Abstract
Objective
The aim of the present study was to determine the quality marker (Q-Markers) of Sparganii Rhizoma against thrombus through an integration of investigations on its antithrombotic effect, content determination and spectrum-effect correlation analysis.
Methods
Based on the concept of Q-Marker, Sparganii Rhizoma was investigated for the identification of chemical component. The pharmacological effects on arachidonic acid-induced thrombosis in zebrafish were also investigated. The material basis in ethanol extract was determined by HPLC-UV. Furthermore, the potential Q-Markers were analyzed and predicted according to the effect-chemical correlation analysis. Finally, the anti-thrombotic Q-Markers were verified through the anti-thrombotic test of monomer components.
Results
The model of thrombosis zebrafish was established with larvae exposed to 100 µmol/L arachidonic acid for 1 h. Nine ingredients in Sparganii Rhizoma were identified as 5-hydroxymethylfurfural, vanillic acid, ferulic acid, p-hydroxybenzaldehyde, p-hydroxybenzoic acid, vanillin, protocatechuic acid, p-coumaric acid and isoferulic acid. According to the determination effect of zebrafish thrombosis model and HPLC content analysis results, all the other contents present positive correlation except 5-hydroxymethylfurfural, and the P values of three representative potential Q-Markers (ferulic acid, protocatechuic acid and p-coumaric acid) were 0.002, 0.001 and 0.026, respectively.
Conclusion
Sparganii Rhizoma showed a dose-dependent effect on the recovery of reducing cardiac red blood cell on zebrafish model. Three phenolic acids (ferulic acid, protocatechuic acid and p-coumaric acid) were proved to possess the anti-thrombotic effects which could be regarded as the potential Q-Markers for quality assessment of Sparganii Rhizoma.
Keywords: anti-thrombotic activity, Q-Marker, Sparganii Rhizoma, zebrafish
1. Introduction
Traditional Chinese medicine (TCM) has been widely used in China for thousands of years in the prevention and treatment of human diseases based on the theories of Chinese medical science. The quality of TCM is the basis of the healthy and sustainable development of TCM industry. However, the emerging limitations, such as unclearness of complex chemical material, unsatisfactory quality control and indefinable therapeutic mechanisms, impede the development of TCM.
In view of this situation, academician Chang-xiao Liu proposed a new concept of quality marker (Q-Marker) based on the theory of TCM and principle of multi-flavor formula used to evaluate the quality of TCM (Liu et al., 2016, Liu, 2016). According to the concept, Q-Marker is defined as a chemical component with five characteristics: 1) Q-Markers can be markers of morphological, histological and genetic characteristics of traditional Chinese medicinal herbs and decoction pieces, or markers of functional qualities from TCM herbs, decoctions, extracts, single or compound agents; 2) Q-Markers are substances that can be qualitatively and quantitatively determined by chemical analysis and biological assay; 3) Q-Markers have specificity in biological effects; 4) Q-Markers have traceability and transmission of industry process; 5) Under the guidance of TCM theories, Q-Markers can observe the rules of prescription combination (Liu, 2017). The first four elements are suitable for the Q-Marker search of single herb.
Several strategies have been applied for the discovery and identification of Q-Markers successfully since the concept of Q-marker was proposed. The search for Q-Markers mainly includes the following research methods: 1) Search for active compounds as Q-Markers through literature search (Ye et al., 2018); 2) Use stoichiometric method to calculate and analyze the Q-Markers (Zhong et al., 2018, Liu et al., 2018, Zhang et al., 2018b, Zhang et al., 2018a) 3) Construct a composition-activity-target network through network pharmacology to search for Q-Markers (Li et al., 2019); 4) Perform pharmacodynamic experiments, conduct composition analysis, and obtain Q-Markers through spectral correlation studies (Qi et al., 2017, Wu et al., 2018, Feng et al., 2018); 5) Q-Markers are obtained through pk research. These methods are all useful attempts to obtain the corresponding Q-Markers from different angles (Zhang et al., 2018b, Zhang et al., 2018a, Nie et al., 2018, Li et al., 2018, He et al., 2018). Besides these researches, Jiang et al. developed a ‘Spider-web’ mode based on discrimination and identification of Q-Markers for quality control of TCM (Jiang et al., 2018). The Q-Markers selected have the optimal comprehensive properties of content, stability, pharmacokinetics and pharmacology in many complex TCM. Kang et al. established a Q-Marker system of burdock in morphology, chemistry and biology (Kang et al., 2019).
