carrasco, Silva, and Silva (2017), the image of cytological analysis is from Palma-Rojas et al. (2017), the image of protein level analysis is from Kang and Huang (2002), and the images of DNA level analysis and the cluster diagram are from Pang, Chen, Song, Wang, and Li (2018).
3. Cultivation of Chinese herbal medicine
3.1. Germination of seeds and growth of seedlings
The papers of Chinese Traditional and Herbal Drugs addressed the research and development of seeds and seedlings of CHM, and these papers can be divided into three directions. First, a study sought to investigate the regulation mechanism of enzymes and endogenous hormones at different stages of seed germination (Su et al., 2017). Second, determining conditions for seed germination and seedling growth was an important topic of investigation. The influences of temperature, endogenous hormones, and pollination methods for seed germination and emergence of Paris polyphylla var. yunnanensis were explored, and a technical system was established to promote the germination of its seeds (Zhang et al., 2017). Third, improving the stress tolerance of seeds and seedlings of medicinal plants was investigated. 5-Aminolevulinic acid (ALA) was used to treat Belamacanda chinensis seeds under salt stress (100 mmol/L NaCl). It was found that the exogenous ALA improved the germination index of B. chinensis seeds and effectively alleviated the damage of salt stress on PSII system of B. chinensis seedlings, which improved the ability of plants to resist stress (Yang, He, Duan, & Xu, 2014).
3.2. Effects of environmental factors on Chinese herbal medicine
The growth and quality of medicinal plants are affected by their living environment, including geography, climate, soil, and biological factors, and their own reproductive mode. An altitude effect study was performed on Angelic sinensis at 2300−2800 m in Chabu countryside, Min County, Gansu province, China. The study used a combination of field trials and correlation analysis to explore the impact of altitude on the formation of A. sinensis and identified the key factors affecting yield (Wang et al., 2013).
Light, temperature, and precipitation are key climatic factors affecting plant growth. The physiological indexes of Paris polyphylla treated at different temperatures were measured to find the optimum temperature suitable for its growth (Zhang, Ma, Hu, & Lan, 2018). The effects of growth factors, altitude, and illumination (shady slope and sunny slope) on anthraquinones and tannins of medicinal rhubarb were studied to find the theoretical basis for choosing the optimum growth conditions (Yan et al., 2017).
Soil provides basic conditions for plant growth and development. The nutrients and water in the soil are related to the quality of medicinal plants, and the microbial diversity in the soil is connected to the plant's disease resistance. Therefore, studies focusing on plant growth (Qiang, Wang, Wang, & Li, 2015; Shao et al., 2018) and soil microbial composition (Li, Song, Wang, & Li, 2013) were also reported in Chinese Traditional and Herbal Drugs. However, there are few reports on CHM cultivation, especially field experiments due to the long experiment cycles and unstable conditions.
Endophytic fungi of medicinal plants, an active area of current research, are fungi that live within plants and do not cause obvious diseases in host tissues. Endophytic fungi of medicinal plants can produce special active substances on their own and induce and promote the synthesis and accumulation of secondary metabolites of host plants. As for CHM, it is important to screen out endophytic fungi that produce bacteriostatic substances so that the safe and effective biological measures can be found to inhibit the diseases of CHM. The endophytic fungi were isolated from Glehnia littoralis by the tissue isolation, and their antibacterial activity was investigated (Hou et al., 2015). Arbuscular mycorrhizal (AM) fungus could increase the survival rate of Paris polyphylla var. yunnanensis seedlings, and the multiple AM fungi strains affect the content of endogenous hormones of this plant (Zhou, Li, & Luo, 2017). However, only the isolation and identification of endophytic fungi were reported, and the effects of endophytic fungi on secondary metabolites of medicinal plants have not been reported.
3.3. Harvesting and processing of Chinese herbal medicine
Quantitative determination of berberine and phellodendrine by HPLC was carried out to analyze the changes of berberine and phellodendrine content in different years, different harvest seasons, and different portions of Phellodendron chinense, in order to provide a scientific basis for harvesting P. chinense (Ji, Xie, Cai, & Zeng, 2014). The effects of different drying methods on composition and content of five active constituents in root bark and root of Polgala tenuifolia were investigated. This work provided the basis for choosing the drying methods of P. tenuifolia with different specifications (Peng et al., 2018).
3.4. Research gaps in CHM cultivation
The articles of this journal covered the effects of various biological and abiotic factors on the cultivation of CHM during 2013−2018, but few of them studied the molecular mechanisms. It is well known that the continuous cropping obstacle is one of the major problems in the cultivation of medicinal plants, and the molecular techniques can be combined to analyze the causes of continuous cropping obstacles of medicinal plants. The succession of the bacteria community structure in soil of long-term continuous cotton cropping was studied using high-throughput DNA sequencing (Wei & Yu, 2018). Furthermore, the formation of genuine herbs is also a hot research topic in medicinal plants cultivation. However, there are few articles concerning about these aspects.
4. Quality analysis and evaluation of Chinese herbal medicine
The quality and safety of CHM, including the authenticity and quality evaluation, have been of concern of many CHM scholars. The main contents of identification and quality evaluation of TCM are shown in Fig. 2. More than one-third of the articles focused on the identification and quality analysis of CHM to ensure the drug safety. Among them, quality analysis articles account for the vast majority.
Fig. 2.
Identification and quality evaluation of Chinese herbal medicine
Note: The images of origin and macroscopic and microscopic identification are from Yi, Wu, Zhang, Wu, and Huang (2015); the images of physicochemical identification are from Liu, Shi, and Lei (2017) and Yang et al. (2016); the image of molecular identification is from Yu et al. (2014); the image of mycotoxin is from Díaz Nietoa, Granerob, Alicia Zon, and Fernández (2018).
4.1. Identification of Chinese herbal medicine
4.1.1. Classical identification methods
The classical identification methods, including origin identification, macroscopic identification, microscopic identification, and physicochemical identification, were adopted in some Chinese Traditional and Herbal Drugs articles to identify CHM plants. HPLC fingerprints were used for the identification of Ophiocordyceps sinensis and its fake burnet (Huang et al., 2017), Lonicerae Japonicae Flos, Lonicera Flos (Liu, Li, Jin & Ma, 2017; Wang, Deng, Ma, & Yin, 2017), and Crocus sativus (Yao, Jin, He, & Wang, 2015). Spectral identification technology was used in the identification of several CHM compounds. Methods of classification and identification of Rhodiola quadrifida and Rhodiola crenulata were established based on nuclear magnetic resonance spectroscopy and 1H NMR fingerprint-chemical pattern recognition techniques (Li, Su, Li, Li, & Si, 2018). The aqueous extracts of donkey-hide and its related compounds (horse-hide and mule-hide) were identified using 1H NMR metabolomics (Tian et al., 2015). Fourier transform infrared spectroscopy (FT-IR) and two-dimension IR correlation infrared spectroscopy (2D-IR) were used to identify Paeoniae Radix Alba, Paeoniae Radix Rubra, and their alcohol extracts (Yang et al., 2016). Guang Citrus Reticulata Pericarpium (GCRP) samples were identified by near infrared spectroscopy techniques (Yan et al., 2015). The monosaccharide fingerprint technology was used to determine the differences in the composition and amount of monosaccharides in the cytoplasm of wild Astragalus and fast-growing Astragalus; Their monosaccharide ratio was distinct, which is useful in the identification of Astragalus with different growth modes (Gao, Li, Hao, Wang, & Qin, 2015).