Blood stasis is a complex syndrome with blood flow retardation or cessation. The classic TCM, Sparganii rhizoma (SR), showed promising effects on this disease, and especially effective when used in combination with Curcumae Rhizoma. Sparganii Rhizoma, derived from the roots of Sparganium stoloniferum Buch.-Ham, is a herbal medicine with antioxidant, anti-thrombotic, analgesic, anti-inflammatory and anti-cancer activities (Wang et al., 2013b, Wang et al., 2013a). Phytochemical investigations have resulted in the isolation and identification of many compounds such as phenolic compounds, flavonoids, coumarins and some rare stilbenes (Wang et al., 2013b, Wang et al., 2013a, Wang et al., 2012). In Chinese Pharmacopoeia (Edition 2015), traits, microscopic and TLC identification are used for SR source identification and quality control. However, the composition and content of SR from different producing areas have great differences. Therefore, it is necessary to establish a standard of composition that can be used for quality control. In this study, we used the method of spectral correlation to search for the Q-Markers that could represent the anti-thrombotic activity of Sparganii Rhizoma.
2. Materials and methods
2.1. Chemicals, drugs and reagents
Arachidonic acid (AA) were purchased from TCI (Shanghai) Development Co., Ltd., dimethyl sulfoxide (DMSO) were purchased from Sangon Biotech (Shanghai) Co., Ltd., o-dianisdine, 1-phenyl-2-thiourea (PTU), paraformaldehyde and aspirin (1002525379) were bought from Sigma-Aldrich Inc. (St. Louis, MO, USA). 5-Hydroxymethylfurfural (5-HMF), vanillic acid (VA), ferulic acid (FA), p-hydroxybenzaldehyde (PH), p-hydroxybenzoic acid (PHA), vanillin (VN), protocatechuic acid (PA), p-coumaric acid (PCA) and isoferulic acid (IA) were purchased from Sichuan Vicky Biotechnology Co., Ltd. (Sichuan, China). Twenty batches samples of SR were provided by which Pharmaceutical Co., Ltd shown in Table1. The glycerol, 30% H2O2, sodium acetate and ethanol were purchased from Sinopharm chemical reagent Co., Ltd. (Shanghai, China). 4% Paraformaldehyde fix solution was obtained from Wuhan Saiweier Biotechnology Co., Ltd. (Wuhan, China). Pure water was obtained from Hangzhou Wahaha group Co., Ltd (Hangzhou, China).
Table 1.
Sourses of 20 batches of Sparganii Rhizoma.
| Sample number | Locality of growth | Batch number |
|---|---|---|
| 1 | Hebei | 120,801 |
| 2 | Henan | 20,171,220 |
| 3 | Liaoning | 20,160,620 |
| 4 | Hubei | 20,160,806 |
| 5 | Zhejiang | 20,120,301 |
| 6 | Hunan | 20,140,401 |
| 7 | Guangxi | 150,501 |
| 8 | Henan | 20,170,510 |
| 9 | Shanxi | 17,031,001 |
| 10 | Jiangsu | 15,050,115 |
| 11 | Guangxi | 15,050,409 |
| 12 | Hebei | 16,110,201 |
| 13 | Zhejiang | D5070101 |
| 14 | Shandong | 1,603,001 |
| 15 | Neimenggu | 160,503 |
| 16 | Jiangsu | 160,901 |
| 17 | Anhui | 20,171,201 |
| 18 | Hebei | 4,011,811 |
| 19 | Shanxi | 161,108,008 |
| 20 | Anhui | 20,170,515 |
2.2. Zebrafish feeding and maintenance
AB strain zebrafish were purchased from Chinese zebrafish resource center in Wuhan, and bred in the circulation system in the laboratory in Institute of Biology of Shandong Academy of Sciences (Jinan, China). Adult AB zebrafish are raised in light and temperature control of breeding facilities, standard illumination for 14 h/day, live feed brine shrimp, twice a day to dry flake once a day. 3dpf zebrafish larvae were used to complete all of the experiments. One night before collecting the embryos, healthy and mature zebrafish were selected and placed in a mating tank at a ratio of 1:2 in male and female, and embryos were obtained the next morning. The embryos were washed with zebrafish-specific fish water (5 mmol/L NaCl, 0.17 mmol/L KCl, 0.4 mmol/L CaCl2, 0.16 mmol/L MgSO4), and then methylene blue was added for disinfection. To faciliate to microscopic observation, PTU was added to inhibit melanogenesis. The embryos were placed in a 28 °C constant temperature light incubator, and the fish water was changed once a day, at the same time the dead embryos were taken out. Three days later, zebrafish larvae were obtained to perform the bioactivity assay.
2.3. Experimental protocol
For all experiments, zebrafish were kept in fish water containing PTU at a final concentration of 2.5 µmol/L. VA, FA, PH, PHA, VN, PA, PCA and IA were dissolved in DMSO to prepare stock solutions, and then further diluted to the working solution with fish water. Water containing PTU was used to dilute SR extract to working concentration.
The final concentration of DMSO remained below 0.5%. Zebrafish larvae were all 3 DPF. In all experiments, zebrafish were exposed to 24-well plates of 10 fish per plate, and two parallel plates were set for each group. Temperature was 28 °C for all exposure times. The control group received DMSO working solution containing the same DMSO concentration as the other groups in the same experiment, and 100 µmol/L AA was used for the modeling of thrombosis.
AA stock solution: 100 mg arachidonic acid was accurately weighed and dissolved in 41.1 mL water to obtain 8 mmol/L arachidonic acid stock solution, which was diluted at the time of molding.
O-dianisidine working solution: The o-dianisidine stock solution was dissolved by ethanol to the concentration of 5.85 mmol/L, and then diluted by water 1 mL/mL, 0.1 mol/L NaOAc (pH = 4.5) 250 µL/mL, 30% H2O2 50 µL/mL into the working solution.
2.4. Drug efficiency assessment
Healthy 3dpf AB zebrafish larvae were choosen in 24-well plates and 10 zebrafish were placed in each well. Twenty-three experimental groups were set: blank control group, thrombus model group, positive control group, and different drug groups. The thrombus model group was treated with fish water for the first 6 h; The positive control group was treated with 22.5 umol/L aspirin for 6 h; The drug group was treated with 20 batches of SR extracts (600 μg/L) for 6 h; Fish water was added to the blank group for 6 h. All zebrafish were placed in a light incubator at 28 °C for the first 6 h. After 6 h, the zebrafish except the blank control group were added with 100 umol/L AA, and immediately transferred to a 28 °C light incubator lasted 1 h.
2.5. Staining and fixing
Discard all incubation liquid after processing, transferring zebrafish to 2.5 mL epoxide tube, 1 mL o-dianisidine working solution dyeing 3 min without light. Then discard the o-diphenylamine working solution and wash the zebrafish three times. Zebrafish were finally fixed with 4% paraformaldehyde for 12 h and stored in 90% glycerin at 4 °C.
2.6. Image capture and quantitative analysis of zebrafish thrombosis
Cardiac red blood cell intensity of 10 zebrafish in each group was quantitatively analyzed. All images were taken using an inverted fluorescence microscope (Olympus IX53 Tokyo, Japan). Image-pro Plus 5.1 (Media Cybernetios Inc., USA) was used for quantitative analysis of cardiac RBC area, and SPSS Statistics 20.0 (International Business Machines Corporation, USA) was used for statistical analysis. One-way anova was used to compare the differences between the groups, and it was considered that P < 0.05 was statistically significant compared with the model group and the control group. T test was used to compare the statistical significance of the difference in efficacy between SR batches at the level of 0.01, and the inhibition rate (IR) was calculated with the following formulation: IR = [E(drug) − E(model)] × 100% / [E(control) − E(model)] (Qi, et al., 2017).