The combination of several classical identification methods was used in a few papers. To distinguish Anoectochilus roxburghii, A. formosanus, and A. chapaensis, the methods of freehand section, microexamination, and digital photography were combined to compare the plant morphology, tissue structures of root, stem and leaf transverse, and dried medicinal powder (Yi, Wu, Zhang, Wu, & Huang, 2015). Qualitative and quantitative studies on Vitex negundo var. cannabifolia and Vitex negundo were carried out using original plant identification, morphological identification, microscopic identification, TLC, and HPLC identifications (Luo et al., 2017). Some advanced technology, such as SEM and bionic systems, were also used in several studies. The bionic olfactory system (electronic nose, PEN3) was used to measure the odor of Aurantii Fructus from different growing areas (Zhou, Li, & Luo, 2017). Five pungent-taste herbs were recognized by electronic nose and electronic tongue (Cao et al., 2016). The structures of the flower of Lonicerae Japonicae Flos and Lonicera Flos were observed using SEM (Wu, Feng, & Zeng, 2014).
4.1.2. DNA molecular identification
Three major DNA identification technologies are related with molecular hybridization signal (RFLP), PCR amplification fingerprint (RAPD, etc.), and nucleic acid sequence analysis (ITS, rbcL, etc.), respectively. Three studies are based on molecular hybridization signals and PCR amplification of fingerprints. The deer blood was identified using RFLP techniques; DNA sequence differences can be used to generate specific restriction enzyme sites that result in changes in the length or the amount of the enzyme fragment for identification (Wang, Chen, Ren, & Wang, 2018). RAPD technology was used to identify the donkey skin (Tian et al., 2013) and Fritillaria thunbergii (Li, Huang, Zhao, & Chen, 2014). In addition, some studies used the DNA barcoding of medicinal plants, e.g., Scutellaria baicalensis (Xia, Feng, Gao, Li, & Zhang, 2014), Atractylodes lancea (Shao et al., 2015), and Bupleuri Radix (Wang et al., 2017). Molecular identification technologies, especially DNA barcoding technology, have been an effective supplement to conventional identification methods (Chen et al., 2014).
4.2. Quality evaluation of Chinese herbal medicine
CHM is rich in various active compounds, synergistic components, ineffective components, and toxic components. These ingredients may change due to germplasm resources, adulteration, ecological environment, cultivation mode, harvesting time, processing, storage conditions, and other factors, thus affecting the quality of CHM. Therefore, comparing active ingredients of the same species in different environments or harvesting periods, or with different processing methods is also crucial for the quality control of CHM.
4.2.1. Analysis of active ingredients in Chinese herbal medicine
Most articles focused on the determination of the active ingredients of CHM, including determining the content of effective components, establishing a fingerprint of CHM, and the influence of different processing methods on the effective components, etc. Numerous studies were conducted to establish the fingerprint of CHM for the quality control or the comparison between different breeds. For example, on the HPLC fingerprint of Anoectochilus candidus, there were 22 characteristic peaks that distinguish active components from inactive ones. This fingerprint provides a basis for the comprehensive evaluation of the quality of A. candidus (Wu & Huang, 2015). Eupolyphaga Steleophagai contains 18 specific amino acids, and the HPLC fingerprints of E. Steleophagai and their adulterants were established using the OPA-FMOC online derivatization method (Wang et al., 2016). From the HPLC fingerprint, it can be concluded that the active ingredient content of Lindera aggregate produced in Tiantai, Zhejiang Province is higher than those of the other five producing areas (Fang, Chen, Yu, Jin, & Huang, 2013).
In addition to fingerprint technology, the “Quantitative Analysis of Multi-components by Single marker (QAMS)” method was also adopted in some studies. For instance, the QAMS method was used to determine five active constituents of Draconis Resina, and the results were comparable to those determined by the external standard method (Wan, Wang, Fang, Xiong, & Mei, 2017). Although QAMS was first proposed in 2006 (Wang, Gao, Fu, & Wang, 2006), no enough attention was paid to it, and only a handful of articles were published in Chinese Traditional and Herbal Drugs from 2006 to 2015. The new concept of “quality marker (Q-Marker)”, first proposed by Chang-xiao Liu, was published in Chinese Traditional and Herbal Drugs in 2016 (Liu et al., 2016). Orydalis ohizoma was taken as an example to illustrate research based on the concept of Q-marker (Zhang et al., 2016). The research group of Xiao-he Xiao put forward the new concept of “effect-equivalent” (Zhang, Xiao, Wang, & Wang, 2015). Both groups carried out a large number of studies and provided new ideas for the quality evaluation of CHM.
4.2.2. Analysis of inorganic elements in Chinese herbal medicine
In addition to active ingredients, the efficacy of CHM is also related with trace elements found in some herbs. Since 2013, Chinese Traditional and Herbal Drugs has published five articles on the analysis of inorganic elements in CHMs. The inorganic elements in Polygoni Multiflori Radix were analyzed by ICP-MS, and the inorganic elements were compared between different origins and commercial herbs (Luo et al., 2015). Inorganic elements can directly participate in the regulation of essential elements in vivo and produce synergistic effects with the medicinal organic components to enhance their curative effects. However, excessive heavy metal elements affect the safety of CHMs. The same study found that heavy metal elements, including Hg, As, and Cr, exceeded the standard of “green industry for import and export of medicinal plants and preparations” (Luo et al., 2015).
4.2.3. Analysis of pesticide residues and mycotoxin in Chinese herbal medicine
With the growth of artificial cultivation of CHM, the toxicity of pesticide residues is also drawing close attention due to safety concerns. A few articles reported the detection of pesticide residues and aflatoxin. Eight kinds of organochlorine pesticide residues in Anoectochili Roxburghii Gemma Terminalis, i.e., BHC (α-BHC, β-BHC, γ-BHC, and δ-BHC) and DDT (PP'-DDE, PP'-DDD, OP'-DDT, and PP'-DDT), were detected using gas chromatography (GC) (Shen et al., 2016). The immunoaffinity column HPLC method with post column photochemical derivatization and fluorescence detection was used to determine the aflatoxin residue of animal medicines, and a few animal drugs were found to have the aflatoxin contamination (Liu et al., 2017). This study suggested that the standardization of CHM storage should be immediately considered to prevent mildew and reduce safety risks for drug use.