2.7. Multicomponent determination of Sparganii Rhizoma
Twenty batches of SR were quantitative content analyzed by HPLC on an HPLC system (Shimadzu LC-2030C, Japan) with a UV detector using an InertSustain C18 column (250 mm × 4.6 mm, 5 µm, Shimadzu, USA). Column oven temperature was maintained at 40 °C. Sample compartment temperature was 4 °C. 254 nm was choosen for UV detection. Flow rate of 0.8 mL/min and a gradient elution of mobile phase (A: 0.1% aqueous formic acid in water, B: methanol) were used. The gradient was started at 5% B, then rised to 100% B at 60 min. and returns to the initial conditions after 10 min.
Standard curve method was used to quantify nine contents (5-HMF, VA, FA, PH, PHA, VN, PA, PCA, IA). Bivariate correlation analysis between anti-thrombotic activity and the main components in SR was conducted by SPSS 19.0 software, which was represented by Spearman correlation coefficient (R) and significance (P).
3. Results
3.1. Establishment of zebrafish thrombosis model
After exposing zebrafish to 100 µmol/L AA for 1 h, the zebrafish thrombosis model was established. Representative images of o-dianisidine stained zebrafish were taken by stereoscopic microscope. Compared with control group, the visible RBC's almost disappeared in the hearts of the AA-treated zebrafish (Fig. 1, marked by the red line). These results indicate that the zebrafish thrombosis model has been successfully established. At the same time, the treatment of aspirin at 45 g/mL can restore the number of cardiac red blood cells significantly.
Fig. 1.
Heart red blood cells stained with o-dianisidine in zebrafish model. A: Control group; B: AA group (100 µmol/L); C: Positive drug group (aspirin 45 µg/mL); D: SR group (600 µg/mL).
The effect of SR on AA-induced thrombosis in zebrafish was quantitatively evaluated by measuring RBC intensity in the cardiac area. The results of SR extraction indicated that the pre-incubation dose of 100, 150, 200, 400, 600 and 800 µg/mL could restore the cardiac RBC in a dose-dependent manner (Fig. 2). These findings suggest that this zebrafish model is suitable for the assessment of the efficacy of antithrombotic therapy in SR extracted at doses of 600 µg/mL or higher, and it significantly restored cardiac RBC compared to the AA-induced thrombus formation in zebrafish. Therefore, we selected a concentration of 600 µg/mL to evaluate the efficacy of 20 batches of SR.
Fig. 2.
Heart red blood cells intensity in the zebrafish of control group, model group, positive drug group and SR group. Compared to model group: **P < 0.01, *P < 0.05; Compared to positive drug group: ##P < 0.01, #P < 0.05. n = 10. Control: Control group; Model: AA group (100 µmol/L); Positive: Positive drug group (Aspirin 45 µg/mL); SR group of different concentrations (100–800 µg/mL).
3.2. Speculation on anti-thrombotic components in Sparganii Rhizoma
3.2.1. Multicomponent determination of Sparganii Rhizoma
Thrombosis model and HPLC were used to evaluate the differences of efficacy and compound content between different SR batches, respectively, and compounds that could be used as Q-Markers were screened out. According to the analysis by HPLC, there was a significant difference in the content of compounds between SR batches, as shown in Fig. 3. The peaks of VA, FA, PH, PHA, VN, PA, PCA and IA were labeled for qualitative analysis. The content of major compounds of VA, FA, PH, PHA, VA, PA, PCA, IA were (1.2960 ± 0.8655), (16.4304 ± 7.5170), (2.2035 ± 1.8023), (19.0602 ± 7.9850), (8.8453 ± 6.1880), (28.6204 ± 11.8747), (4.9063 ± 2.4669) and (1.0704 ± 0.9762) µg/mL in SR, respectively.
Fig. 3.
HPLC chromatogram of SR extract. 1. 5-hydroxymethylfurfural; 2. vanillic acid; 3. ferulic acid; 4. p-hydroxybenzaldehyde; 5. p-hydroxybenzoic acid; 6. vanillin; 7. protocatechuic acid; 8. p-coumaric acid; 9. isoferulic acid.
3.2.2. Anti-thrombotic effects of 20 batches of Sparganii Rhizoma
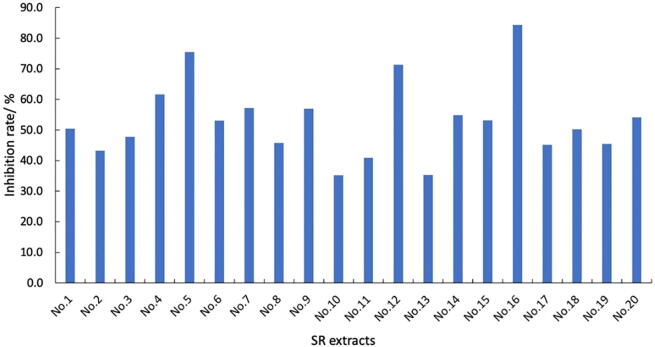
The antithrombotic effects of 20 batches of SR were investigated in zebrafish models of AA-induced thrombosis. At a concentration of 600 µg/mL, different batches of SR showed different degrees of antithrombotic activity compared with the control group and the positive drug group. The anti-thrombotic results were showed in Fig. 4.
Fig. 4.
Anti-thrombotic effects of 20 batches of Sparganii Rhizoma.
3.3. Correlation coefficients between antithrombotic effect and main compounds of SR
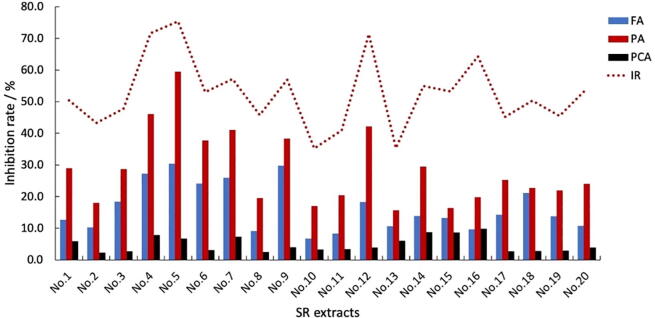
The correlation coefficients between the antithrombotic effect and the main compounds in SR were calculated, as shown in Table 2. It can be seen that except 5-HMF, all the others are positively correlated. As shown in Fig. 5, the efficacy of SR samples from different production batches was significantly different, which was consistent with the content analysis results in Fig. 5. Based on active compounds and content analysis by HPLC, three represents compounds (FA, PA and PCA) can be determined as potential Q-markers, and the total content of these three compounds was proved to be significantly unique (Fig. 5), in accordance with the efficacy of SR, this showed that the FA, PA and PCA could be considered in the quality assessment of potential Q-Markers.
Table 2.
Correlation coefficients between anti-thrombotic effect and contents for major compounds in SR.
| Number | Composition | Correlation coefficient | P-value |
|---|---|---|---|
| 1 | 5-HMF | −0.025 | 0.916 |
| 2 | VA | 0.188 | 0.428 |
| 3 | FA** | 0.657 | 0.002 |
| 4 | PH | 0.205 | 0.385 |
| 5 | PHA | 0.422 | 0.064 |
| 6 | VN | 0.443 | 0.051 |
| 7 | PA** | 0.802 | 0.001 |
| 8 | PCA* | 0.497 | 0.026 |
| 9 | IA | 0.244 | 0.301 |
Note: * significant correlation at 0.05 level; ** significant correlation at 0.01 level.
Fig. 5.
Distinction of concentration of FA, PA and PCA in accordance with inhibition rate between 20 batches of SR samples.
3.4. Identification of Q-Markers (anti-thrombotic components) of Sparganii Rhizoma
Active substances that can be labeled as Q-Markers of SR were identified. Ferulic acid (FA), protocatechuic acid (PA) and p-coumaric acid (PCA) were evaluated. As the statistical results showed in Fig. 6, all compounds proved to be significantly effective on this model and the activity was dose-dependent.
Fig. 6.
Heart red blood cells intensity in zebrafish of control, model (AA 100 µmol/L), FA, PA and PCA groups.
4. Discussion
In China, traditional Chinese medicine formulations are important tools for TCM treatment of diseases, and TCM is the basic component of TCM formulations. Therefore, the quality control of TCM, especially the processed traditional Chinese medicine, is the premise to ensure the quality and efficacy of TCM formulations. As an important medicine for breaking blood and removing blood stasis, Sparganii Rhizoma is often used in formulations for the treatment of stagnation and blood stasis syndrome. As we all know, traditional Chinese medicine often comes from many sources. Take Sparganii Rhizoma for example, its sources include Jiangsu, Henan, Shandong, Jiangxi, Anhui, Guangxi and other provinces. There are great differences in the kinds and contents of its constituents in different producing areas. The correlation between those ingredients and the quality of the medicine has not been studied. Therefore, it is meaningful to establish a standard of composition that can be used for quality control of Sparganii Rhizoma.