4.3. Research gap in quality analysis and evaluation of Chinese herbal medicine
The authenticity of CHM is the basis for ensuring their clinical use. Most articles use molecular techniques for the identification of CHM, and only a few articles use classical identification methods. The identification methods have become more and more convenient and accurate. However, these methods are not suitable for the identification of large quantities of medicinal materials, and it is time-consuming and laborious to regulate and monitor the medicinal materials on the market. Therefore, it is recommended that new high throughput technologies and methods should be developed and applied. In addition, the detection of active ingredients is to ensure the effectiveness of CHM, and the detection of toxic and hazardous substances is to ensure the safety of CHM. However, papers on pesticide residues, mycotoxin and toxic ingredients detection are too few as compared with those of the active components. Pyrrolizidine alkaloids (PAs), aristolochic acids (AAs) and some other toxic compounds are found in a variety of CHMs, and are harmful to human health if used improperly (Li, Xia, Ruan, Fu, & Lin, 2011; Vanherweghem, Depierreux, Tielemans, Abramowicz, & Vanhaelen-Fastre, 1993). These toxic compounds should be quantified to ensure the medication safety of the relevant CHMs. Therefore, the detection of toxic ingredients should be given priority.
5. Bioengineering of Chinese herbal medicine
5.1. Cell engineering
Tacca chantrieri Andre is a national tertiary protection plant. The best combinations of explants and hormones were selected to induce the callus and establish an asexual system for T. chantrieri. This research provided technical support for the protection and scientific development of T. chantrieri and provided a reference for its further study (Wei et al., 2013). With the leaf of Saposhnikovia divaricate as the explant, the S. divaricate callus was successfully induced and a stable and efficient plant regeneration system was established (Fu, Huang, Wang, Hui, & Yang, 2018). However, plant cell cultures require high conditions and batch results varied widely. The cell growth was slow and the yield was low. Currently there are less studies of plant cell engineering.
5.2. Genetic engineering
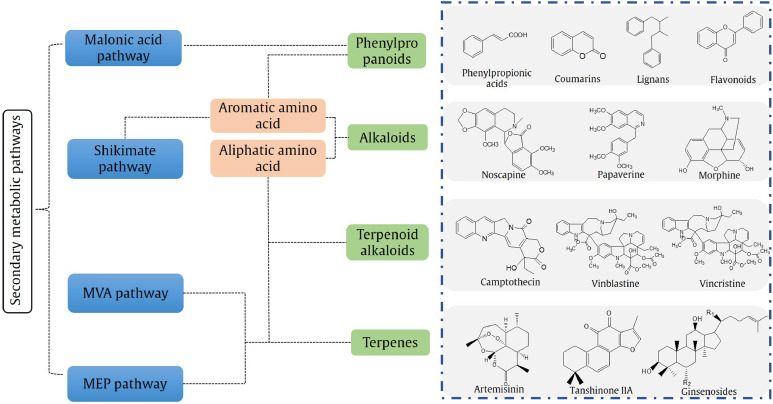
Genetic engineering is a very important topic in Chinese Traditional and Herbal Drugs. Although natural products are complex in structure, most of them are synthesized through several main metabolic pathways from a few precursor substances, such as acetic acid and amino acids; MVA, MEP, the shikimate pathway and the malonic acid pathway are especially highlighted. The main synthetic routes of CHM natural products are shown in Fig. 3. There are many key enzymes in each pathway. Although different plants may contain the same components, the enzymatic characteristics and the regulatory mechanisms of these key enzymes are distinct in different plants. More than 100 papers studied the cloning and expression of key enzymes in different medicinal plants, and most of them are related to MVA or MEP pathways (e.g., FPPS in Atractylodes lancea (Jiang et al., 2017) and sesquiterpene synthase (SES) in Dendrobium officinale (Shen et al., 2017)). Key enzymes in shikimate and malonic acid pathways, such as chalcone isomerase (CHI) in Fagopyrum dibotrys (Luo et al., 2013) and Scutellaria baicalensis (Guo, Cheng, Yang, Liu, & Han, 2015), are also investigated, as well as the synthesis pathways of alkaloids (Wang et al., 2016) and the aspartic acid metabolic pathway (Wang et al., 2017).
Fig. 3.
Main synthetic route of natural products of CHM
Note: The structures are the natural active components that has been widely studied in recent years.
The screening and analysis of internal reference genes and the analysis of key enzymes involved in plant growth under stress are also important. The phloem development (APL) genes from Taxus chinensis were cloned and altered, and their potential regulatory role in tissue regeneration after bark girdling was revealed by investigating their expression profiles (Li, Yang, Liu, Wang, & Qiu, 2015). Three actin gene sequences were cloned from Pseudostellaria heterophylla for the first time, and the PhACT2 gene was determined to be a suitable internal standard gene for the expression analysis of functional genes in P. heterophylla (Ding et al., 2016). An inorganic phosphate transporter (PiT) gene was cloned from Polyporus umbellatus and bioinformatics and expression mode analyses were performed. The results laid the foundation for further functional determination of genes involved in phosphorus translocation regulation and symbiotic process (Liu, Xin, Wang, & Guo, 2017).
There are 17 articles related to transcriptomics, most of which were published in 2017 and 2018. Armillaria mellea and Zhaotong Gastrodia elata vegetative stems were used as the experimental material to reveal the symbiosis mechanism of A. mellea infecting G. elata through the comparative transcriptome sequencing analysis (Tan et al., 2018). Illumina HiSeq 2500 sequencing was used to sequence the leaf transcriptome of Tussilago farfara at different stages, and the expressions of phenylpropanoid biosynthesis genes at different growth stages were compared to predict the optimal time for the synthesis and accumulation of phenylpropanoids (Nie et al., 2018). Transcriptome analysis is an important part of herbgenomics (bencao genomics), and the use of omics technology will be expanded in the future (Xin et al., 2019)
5.3. Research gap in bioengineering of Chinese herbal medicine
The development of cell engineering technology can increase the resources of medicinal plants to some extent. The plant cell culture and adventitious root culture technology were used to establish the first industrialized production line of CHM cells, which enables the industrial production of endangered medicinal plants such as Saussurea involucrata and Panax ginseng (Huang, 2016). However, the articles of basic research are too few, and no papers are about the application of cell engineering in the large-scale production and industrial applications. In addition, the genetic engineering studies of the key enzyme genes were mainly about the upstream pathways, and the key enzymes in the downstream pathway have not been reported. In order to promote the sustainable use of medicinal plants, it is essential to have a complete understanding of the biosynthesis of natural active ingredients in CHM plants.