Many researchers have used different research methods to study the Q-Markers, but this study believes that the research method based on spectral correlation may be more suitable. Because, through literature search directly or search network pharmacology active substances as Q-Markers will have certain one-sidedness, because a lot of research is the in vitro experiment, and the effectiveness of the in vitro experiment could not judge the body effectively, likewise, in vitro experiment ineffective ingredients into the body could be effective, so the literature search results are controversial. Using chemometrics methods to analyze the component-effect correlation, together with the analysis of pharmacokinetic parameters, and search for Q-Markers is a reliable research idea.
The role of TCM is characterized by the holistic therapeutic effect of multiple components and multiple targets. Due to the lack of organ structure and the limited value of detection in vitro pharmacological model, it is usually challenging to extrapolate these results to the whole organism. Traditional mammalian models of thrombosis in vivo are often laborious, expensive and time-consuming, limiting their use in early screening (Zhu et al., 2016, Chen et al., 2018). Zebrafish is a reasonable model that could mimic mammalian hemostasis and thrombosis since it shares most of the central factors of platelet adhesion, activation, aggregation, and release reaction with humans, and possesses coagulation factors and thrombocyte receptors, and responds to anticoagulant and antiplatelet drugs commonly used in clinical treatment (Weyand and Shavit, 2014, Gregory et al., 2002, Khandekar et al., 2012, Sheng et al., 2020).
The present study intended to identify bioactive Q-Markers for the quality assessment of SR through a zebrafish model based on the efficacy of SR. As for SR, it was reported that it could prevent the occurrence of thrombosis and the aggregation of platelets. We chosed 3 dpf (days post fertilization) zebrafish as the appropriate modeling stage, since zebrafish developed functional platelets and coagulation factors through 36 hpf (hours post fertilization). Qi et al. verified that the length of zebra fish tail vein thrombosis and the heart red blood cell area could be used to evaluate the severity of thrombosis (Qi, et al., 2017). Since the zebrafish tail venous thrombus was reported to be reversely correlated with the RBC intensity, which could be positively correlated with the area of heart red blood cell (RBC) on the same direction, we chose the area of the heart RBC to represent the thrombus severity. After AA treatment, zebrafish heart images and statistical results showed that red blood cells were significantly reduced, indicating that the model has been successfully established. Aspirin and SR significantly improved the damage caused by AA, respectively.
Firstly, we studied the dose–effect relationship of SR in a single batch and found that the strength of erythrocytes in the heart could be significantly improved when the dose was 600 µg/mL. Therefore, 600 µg/mL was used as the dose for the spectrum effect correlation study. Then, on the basis of this dose, we tested the anti-thrombotic activity of 20 batches of SR, established the corresponding HPLC fingerprint, and identified the main peak area components and determined the content of multiple components on the basis of previous studies. Further SPSS analysis was adopted to analyze the spectral-effect correlation coefficient based on the activity and component content, and it was found that the P value of correlation coefficient of FA, PA and PCA was <0.05. Therefore, we took them as Q-Markers candidates. Finally, the activity verification of the these three components showed that the they had significant anti-thrombotic activity, and these three components basically satisfied the four key elements of Q-Marker proposed by academician Chang-xiao Liu. Despite our preliminary study on rat metabolism of SR, there was no presence of PA in plasma, but considering that some components were not functioning in prototype, we still identified PA as a Q-Marker. Therefore, we determined that FA, PA and PCA are Q-Markers for SR anti-thrombotic activity.
Nevertheless, this study still has some limitations. We have only selected one pharmacological model for research, and in subsequent studies, we will further validate it in other models. Meanwhile, we have identified Q-Markers only based on anti-thrombotic activity, not its other activity, such as antioxidant, analgesic, anti-inflammatory, anticancer activity, and so on. Therefore, FA, PA and PCA are Q-Markers just for SR anti-thrombotic activity, not represent the overall quality of SR. In the next study, we will gradually study other active Q-Markers, and strive to provide a Q-Markers system that can express SR quality comprehensively.
5. Conclusions
The zebrafish thrombosis model was utilized to validate the anti-thrombotic effect of SR for the first time. The SR, as well as several constituents, namely PA, PCA, IA, proved to possess significant anti-thrombotic effect. Combining the efficacy assessment and content analysis of the 20 batches of SR extracts, PA, PCA, IA, were identified as the potential efficacy-based components of SR.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study is supported by the Shandong Provincial Chinese Medicine Science and Technology Development Project (2017-165) and Shandong Provincial Natural Science Fund Project (ZR2015HL117).
References
- Chen Y., Chen P.D., Bao B.H., Shan M.Q., Zhang K.C., Cheng F.F., Ding A.W. Anti-thrombotic and pro-angiogenic effects of Rubia cordifolia extract in zebrafish. Journal of Ethnopharmacology. 2018;219:152–160. doi: 10.1016/j.jep.2017.11.005. [DOI] [PubMed] [Google Scholar]
- Feng G., Chen Y.L., Li W., Li L.L., Wu Z.G., Wu Z.J., He X. Exploring the Q-marker of “sweat soaking method” processed Radix Wikstroemia Indica: Based on the “effect-toxicity-chemicals” study. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2018;45:49–58. doi: 10.1016/j.phymed.2018.03.063. [DOI] [PubMed] [Google Scholar]
- Gregory M., Hanumanthaiah R., Jagadeeswaran P. Genetic analysis of hemostasis and thrombosis using vascular occlusion. Blood Cells, Molecules & Diseases. 2002;29(3):286–295. doi: 10.1006/bcmd.2002.0568. [DOI] [PubMed] [Google Scholar]
- He J., Feng X., Wang K., Liu C., Qiu F. Discovery and identification of quality markers of Chinese medicine based on pharmacokinetic analysis. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2018;44:182–186. doi: 10.1016/j.phymed.2018.02.008. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Yang J., Wang Y. Discrimination and identification of Q-markers based on 'Spider-web' mode for quality control of traditional Chinese medicine. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2018;44:98–102. doi: 10.1016/j.phymed.2017.12.034. [DOI] [PubMed] [Google Scholar]
- Kang T., Dou D., Xu L. Establishment of a quality marker (Q-marker) system for Chinese herbal medicines using burdock as an example. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2019;54:339–346. doi: 10.1016/j.phymed.2018.04.005. [DOI] [PubMed] [Google Scholar]
- Khandekar G., Kim S., Jagadeeswaran P. Zebrafish thrombocytes: Functions and origins. Advances in Hematology. 2012;2012:1–9. doi: 10.1155/2012/857058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Polachi N., Wang X., Chu Y., Wang Y., Tian M.…Liu C. A quality marker study on salvianolic acids for injection. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2018;44:138–147. doi: 10.1016/j.phymed.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Li Y., Zhang Y., Wang Y., Li Y., Yang F., Zhang P.…Liu C. A strategy for the discovery and validation of toxicity quality marker of Chinese medicine based on network toxicology. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2019;54:365–370. doi: 10.1016/j.phymed.2018.01.018. [DOI] [PubMed] [Google Scholar]
- Liu C.X. Recognizing healthy development of Chinese medicine industry from resources- quality-quality markers of Chinese medicine. Chinese Traditional and Herbal Drugs. 2016;47(18):3149–3154. [Google Scholar]
- Liu C.X. Construction of traceability system of Chinese materia medica product quality based on quality marker of Chinese materia medica. Chinese Traditional and Herbal Drugs. 2017;48(18):3669–3676. [Google Scholar]
- Liu C.X., Chen S.L., Xiao X.H., Zhang T.J., Hou W.B., Liao M.L. A new concept on quality marker of Chinese materia medica: Quality control for Chinese medicinal products. Chinese Traditional and Herbal Drugs. 2016;47(9):1443–1457. [Google Scholar]
- Liu W.L., Zhang X.L., Fan S.Q., Zhu J.P., Liang H.H., Zhang Y.T., Xiao X.Q. A novel concept of Q-markers: Molecular connectivity index. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2018;45:36–40. doi: 10.1016/j.phymed.2018.03.015. [DOI] [PubMed] [Google Scholar]