6. Discussion and perspective of CHM resources
Chinese Traditional and Herbal Drugs has an archive of 781 articles related to CHM resources. The articles can be divided into four categories: germplasm resources of CHM, cultivation of CHM, quality analysis and evaluation of CHM, and bioengineering of CHM. The classification of the articles is shown in Fig. 4. The articles relating to quality evaluation and analysis of CHM are most abundant, indicating that the effectiveness and safety of CHM are the primary focus of current studies. Articles relating to bioengineering are also of significant importance due to the application of advanced technologies.
Fig. 4.
Classification of articles in Chinese Traditional and Herbal Drugs in 2013−2018
Note: The Numbers represent the number and proportion of articles in each section.
6.1. Constructing core germplasm of medicinal plants and expanding germplasm resources
The genetic diversity analysis of medicinal plant resources can provide guidance for the collection, preservation, classification, evaluation, and establishment of core germplasm of medicinal plant resources. Frankle (1984) first proposed the core germplasm, which could improve the utilization efficiency of germplasm bank and facilitate its management. China's first national CHM germplasm resource database was completed and launched at the Institute of Medicinal Plant Development (IMPLAD), Chinese Academy of Medical Sciences (CAMS) in 2007 (Liu et al., 2007). The establishment of the database was of great significance for ensuring the quality of medicinal materials, discovering new varieties and breeding improved varieties, preserving biodiversity, and ensuring the sustainable use of medicinal resources. However, the improvement and operation of the medicinal plant germplasm resource bank still faces many difficulties, such as difficulties in collecting germplasm of wild medicinal plants and loss of viability during its cryopreservation. A complete system for the collection and preservation of medicinal plant germplasm needs to be established, and the strength of domestic and international scholars should be combined to expand the range of medicinal plant species and populations. A follow up test of the germplasm viability and a timely update of the germplasm will also be crucial to the long-term development of germplasm resources.
6.2. Combining molecular research with field experiments and promoting the in-depth study of cultivation of CHM plants
The cultivation of medicinal plants is directly related to the quality and yield of CHM. It is limited by many conditions, including biological factors and abiotic factors. The traditional field experiment takes a long time and has many uncontrollable factors. To draw a reliable conclusion, repeated experiments are warranted. Research of the interaction between various factors and the determination of main influential factors at the molecular level may be a future direction. To study the influences of allelochemicals on the microbial community of ginseng cultivating soil, the carbon metabolic ability and genetic polymorphisms were analyzed by Biolog and RAPD methods, respectively; It was found that allelochemicals significantly reduced the genetic diversity and carbon metabolism of soil microbes in the new reforestation forest of ginseng (Li, Ying, Zhao, & Ding, 2014). The comparative genome and transcriptomic analyses revealed common and species-specific desiccation tolerance strategies in Selaginella tamariscina, providing significant insights into the desiccation tolerance mechanism and the evolution of resurrection plants (Xu et al., 2018). The effects of Bacillus pumilus (endophytes) inoculation on growth, metabolite accumulation, and related protein expression were probed, and the antioxidant defense mechanisms of Glycyrrhiza uralensis Fisch. with respect to the endophyte were investigated. The findings demonstrated that B. pumilus improved G. uralensis growth under drought stress through the modification of antioxidant accumulation and enhanced glycyrrhizic acid content by the incremental expression of key enzymes (Xie, Chu, Zhang, Lang, & Zhang, 2019). However, basic molecular research is not enough; Field experiments should follow after the molecular mechanism is elucidated.
6.3. Improving quality evaluation methods and strengthening the identification of Chinese patent medicine
The quality of CHM is directly related to clinical efficacy, and the chemical components are the material base of CHM to exert its efficacy. The efficacy and safety of drugs can be guaranteed only when the effective components and toxic components of CHM meet the defined standards; then these drugs can be used in clinical practice. The method of determining the content of single or several components to evaluate the quality of CHM has limitations. The ideal CHM quality control is to analyze and detect the active components under conditions that ensure the pharmacodynamic substances of CHM are stable. However, it is very difficult to study the basic pharmacodynamic materials, and it is more difficult to elucidate the pharmacodynamic basis of CHM accurately. Q-marker, the quality control of CHM based on the biological effect benchmark, may be a beneficial supplement to the quality standard of CHM, providing a new reference for the research of CHM quality control (Liu, 2019).
Pesticide residues and heavy metal elements introduced by improper cultivation and processing, and mycotoxins caused by improper storage, will affect the efficacy of CHM and hinder the use and development of CHM. A review summarized the extrinsic harmful residues contaminating CHMs, as well as their types, detection methods, national and international regulations (Liu, Qin, Dou, Yang, & Sun, 2018). The pesticide contamination of 313 samples, including Paeoniae Radix Alba, Chaenomelis Fructus, and Moutan Cortex, were tested by the QuEChERS-UPLC-MS/MS method. The pesticides detected were below standard levels, but several banned pesticides were detected. Future studies should focus on mixed matrices and PFs following decocting (Xiao et al., 2019). The detection of toxic components should also be included in the quality evaluation of CHM.
Chinese patent medicine is also an important component of CHM. “Chinese pharmacopeia” contains 1493 kinds of Chinese patented medicines. The composition of Chinese patented medicines is complex and the dosage form varies. Counterfeit medicines and other impurities will affect its quality and endanger human health. Lacking of accurate identification methods of Chinese patented medicines will restrict the application of CHM throughout the world. Therefore, improving the identification standard of Chinese patented medicines and strengthening quality control are also the main task of the CHM identification. The high-throughput sequencing technology was used to identify the biological components of Liuwei Dihuang Wan (Cheng et al., 2014) and Jiuwei Qianghuo Wan (Xin et al., 2018). With the development of high-throughput sequencing technology and molecular technology, more and more mature and convenient systems will be available for the identification of CHM.
6.4. Promoting the sustainable development of CHM by bioengineering and synthetic biology
“Herbgenomics (bencao genomics)” was proposed (Chen & Song, 2016), including structural genomics, functional genomics, and epigenomics, etc. The concept of “model medicinal species” made a breakthrough in the development of medicinal plants, and is the key foundation of “herbal genomics effect” in the “omics” era (Liu, 2016). The biosynthesis and regulation of active compounds in Salvia miltiorrhiza, a model medicinal plant, have been the research hotspot (Xu, Ji, Zhang, Song, & Chen, 2016). As above mentioned, most natural products are synthesized from a few precursor substances, however, relevant enzymes in the downstream pathway need to be fully identified. So far, the biosynthetic pathways of many well-known natural active ingredients, such as artemisinin, taxol, and tanshinone, have not been completely analyzed. With the development of high-throughput sequencing technology, the analysis of the synthetic pathway of active ingredients has been accelerated and has provided abundant information for the synthetic biology of natural products.
The synthetic biology can be very helpful in the sustainable utilization of CHM resources, and bioengineering is an important supporting technology of synthetic biology. Producing active ingredients by synthetic biology is not affected by changes in various environmental factors, such as climate, pests, geography and seasonal restrictions, and the standardized production system can be used, with shorter production cycle than whole plants, and more stable quality and production. At present, the synthetic biology studies of artemisinin (Martin, Pitera, Withers, Newman, & Keasling, 2003; Ro et al., 2006), taxol (Ajikumar et al., 2010), baicalein, and scutellarein (Li et al., 2019) have been successful. The synthetic biology could help solve the problem of CHM exhaustion and contribute to the sustainable development of CHM resources.
Declaration of Competing Interest
The authors declare no conflict of interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81874339) and Sichuan Science and Technology Program (No. 2018SZ0061).
References
- Ajikumar P.K., Xiao W.H., Tyo K.E.J., Wang Y., Simeon F., Leonard E. Isoprenoid pathway optimization for taxol precursor overproduction in Escherichia coli. Science (New York, N.Y.) 2010;330(6000):70–74. doi: 10.1126/science.1191652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Zhang T.J., Zhang J.Y., Gong S.X., Xu J., Liu C.X. Characterization of smell and taste of pungent-taste herbs based on electronic nose and electronic tongue. Chinese Traditional and Herbal Drugs. 2016;47(11):1962–1967. [Google Scholar]
- Chan K., Liu H.C., Li J.L., Luo F.L., Wang H.L., Huang M.J., et al. Genetic diversity of Gastrodia elata based on srap analysis. Chinese Traditional and Herbal Drugs. 2014;45(20):2974–2981. [Google Scholar]
- Chen S.L., Pang X.H., Song J.Y., Shi L.C., Yao H., Han J.P., et al. A renaissance in herbal medicine identification: From morphology to DNA. Biotechnology Advances. 2014;32(7):1237–1244. doi: 10.1016/j.biotechadv.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Chen S.L., Song J.Y. Herbgenomics. China Journal of Chinese Materia Medica. 2016;41(21):3881–3889. doi: 10.4268/cjcmm20162101. [DOI] [PubMed] [Google Scholar]
- Cheng X.W., Su X.Q., Chen X.H., Zhao H.X., Bo C.P., Xu J., et al. Biological ingredient analysis of traditional Chinese medicine preparation based on high-throughput sequencing: The story for liuwei dihuang wan. Scientfic Reports. 2014;4:5147. doi: 10.1038/srep05147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz Nietoa C.H., Granerob A.M., Alicia Zon M., Fernández H. Sterigmatocystin: A mycotoxin to be seriously considered. Food and Chemical Toxicology. 2018;118:460–470. doi: 10.1016/j.fct.2018.05.057. [DOI] [PubMed] [Google Scholar]
- Ding L., Li J., Zhou T., Zheng W., Long D.K., Jiang W.K. Cloning and sequence analysis of three actin gene fragments from Pseudostellaria heterophylla. Chinese Traditional and Herbal Drugs. 2016;47(11):1935–1942. [Google Scholar]
- Fang L., Chen F.L., Yu C.Q., Jin Z.M., Huang R.P. HPLC fingerprint analysis of Linderae Radix from different habitats. Chinese Traditional and Herbal Drugs. 2013;44(2):229–231. [Google Scholar]
- Frankle O.H. Genetic Manipulation: Impact on Man and Society. Cambridge University Press; 1984. Genetic perspectives of germplasm conservation; pp. 161–170. [Google Scholar]
- Fu H., Huang H.Q., Wang Y., Hui F., Yang S.H. Callus induction and establishment of plant regeneration system of Saposhnikovia divaricate. Chinese Traditional and Herbal Drugs. 2018;49(13):3127–3133. [Google Scholar]
- Gao F.R., Li K., Hao X., Wang G.Z., Qin X.M. Identification of cultured and natural Astragali Radix based on fingerprint of monosaccharides. Chinese Traditional and Herbal Drugs. 2015;46(14):2134–2142. [Google Scholar]
- Guo S.S., Cheng L., Yang L.M., Liu C.J., Han M. Cloning and bioinformatic analysis of chalcone isomerase in Scutellaria baicalensis. Chinese Traditional and Herbal Drugs. 2015;46(10):1506–1511. [Google Scholar]
- Hou X.Q., Ren X.Y., Fu Y.J., Wang H., Zuo X.L., Lv H.C., et al. Study on antimicrobial activity and classification of endophytic fungi from Glehnia littoralis. Chinese Traditional and Herbal Drugs. 2015;46(19):2932–2936. [Google Scholar]
- Huang B., Cheng Y.L., Cao X.J., Li Y., Chen R., Cao J., et al. HPLC fingerprint of Cordyceps sinensis and its confused species and identification of common composition. Chinese Traditional and Herbal Drugs. 2017;48(5):991–996. [Google Scholar]
- Huang L.Q. Dalian Practical Biotechnology Company Ltd; Liaoning: 2016. Research and industrialization of rare and endangered medicinal plant cells and adventitious root cultures such as Saussurea involucrata and Panax ginseng. [Google Scholar]
- Ji K.K., Xie H.Q., Cai S., Zeng J.G. Accumulation of main alkaloids composition in growth process of different parts of Phellodendron chinense. Chinese Traditional and Herbal Drugs. 2014;45(23):3462–3466. [Google Scholar]
- Jiang L., Gu W., Chao J.G., Sang X.H., Han Y., Liu Q.Z., et al. Gene cloning of farnesyl pyrophosphate synthase in Atractylodes lancea and its expression pattern analysis. Chinese Traditional and Herbal Drugs. 2017;48(4):760–766. [Google Scholar]
- Kang M., Huang H.W. Allozymic variation and genentic diversity in Malus hupehensis (Rosaceae) Biodiversity Science. 2002;10(4):376–385. [Google Scholar]
- Li J.H., Tian C.F., Xia Y.H., Mutanda I., Wang K.B., Wang Y. Production of plant-specific flavones baicalein and scutellarein in an engineered E. coli from available phenylalanine and tyrosine. Metabolic Engineering. 2019;52:124–133. doi: 10.1016/j.ymben.2018.11.008. [DOI] [PubMed] [Google Scholar]