- Nie C., Zhang F., Ma X., Guo R., Zhou S., Zhao L.…Wang Z. Determination of quality markers of Xuezhiling tablet for hyperlipidemia treatment. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2018;44:231–238. doi: 10.1016/j.phymed.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Qi Y., Zhao X., Liu H., Wang Y., Zhao C., Zhao T.…Wang Y.i. Identification of a quality marker (Q-Marker) of Danhong Injection by the zebrafish thrombosis model. Molecules (Basel, Switzerland) 2017;22(9):1443. doi: 10.3390/molecules22091443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng J., Meng Q., Yang Z., Guan J., Zhao Y., Zhang J.…Wang Y. Identification of cryptotanshinone from Tongmai to inhibit thrombosis in zebrafish via regulating oxidative stress and coagulation cascade. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2020;76 doi: 10.1016/j.phymed.2020.153263. [DOI] [PubMed] [Google Scholar]
- Wang X., Wu Q.-n., Wu Y., Wu C., Yue W., Liang Q. Determination of seven phenolic compounds in Rhizoma Sparganii by RP-HPLC. Journal of Chromatographic Science. 2013;51(4):371–375. doi: 10.1093/chromsci/bms150. [DOI] [PubMed] [Google Scholar]
- Wang X., Wu Q., Wu Y., Chen G., Yue W., Liang Q. Response surface optimized ultrasonic-assisted extraction of flavonoids from Sparganii Rhizoma and evaluation of their in vitro antioxidant activities. Molecules (Basel, Switzerland) 2012;17(6):6769–6783. doi: 10.3390/molecules17066769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wu Y., Chen G., Yue W., Liang Q., Wu Q. Optimisation of ultrasound assisted extraction of phenolic compounds from Sparganii Rhizoma with response surface methodology. Ultrasonics Sonochemistry. 2013;20(3):846–854. doi: 10.1016/j.ultsonch.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Weyand A.C., Shavit J.A. Zebrafish as a model system for the study of hemostasis and thrombosis. Current Opinion in Hematology. 2014;21(5):418–422. doi: 10.1097/MOH.0000000000000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Zhang H., Fan S., Zhang Y., Yang Z., Fan S.…Zhang Y. Quality markers based on biological activity: A new strategy for the quality control of traditional Chinese medicine. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2018;44:103–108. doi: 10.1016/j.phymed.2018.01.016. [DOI] [PubMed] [Google Scholar]
- Ye J.i., Gao Y., Tian S., Su J., Zhang W. A novel and effective mode-switching triple quadrupole mass spectrometric approach for simultaneous quantification of fifteen ginsenosides in Panax ginseng. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2018;44:164–172. doi: 10.1016/j.phymed.2018.02.007. [DOI] [PubMed] [Google Scholar]
- Zhang F., Zhang Y.u., Li X., Zhang S., Zhu M., Du W., Xiao X. Research on Q-markers of Qiliqiangxin capsule for chronic heart failure treatment based on pharmacokinetics and pharmacodynamics association. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2018;44:220–230. doi: 10.1016/j.phymed.2018.03.003. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-T., Xiao M.-F., Deng K.-W., Yang Y.-T., Zhou Y.-Q., Zhou J.…Liu W.-L. Novel mathematic models for quantitative transitivity of quality-markers in extraction process of the Buyanghuanwu decoction. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2018;45:68–75. doi: 10.1016/j.phymed.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Zhong Y.i., Zhu J., Yang Z., Shao Q., Fan X., Cheng Y. Q-marker based strategy for CMC research of Chinese medicine: A case study of Panax notoginseng saponins. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2018;44:129–137. doi: 10.1016/j.phymed.2018.01.023. [DOI] [PubMed] [Google Scholar]
- Zhu X.Y., Liu H.C., Guo S.Y., Xia B.O., Song R.S., Lao Q.C., Li C.Q. A Zebrafish thrombosis model for assessing antithrombotic drugs. Zebrafish. 2016;13(4):335–344. doi: 10.1089/zeb.2016.1263. [DOI] [PubMed] [Google Scholar]