- Li M., Huang L.M., Zhao X., Chen Q. Specific pcr identification of Fritillaria thunbergii. Chinese Traditional and Herbal Drugs. 2014;45(12):1754–1757. [Google Scholar]
- Li N., Xia Q., Ruan J., Fu P., Lin G. Hepatotoxicity and tumorigenicity induced by metabolic activation of pyrrolizidine alkaloids in herbs. Current Drug Metabolism. 2011;12:823–834. doi: 10.2174/138920011797470119. [DOI] [PubMed] [Google Scholar]
- Li T., Song B., Wang C.Q., Li T.H. Analysis on soil fungal community structures in propagation of Cordyceps sinensis by denaturing gradient gel electrophoresis. Chinese Traditional and Herbal Drugs. 2013;44(4):478–481. [Google Scholar]
- Li T., Su C., Li L.X., Li C., Li C., Si M.X. Identification of Rhodiola quadrifida and Rhodiola crenulata based on NMR fingerprint and chemical pattern recognition method. Chinese Traditional and Herbal Drugs. 2018;49(16):3918–3925. [Google Scholar]
- Li Y.Y., Yang M.F., Liu H.W., Wang S., Qiu D.Y. Cloning and expression analysis of tcapls in Taxus chinensis. Chinese Traditional and Herbal Drugs. 2015;46(10):1512–1519. [Google Scholar]
- Li Y., Ying Y.X., Zhao D.Y., Ding W.L. Influence of allelochemicals on microbial community in ginseng cultivating soil. Chinese Herbal Medicines. 2014;6:313–318. [Google Scholar]
- Liu C.M., Qin J.A., Dou X.W., Yang M.H., Sun X.B. Extrinsic harmful residues in Chinese herbal medicines: Types, detection and safety evaluation. Chinese Herbal Medicines. 2018;10:117–136. [Google Scholar]
- Liu C.X. Medicinal model plants: Breaking the traditional medicine research methods. Chinese Herbal Medicines. 2016;8:1–2. [Google Scholar]
- Liu C.X. Develop theoretical methods and strategies of Q-marker, to research and improve the scientific and technological level of TCM. Acta Pharmaceutica Sinica. 2019;54(2):15–16. [Google Scholar]
- Liu C.X., Chen S.L., Xiao X.H., Zhang T.J., Hou W.B., Liao M.L. A new concept on quality marker of Chinese materia medica: Quality control for chinese medicinal products. Chinese Traditional and Herbal Drugs. 2016;47(9):1443–1457. [Google Scholar]
- Liu L.N., Li Y.L., Jin H.Y., Ma S.C. Determination of aflatoxins in animal medicines by immunoaffinity column and HPLC-FLD with photochemical derivatization fluorescence detection. Chinese Traditional and Herbal Drugs. 2017;48(6):1220–1224. [Google Scholar]
- Liu M.M., Xin Y.M., Wang A.R., Guo S.X. Molecular cloning and characterization of an inorganic phosphate transporter protein encoding gene in Polyporus umbellatus. Chinese Traditional and Herbal Drugs. 2017;48(22):4734–4739. [Google Scholar]
- Liu Y.N., Shi D.H., Lei L.C. HPLC fingerprint identification of Lonicera japonica flos and Lonicera flos. Chinese Traditional and Herbal Drugs. 2017;48(4):773–776. [Google Scholar]
- Liu Z.L., Wei J.H., Chen S.L., Zhang S.F., Yu J., Li X.E., et al. The analysis of the construction technology of the national medicinal plant gene bank. Modernization of Traditional Chinese Medicine and Materia-MedicWorld Science and Technology. 2007;9(5):72–76. [Google Scholar]
- Luo G.L., Wang Y., Li H.Q., Shu Z.H., Qin L.P., Zheng C.J. Qualitative and quantitative identification of Vitex negundo var. cannabifolia fruits and Vitex negundo fruits. Chinese Traditional and Herbal Drugs. 2017;48(17):3624–3628. [Google Scholar]
- Luo X.P., Bai Y.C., Gao F., Li C.L., Cheng H., Wu Q. Gene cloning and expression level of chalcone isomerase during florescence and content of flavonoids in Fagopyrum dibotrys. Chinese Traditional and Herbal Drugs. 2013;44(11):1481–1485. [Google Scholar]
- Luo Y.Y., Liu J.X., Hou Y., Liu X.H., Lan C.W., Ma Y., et al. ICP-MS analysis on inorganic elements in Polygoni multiflori radix from different habitats and commercial herbs. Chinese Traditional and Herbal Drugs. 2015;46(7):1056–1064. [Google Scholar]
- Martin V.J.J., Pitera D.J., Withers S.T., Newman J.D., Keasling J.D. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nature Biotechnology. 2003;21(7):796–802. doi: 10.1038/nbt833. [DOI] [PubMed] [Google Scholar]
- Minami M., Yasueda M., Shirako T., Murakami T., Mori T., Fujii T., et al. Ecological, phylogenetical, and pharmacognostical characteristics of Aconitum kiyomiense endemic to Hida Highlands, Takayama City, Gifu Prefecture, Japan. Journal of Natural Medicine. 2019;73(3):523–532. doi: 10.1007/s11418-019-01296-6. [DOI] [PubMed] [Google Scholar]
- Nie J.H., Zhang F.S., Tian D., Guo D., Lei Z.H., Qin X.M., et al. Comparative transcriptomic analysis of leaves of Tussilago farfara in different development stages. Chinese Traditional and Herbal Drugs. 2018;49(13):3095–3101. [Google Scholar]
- Palma-Rojas C., Gonzalez C., Carrasco B., Silva H., Silva R.H. Genetic, cytological and molecular characterization of chia (Salvia hispanica L.) provenances. Biochemical Systematics and Ecology, 73. 2017:16–21. [Google Scholar]
- Pang Y., Chen D.X., Song X.H., Wang Y., Li L.Y. Genetic diversity of cultivated Gardenia jasminoides germplasms detected by scot markers. Chinese Traditional and Herbal Drugs. 2018;49(14):3376–3381. [Google Scholar]
- Peng L., Yang B.Y., Cheng H.Y., Zhang G., Sun T., Zhang M.Y., et al. Effects of different drying methods on active constituents of root bark and root of Polygala tenuifolia. Chinese Traditional and Herbal Drugs. 2018;49(21):5010–5017. [Google Scholar]
- Qiang Z.Z., Wang Y., Wang M.W., Li C.Y. Correlation of contents between soil nutrients and calycosin and formononetin in Hedysari Radix. Chinese Traditional and Herbal Drugs. 2015;46(22):3409–3413. [Google Scholar]
- Ro D.K., Paradise E.M., Ouellet M., Fisher K.J., Newman K.L., Ndungu J.M., et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440:940–943. doi: 10.1038/nature04640. [DOI] [PubMed] [Google Scholar]
- Shao J., Gu W., Chao J.G., Geng C., Sun H.M., Li M.Y. Molecular identification of Atractylodes lancea and its closely related species based on ITS2 sequence. Chinese Traditional and Herbal Drugs. 2015;46(8):1209–1215. [Google Scholar]
- Shao Q.Q., Li D., Jiang P., Zeng C.Y., Ade J.J., Chen Y., et al. Effects of combined application of N, P, and k on yield and quality of Chuanmingshen violaceum. Chinese Traditional and Herbal Drugs. 2018;49(16):3926–3932. [Google Scholar]
- Shen T.M., Liu Z.Y., Wu Z.Y., Shi X.H., Wu J.J., Huang C.Q., et al. Determination of 666 and ddt pesticide residues in Anoectochili Roxburghii Gemma Terminalis by gas chromatography. Chinese Traditional and Herbal Drugs. 2016;47(22):4082–4084. [Google Scholar]
- Shen W.Q., Chen L.L., Zhang C.M., Lin Y., Cai Y.P., Fan H.H. Cloning and expression analysis of sesquiterpene synthase gene in Dendrobium officinale. Chinese Traditional and Herbal Drugs. 2017;48(23):4963–4969. [Google Scholar]
- Su H.L., Zhou X.Z., L X., Chen M.J., Zhou J.T., Tang J.Y., et al. Physicochemical changes of Paris polyphylla var. chinensis seed during different stages of germination. Chinese Traditional and Herbal Drugs. 2017;48(22):4755–4763. [Google Scholar]
- Tan Y.W., Bao Y., Cao J.J., Zhang H.L., Chen L.M., Xu H.N., et al. Transcriptome analysis on symbiotic molecular mechanism of Armillaria mellea and Gastrodia elata. Chinese Traditional and Herbal Drugs. 2018;49(17):4125–4130. [Google Scholar]
- Tian J.S., Na L.D., Xiang H., Chen J.L., Ma X.Q., Qin X.M. Identification of donkey-hide and its counterfeits based on 1H NMR metabolomics. Chinese Traditional and Herbal Drugs. 2015;46(2):255–261. [Google Scholar]
- Tian J.S., Shi B.Y., Zhang F.S., Li X.W., Geng W.H., Yang Y., et al. Establishment of rapd for analysis on furs of Equus asinus and discrimination from Equus caballus orientalis. Chinese Traditional and Herbal Drugs. 2013;44(3):354–358. [Google Scholar]
- Vanherweghem J.L., Depierreux M., Tielemans C., Abramowicz D., Vanhaelen-Fastre R. Rapidly progressive interstitial renal fibrosis in young women: Association with slimming regimen including Chinese herbs. The Lancet. 1993;341:387–391. doi: 10.1016/0140-6736(93)92984-2. [DOI] [PubMed] [Google Scholar]
- Wan Q., Wang W.Q., Fang J.G., Xiong H., Mei Z.N. Determination of five active components in Draconis Resina by qams method. Chinese Traditional and Herbal Drugs. 2017;48(21):4541–4545. [Google Scholar]
- Wang F.X., Chen Y.Y., Ren G.Q., Wang C.M. Identification of deer blood by PCR-RFLP method. Chinese Traditional and Herbal Drugs. 2018;49(8):1914–1918. [Google Scholar]
- Wang H.B., Deng L., Ma Y.C., Yin C.P. Study on identification and determination of Lonicerae Japonicae Flos and Lonicerae Flos by uhplc. Chinese Traditional and Herbal Drugs. 2017;48(12):2516–2521. [Google Scholar]
- Wang H.Z., Zhang E.H., Gao S.F., Zhang Y.H., Jin L., Li Y.D. Regulation and control of Angelic sinensis yield by altitude and analysis on key influence factors. Chinese Traditional and Herbal Drugs. 2013;44(14):1990–1994. [Google Scholar]
- Wang X.L., Li Q., Li B.H., Hui Y., Chen Y.Y., Bi K.S. HPLC fingerprint analysis for Eupolyphaga steleophaga and its adulterants. Chinese Traditional and Herbal Drugs. 2016;47(10):1780–1784. [Google Scholar]
- Wang Y.D., Han X.N., Zhao Y.D., Lei T.L., Han L., Zhang Y.F. Identification of commercial Bupleuri Radix and its adulterants based on ITS2 barcode. Chinese Traditional and Herbal Drugs. 2017;48(17):3590–3596. [Google Scholar]
- Wang Y.F., Zhang L., Han H.X., Yu Y., Zang P., Mao X.X., et al. Cloning of full-length cDNA of gene encoding dihydrodipicolinate synthase from Carthamus tinctorius and construction of plant expression vector. Chinese Traditional and Herbal Drugs. 2017;48(8):1629–1634. [Google Scholar]
- Wang Z.M., Gao H.M., Fu X.T., Wang W.H. Multicomponents quantitation by one marker new method for quality evaluation of Chinese herbal medicine. China Journal of Chinese Materia Medica. 2006;31(23):1925–1928. [PubMed] [Google Scholar]
- Wang Z.P., Qin B.F., Qiang W., Qiu F., Hou Y.L., Cheng M., et al. Cloning and expression analysis of sesquiterpene synthase gene in Dendrobium officinale. Chinese Traditional and Herbal Drugs. 2016;47(15):2734–2740. [Google Scholar]
- Wei Y., Huang X.Y., Wei K.H., Huang B.Y., Li C., Zhang Z.J. Callus induction and asexual line establishment of Tacca chantrieri. Chinese Traditional and Herbal Drugs. 2013;44(17):2466–2470. [Google Scholar]
- Wei Z., Yu D. Analysis of the succession of structure of the bacteria community in soil from long-term continuous cotton cropping in Xinjiang using high-throughput sequencing. Archives of Microbiology. 2018;200:653–662. doi: 10.1007/s00203-018-1476-4. [DOI] [PubMed] [Google Scholar]
- Wu F.Y., Feng S.G., Zeng J.G. Identification and attribution of Lonicerae Japonicae Flos and Lonicera Flos. Chinese Traditional and Herbal Drugs. 2014;45(8):1150–1156. [Google Scholar]
- Wu P.P., Huang L.Y. Fingerprint analysis of Anoectochili Roxburghii Gemma Terminalis by HPLC. Chinese Traditional and Herbal Drugs. 2015;46(13):1975–1979. [Google Scholar]
- Xia Z., Feng C.Y., Gao Z.M., Li H.M., Zhang H.R. Authentication of dna barcoding of Scutellaria baicalensis and its related species. Chinese Traditional and Herbal Drugs. 2014;45(1):107–112. [Google Scholar]
- Xia Z., Huang Y., Li H.M., Zhou Y., Gao Z.M. Origin of cultivated Rehmannia glutinosa based on chloroplast gene psbA-trnH, trnS-trnG, and nuclear its sequences. Chinese Traditional and Herbal Drugs. 2018;49(2):423–430. [Google Scholar]
- Xiao J.J., Xu X., Wang F., Ma J.J., Liao M., Shi Y.H., et al. Analysis of exposure to pesticide residues from traditional Chinese medicine. Journal of Hazardous Materials. 2019;44(1):48–52. doi: 10.1016/j.jhazmat.2018.11.075. [DOI] [PubMed] [Google Scholar]
- Xie Z.C., Chu Y.K., Zhang W.J., Lang D.Y., Zhang X.H. Bacillus pumilus alleviates drought stress and increases metabolite accumulation in Glycyrrhiza uralensis fisch. Environmental and Experimental Botany. 2019;158:99–106. [Google Scholar]
- Xin T.Y., Xu Z.C., Jia J., Leon C., Hu S.N., Lin Y.L., et al. Biomonitoring for traditional herbal medicinal products using dna metabarcoding and single molecule, real-time sequencing. Acta Pharmaceutica Sinica B. 2018;8(3):488–497. doi: 10.1016/j.apsb.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin T.Y., Zhang, Pu Y., Gao R.R., Xu Z.C., Song J.Y. Trends in herbgenomics. Science China Life Sciences. 2019;62(3):288–308. doi: 10.1007/s11427-018-9352-7. [DOI] [PubMed] [Google Scholar]
- Xu Z.C., Ji A.J., Zhang X., Song J.Y., Chen S.L. Biosynthesis and regulation of active compounds in medicinal model plant Salvia miltiorrhiza. Chinese Herbal Medicines. 2016;8(1):3–11. [Google Scholar]
- Xu Z.C., Xin T.Y., Bartels D., Li Y., Gu W., Yao H., et al. Genome analysis of the ancient tracheophyte Selaginella tamariscina reveals evolutionary features relevant to the acquisition of desiccation tolerance. Molecular Plant. 2018;11:983–994. doi: 10.1016/j.molp.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Yan K.W., Wang F., Mei G.R., Lu J.Y., Zhang L., Fu G.L., et al. Application of near infrared spectroscopy in identification of Citrus reticulata. Chinese Traditional and Herbal Drugs. 2015;46(20):3096–3099. [Google Scholar]
- Yan Y.G., Wang H.Y., Deng C., Zhang G., Chen Y., Shen X., et al. Effects of growth years, altitude, and light factors on contents of eight components in Rheum officinale. Chinese Traditional and Herbal Drugs. 2017;48(11):2285–2291. [Google Scholar]
- Yang M., He P., Duan C.X., Xu M.P. Effects of exogenous 5-aminolevulinic acid on seed germination and seedling photosynthetic characteristics of Belamacanda chinensis. Chinese Traditional and Herbal Drugs. 2014;45(15):2235–2241. [Google Scholar]
- Yang Y.F., Wang J.Y., Zhang G.J., Sun S.Q., Wu H.Z., Guo Y.Z., et al. Analysis and discrimination of integral structure of Paeoniae Radix alba and Paeoniae Radix rubra and their alcohol extracts by infrared spectroscopy. Chinese Traditional and Herbal Drugs. 2016;47(19):3508–3512. [Google Scholar]
- Yao J.B., Jin H.H., He H.H., Wang R.W. Study on specific chromatograms of Crocus sativus and authenticity identification. Chinese Traditional and Herbal Drugs. 2015;46(9):1378–1380. [Google Scholar]
- Yi J., Wu J.G., Zhang X.C., Wu Y.B., Huang Z.H. Pharmaceutical identification of three original plants of Anoectochilus. Chinese Traditional and Herbal Drugs. 2015;46(23):3570–3576. [Google Scholar]
- Yu C., Liang X., Chen J.J., Sheng M.J., Feng Y.B., Wang Z.H. Identification of plants in Fritillariae L. by DNA barcoding technology. Chinese Traditional and Herbal Drugs. 2014;45(11):1613–1619. [Google Scholar]
- Zhang D., Ren M.Y., Zhang Y.D., Guan X. Genetic diversity research on Anisodus tanguticus based on ISSR molecular markers. Chinese Traditional and Herbal Drugs. 2018;49(1):219–226. [Google Scholar]
- Zhang H.Z., Xiao X.H., Wang J.B., Wang J. Consistency of efficacy-equivalent: Key essential point of quality control for Chinese Materia Medica. Chinese Traditional and Herbal Drugs. 2015;46(11):1571–1575. [Google Scholar]
- Zhang J.L., Ma Y.Z., Hu W.L., Lan G.Y. Effects of high temperature stress on physiological indicators of Paris polyphylla var. yunnanensis. Chinese Traditional and Herbal Drugs. 2018;49(17):4131–4137. [Google Scholar]
- Zhang L.D., Gong J.Y. Protection and sustainable reuse of traditional Chinese medicine resources. World Latest Medcine Information. 2016;16(18):203–204. [Google Scholar]
- Zhang S.S., Liu X., Wang J.F., Yu M.J., Huang Z.J., Liu Y., et al. Effect of multiple factors on seeds germination of Paris polyphylla var. yunnanensis and polygerm varieties. Chinese Traditional and Herbal Drugs. 2017;48(10):2111–2115. [Google Scholar]
- Zhang T.J., Xu J., Han Y.Q., Zhang H.B., Gong S.X., Liu C.X. Quality markers research on Chinese materia medica: Quality evaluation and quality standards of Corydalis rhizoma. Chinese Traditional and Herbal Drugs. 2016;47(9):1458–1467. [Google Scholar]
- Zhou H.Y., Li Z., Luo D.H. Identification of Aurantii fructus from different growing areas based on bionic olfaction technology. Chinese Traditional and Herbal Drugs. 2017;48(19):4068–4072. [Google Scholar]
- Zhou N., Zhang J., Pan X.J., Du H.H., Guo D.Q., Ding B., et al. Effect of arbuscular mycorrhizal fungi on endogenous hormones in Paris polyphylla var. yunnanensis. Chinese Traditional and Herbal Drugs. 2017;48(23):4970–4978. [Google Scholar]
- Zhu W.Q., Wang L.J., Liang P., He Z.L., Yan G.H. Analysis of the status quo and future prospects of Chinese medicinal resources sustainable development. World Chinese Medcine. 2018;13(7):1752–1755. [Google Scholar]